Abstract
We previously showed that treatment with tadalafil, a long-acting phosphodiesterase-5a (PDE5a) inhibitor, effectively prevented adverse left ventricular (LV) remodeling of the infarcted heart. We hypothesized that short-hairpin RNA (shRNA) therapy targeting PDE5a would simulate the effects of pharmacological intervention for treatment of postinfarction LV remodeling and dysfunction. Experimental model of myocardial infarction was developed in female mice by permanent ligation of left coronary artery. Immediately after that, an adenoviral vector encoding for shRNA sequence targeting PDE5a (Ad-shPDE5a) was injected intramyocardially, which specifically inhibited PDE5a in the heart. Four weeks later, Ad-shPDE5a treated mice showed significant mitigation of the left ventricle (LV) dilatation and dysfunction as indicated by smaller LV cavity and more preserved ejection fraction and fractional shortening. Infarction size and fibrosis were significantly reduced in Ad-shPDE5a-treated mice. Additionally, more salvaged cardiomyocytes, significantly reduced collagen contents, and higher blood vessel density were observed in Ad-shPDE5a-treated mice. The cytoprotective effects of Ad-shPDE5a were demonstrated in vitro in Ad-shPDE5a transfected cardiomyocytes cultured under oxygen glucose deprivation. Among downstream mediators of PDE5a signaling, cyclic GMP (cGMP) and cGMP-dependent protein kinase G (PKG) were activated with concomitant reduction in caspase-3 activity. However, no significant change in PKA and cAMP activities were observed in Ad-shPDE5a-treated hearts. Inhibition with shRNA improved cardiac remodeling and dysfunction by reducing infarction size and cardiac fibrosis and increased cGMP and PKG activity. These findings suggest that PDE5 inhibition with Ad-shPDE5a is a novel approach for treatment of myocardial infarction.
Keywords: gene therapy, heart, infarction, myocardial
chronic heart failure is a leading cause of mortality and morbidity, worldwide. At present, patients with chronic heart failure have poor prognosis (21). The most common cause of heart failure is myocardial infarction induced remodeling of the left ventricle (LV), which is characterized by LV dilatation and diminished cardiac performance (6, 12). Therefore, it is essential to develop therapies to effectively inhibit LV remodeling for better clinical outcome in patients with myocardial infarction.
Phosphodiesterases (PDEs) belong to a complex and diverse superfamily of 11 structurally related gene families (PDE1–PDE11) (8, 27). At least 22 genes encoding for more than 50 different PDE isoforms have been identified, and these PDE variants are selectively expressed in different tissues, cells, and subcellular compartments (11). PDE5a was first found in platelets and has three isoforms, PDE5a1, PDE5a2, PDE5a3, which differ only in the initial portion of exon 1 in the NH2 terminus with no known functional differences between these isoforms (19). PDE5a is generally considered to be a cytosolic protein. In rodents, relatively high levels of PDE5a mRNA have been localized to vascular smooth muscle, heart, placenta, skeletal muscle, pancreas, brain, liver, gastrointestinal tissues, and lung (25, 33). The cyclic nucleotides cAMP and cGMP both play central role in cardiovascular regulation, influencing function, gene expression, and morphology (19). Cardiac cGMP is synthesized by activation of soluble guanylate cyclase by nitric oxide (NO) and by the binding of natriuretic peptides to their receptors (NPR-1 and NPR-2) (7, 35). cGMP is hydrolyzed by members of PDE family of enzymes (4) of which PDE5a acts more specifically (27). PDE5a plays an important role in the pulmonary vasculature where its inhibition benefits patients with pulmonary hypertension (3). In the heart, PDE5a signaling appears to be compartmentalized, and its inhibition is cardioprotective against blunt acute adrenergic contractile stimulation (32), ischemia-reperfusion injury (1, 31), and suppresses chronic hypertrophy and dysfunction attributable to pressure-overload (34). Myocardial PDE5a expression has been shown to increase in patients with advanced heart failure and contributes to LV remodeling subsequent to myocardial infarction (28). Previous studies have shown that pharmacological inhibition of PDE5 in the heart with sildenafil treatment significantly attenuated infarct size expansion and preserved global cardiac function (30). Given the short biological half-life of sildenafil, we have previously shown that one-time administration of long-acting member of PDE5 inhibitors, tadalafil, had cytoprotective effects spanned over 36 h and attenuated infarct size expansion (1). Similarly, treatment of stem cells with tadalafil protected the cells against oxidant stress in vitro and provided the cells a longer window of protection posttransplantation in the infarcted heart (15). We therefore determined the feasibility of continuous inhibition of PDE5a enzyme and study its beneficial effects in prevention of postinfarction cardiac remodeling and dysfunction. We hypothesized that prolonged inhibition of PDE5a through short hairpin RNA (shRNA) interference via adenoviral vectors (Ad) would relieve cardiac remodeling and dysfunction following myocardial infarction.
MATERIALS AND METHODS
Recombinant adenoviral shRNA vectors.
PDE5a-specific shRNA was designed based on rat PDE5a sequence; two complementary oligonucleotides of PDE5a were: for 5′-gatccgg agcagcagtcattggaagtcgaaacttccaatgactgctgctccttttttg-3′ and rev5′-aattcaaaaaaggag cagcagtcattggaagtttcgacttccaatgactgctgctccg-3′. The ds oligonucleotides were ligated into RNAi-Ready pSIREN-DNR-DsRed-Express Vector (Clontech, Mountain View, CA). Then the Donor Vector was inserted into Adeno-X LP CMV Vector (Clontech) to be constructed as the recombinant pLP-Adeno-X PDE5a shRNA (Ad-shPDE5a). The Ad vector without therapeutic shRNA was prepared as control adenovirus (Ad-Null). These replication-deficient Ad-shPDE5a and Ad-Null vectors were propagated in HEK-293 cells using Dulbecco's modified Eagle's medium (DMEM; Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS, Sigma). At the stipulated time, the supernatant from 293 cells or 293 cells were collected for adenovirus purification with Adeno-X Maxi Purification Kit (Clontech).
Preparation and transduction of cultured cardiomyocytes.
Cardiomyocytes were isolated from 1-day-old neonatal C57BL/6J mice as reported previously (37). The cardiomyocytes were plated on laminin-coated dishes and incubated in DMEM containing 10% FBS at 37°C for 24 h. The cells were exposed to infection medium containing 1 × 108 Ad-vector particles/ml for 16 h, followed by maintenance in normal medium for 56 h. The cells were used for biochemical analyses. Proteins extracted from cardiomyocytes were used for Western blotting and ELISA.
Ad-shPDE5a transduction and cardiomyocytes survival.
For cardiomyocyte survival studies, the cells were transduced with Ad-shPDE5a or Ad-Null or treated with DMEM for 16 h followed by maintenance in the viral vector-free DMEM for 56 h. For oxygen and glucose deprivation (OGD), cell culture medium was replaced with glucose- and serum-free DMEM, and the cells were kept in airtight anoxia chamber (InVivo 500, Ruskinn Life Science) saturated with 95% N2/5% CO2. After 4 or 8 h of incubation, the supernatant from each Petri dish was removed for lactate dehydrogenase (LDH) leakage using the Homogeneous Integrity Assay kit (Promega, Madison, WI) while the cells with 8 h incubation were fixed in 4% paraformaldehyde for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL).
Animal experiments.
This study was approved by Institutional Animal Research Committee and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996). The experimental animal model of acute coronary artery ligation was developed in 10-wk-old male C57BL/6J mice (Harlan) as previously described (23). Immediately after coronary artery ligation, 1 × 1010 Ad-shPDE5a particles were injected at four or five sites per mouse heart along the anterior and posterior LV wall (17). Ad-Null or DMEM was injected in the same manner in the control animals. In sham-operated mice (n = 6 animals), the suture was only passed but not tied. The animals were followed up for 1 wk (n = 6 animals per group) and 4 wk (n = 12 animals per group).
Heart function studies.
Echocardiograms were recorded 4 wk postmyocardial infarction using an echocardiographic system (HDI-5000 SONOS CT) equipped with Compact Linear Array probe CL10-5. The heart was imaged in M-Mode, and recordings were obtained from parasternal long-axis view at papillary muscles level.
Histological studies.
After the heart function studies, the animals were euthanized and their hearts were removed. For measurement of infarction size and area of fibrosis, the heart was arrested in diastole by intravenous injection of cadmium chloride, excised, and fixed in formalin. The heart was then excised, cut transversely, embedded in paraffin, and used for histological studies. Tissue sections of 4 μm thickness were cut and used for hematoxylin-eosin and Masson's trichrome staining to visualize muscle architecture and LV wall thickness as described earlier (36). Infarct size was defined as the sum of the epicardial and endocardial infarct circumference divided by the sum of the total LV epicardial and endocardial circumferences using computer-based planimetry with Image J analysis software (version 1.6065, NIH). Fibrosis and total LV area of each image were measured using the Image-Pro Plus (Media Cybernetics).
Immunohistochemical studies.
Blood vessel density was assessed as previously described (2). Briefly, deparaffinized 4 μm thick sections were immunostained using specific antibodies for von Willebrand factor-VIII (vWF) or smooth muscle actin (SMA) (all from DAKO, Carpinteria, CA) and detected by specific secondary antibody conjugated with Alexa Fluor 488 or 546 (Molecular Probes, Carlsbad, Invitrogen, CA). The number of blood vessels positive for vWF-VIII and SMA were counted in both infarct and peri-infarct regions. At least 5 high-power fields (HPF) (×400) in infarct or peri-infarct region of each animal (n = 4 animals per group) were randomly selected and counted. Blood vessel density was expressed as the number of vessels per HPF (×400). Blood vessel maturation was assessed by calculating SMA-positive blood vessels in relation to the vWF-VIII-positive vessels.
Apoptosis was evaluated using the TUNEL method with an In-Situ Cell Death Detection kit (Roche, Indianapolis, IN) according to the supplier's instructions. TUNEL was performed on the cultured cardiomyocytes or deparaffinized 4 μm thick sections. The degree of apoptotic cell death was determined by counting the total number of TUNEL-positive nuclei per HPF (×400). For double immunofluorescent labeling, tissue sections were first stained with desmin (Novus Biologicals, Littleton, CO) and then labeled with In-Situ Cell Death Detection kit followed by Alexa Fluor 488. 4′,6-Diamidino-2-phenylindole (DAPI) staining was performed to stain the nuclei.
Western blotting and ELISA.
Proteins extracted from the cultured cardiomyocytes or LV of the heart were electrophoresed on 14% polyacrylamide gel and transferred to polyvinylidene difluoride membranes as described earlier (2). The membranes were then probed with a primary antibody against PDE5a (Cell Signaling, Danvers, MA). Anti-PDE1a (Abcam), anti-phosphorylated glycogen synthase kinase (pGSK)3β, anti-phosphorylated extracellular signal-regulated kinase (pERK)1/2 (Cell Signaling). Three hearts from each group were subjected to the blotting. The blots were visualized using enhanced chemiluminescence with Amersham ECL plus (GE Healthcare Biosciences), and the signals were quantified by densitometry. Actin (Santa Cruz Biotechnology, Santa Cruz, CA) served as the loading control.
Levels of cyclic GMP (cGMP) or cGMP-dependent protein kinase G (PKG) activity in the cells and LV were assayed with cyclic GMP Complete Kit or cyclic GMP dependent protein kinase Assay Kit (CycLex, Nagano, Japan). Three to five hearts from each group were used for these assays.
PKA activity assay.
PKA activity assay was performed with PepTag assay for nonradioactive detection of cAMP-dependent protein kinase (Promega). Briefly, 5 × 106 cardiomyocytes were harvested with 0.5 ml of cold PKA extraction buffer, homogenized, and centrifuged to collect the lysate samples. PKA assay was performed by incubation of lysate samples with PKA-specific peptide substrate PepTag A1 Peptide at room temperature for 30 min per manufacturer's protocol. Phosphorylated peptide was separated on 0.8% agarose gel using 50 mM Tris·HCl (pH 8.0) as running buffer. Gel images were obtained with gel-doc imaging system and densitometry was performed with Image-J. Density of phosphorylated peptide represented the relative PKA activity.
cAMP immunoassay.
cAMP assay was performed with cAMP immunoassay kit from R&D systems. Briefly, a monoclonal antibody specific for cAMP was coated on 96-well plate. Samples were collected per manufacturer's protocol. cAMP present in the samples competed with a fixed amount of horseradish peroxidase-labeled cAMP for sites on the monoclonal antibody during incubation. Substrate solution was added to the wells to determine the bound enzyme activity. The color development was stopped, and the absorbance was read at 450 nm. The intensity of the color was inversely proportional to the concentration of cAMP in the sample. Serially diluted cAMP standards were assayed simultaneously and a standard curve was created for absolute quantifications of cAMP in the samples.
Statistical analysis.
Values are shown as means ± SE. Survival was analyzed using Kaplan-Meier method with log-rank Cox-Mantel method. The significance of differences was evaluated by t-tests or one-way ANOVA and Newman-Keul's multiple comparison test. Values of p < 0.05 were considered significant.
RESULTS
Effect of intramyocardial injection of Ad-shPDE5a in the hearts.
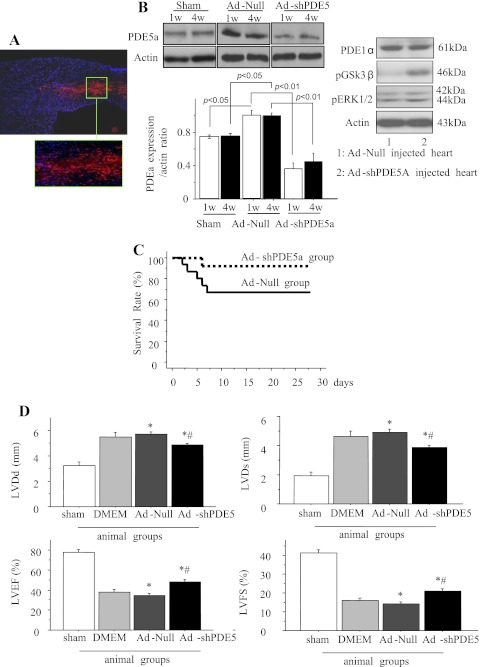
Histological sections were observed for DS-Red (red fluorescence) in Ad-shPDE5a-treated animals at the site of injection. Figure 1A shows a typical injection site indicated by red fluorescence on day 7 in Ad-shPDE5a-treated animal heart. The nuclei were visualized by DAPI stain (blue). Western blot studies showed that PDE5a expression was significantly increased in Ad-Null treated hearts following infarction at 1 wk and 4 wk compared with the sham-operated control animal hearts (p < 0.05, n = 7 each at 1 wk and 4 wk). Ad-shPDE5a treatment significantly abrogated PDE5a expression in the infarcted hearts both 1 wk and 4 wk after treatment compared with Ad-Null-treated animal hearts (P < 0.01, Fig. 1B). Specificity of Ad-shPDE5a treatment was confirmed by assessing expression of PDE1a isoform in the animal hearts with their respective treatment, which did not show any expression change (Fig. 1B). Western blot analysis of the cardiac tissue samples showed that Ad-shPDE5a treatment did not alter phosphorylation of ERK in the infarcted heart compared with Ad-Null-treated animal hearts. However, there was significant change in GSK3β activation (Fig. 1B).
Fig. 1.
Expression of phosphodiesterase-5a (PDE5a) in vivo, survival curves, and left ventricular (LV) heart function in various treatment groups of animals. A: a typical injection site in adenoviral vector encoding for short hairpin RNA sequence targeting PDE5a (Ad-shPDE5a)-injected animal heart in the peri-infarct region identified by DS Red (red fluorescence) on day 7 after treatment. 4′,6-Diamidino-2-phenylindole (DAPI) was used to visualize the nuclei (original magnification ×10). Area in the green box has been magnified for clarity. B: Western blot showing PDE5a expression in the LV of infarcted hearts at 1 and 4 wk after treatment with adenoviral vector without therapeutic shRNA (Ad-Null) or Ad-shPDE5a using sham-operated animals as a baseline control. Graph shows PDE5a expression increased significantly in Ad-Null-treated infarcted hearts compared with sham-operated heart. Ad-shPDE5a abrogated PDE5a expression both at 1 wk and 4 wk after treatment (P < 0.01 vs. both Ad-Null and sham-operated animals). No change in PDE1a was observed in the animal hearts with their respective treatment. C: survival curves from Ad-Null and Ad-shPDE5a treatment groups of animals. Compared with 67% in the Ad-Null-treated group, the survival rate was 92% in the Ad-shPDE5a-treated group (P = 0.11) D: echocardiographic data for LV geometry and function 4 wk posttreatment with Ad-Null and Ad-shPDE5a. LV diastolic diameter (LVDd), LV end-systolic diameter (LVDs), LV ejection fraction (LVEF), and LV fractional shortening (LVFS) were significantly preserved in Ad-shPDE5a-treated animal hearts. Values are means ± SE. *P < 0.05 vs. DMEM-treated mice; #P < 0.05 vs. sham-operated mice.
Four weeks after coronary artery ligation, five (33%) of the Ad-Null, three (37%) of DMEM, and one (8%) of the Ad-shPDE5a-treated mice died. The survival rate 4 wk postmyocardial infarction was 67% in Ad-Null-treated and 92% in Ad-shPDE5a-treated animals (p = 0.11, Fig. 1C). Echocardiography at 4 wk posttreatment showed that compared with sham-operated mice, Ad-Null-treated mice had enlargement of the LV cavity and reduced cardiac function, as indicated by increased LV end-systolic and end-diastolic diameter and reduced LV percent fractional shortening and ejection fraction (Fig. 1D). All of these functional parameters showed modest but significant preservation in Ad-shPDE5a-treated mice (Fig. 1D), thus suggesting that Ad-shPDE5a mitigated postmyocardial infarction remodeling and cardiac dysfunction through PDE5a inhibition. There was no significant difference in cardiac function 4 wk after myocardial infarction between the Ad-Null- and DMEM-treated mice, indicating a negligible effect of Ad-Null treatment on cardiac function.
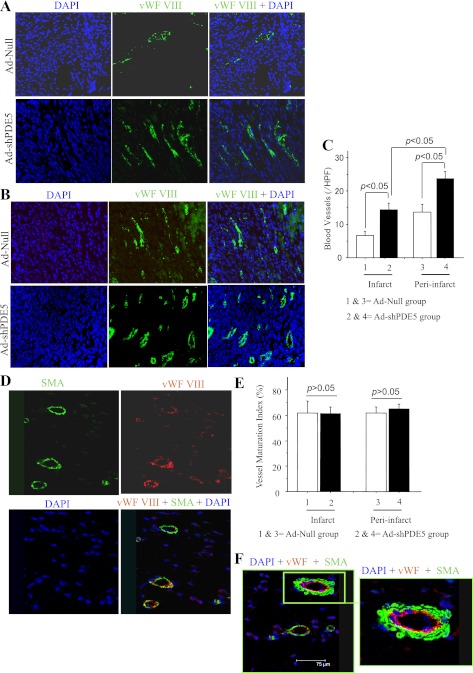
Histological sections from animals harvested on day 3 showed significant reduction in the number of TUNEL+ cells in Ad-Null-treated group 1 as compared Ad-shPDE5a-treated animal hearts (P < 0.05) (Fig. 2A). Ad-Null treated mice 4 wk after myocardial infarction showed marked LV dilatation with a thin infarcted segment, while Ad-shPDE5a-treated mice showed smaller LV cavities and thicker infarcted segments (Fig. 2B). The viable cardiomyocytes were identified in the LV scar tissue of Ad-shPDE5a-treated mice. Compared with Ad-Null and DMEM groups, abundant islands of viable cardiomyocytes were observed in the center of the infarct in Ad-shPDE5a-treated animals (Fig. 2C). Histological slides stained with Masson's trichrome were analyzed for cardiomyocytes and collagen contents in the infarct area under low magnification (×200) using Image-Pro plus 6.0 software (Fig. 2C). The area of the infarct had more residual cardiomyocytes, and collagen contents were significantly reduced in the Ad-shPDE5a-treated animal groups compared with the Ad-Null-treated animal group (Fig. 2D). The size of the infarct was significantly reduced in Ad-shPDE5a-treated animals (25.4 ± 2.9%, P < 0.05) compared with Ad-Null (42.0 ± 3.2%) and DMEM groups (43.3 ± 2.6%) (Fig. 2E). Severe fibrosis of the myocardium was observed in Ad-Null (34.6 ± 2.2%) and DMEM groups (35.1 ± 2.5%) compared with which, Ad-shPDE5a-treated animals had less fibrosis (26.5 ± 1.9%, P < 0.05) (Fig. 2E). The number of vWF-positive blood vessels in the infarct and peri-infarct regions was also greater in Ad-shPDE5a-treated mice (14.3 ± 2.0 and 23.6 ± 2.1, P < 0.05) than Ad-Null-treated mice (6.6 ± 1.2 and 13.7 ± 2.4) (Fig. 3, A–C). Double fluorescent immunostaining for vWF-VIII and SMA revealed that most of the newly formed vessels were mature, having an SMA covering; no significant difference in maturation index was found between groups (Fig. 3, D–F). Figure 3F shows a typical blood vessel stained for endothelial (red) and α-SMA expression (green) in the normal myocardium.
Fig. 2.
Gross morphology and histology of hearts in different treatment groups of animals at 4 wk after treatment. A: terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining of histological section on day 3 after treatment. The number of TUNEL+ cells was significantly lower in Ad-shPDE5A-treated group 2 compared with Ad-Null-treated animal hearts. B: transverse ventricular sections of mice hearts treated with DMEM, Ad-Null, and Ad-shPDE5a. Sections were stained with Masson's trichrome. Note the smaller LV cavity, shorter infarct segments, and thicker infarct walls in Ad-shPDE5a-treated heart. C: salvaged cardiomyocytes (residual cardiomyocytes) in the infarcted wall of hearts from different treatment groups stained with Masson's trichrome. Islands of abundant viable cardiomyocytes were observed in the infarcted wall of Ad-shPDE5a-treated mice. Magnification ×1000. D: infarct size and fibrosis were significantly reduced in Ad-shPDE5a-treated mice. Values are means ± SE. *P < 0.05 vs. Ad-Null-treated mice; #P < 0.05 vs. DMEM-treated mice.
Fig. 3.
Blood vessel density and maturation index in infarct and peri-infarct regions of heart from different treatment groups. Photomicrographs showing von Willebrand factor (vWF)-positive vessels (green) in infarct (A) and peri-infarct areas (B) at 4 wk after treatment. DAPI, 4′,6-diamidino-2-phenylindole. C: graph comparing the number of vWF-positive blood vessels between groups. *P < 0.05 vs. Ad-Null-treated mice in infarct area; #P < 0.05 vs. Ad-Null-treated mice in peri-infarct area. HPF, high-power field (×400). D, E: representative photomicrographs of tissue sections from Ad-shPDE5a-treated heart immunolabeled for vWF-VIII (red) and α-smooth muscle actin (SMA, green). Graph for blood vessel maturation index showing insignificant change in maturation index between Ad-shPDE5a- and Ad-Null-treated animal hearts in both infarct and peri-infarct regions. Values in the graph are means ± SE. F: double fluorescence immunostaining of the LV in the normal myocardium for vWF-VIII (red) and α-SMA (green) expression showing a typical blood vessel depicting endothelial (red) and SMA (green) staining (original magnification ×20).
Cytoprotective effects of Ad-shPDE5a.
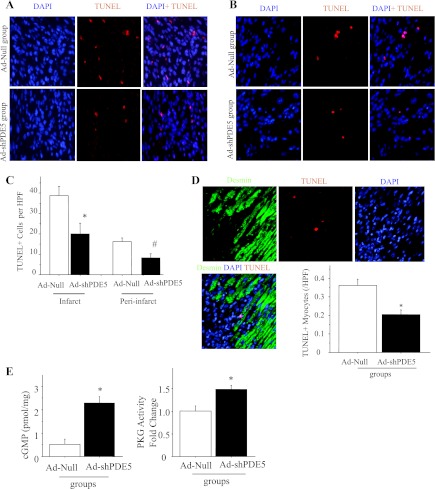
To elucidate the mechanisms responsible for protection by Ad-shPDE5a, we evaluated cell apoptosis in infarct and peri-infarct areas 1 wk postmyocardial infarction i.e., 1 wk after adenovirus injection. Immunohistochemical evaluation of TUNEL showed that Ad-shPDE5a-treated animals had smaller numbers of apoptotic cells in infarct and peri-infarct areas compared with Ad-Null group, suggesting Ad-shPDE5a treatment reduced the incidence of apoptosis (Fig. 4, A–C). Further analysis using double immunofluorescent labeling for desmin and TUNEL revealed that Ad-shPDE5a treatment significantly reduced the incidence of TUNEL positivity in the cardiomyocytes in the peri-infarct area (Fig. 4D). cGMP and PKG activity was significantly upregulated in the LV of mice 1 wk after treatment with Ad-shPDE5a, which implied that Ad-shPDE5a upregulated cGMP and PKG activity through silencing PDE5a expression (Fig. 4E). The higher cGMP contributed substantially to the beneficial effects against LV remodeling.
Fig. 4.
TUNEL positivity and cyclic GMP/cGMP-dependent protein kinase G (cGMP/PKG) activity in the infarct and peri-infarct areas. A, B: photomicrographs showing TUNEL-positive cells (red fluorescence) in infarct and peri-infarct areas. C: graph comparing TUNEL positivity between various treatment groups. Values are means ± SE. *P < 0.05 vs. Ad-Null-treated mice in infarct area; #P < 0.05 vs. Ad-Null-treated mice in peri-infarct area. HPF, ×400. D: photomicrographs of tissue sections from Ad-Null-treated heart probed by TUNEL (red fluorescence) and immunostained for desmin expression (green fluorescence). Graphs showing the incidences of TUNEL positivity evaluated in cardiomyocytes. E: myocardial cGMP and PKG activity assay 1 wk after treatment with Ad-shPDE5a and Ad-Null treatment. Values are means ± SE. *P < 0.05 vs. Ad-Null-treated mice. HPF ×400.
Effect of Ad-shPDE5a treatment on cultured cardiomyocytes.
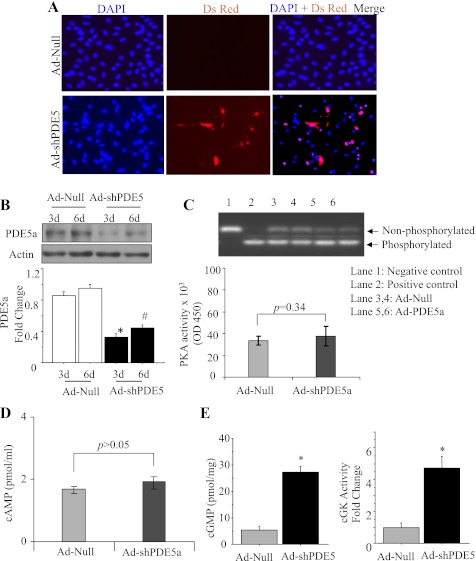
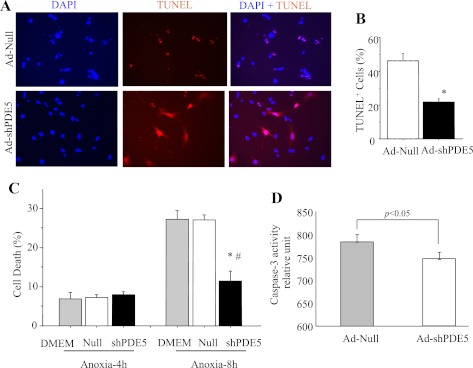
Successful transduction of cardiomyocytes with Ad-shPDE5a was performed for abrogation of PDE5a expression (Fig. 5A). Western blot showed that 16 h transduction with Ad-shPDE5a significantly inhibited PDE5a expression in the cardiomyocytes until 6 days of observation (Fig. 5B). We performed PKA and cAMP activity assays on cardiomyocytes transduced with Ad-shPDE5a using Ad-Null transduced cardiomyocytes as control (Fig. 5, C and D). Activity assays showed that PKA (P = 0.34 vs. control) and cAMP (P > 0.05 vs. control) activities changed insignificantly in the cardiomyocytes with PDE5a inhibition compared with the control cells. However, ELISA-based activity assay showed that cGMP and PKG activities were markedly increased in Ad-shPDE5a-treated cardiomyocytes on day 3 after transduction (Fig. 5E). The cytoprotective effects of these molecular changes were observed by subjecting the cells to OGD for 8 h (Fig. 6A). The number of TUNEL-positive cells significantly increased in Ad-Null-treated cardiomyocytes compared with Ad-shPDE5a-treated myocytes after 4 h and 8 h OGD (Fig. 6, A and B). These results were substantiated by LDH release assay from the cells, which was significantly increased in Ad-Null-treated cells, thus revealing their increased susceptibility to damage compared with Ad-shPDE5a-treated cells under 8 h OGD (Fig. 6C). Caspase-3 activity assay was performed on the cardiomyocytes transduced with Ad-shPDE5a. We observed significantly higher caspase-3 activity in Ad-Null transduced cardiomyocytes compared with Ad-shPDE5a transduced cells (Fig. 6D).
Fig. 5.
Genetic manipulation of cardiomyocytes with Ad-shPDE5a. A: the cultured cardiomyocytes transduced with Ad-shPDE5a (DsRed positive, red fluorescence) at 72 h after transduction. B: Western blot showing successful abrogation of PDE5a expression in cultured cardiomyocytes observed on day 3 and day 6 after transduction with Ad-ShPDE5a. Values plotted in the graph are means ± SE. *P < 0.05 vs. Ad-Null-treated group on day 3; #P < 0.05 vs. Ad-Null-treated group on day 6. C, D: activity assays showing insignificantly increased PKA and cAMP activity in the cardiomyocytes transduced with Ad-shPDE5a compared with Ad-Null treated cardiomyocytes. E: cGMP and PKG activity assays in cultured cardiomyocytes on day 3 after transduction with Ad-shPDE5a. Values are means ± SE. *P < 0.05 vs. Ad-Null-treated group.
Fig. 6.
Cytoprotective effects of Ad-shPDE5a on cultured cardiomyocytes. A: photomicrographs showing TUNEL positivity (red fluorescence) in different treatment groups of cardiomyocytes subsequent to 8 h exposure to oxygen and glucose deprivation (OGD). B: graphs showing TUNEL positivity after 8 h OGD and lactate dehydrogenase (LDH) release assay after 4 h and 8 h OGD. Values are means ± SE. *P < 0.05 vs. Ad-Null treated group; #P < 0.05 vs. DMEM treated group. C: LDH release assay showing significantly higher LDH leakage from Ad-Null transduced and DMEM-treated cardiomyocytes cultured under lethal anoxia for 8 h. D: caspase-3 activity was significantly increased in Ad-Null transduced cardiomyocytes compared with Ad-shPDE5a transduced cardiomyocytes.
DISCUSSION
We provide the first evidence that postinfarction interference therapy with Ad-shRNA targeting PDE5a relieved the adverse effects on LV geometry and contractile function during the chronic stage. The major findings of the present study include: 1) shRNA targeting PDE5a consistently exerted its knock-down effect on PDE5a gene expression for up to 6 days of observation in vitro or 4 wk in vivo; 2) inhibition of PDE5a expression by Ad-shPDE5a upregulated cGMP and PKG activity in the cardiomyocytes in vitro as well as in the infarcted heart; 3) direct intramyocardial injection of Ad-shPDE5a improved LV function and reduced infarction size and cardiac fibrosis; and 4) antiapoptotic and cytoprotective effects of Ad-shPDE5a were observed by in vivo and in vitro experiments.
RNA interference is an innate biological phenomenon that has evolved during the mammalian evolution (14). In the biological system, RNA interference is critical for transient and long-term abrogation of protein expression. It is achieved by loading the RNA interference silence complex with a short single-stranded antisense RNA that is complementary to a target mRNA (13). Instead of using small interfering RNA fragments, which possess only short-term stability in vivo, we constructed a PDE5a-specific shRNA adenoviral vector. The plasmid activity of shRNA has been observed to continue from 1 to 4 wk of observation in vivo (16). Based on our in vitro and in vivo experimental findings, we hypothesized that the choice of shRNA targeting of some critical genes might be a useful strategy for cardioprotective therapy. Compared with the contemporary therapeutic approaches for treatment of ischemic heart disease, our novel strategy based on inhibition of PDE5a offers the benefit of protective effects for extended time duration without the need for repeated treatment. The role of PDE5a in regulating smooth muscle tone in the systemic and pulmonary vasculature has long been recognized (22). Although PDE5a expression in the cardiomyocytes as well as in the heart under physiological conditions is low, more recent studies have established the importance of PDE5a in cardiac pathologies in response to perturbations in NO and natriuretic peptide signaling (38, 20). The LV of the patients with cardiomyopathy showed elevated PDE5 expression/activity and contributed to adverse LV remodeling after myocardial infarction (28). Our results showed that PDE5a expression in Ad-Null-treated infarcted animal hearts increased significantly compared with the sham-operated animal hearts. We therefore selected PDE5a as target gene for knock-down using Ad-shPDE5a. We observed abrogation of PDE5a in Ad-shPDE5a-treated hearts compared with Ad-Null and sham-operated animal hearts.
One important outcome of Ad-shPDE5a therapy was the significantly attenuation of fibrosis. Myocardial fibrosis subsequent to ischemic episode contributes to both systolic and diastolic dysfunction (18). Ad-shPDE5a mitigated LV remodeling, which was evident from the preserved LV dimensions during both systole and diastole. Ad-shPDE5a treatment also remarkably contributed to the preserved myocardial function by maintenance of the viability of the residual cardiomyocytes (26). Apoptosis is the consequence of a genetically determined cell death program that can be initiated by a number of stimuli such as growth factor withdrawal, signaling through apoptotic receptors, or cell-damaging stress (29). TUNEL combined with immunofluorescence showed that Ad-shPDE5a treatment significantly reduced apoptosis of cardiomyocytes in peri-infarct and infarct areas. The antiapoptotic and cytoprotective effects of Ad-shPDE5a via shRNA interference were substantiated by our in vitro study results. These observations were consistent with the earlier report that pharmacological inhibition of PDE5a prevented cardiomyocyte apoptosis under ischemic stress (9, 15). We have provided convincing evidence that pharmacological inhibition of PDE5 with long-acting tadalafil significantly protected stem cells as well as cardiomyocytes (1, 15). One-time administration of tadalafil, a known pharmacological inhibitor of PDE5, effectively protected the heart against ischemic insult for 36–48 h, in terms of reduced infarct size and preserved hemodynamic parameters. Similarly, we have also shown that pharmacological inhibition of PDE5 with tadalafil initiated cGMP/PKG signaling, which was blocked by pretreatment with PKG1 blockers KT-5823 or K-252a (15). Cardiac cGMP is selectively hydrolyzed by enzymatic activity of PDE5a (27). PDE inhibition prevents the breakdown of cGMP into 5′-GMP that leads to cGMP accumulation. This strategy is currently being exploited as a novel strategy to augment cGMP signaling in cardiovascular therapeutics to prevention of cardiac remodeling and attenuation of cardiac fibrosis (5). PKG is a serine/threonine protein kinase and is one of the major intracellular receptors for cGMP. It has been reported that cGMP/PKG pathway has antiapoptotic effect on the cardiomyocytes (10). Accumulation of cGMP in response to PDE5a inhibition counters the ill effects of adrenergic stimulation and prevents cardiomyocyte hypertrophy and pressure-induced remodeling of the heart (34). Furthermore, there is mounting evidence in the literature that the positive ionotrophic effects of adrenergic stimulation on the heart are countered by cGMP and are mediated via reduction in Ca2+ flux, shortening of action potential duration, and inhibition of Ca2+-dependent action potentials (24). During the present study, PDE5a enzyme activity was significantly inhibited in the LV after Ad-shPDE5a treatment, which was accompanied by strong elevation of cGMP and PKG activity. These molecular changes substantially reduced the incidence of cardiomyocyte apoptosis in Ad-shPDE5a-treated animal hearts. Besides antiapoptotic activity, increased blood vessel density was also observed in response to Ad-shPDE5a treatment, which was necessary to support the surviving cardiomyocytes.
Although treatment with Ad-shPDE5a produced encouraging results, the study has some limitations. First, given the massive loss of cardiomyocytes during the myocardial infarction, gene therapy alone may be insufficient to achieve myocardial repair process. Therefore, it would be prudent to combine Ad-shPDE5a treatment with stem cell transplantation to support myocardial regeneration. Additionally, we and other research groups have previously provided convincing evidence that pharmacological inhibition of PDE5a by treatment with tadalafil and sildenafil is cardioprotective (1, 9, 15, 30). Future studies are warranted to directly compare PDE5a inhibition using pharmacological intervention vs. Ad-shPDE5a in the same experimental settings for cytoprotective and cardioprotective effects. Besides the antiapoptotic effect, the effect of Ad-shPDE5A treatment on cardiomyocyte hypertrophy should be determined. Moreover, future studies would be required to determine the long-term benefits of the treatment. Lastly, although we found enhanced blood vessel density in response to our therapeutic intervention, we did not determine the functional status of the newly formed blood vessels. In conclusion, postinfarction PDE5a inhibition through shRNA interference successfully attenuated infarction size expansion and cardiac fibrosis via antiapoptotic actions of cGMP/PKG signaling. These findings imply a novel strategy for the treatment of patients with myocardial infarction.
GRANTS
This work was supported by National Institutes of Health Grants R37-HL-074272, HL-095375, and HL-087246 (to M. Ashraf) and HL-087288, HL-089535, and HL-106190-01 (to H. K. Haider).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Ahmad N, Wang Y, Ali AK, Ashraf M. Long-acting phosphodiesterase-5 inhibitor, tadalafil, induces sustained cardioprotection against lethal ischemic injury. Am J Physiol Heart Circ Physiol 297: H387–H391, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed RP, Haider KH, Shujia J, Afzal MR, Ashraf M. Sonic Hedgehog gene delivery to the rodent heart promotes angiogenesis via iNOS/netrin-1/PKC pathway. PLoS One 5: e8576, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldashev AA, Kojonazarov BK, Amatov TA, Sooronbaev TM, Mirrakhimov MM, Morrell NW, Wharton J, Wilkins MR. Phosphodiesterase type 5 and high altitude pulmonary hypertension. Thorax 60: 683–687, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58: 488–520, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Boerrigter G, Lapp H, Burnett JC. Modulation of cGMP in heart failure: a new therapeutic paradigm. Handb Exp Pharmacol: 485–506, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourassa MG, Gurne O, Bangdiwala SI, Ghali JK, Young JB, Rousseau M, Johnstone DE, Yusuf S. Natural history and patterns of current practice in heart failure. The Studies of Left Ventricular Dysfunction (SOLVD) Investigators. J Am Coll Cardiol 22: 14A–19A, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Cerra MC, Pellegrino D. Cardiovascular cGMP-generating systems in physiological and pathological conditions. Curr Med Chem 14: 585–599, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 76: 481–511, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Das A, Xi L, Kukreja RC. Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J Biol Chem 280: 12944–12955, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Fiedler B, Feil R, Hofmann F, Willenbockel C, Drexler H, Smolenski A, Lohmann SM, Wollert KC. cGMP-dependent protein kinase type I inhibits TAB1-p38 mitogen-activated protein kinase apoptosis signaling in cardiac myocytes. J Biol Chem 281: 32831–32840, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Francis SH, Turko IV, Corbin JD. Cyclic nucleotide phosphodiesterases: relating structure and function. Prog Nucleic Acid Res Mol Biol 65: 1–52, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Gheorghiade M, Sopko G, De Luca L, Velazquez EJ, Parker JD, Binkley PF, Sadowski Z, Golba KS, Prior DL, Rouleau JL, Bonow RO. Navigating the crossroads of coronary artery disease and heart failure. Circulation 114: 1202–1213, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Gou D, Narasaraju T, Chintagari NR, Jin N, Wang P, Liu L. Gene silencing in alveolar type II cells using cell-specific promoter in vitro and in vivo. Nucleic Acids Res 32: e134, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, Marion P, Salazar F, Kay MA. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature 441: 537–541, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Haider H, Lee YJ, Jiang S, Ahmed RP, Ryon M, Ashraf M. Phosphodiesterase inhibition with tadalafil provides longer and sustained protection of stem cells. Am J Physiol Heart Circ Physiol 299: H1395–H1404, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang M, Chan DA, Jia F, Xie X, Li Z, Hoyt G, Robbins RC, Chen X, Giaccia AJ, Wu JC. Short hairpin RNA interference therapy for ischemic heart disease. Circulation 118: S226–S233, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huusko J, Merentie M, Dijkstra MH, Ryhanen MM, Karvinen H, Rissanen TT, Vanwildemeersch M, Hedman M, Lipponen J, Heinonen SE, Eriksson U, Shibuya M, Yla-Herttuala S. The effects of VEGF-R1 and VEGF-R2 ligands on angiogenic responses and left ventricular function in mice. Cardiovasc Res 86: 122–130, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Jalil JE, Doering CW, Janicki JS, Pick R, Shroff SG, Weber KT. Fibrillar collagen and myocardial stiffness in the intact hypertrophied rat left ventricle. Circ Res 64: 1041–1050, 1989 [DOI] [PubMed] [Google Scholar]
- 19.Kass DA, Champion HC, Beavo JA. Phosphodiesterase type 5: expanding roles in cardiovascular regulation. Circ Res 101: 1084–1095, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Kass DA, Takimoto E, Nagayama T, Champion HC. Phosphodiesterase regulation of nitric oxide signaling. Cardiovasc Res 75: 303–314, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med 347: 1397–1402, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Lewis GD, Semigran MJ. Type 5 phosphodiesterase inhibition in heart failure and pulmonary hypertension. Curr Heart Fail Rep 1: 183–189, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Li L, Okada H, Takemura G, Kosai K, Kanamori H, Esaki M, Takahashi T, Goto K, Tsujimoto A, Maruyama R, Kawamura I, Kawaguchi T, Takeyama T, Fujiwara T, Fujiwara H, Minatoguchi S. Postinfarction gene therapy with adenoviral vector expressing decorin mitigates cardiac remodeling and dysfunction. Am J Physiol Heart Circ Physiol 297: H1504–H1513, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Lohmann SM, Fischmeister R, Walter U. Signal transduction by cGMP in heart. Basic Res Cardiol 86: 503–514, 1991 [DOI] [PubMed] [Google Scholar]
- 25.Loughney K, Hill TR, Florio VA, Uher L, Rosman GJ, Wolda SL, Jones BA, Howard ML, McAllister-Lucas LM, Sonnenburg WK, Francis SH, Corbin JD, Beavo JA, Ferguson K. Isolation and characterization of cDNAs encoding PDE5A, a human cGMP-binding, cGMP-specific 3′,5′-cyclic nucleotide phosphodiesterase. Gene 216: 139–147, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Miyagawa S, Sawa Y, Taketani S, Kawaguchi N, Nakamura T, Matsuura N, Matsuda H. Myocardial regeneration therapy for heart failure: hepatocyte growth factor enhances the effect of cellular cardiomyoplasty. Circulation 105: 2556–2561, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res 100: 309–327, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Pokreisz P, Vandenwijngaert S, Bito V, Van den Bergh A, Lenaerts I, Busch C, Marsboom G, Gheysens O, Vermeersch P, Biesmans L, Liu X, Gillijns H, Pellens M, Van Lommel A, Buys E, Schoonjans L, Vanhaecke J, Verbeken E, Sipido K, Herijgers P, Bloch KD, Janssens SP. Ventricular phosphodiesterase-5 expression is increased in patients with advanced heart failure and contributes to adverse ventricular remodeling after myocardial infarction in mice. Circulation 119: 408–416, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rich T, Watson CJ, Wyllie A. Apoptosis: the germs of death. Nat Cell Biol 1: E69–E71, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Salloum FN, Abbate A, Das A, Houser JE, Mudrick CA, Qureshi IZ, Hoke NN, Roy SK, Brown WR, Prabhakar S, Kukreja RC. Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. Am J Physiol Heart Circ Physiol 294: H1398–H1406, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Salloum FN, Chau VQ, Hoke NN, Abbate A, Varma A, Ockaili RA, Toldo S, Kukreja RC. Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase g-dependent generation of hydrogen sulfide. Circulation 120: S31–S36, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Senzaki H, Smith CJ, Juang GJ, Isoda T, Mayer SP, Ohler A, Paolocci N, Tomaselli GF, Hare JM, Kass DA. Cardiac phosphodiesterase 5 (cGMP-specific) modulates beta-adrenergic signaling in vivo and is down-regulated in heart failure. FASEB J 15: 1718–1726, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Stacey P, Rulten S, Dapling A, Phillips SC. Molecular cloning and expression of human cGMP-binding cGMP-specific phosphodiesterase (PDE5). Biochem Biophys Res Commun 247: 249–254, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med 11: 214–222, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Tremblay J, Desjardins R, Hum D, Gutkowska J, Hamet P. Biochemistry and physiology of the natriuretic peptide receptor guanylyl cyclases. Mol Cell Biochem 230: 31–47, 2002 [PubMed] [Google Scholar]
- 36.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res 98: 1414–1421, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Feng ZP, Kondo CS, Sheldon RS, Duff HJ. Developmental changes in the delayed rectifier K+ channels in mouse heart. Circ Res 79: 79–85, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Zhang M, Koitabashi N, Nagayama T, Rambaran R, Feng N, Takimoto E, Koenke T, O'Rourke B, Champion HC, Crow MT, Kass DA. Expression, activity, and pro-hypertrophic effects of PDE5A in cardiac myocytes. Cell Signal 20: 2231–2236, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]