Abstract
Background
Endothelial progenitor cells (EPCs) are involved in the endothelium repair. Low circulating EPC levels are predictive of cardiovascular events in HIV-negative subjects. The impact of HIV infection on EPCs, and the role of EPCs in HIV-associated cardiovascular disease, is not known. We hypothesized that circulating EPCs would be inversely associated with carotid artery intima-media thickness (c-IMT) changes in HIV-infected subjects.
Methods
EPCs (CD34+/KDR+, CD133+/KDR+ and CD34+/CD133+/KDR+) were defined retrospectively by flow cytometry in cryopreserved peripheral blood mononuclear cells collected longitudinally from 66 chronic HIV-infected subjects and cross-sectionally from 50 at-risk HIV-negative subjects. The HIV-infected subjects participated in the Study of the Consequences of the Protease Inhibitor Era (SCOPE) cohort, were receiving antiretroviral therapy (59/66) and had two sequential measurements of c-IMT 1 year apart. Two distinct groups of HIV-infected subjects were identified a priori: rapid c-IMT progressors (subjects with rapid c-IMT progression, n=13, Δc-IMT>0.2 mm) and slow c-IMT progressors (subjects with slow or no c-IMT progression, n=53, Δc-IMT<0.2 mm).
Results
Although cryopreservation reduced sensitivity of detection, EPC frequency in HIV-infected subjects was still significantly higher compared to at-risk HIV-negative subjects (CD34+/KDR+; P=0.01) and correlated positively with CD4+ T-cell count (CD34+/KDR+, r=0.27; P=0.03). No association was found between the change of EPC frequencies over time (ΔEPC) and Δc-IMT or between EPC frequencies and c-IMT or Δc-IMT.
Conclusions
The lack of an association between EPCs and c-IMT in our cohort does not support HIV-associated reductions in EPC frequency as a cause of accelerated atherosclerosis.
Introduction
HIV infection increases cardiovascular risk by promoting early atherosclerosis via endothelial activation [1,2]. Introduction of antiretroviral therapy (ART) does not completely normalize endothelial function [3,4].
Measurement of carotid artery intima-media thickness (c-IMT) with high-resolution B-mode ultrasound is a well-validated surrogate marker of atherosclerosis and cardiovascular risk, even after adjustment for other risk factors [5]. As shown by our group [1], HIV-infected patients have increased c-IMT compared with healthy age-matched control subjects, and exhibit rapid progression of c-IMT.
Endothelial progenitor cells (EPCs) – a heterogeneous population of cells found in the peripheral circulation [6–9] – are actively recruited at sites of new vessel growth [10], take part in compensatory angiogenesis in ischemic tissues [10] and are considered as a biomarker for cardiovascular disease [11,12]. Flow cytometry identifies EPCs by the expression of both haematopoietic stem cells (CD34 and CD133) and endothelial cell markers (kinase insert domain receptor [KDR]) [13–15], although recently, it has been suggested that CD133+ cells include haematopoietic rather than endothelial progenitors [9,16]. Colony-forming assays identify EPCs as colony-forming unit endothelial cells (CFU-EC) expressing endothelial and myeloid markers (CD45 and CD14) with slow proliferative ability and of haematopoietic/monocytic origin (not usually considered as a ‘progenitor’ population), and endothelial colony-forming cells (ECFC) exhibiting a ‘classic’ endothelial phenotype and high proliferative capacity [9,17].
We conducted a retrospective study in cryopreserved peripheral blood mononuclear cells (PBMCs) with matched c-IMT progression data in order to investigate the effect of HIV infection on EPC frequencies and the association between EPC frequencies and c-IMT changes.
Methods
Participants
Cryopreserved PBMCs were retrospectively selected from 66 HIV-positive and 50 at-risk HIV-negative individuals. HIV-positive subjects were enrolled in the Study of the Consequences of the Protease Inhibitor Era (SCOPE) cohort, were receiving ART (59/66), underwent two measurements of c-IMT 1 year apart and were segregated a priori – based on c-IMT progression over 1 year (Δc-IMT) – to rapid c-IMT progressors (subjects with rapid c-IMT progression, n=13, Δc-IMT>0.2 mm) and slow c-IMT progressors (subjects with slow or no c-IMT progression, n=53, Δc-IMT<0.2 mm). HIV-negative subjects were randomly sampled from a study of persons seeking post-exposure prophylaxis following unprotected sex with an HIV-positive or status-unknown partner. Their HIV-negative status was confirmed at the time of sampling by a non-reactive antibody test for HIV and undetectable plasma HIV-1 RNA. Informed consent was obtained from all participants. The study protocol was approved by the Institutional Review Boards of the authors’ institutions.
Carotid artery intima-media assessment
Carotid ultrasound assessments at baseline and follow-up were made using a GE Vivid 7 machine (Universal Diagnostic Solutions, Oceanside, CA, USA) and following a previously described standardized protocol [1]. c-IMT was measured in 12 segments on both the right and left side including the near and far wall of the common carotid, the near and far wall of the bifurcation region and the near and far wall of the internal carotid by an experienced vascular technician who was blinded to the patient’s HIV status and clinical features.
Flow cytometry analysis
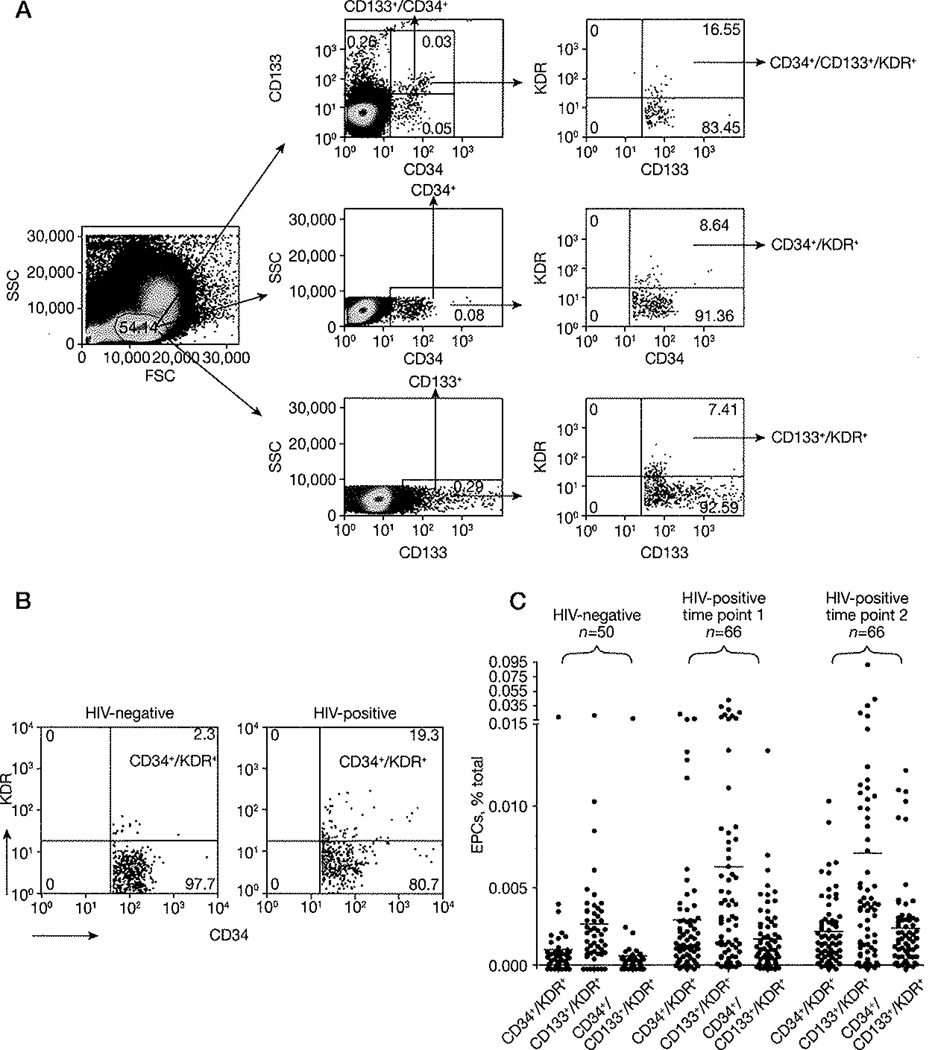
Cryopreserved PBMC were stained as previously described [13] with anti-human monoclonal antibody including CD34-fluorescein isothiocyanate (FITC; BD Biosciences, San Jose, CA, USA), CD133-phycoerythrin (PE; Miltenyi Biotech, Auburn, CA, USA) and KDR-allophycocyanin (APC; R&D Biosystems, Minneapolis, MN, USA), and mouse isotype controls including IgG1k-FITC (BD Biosciences), IgG1-PE (BD Biosciences) and IgG1-APC (R&D Biosystems). A 9-colour CyAn cytofluorimeter (Cytomation, Fort Collins, CO, USA) was used for data collection in 2–3×lO6 live lymphocytes (defined by size and granularity in forward scatter and side scatter) and the FloJo software (Tree Star, San Carlos, CA, USA) was used for analysis. EPC subpopulations were defined following manual definition of the lymphocyte gate and as suggested by Fadini et al. [13] (Figure 1A). Thresholds were set by isotype-matched negative controls and unstained cells. In the text, EPCs frequencies represent percentage of total cells. Analysis was done for all EPC subsets. As CD34+/KDR+ cells are considered as the major constituent of the circulating EPCs and are more strictly linked to cardiovascular damage [13], the term ‘EPC’ is being used in the text primarily for CD34+/KDR+, although findings were also confirmed for CD133+/KDR+ and CD34+/CD133+/KDR+ cells. Preliminary experiments measured EPCs in fresh venous blood, fresh PBMC and cryopreserved PBMC.
Figure 1. Increased levels of CD34+/KDR+ cells in HIV-1-infected subjects on antiretroviral therapy.
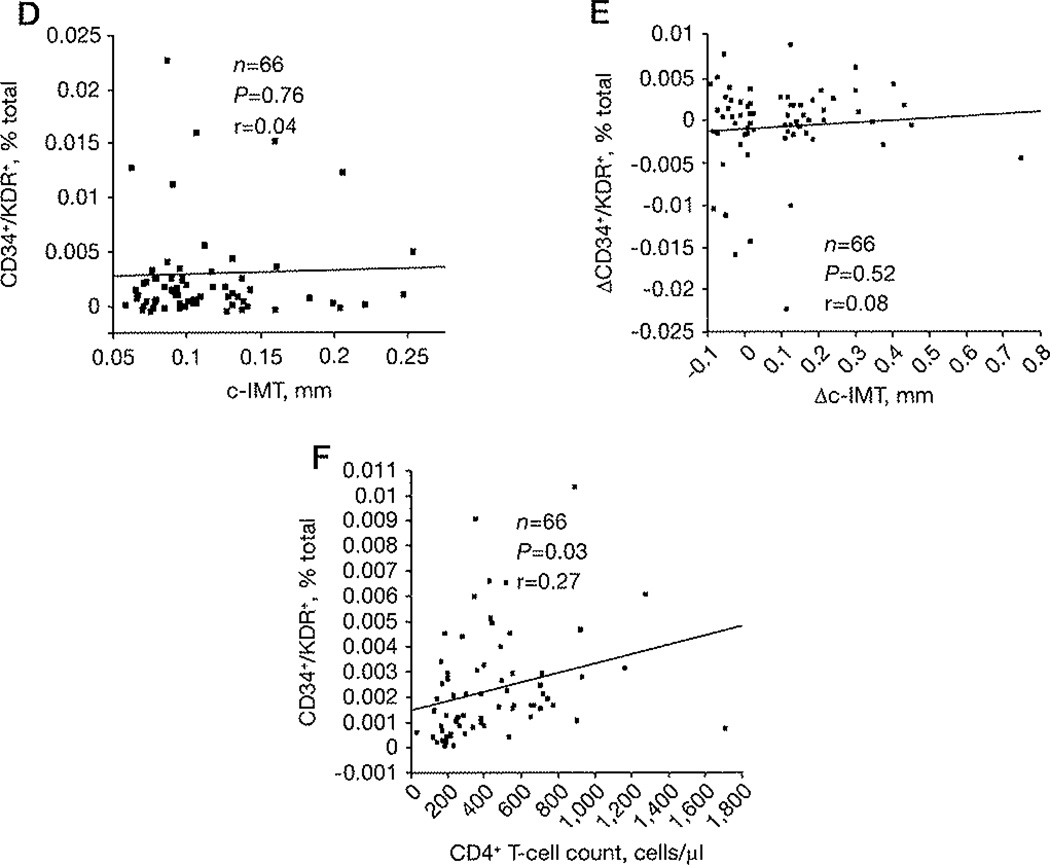
(A) Gating approach, Scatter plots illustrating the gating approach used to quantify peripheral blood progenitor cells on the basis of the cell surface expression of CD34, CD133 and KDR molecules. Thresholds were set by combined usage of isotype-matched negative controls and unstained cells. (B) Scatter plots of a representative HIV-negative and a representative HIV-positive subject showing CD134+/KDR+ cells (thresholds were set by combined usage of isotype-matched negative controls and unstained cells). (C) CD34+/KDR+/CD133+/KDR+ and CD34+/CD133+/KDR+ percentages of total cells are shown for HIV-negative (n=50) and HIV-positive subjects for time points 1 (baseline, n=66) and 2 (1 year, n=66), respectively (HIV-negative versus HIV-positive time point 1: CD34+/KDR+ P=0.011, CD133+/KDR+ P=0.008 and CD34+/CD133+/KDR+ P=0.018; HIV-negative versus HIV-positive time point 2: CD34+/KDR+ P=0.015, CD133+/KDR+ P=0.025 and CD34+/CD133+/KDR+ P=0.0008). (D) Correlation between the CD34+/KDR+ percentage of total cells and carotid artery intima-media thickness (c-IMT) at time point 1. (E) Correlation between the change of CD34+/KDR+ percentage of total cells (ΔCD34+/KDR+) and Δc-IMT between time points 1 and 2. (F) Correlation between the CD34+/KDR+ percentage of total cells and CD4+ T-cell count at time point 2. Data in (C) is shown for all subjects together with mean of the distribution, whereas data in panels (D–F) are shown as regression lines, with correlation and P-values. EPC, endothelial progenitor cell; FSC, forward scatter; SSC, side scatter.
Statistical analyses
Data are described as medians with 25th and 75th percentiles in parentheses. Variable distributions were analysed for normality using the Shapiro–Wilk W test (P>0.05). Depending on data distribution, Student’s t-test or Wilcoxon/Kruskal–Wallis test (rank sums) were used for between-groups comparisons and non-parametric Wilcoxon signed-rank test or paired Student’s t-tests for between-time-points comparisons. Correlation analysis was done using Spearman or pairwise correlation tests. Statistical analysis was performed using JMP 7.0 (SAS Institute, Cary, NC, USA).
Results
The median progression of c-IMT was 0.30 mm (0.22–0.41) for the rapid c-IMT progressors (n=13) and 0.01 mm (−0.03–0.12) for the slow c-IMT progressors (n=53), while the median CD4+ T-cell count at the first visit was 354 cells/µl (Table 1). Despite ART, CD4+ T-cell count <350 cells/µ1 was found in 10/66 and 15/66 subjects at time points 1 and 2, respectively. This could be attributed to low levels of CD4+ T-cell count at first presentation [18] or could reflect a suboptimal immune recovery in this subset of patients.
Table 1.
Demographic and clinical information of HIV-negative and HIV-positive subjects
| Variable | Value |
|---|---|
| HIV-negative | |
| Time point 1 | |
| Male sex, % | 100 |
| Caucasian ethnicity, % | 74 |
| Age, years | 48 (45–55) |
| HIV-positive | |
| Time point 1 | |
| Male sex, % | 89 |
| Caucasian ethnicity, % | 59 |
| CD4+ T-cell nadir, cells/µl | 77 (37–156) |
| C-reactive protein, mg/l | 1.5 (0.6–4.9) |
| Age, years | 48 (43–54) |
| CD4+ T-cells, cells/µl | 354 (221–651) |
| Plasma HIV-1 RNA, copies/ml | 75 (50–1,592) |
| c-IMT, mm | 0.94 (0.78–1.3) |
| Total cholesterol, mg/dl | 191 (165–231) |
| HDL, mg/dl | 43 (34–53) |
| LDL, mg/dl | 104 (85–134) |
| Triglycerides, mg/dl | 148 (109–294) |
| Subjects on ART, n | 59/66 |
| Subjects with high c-IMT, n | 41/66 |
| Subjects with hypertension diagnosis, n | 17/66 |
| Subjects with hypertension diagnosis on medication, n | 12/17 |
| Subjects who smoked, n | 40/66 |
| Packets/day × years of smoking | 19 (10–37) |
| Time point 2 | |
| Age, years | 49 (44–55) |
| CD4+ T-cells, cells/µl | 383 (205–583) |
| Plasma HIV-1 RNA, copies/ml | 75 (75–1,552) |
| c-IMT, mm | 1.02 (0.87–1.49) |
| Total cholesterol, mg/dl | 192 (169–239) |
| HDL, mg/dl | 46 (35–58) |
| LDL, mg/dl | 106 (84–140) |
| Triglycerides, mg/dl | 148 (107–243) |
| Subjects on ART, n | 60/66 |
| Subjects with hypertension diagnosis, n | 18/66 |
| Subjects with hypertension diagnosis on medication, n | 13/18 |
| Time point 2 minus time point 1 | |
| Number of rapid c-IMT progressors, na | 13/66 |
| Days between time point 1 and time point 2 | 374 (357–391) |
| Δc-IMT, mmb | 0.1 (−0.025–0.1688) |
Data are median (25th and 75th percentile) unless indicated otherwise.
Subjects with change in carotid artery intima-media thickness (c-IMT)>0.2 mm.
c-IMT at time point 2 minus c-IMT at time point 1.
ART, antiretroviral therapy; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Preliminary experiments confirmed that cryopreservation can result in a decreased EPC detection, yet both cryopreserved and fresh PBMC suggested higher EPC frequencies in HIV-infected as compared to HIV-negative subjects (Table 2). This rise in EPC frequencies was further confirmed in cryopreserved PBMCs from 116 subjects as the frequency of detected CD34+/KDR+, CD133+/KDR+ or CD34+/CD133+/KDR+ cells was significantly higher in HIV-positive (n=66) as compared to HIV-negative (n=50) subjects at either of both independent time points measured (Figure 1B and 1C), suggesting a sustained higher EPC frequency in HIV-positive subjects. HIV-positive subjects maintained higher EPC frequency when compared to HIV-negative subjects irrespective of the presence of ART (that is, CD34+/KDR+ cell frequency at time point 1 for subjects on ART [n=59], P=0.01 and off ART [n=7], P=0.009; and at time point 2 for subjects on ART [n=60], P=0.017 and off ART [n=6], P=0.004), or levels of CD4+ T cell count (data not shown). No difference in EPCs was detected between ART-treated and untreated HIV-positive subjects (data not shown).
Table 2.
EPC levels in matched whole blood, fresh PBMC and cryopreserved PBMC derived from an HIV-positive and an HIV negative subject
| Subject | EPC | Whole blood | Fresh PBMC | Cryopreserved PBMC |
|---|---|---|---|---|
| HIV-negative | CD34+/KDR+ | 0.001 | 0.0017 | 0.0007 |
| CD133+/KDR+ | 0.001 | 0.0029 | 0.0181 | |
| CD34+/CD133+/KDR+ | 0.0006 | 0.0006 | 0.0002 | |
| HIV-positive | CD34+/KDR+ | 0.0009 | 0.0056 | 0.0022 |
| CD133+/KDR+ | 0.0015 | 0.0044 | 0.00191 | |
| CD34+/CD133+/KDR+ | 0.0005 | 0.0028 | 0.0018 |
Data are the percentage of total cells. EPC, endothelial progenitor cells; PBMC, peripheral blood mononuclear cells.
EPC frequencies were not detected to be associated with c-IMT (Figure 1D), total levels of triglycerides, total cholesterol, high-density lipoprotein, low-density lipoprotein and C-reactive protein (data not shown). No difference in baseline EPC frequencies between rapid and slow c-IMT progressors (data not shown) was detected in the HIV-positive cohort, consistent with no correlation between baseline EPC frequencies and Δc-IMT (r=−0.105, P=0.4), or between ΔEPC (difference of EPC frequency between time point 1 and 2) and Δc-IMT (Figure 1E). Finally, CD34+/KDR+ cells frequency was positively associated with peripheral CD4+ T-cell count (n=66, r=0.27, P=0.03; Figure 1F).
Discussion
Current models of atherosclerosis pathogenesis suggest that endothelial damage stems from a variety of insults and results in a positive feedback mechanism, in which the production of EPCs in the bone marrow is increased, leading to partial reconstitution of normal endothelium.
We observed higher EPC frequencies in HIV-positive as compared to HIV-negative subjects, a finding that supports our previous observations indicating an increased endothelial stress in HIV-infected subjects [19]. In contrast to other studies showing lower levels of EPCs and CFU-ECs together with similar frequency of ECFCs in untreated HIV-positive subjects as compared to HIV-negative subjects [20,21], we observed higher EPC frequencies in HIV-positive as compared to HIV-negative subjects irrespective of the presence of ART, or subsequent change in c-IMT. With regards to the impact of ART on EPCs, our lack of a difference between ART-treated and untreated groups is limited by the smaller number of subjects on ART, as our study was focused on a priori selected subjects based on prospective c-IMT change rather than balanced for ART versus non-ART subjects at baseline.
EPCs can serve as predictors of early subclinical atherosclerosis [22] or cardiovascular events [23] in HIV-negative subjects, while low CD4+ T-cell count has been reported as a robust risk factor for increased c-IMT in HIV-positive subjects [24]. In this study, despite higher EPC frequencies in HIV-positive as compared to HIV-negative subjects, c-IMT was not associated with EPC frequencies or CD4+ T-cell count. Interestingly though, there was a positive association between EPC frequencies and CD4+ T-cell count. One explanation for this finding could be that although failure to produce EPCs may not be a major cause of HIV-associated atherosclerosis, increased EPC frequencies in HIV infection may represent the enrichment of dysfunctional EPCs; therefore they are not associated with direct c-IMT changes. It is also possible as suggested by Chironi et al. [25] that c-IMT, measured in the common carotid segment free from atherosclerosis, is not a specific marker of atherosclerosis and may represent medial hypertrophy that is a non-atherosclerotic process [25]. A potential for future EPC functional analysis could include matched flow-mediated dilation measures of the brachial artery, widely used as a measure of endothelial function [26,27], as this was not performed in this cohort.
Our study has several limitations. First, our sample size was too small to rule out the presence of a clinically meaningful effect of EPCs on outcome. A larger study including a group of atherosclerotic HIV-negative subjects may be necessary to distinguish between the effects induced by vascular damage and those induced by HIV infection. Second, cryopreservation resulted in 50% decrease in EPC frequency, and as a result although cryopreserved PBMC were as informative as fresh PBMC from the same donors in documenting an EPC frequency rise in HIV-infected subjects when compared to HIV-negative subjects, they may be limiting to evidence weak associations with c-IMT, if present. Third, as c-IMT reflects a cumulative exposure to risk factors while circulating EPCs may reflect acute ongoing endothelial stress, additional functional studies and long-term follow-up of subjects with increased EPC frequencies will be needed to address if EPCs are functional, if they are associated with cardiovascular events independently of c-IMT changes and if a cardiac clinical event in HIV-infected subjects is preceded by a decrease of the otherwise increased EPC frequencies.
In summary, our data showing increased levels of steady-state circulating EPCs independently of c-IMT change support the hypothesis that HIV infection does not impede EPCs from emerging in circulation as an indication of chronic vascular stress irrespective of ART-mediated suppression.
Acknowledgements
We thank the HIV-1-positive and HIV-negative subjects who participated in the study and their providers. This work was primarily supported by a grant to LJM by the National Institute of Allergy and Infections Disease (NIH AI48398, AI051986, AI065279), the Philadelphia Foundation (Robert I Jacobs Fund), the Stengel-Miller family, as well as AIDS funds from the Commonwealth of Pennsylvania and from the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health. Support for PH was provided by grants from NIAID (5K23AI066885) and NHLBI (5R01HL095130). Support to SGD and JNM was provided by grants from the NIAID (K24 AI069994), the UCSF/Gladstone Center for AIDS Research (P30 AI27763, P30 MH59037), the Center for AIDS Prevention Studies (P30 MH62246) and the UCSF Clinical and Translational Science Institute (UL1 RR024131-01). The funding sources had no involvement in the study design, collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
EP was involved in study design, planning and overseeing of the experiments, data analysis and manuscript preparation. PH was involved in study design, planning, patient recruitment, c-IMT assessment, blood sampling and manuscript preparation. GR, MP and AH provided technical assistance. JNM, SGD and LJM were involved in study design and manuscript preparation.
Footnotes
Disclosure statement
The authors declare no competing interests.
References
- 1.Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109:1603–1608. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 2.Oliviero U, Bonadies G, Apuzzi V, et al. Human immunodeficiency virus per se exerts atherogenic effects. Atherosclerosis. 2009;204:586–589. doi: 10.1016/j.atherosclerosis.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Torriani FJ, Komarow L, Parker RA, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52:569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf K, Tsakiris DA, Weber R, Erb P, Battegay M. Antiretroviral therapy reduces markers of endothelial and coagulation activation in patients infected with human immunodeficiency virus type 1. J Infect Dis. 2002;185:456–462. doi: 10.1086/338572. [DOI] [PubMed] [Google Scholar]
- 5.Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS) Stroke. 2006;37:87–92. doi: 10.1161/01.STR.0000196964.24024.ea. [DOI] [PubMed] [Google Scholar]
- 6.Asahara T, Murobara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 7.Goligorsky MS, Kuo MC, Patschan D, Verhaar MC. Review article: endothelial progenitor cells in renal disease. Nephrology (Carlton) 2009;14:291–297. doi: 10.1111/j.1440-1797.2009.01112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106:1525–1531. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- 9.Sabatier F, Camoin-Jau L, Anfosso F, Sampol J, Dignac-George F. Circulating endothelial cells, microparticles and progenitors: key players towards the definition of vascular competence. J Cell Mol Med. 2009;13:454–471. doi: 10.1111/j.1582-4934.2008.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 11.Rosenzweig A. Circulating endothelial progenitors--cells as biomarkers. N Engl J Med. 2005;353:1055–1057. doi: 10.1056/NEJMe058134. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt-Lucke C, Rossig L, Fichtlscherer S, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 13.Fadini GP, Sartore S, Albiero M, et al. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vase Biol. 2006;26:2140–2146. doi: 10.1161/01.ATV.0000237750.44469.88. [DOI] [PubMed] [Google Scholar]
- 14.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 15.Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109:337–346. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Case J, Mead LE, Bessler WK, et al. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Althoff KN, Gange SJ, Klein MB, et al. Late presentation for human immunodeficiency virus care in the United States and Canada. Clin Infect Dis. 2010;50:1512–1520. doi: 10.1086/652650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papasavvas E, Azzoni L, Pistilli M, et al. Increased soluble vascular cell adhesion molecule-1 plasma levels and soluble intercellular adhesion molecule-1 during antiretroviral therapy interruption and retention of elevated soluble vascular cellular adhesion molecule-1 levels following resumption of antiretroviral therapy. AIDS. 2008;22:1153–1161. doi: 10.1097/QAD.0b013e328303be2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.da Silva EF, Fonseca FA, Franca CN, et al. Imbalance between endothelial progenitors cells and microparticles in HIV-infected patients naive for antiretroviral therapy. AIDS. 2011;25:1595–1601. doi: 10.1097/QAD.0b013e32834980f4. [DOI] [PubMed] [Google Scholar]
- 21.Teofili L, Iachininoto MG, Capodimonti S, et al. Endothelial progenitor cell trafficking in human immunodeficiency virus-infected persons. AIDS. 2010;24:2443–2450. doi: 10.1097/QAD.0b013e32833ef79d. [DOI] [PubMed] [Google Scholar]
- 22.Fadini GP, Coracina A, Baesso I, et al. Peripheral blood CD34+KDR+ endothelial progenitor cells are determinants of subclinical atherosclerosis in a middle-aged general population. Stroke. 2006;37:2277–2282. doi: 10.1161/01.STR.0000236064.19293.79. [DOI] [PubMed] [Google Scholar]
- 23.Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS. 2008;22:1615–1624. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chironi G, Walch L, Pernollet MG, et al. Decreased number of circulating CD34+KDR+ cells in asymptomatic subjects with preclinical atherosclerosis. Atherosclerosis. 2007;191:115–120. doi: 10.1016/j.atherosclerosis.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 26.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 27.Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40:505–510. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]