Abstract
Group IVA cytosolic phospholipase A2 (cPLA2α), which preferentially cleaves arachidonic acid from phospholipids, plays a role in apoptosis and tissue injury. Downstream signals in response to tumor necrosis factor (TNF)-α, a mediator of myocardial ischemia-reperfusion (I/R) injury, involve cPLA2α activation. This study examined the potential role of cPLA2α and its mechanistic link with TNF-α in myocardial I/R injury using cPLA2α knockout (cPLA2α−/−) mice. Myocardial I/R was created with 10-wk-old male mice by 1 h ligation of the left anterior descending coronary artery, followed by 24 h of reperfusion. As a result, compared with wild-type (cPLA2α+/+) mice, cPLA2α−/− mice had a 47% decrease in myocardial infarct size, preservation of echocardiographic left ventricle (LV) function (%fractional shortening: 14 vs. 21%, respectively), and lower content of leukotriene B4 and thromboxane B2 (62 and 50% lower, respectively) in the ischemic myocardium after I/R. Treatment with the TNF-α inhibitor (soluble TNF receptor II/IgG1 Fc fusion protein, sTNFR:Fc) decreased myocardial I/R injury and LV dysfunction in cPLA2α+/+ mice but not cPLA2α−/− mice. sTNFR:Fc also suppressed cPLA2α phosphorylation in the ischemic myocardium after I/R of cPLA2α+/+ mice. Similarly, sTNFR:Fc exerted protective effects against hypoxia-reoxygenation (H/R)-induced injury in the cultured cardiomyocytes from cPLA2α+/+ mice but not cPLA2α−/− cardiomyocytes. H/R and TNF-α induced cPLA2α phosphorylation in cPLA2α+/+ cardiomyocytes, which was reversible by sTNFR:Fc. In cPLA2α−/− cardiomyocytes, TNF-α induced apoptosis and release of arachidonic acid to a lesser extent than in cPLA2α+/+ cardiomyocytes. In conclusion, disruption of cPLA2α attenuates myocardial I/R injury partly through inhibition of TNF-α-mediated pathways.
Keywords: phospholipase A2, gene knockout mouse, leukotriene, arachidonic acid, Etanercept
it has long been implicated that phospholipases A2 (PLA2) plays critical roles in the pathogenesis of myocardial ischemia (7, 13, 31). PLA2 are causally related to membrane phospholipid degradation and cellular damage, leading to electrophysiological dysfunction and myocytic necrosis or apoptosis during myocardial ischemia-reperfusion (I/R) (7, 11, 12, 13, 31, 32, 39). Lipid mediators produced by PLA2 induce further myocyte damage and death (7, 11, 12, 13, 31, 32, 39). At present, several PLA2 have been identified and classified into different families based on their biochemical features and primary structures (1, 9, 28, 38). Human PLA2 are classified into four main categories: intracellular cytosolic PLA2 (cPLA2); Ca2+-independent PLA2s (iPLA2); PAF acetylhydrolases; and secretory PLA2 (sPLA2) (1, 9, 28, 38). Among human PLA2, cPLA2α is the most ubiquitously expressed enzyme and plays a crucial role in a variety of physiological, inflammatory, and pathophysiological states (4, 22, 26, 33). We have previously shown that cPLA2 modulates group V sPLA2 action in a synergistic manner in myocardial I/R injury (39), indicating a central role for cPLA2 in the release of arachidonic acid for eicosanoid production in many pathological conditions. Previous reports showed that cPLA2 significantly contributed to ischemic brain injury, but the precise role of cPLA2 in myocardial I/R injury remains undefined (3, 35).
Several previous studies showed that tumor necrosis factor (TNF)-α is produced in ischemic myocardium and contributes to myocardial I/R injury (10, 14, 15, 23, 27, 34, 37). Several lines of evidence suggest that downstream signaling in response to TNF-α involves cPLA2 activation in different cell types (18, 20, 24). Thus, this study examined a potential role of cPLA2 and its mechanistic link with TNF-α in myocardial I/R injury using cPLA2α knockout (cPLA2−/−) mice.
MATERIALS AND METHODS
Materials.
Anti-human cPLA2α (catalog no. 2832) and phospho-cPLA2 (Ser505) (catalog no. 2831) polyclonal antibodies for immunoblot analysis were purchased from Cell Signaling Technology (Danvers, MA). Also, antibodies for protein kinase B (AKT) (catalog no. 9272), phospho-AKT (4058S), p42/p44 extracellular signal-regulated kinases (ERK1/2) (9102S), phospho-ERK1/2 (4377S), p38 mitogen-activated protein kinase (p38 MAPK) (9212), phospho-p38 MAPK (9211S), c-Jun NH2-terminal kinase (JNK) (9252), and phospho-JNK (9251S) were from Cell Signaling Technology. Anti-β-tubulin polyclonal antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Cell culture reagents were from Sigma (Tokyo, Japan) and Invitrogen (Carlsbad, CA). The soluble TNF receptor (p75) linked to the Fc portion of human IgG1 (sTNFR II/IgG1 Fc fusion protein: sTNFR:Fc, Etanercept), a TNF-α inhibitor, was from Takeda Pharmaceutical (Tokyo, Japan). AACOCF3 and MAFP (inhibitors of cPLA2 activity) and U-46619 (a TXA2-mimetic agent) were from Cayman Chemical (Ann Arbor, MI). Other chemicals were purchased from Sigma unless otherwise indicated.
Mice.
The experimental protocol was approved by the University of Yamanashi Animal Care and Use Committee, and procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (1996). Male cPLA2α−/− (systemic deficiency in cPLA2α) mice with a C57BL/6 background from F13∼F15 (10–12 wk old, 20–25 g) were analyzed (22, 35), and littermate cPLA2α+/+ male mice were used as wild-type controls.
Myocardial I/R in vivo.
cPLA2α+/+ and cPLA2α−/− mice were subjected to 1 h of myocardial ischemia and 24 h of reperfusion (I/R) (11, 39). Mice were anesthetized with pentobarbital sodium (50 mg/kg body wt) with buprenorphine (0.05 mg/kg; Otsuka Pharmaceutical, Tokyo, Japan) subcutaneously administered 30 min before surgery and every 8 h after surgery for analgesia. The adequacy of anesthesia and analgesia was determined by limb muscular relaxation and the absence of reaction to a toe pinch test. Mice were intubated and ventilated on a respirator (model SN-480–7; Shinano, Tokyo, Japan) with electrocardiographic (ECG) monitoring (surface ECG, lead II). Ischemia was achieved by ligating the left anterior descending coronary artery (LAD) using an 8–0 nylon suture with a section of PE-10 tubing placed over the LAD, 1 mm from the tip of the normally positioned left atrium. After occlusion for 1 h, reperfusion was initiated by releasing the ligature and removing the PE-10 tubing. Successful coronary occlusion and reperfusion were demonstrated by visual changes in the ischemic region and significant ECG changes. The chest wall was then closed, and the animal was extubated. During the procedure, body temperature was maintained with a 37°C warming plate. After 24 h of reperfusion, mice were again anesthetized with 5% isoflurane in an anesthetic chamber to the point where they were nonresponsive to toe pinch, and the chest wall was reopened. The loosened suture was left in place and then retied for purposes of evaluating the ischemic area. The myocardial infarct size was assessed as described below. In some experiments, hearts were harvested at the indicated time points of reperfusion following 1 h ischemia.
When the effects of the TNF-α inhibitor sTNFR:Fc or cPLA2α inhibitors on the myocardial I/R injury were examined, sTNFR:Fc, cPLA2α inhibitors, or vehicle was administered once intraperitoneally at the indicated dose 1 h before the ischemia.
Assessment of area at risk and infarct size.
After 1 h of myocardial ischemia and 24 h reperfusion, the LAD was reoccluded at the same position, and 0.5 ml of 1% Evans blue dye was administered through a 26-gauge needle inserted in the left ventricle (LV) (11, 39). The Evans blue dye was uniformly distributed to those areas of the myocardium proximal to the ligature. Upon removal, the LV was cut transversely into five sections, and each section was photographed. The area of the myocardium that was not stained with Evans blue was defined as the area at risk (AAR). The sections were weighed and incubated in 1% triphenyltetrazolium chloride for 10 min at 37°C and rephotographed. The second photograph showed that viable myocardium was stained brick red and the infarct remained a pale white. Computerized planimetry (NIH Image J analysis software) of the photograph was used to analyze all sections of the slices, including the AAR and infarcted areas. The sizes of the AAR and infarcts in proportion to the total size of the slices were calculated and multiplied by the weight of each slice to determine the AAR and infarct weight per slice. Infarct size was expressed as a percentage of LV mass and a percentage of AAR.
In vivo transthoracic echocardiography.
M-mode echocardiography was performed at baseline and after 1 h of ischemia followed by 24 h of reperfusion under ketamine-xylazine anesthesia using a 15-MHz phased-array probe connected to a Sonos 5500 echocardiograph (Philips Medical Imaging, Andover, MA) (11, 39). In brief, an M-mode cursor was positioned in the parasternal short-axis view perpendicular to the interventricular septum and posterior wall of the LV at the level of the papillary muscles, and M-mode images were obtained for measurement of LV end-diastolic and end-systolic dimension (LVDd and LVDs, respectively). The percentage of fractional shortening (%FS) was calculated from the equation: %FS = [(LVDd − LVDs)/LVDd] × 100. All data were averaged from five cardiac cycles/experiments. The individuals who performed the echocardiography and the resulting calculations were blinded to the mouse genotypes.
Measurements of mRNA and protein expression levels.
Total RNA was extracted from tissues and cells with a Qiagen RNeasy kit and DNase I (QIAGEN, Tokyo, Japan). The mRNA expression levels were quantified by a real-time two-step reverse transcriptase PCR assay using SYBR Green I chemistry and with the use of a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA) (11, 39). The PCR primers are listed in Table 1. The GAPDH housekeeping gene was used for normalization of gene expression.
Table 1.
Sequences of PCR primers
| Forward Primers | Reverse Primers | |
|---|---|---|
| sPLA2-IIC | 5′-CCGGGATCCTAGAAAACACA-3′ | 5′-TGTCCCGAACATCCTCTTTC-3′ |
| sPLA2-IID | 5′-GAACCACCGGCCTAATTACA-3′ | 5′-GATGAAGGTAGGCTGGGTCA-3′ |
| sPLA2-IIE | 5′-CCTGCGAGTGTGACAAGAGA-3′ | 5′-ATGAGTCTGCTGGGAGAGGA-3′ |
| sPLA2-IIF | 5′-AACACTCCACTGGACGGAAG-3′ | 5′-GTAGCCCACAAAGGACAGGA-3′ |
| sPLA2-III | 5′-GGCTGAGGCCACCTCATATACTTC-3′ | 5′-TCCTTTGCCCTCAGCACAGTCAAG-3′ |
| sPLA2-V | 5′-GATGCACGACCGTTGTTATGG-3′ | 5′-AGGAGTCGTGTTCGCAGATGA-3′ |
| sPLA2-X | 5′-TTCAGCGAAGCAACCAGGA-3′ | 5′-CACCAAGGCCACAATAACAGC-3′ |
| cPLA2α | 5′-CTGGCCAACATCAACTTCAG-3′ | 5′-GCCAGTCTCTTCTGCATAGT-3′ |
| cPLA2β | 5′-GTGATTGCCGTGATGGCCACTG-3′ | 5′-CCGGCCAGCTGCCCATACAAAG-3′ |
| cPLA2γ | 5′-GGACCAGTGACATACTCAGAAG-3′ | 5′-ACTAAATCCAAGAGCATTGC-3′ |
| iPLA2 | 5′-TCAGGATCTCATGCCCATCTCT-3′ | 5′-TGGTCGTGACTCCGCTTCTC-3′ |
| GAPDH | 5′-TGCACCACCAACTGCTTAG-3′ | 5′-GATGCAGGGATGATGTTC-3′ |
sPLA2, secretory phospholipase A2 (PLA2); cPLA2, cytosolic PLA2; iPLA2, Ca2+-independent PLA2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
For immunoblot analysis, 15 μg protein from the cells and tissue extracts were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane (11, 39). The intensity of the β-tubulin band was used as a loading control between the tissue samples.
dUTP nick end-labeling assay and caspase-3 activity.
Hearts were harvested after 24 h reperfusion following 1 h ischemia (I/R), fixed in 4% paraformaldehyde solution, and embedded in paraffin, and the midventricular tissue was sliced into 5-μm-thick sections. For the cultured cardiomyocytes, after various treatments, the cells were fixed with 1% paraformaldehyde solution for 10 min at room temperature. Apoptotic cells in the noninfarct myocardium in ischemic areas and in cultured cardiomyocytes were identified by dUTP nick end-labeling (TUNEL) staining using an apoptosis detection kit according to the manufacturer's protocol (Millipore, Billerica, MA) (39). Slides were covered with a glass cover slide applied with mounting medium containing DAPI. For each experiment, TUNEL-positive cells were counted in six fields of five separate sections or chambers at ×400 magnification by a blinded observer and are presented as a percentage of the total number of cardiomyocytes (nuclei). For colocalization analysis, subsequent immunofluorescence analysis for α-actin was performed. After the TUNEL staining, myocardial sections were incubated with anti-α-actin antibody (rabbit polyclonal antibody, 1:500; Abcam, Cambridge, UK) followed by the secondary antibody with Alexa Fluor 594 (goat polyclonal antibody, 1:500; Invitrogen) for 60 min at room temperature. The slides were examined using a confocal microscope (FV-1000; Olympus, Tokyo, Japan) equipped with a ×100/1.0-numerical aperture oil-immersion objective.
Myocardial caspase-3 activity was measured by fluorogenic assays using the corresponding fluorogenic substrate peptide, DEVD- 7-amino-4-trifluoromethyl coumarin (AFC) (ApoAlert Caspase Fluorescent Assay Kits; Clontech) (39). Hearts were harvested after I/R, and the noninfarcted myocardium in the ischemic area was homogenized in the lysis buffer. The protein lysates were incubated with the reaction reagent containing the respective substrates, and the myocardial caspase-3 activity was expressed as relative fluorescence units using the calibration curve obtained with known concentrations of AFC.
Measurement of myeloperoxidase activity in infarcted hearts.
The myeloperoxidase (MPO) assay procedures are similar to the methods described in our previous report (11). Hearts were exercised after 3 h reperfusion following 1 h of ischemia and divided into ischemic and nonischemic parts. The ischemic tissues were weighted, homogenized, and sonicated in 0.5% hexadecyltrimethyl-ammonium bromide in 50 mM potassium phosphate buffer, pH 6.0. The mixture was centrifuged at 12,500 rpm for 30 min at 4°C. The supernatants were then collected and reacted with 0.167 mg/ml of ο-dianisidine dihydrochloride and 0.0005% H2O2 in 50 mM phosphate buffer at pH 6.0. The change in absorbance was measured spectrophotometrically at 460 nm. One unit of MPO activity is defined as the quantity of enzyme hydrolyzing 1 mmol peroxide/min at 25°C.
Preparation and culture of mouse cardiomyocytes.
Primary cultures of adult mouse cardiomyocytes were prepared by collagenase digestion from the ventricles of 10- to 12-wk-old mice as described previously (11, 39). Mice were anesthetized with 5% isoflurane in an anesthetic chamber to the point where they were nonresponsive to toe pinch and then killed by cervical dislocation. Adult hearts from cPLA2α+/+ and cPLA2α−/− mice were rapidly excised and placed on a cannula for perfusion through the aorta. Perfusion was performed for 5 min with PBS including 10 mM 2,3-butanedione monoxime (BDM), followed for 10 min with perfusion buffer containing Liberase blendzyme 1 (0.25 mg/ml), trypsin (0.14 mg/ml), and CaCl2 (12.5 μM). After mechanical dissection of the LV cells, the cardiomyocytes were resuspended with myocyte plating MEM containing 5% FBS, BDM (10 mM), and l-glutamine (2 mM) and plated on laminin-coated culture dishes (BD Biosciences). After incubation at 37°C, 2% CO2 for 1 h, the medium was exchanged to myocyte culture MEM (0.1 mg/ml BSA, 10 mM BDM, 5 μg/ml insulin, 5 μg/ml transferrin, and 5 ng/ml selenium). Our cell preparations yielded at least 80% of the cardiomyocytes with rod shape morphology, clear sarcomeric striations, and clear peripheral margins. Each experiment using cultured cardiomyocytes was performed 2 h after the incubation.
Experiments using cultured cardiomyocytes.
The cultured mouse myocardial cells were exposed to hypoxia-reoxygenation (H/R); the cells were placed in a hypoxia chamber (Ikemoto Scientific Technology, Tokyo, Japan) filled with 1% O2-2% CO2-97% N2 at 37°C for 40 min, followed by reoxygenation by placing the cells in normoxic conditions (98% atmosphere-2% CO2) for 12 h (11). As a control, some cells were placed in normoxic conditions for the same period. In some experiments, the cells were treated for 12 h with TNF-α instead of H/R. In some experiments, cultured cardiomyocytes were coincubated with sTNFR:Fc or vehicle during exposure to H/R or TNF-α.
Cell viability was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) method (11) where 200 μl MTT solution were added to 1 ml culture medium after the treatment (TACS MTT Assays; R&D Systems). After incubation at 37°C for 4 h, cells were solubilized with solubilization solution included in the assay kit. The absorbance at 570 nm for each aliquot was determined using a microplate reader. The reduction in MTT activity in the treated cells was presented as the percentage of nontreated control cells.
Experiments using isolated neutrophils.
The peritoneal neutrophils were isolated as in our previous report (11). Briefly, after intraperitoneal injection of 2 ml of 2% casein, the peritoneal fluid was collected. Neutrophils were purified from the peritoneal cells by Ficoll-Paque gradients followed by hypotonic lysis of erythrocytes. The number of neutrophils recovered from one mouse was 1∼2 × 107 cells, and the purity was >98% by Türk stain (0.01% of methilrosaniline chloride and 1.0% acetic acid), neutrophil alkaline phosphatase, and neutrophil esterase stain. After 1 h of suspension in serum-free medium 199 at 4°C, the isolated neutrophils were used in the experimental protocols for arachidonic acid release.
Assay for arachidonic acid release.
The release of arachidonic acid in response to H/R or TNF-α was measured using cultured cardiomyocytes or neutrophils prelabeled with 0.5 μCi/ml [3H]arachidonic acid (11, 39). Briefly, the isolated cardiomyocytes and neutrophils were incubated overnight and for 30 min, respectively, with the culture medium containing 0.5 μCi/ml [3H]arachidonic acid and 0.1 mg/ml fatty acid-free BSA. After being washed with culture medium three times, the labeled cells were incubated for 15 min at 37°C with culture medium and 0.1 mg/ml fatty acid-free BSA. Next, the cells were exposed to H/R or TNF-α, and the culture medium and the cells were collected separately. The radioactivity in the culture medium was expressed as a percentage of the total radioactivity incorporated into the cells.
Assays for leukotriene B4 and thromboxane B2 levels in myocardium.
Concentrations of leukotriene B4 (LTB4) and thromboxane B2 (TXB2) in the homogenates of ischemic myocardium after 24 h reperfusion following 1 h ischemia were determined by enzyme immunoassay kits (Amersham Pharmacia Biotech, Piscataway, NJ) (39). Protein concentrations of myocardial homogenates were determined by the bicinchoninic acid protein assay.
Statistical analysis.
Results are expressed as means ± SE. Mean values were compared between two groups with an unpaired t-test. Comparisons among more than three groups were performed by one-way ANOVA with the Scheffé F procedure for post hoc analysis. P < 0.05 was considered statistically significant.
RESULTS
Characterization of cPLA2α−/− mice.
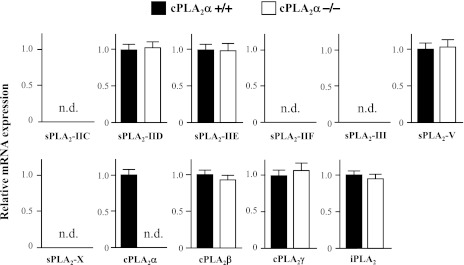
There was no significant difference in body weight, heart size, heart rate, blood pressure, and complete blood count at baseline (12 wk old) between the cPLA2α+/+ and cPLA2α−/− mice (Table 2). Myocardial expression levels of mRNA for sPLA2-IID, sPLA2-IIE, sPLA2-V, cPLA2β, cPLA2γ, and iPLA2β (VI) at baseline were similar between the cPLA2α+/+ and cPLA2α−/− mice (Fig. 1). Myocardial mRNA expression of sPLA2-IIC, -IIF, -III, and -X was not detectable in either the cPLA2α+/+ or cPLA2α−/− mice. sPLA2-IIA was naturally disrupted by a frameshift mutation in C57BL/6J background mice.
Table 2.
Comparison of body weight, heart weight, blood pressure, heart rate, and complete blood count between cPLA2α+/+ and cPLA2α−/− male mice at baseline (12 wk old)
| cPLA2α+/+ | cPLA2α−/− | |
|---|---|---|
| Body wt, g | 25.9 ± 1.7 | 24.2 ± 1.8 |
| Heart wt, mg | 116 ± 7.4 | 124 ± 6.5 |
| Heart wt/body wt, ×10−3 | 4.93 ± 0.14 | 5.07 ± 0.17 |
| sBP, mmHg | 83.1 ± 1.8 | 89.1 ± 4.6 |
| dBP, mmHg | 53.5 ± 6.4 | 52.3 ± 5.3 |
| HR, beats/min | 680 ± 12 | 683 ± 14 |
| WBC, μl−1 | 2,686 ± 435 | 2,969 ± 473 |
| Platelet, ×104/mm3 | 41.6 ± 10 | 46.0 ± 11 |
| RBC, ×104/mm3 | 907.1 ± 14.7 | 962 ± 14.8 |
| Hb, g/dl | 13.6 ± 0.60 | 14.1 ± 0.40 |
| Hct, % | 48.8 ± 1.6 | 51.3 ± 1.6 |
Data are expressed as means ± SE; n = 5 mice in each group. cPLA2α+/+, knockout cPLA2 mice; cPLA2α−/−, wild-type cPLA2 mice; sBP, systolic blood pressure; dBP, diastolic blood pressure; HR, heart rate; WBC, white blood cell; RBC, red blood cell; Hb, hemoglobin; Hct, hematocrit; There was no significant difference in each parameter between the 2 genotypes.
Fig. 1.
Comparison of myocardial mRNA expression levels of various types of phospholipase A2 (PLA2) between cytosolic PLA2 (cPLA2) wild-type (cPLA2α+/+) and knockout (cPLA2α−/−) mice at baseline. Real-time quantitative PCR analysis of various types of PLA2 mRNA expression was performed in the left ventricle (LV) from cPLA2α+/+ (filled bars) and cPLA2α−/− (open bars) mice. The levels of mRNA expression normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression and expressed relative to the LV cPLA2α+/+ mice (=1). nd, Not detectable; n = 5∼7 mice. sPLA2, secretory PLA2; iPLA2, Ca2+-independent PLA2.
Myocardial infarct size and echocardiography.
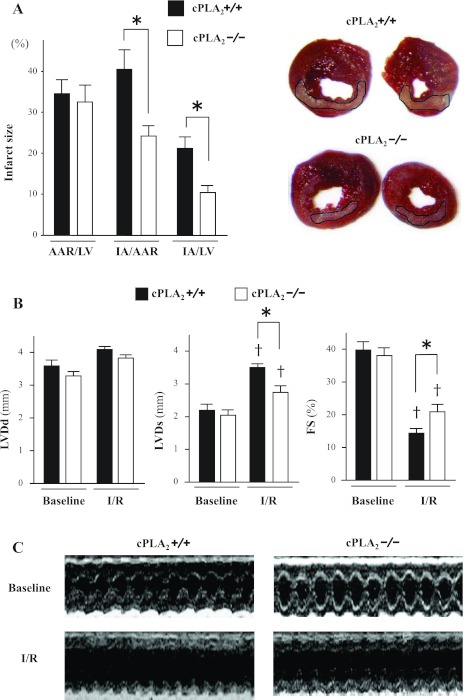
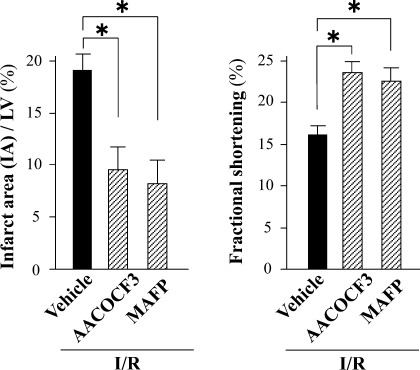
In I/R experiments in vivo, the infarct area (IA)/AAR and IA/LV were significantly smaller in cPLA2α−/− mice than in cPLA2α+/+ mice (IA/AAR: 23 ± 2.3 vs. 41 ± 4.5%, respectively, P < 0.01; IA/LV: 11 ± 1.3 vs. 21 ± 2.3%, respectively, P < 0.01) (Fig. 2A). Echocardiographic parameters at baseline were comparable between the two mouse genotypes. Smaller decreases in fractional shortening (FS) after myocardial I/R injury were observed in cPLA2α−/− mice compared with cPLA2α+/+ mice (%FS: 21 ± 1.6 vs. 14 ± 1.3%, respectively, P < 0.01) (Fig. 2, B and C). In cPLA2α+/+ mice, treatment with either AACOCF3 (20 mg/kg) or MAFP (3 mg/kg), inhibitors of cPLA2α activity, attenuated myocardial infarct size (IA/LV: vehicle, 19 ± 1.6%; AACOCF3, 9.8 ± 1.9%; MAFP, 8.2 ± 1.9%, n = 7 in each experiment, P < 0.01) and preserved FS (vehicle, 16 ± 1.0%; AACOCF3, 23 ± 1.2%; MAFP, 22 ± 1.3%, n = 6 in each experiment, P < 0.01) after I/R (Fig. 3).
Fig. 2.
Myocardial infarct size and echocardiography. A: comparison of myocardial infarct size between cPLA2α+/+ mice (filled bars) and cPLA2α−/− mice (open bars). Right, representative photomicrographs of LV sections from cPLA2α+/+ and cPLA2α−/− mice after 1 h of left anterior descending coronary artery (LAD) occlusion followed by 24 h reperfusion (I/R). Viable myocardium is stained red upon reaction with triphenyltetrazolium chloride (TTC). The infarct area (IA), which is demarcated, appears pale due to a lack of TTC staining. These representative sections were fixed in 10% phosphate-buffered formalin after TTC staining to enhance the contrast of the TTC stain. Left, data indicating the area at risk (AAR) as a percentage of LV mass (AAR/LV), IA as a percentage of AAR (IA/AAR), and IA as a percentage of LV mass (IA/LV) in cPLA2α+/+ and cPLA2α−/− mice (n = 12∼14 for each genotype). *P < 0.01. B: in vivo transthoracic echocardiography at baseline and after I/R. M-mode images were obtained for measurement of LV end-diastolic and end-systolic dimensions (LVDd and LVDs, respectively). The fractional shortening (%FS) was calculated as described in the text (n = 10 for each genotype). †P < 0.01 compared with the respective baselines. *P < 0.01. C: representative M-mode images of LV.
Fig. 3.
Effects of cPLA2 inhibitors on the myocardial infarct size and echocardiographic LV function after I/R in the cPLA2 (cPLA2α+/+) mice. Myocardial I/R injury was made as in Fig. 2 in the cPLA2α+/+ mice. AACOCF3 (20 mg/kg) or MAFP (3 mg/kg), inhibitors of cPLA2α activity, was administered ip 1 h before ischemia in cPLA2α+/+ mice. Myocardial infarct size and echocardiographic LV function were determined as in Fig. 2. Left: myocardial IA/LV. Right: echocardiographic LV function (%FS); n = 6 in each experiment. *P < 0.05 compared with treatment with vehicle.
TUNEL staining, caspase-3 activity, LTB4 and TXB2 content, and MPO activity in myocardium.
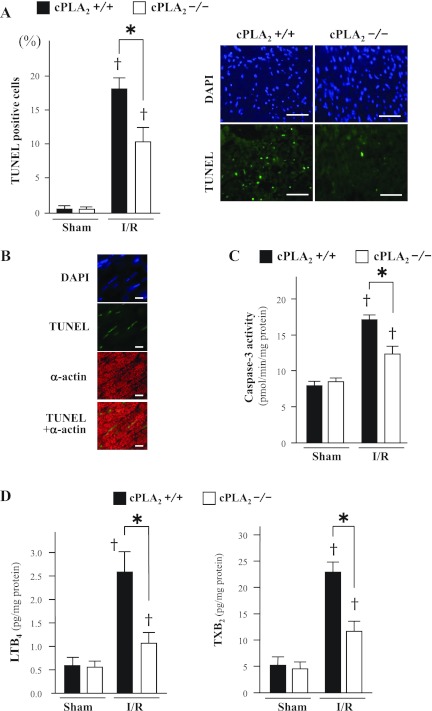
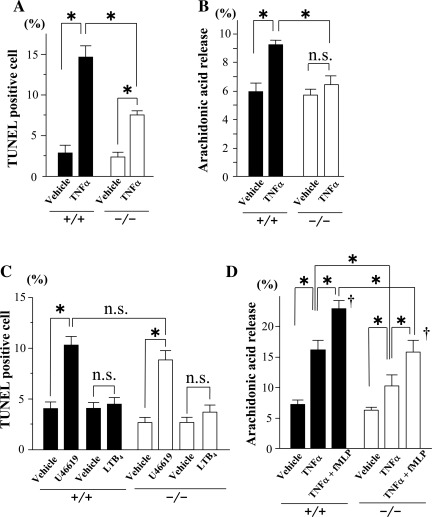
The frequency of TUNEL-positive cells and the caspase-3 activity in ischemic myocardium after I/R were lower in cPLA2α−/− mice than cPLA2α+/+ mice (TUNEL-positive cells: 10 ± 2.0 vs. 18 ± 1.8%, respectively, P < 0.01; caspase-3: 12 ± 0.9 vs. 17 ± 0.7 pmol·min−1·mg protein−1, respectively, P < 0.01) (Fig. 4, A and C). The double-immunofluorescence analysis shows that the TUNEL-positive cells were identified as cardiomyocytes (Fig. 4B).
Fig. 4.
dUTP nick end-labeling (TUNEL) assay, caspase activity, and leukotriene B4 (LTB4) and thromboxane B2 (TXB2) content in myocardium. A: left, percentage of TUNEL-positive nuclei in sham-operated myocardium (Sham) and in noninfarcted myocardium in the ischemic area after 24 h reperfusion following 1 h ischemia (I/R). The TUNEL-positive nuclei were counted in 6 fields of 5 separate sections at ×400 magnification and are presented as a percentage of the total number of cardiomyocytes (nuclei). Right, representative TUNEL staining in noninfarcted myocardium in the ischemic area after I/R. TUNEL-positive nuclei are stained green [fluorescein isothiocyanate (FITC)]. The entire cell population was visualized under a fluorescence microscope with a DAPI filter. Scale bars = 50 μm. B: representative immunofluorescence microscopic images of ischemic myocardium of cPLA2α+/+ mice stained for TUNEL (green) and α-actin (red). Scale bars = 10 μm. C: caspase-3 activity in sham-operated myocardium and in noninfarcted myocardium in the ischemic area after I/R. D: content of LTB4 and TXB2 in homogenates of noninfarcted myocardium in the ischemic area after I/R. *P < 0.05 and †P < 0.01 compared with the respective sham-operated mice (n = 6∼12 mice in each experiment).
The LTB4 and TXB2 levels in the ischemic lesion were increased in both genotyped mice after I/R compared with sham-operated mice but were lower in cPLA2α−/− mice compared with cPLA2α+/+ mice (LTB4: 1.0 ± 0.2 vs. 2.6 ± 0.4 pg/mg protein, respectively, P < 0.01; TXB2: 11 ± 2.1 vs. 22 ± 2.1 pg/mg protein, respectively, P < 0.01) (Fig. 4D).
The MPO activity in the ischemic myocardium was significantly increased in both genetic types of mice compared with sham-operated mice, and the MPO activity in the ischemic myocardium tended to be lower in cPLA2α−/− mice than that in cPLA2α+/+ mice, but it did not reach statistical significance (2.4 ± 0.3 vs. 2.9 ± 0.3 U/100 mg tissue, respectively, n = 6 in each experiment, P = 0.09).
Expression and phosphorylation of cPLA2α in the ischemic myocardium and cultured cardiomyocytes from cPLA2α+/+ mice.
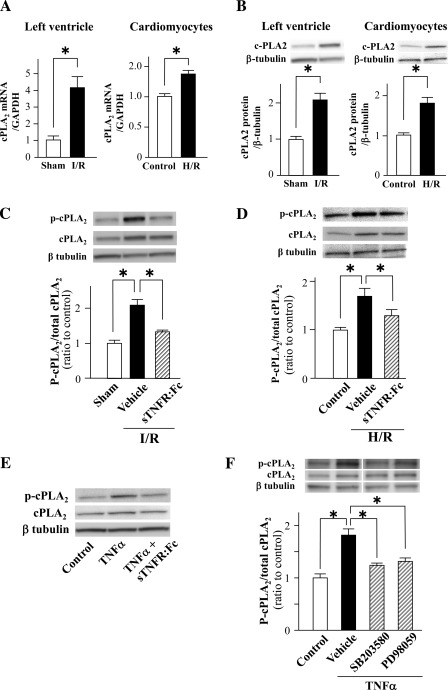
Expression of cPLA2α mRNA and protein was upregulated in the ischemic myocardium after 24 h reperfusion following 1 h ischemia and in cultured cardiomyocytes after H/R (Fig. 5, A and B). I/R induced phosphorylation of cPLA2α in the ischemic myocardium, which was reversed by pretreatment with sTNFR:Fc (10 mg/kg) (Fig. 5C). Similarly, H/R and the incubation with TNF-α (50 ng/ml) induced cPLA2α phosphorylation in cultured cardiomyocytes, which was also reversed by coincubation with sTNFR:Fc (5 μg/ml) (Fig. 5, D and E). The TNF-α-induced cPLA2α phosphorylation was attenuated by coincubation with either SB-203580 (25 μM), p38 MAPK inhibitor, or PD-98059 (25 μM), ERK1/2 inhibitor (Fig. 5F), in cultured cardiomyocytes.
Fig. 5.
Expression and phosphorylation of cPLA2α in the ischemic myocardium and in cultured cardiomyocytes from cPLA2α+/+ mice. Expression of cPLA2α mRNA (A) and protein (B) in the ischemic myocardium after 24 h reperfusion following 1 h ischemia (I/R) and cultured cardiomyocytes after hypoxia-reoxygenation (H/R) from cPLA2α+/+ mice. A: expression levels of cPLA2α mRNA were quantified by real-time quantitative PCR, normalized to GAPDH mRNA expression, and expressed relative to the sham-operated myocardium or control cells (=1). B: expression levels of cPLA2α protein; homogenates (15 μg protein) of tissue or cells were subjected to immunoblot analysis using anti-human cPLA2α polyclonal antibody. The intensity of the β-tubulin band was used as a loading control between samples. The protein levels are expressed relative to the sham-operated myocardium or control cells (=1). Insets on top show representative immunoblots. C: cPLA2α phosphorylation in the ischemic myocardium after 6 h reperfusion following 1 h ischemia and the effect of tumor necrosis factor (TNF)-α blocker, soluble TNF receptor II/IgG1 Fc fusion protein (sTNFR:Fc, 10 mg/kg). Insets show representative immunoblots. D: cPLA2α phosphorylation in the cultured cardiomyocytes after H/R and coincubation with sTNFR:Fc (5 μg/ml). Insets show representative immunoblots. E: immunoblots of cPLA2α phosphorylation in cultured cardiomyocytes after incubation for 12 h with TNF-α (50 ng/ml). F: effects of coincubation with p38 mitogen-activated protein kinase (MAPK) inhibitor (SB-203580, 25 μM) or extracellular signal-regulated kinase (ERK) 1/2 inhibitor (PD-98059, 25 μM) on TNF-α-induced cPLA2α phosphorylation in cultured cardiomyocytes. *P < 0.05; n = 6 mice in each experiment.
Comparison of phosphorylation status of AKT, ERK1/2, p38 MAPK, and JNK in the ischemic myocardium.
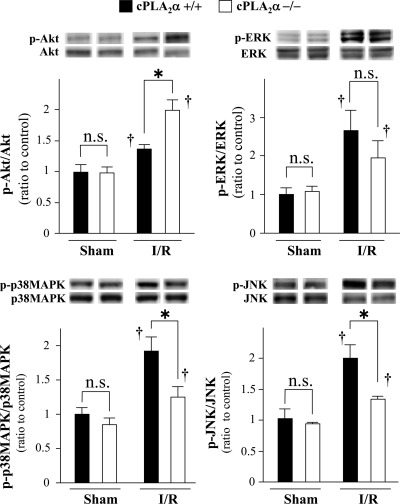
The phosphorylation status of all kinases examined was increased in the ischemic myocardium after 6 h reperfusion following 1 h ischemia. The AKT phosphorylation in the ischemic myocardium was greater in cPLA2α−/− mice than cPLA2α+/+ mice (Fig. 6). In contrast, the phosphorylation of p38 MAPK and JNK in the ischemic myocardium was lower in cPLA2α−/− mice compared with cPLA2α+/+ (Fig. 6). The ERK1/2 phosphorylation in the ischemic myocardium was not statistically different between cPLA2α−/− mice and cPLA2α+/+ mice.
Fig. 6.
Comparison of phosphorylation status of protein kinase B (AKT), ERK1/2, p38 MAPK, and c-Jun NH2-terminal kinase (JNK) in the ischemic myocardium between cPLA2α+/+ and cPLA2α−/− mice. Hearts from cPLA2α+/+ mice (filled bars) and cPLA2α−/− mice (open bars) were harvested at 6 h reperfusion following 1 h ischemia. The expression of total and phosphorylated (p) kinases was assessed by immunoblotting. Insets on top show representative immunoblots. Levels of phosphorylated kinases were expressed as a ratio of the intensity of the respective total protein band and normalized to the sham-operated cPLA2α+/+ myocardium (=1); n = 6 in each experiment. *P < 0.05 and †P < 0.05 compared with the respective sham-operated mice. ns, Not statistically significant.
Effects of TNF-α inhibitor sTNFR:Fc on myocardial I/R injury.
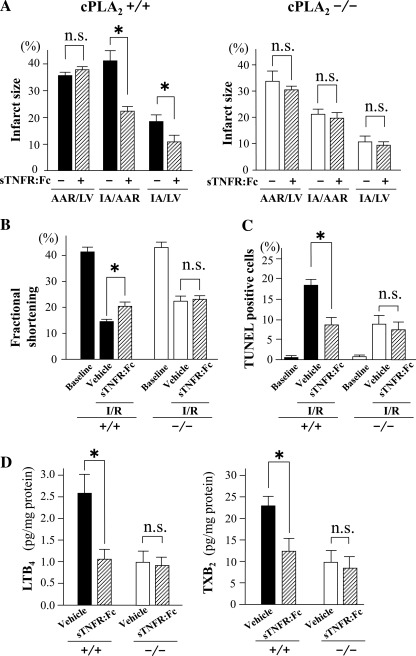
Administration of sTNFR:Fc (10 mg/kg) decreased the IA/LV mass, the IA/AAR, the frequency of TUNEL-positive cells, and the myocardial content of LTB4 and TXB2 after I/R in cPLA2α+/+ mice but had no effect on cPLA2α−/− mice (Fig. 7, A, C, and D). Also, echocardiography showed that I/R decreased LV FS to a lesser extent after sTNFR:Fc administration compared with vehicle in cPLA2α+/+ mice, whereas I/R decreased LV FS to a similar extent after sTNFR:Fc compared with vehicle in cPLA2α−/− mice (Fig. 7B).
Fig. 7.
Effects of sTNFR:Fc on the myocardial infarct size, echocardiographic LV function, TUNEL assay, and myocardial content of LTB4 and TXB2. Myocardial I/R injury was induced as in Fig. 2. The TNF-α blocker sTNFR:Fc or vehicle was administered ip at a dose of 10 mg/kg 1 h before ischemia. Myocardial infarct size and echocardiographic LV function were determined as in Fig. 2. A: myocardial infarct size. Right, data in cPLA2α−/−. Left, data in cPLA2α+/+. B: echocardiographic LV function (%FS). C: TUNEL assay. The TUNEL-positive nuclei are presented as a percentage of the total no. of cardiomyocytes (nuclei). D: LTB4 and TXB2 content in the ischemic myocardium; n = 8∼10 in each experiment. *P < 0.05. +/+, cPLA2α+/+ mice; −/−, cPLA2α−/− mice.
Cultured cardiomyocytes after H/R and incubation with TNF-α, LTB4, and TXA2 analog U-46619.
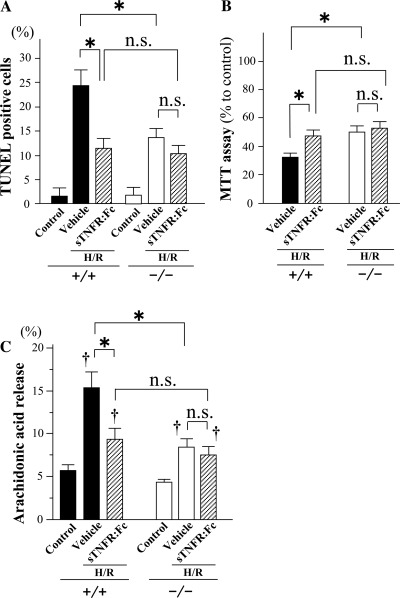
Because cPLA2α is known to be expressed in a variety of constituent cells in myocardium and blood (4, 22, 26, 33), we examined the contribution of cPLA2α in cardiomyocytes to myocardial I/R injury in in vitro experiments using cultured cardiomyocytes. In cardiomyocytes cultured from cPLA2α−/− mice, H/R induced fewer TUNEL-positive cells, lessened hypoxic injury as evaluated by MTT assay, and resulted in a smaller amount of arachidonic acid release than that of cardiomyocytes cultured from cPLA2α+/+ mice (Fig. 8). Coincubation with sTNFR:Fc (5 μg/ml) suppressed the induction of the TUNEL-positive cells, hypoxic injury, and release of arachidonic acid after H/R in cPLA2α+/+ cardiomyocytes but not cPLA2α−/− cardiomyocytes (Fig. 8). Treatment with TNF-α (50 ng/ml) induced TUNEL-positive cells and the release of arachidonic acid in cPLA2α+/+ cardiomyocytes (Fig. 9, A and B). In contrast, TNF-α induced fewer TUNEL-positive cells and had no significant effect on the release of arachidonic acid in cPLA2α−/− cardiomyocytes (Fig. 9, A and B). The incubation with U-46619 (100 nM), a TXA2 analog, induced TUNEL-positive cells in either cPLA2α+/+ cardiomyocytes or cPLA2α−/− cardiomyocytes (Fig. 9C), whereas LTB4 (100 nM) did not induce TUNEL-positive cells. The proapoptotic effect of U-46619 was similar between cPLA2α+/+ and cPLA2α−/− cardiomyocytes (Fig. 9C).
Fig. 8.
Effects of sTNFR:Fc on cultured cardiomyocytes after H/R. Cultured cardiomyocytes were exposed to H/R in the presence of vehicle or sTNFR:Fc (5 μg/ml). A: TUNEL assay in cultured cardiomyocytes. The TUNEL-positive nuclei were counted in 6 fields of 5 separate chambers on glass slides at ×400 magnification and are presented as a percentage of the total number of cardiomyocytes (nuclei). B: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. The reduction in MTT activity in the treated cells is presented as a percentage of nontreated control cells. C: extracellular release of arachidonic acid from cultured cardiomyocytes. Cardiomyocytes prelabeled with 0.5 μCi/m [3H]arachidonic acid were exposed to H/R in the presence of vehicle or sTNFR:Fc. The radioactivity in the supernatant was measured in a scintillation spectrometer. The activity was expressed as the percentage of total cell radioactivity. *P < 0.01 and †P < 0.01 compared with the respective nontreated control cells (Control); n = 6∼8 mice in each experiment.
Fig. 9.
Effects of TNF-α, LTB4, and TXA2 analog U-46619 in cultured cardiomyocytes and isolated neutrophils. A: TNF-α (50 ng/ml)-induced TUNEL-positive nuclei in cultured cardiomyocytes. TUNEL-positive nuclei were counted after incubation for 12 h with vehicle or TNF-α. B: extracellular release of arachidonic acid from cultured cardiomyocytes in response to TNF-α. Cardiomyocytes prelabeled with 0.5 μCi/ml [3H]arachidonic acid were treated for 12 h with vehicle or TNF-α (50 ng/ml). The radioactivity in the supernatant was measured in a scintillation spectrometer. The activity was expressed as the percentage of total cell radioactivity. The content was calculated by dividing by the amount of cellular protein. C: TUNEL-positive nuclei in cultured cardiomyocytes after incubation for 12 h with TXA2 analog U-46619 (100 nM) or LTB4 (100 nM). D: extracellular release of arachidonic acid from isolated peritoneal neutrophils in response to TNF-α (50 ng/ml) alone for 20 min or in combination with N-formyl-methionyl-leucyl phenylalanine (fMLP, 1 μM) for an additional 20 min. *P < 0.01 and †P < 0.05 compared with vehicle; n = 6∼8 mice in each experiment.
Arachidonic acid release from isolated neutrophils.
Treatment with TNF-α (50 ng/ml) alone for 20 min or in combination with N-formyl-methionyl-leucyl phenylalanine (1 μM) for an additional 20 min induced a smaller amount of arachidonic acid release in cPLA2α−/− neutrophils than cPLA2α+/+ neutrophils (Fig. 9D).
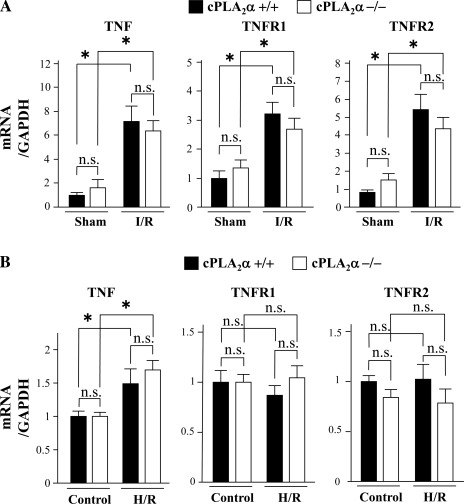
Expression of mRNA of TNF-α, TNF receptor-1, and TNF receptor-2 in the ischemic myocardium after I/R and in cultured cardiomyocytes after H/R.
Expression of mRNA of TNF-α, TNF receptor (TNFR)-1, and TNFR-2 was upregulated in the ischemic myocardium after I/R in both mouse genotypes, and the extent of their expression did not differ in either sham-operated or ischemic myocardium after I/R between cPLA2α−/− and cPLA2α+/+ mice (Fig. 10A). Expression of TNF-α but not TNFR-1 or TNFR-2 was upregulated in cultured cardiomyocytes after H/R, and their expression levels were similar in control cells or cells after H/R between cPLA2α−/− and cPLA2α+/+ cardiomyocytes (Fig. 10B).
Fig. 10.
Expression levels of mRNA of TNF-α, TNF receptor (TNFR)-1, and TNFR-2 in the ischemic myocardium and cultured cardiomyocytes. Expression levels of mRNA of TNF-α, TNFR-1, and TNFR-2 were quantified by real-time quantitative PCR, normalized to GAPDH mRNA expression, and expressed relative to the sham-operated myocardium (=1). Ischemic myocardium at 24 h reperfusion following 1 h ischemia (A) and cultured cardiomyocytes after H/R (B) from cPLA2α+/+ mice (filled bars) and cPLA2α−/− mice (open bars); n = 6 in each experiment. *P < 0.05.
DISCUSSION
The present study demonstrated that myocardial I/R injury and apoptosis were attenuated in cPLA2α−/− mice in association with a reduction in myocardial content of LTB4 and TXB2, which potentially exert deleterious effects on ischemic myocardium compared with cPLA2α+/+ mice. Pretreatment with the TNF-α antagonist sTNFR:Fc decreased myocardial I/R injury, apoptosis, and content of LTB4 and TXB2 in cPLA2α+/+ mice, but not in cPLA2α−/− mice. sTNFR:Fc inhibited I/R-induced phosphorylation of cPLA2α in the ischemic myocardium in cPLA2α+/+ mice. Together, these results suggest that cPLA2α, in concert with TNF-α, plays a pathogenic role in myocardial I/R injury. Consistent with these in vivo data, sTNFR:Fc exerted protective effects against H/R-induced injury and apoptosis in cPLA2α+/+ cardiomyocytes but not cPLA2α−/− cardiomyocytes. Incubation with TNF-α as well as H/R induced cPLA2α phosphorylation in cPLA2α+/+ cardiomyocytes, which was reversible by sTNFR:Fc. TNF-α incubation increased the amount of apoptosis and release of arachidonic acid in cPLA2α+/+ cardiomyocytes, but it had smaller or no effects in cPLA2α−/− cardiomyocytes. These results indicate that the signaling of endogenous TNF-α may be an upstream regulator of cPLA2 activation during H/R and that cPLA2 activation works cooperatively with TNF-α signaling in H/R injury in cultured cardiomyocytes. Taken together, deletion of cPLA2α suppressed myocardial I/R injury partly by inhibiting TNF-α-mediated pathways.
The present study could not determine how cPLA2α exerted cytotoxicity in the ischemic myocardium, but several mechanisms are considered (5, 29, 36). Arachidonic acid, generated by cPLA2α, is a potent inducer of apoptosis through the mechanisms of induction of mitochondrial permeability transition and an increase in ceramide levels. In addition, arachidonic acid metabolites, 15-hydroxyeicosatetraenoic acid, 12-epoxyeicosatrienoic acids, and 19-hydroxyeicosatetraenoic acid, can induce apoptosis. The present study showed that the content of TXB2 and LTB4 in the ischemic myocardium was greater in cPLA2α+/+ mice and that TXA2 analog U-46619 exerted a direct proapoptotic effect. Also, LTB4 is known to have a proinflammatory effect (30) that potentially leads to inflammatory cell-mediated cytotoxic effects on myocardium. In addition, arachidonic acid exerts necrotic action by loss of cell membrane integrity, namely induction of lipid peroxidation or by detergent action, and preventing ATP generation. The present study showed that cPLA2α deficiency suppressed the activation of proapoptotic kinases, p38 MAPK and JNK, in the ischemic myocardium, whereas cPLA2α deficiency augmented the activation of AKT, an anti-apoptotic kinase. These results may partly explain for the reduction in apoptosis in the ischemic myocardium in cPLA2α−/− mice. The increased activation of AKT in the ischemic myocardium was in agreement with the enhanced signaling pathway of insulin-like growth factor (IGF)-I-phosphatidylinositol-3-OH kinase-AKT in cPLA2α−/− mice as shown in a previous report (16). Whether the activation of IGF-I signaling may be directly related to attenuation of myocardial I/R injury and apoptosis in cPLA2α−/− mice remains to be determined. It is possible that cPLA2α−/− myocardium after I/R may have a lower concentration of arachidonic acid that leads to suppression of arachidonic acid-induced activation of p38 MAPK and JNK compared with cPLA2α+/+ myocardium (19). However, the precise mechanisms for lower phosphorylation of p38 MAPK and JNK in cPLA2α−/− myocardium after I/R remain to be determined.
There are conflicting results regarding the role of TNF-α and its receptors in myocardial I/R injury (6, 10, 23, 27). Preischemic neutralization of TNF-α with TNF-α antibody or soluble TNFR-1 consistently reduced myocardial I/R injury (14, 15, 34, 37), which is in agreement with the present data. In contrast, permanent inhibition of TNF-α action using gene knockout of TNF-α resulted in different outcomes (6, 10, 27). The mechanisms responsible for these differences remain unclear, but deletion of the TNF-α gene product might induce unknown compensatory mechanisms that may cause different myocardial response to I/R insults (23). The TNF-α signaling pathway contributes to cardioprotection by ischemic preconditioning, but the cardioprotective effects of TNF-α depend on its concentration, duration of exposure, and localization (23, 25). It has been previously shown that TNF-α might participate in multiple signaling pathways leading to cPLA2α activation (21, 24) and that these may involve both TNFR-1 and TNFR-2 in a manner that is either dependent or independent of p38 MAPK and ERK1/2 (21, 24). It is reported that TNFR-1 stimulates p38 MARK, ERK1/2, and JNK through interaction with TNFR-1-associating adaptor proteins and that all of these kinases could phosphorylate and activate cPLA2α (21, 24). In line with these previous reports (21, 24), the present inhibitors studied showed that p38 MAPK and ERK1/2 may play a role in TNF-α-induced cPLA2α phosphorylation in cultured cardiomyocytes. Although TNFR-2 mediates cPLA2α activation to a smaller extent than TNFR-1, TNFR-2 is reported to mediate cPLA2α activation independently of kinase phosphorylation (21, 24). In addition, upregulation of TNF-α and its receptors and cPLA2α expression in the ischemic myocardium might also modulate signaling pathways between TNF-α and cPLA2α leading to myocardial I/R injury. The signaling mechanisms between TNF-α and cPLA2 activation in myocardial ischemic injury thus appear to be very complex, and the exact mechanisms still remain to be determined.
It has been shown that cPLA2α activation is a key downstream regulator of many cytokines to generate eicosanoids in inflammatory diseases and tissue injury (4, 22, 26, 33). Also, we have recently shown that cPLA2α has cross talk signaling with sPLA2-V to produce detrimental eicosanoids following myocardial I/R injury (39). Thus, divergent external stimuli may be mediated through cPLA2α activation in inflammatory diseases or ischemic injury. In this sense, cPLA2α may be an important therapeutic target in inflammatory and ischemic diseases. In line with this idea, the present study showed that pharmacological inhibitors of cPLA2 activity exerted protective effects for myocardial I/R injury. However, it should be noted that some arachidonic acid-derived lipid mediators, such as prostaglandin E2 and epoxyeicosatrienoic acids, have cytoprotective properties (12). A recent report showed that the protective effects of pioglitazone, an insulin-sensitizing thiazolidinedione, against myocardial I/R injury were associated with upregulation of cPLA2α expression (2). Thus, cPLA2α may have a dual role (40), i.e., having both protective and detrimental effects in inflammatory or ischemic diseases. Although the present study indicates that cPLA2α has a causative role in myocardial I/R injury during the acute phase, which role cPLA2α may play in cardiac remodeling during the chronic phase after I/R remains to be determined.
The present study used mice with systemic but not cardiomyocyte-specific deletion of cPLA2α. Thus, the present study cannot dissect the effects of myocardial cPLA2α from that of cPLA2α expressed by blood and other cells. In the present study, the experiments using the isolated cultured cardiomyocytes showed consistent results with those in in vivo experiments, indicating a role of cPLA2α in cardiomyocytes in myocardial I/R injury. Although the number of the infiltrated neutrophils in the ischemic myocardium was not statistically different between cPLA2α+/+ and cPLA2α−/− mice as estimated with MPO assay, the amount of arachidonic acid release in response to TNF-α was less in cPLA2α−/− mice than cPLA2α+/+ mice. This indicated that cPLA2α in the infiltrated neutrophils may have a role in the production of arachidonic acid and its harmful metabolites, leading to myocardial I/R injury.
In conclusion, disruption of cPLA2 attenuated myocardial I/R injury partly through inhibition of TNF-α-mediated pathways.
GRANTS
This work was supported by the Ministry of Education, Culture, Sports, Science, and Technology, Health, Tokyo, Japan (grants-in-aid for B2-19390209 and B-22390158, Priority Areas C Medical Genome Science 15012222 to K. Kugiyama), and Health and Labor Sciences Research Grants for Comprehensive Research on Aging and Health, Tokyo, Japan (H15-Choju-012 to K. Kugiyama).
DISCLOSURES
None declared.
AUTHOR CONTRIBUTIONS
Author contributions: Y.S., K.W., D.F., T.N., J.-e.O., K.k., Y.W., H.M., S.T., Y.K., T.S., and K.K. conception and design of research; Y.S., K.W., D.F., Y.W., H.M., S.T., Y.K., and T.S. performed experiments; Y.S., K.W., D.F., T.N., J.-e.O., K.k., Y.W., H.M., S.T., and K.K. analyzed data; Y.S., K.W., D.F., T.N., J.-e.O., K.k., Y.W., H.M., S.T., Y.K., T.S., and K.K. interpreted results of experiments; Y.S. and K.K. prepared figures; Y.S. and K.K. drafted manuscript; Y.S. and K.K. edited and revised manuscript; Y.S., K.W., D.F., T.N., J.-e.O., K.k., Y.W., H.M., S.T., Y.K., T.S., and K.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge the technical assistance of M. Nakamura, A. Watanabe, and H. Watanabe.
REFERENCES
- 1. Balsinde J, Winstead MV, Dennis EA. Phospholipase A2 regulation of arachidonic acid mobilization. FEBS Lett 531: 2–6, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Birnbaum Y, Long B, Qian J, Perez-Polo JR, Ye Y. Pioglitazone limits myocardial infarct size, activates Akt, and upregulates cPLA2 and COX-2 in a PPAR-γ-independent manner. Basic Res Cardiol 106: 431–446, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Bonventre JV, Huang Z, Taheri MR, O'Leary E, Li E, Moskowitz MA, Sapirstein A. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature 390: 622–625, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Bonventre JV, Sapirstein A. Group IV cytosolic phospholipase A2 (PLA2) function: insights from the knockout mouse. Adv Exp Med Biol 507: 25–31, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Caro AA, Cederbaum AI. Role of cytochrome P450 in phospholipase A2- and arachidonic acid-mediated cytotoxicity. Free Radic Biol Med 40: 364–375, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Dawn B, Guo Y, Rezazadeh A, Wang OL, Stein AB, Hunt G, Varma J, Xuan YT, Wu WJ, Tan W, Zhu X, Bolli R. Tumor necrosis factor-α does not modulate ischemia/reperfusion injury in naive myocardium but is essential for the development of late preconditioning. J Mol Cell Cardiol 37: 51–61, 2004 [DOI] [PubMed] [Google Scholar]
- 7. De Windt LJ, Reneman RS, Van der Vusse GJ, Van Bilsen M. Phospholipase A2-mediated hydrolysis of cardiac phospholipids: the use of molecular and transgenic techniques. Mol Cell Biochem 180: 65–73, 1998 [PubMed] [Google Scholar]
- 8. Defer N, Azroyan A, Pecker F, Pavoine C. TNFR1 and TNFR2 signaling interplay in cardiac myocytes. J Biol Chem 282: 35564–35573, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Diaz BL, Arm JP. Phospholipase A2. Prostaglandins Leukot Essent Fatty Acids 69: 87–97, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Flaherty MP, Guo Y, Tiwari S, Rezazadeh A, Hunt G, Sanganalmath SK, Tang XL, Bolli R, Dawn B. The role of TNF-alpha receptors p55 and p75 in acute myocardial ischemia/reperfusion injury and late preconditioning. J Mol Cell Cardiol 45: 735–741, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fujioka D, Saito Y, Kobayashi T, Yano T, Tezuka H, Ishimoto Y, Suzuki N, Yokota Y, Nakamura T, Obata J, Kanazawa M, Kawabata K, Hanasaki K, Kugiyama K. Reduction in myocardial ischemia/reperfusion injury in group X secretory phospholipase A2-deficient mice. Circulation 117: 2977–2985, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Gross GJ, Falck JR, Gross ER, Isbell M, Moore J, Nithipatikom K. Cytochrome P450 and arachidonic acid metabolites: role in myocardial ischemia/reperfusion injury revisited. Cardiovasc Res 68: 18–25, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Gross RW. Myocardial phospholipase A2. J Lipid Mediat Cell Signal 12: 131–137, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Gu Q, Yang XP, Bonde P, DiPaula A, Fox-Talbot K, Becker LC. Inhibition of TNF-α reduces myocardial injury and proinflammatory pathways following ischemia-reperfusion in the dog. J Cardiovasc Pharmacol 48: 320–328, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Gurevitch J, Frolkis I, Yuhas Y, Lifschitz-Mercer B, Berger E, Paz Y, Matsa M, Karmer A, Mohr R. Anti-tumor necrosis factor-alpha improves myocardial recovery after ischemia and reperfusion. J Am Coll Cardiol 30: 1554–1561, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Haq S, Kilter H, Michael A, Tao J, O'Leary E, Sun XM, Walters B, Bhattacharya K, Chen X, Cui L, Andreucci M, Rosenzweig A, Guerrero JL, Patten R, Liao R, Molkentin J, Picard M, Bonventre JV, Force T. Deletion of cytosolic phospholipase A2 promotes striated muscle growth. Nat Med 9: 944–951, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res 61: 448–460, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Hayakawa M, Ishida N, Takeuchi K, Shibamoto S, Hori T, Oku N, Ito F, Tsujimoto M. Arachidonic acid-selective cytosolic phospholipase A2 is crucial in the cytotoxic action of tumor necrosis factor. J Biol Chem 268: 11290–11295, 1993 [PubMed] [Google Scholar]
- 19. Hii CS, Huang ZH, Bilney A, Costabile M, Murray AW, Rathjen DA, Der CJ, Ferrante A. Stimulation of p38 phosphorylation and activity by arachidonic acid in HeLa cells, HL60 promyelocytic leukemic cells, and human neutrophils. Evidence for cell type-specific activation of mitogen-activated protein kinases. J Biol Chem 273: 19277–19282, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Hoeck WG, Ramesha CS, Chang DJ, Fan N, Heller RA. Cytoplasmic phospholipase-A2 activity and gene-expression are stimulated by tumor-necrosis-factor: dexamethasone blocks the induced synthesis. Proc Natl Acad Sci USA 90: 4475–4479, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jupp OJ, Vandenabeele P, MacEwan DJ. Distinct regulation of cytosolic phospholipase A2 phosphorylation, translocation, proteolysis and activation by tumour necrosis factor-receptor subtypes. Biochem J 374: 453–461, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kita Y, Ohto T, Uozumi N, Shimizu T. Biochemical properties and pathophysiological roles of cytosolic phospholipase A2s. Biochim Biophys Acta 1761: 1317–1322, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Kleinbongard P, Heusch G, Schulz R. TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther 127: 295–314, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Krönke M, Adam-Klages S. Role of caspases in TNF-mediated regulation of cPLA(2). FEBS Lett 531: 18–22, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Lecour S, Suleman N, Deuchar GA, Somers S, Lacerda L, Huisamen B, Opie LH. Pharmacological preconditioning with tumor necrosis factor-alpha activates signal transducer and activator of transcription-3 at reperfusion without involving classic prosurvival kinases (Akt and extracellular signal-regulated kinase). Circulation 112: 3911–3918, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Leslie CC. Properties and regulation of cytosolic phospholipase A2. J Biol Chem 272: 16709–16712, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Maekawa N, Wada H, Kanda T, Niwa T, Yamada Y, Saito K, Fujiwara H, Sekikawa K, Seishima M. Improved myocardial ischemia/reperfusion injury in mice lacking tumor necrosis factor-α. J Am Coll Cardiol 39: 1229–1235, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Murakami M, Kudo I. Secretory phospholipase A2. Biol Pharm Bull 27: 1158–1164, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Nakanishi M, Rosenberg DW. Roles of cPLA2alpha and arachidonic acid in cancer. Biochim Biophys Acta 1761: 1335–1343, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poeckel D, Funk CD. The 5-lipoxygenase/leukotriene pathway in preclinical models of cardiovascular disease. Cardiovasc Res 86: 243–253, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Prasad MR, Popescu LM, Moraru II, Liu XK, Maity S, Engelman RM, Das DK. Role of phospholipases A2 and C in myocardial ischemic reperfusion injury. Am J Physiol Heart Circ Physiol 260: H877–H883, 1991 [DOI] [PubMed] [Google Scholar]
- 32. Sen A, Buja LM, Willerson JT, Chien KR. Membrane phospholipid metabolism during myocardial ischaemia: past, present and future. Basic Res Cardiol 82, Suppl 1: 121–125, 1987 [DOI] [PubMed] [Google Scholar]
- 33. Shimizu T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev Pharmacol Toxicol 49: 123–125, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Sugano M, Hata T, Tsuchida K, Suematsu N, Oyama J, Satoh S, Makino N. Local delivery of soluble TNF-alpha receptor 1 gene reduces infarct size following ischemia/reperfusion injury in rats. Mol Cell Biochem 266: 127–132, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Tabuchi S, Uozumi N, Ishii S, Shimizu Y, Watanabe T, Shimizu T. Mice deficient in cytosolic phospholipase A2 are less susceptible to cerebral ischemia/reperfusion injury. Acta Neurochir Suppl 86: 169–172, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Taketo MM, Sonoshita M. Phospolipase A2 and apoptosis. Biochim Biophys Acta 1585: 72–76, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Toufektsian MC, Robbez-Masson V, Sanou D, Jouan MG, Ormezzano O, de Leiris J, Boucher F. A single intravenous sTNFR-Fc administration at the time of reperfusion limits infarct size–implications in reperfusion strategies in man. Cardiovasc Drugs Ther 22: 437–442, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Valentin E, Ghomashchi F, Gelb MH, Lazdunski M, Lambeau G. On the diversity of secreted phospholipases A2. Cloning, tissue distribution, and functional expression of two novel mouse group II enzymes. J Biol Chem 274: 31195–31202, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Yano T, Fujioka D, Saito Y, Kobayashi T, Nakamura T, Obata JE, Kawabata K, Watanabe K, Watanabe Y, Mishina H, Tamaru S, Kugiyama K. Group V secretory phospholipase A2 plays a pathogenic role in myocardial ischaemia-reperfusion injury. Cardiovasc Res 90: 335–343, 2011 [DOI] [PubMed] [Google Scholar]
- 40. Yedgar S, Cohen Y, Shoseyov D. Control of phospholipase A2 activities for the treatment of inflammatory conditions. Biochim Biophys Acta 1761: 1373–1382, 2006 [DOI] [PubMed] [Google Scholar]