Abstract
It is believed that increased transmural pressure exerts force on vascular smooth muscle cells (VSMCs) and triggers Ca2+ signaling as an initiating event responsible for the arteriolar myogenic response. However, the mechanisms linking the pressure increase to Ca2+ signaling are unclear. We have shown previously using atomic force microscopy (AFM) that mechanical force induces a VSMC contractile response when applied to single fibronectin (FN; Sun Z, Martinez-Lemus LA, Hill MA, Meininger GA. Am J Physiol Cell Physiol 295; C268–C278, 2008) focal adhesion sites. This current study seeks to determine whether application of force to single focal adhesions can cause a change in VSMC Ca2+. Experiments were performed in low passage (p3∼10) as well as in freshly isolated skeletal muscle arteriole VSMCs. AFM-attached microbeads (5 μm) were coated with FN or collagen type I (CN-I) or type IV (CN-IV) and placed on a VSMC for 20 min, resulting in formation of a focal adhesion between the cell and the microbead. In low passage VSMCs, mechanically pulling on the FN-coated beads (800∼3000 pN) did not induce a Ca2+ increase but did cause a contractile response. In freshly isolated VSMCs, application of an FN or CN-I-coated bead onto the cell surface induced global Ca2+ increases. However, these Ca2+ increases were not correlated with the application of AFM pulling force to the bead or with the VSMC contractile responses to FN-coupled pulling. Chelating cytosolic Ca2+ using BAPTA loading had no negative effect on the focal adhesion-related contractile response in both freshly isolated and low passage VSMCs, while the Rho-kinase inhibitor Y27632 abolished the micromyogenic response in both cases. These observations suggest that, in freshly isolated and cultured VSMCs, application of mechanical force to a focal adhesion does not invoke an acute global Ca2+ increase. On the other hand, our data support a role for Rho-linked signaling mechanism involved in mechanotransduction leading to focal contraction that is independent of the need for a global increase in VSMC Ca2+.
Keywords: extracellular matrix proteins, myogenic response, microcirculation, atomic force microscopy, mechanosensation, cell adhesion
ca2+-mediated interaction between actin and myosin filaments plays a central role in the contractile function of vascular smooth muscle cells (VSMCs). A rise of VSMC intracellular Ca2+ concentration ([Ca2+]i) has been observed before vasoconstriction in the myogenic response of arterioles (7, 26) and when single VSMCs are longitudinally stretched (9), and it has been widely assumed that the global Ca2+ elevation plays a central role in triggering myogenic response. The mechanisms that couple the Ca2+ increase to intraluminal pressure and/or stretch remain incompletely resolved. An important aspect of this problem is identification of the receptors that are linked to the signaling pathways that couple the mechanical stimulus with changes in Ca2+ handling.
Integrins are an important family of trans-membrane adhesion molecules that physically link the extracellular matrix (ECM) with the cytoskeleton. Integrin binding with ECM proteins leads to the formation of focal adhesions that can initiate multiple cell signaling cascades. The focal adhesion is one of the major functional units mediating cell-substrate interactions and a leading candidate site for mediating transmission of mechanical force into/or out of the cell (1, 2, 10, 15, 29). We have previously shown that α5β1- and αvβ3-integrins are involved in the myogenic response (25). Furthermore, we have demonstrated that application of force to a single fibronectin (FN) focal adhesion site will cause a mechanical response in VSMCs (30). A major goal of this investigation was to investigate the ability of focal adhesions to different ECM proteins to induce changes in VSMC Ca2+ in response to applied force at the focal adhesion site. It has been shown in isolated arteriolar VSMCs that binding of FN to α5β1-integrin can activate L-type voltage-gated calcium channels (L-VGCC) through activation of Src and PKC, while the binding of vitronectin (33) to αvβ3-integrin decreased the activity of L-VGCC (36). These observations have provided support for a link between integrins and modulation of the activity of L-VGCC, thereby providing one mechanism that could account for changes in cytosolic Ca2+ concentration.
In addition to the Ca2+-dependent contractile mechanisms, Ca2+-independent or -sensitization pathways also appear to play a role in the mechanotransduction process involved in generating the myogenic response (5, 8). Evidence for a Ca2+-independent pathway has come from studies of the relationship between VSMC calcium level and vessel myogenic tone in isolated mesenteric arteries (32), in cremaster muscle arterioles (20), in skeletal muscle resistance arteries (3), in hamster cheek pouch arterioles (6), and in cerebral artery (17). Recently, evidence (14, 21) has been provided that the level of phopsphorylated myosin light chain phosphatase targeting subunit 1 increased in pressurized rat cerebral resistance arteries, and the process was likely mediated by Rho kinase. These studies support an important role for the calcium-sensitization mechanism in the myogenic response. An integrin-based link to the Ca2+-sensitizaton pathway in VSMCs has not been explored in the context of the myogenic response, and it could provide another plausible signaling pathway linking mechanical signaling through the focal adhesion to the contractile apparatus.
In this study, we isolated VSMCs from skeletal muscle arterioles and determined the sensitivity of ECM-integrin focal adhesions to mechanical force by assessing changes in VSMC Ca2+ using confocal microscopy and localized cell mechanical behavior using atomic force microscopy (AFM).
MATERIAL AND METHODS
Cell isolation and cell culture.
All animals were handled in accordance with the guidelines of the Animal Care and Use Committee of the University of Missouri using approved protocols. Micro-VSMCs were isolated from the first order feed arteriole (100- to 150-μm diameter) of Sprague-Dawley rat cremaster skeletal muscles using previously described methods (36). Isolated VSM cells were plated in glass-bottomed dishes (Willco Wells) and were cultured in DMEM/F-12 that was supplemented with 20% FBS, 10 mM HEPES, 2 mM l-glutamine, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B. In cultured cell experiments, low passage VSM cells (passage 3∼10) were used and were maintained in media with 10% FBS. For fresh cell experiments, cells were maintained in a humidified incubator (Heraeus Instruments, Newtown, CT) with 5% CO2 at 37°C for 48 h before the AFM experiments. All cell culture reagents were purchased from Invitrogen (Carlsbad, CA).
Instruments.
A Bioscope AFM System (IVa, Santa Barbara, CA) was mounted on an Axiovert 100 TV inverted microscope (Carl Zeiss, Thornwood, NY). A Bioscope II System was mounted on an Fluoview 1000 confocal microscope (Olympus, Thornwood, NY). In addition, a separate system was employed to perform AFM measurements at 34°C. The thermally isolated system was composed of a MFP-3D AFM system (Asylum Research) coupled to an IX-81 microscope and an ultra-sensitive Evolve CCD camera (Photometrics) for simultaneous fluorescence imaging. The AFM data were collected and analyzed using Nanoscope (Digital Instruments) and Matlab (MathWorks, Natick, MA) software.
Protein coating of beads.
AFM probes with glass beads were purchased form Novascan (Ames, IA) with spring constant of 0.01 N/m. FN, collagen type I (CN-I) or type IV (CN-IV) or BSA (all at 1 mg/ml) were incubated with the AFM probes for 5 min at room temperature. The probes were then washed with Dulbecco's PBS (5×) to remove the unbound proteins. For application of high pulling force (2,000∼3,000 pN), bead-decorated probes (Novascan) with spring constant of 0.2 N/m were used.
AFM force application and measurement.
The application of force and measurement of force with the AFM were performed as described previously (30). Briefly, the AFM was operated in contact mode with the scan size set to 0.1 nm at room temperature, and VSMCs were incubated in DMEM/F12. After thermal equilibration, the protein-coated beads were brought into contact with the cell surface and were kept in a stable force neutral position on the cell surface for 20 min. The deflection set point was then manually adjusted to apply step increases of pulling force (800∼3,000 pN) to the VSMCs at the site of bead contact. The pulling force was generated by the bending of AFM cantilever and was calculated according to Hooke's law: F = d × k, where F is force in the unit of pN, d is cantilever deflection in nm, and k is the cantilever spring constant in pN/nm. Bead displacements were recorded for 4 min in the contact mode. Height data were recorded continuously and analyzed to obtain quantifiable changes in the magnitude of the micromechanical event of the VSMCs following the upward pull by AFM.
Fluo 4-AM and fura 2-AM loading of VSMCs.
Cells cultured on glass-bottomed tissue culture dishes were washed with loading buffer (150 mM NaCl, 5 mM KCl, 1 mM MgCl, 10 mM glucose, and 20 mM HEPES pH 7.4) twice and then incubated with 2.5 μM fluo 4-AM or fura 2-AM (Invitrogen) in loading buffer supplemented with 2% BSA and 0.01% Pluronic F-127 (BASF) for 25 min at room temperature on a rolling plate. Cells were then washed with loading buffer twice and then incubated in OPTI-MEMI (Invitrogen) for another 20 min to allow the deesterification of fluo 4-AM and fura 2-AM. The cells were then washed and incubated in DMEM/F12 or Hank's buffer.
Fluorescence imaging.
To measure the changes of Ca2+ signals in cells subjected to focal adhesion-associated forces, calcium fluorescence images were collected continuously, starting a few seconds before the AFM pulling was applied, and ended ∼50 s after the pulling. Fluo-4-loaded VSMCs was visualized using a confocal microscope (FV1000) equipped with a 488 laser, and the signal was collected at 510∼550 nm. The focal plane was set at the bottom of the cell. For AFM experiments at 34°C, the fluo-4-loaded VSMCs was visualized through an IX-81 microscope coupled with fluo 4 filter sets (Olympus) and an ultra-sensitive Evolve CCD camera. A Delta V Calcium Imaging System (Photon Technology International, London, Canada) was used for fura 2 imaging of cultured VSMCs; ×40 oil immersion objectives were used for all imaging experiments. Ratios of fura 2 images (340/380) were analyzed using the ImageMaster Software (PTI), fluo 4 images were initially analyzed using Fluoview software (Olympus) and were subsequently processed in Matlab Software (Mathworks) for correction of photobleaching and mapping of localized Ca2+ transients.
Reagents.
Human plasma FN, BAPTA-AM, and fluo 4-AM were purchased from Invitrogen, CN-I was purchased from BD Bioscience (San Jose, CA), and CN-IV was purchased from ABcam (Cambridge, MA). The Rho kinase inhibitor (Y-27632) and BSA were purchased from Sigma.
Statistical analysis.
Results were compared using paired or unpaired student t-test. Significance was assumed at a P value ≤0.05.
RESULTS
AFM pulling force applied on cultured VSMCs.
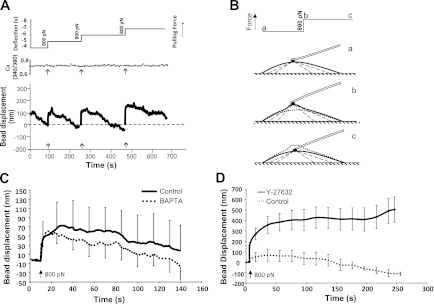
To determine if application of force to a FN-induced focal adhesion site would alter cytosolic Ca2+, VSMCs (passage 3∼10) were loaded with fura 2-AM, and the Ca2+ was measured by fluorescence ratio imaging. Ca2+ imaging was performed while simultaneously using the AFM to apply pulling forces to surface of VSMCs. VSMCs generated localized contractile force in response to pulling (∼800 pN) as evidenced by exerting a downward force against the AFM probe with the attached FN-coated bead (Fig. 1, A and B). This contractile response to pull was observed in 8 out of 10 cells sampled. However, there was no change in Ca2+ associated with the pull in the responding or nonresponding cells (Fig. 1A). To further investigate the Ca2+ requirements of this mechanical response, the experiment was repeated in cells loaded with BAPTA-AM to chelate intracellular Ca2+. Cells loaded with BAPTA-AM exhibited an enhanced contractile response to mechanical pulling (Fig. 1C), indicating that the response could persist in the presence of very low intracellular Ca2+ and that the responses were not associated with an increase of Ca2+.
Fig. 1.
Pulling force applied through fibronectin (FN) focal adhesion induced no calcium increase in low passage (p3∼10) vascular smooth muscle cells (VSMC). A: displacement of a FN-coated bead attached to the VSMC surface in response to step increases of pulling force applied by atomic force microscopy (AFM). An FN-coated bead fused on AFM cantilever was allowed to establish a focal adhesion with cell. Pulling force (Z-direction) was then applied to the bead using AFM (top). Z-direction movement of FN-coated bead reflects the movement of FN-VSMC connection site (bottom). Arrows depict the application of step increase of pulling force. Cells were loaded with fura 2-AM, and concurrent measurement of relative Ca2+ of VSMCs during the time course is shown at top. B: diagrammatic picture of the VSMC response to a step increase of pulling force. a: before force applied; b: right after force applied; c: 4 min after force applied. C: averaged displacement of FN-coated bead on control (n = 9, solid line) and BAPTA-loaded VSMCs (n = 9, dotted line) in response to a step increase of pulling force (800 pN). D: averaged displacement of FN-coated bead on control (n = 9, dotted line) and Y-27632-treated VSMCs (n = 9, solid line) in response to a step increase of pulling force (800 pN). Data are presented as means ± SE.
In an effort to better understand the contractile signaling pathways involved in the force induced contractile response, a specific Rho kinase inhibitor Y-27632 was applied to inhibit Rho kinase activity in VSMCs, and the micromyogenic event was evaluated by AFM. As shown in Fig. 1D, Y-27632 abolished VSMCs contractile response, suggesting the Rho kinase signaling pathway plays an important role in the VSMC micromyogenic events.
AFM pulling force applied on freshly isolated VSMCs.
It is well known that VSMCs dedifferentiate when kept in culture and change to proliferative phenotype accompanied by changes in protein expression. For example, it has been reported that L-VGCC expression progressively decreases during culture (18). We therefore considered the possibility that the absence of Ca2+ change during localized contractile response (i.e., micromyogenic event) was influenced by cell passage. Therefore, we tested the cytosolic Ca2+ changes and micromyogenic response in nonpassaged, freshly isolated VSMCs. VSMCs were isolated from rat cremaster first order arteriole as previously described and were maintained in a cell culture incubator for 2 days to allow development of firm adhesions between cell and the substrate. Although cultured for 2 days, these cells will still be referred to as “fresh VSMCs” in the following context, to be differentiated from the low-passage cultured VSMCs. The cells were loaded with fluo 4-AM for calcium imaging and were subjected to the AFM pulling protocol.
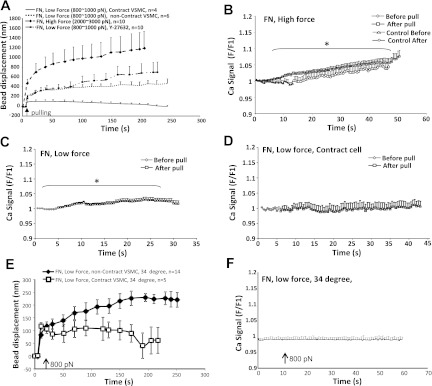
As shown in Fig. 2A, low pulling force (800 pN) applied through FN-coated beads induced contractile response in 40% (4 out of 10) of the cells tested. When higher pulling force (2,000∼3,000 pN) was applied through the AFM to the FN-coated bead, a large initial bead displacement was induced, but no cellular contractile response was observed (14 out of 14). The presence of Y-27632 abolished the contractile response of fresh VSMCs (10 out of 10), further suggesting that Rho kinase signaling pathway was involved in the VSMC mechanotransduction. When high pulling forces (2,000∼3,000 pN) were applied through FN-focal adhesion, a significant global Ca2+ increase was observed in fresh VSMCs (Fig. 2B). However, the same trend of calcium increase was also observed in between the application of pulling forces (Fig. 2B, control), suggesting that the Ca2+ increase was not temporally associated with the pulling force. A low-level global Ca2+ increase was also observed when low pulling force (800∼1,000 pN) was applied to FN-focal adhesions (Fig. 2C). However, in control experiments when FN-bead was loaded on fresh VSMCs but without any pulling, a slow Ca2+ increase was also observed (data not shown); thus at least part of the Ca2+ increase can be attributed to the FN-focal adhesion but not the pulling force. When the fresh VSMCs developed contraction in the presence of pulling force, there was no significant increase of Ca2+ observed (Fig. 2D). By comparing the various VSMC Ca2+ changes and mechanical responses, one can deduce that a global Ca2+ increase was not necessary for the fresh VSMCs to develop contraction in response to AFM pulling. This observation was similar to what was observed from the cultured VSMCs.
Fig. 2.
Pulling forces applied through FN-focal adhesions. A: VSMC response to low (800 pN) and high (2,000∼3,000 pN) pulling force applied through FN-coated bead. High force resulted in large bead displacement with no micromyogenic event, while low force induced micromyogenic response in 40% of cells tested. The presence of Y-27632 (10 μM) abolished the micromyogenic response in fresh VSMCs. B: high pulling force (2,000∼3,000 pN) induced cytosolic Ca2+ increase in VSMCs. VSMCs were loaded with fluo 4, and Ca2+ signal was measured at cell bottom focal plane by confocal fluorescent microscopy. Cytosolic Ca2+ changes were calculated by pixel-by-pixel dividing of each subsequent image with image 1 (F/F1). Control Ca2+ data were collected in the same set of experiments but were in between the force applications. C: VSMCs that did not exhibit a localized contractile response to pulling, the low pulling force also induced cytosolic Ca2+ increase. D: cytosolic Ca2+ concentration was not increased when VSMCs developed contraction in response to the pulling force. E: VSMC mechanical response to low (800 pN) pulling force applied through FN-coated bead at 34°C. F: no cytosolic Ca2+ increase was observed in the VSMCs tested including both contract and noncontract cells at 34°C. *P < 0.05, comparison between before and after the application of pulling force. Data are presented as means ± SE.
It is plausible that the VSMC contractile response and VSMC Ca2+ changes might be uncoupled at room temperature. Thus a set of AFM pulling experiments were performed at 34°C using FN-coated bead and fresh VSMCs. As shown in the Fig. 2F, no global Ca2+ change was observed during the AFM pulling, while a smaller portion of VSMCs developed contraction in response to pulling forces (800∼1,000 pN; Fig. 2E).
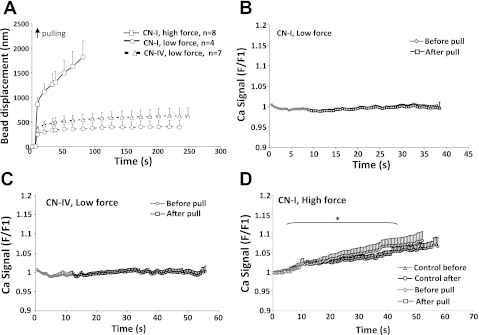
CN-I- or CN-IV-coated beads were also used for experiments to test the cellular response to pull and any associated Ca2+ signaling in fresh VSMCs. No micromyogenic events were observed when low pulling force (800 pN) were applied through CN-I (4 out of 4 cells) or CN-IV (7 out of 7 cells) coated beads (Figure 3A). No Ca2+ increase was observed when low pulling force was applied through CN-I or CN-IV-coated beads (Fig. 3, B and C). To determine if a higher magnitude force would elicit a cellular response, a 2,000∼3,000 pN force was applied to a group of cells (n = 8) through CN-I-coated beads. A large bead displacement was observed in response to the high pulling force; however, no cell contractile response was observed following the initial bead displacement. A significant increase in global Ca2+ was observed with the high-pulling force (Fig. 3D); however, in the following recordings in between force steps, a similar Ca2+ increase was also observed without stepwise raising the pulling force (Fig. 3D, control), implying that the Ca2+ increase was not induced by the acute high pulling force. When CN-I bead was brought into contact with fresh VSMCs but not followed by subsequent AFM pulling, a global Ca2+ increase was also observed but at a much more slower rate (data not shown). Putting these data together, a significant global Ca2+ increase occurred in fresh VSMCs exposed to high pulling force (2,000∼300 pN) applied through CN-I focal adhesions; however, the Ca2+ increase was not accompanied by a VSMC contractile response.
Fig. 3.
Low and high pulling forces applied through collagen type I (CN-I) and type IV (CN-IV) focal adhesions in freshly isolated VSMCs. A: low (800∼1,000 pN) and high (2,000∼3,000 pN) pulling forces were applied through CN-I- and CN-IV-coated beads. High force resulted in larger initial bead displacement. No micromyogenic responses were observed in cells tested. B and C: low pulling force applied through CN-I- and CN-IV-coated beads induced no change in cytosolic Ca2+ concentration. D: high pulling force applied through CN-I bead caused a global increase of Ca2+ in VSMCs. *P < 0.05, statistic comparison was made between Ca2+ signals before the application of pulling force (0∼12 s) and Ca2+ signals after the application of pulling force (15∼55 s). Data are presented as means ± SE.
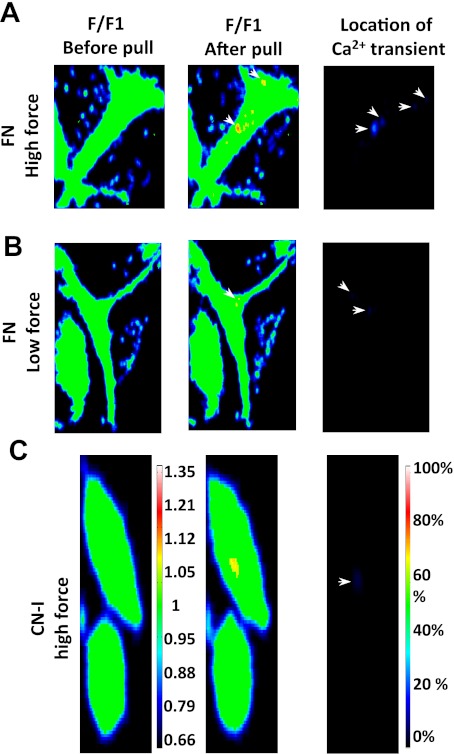
In addition to the global Ca2+ changes in fresh VSMCs, localized Ca2+ events may also play a role in the FN- and CN-I- mediated mechanotransduction. As illustrated in Fig. 4, localized Ca2+ increases were observed when AFM pulling force was applied through FN- or CN-I focal adhesions. Many of the localized events were transient and could last anywhere from 1 to 20 s. No localized Ca2+ increase was detected when VSMCs developed contraction in response to pulling forces. However, it should be noted that small Ca2+ changes beyond the sensitivity of our imaging and mapping system may not have been detected.
Fig. 4.
Pulling force applied through FN- and CN-I-coated beads induced localized calcium events in fresh VSMCs. A: VSMC Ca2+ transients induced by high pulling force applied through FN-coated bead. VSMCs were loaded with fluo-4, and calcium signal was measured at cell bottom focal plane by confocal fluorescent microscopy. Pseudocolored ratio images were calculated by dividing each subsequent image with image 1 (F/F1), and the localized calcium increase was detected as yellow or red color. Location of calcium transients was mapped over the consecutive images that were collected after the pull. Location images were color coded to show the portion of frames that a calcium transient was detected in the same location as a percentage of the total collected frames. B: Ca2+ transients induced by low pulling force applied through FN-coated bead. C: Ca2+ transients induced by high pulling force applied through CN-I-coated bead. Color bar at left encodes scales of Ca2+ transients in a single frame, and color bar at right encodes how frequently a Ca2+ transients appeared at a location.
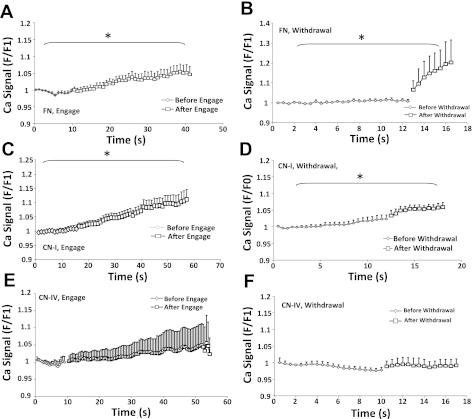
We also observed that making contact with the freshly isolated VSMCs with the AFM probe with a FN- or CN-I coated bead elicited a Ca2+ increase (Fig. 5, A and C). In addition, withdrawal of FN- or CN-I beads from the VSMC surface also induced a significant increase in Ca2+ (Fig. 5, B and D). It is worth to pointing out that these Ca2+ increases may have physiological relevance. For example, during the vessel pressurization, there is very likely force-induced formation of new focal contacts and abolition or remodeling of existing adhesions at the interface between VSMCs and surrounding ECM proteins. These events could conceivably then trigger Ca2+ signals in VSMCs. In comparison, cell contact or withdrawal of CN-IV-coated beads from VSMCs induced no Ca2+ change. BSA-coated beads were used as a negative control for ECM-proteins, and no Ca2+ changes were observed upon cell contact, pulling, or withdrawal of the BSA-coated beads (data not shown).
Fig. 5.
Changes in Ca2+ induced by the initial contact of FN- , CN-IV, and CN-I coated bead with VSMCs and by withdrawal of the bead from the VSMCs. A–D: contact and withdrawal of FN- and CN-I-coated beads induced cellular Ca2+ increase in freshly isolated VSMCs. E and F: contact and withdrawal of CN-IV-coated beads resulted in no cellular Ca2+ increase. *P < 0.05, comparison between before and after the contact or withdrawal of beads. Data are presented as means ± SE.
Micromyogenic events in BAPTA-loaded VSMCs.
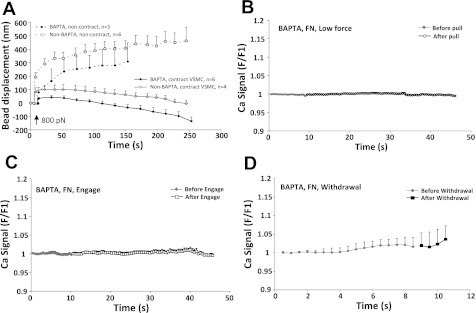
To determine whether the micromyogenic events were independent of Ca2+ increase in fresh VSMCs, cells were loaded with both BAPTA-AM (5 μM) and fluo 4 and were tested for cell response to AFM-pulling forces and the cellular calcium level. The effectiveness of BAPTA was confirmed by observing that it significantly buffered ionomycin-induced Ca2+ increase in the fresh VSMCs (data not shown). As shown in Fig. 6, no Ca2+ increase was observed during the contact, pulling, and withdrawal of FN-coated beads in BAPTA-loaded VSMCs. However, the magnitude of force-induced contraction of BAPTA-loaded VSMCs was comparable or even enhanced compared with that of non-BAPTA-loaded cells and so was the number of cells that demonstrated micro-contractile events (6 out of 9 compare to 4 out of 10). These results are consistent with our observations in cultured VSMCs.
Fig. 6.
Fresh VSMCs loaded with BAPTA developed contraction in response to pulling forces applied through FN-focal adhesions. A: comparison of pulling force (800 pN) induced FN-bead displacement in BAPTA-loaded and non-BAPTA-loaded VSMCs. B–D: pull, engage, and withdrawal of FN-coated bead did not induce any cytosolic Ca2+ changes in BAPTA-loaded VSMCs. Data are presented as means ± SE.
DISCUSSION
This study aimed to determine whether global Ca2+ signals were associated with the cellular contractile response to pulling at the level of a single focal adhesion site and to determine if there were ECM protein-dependent differences associated with Ca2+ signaling in isolated VSMCs. The results of this study reveal that the relationship among Ca2+, ECM proteins, and applied force is complex. An increase in cytosolic Ca2+ level occurs when FN or CN-I-coated beads were brought into contact with VSMC surface and subsequently removed from the cell, indicating that the VSMCs reacts to initial contact with FN and CN-I and to breaking contact with these ECM proteins, but similar observations were not found with CN-IV. Furthermore, the results demonstrated that there is an increase in cellular Ca2+ activity evidenced as localized Ca2+ increases when pulling force was applied to single focal adhesion sites in contact with FN- or CN-I-coated beads but not for CN-IV. The stepwise high pulling forces (2,000∼3,000 pN) also induced global Ca2+ increases in fresh VSMCs. However, of importance was the observation that Ca2+ was not increased in cells that exhibited the localized contractile events in response to pulling, suggesting that this mechanical response does not require an increase in global Ca2+ to occur. Loading VSMCs with BAPTA to buffer the intracellular Ca2+ demonstrated no negative effect on the VSMC contractile responses to pull, further supporting the relative Ca2+ independence of this processes.
The fact that engagement of both FN- and CN-I-coated beads with the cell surface induced Ca2+ increase indicates that integrin interactions with FN or CN-I are capable triggers of VSMC Ca2+ signals. The rise in Ca2+ following contact may be associated with development and maturation of the focal adhesion site with the bead. Similar integrin-mediated calcium signals have been reported in other cell types either with or without force applied. For example, Glogauer et al. (16) showed that initial placement of CN-I-coated paramagnetic beads induced a global Ca2+ increase in cultured human fibroblast, while magnetic pulling force applied through the CN-I coated beads induced an additional Ca2+ increase in these cells. Similarly, magnetic forces applied through paramagnetic beads coated antibodies against integrin β1 or α2 (primary CN-I binding receptors) were also shown to induce global Ca2+ increase in cultured osteogenic cells (27). However, there was no information regarding whether the cells mechanically responded to the pulling and its potential relationship with Ca2+. In this study, we demonstrated that the force induced VSMC contraction did not correlate with the global increase of Ca2+.
It has been observed that mechanical pulling a single VSMC lengthways can trigger robust cellular Ca2+ signals. For example, Davis et al. (9) have shown that longitudinal stretching of isolated VSMCs induced a strong global Ca2+ increase through release of Ca2+ from intracellular stores as well as through activity of L-VGCC or other mechanical sensitive cation channels. As these cells were stretched with two attached patch pipettes, it is unlikely integrins were involved and that the stretching force was affecting membrane and cytoskeletal components directly. This may explain the observed Ca2+ increase induced by high pulling forces applied through FN or CN-I-bead (Figs. 2B and 3D). Alternatively, the stretching forces may cause a slow membrane leak that led to the Ca2+ increase. The fact that these Ca2+ increase was not associated with VSMC contractile response was puzzling, one possibility may be that the pulling force (2,000∼3,000 pN) was too high for the underlying cytoskeleton to develop contraction.
We have observed here that both the freshly isolated VSMCs and low passage cultured VSMCs can generate active contractile forces in response to the externally applied pulling forces. In addition to VSMCs, the cellular mechanical responses to external forces have also been reported in a variety of other cells types. For example, magnetic twisting cytometry has been employed to apply torque forces on endothelial cells (34), pulmonary vascular smooth muscle cells (24), and lately on epithelial cells (23), and each cell type showed an increase of local resistance to the torque forces. Choquet et. al. (4) have shown that fibroblast cells can generate sufficient force to drag away the FN-coated beads that were engaged on cell surface and were mechanically held by an optical tweezer. Heidemann et al. (19) have shown that fibroblast cells tethered on laminin-treated needles developed active contraction in response to the stretching force applied through the needle. Together, these results suggest that the ability of a cell to generate mechanical response to external forces is not unique to VSMCs, and future work will need to address whether there is a common molecular mechanism that is shared by different cell types to develop the mechanical responses.
In previous work from our laboratories (35, 36), FN-coated beads applied to α5β1-integrin triggered activation of L-VGCC in freshly isolated VSMCs, which required the activities of focal adhesion kinase and Src, as well as focal adhesion proteins paxillin and vinculin. When loading FN- or CN-I-coated beads on the surface of VSMCs, clusters of α5β1-integrins can be observed to cluster around the bead within 10 min (31; and unpublished observations). It is possible that the initial VSMC Ca2+ increase was mediated through activation of L-VGCC that was induced by binding and activation of integrins α5β1 by the FN- and CN-I-coated beads. Recently, transient receptor potential channels have been proposed to function as mechanical sensitive nonselective cation channels in VSMCs (12, 13). In this model, force induced membrane stretching can activate transient receptor potential channels, which could be another route leading to depolarization with subsequent activation of L-VGCC and a Ca2+ increase.
In our experiments, the mechanical pull applied to the VSMCs is normal to the cell membrance as opposed to longitudinal. The stretch does cause significant membrane deformation during AFM pulling. Whether the axis of pull is an important variable remains to be determined. With respect to the application of force per se, a number of events associated with the pulling could play a role, such as: 1) rupture of integrin-FN or -CN-I adhesions, 2) breakage of the cytoskeletal connection with the bead adhesion sites and/or inside of the cell, 3) deformation of cell membrane, and 4) deformation of intracellular organelles (i.e., ER and mitochondria) and/or conformation changes in cytoskeletal or focal adhesion proteins that alters activity within the focal adhesion. Further work will be required to determine the role these effects may play in linking mechanical force to the contractile response and to Ca2+ in the VSMCs.
The evidence that the Ca2+ signal is uncoupled from the VSMC contractile response to pull suggests that a “Ca2+-sensitization” process may play an important role. Karibe et al. (22) have shown that treatment with PKC inhibitor abolished myogenic response of rat skeletal muscle small arteries. Furthermore, translocation of PKC-α has been detected in VSM cells during the myogenic response of ferret coronary arterioles (11). On the other hands, Gokina et al. (17) reported that inhibition of Rho kinase activity using Y-27623 abolished the myogenic response in cannulated rat cerebral arteries. Consistent with these vessel studies, we observed that Rho-kinase inhibition abolished the micromyogenic event in both cultured and fresh VSMCs (Figs. 1D and 2A). This evidence supports the possible role of Ca2+-sensitization mediated by Rho kinase in our model system as a mechanism for the micromyogenic events.
In conclusion, we have demonstrated that a global Ca2+ increase could be induced by loading of FN- or CN-I-coated bead on VSMC surface or by application of high pulling force through the FN- or CN-I-focal adhesions. The Ca2+ signal appears to depend on cellular contact with FN and CN-I, as well as on exposure to high force. However, the global Ca2+ increase is not correlated with the cellular pulling response (micromyogenic response) to externally applied force with the AFM through FN-focal adhesions.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-058690 and 1P01-HL-095486 (to G. A. Meininger) and American Heart Association Grants 0765481Z and 0835676N (to Z. Sun).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.S. and G.A.M. conception and design of research; Z.S. and Z.L. performed experiments; Z.S. and Z.L. analyzed data; Z.S. and G.A.M. interpreted results of experiments; Z.S. prepared figures; Z.S. drafted manuscript; Z.S. and G.A.M. edited and revised manuscript; Z.S., Z.L., and G.A.M. approved final version of manuscript.
REFERENCES
- 1. Alenghat FJ, Ingber DE. Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Sci STKE 2002: PE6, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol 19: 677–695, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Bolz SS, Vogel L, Sollinger D, Derwand R, de Wit C, Loirand G, Pohl U. Nitric oxide-induced decrease in calcium sensitivity of resistance arteries is attributable to activation of the myosin light chain phosphatase and antagonized by the RhoA/Rho kinase pathway. Circulation 107: 3081–3087, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin- cytoskeleton linkages. Cell 88: 39–48, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Cole WC, Welsh DG. Role of myosin light chain kinase and myosin light chain phosphatase in the resistance arterial myogenic response to intravascular pressure. Arch Biochem Biophys 510: 160–173, 2011 [DOI] [PubMed] [Google Scholar]
- 6. D′Angelo G, Davis MJ, Meininger GA. Calcium and mechanotransduction of the myogenic response. Am J Physiol Heart Circ Physiol 273: H175–H182, 1997 [DOI] [PubMed] [Google Scholar]
- 7. D′Angelo G, Mogford JE, Davis GE, Davis MJ, Meininger GA. Integrin-mediated reduction in vascular smooth muscle [Ca2+]i induced by RGD-containing peptide. Am J Physiol Heart Circ Physiol 272: H2065–H2070, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Davis MJ, Meininger GA, Zawieja DC. Stretch-induced increases in intracellular calcium of isolated vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 263: H1292–H1299, 1992 [DOI] [PubMed] [Google Scholar]
- 10. Davis MJ, Wu X, Nurkiewicz TR, Kawasaki J, Davis GE, Hill MA, Meininger GA. Integrins and mechanotransduction of the vascular myogenic response. Am J Physiol Heart Circ Physiol 280: H1427–H1433, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Dessy C, Matsuda N, Hulvershorn J, Sougnez CL, Sellke FW, Morgan KG. Evidence for involvement of the PKC-α isoform in myogenic contractions of the coronary microcirculation. Am J Physiol Heart Circ Physiol 279: H916–H923, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Earley S, Straub SV, Brayden JE. Protein kinase C regulates vascular myogenic tone through activation of TRPM4. Am J Physiol Heart Circ Physiol 292: H2613–H2622, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 95: 922–929, 2004 [DOI] [PubMed] [Google Scholar]
- 14. El-Yazbi AF, Johnson RP, Walsh EJ, Takeya K, Walsh MP, Cole WC. Pressure-dependent contribution of Rho kinase-mediated calcium sensitization in serotonin-evoked vasoconstriction of rat cerebral arteries. J Physiol 588: 1747–1762, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nat Rev Mol Cell Biol 2: 793–805, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Glogauer M, Ferrier J, McCulloch CA. Magnetic fields applied to collagen-coated ferric oxide beads induce stretch-activated Ca2+ flux in fibroblasts. Am J Physiol Cell Physiol 269: C1093–C1104, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Gokina NI, Park KM, McElroy-Yaggy K, Osol G. Effects of Rho kinase inhibition on cerebral artery myogenic tone and reactivity. J Appl Physiol 98: 1940–1948, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Gollasch M, Haase H, Ried C, Lindschau C, Morano I, Luft FC, Haller H. L-type calcium channel expression depends on the differentiated state of vascular smooth muscle cells. FASEB J 12: 593–601, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Heidemann SR, Kaech S, Buxbaum RE, Matus A. Direct observations of the mechanical behaviors of the cytoskeleton in living fibroblasts. J Cell Biol 145: 109–122, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hill MA, Zou H, Davis MJ, Potocnik SJ, Price S. Transient increases in diameter and [Ca2+]i are not obligatory for myogenic constriction. Am J Physiol Heart Circ Physiol 278: H345–H352, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Johnson RP, El-Yazbi AF, Takeya K, Walsh EJ, Walsh MP, Cole WC. Ca2+ sensitization via phosphorylation of myosin phosphatase targeting subunit at threonine-855 by Rho kinase contributes to the arterial myogenic response. J Physiol 587: 2537–2553, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karibe A, Watanabe J, Horiguchi S, Takeuchi M, Suzuki S, Funakoshi M, Katoh H, Keitoku M, Satoh S, Shirato K. Role of cytosolic Ca2+ and protein kinase C in developing myogenic contraction in isolated rat small arteries. Am J Physiol Heart Circ Physiol 272: H1165–H1172, 1997 [DOI] [PubMed] [Google Scholar]
- 23. le Duc Q, Shi Q, Blonk I, Sonnenberg A, Wang N, Leckband D, de Rooij J. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol 189: 1107–1115, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee KM, Tsai KY, Wang N, Ingber DE. Extracellular matrix and pulmonary hypertension: control of vascular smooth muscle cell contractility. Am J Physiol Heart Circ Physiol 274: H76–H82, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Martinez-Lemus LA, Crow T, Davis MJ, Meininger GA. alphavbeta3- and alpha5beta1-integrin blockade inhibits myogenic constriction of skeletal muscle resistance arterioles. Am J Physiol Heart Circ Physiol 289: H322–H329, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Meininger GA, Zawieja DC, Falcone JC, Hill MA, Davey JP. Calcium measurement in isolated arterioles during myogenic and agonist stimulation. Am J Physiol Heart Circ Physiol 261: H950–H959, 1991 [DOI] [PubMed] [Google Scholar]
- 27. Pommerenke H, Schreiber E, Durr F, Nebe B, Hahnel C, Moller W, Rychly J. Stimulation of integrin receptors using a magnetic drag force device induces an intracellular free calcium response. Eur J Cell Biol 70: 157–164, 1996 [PubMed] [Google Scholar]
- 28. Schaffner-Reckinger E, Gouon V, Melchior C, Plancon S, Kieffer N. Distinct involvement of beta3 integrin cytoplasmic domain tyrosine residues 747 and 759 in integrin-mediated cytoskeletal assembly and phosphotyrosine signaling. J Biol Chem 273: 12623–12632, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nat Cell Biol 4: E65–68, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Sun Z, Martinez-Lemus LA, Hill MA, Meininger GA. Extracellular matrix-specific focal adhesions in vascular smooth muscle produce mechanically active adhesion sites. Am J Physiol Cell Physiol 295: C268–C278, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun Z, Martinez-Lemus LA, Trache A, Trzeciakowski JP, Davis GE, Pohl U, Meininger GA. Mechanical properties of the interaction between fibronectin and alpha5beta1-integrin on vascular smooth muscle cells studied using atomic force microscopy. Am J Physiol Heart Circ Physiol 289: H2526–H2535, 2005 [DOI] [PubMed] [Google Scholar]
- 32. VanBavel E, Wesselman JP, Spaan JA. Myogenic activation and calcium sensitivity of cannulated rat mesenteric small arteries. Circ Res 82: 210–220, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Vassbotn FS, Havnen OK, Heldin CH, Holmsen H. Negative feedback regulation of human platelets via autocrine activation of the platelet-derived growth factor alpha-receptor. J Biol Chem 269: 13874–13879, 1994 [PubMed] [Google Scholar]
- 34. Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science 260: 1124–1127, 1993 [DOI] [PubMed] [Google Scholar]
- 35. Wu X, Davis GE, Meininger GA, Wilson E, Davis MJ. Regulation of the L-type calcium channel by alpha 5beta 1 integrin requires signaling between focal adhesion proteins. J Biol Chem 276: 30285–30292, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Wu X, Mogford JE, Platts SH, Davis GE, Meininger GA, Davis MJ. Modulation of calcium current in arteriolar smooth muscle by alphav beta3 and alpha5 beta1 integrin ligands. J Cell Biol 143: 241–252, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]