Abstract
The discovery of taste and nutrient receptors (chemosensors) in the gut has led to intensive research on their functions. Whereas oral sugar, fat, and umami taste receptors stimulate nutrient appetite, these and other chemosensors in the gut have been linked to digestive, metabolic, and satiating effects that influence nutrient utilization and inhibit appetite. Gut chemosensors may have an additional function as well: to provide positive feedback signals that condition food preferences and stimulate appetite. The postoral stimulatory actions of nutrients are documented by flavor preference conditioning and appetite stimulation produced by gastric and intestinal infusions of carbohydrate, fat, and protein. Recent findings suggest an upper intestinal site of action, although postabsorptive nutrient actions may contribute to flavor preference learning. The gut chemosensors that generate nutrient conditioning signals remain to be identified; some have been excluded, including sweet (T1R3) and fatty acid (CD36) sensors. The gut-brain signaling pathways (neural, hormonal) are incompletely understood, although vagal afferents are implicated in glutamate conditioning but not carbohydrate or fat conditioning. Brain dopamine reward systems are involved in postoral carbohydrate and fat conditioning but less is known about the reward systems mediating protein/glutamate conditioning. Continued research on the postoral stimulatory actions of nutrients may enhance our understanding of human food preference learning.
Keywords: conditioned flavor acceptance and preference, taste receptors, gut nutrient sensors, carbohydrate, fat, protein, intragastric infusion
the flavor of food, that is, its taste, odor, and texture, provides animals with information about the nutritional quality of the food, elicits cephalic phase digestive responses that facilitate food utilization, and may induce a hedonic experience that stimulates eating even in the absence of homeostatic need (29, 215, 222). Taste stimulation in particular can have direct effects on brain reward circuits that drive eating, while odor and texture stimuli provide complexity to the flavor experience and along with taste cues can serve as conditioned reward signals (102, 265). Among the so-called basic tastes, the sweet taste of sugar is the most intimately associated with food hedonics. As noted by Young (278), “the adjective ‘hedonic’ is derived from a Greek root (hedon) with two basic meanings: (1) ‘sweet tasting’ and (2) ‘pleasant’.” The flavor of fat is also a source of food pleasure, which now appears to have a taste component that determines the initial preference for fatty foods for some species (122). A third more subtle flavor component is umami, the taste of glutamate and certain nucleotides that adds a savory flavor to foods (264). While many mammals have an innate preference for sweet and perhaps for fatty and umami tastes, most flavor preferences are learned, based in part on the nutritional properties of food (198, 266, 270). Even the innate preference for sweets can be enhanced by the postoral actions of sugars or converted to an aversion if associated with toxic consequences (157, 169).
Food learning can involve multiple learning processes that involve flavor, other food-related stimuli (e.g., color, food packaging), and social cues (270). Flavor learning is usually viewed as a form of Pavlovian conditioning in which a flavor (the conditioned stimulus, CS+) is associated with the oral and/or postoral properties of nutrients (the unconditioned stimulus, US). Flavor-taste (or flavor-flavor) learning refers to the process by which a preference or aversion develops for a neutral target flavor (e.g., almond flavor) that is mixed with an already preferred or avoided taste (e.g., sweet or bitter taste) (104, 241). Flavor-postoral (or flavor-consequence) learning refers to the process by which a preference or aversion develops for a flavor that is associated with positive (e.g., nutrient feedback from sucrose) or negative (e.g., visceral discomfort from a food toxin) postoral consequence (241, 270). Both forms of conditioning may operate during an eating experience: we learn to prefer the flavor of chocolate because of its association with both the sweet taste and postoral nutrient effects of the food (270). Flavor-postoral learning is adaptive in allowing humans and other animals to select nutrient-rich foods and avoid potentially dangerous foods. However, in today's “obesifying” food environment, flavor-postoral nutrient learning may have a deleterious effect by enhancing the preference for and consumption of sugar- and fat-rich foods (29, 120, 270). Flavor-avoidance learning has been extensively studied and reviewed elsewhere [e.g., (184)]. The present review focuses on flavor-preference learning based on the postoral actions of nutrients. In the case of sugar and fat the postoral conditioning process can have profound effects on preference and intake. For example, rats develop strong and long-lasting preferences for flavors associated with these nutrients and, when allowed to self-administer a flavored solution paired with an intragastric (IG) sugar or fat infusion, may overconsume the solution, resulting in increased total energy intake and weight gain (73, 132, 211, 259).
Our understanding of orosensory detection of sugar, fat, and glutamate has been greatly advanced in the last decade with the identification of the gustatory receptors (or putative receptors) and intracellular signaling pathways for these food components (21, 267). This was soon followed by the discovery that the same receptor and signaling proteins as well as bitter taste receptors exist in diffusely distributed epithelial cells in the alimentary canal (70, 188). The function of these gut “taste” receptors is under intense investigation. [The term taste is reserved here for sensations arising from gustatory receptors in the mouth; reference to these receptors in the gut will be delimited with quotation marks (78).] Even before “taste” receptors in the gut were identified, gut nutrient sensing was a hot topic, as indicated by a series of review papers published in the American Journal of Physiology-Regulatory, Integrative and Comparative Physiology more than a decade ago (38, 84, 118, 182, 216). Most of the attention on gut “taste” and nutrient sensors (or chemosensors) has focused on their role in physiological processes involving digestive and metabolic functions such as gastric emptying, nutrient absorption, mucosal defenses, and glucose homeostasis (14, 66, 167, 221, 228, 279). The role of gut chemosensors in the satiation and satiety processes that suppress feeding during and after a meal is also the subject of considerable interest (52, 89, 224). A third possible function of gut chemosensors, which is the topic of the present review, relates to flavor-postoral nutrient learning. Although it has long been assumed that gut satiety signals enhance the reward value of food (28), the available evidence suggests that satiety signals do not directly mediate flavor preference learning and may actually suppress learning in some cases (200, 229). Thus it may be that gut chemosensors generate separate signals that have appetite stimulatory and satiating actions.
Oral and Postoral Carbohydrate Sensing and Preference
Carbohydrates in the form of sugar and starch are an abundant source of food energy. Sugar is readily detected by taste receptors and strongly preferred by many animal species (22). Dietary sugars and glucose produced by starch digestion are also detected by chemosensors in the gut and at postabsorptive sites (liver, pancreas, brain), which may be the source of the positive feedback signals responsible for carbohydrate-conditioned flavor preferences.
Carbohydrate taste.
In many mammalian species, sugars are detected by the heterodimeric T1R2+T1R3 sweet receptor located in taste cells on the tongue and palate (22, 247). The same receptor responds to nonnutritive sweeteners and to some proteins and amino acids. When stimulated, the sweet receptor activates an intracellular signaling cascade that includes the G protein α-gustducin phospholipase Cβ2 (PLCβ2), inositol 1,4,5-trisphosphate receptor 3 (IP3R3), and the transient receptor potential cation channel Trpm5 (47). The critical importance of the T1R2 and T1R3 receptor components for the detection of and preference for sugars and nonnutritive sweeteners was demonstrated in seminal studies of knockout (KO) mice missing one or both of these signaling elements (54, 282). Electrophysiological experiments revealed that gustatory nerves in T1R2 KO, T1R3 KO, or T1R2+T1R3 double KO mice do not respond to nonnutritive sweeteners (saccharin, sucralose) and show at most weak responses to concentrated sugar solutions compared with wild-type (WT) control mice (54, 282). Consistent with these findings, results from brief-access lick tests, which minimize postoral feedback, demonstrated that T1R2, T1R3, and double KO mice show essentially no enhanced licking response to nonnutritive sweeteners and minimal or no response to concentrated sugar solutions (245, 246, 282, 284). T1R3 KO mice also fail to prefer nonnutritive sweeteners or dilute sucrose solutions in 24-h sweetener versus water choice tests (54, 284). However, T1R3 KO mice develop preferences for concentrated (≥8%) sucrose solutions in 24-h tests, which has been attributed to postoral nutritive effects (see Postoral sugar-sensing and food preferences.) (282, 284, 285). In addition to T1R2 and T1R3 KO mice, sweet taste deficits have been reported in KO mice missing the downstream signaling elements gustducin, PLCβ2, IP3R3, and Trpm5 and the gustatory nerve ATP receptor P2X2/P2X3 (53, 77, 107, 190, 281).
Whereas the T1R2+T1R3 heterodimer appears to be the primary sweet taste receptor, there may be other sugar sensors in taste cells (162). Potential candidates include glucose transporters (e.g., SGLT1, GLUT2) and the ATP-gated K+ metabolic sensor recently identified in some taste receptor cells (150, 243, 269). These transporters/sensors are located throughout the intestinal tract, and their presence in lingual taste cells further points to the functional similarity of lingual and intestinal cells, as does the presence of intestinal hormones [glucagon-like peptide-1 (GLP-1), peptide YY (PYY), cholecystokinin (CCK)] in isolated taste cells (13, 74, 217, 220). However, the signaling role, if any, of these glucose transporters/sensors in taste cells remains to be established.
In addition to sugar taste, there is considerable evidence that rodents have a separate taste for starch-derived maltodextrins (197, 199). Consistent with this view, recent studies demonstrate that T1R2, T1R3, and double KO mice, which show little or no response to sucrose solutions, display normal or near-normal responses to maltodextrin solutions (245, 246, 284). However, maltodextrin preferences are attenuated in gustducin KO and Trpm5 KO mice, which suggests that an as yet unidentified maltodextrin taste receptor utilizes the same downstream signaling pathway as does the sweet taste receptor (see Ref. 214, but also see Ref. 96). Recent exercise studies suggest that humans may have an oral maltodextrin chemosensor that improves motor performance without producing a reported taste perception (44, 86, 116). Thus rodents and other species may have multiple carbohydrate chemosensors in the mouth, although the T1R2+T1R3 sweet receptor is the only one identified to date.
Postoral sugar-sensing and food preferences.
Carbohydrate sensing begins with taste receptors in the mouth and continues after ingestion with the stimulation of sugar sensors in the gut and beyond (Fig. 1A). The localization of sweet taste signaling elements including T1R2, T1R3, gustducin, and Trpm5 in the gut has been the subject of intense interest during the past several years. Initial studies indicated that gut sweet “taste” receptors mediate incretin hormone (GLP-1, GIP) release from enteroendocrine cells and promote glucose absorption via increased expression of intestinal SGLT1 and GLUT2 transporters (115, 139, 140, 223). These studies include the novel finding that nonnutritive sweeteners produce gut effects similar to glucose (115, 139, 140, 223). However, subsequent research reported inconsistent results concerning the involvement of gut T1R2+T1R3 receptors in the release of incretin hormones (35, 79, 81, 137, 138, 153, 155, 186). Instead, different intestinal sugar sensors, including SGLT1 and SGLT3, were proposed to mediate incretin hormone release (62, 183, 185), although this view has been questioned (221). The sodium glucose cotransporter SGLT1, the primary transporter of glucose in intestinal enterocytes, may also serve as a glucose sensor in enteroendocrine cells (228). The related protein SGLT3 has little or no transport function and is thought to function primarily as a glucose sensor (228). Although originally located in enteric neurons (64), it may also exist in intestinal cells where it could detect luminal glucose (80, 258). There are many comprehensive reviews of this fast-developing field (25, 30, 71, 79, 183, 221, 224, 228, 247, 279), and the present paper focuses instead on the postoral sugar-sensing processes that may mediate carbohydrate-conditioned flavor preferences.
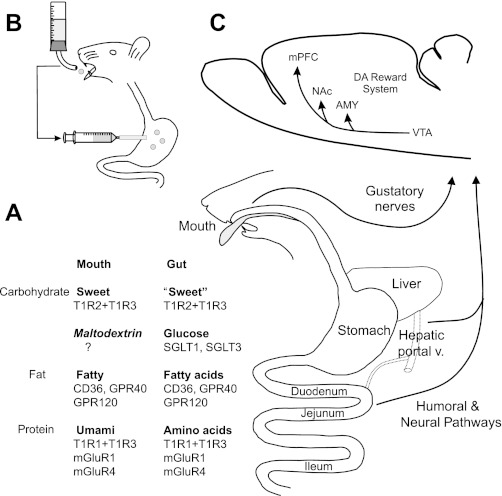
Fig. 1.
Oral and postoral nutrient sensing and food reward. A: schematic representation of the alimentary canal with a list of oral (mouth) and postoral (gut) nutrient chemosensors. The oral chemosensors include known (sweet and umami) and putative (fatty) taste receptors; an unidentified maltodextrin taste receptor is indicated based on evidence discussed in the text. The gut chemosensors includes a partial list of known and putative nutrient receptors (see Ref. 228). To date, none of the listed gut chemosensors have been identified as being responsible for postoral nutrient preference conditioning. Also unidentified are the nutrient sensors that mediate postabsorptive (e.g., hepatic portal vein, liver) nutrient conditioning. Postoral nutrient conditioning involves both humoral and neural (vagal) gut-brain pathways. B: schematic representation of the intragastric (IG) self-infusion system for postoral nutrient conditioning. The animal is fitted with a chronic intragastric (IG) catheter connected to a syringe pump via a head-mounted port (not shown). As the animal drinks a flavored solution, a computerized lickometer activates the pump which infuses a nutrient solution into the stomach. The system can be modified to infuse nutrients into the intestinal tract, hepatic portal vein or jugular vein. C: schematic representations of brain dopamane (DA) reward system stimulated by oral and postoral sensing of sugar and fat. DA involvement in protein/umami appetite is uncertain. Opioid and other reward systems mediate nutrient appetite but their involvement in postoral preference conditioning has not been established. VTA, ventral tegmental area; NAc, nucleus accumbens; AMY, amygdala, mPFC, medial prefrontal cortex.
The postoral enhancement of carbohydrate preference has been studied using a variety of experimental procedures and in species as diverse as flies (39, 69), mice (209), rats (108), sheep (40, 257), and humans (32, 37, 272). The most direct evidence for postoral preference conditioning is provided by studies in which the intake of an arbitrary flavor is paired with IG or intraduodenal (ID) carbohydrate infusions. For example, in one experiment we trained food-restricted rats fitted with chronic IG catheters to drink a flavored saccharin solution (CS+, e.g., grape) paired with a concurrent IG infusion of 16% glucose (US) and a different flavored solution (CS−, e.g., cherry) paired with an IG water infusion during alternating daily training sessions (30 min/day). After 6 training sessions the rats displayed a 91% preference for the CS+ over the CS− solution in a two-choice test conducted without IG infusions (206). In this and many other studies an automated “IG self-infusion” procedure was used (5) (Fig. 1B). As the animal licks the sipper tube containing the CS+ or CS− solution, a computer interfaced to a lickometer turns on the IG infusion pump and turns it off with a short latency (≤3 s) when the animal stops licking. Thus the animal controls the amount and timing of the infusion, which mixes with the consumed CS solution in the stomach and empties normally into the intestines. Flavor preferences are also conditioned by fixed-volume IG infusions that begin as the animal starts licking the CS solution or with a delay up to 1 h after the animal has ended its CS drinking session (2). Thus, as in the case of flavor aversion learning (87), animals can learn to prefer a flavor paired with immediate as well as delayed postoral nutrient feedback.
There are other notable features of preference conditioning by IG carbohydrate infusions. In particular, flavor conditioning does not require multiple training sessions; a preference can be conditioned in rats by a single IG glucose infusion (3, 156). Flavor conditioning also does not require a state of energy need. IG maltodextrin infusions conditioned equivalent flavor preferences in food-restricted and unrestricted rats (273, 274). Finally, once established, carbohydrate conditioned flavor preferences are very resistant to extinction and persist over several days to weeks of testing without IG infusions (67, 73, 156). This demonstrates that conditioning produced a long-lasting increase in the reward value of the CS+ flavor.
In addition to conditioning a preference, the postoral actions of carbohydrates can stimulate the absolute intake, i.e., “acceptance,” of a flavored solution (67, 173, 178, 179, 208, 209, 274). For example, in one experiment, C57BL/6J (B6) mice given ad libitum access to chow consumed nearly twice as much of a CS+ solution paired with IG self-infusion of 16% sucrose than of the CS− solution paired with IG water during 24-h one-bottle training sessions (209). We recently reported that postoral sugar stimulation of intake can occur within minutes into the very first test session (283). Food-restricted B6 mice were adapted to lick a flavored 0.025% saccharin solution (CS−) paired with IG water self-infusion for three 1-h sessions (days 1–3) followed by three sessions (days 4–6) with a different flavored saccharin solution (CS+) paired with IG self-infusion of 16% glucose. The glucose self-infusion stimulated CS+ licking within 15 min in the first session and increased total 1-h licks by 44% compared with the prior CS− sessions (Fig. 2A). CS+ licks were increased by 90% in the next two glucose sessions (283). In these later sessions, lick rates were high during the initial minutes of drinking, indicating a conditioned enhancement of the CS+ flavor, and rapidly declined later in the session as the animals experienced the satiating action of the self-infused glucose. In a subsequent choice test without IG infusions, the mice significantly preferred the CS+ to the CS−, further demonstrating the postoral conditioning effect of the glucose. The glucose stimulation of intake observed in this study is in marked contrast to the more commonly reported suppression of intake produced by gastric or intestinal sugar infusions (e.g., 275, 280). To observe stimulation rather than suppression of intake, it is critical to offer animals a minimally attractive CS solution (e.g., a dilute saccharin solution) so that baseline CS intake is relatively low, leaving room for increased consumption. If rodents are instead offered a highly palatable solution (e.g., 3% glucose + 0.2% saccharin), then baseline intakes are high and glucose self-infusion rapidly suppresses rather than increases intake (e.g., 206). The ability to observe glucose-induced increased licking early in a feeding session provides a window into the postoral stimulatory action of the sugar before the onset of its satiating action that terminates ingestion.
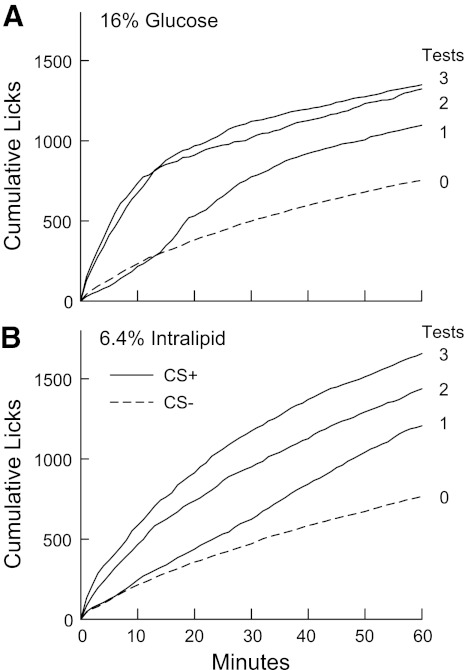
Fig. 2.
Rapid stimulation of licking by IG nutrient self-infusion in B6 mice (283). Food-restricted mice were trained to drink (60 min/day) a conditioned stimulus (CS−) solution paired with IG self-infusion of water for three one-bottle sessions (days 1–3) followed by a CS+ solution paired with IG self-infusion of nutrient solution for three sessions (days 4–6). The CS solutions contained 0.025% saccharin and were flavored with 0.01% ethyl acetate or propyl acetate. Cumulative licks are plotted for the average of the last two CS− sessions (Test 0) and for the three CS+ sessions (Tests 1-3). A, top: mice (n = 11) were trained with the CS+ solution paired with IG self-infusion of 16% glucose. B, bottom: mice (n = 13) were trained with the CS+ solution paired with IG self-infusion of 6.4% Intralipid (soybean oil emulsion), which is isocaloric to 16% glucose.
The postoral processes responsible for flavor conditioning by carbohydrates are incompletely understood, but the upper intestinal tract appears to be an important site of action. This is indicated by the findings that 1) IG glucose infusions conditioned flavor preferences only if the glucose was allowed to empty into the intestine (68); 2) flavor preferences were conditioned by glucose infusions into the duodenum and jejunum but not into the ileum (12, 68); and 3) hepatic-portal or jugular vein glucose infusions failed to condition preferences for flavored nonnutritive solutions, i.e., saccharin or sham-fed sucrose (12, 97, 98). In contrast to these results, two studies reported that rats learned to prefer a flavored chow paired with jugular vein or hepatic-portal glucose infusions over a different flavored chow paired with saline infusions (143, 230). Taken together, these findings suggest that intravascular glucose can reinforce a flavor preference when it is associated with the preabsorptive nutrient stimulation as provided by chow. However, a recent study indicates that intravascular glucose alone is sufficient to condition a behavioral response (163). In this study, thirsty and hungry rats trained to drink from two water bottles developed a preference for the bottle position that was paired with an intravascular glucose infusion. Glucose infused into the hepatic-portal vein was effective at a lower dose than a jugular infusion, which suggested the involvement of intra-abdominal glucose sensors (see also Ref. 230). Whether the same intravascular glucose infusions are sufficient to condition a flavor preference in rats drinking a nonnutritive solution remains to be established.
It is important to note that carbohydrates vary in their flavor conditioning effects. In rats and mice glucose and glucose-containing carbohydrates (sucrose, maltose, and maltodextrin) condition stronger preferences than does fructose (3, 6, 10, 11, 20, 201, 205, 206, 283). In particular, whereas IG glucose infusion conditions flavor preferences in short sessions (10–60 min), fructose is effective only in long sessions (20–24 h) and under more restrictive training conditions. The reduced flavor conditioning produced by fructose may occur because, unlike glucose, it acts only at a postabsorptive site to reinforce flavor preferences (11). Intestinal fructose is involved in other functions such as promoting SGLT1 expression and GLP-1 secretion via activation of gut “sweet taste” or other sensors (100, 223). IG lactose and galactose infusions, in contrast, condition flavor avoidance rather than preference in rats, which may be secondary to incomplete digestion or metabolism of these sugars (206, 238). As discussed next, the differential conditioning effects of these various carbohydrates provide insight into the postoral chemosensors involved in flavor learning.
The presence of taste signaling proteins (T1R2, T1R3, gustducin, Trpm5) in the intestinal tract suggests the intriguing idea that the same sensor system that mediates sweet taste reception in the mouth is responsible for flavor conditioning by sugar in the gut (28). However, several findings argue against this possibility. First, not all ligands of the sweet taste receptor condition flavor preferences when infused IG. As noted above, glucose is more effective than fructose in conditioning flavor preferences (11, 206), although the two sugars are closely matched in palatability in brief taste tests (41, 212). Furthermore, whereas B6 mice strongly prefer the artificial sweetener sucralose to water in two-bottle tests, the mice failed to prefer a flavor paired with IG sucralose self-infusion (207). Second, the T1R3 receptor is not essential for postoral sugar conditioning. T1R3 KO mice with a substantially impaired sucrose taste preference display a normal flavor conditioning response to IG sucrose self-infusions (207) (Fig. 3). Gustducin KO and Trpm5 KO mice also learn to strongly prefer flavors paired with IG sucrose or glucose infusions (95, Sclafani A, unpublished findings; Fig. 3). In addition, Trpm5 KO mice acquire a bottle side preference based on the postoral effects of sucrose but not sucralose (56). The postoral sugar conditioning response of sweet-ageusic T1R3 KO mice can explain why these mice develop strong sugar preferences in 24-h two-bottle tests (284). That is, the KO mice can learn to associate the T1R3-independent orosensory properties of sucrose (residual taste, odor, texture) with the sugar's postoral reinforcing actions (285).
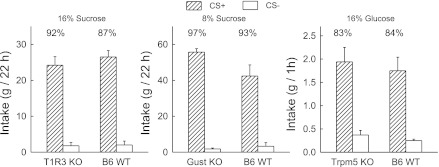
Fig. 3.
Flavor conditioning produced by IG sugar self-infusions in T1R3, gustducin, and Trpm5 KO mice. During training (not shown) intake of the CS+ solution was paired with IG self-infusion of the sugar, while intake of the CS− solution was paired with IG self-infusion of water, in alternating sessions. Intake values are expressed as means + SE total intakes (oral+IG) per 22- or 1-h session averaged for the four two-bottle test days. Numbers atop bars represent mean percent preference for the CS+ solutions. Left, T1R3 knockout (KO) (n = 9) and B6 wild-type (WT) (n = 11) mice were trained with 16% sucrose infusions, with six alternating one-bottle training sessions (22 h/day) with the CS+ and CS− before the test shown here (207). The CS solutions contained 1% Intralipid and were flavored with 0.05% grape or cherry Kool-Aid. Middle, gustducin KO (Gust KO, n = 8) and B6 WT (n = 6) mice were trained with 8% sucrose infusions, with six alternating one-bottle training sessions (22 h/day) with the CS+ and CS− before the test shown here (Sclafani A, unpublished findings). The CS solutions contained 0.625% Intralipid and were flavored with 0.05% orange or lemon-lime Kool-Aid. The mice had extensive prior experience with carbohydrate solutions and oil emulsions but not with IG sugar infusions (214). Right, Trpm5 KO (n = 10) and B6 WT (n = 9) mice were trained with 16% glucose infusions, with eight alternating one-bottle training sessions (1 h/day) with the CS+ and CS− before the test shown here (95). The CS solutions contained 0.05% grape or cherry Kool-Aid in water, and the animals were trained while water restricted to induce them to drink the solutions. The two-bottle tests were conducted while the animals were food restricted. Note that T1R3 KO and Gust KO mice do not prefer saccharin but are attracted to fat emulsions and therefore were trained with flavored Intralipid. Trpm5 mice are indifferent to both Intralipid and saccharin and were therefore trained with flavored water.
Rather than intestinal T1R2+T1R3 “taste” receptors, other gut chemosensors must mediate the flavor conditioning produced by IG and ID glucose infusions. The putative sugar sensors SGLT1 and SGLT3 are attractive candidates because they bind to glucose but not fructose (262). The two sensors differ in that SGLT1 but not SGLT3 binds to galactose (262). In rats and mice IG galactose failed to condition flavor preferences (201, 206), which would appear to favor SGLT3 as the chemosensor responsible for intestinal glucose conditioning. However, the incomplete postabsorptive metabolism of galactose may limit its ability to reinforce flavor preferences in rodents (201, 206), although a recent study reported that IG galactose has a postoral place preference conditioning action in mice (143). We are currently reexamining the flavor conditioning effects of galactose in mice as well as the conditioning effects of the nonmetabolizable glucose analogs α-methyl-d-glucopyranoside and 3-O-methyl-glucose, which selectively bind to SGLT1 and SGLT3 (80).
Another issue to be resolved is how the flavor conditioning signals generated by intestinal and/or postabsorptive glucose sensors reach the brain. Vagal and splanchnic afferent fibers are activated by intestinal glucose and contribute to sugar-induced satiety and the control of gastric emptying (28, 33). However, lesioning vagal and nonvagal neural afferents by abdominal vagotomy, selective afferent abdominal vagotomy, celiac-superior mesenteric ganglionectomy, or systemic capsaicin treatment did not block flavor preference conditioning by IG or ID maltodextrin infusions [(134, 203, 210), see also Ref. 283]. These findings indicate that visceral afferent fibers are not essential for flavor conditioning by the intestinal actions of glucose, but the role of such afferents in the postabsorptive conditioning effects of glucose remains to be determined. Vagal afferents are implicated in fructose-conditioned flavor preferences (231).
Hormonal signaling is the alternate pathway for peripheral glucose sensors to activate brain reward circuits implicated in flavor conditioning (12). However, most gut hormones (e.g., CCK, GLP-1, PYY) released by carbohydrates inhibit feeding and in some cases induce flavor aversions (48, 191, 280), actions that argue against a role in glucose-stimulated intake and preference conditioning. Nevertheless, certain “satiety” hormones may have biphasic effects. We previously reported that a low dose of exogenous CCK, which did not suppress intake, conditioned a mild flavor preference whereas higher CCK doses, which suppressed intake, were ineffective or produced a flavor aversion (174). A subsequent study revealed that CCK receptor antagonism blocked feeding inhibition but not flavor conditioning by ID maltodextrin infusions, indicating CCK involvement in postoral carbohydrate satiation but not preference learning (172). Ghrelin is one gut hormone that stimulates feeding and is implicated in food reward (149, 165), but glucose acts in the intestine to suppress ghrelin release (260), which seems inconsistent with a ghrelin role in glucose-stimulated intake and flavor conditioning. Insulin has been suggested to be a humoral signal for postoral glucose reward (249). However, we observed glucose-conditioned flavor preferences in insulin-deficient streptozotocin-diabetic rats (10). Other findings indicate that insulin acts in the brain to reduce sugar intake and reward (76, 149). A possible role of GIP in glucose-conditioned preference and intake warrants investigation. Glucose stimulates the release of GIP from L cells in the upper intestine via SGLT1 (50, 168), and GIP infusions were reported to increase appetite in humans (55). However, this was not confirmed in a subsequent study (18), and treatment with a long-acting GIP agonist did not alter food intake in mice (112). Thus the hormonal mediation of postoral glucose conditioning remains an open question that requires further study.
Oral and Postoral Fat Sensing and Preference
Dietary fat is the most energy-dense nutrient source and, like sugars, enhances the palatability of food. The oral and postoral sensing of dietary fat have been extensively reviewed in recent publications (49, 130, 145, 152, 225), and the present review provides a brief overview and highlights postoral fat conditioning.
Orosensory detection of fat.
It was long assumed that dietary fat is “tasteless” (148) and fat flavor was attributed primarily to texture and secondarily to olfactory cues. As reviewed in detail elsewhere (7), studies using nonnutritive mineral oil or petrolatum jelly confirm that rodents are attracted to foods and fluids with greasy or oily textures. With experience, however, foods and fluids containing nutritive fats are preferred to those containing nonnutritive fat substitutes, indicating an important role for postoral sensing in dietary fat preference. Several studies have investigated the role of olfaction in fat detection and preference and generally agree that odor cues can influence fat detection at low concentrations in rodents but are not essential for the selection of fat-rich foods or fluids (83, 126, 177, 192, 227, 252).
Evidence for the existence of a fatty acid taste has accumulated over the last 15 years (1, 82, 93, 127, 141, 144). In rodents, long-chain fatty acids such as linoleic acid are sensed and preferred at low concentrations (83, 119, 127, 175, 251, 277). In addition, rats are more attracted to a pure triglyceride when lingual lipase can act to release fatty acids within taste papillae, although their preference for corn oil does not appear to depend upon lipolysis in the oral cavity (119). Humans also appear to detect fatty acids at low concentrations, although, unlike rodents, they are not attracted to the taste (43).
Several fatty acid receptor elements, including CD36, GPR120, and GPR40 have been proposed to mediate fatty acid taste (94). Critical evidence that CD36 is involved in fatty acid taste was provided by the report that CD36 KO mice, unlike WT mice, failed to prefer a 2% linoleic suspension (127, 142). We observed that naïve CD36 KO mice are indifferent to dilute soybean oil emulsions as well as linoleic acid (202). However, after experience with concentrated soybean oil, CD36 KO mice displayed significant preferences for dilute to concentrated oil emulsions as well as for linoleic acid (202). These experience-dependent preferences in CD36 KO mice are attributed to postoral fat conditioning, as discussed below. GPR120 KO and GPR40 KO mice are also reported to show little or no preference for linoleic acid or oleic acid (42). CD36 and GPR120 are colocalized in taste buds and may function as coreceptors in fatty acid taste transduction (42, 94, 141). Trpm5 is also implicated in fat taste processing, because Trpm5 KO mice are deficient in their preference response for soybean oil and linoleic acid (131, 214). The role of GPR40, on the other hand, as a fat taste sensor is questionable because of inconsistent data on its presence in taste cells (57).
Postoral fat-sensing and food preferences.
There is considerable evidence for the role of learning in fat preference. Early studies reported that rats developed a preference for a CS+ flavor added to a corn oil emulsion over a CS− flavor presented in a fat-free solution (72, 146). Children given repeated exposures to distinctly flavored high-fat and low-fat or nonfat yogurt drinks matched for orosensory characteristics increased their preference for the high-fat flavor (117, 121).
Direct evidence for flavor conditioning by the postoral actions of fat is provided by experiments in which a CS+ flavor is paired with IG or ID fat infusions (7). Postoral fat conditioning has some features in common with carbohydrate conditioning. IG fat infusions condition flavor preferences in freely fed as well as food-restricted animals (273), and these preferences are resistant to extinction (133). Fat sources vary in the strength of the conditioned preferences they support as a function of their fatty acid composition (4). In particular, IG infusion of a long-chain triglyceride (corn oil) conditioned a stronger flavor preference than did a medium chain triglyceride. Among various long-chain triglyceride sources (corn oil, safflower oil, beef tallow, vegetable shortening), fats with high polyunsaturated content and/or lower saturated fat content were the most reinforcing.
In rats, even effective fat sources, such as corn oil, have a weaker postoral conditioning effect than glucose-based carbohydrates. For example, IG fat self-infusion was less effective at stimulating saccharin intake (180) and produced weaker CS+ flavor preferences than did IG carbohydrate self-infusions (135). In addition, rats preferred a carbohydrate-paired CS+ flavor over a fat-paired CS+ flavor in direct choice tests (135, 171, 244). Relative to glucose conditioned preferences, which can be observed after a single training trial, fat-conditioned preferences required multiple training trials (3, 156). These differences may reflect the slower digestion and signal detection of fat compared with rapidly digested carbohydrates. In contrast to these findings obtained with pure nutrient infusions are results obtained with complete diets. IG self-infusion of a high-fat liquid diet conditioned a stronger preference than did self-infusion of high-carbohydrate liquid diet, which was attributed to the reduced satiating effect of the high-fat diet (132).
Mice appear to be more sensitive than rats to the postoral actions of fat. Isocaloric sucrose and soybean oil self-infusions in B6 and 129P3/J mice stimulated the overconsumption of a CS+ flavored solution to the same degree and conditioned comparable CS+ preferences (∼95%) relative to a CS− flavor (209). Also, B6 mice rapidly detected an IG fat self-infusion and increased their rate of licking within minutes of the first 1-h training session with a CS+ solution paired with the fat infusion (Fig. 2B) (283). The fat self-infusions increased 1-h licks (and intakes) more than did isocaloric glucose infusions (see Fig. 2).
The requisite sites and sensors for postoral fat conditioning have not yet been determined. The stomach does not appear to be essential, because intestinal fat infusions, like gastric infusions, condition flavor preferences in rats (134). The rapid stimulation of licking in mice self-infusing fat (283) suggests a preabsorptive site of action if mice, like rats, do not absorb fat within the first 30 min of the infusion (99, 276). However, no studies have compared flavor conditioning by intestinal versus intravenous fat infusions, and a role for postabsorptive fat sensing cannot be excluded. There is evidence for multiple fatty acid sensors in the intestine (225), including the three (CD36, GPR120, and GPR40) implicated as fatty acid taste receptors in the mouth (42, 127) (Fig. 1A). We investigated the role of intestinal CD36 in the postoral conditioning response to fat by training CD36 KO and WT mice to associate CS+ and CS− flavors with IG self-infusions of 5% soybean oil (Intralipid) and water, respectively. The CD36 KO mice, like the WT mice, learned a near-total preference (99%) for the fat-paired CS+ flavor (202). This intact postoral conditioning response can explain how CD36 KO mice, despite their fatty acid taste deficit, develop strong preferences for fat emulsions after experiencing their postoral consequences (202). While gut CD36 is not involved in postoral fat conditioning, it is implicated in fat-induced satiety (196). The role of GPR40, GPR120, and other gut lipid sensors (62, 130) in postoral flavor conditioning by fat remains to be determined.
As in the case of carbohydrate conditioning, how the postoral conditioning signals generated by fat reach the brain is not certain. Vagal afferents are activated by intestinal lipid and fatty acid and are involved in fat-induced satiation (181, 195). In two studies we investigated the role of visceral afferents in fat-conditioned flavor preferences (134, 203). Vagal deafferentation produced by systemic capsaicin treatment or selective afferent vagotomy did not prevent flavor conditioning by intestinal fat infusions in rats, although the feeding suppressive effect of the fat was blocked (134, 203). Rats with selective afferent vagotomy combined with celiac-superior mesenteric ganglionectomy did not display a significant fat-conditioned flavor preference (57%), although the results are not conclusive because the sham control rats in this experiment displayed a rather weak preference (66%). These data suggest that vagal afferents do not mediate postoral fat conditioning, but the role of extra-vagal sensory fibers remains to be established.
Fat in the gut releases a variety of intestinal hormones, including CCK, GLP-1, PYY, apolipoprotein A-IV, and enterostatin, but these are all implicated in the satiating action (154) rather than the stimulating action of fat. As previously noted, a low dose of CCK was found to condition a weak flavor preference in rats (174), but flavor conditioning by ID fat infusions was not blocked by a CCK antagonist (Perez C, Lucas F, and Sclafani A, unpublished data). Fatty acids in the intestine stimulate GIP release (50, 168) which may contribute to the feeding response to dietary fat (17, 151). Recent findings suggest that intestinal endocannabinoids (anandamide, 2-arachidonoylglycerol) may function as a “hunger” signal, are modulated by oral exposure to dietary fat, and stimulate fat ingestion (65, 114). However, there is no evidence that intestinal exposure to fat increases endocannabinoid activity; instead food deprivation and refeeding are reported to increase and decrease intestinal endocannabinoid activity, respectively (114). Nevertheless, given the well-known effects of endocannabinoids on enhancing food reward and intake, the involvement of intestinal endocannabinoid activity in postoral fat conditioning warrants some attention (51).
Oral and Postoral Protein Sensing and Preference
Regular intake of dietary protein is necessary because, unlike fat and carbohydrate, excess amino acids are not stored (88). Thus it is not unreasonable to expect animals to detect and respond to dietary protein sources. Early studies proposed that protein-deprived rats have an unlearned preference for the odor of some dietary proteins (61, 106), but this has not been further documented. The detection of protein foods could be accomplished by gustation, as suggested by the recent recognition of umami/glutamate as a basic taste quality. Glutamate, which is among the commonest amino acids, could serve as a reasonable general index of protein content (124). Monosodium glutamate (MSG) is a prototype for umami, the taste quality sometimes described as “delicious” or “savory.” For humans, MSG alone is not preferred, but it enhances the flavors of some foods (103). Discussion of umami as a basic taste is generally accompanied by the interpretation that it is a means of detecting protein (e.g., Refs. 34, 110, 232, 233). However, it has not been demonstrated that protein-deficient animals show an increase in MSG preference (233), but this has not been studied in detail. Other studies have found no indication that animals automatically recognize protein sources but rather learn to select protein-rich foods when in need (85).
Glutamate/umami taste.
The attractiveness of MSG has been assessed by various measures, some of which separate oral and postoral contributions. Some data suggest that rats are only modestly attracted to MSG solutions over a limited concentration range (e.g., Refs. 123, 161). However, MSG stimulation of intake depends strongly on the test conditions: rats accepted a wider range of concentrations in brief access tests than in 24-h sessions (101, 161). These differences suggest postoral influences on MSG intake. In mice, attraction to MSG is strongly influenced by experience with sapid solutions. In 24-h tests, mice preferred MSG at 1–300 mM concentrations with intakes peaking at 300 mM. However, these mice had prior experience with other sapid solutions; naïve mice did not prefer 300 mM MSG and consumed markedly less than the experienced mice (23). Subsequent experiments also revealed that prior tastant experience influenced MSG preference (8, 189). These studies indicate that MSG preference is very influenced by prior tastant/nutrient experience, which should be considered in the design of future studies. The source (oral vs. postoral) of this experiential effect requires further investigation.
Gustatory detection of amino acids.
Oral amino acid sensing involves multiple G protein-coupled receptors, and it is not yet clear how each contributes to behavioral responses to protein. The heterodimeric T1R1+T1R3 receptor has been identified as a major umami sensor in taste cells. The T1R1+T1R3 receptor is very specific to glutamate in humans (128, 158, 282) but is more broadly tuned in rodents (158). Rats treat the tastes of some amino acids (glycine, proline, low concentrations of serine) as similar to that of glutamate (60). In addition to T1R1+T1R3, there are oral glutamate sensors that belong to the metabotropic glutamate receptor family, including mGluR1 (193, 242) and mGluR4 (45). A recent electrophysiological study indicates that umami taste in mice is mediated by T1R1+T1R3 receptors in sweet-best gustatory nerve fibers and mGluR receptors in glutamate-best gustatory fibers (268). A classic feature of umami is the synergy of MSG with the ribonucleotides inosine and guanosine 5′-monophosphate (IMP and GMP) (54). This effect, in which responses to the combination of MSG and a ribonucleotide are greater than the sum of responses to each component of the mixture, is attributed to the T1R1+T1R3 receptor rather than mGluR receptors (46, 263).
Umami taste has been studied in knockout mice missing different taste signaling components. Conflicting results are reported with T1R3 KO mice: one study found that they showed no licking response to MSG in brief tests (282), but another found only minor differences between T1R3 KO and WT mice in 24-h two-bottle preference tests (54). Conflicting data have also been reported for T1R1 KO mice (125, 282). Differences in the KO constructs and/or behavioral test procedures may account for the discrepant findings (see Ref. 59). Gustducin KO mice have partial deficits in their behavioral response to MSG in brief licking and 24-h two-bottle tests (96, 105, 189). Trpm5 KO mice show a complete loss of response to MSG in brief taste tests and at low concentrations in 24-h tests (53, 281), but significantly preferred 100–300 mM MSG in 24-h tests, which may be due to postoral actions (53). Residual MSG responses in Trpm5 KO mice can also be attributed to activation of the mGluR receptors, which utilize a Trpm5-independent signaling pathway (268). P2X2/P2X3 KO mice also failed to respond to MSG in brief taste tests (77). Thus, as in knockout studies of sweet taste, brief access tests with MSG reveal more profound deficits than do 24-h tests, in which postoral nutrient effects can influence intake and preference.
Postoral protein/glutamate-sensing and food preferences.
There is considerable evidence that protein selection is accomplished by learning to associate orosensory and postoral characteristics of the diet. Not discussed here is the extensive literature on learning to avoid amino acid imbalanced diets (92). Animals readily learn about complete amino acid proteins; for example, protein-restricted hamsters learned to prefer a CS+ flavor added to a protein-rich diet (63). Humans also learned to associate flavors with low- and high-protein foods and expressed a preference for the high-protein flavor after consuming a low-protein drink (91). Some findings also suggest that postoral factors may contribute to the preferences displayed by humans for umami-tasting foods (176, 271).
Direct evidence for flavor learning based on the postoral actions of protein is provided by IG infusion studies. Specific deprivation of protein is not required for such learning, because rats maintained on standard chow with adequate protein prefer a CS+ flavor paired with IG casein infusion over a CS− flavor paired with noncaloric infusion (24). These animals were trained and tested after a 4-h food deprivation, which the authors assumed produced a mild protein need. However, food restriction is not required for protein conditioning and ad libitum-fed and food-restricted rats displayed similar preferences for a CS+ flavor paired with IG casein (74% vs. 78%) (273). Food-restricted rats also acquired separate preferences for a flavor (CS+P) paired with IG protein (casein) and a flavor (CS+C) paired with isocaloric IG carbohydrate (maltodextrin) over a CS− paired with water (170). When these animals were given an oral CS plus IG or IG-only preload of casein, they preferred CS+C over CS+P, and preferred CS+P after a maltodextrin preload. This indicates that protein-based flavor preferences are not simply based on the provision of energy but rather reflect specific association with protein (see also Refs. 24, 90, 256).
The mechanism for postoral protein learning is not yet clear but is likely based on amino acid detection. Recent studies have demonstrated flavor preference conditioning using IG MSG infusions (253, 254). Water-restricted rats displayed a 70% preference for a CS+ flavor paired with IG self-infusion of 60 mM (1%) MSG over a CS− flavor paired with IG water infusions during 30-min daily sessions. The sodium component of the MSG was not responsible for this conditioned preference because a second group of rats infused with IG 60 mM NaCl did not acquire a CS+ preference. A CS+ preference was also not conditioned by IG self-infusions of isocaloric glucose (60 mM, 1%), which indicated that the MSG-induced preference was not due to the energy value of the MSG or, more specifically, to its conversion to glucose. The failure of IG 1% glucose to condition a preference does not contradict prior studies that used more concentrated glucose infusions (≥8%, e.g., Ref. 2). Based on these results, the authors proposed that the MSG-conditioned preference was mediated by glutamate sensing in the gut (253).
In a follow-up study, we observed CS+ preferences for a flavor paired with IG self-infusions of 60 mM MSG in rats that were water restricted or food restricted (9). In addition, preferences were conditioned by MSG self-infusions into the duodenum, suggesting that gastric MSG sensing was not critical for the learned response. Increasing the concentration of the IG MSG infusion to 120 or 240 mM decreased rather than increased the magnitude of the CS+ preference in food-restricted rats, suggesting that 60 mM may be an optimal concentration; however, lower concentrations were not tested. The addition of 2 mM IMP to the IG 60 mM MSG infusion also did not enhance CS+ conditioning, suggesting a lack of MSG+IMP synergism. However, only one concentration of each compound was tested and additional concentrations should be examined before ruling out a role for synergy, and therefore a role for T1R1+T1R3 as the sensor in postoral MSG reward (9). T1R1+T1R3 sensing is implicated in other gut functions, such as duodenal mucosal defense responses, as shown by the synergism of IMP with glutamate or other amino acids (16). Further evidence for postoral MSG conditioning is provided by the finding that P2X2/P2X3 KO mice, which do not taste MSG, acquired a preference for a flavor mixed into a 150 mM MSG solution (226).
Relative to oral detection, less is known about postoral detection of glutamate, other amino acids and umami compounds. T1R1 and T1R3, along with gustducin and Trpm5 are present in multiple locations in the rodent gut and have been implicated in amino acid detection (31) (Fig. 1A). In addition, mGluR receptors are found in the gut (16, 194). Thus, as reviewed elsewhere (15, 26, 113), all the major amino acid-sensing elements of the oral cavity are present in more distal areas of the gastrointestinal tract. It is possible that, like current conjectures about multiple umami taste receptors in the mouth, the presence of glutamate and other amino acids in the gut may be detected in multiple locations with different receptors.
Vagal transmission appears to mediate postoral MSG learning. Uematsu et al. (254) reported that rats with total abdominal vagotomy (TVX) or with abdominal vagotomy with the common hepatic branch intact, unlike rats with hepatic vagotomy or sham surgery, failed to learn a preference for a CS+ flavor paired with IG self-infusions of 60 mM MSG. The effects of TVX were selective to MSG conditioning because vagotomized rats learned to prefer a CS+ flavor paired with IG self-infusion of glucose (460 mM, 8.6%), consistent with prior findings (203, 210). Also consistent with the vagotomy results are many studies reporting vagal stimulation by gastric or intestinal infusions of glutamate and other amino acids (147, 159). One study reported that IG infusions of MSG, but not other amino acids or sodium chloride, stimulated gastric vagal afferent activity when the pylorus was occluded to confine the infusate to the stomach (255), but gastric detection of MSG was questioned by another report (109). The selective vagal response to MSG reported by Uneyama et al. (255) raises the question as to whether flavor conditioning is specific to glutamate; alternately, IG infusions of other amino acids may also condition CS+ preferences. Related questions concern the role of glutamate and vagal transmission in the flavor conditioning response to IG protein infusions. In particular, does glutamate act alone or do other amino acids contribute to the flavor conditioning produced by IG casein infusions? Also, does IG casein conditioning, like MSG conditioning, require an intact vagus nerve?
Brain integration of oral and postoral nutrient sensing.
The previous sections of this review have focused on the taste and nutrient chemosensors in the mouth and gut that influence food preference and acceptance. The signals generated by these chemosensors are forwarded to the brain where the central processing of food reward and feeding decisions are made and flavor memories are stored. Much is known about the brain circuits that process taste and olfactory stimuli and form flavor memories (136, 160, 218, 261). Much less is known about the central processing of viscerosensory signals generated by the postoral actions of nutrients that condition flavor preference and increase intake. Nevertheless, some progress has been made in elucidating the brain sites and mechanisms involved in postoral nutrient conditioning.
In the case of carbohydrates, it is well documented that stimulation of oral sweet taste receptors promotes dopamine release in the nucleus accumbens (NAc), a brain site implicated in food and other rewards (102). Recent studies also indicate that postoral (gastric, hepatic-portal) glucose infusions increase NAc dopamine (DA) activity in rodents (163, 187). An abdominal rather than a brain site of action is suggested by the finding that hepatic-portal glucose infusion was more effective than jugular vein infusion in promoting NAc DA release (163). IG glucose infusion in rats is also reported to activate the NAc as well as the amygdala (AMG), another reward-related area, in fMRI and c-fos experiments (166, 249). Vagotomy did not alter the fMRI-recorded brain response to IG glucose, which is relevant to the finding that vagotomy does not block IG glucose-conditioned flavor preferences (203, 210, 254). The importance of dopamine signaling in the NAc and AMG as well as the medial prefrontal cortex (mPFC) to glucose conditioning is indicated by the findings that microinfusions of a DA D1 receptor antagonist (SCH23390) in these areas blocked flavor learning by IG glucose infusions (234–236) (Fig. 1C). Consistent with these results, lesions of the AMG impaired flavor conditioning by IG maltodextrin infusions (239). Lesions of the pontine parabrachial nucleus, which relays taste and viscerosensory information to the forebrain, also impaired maltodextrin-conditioned flavor preferences (204), whereas lesions of the taste and viscerosensory regions of the insular cortex were without effect (240). In addition to DA, brain opioid signaling modulates sugar reward (164) but it does not appear to have a direct role in sugar-conditioned flavor preferences (213). In particular, opioid receptor antagonism did not block the acquisition or expression of flavor preferences conditioned by IG sugar infusions (19, 27). The role of the other neurochemical systems implicated in food reward (e.g., endocannabinoid, benzodiazepine, orexin, and ghrelin circuits) in postoral sugar conditioning remains to be investigated.
Less is known about the central neural mechanisms involved in postoral fat conditioning. In rats oral exposure to fat promotes DA release in the NAc, and recent findings indicate that IG fat infusions also increase NAc DA levels (75, 129). A rat fMRI study also revealed that IG corn oil infusion activates brain regions involved in the DA reward system including the ventral tegmental area (VTA), AMG, and mPFC as well as other brain areas (250). This activation occurred during a 10-min oil infusion before any significant fat absorption occurred, suggesting that it was triggered by intestinal hormonal or neural signals. Excitotoxic lesions of the AMG blocked flavor conditioning by IG corn oil infusions, whereas lateral hypothalamus (LH) lesions reduced but did not eliminate the conditioned preference (237, 239). The effects of DA receptor antagonism in DA brain reward sites (NAc, AMG, mPFC, VTA) on flavor conditioning by IG fat infusions have not been investigated. Systemic treatment with D1 and D2 receptor antagonists did not prevent flavor conditioning by orally consumed corn oil in rats (58). although systemic D1 antagonism blocked fat-induced place preference conditioning in mice (111). Systemic injection of a D2 antagonist (haloperidol) was recently reported to alter the operant licking for IG Intralipid infusions in mice (75).
No studies have investigated central neural mediation of protein/glutamate conditioning, although recent fMRI and c-fos data suggest areas of interest. That is, an IG infusion of 60 mM MSG, which conditions flavor preferences in rats, activated areas of the habenular nucleus (HbN), central nucleus of amygdala (CeA), medial preoptic area (mPOA), and dorsomedial hypothalamus (DMH) (166, 248). A subsequent fMRI study revealed that vagotomy attenuated MSG-induced activation in the HbN, mPOA, DMH, LH, and ventromedial hypothalamus (249). These brain regions are of special interest given that vagotomy blocked flavor conditioning by IG MSG infusions (254). Missing from this list of brain sites activated by IG MSG are the NAc and VTA. Lesions of VTA DA neurons were found to suppress sucrose intake but not MSG intake in rats (219). Taken together, these findings suggested that the oral and postoral glutamate reward processes may be independent of the VTA DA projections (166).
Summary
Taste and nutrient sensors in the upper (oral) and lower (gastric, intestinal) regions of the alimentary canal are critical for the stimulation and inhibition of feeding behavior. Originally, the mouth was assumed to be the only site of feeding stimulation via the activation of taste, odor, and texture receptors, whereas the gut was considered to be primarily a source of inhibitory (satiation) signals that suppress feeding. It is now clear, however, that nutrients acting in the gut and/or at postabsorptive sites also generate positive feedback signals that can condition flavor preferences and, in some cases, substantially stimulate nutrient intake. This is most extensively documented in rats and mice, but studies conducted with flies, sheep, and humans also reveal postoral nutrient conditioning. Much remains to be discovered about the gut and postabsorptive nutrient chemosensors and signaling pathways that mediate postoral nutrient conditioning. Recent progress in the identification of gut “taste” and nutrient sensors should facilitate this research. Postoral nutrient conditioning occurs in humans, but the importance of this process, compared with orosensory, environmental, and cognitive factors in human feeding regulation and dysregulation is not certain (36, 270). Discovery of the critical chemosensors and signaling pathways responsible for postoral nutrient learning in experimental animals may enhance our understanding of this learning process in humans.
GRANTS
The research conducted in the authors' laboratory was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-031135 and DK-071761 and a grant from Ajinomoto Amino Acid Research Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.S. and K.A. conception and design of research; A.S. prepared figures; A.S. and K.A. drafted manuscript; A.S. and K.A. edited and revised manuscript; A.S. and K.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank John I. Glendinning and Khalid Touzani for helpful comments on this paper.
REFERENCES
- 1. Abumrad NA. CD36 may determine our desire for dietary fats. J Clin Invest 115: 2965– 2967, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ackroff K, Drucker DB, Sclafani A. The CS-US delay gradient in flavor preference conditioning with intragastric carbohydrate infusions. Physiol Behav 105: 168– 174, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ackroff K, Dym C, Yiin YM, Sclafani A. Rapid acquisition of conditioned flavor preferences in rats. Physiol Behav 97: 406– 413, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ackroff K, Lucas F, Sclafani A. Flavor preferences conditioning as a function of fat source. Physiol Behav 85: 448– 460, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Ackroff K, Sclafani A. Conditioned flavor preferences: evaluating the postingestive reinforcing effects of nutrients. In: Current Protocols in Neuroscience, edited by Crawley J, Gergen C, McKay R, Rogawski M, Sibley D, Skolnick P. New York: Wiley, 1999 [Google Scholar]
- 6. Ackroff K, Sclafani A. Fructose-conditioned flavor preferences in male and female rats: effects of sweet taste and sugar concentration. Appetite 42: 287– 297, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Ackroff K, Sclafani A. Oral and post-oral determinants of dietary fat appetite. In: Fat Detection: Taste, Texture, and Post Ingestive Effects, edited by Montmayeur J-P, le Coutre J. Boca Raton, FL: Taylor and Francis, 2010, p. 295–321 [PubMed] [Google Scholar]
- 8. Ackroff K, Sclafani A. Experience with sapid fluids stimulates MSG solution preference in mice. Chem Senses 36: A19, 2011 [Google Scholar]
- 9. Ackroff K, Sclafani A. Flavor preferences conditioned by post-oral infusion of monosodium glutamate in rats. Physiol Behav 104: 488– 492, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Ackroff K, Sclafani A, Axen KV. Diabetic rats prefer glucose-paired flavors over fructose-paired flavors. Appetite 28: 73– 83, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Ackroff K, Touzani K, Peets TK, Sclafani A. Flavor preferences conditioned by intragastric fructose and glucose: differences in reinforcement potency. Physiol Behav 72: 691– 703, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Ackroff K, Yiin YM, Sclafani A. Post-oral infusion sites that support glucose-conditioned flavor preferences in rats. Physiol Behav 99: 402– 411, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Acosta A, Hurtado MD, Gorbatyuk O, La Sala M, Duncan D, Aslanidi G, Campbell-Thompson M, Zhang L, Herzog H, Voutetakis A, Baum BJ, Zolotukhin S. Salivary PYY: A putative bypass to satiety. PLos One 6: e26137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akiba Y, Kaunitz JD. Duodenal chemosensing and mucosal defenses. Digestion 83: 25– 31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Akiba Y, Kaunitz JD. Luminal chemosensing in the duodenal mucosa. Acta Physiol (Oxf) 201: 77– 84, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Akiba Y, Watanabe C, Mizumori M, Kaunitz JD. Luminal l-glutamate enhances duodenal mucosal defense mechanisms via multiple glutamate receptors in rats. Am J Physiol Gastrointest Liver Physiol 297: G781– G791, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Althage MC, Ford EL, Wang S, Tso P, Polonsky KS, Wice BM. Targeted ablation of glucose-dependent insulinotropic polypeptide-producing cells in transgenic mice reduces obesity and insulin resistance induced by a high fat diet. J Biol Chem 283: 18365– 18376, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Asmar M, Tangaa W, Madsbad S, Hare K, Astrup A, Flint A, Bûlow J, Holst JJ. On the role of glucose-dependent insulintropic polypeptide in postprandial metabolism in humans. Am J Physiol Endocrinol Metab 298: E614– E621, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Azzara AV, Bodnar RJ, Delamater AR, Sclafani A. Naltrexone fails to block the acquisition or expression of a flavor preference conditioned by intragastric carbohydrate infusions. Pharmacol Biochem Behav 67: 545– 557, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Azzara AV, Sclafani A. Flavor preferences conditioned by intragastric sugar infusions in rats: maltose is more reinforcing than sucrose. Physiol Behav 64: 535– 541, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Bachmanov AA, Beauchamp GK. Taste receptor genes. Annu Rev Nutr 27: 389– 414, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bachmanov AA, Bosak NP, Floriano WB, Inoue M, Li X, Lin C, Murovets VO, Reed DR, Zolotarev VA, Beauchamp GK. Genetics of sweet taste preferences. Flavour Fragr J 26: 286– 294, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bachmanov AA, Tordoff MG, Beauchamp GK. Intake of umami-tasting solutions by mice: a genetic analysis. J Nutr 130: 935S– 941S, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baker BJ, Booth DA, Duggan JP, Gibson EL. Protein appetite demonstrated: Learned specificity of protein-cue preference to protein need in adult rats. Nutr Res 7: 481– 487, 1987 [Google Scholar]
- 25. Behrens M, Meyerhof W. Gustatory and extragustatory functions of mammalian taste receptors. Physiol Behav 105: 4– 13, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Behrens M, Meyerhof W, Hellfritsch C, Hofmann T. Sweet and umami taste: natural products, their chemosensory targets, and beyond. Angew Chem Int Ed 50: 2220– 2242, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Bernal SY, Touzani K, Gerges M, Abayev Y, Sclafani A, Bodnar RJ. Opioid receptor antagonism in the nucleus accumbens fails to block the expression of sugar-conditioned flavor preferences in rats. Pharmacol Biochem Behav 95: 56– 62, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil 20: 64– 72, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berthoud HR, Lenard NR, Shin AC. Food reward, hyperphagia, obesity. Am J Physiol Regul Integr Comp Physiol 300: R1266– R1277, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bertrand PP. The cornucopia of intestinal chemosensory transduction. Front Neurosci 3: 48 doi: 10.3389/neuro.21.003.2009: 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bezençon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses 32: 41– 49, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Birch LL, McPhee L, Steinberg L, Sullivan S. Conditioned flavor preferences in young children. Physiol Behav 47: 501– 505, 1990 [DOI] [PubMed] [Google Scholar]
- 33. Blackshaw LA, Young RL. Detection and signaling of glucose in the intestinal mucosa-vagal pathway. Neurogastroenterol Motil 23: 591– 594, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Breslin PAS, Spector AC. Mammalian taste perception. Curr Biol 18: R148– R155, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Brown RJ, Walter M, Rother KI. Ingestion of diet soda before a glucose load augments GLP-1 secretion. Diabetes Care 32: 2184– 2186, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brunstrom JM. Dietary learning in humans: directions for future research. Physiol Behav 85: 57– 65, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Brunstrom JM, Mitchell GL. Flavor-nutrient learning in restrained and unrestrained eaters. Physiol Behav 90: 133– 141, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Buchan AMJ. Nutrient tasting and signaling mechanisms in the gut. III. Endocrine cell recognition of luminal nutrients. Am J Physiol Gastrointest Liver Physiol 277: G1103– G1107, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Burke CJ, Waddell S. Remembering nutrient quality of sugar in Drosophila. Curr Biol 21: 746– 750, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burritt EA, Provenza FD. Lambs form preferences for nonnutritive flavors paired with glucose. J Anim Sci 70: 1133– 1136, 1992 [DOI] [PubMed] [Google Scholar]
- 41. Cagan RH, Maller O. Taste of sugars: brief exposure single-stimulus behavioral method. J Comp Physiol Psychol 87: 47– 55, 1974 [DOI] [PubMed] [Google Scholar]
- 42. Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N, le Coutre J, Ninomiya Y, Damak S. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci 30: 8376– 8382, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chale-Rush A, Burgess JR, Mattes RD. Multiple routes of chemosensitivity to free fatty acids in humans. Am J Physiol Gastrointest Liver Physiol 292: G1206– G1212, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Chambers ES, Bridge MW, Jones DA. Carbohydrate sensing in the human mouth: effects on exercise performance and brain activity. J Physiol 587: 1779– 1794, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chaudhari N, Landin MA, Roper SD. A metabotropic glutamate receptor variant functions as a taste receptor. Nat Neurosci 3: 113– 119, 2000 [DOI] [PubMed] [Google Scholar]
- 46. Chaudhari N, Pereira E, Roper SD. Taste receptors for umami: the case for multiple receptors. Am J Clin Nutr 90: 738S- 7742, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol 190: 285– 296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chelikani PK, Haver AC, Reidelberger RD. Dose-dependent effects of peptide YY(3–36) on conditioned taste aversion in rats. Peptides 27: 3193– 3201, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Chevrot M, Martin C, Passilly-Degrace P, Besnard P. Role of CD36 in oral and postoral sensing of lipids. In: Appetite Control, Handbook of Experimental Pharmacology, edited by Joost H-G. Berlin, Heidelberg: Springer, vol. 209, 2012, p. 295–307 [DOI] [PubMed] [Google Scholar]
- 50. Cho YM, Kieffer TJ. K-cells and glucose-dependent insulinotropic polypeptide in health and disease. In: Vitamins & Hormones: Incretins and Insulin Secretion, edited by Liwack G. London: Academic, 2010, p. 111–150 [DOI] [PubMed] [Google Scholar]
- 51. Cota D, Tschop MH, Horvath TL, Levine AS. Cannabinoids, opioids and eating behavior: the molecular face of hedonism? Brain Res Rev 51: 85– 107, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest 117: 13– 23, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Perez CA, Shigemura N, Yoshida R, Mosinger B, Jr, Glendinning JI, Ninomiya Y, Margolskee RF. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses 31: 253– 264, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 301: 850– 853, 2003 [DOI] [PubMed] [Google Scholar]
- 55. Daousi C, Wilding JP, Aditya S, Durham BH, Cleator J, Pinkney JH, Ranganath LR. Effects of peripheral administration of synthetic human glucose-dependent insulinotropic peptide (GIP) on energy expenditure and subjective appetite sensations in healthy normal weight subjects and obese patients with type 2 diabetes. Clin Endocrinol 71: 195– 201, 2009 [DOI] [PubMed] [Google Scholar]
- 56. De Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MAL, Simon SA. Food reward in the absence of taste receptor signaling. Neuron 57: 930– 941, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Degrace-Passilly P, Besnard P. CD36 and taste of fat. Curr Opin Clin Nutr Metab Care 15: 107– 111, 2012 [DOI] [PubMed] [Google Scholar]
- 58. Dela Cruz J, Icaza D, Taybali H, Sampson C, Galanopoulos V, Bamshad D, Touzani K, Sclafani A, Bodnar RJ. Roles of dopamine D1 and D2 receptors in the acquisition and expression of fat-conditioned flavor preferences in rats. Neurobiol Learn Mem. 97: 332– 337, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Delay ER, Hernandez NP, Bromley K, Margolskee RF. Sucrose and monosodium glutamate taste thresholds and discrimination ability of T1R3 knockout mice. Chem Senses 31: 351– 357, 2006 [DOI] [PubMed] [Google Scholar]
- 60. Delay ER, Mitzelfelt JD, Westburg AM, Gross N, Duran BL, Eschle BK. Comparison of l-monosodium glutamate and l-amino acid taste in rats. Neuroscience 148: 266– 278, 2007 [DOI] [PubMed] [Google Scholar]
- 61. Deutsch JA, Moore BO, Heinrichs SC. Unlearned specific appetite for protein. Physiol Behav 46: 619– 624, 1989 [DOI] [PubMed] [Google Scholar]
- 62. Diakogiannaki E, Gribble FM, Reimann F. Nutrient detection by incretin hormone secreting cells. Physiol Behav In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. DiBattista D, Mercier S. Role of learning in the selection of dietary protein in the golden hamster (Mesocricetus auratus). Behav Neurosci 113: 574– 586, 1999 [DOI] [PubMed] [Google Scholar]
- 64. Diez-Sampedro A, Hirayama BA, Osswald C, Gorboulev V, Baumgarten K, Volk C, Wright EM, Koepsell H. A glucose sensor hiding in a family of transporters. Proc Natl Acad Sci USA 100: 11753– 11758, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dipatrizio NV, Astarita G, Schwartz G, Li X, Piomelli D. Endocannabinoid signal in the gut controls dietary fat intake. Proc Natl Acad Sci USA 108: 12904– 12908, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dockray GJ. How the gut sends signals in response to food. Int Dairy J 20: 226– 230, 2010 [Google Scholar]
- 67. Drucker DB, Ackroff K, Sclafani A. Nutrient-conditioned flavor preference and acceptance in rats: effects of deprivation state and nonreinforcement. Physiol Behav 56: 701– 707, 1994 [DOI] [PubMed] [Google Scholar]
- 68. Drucker DB, Sclafani A. The role of gastric and postgastric sites in glucose-conditioned flavor preferences in rats. Physiol Behav 61: 351– 358, 1997 [DOI] [PubMed] [Google Scholar]
- 69. Dus M, Min SH, Keene AC, Lee GY, Suh GSB. Taste-independent detection of the caloric content of sugar in Drosophila. Proc Natl Acad Sci USA 108: 11644– 11649, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dyer J, Daly K, Salmon KSH, Arora DK, Kokrashvili Z, Margolskee RF, Shirazi-Beechey SP. Intestinal glucose sensing and regulation of intestinal glucose absorption. Biochem Soc Trans 035: 1191– 1194, 2007 [DOI] [PubMed] [Google Scholar]
- 71. Egan JM, Margolskee RF. Taste cells of the gut and gastrointestinal chemosensation. Mol Interv 8: 78– 81, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Elizalde G, Sclafani A. Fat appetite in rats: flavor preferences conditioned by nutritive and non-nutritive oil emulsions. Appetite 15: 189– 197, 1990 [DOI] [PubMed] [Google Scholar]
- 73. Elizalde G, Sclafani A. Flavor preferences conditioned by intragastric Polycose infusions: a detailed analysis using an electronic esophagus preparation. Physiol Behav 47: 63– 77, 1990 [DOI] [PubMed] [Google Scholar]
- 74. Feng XH, Liu XM, Zhou LH, Wang J, Liu GD. Expression of glucagon-like peptide-1 in the taste buds of rat circumvallate papillae. Acta Histochem 10: 151– 154, 2007 [DOI] [PubMed] [Google Scholar]
- 75. Ferreira JG, Tellez LA, Ren X, Yeckel CW, De Araujo IE. Regulation of fat intake in the absence of flavor signaling. J Physiol 590: 953– 972, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Figlewicz DP, Sipols AJ. Energy regulatory signals and food reward. Pharmacol Biochem Behav 97: 15– 24, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310: 1495– 1499, 2005 [DOI] [PubMed] [Google Scholar]
- 78. Finger TE, Kinnamon SC. Taste isn't just for taste buds anymore. F1000 Biol Rep 3: 20 (doi:10.3410/B3–20), 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ford HE, Peters V, Martin NM, Sleeth ML, Ghatei MA, Frost GS, Bloom SR. Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. Eur J Clin Nutr 65: 508– 513, 2011 [DOI] [PubMed] [Google Scholar]
- 80. Freeman S, Bohan DC, Darcel N, Raybould HE. Luminal glucose sensing in the rat intestine has characteristics of a sodium-glucose co-transporter. Am J Physiol Gastrointest Liver Physiol 291: G439– G445, 2006 [DOI] [PubMed] [Google Scholar]
- 81. Fujita Y, Wideman RD, Speck M, Asadi A, King DS, Webber TD, Haneda M, Kieffer TJ. Incretin release from gut is acutely enhanced by sugar but not by sweeteners in vivo. Am J Physiol Endocrinol Metab 296: E473– E479, 2009 [DOI] [PubMed] [Google Scholar]
- 82. Fukuwatari T, Kawada T, Tsuruta M, Hiraoka T, Iwanaga T, Sugimoto E, Fushiki T. Expression of the putative membrane fatty acid transporter (FAT) in taste buds of the circumvallate papillae in rats. FEBS Lett 414: 461– 464, 1997 [DOI] [PubMed] [Google Scholar]
- 83. Fukuwatari T, Shibata K, Iguchi K, Saeki T, Iwata A, Tani K, Sugimoto E, Fushiki T. Role of gustation in the recognition of oleate and triolein in anosmic rats. Physiol Behav 78: 579– 583, 2003 [DOI] [PubMed] [Google Scholar]
- 84. Furness JB, Kunze WAA, Clerc N. Nutrient tasting and signaling mechanisms in the gut II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Physiol Gastrointest Liver Physiol 277: G922– G928, 1999 [DOI] [PubMed] [Google Scholar]
- 85. Galef BG., Jr Is there a specific appetite for protein? In: Neural and Metabolic Control of Macronutrient Intake, edited by Berthoud H-R, Seeley R. Boca Raton, FL: CRC, 1999, p. 19–28 [Google Scholar]
- 86. Gant N, Stinear CM, Byblow WD. Carbohydrate in the mouth immediately facilitates motor output. Brain Res 1350: 151– 158, 2010 [DOI] [PubMed] [Google Scholar]
- 87. Garcia J, Ervin FR, Koelling RA. Learning with prolonged delay of reinforcement. Psychon Sci 5: 121– 122, 1966 [Google Scholar]
- 88. Geiger E. The role of the time factor in feeding supplementary protein. J Nutr 36: 813– 819, 1948 [DOI] [PubMed] [Google Scholar]
- 89. Geraedts MCP, Troost FJ, Saris WHM. Gastrointestinal targets to modulate satiety and food intake. Obes Rev 12: 470– 477, 2010 [DOI] [PubMed] [Google Scholar]
- 90. Gibson EL, Booth DA. Acquired protein appetite in rats: dependence on a protein-specific state. Experientia 42: 1003– 1004, 1986 [DOI] [PubMed] [Google Scholar]
- 91. Gibson EL, Wainwright CJ, Booth DA. Disguised protein in lunch after low-protein breakfest conditions food-flavor preferences dependent on recent lack of protein intake. Physiol Behav 58: 363– 371, 1995 [DOI] [PubMed] [Google Scholar]
- 92. Gietzen DW, Hao S, Anthony TG. Mechanisms of food intake repression in indispensable amino acid deficiency. Annu Rev Nutr 27: 63– 78, 2007 [DOI] [PubMed] [Google Scholar]
- 93. Gilbertson TA, Fontenot DT, Liu LD, Zhang H, Monroe WT. Fatty acid modulation of K+ channels in taste receptor cells: gustatory cues for dietary fat. Am J Physiol Cell Physiol 272: C1203– C1210, 1997 [DOI] [PubMed] [Google Scholar]
- 94. Gilbertson TA, Yu T, Shah BP. Gustatory mechanisms for fat detection. In: Fat Detection: Taste, Texture, and Post Ingestive Effects, edited by Montmayeur J-P, le Coutre J. Boca Raton, FL: Taylor and Francis, 2010, p. 83–104 [Google Scholar]
- 95. Glass DS, Margolskee RF, Sclafani A. Glucose-conditioned preferences in taste-impaired TRPM5 knockout mice. Appetite 52: 833, 2009 [Google Scholar]
- 96. Glendinning JI, Bloom LD, Onishi M, Zheng KH, Damak S, Margolskee RF, Spector AC. Contribution of α-gustducin to taste-guided licking responses of mice. Chem Senses 30: 299– 316, 2005 [DOI] [PubMed] [Google Scholar]
- 97. Gowans SE. Role of Portal and Plasma Glucose Elevations in Taste-To-Postingestive Consequence Learning (Dissertation). McMaster University, 1992 [Google Scholar]
- 98. Gowans SE, Weingarten HP. Elevations of plasma glucose do not support taste-to-postingestive consequence learning. Am J Physiol Regul Integr Comp Physiol 261: R1409– R1417, 1991 [DOI] [PubMed] [Google Scholar]
- 99. Greenberg D, Kava RA, Lewis DR, Greenwood MRC, Smith GP. The time course for the entry of intestinally infused lipids into the blood of rats. Am J Physiol Regul Integr Comp Physiol 269: R432– R436, 1995 [DOI] [PubMed] [Google Scholar]
- 100. Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 52: 1147– 1154, 2003 [DOI] [PubMed] [Google Scholar]
- 101. Grill HJ, Flynn FW. Behavioral analysis of oral stimulating effects of amino acid and glutamate compounds on the rat. In: Umami: A Basic Taste, edited by Kawamura Y, Kare MR. New York: Marcel Dekker, 1987, p. 461–480 [Google Scholar]
- 102. Hajnal A, Norgren R. Dopamine release by sucrose. In: The Senses: A Comprehensive Reference, edited by Basbaum AI, Kaneko A, Shepherd GM, Westheimer G, Albright TD, Masland RH, Dallos P, Oertel D, Firestein S, Beauchamp GK, Bushnell MC, Kaas JH, Gardner E. New York: Academic, 2008, p. 459–468 [Google Scholar]
- 103. Halpern BP. What's in a name? Are MSG and umami the same? Chem Senses 27: 845– 846, 2002 [DOI] [PubMed] [Google Scholar]
- 104. Havermans RC, Jansen A. Acquired tastes: establishing food (dis-)likes by flavour-flavour learning. In: Handbook of Behavior, Food and Nutrition, edited by Preedy VR, Watson RR, Martin CR. New York: Springer-Verlag, 2011, p. 73–84 [Google Scholar]
- 105. He W, Yasumatsu K, Varadarajan V, Yamada A, Lem J, Ninomiya Y, Margolskee RF, Damak S. Umami taste responses are mediated by α-transducin and α-gustducin. J Neurosci 24: 7674– 7680, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Heinrichs SC, Deutsch JA, Moore BO. Olfactory self-selection of protein-containing foods. Physiol Behav 47: 409– 413, 1990 [DOI] [PubMed] [Google Scholar]
- 107. Hisatsune C, Yasumatsu K, Takahashi-Iwanaga H, Ogawa N, Kuroda Y, Yoshida R, Ninomiya Y, Mikoshiba K. Abnormal taste perception in mice lacking the type 3 inositol 1, 4, 5-trisphosphate receptor. J Biol Chem 282: 37225– 37231, 2007 [DOI] [PubMed] [Google Scholar]
- 108. Holman GL. Intragastric reinforcement effect. J Comp Physiol Psychol 69: 432– 441, 1968 [DOI] [PubMed] [Google Scholar]
- 109. Horn CC, Murat C, Rosazza M, Still L. Effects of gastric distension and infusion of umami and bitter taste stimuli on vagal afferent activity. Brain Res 1419: 53– 60, 2011 [DOI] [PubMed] [Google Scholar]
- 110. Ikeda K. New seasonings. Chem Senses 27: 847– 849, 2002 [DOI] [PubMed] [Google Scholar]
- 111. Imaizumi M, Takeda M, Fushiki T. Effects of oil intake in the conditioned place preference test in mice. Brain Res 870: 150– 156, 2000 [DOI] [PubMed] [Google Scholar]
- 112. Irwin N, Green BD, Gault VA, Cassidy RS, O'Harte FPM, Harriott P, Flatt PR. Effects on glucose homeostasis and insulin secretion of long term activation of the glucose-dependent insulinotropic polypeptide (GIP) receptor by N-AcGIP(LysPAL37) in normal mice. Peptides 27: 893– 900, 2006 [DOI] [PubMed] [Google Scholar]
- 113. Iwatsuki K, Ichikawa R, Uematsu A, Kitamura A, Uneyama H, Torii K. Detecting sweet and umami tastes in the GI tract. Acta Physiol (Oxf) 204: 169– 177, 2011 [DOI] [PubMed] [Google Scholar]
- 114. Izzo AA, Sharkey KA. Cannabinoids and the gut: new developments and emerging concepts. Pharmacol Therapeutics 126: 21– 38, 2010 [DOI] [PubMed] [Google Scholar]
- 115. Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA 104: 15069– 15074, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Jeukendrup AE, Chambers ES. Oral carbohydrate sensing and exercise performance. Curr Opin Clin Nutr Metab Care 13: 447– 451, 2010 [DOI] [PubMed] [Google Scholar]
- 117. Johnson SL, McPhee L, Birch LL. Conditioned preferences for flavors associated with high dietary fat. Physiol Behav 50: 1245– 1251, 1991 [DOI] [PubMed] [Google Scholar]
- 118. Katz DB, Nicolelis MAL, Simon SA. Nutrient tasting and signaling mechanisms in the gut IV. There is more to taste than meets the tongue. Am J Physiol Gastrointest Liver Physiol 278: G6– G9, 2000 [DOI] [PubMed] [Google Scholar]
- 119. Kawai T, Fushiki T. Importance of lipolysis in oral cavity for orosensory detection of fat. Am J Physiol Regul Integr Comp Physiol 285: R447– R454, 2003 [DOI] [PubMed] [Google Scholar]
- 120. Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron 69: 664– 679, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kern DL, McPhee L, Fisher J, Johnson S, Birch LL. The postingestive consequences of fat condition preferences for flavors associated with high dietary fat. Physiol Behav 54: 71– 76, 1993 [DOI] [PubMed] [Google Scholar]
- 122. Khan NA, Besnard P. Oro-sensory perception of dietary lipids: new insights into the fat taste transduction. Biochim Biophys Acta 1791: 149– 155, 2009 [DOI] [PubMed] [Google Scholar]
- 123. Kondoh T, Mori M, Ono T, Torii K. Mechanisms of umami taste preference and aversion in rats. J Nutr 130: 966S– 970S, 2000 [DOI] [PubMed] [Google Scholar]
- 124. Kondoh T, Torii K. Forebrain activation by postoral nutritive substances. In: Handbook of Behavior, Food and Nutrition, edited by Preedy VR, Watson RR, Martin CR. New York: Springer-Verlag, 2011, p. 469–487 [Google Scholar]
- 125. Kusuhara Y, Yasumatsu K, Ohkuri T, Yoshida R, Maeda K, Ninomiya Y. Involvement of mGluRs in umami detection in mice. Neurosci Res 71, Suppl: e358, 2011 [Google Scholar]
- 126. Larue C. Oral cues involved in the rat's selective intake of fats. Chem Senses Flav 3: 1– 6, 1978 [Google Scholar]
- 127. Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest 115: 3177– 3184, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA 99: 4692– 4696, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Liang NC, Hajnal A, Norgren R. Sham feeding corn oil increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol 291: R1236– R1239, 2006 [DOI] [PubMed] [Google Scholar]
- 130. Little TJ, Feinle-Bisset C. Effects of dietary fat on appetite and energy intake in health and obesity-oral and gastrointestinal sensory contributions. Physiol Behav 104: 613– 620, 2011 [DOI] [PubMed] [Google Scholar]
- 131. Liu P, Shah BP, Croasdell S, Gilbertson TA. Transient receptor potential channel type M5 Is essential for fat taste. J Neurosci 31: 8634– 8642, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Lucas F, Ackroff K, Sclafani A. High-fat diet preference and overeating mediated by postingestive factors in rats. Am J Physiol Regul Integr Comp Physiol 275: R1511– R1522, 1998 [DOI] [PubMed] [Google Scholar]
- 133. Lucas F, Sclafani A. Flavor preferences conditioned by intragastric fat infusions in rats. Physiol Behav 46: 403– 412, 1989 [DOI] [PubMed] [Google Scholar]
- 134. Lucas F, Sclafani A. Capsaicin attenuates feeding suppression but not reinforcement by intestinal nutrients. Am J Physiol Regul Integr Comp Physiol 270: R1059– R1064, 1996 [DOI] [PubMed] [Google Scholar]
- 135. Lucas F, Sclafani A. Differential reinforcing and satiating effects of intragastric fat and carbohydrate infusions in rats. Physiol Behav 66: 381– 388, 1999 [DOI] [PubMed] [Google Scholar]
- 136. Lundy RF, Jr, Norgren R. Gustatory system. In: The Rat Nervous System (3rd ed), edited by George P. Burlington: Academic, 2004, p. 891–921 [Google Scholar]
- 137. Ma J, Bellon M, Wishart JM, Young RL, Blackshaw LA, Jones KL, Horowitz M, Rayner CK. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am J Physiol Gastrointest Liver Physiol 296: G735– G739, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ma J, Chang J, Checklin HL, Young RL, Jones KL, Horowitz M, Rayner CK. Effect of the artificial sweetener, sucralose, on small intestinal glucose absorption in healthy human subjects. Br J Nutr 104: 803– 806, 2010 [DOI] [PubMed] [Google Scholar]
- 139. Mace OJ, Affleck JA, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol 582: 379– 392, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Margolskee RF, Dyer J, Kokrashvili Z, Salmon KSH, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA 104: 15075– 15080, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Martin C, Chevrot M, Poirier H, Passilly-Degrace P, Niot I, Besnard P. CD36 as a lipid sensor. Physiol Behav 105: 36– 42, 2011 [DOI] [PubMed] [Google Scholar]
- 142. Martin C, Passilly-Degrace P, Gaillard D, Merlin JF, Chevrot M, Besnard P. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLos One 6: e24014, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Mather P, Nicolaidis S, Booth DA. Compensatory and conditioned feeding responses to scheduled glucose infusions in the rat. Nature 273: 461– 463, 1978 [DOI] [PubMed] [Google Scholar]
- 144. Mattes RD. Accumulating evidence supports a taste component for free fatty acids in humans. Physiol Behav 104: 624– 631, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Mattes RD. Oral fatty acid signaling and intestinal lipid processing: support and supposition. Physiol Behav 105: 27– 35, 2011 [DOI] [PubMed] [Google Scholar]
- 146. Mehiel R, Bolles RC. Learned flavor preferences based on calories are independent of initial hedonic value. Anim Learn Behav 16: 383– 387, 1988 [Google Scholar]
- 147. Mei N. Intestinal chemosensitivity. Physiol Rev 65: 211– 237, 1985 [DOI] [PubMed] [Google Scholar]
- 148. Mela DJ. Dietary Fats: Determinants of Preference, Selection and Consumption. London: Elsevier Applied Science, 1992 [Google Scholar]
- 149. Menzies JRW, Skibicka KP, Egecioglu E, Leng G, Dickson SL. Peripheral signals modifying food reward. In: Appetite Control, Handbook of Experimental Pharmacology, edited by Joost H-G. Berlin Heidelberg: Springer, 2012, vol. 209, p. 131– 158 [DOI] [PubMed] [Google Scholar]
- 150. Merigo F, Benati D, Cristofoletti M, Osculati F, Sbarbati A. Glucose transporters are expressed in taste receptor cells. J Anat 219: 243–252, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, Fujimoto S, Oku A, Tsuda K, Toyokuni S, Hiai H, Mizunoya W, Fushiki T, Holst JJ, Makino M, Tashita A, Kobara Y, Tsubamoto Y, Jinnouchi T, Jomori T, Seino Y. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 8: 738– 742, 2002 [DOI] [PubMed] [Google Scholar]
- 152. Montmayeur JP, le Coutre J. Fat Detection: Taste, Texture, and Post Ingestive Effects. Boca Raton, FL: Taylor and Francis, 2010 [PubMed] [Google Scholar]
- 153. Moran AW, Al Rammahi MA, Arora DK, Batchelor DJ, Coulter EA, Daly K, Ionescu C, Bravo D, Shirazi-Beechey SP. Expression of Na+/glucose co-transporter 1 (SGLT1) is enhanced by supplementation of the diet of weaning piglets with artificial sweeteners. Br J Nutr 104: 637– 646, 2010 [DOI] [PubMed] [Google Scholar]
- 154. Moran TH, Dailey MJ. Intestinal feedback signaling and satiety. Physiol Behav 105: 77– 81, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Moriya R, Shirakura T, Ito J, Mashiko S, Seo T. Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am J Physiol Endocrinol Metab 297: E1358– E1365, 2009 [DOI] [PubMed] [Google Scholar]
- 156. Myers KP. Robust preference for a flavor paired with intragastric glucose acquired in a single trial. Appetite 48: 123– 127, 2007 [DOI] [PubMed] [Google Scholar]
- 157. Myers KP, Sclafani A. Conditioned enhancement of flavor evaluation reinforced by intragastric glucose. I. Intake acceptance and preference analysis. Physiol Behav 74: 481– 493, 2001 [DOI] [PubMed] [Google Scholar]
- 158. Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature 416: 199– 202, 2002 [DOI] [PubMed] [Google Scholar]
- 159. Niijima A. Reflex effects of oral, gastrointestinal and hepatoportal glutamate sensors on vagal nerve activity. J Nutr 130: 971S– 973S, 2000 [DOI] [PubMed] [Google Scholar]
- 160. Núñez-Jaramillo L, Ramírez-Lugo L, Herrera-Morales W, Miranda MI. Taste memory formation: latest advances and challenges. Behav Brain Res 207: 232– 248, 2010 [DOI] [PubMed] [Google Scholar]
- 161. Ohara I, Naim M. Effects of monosodium glutamate on eating and drinking behavior of rats. Physiol Behav 19: 627– 634, 1977 [DOI] [PubMed] [Google Scholar]
- 162. Ohkuri T, Yasumatsu K, Horio N, Jyotaki M, Margolskee RF, Ninomiya Y. Multiple sweet receptors and transduction pathways revealed in knockout mice by temperature dependence and gurmarin sensitivity. Am J Physiol Regul Integr Comp Physiol 296: R960– R971, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Oliveira-Maia AJ, Roberts CD, Walker QD, Luo B, Kuhn C, Simon SA, Nicolelis MA. Intravascular food reward. PLos One 6: e24992, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Olszewski PK, Levine AS. Central opioids and consumption of sweet tastants: When reward outweighs homeostasis. Physiol Behav 91: 506– 512, 2007 [DOI] [PubMed] [Google Scholar]
- 165. Olszewski PK, Schioth HB, Levine AS. Ghrelin in the CNS: from hunger to a rewarding and memorable meal? Brain Res Rev 58: 160– 170, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Otsubo H, Kondoh T, Shibata M, Torii K, Ueta Y. Induction of FOS expression in the rat forebrain after intragastric administration of monosodium l-glutamate, glucose and NaCl. Neuroscience 196: 97– 103, 2011 [DOI] [PubMed] [Google Scholar]
- 167. Parker HE, Reimann F, Gribble FM. Molecular mechanisms underlying nutrient-stimulated incretin secretion. Expert Rev Mol Med 12: e1, 2010 [DOI] [PubMed] [Google Scholar]
- 168. Paschetta E, Hvalryg M, Musso G. Glucose-dependent insulinotropic polypeptide: from pathophysiology to therapeutic opportunities in obesity-associated disorders. Obes Rev 12: 813– 828, 2011 [DOI] [PubMed] [Google Scholar]
- 169. Pelchat ML, Grill HJ, Rozin P, Jacobs J. Quality of acquired responses to tastes by Rattus norvegicus depends on type of associated discomfort. J Comp Psychol 97: 140– 153, 1983 [PubMed] [Google Scholar]
- 170. Pérez C, Ackroff K, Sclafani A. Carbohydrate- and protein-conditioned flavor preferences: effects of nutrient preloads. Physiol Behav 59: 467– 474, 1996 [DOI] [PubMed] [Google Scholar]
- 171. Pérez C, Fanizza LJ, Sclafani A. Flavor preferences conditioned by intragastric nutrients in rats fed chow or a cafeteria diet. Appetite 32: 155– 170, 1999 [DOI] [PubMed] [Google Scholar]
- 172. Pérez C, Lucas F, Sclafani A. Devazepide, a CCKA antagonist,attenuates the satiating but not the preference conditioning effects of intestinal carbohydrate infusions in rats. Pharmacol Biochem Behav 59: 451– 457, 1998 [DOI] [PubMed] [Google Scholar]
- 173. Pérez C, Lucas F, Sclafani A. Increased flavor preference and acceptance conditioned by the postingestive actions of glucose. Physiol Behav 64: 483– 492, 1998 [DOI] [PubMed] [Google Scholar]
- 174. Pérez C, Sclafani A. Cholecystokinin conditions flavor preferences in rats. Am J Physiol Regul Integr Comp Physiol 260: R179– R185, 1991 [DOI] [PubMed] [Google Scholar]
- 175. Pittman DW, Labban CE, Anderson AA, O'Connor HE. Linoleic and oleic acids alter the licking responses to sweet, salt, sour, and bitter tastants in rats. Chem Senses 31: 835– 843, 2006 [DOI] [PubMed] [Google Scholar]
- 176. Prescott J. Effects of added glutamate on liking for novel food flavors. Appetite 42: 143– 150, 2004 [DOI] [PubMed] [Google Scholar]
- 177. Ramirez I. Role of olfaction in starch and oil preference. Am J Physiol Regul Integr Comp Physiol 265: R1404– R1409, 1993 [DOI] [PubMed] [Google Scholar]
- 178. Ramirez I. Stimulus specificity in flavor acceptance learning. Physiol Behav 60: 595– 610, 1996 [DOI] [PubMed] [Google Scholar]
- 179. Ramirez I. Intragastric carbohydrate exerts both intake-stimulating and intake-suppressing effects. Behav Neurosci 111: 612– 622, 1997 [PubMed] [Google Scholar]
- 180. Ramirez I. Stimulation of fluid intake by nutrients: oil is less effective than carbohydrate. Am J Physiol Regul Integr Comp Physiol 272: R289– R293, 1997 [DOI] [PubMed] [Google Scholar]
- 181. Randich A, Tyler WJ, Cox JE, Meller ST, Kelm GR, Bharaj SS. Responses of celiac and cervical vagal afferents to infusions of lipids in the jejunum or ileum of the rat. Am J Physiol Regul Integr Comp Physiol 278: R34– R43, 2000 [DOI] [PubMed] [Google Scholar]
- 182. Raybould HE. Nutrient tasting and signaling mechanisms in the gut. I. Sensing of lipid by the intestinal mucosa. Am J Physiol Gastrointest Liver Physiol 277: G751– G755, 1999 [DOI] [PubMed] [Google Scholar]
- 183. Raybould HE. Gut chemosensing: Interactions between gut endocrine cells and visceral afferents. Auton Neurosci 153: 41– 46, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Reilly S, Schachtman TR. Conditioned Taste Aversion: Behavioral And Neural Processes. Oxford: Oxford University, 2009 [Google Scholar]
- 185. Reimann F. Molecular mechanisms underlying nutrient detection by incretin-secreting cells. Int Dairy J 20: 236– 242, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab 8: 532– 539, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Ren X, Ferreira JG, Zhou L, Shammah-Lagnado SJ, Yeckel CW, De Araujo IE. Nutrient selection in the absence of taste receptor signaling. J Neurosci 30: 8012– 8023, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Rozengurt E, Sternini C. Taste receptor signaling in the mammalian gut. Curr Opin Pharmacol 7: 557– 562, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Ruiz CJ, Wray K, Delay E, Margolskee RF, Kinnamon SC. Behavioral evidence for a role of α-gustducin in glutamate taste. Chem Senses 28: 573– 579, 2003 [DOI] [PubMed] [Google Scholar]
- 190. Ruiz-Avila L, Wong GT, Damak S, Margolskee RF. Dominant loss of responsiveness to sweet and bitter compounds caused by a single mutation in alpha -gustducin. Proc Natl Acad Sci USA 98: 8868– 8873, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Ruttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology 150: 1174– 1181, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Saitou K, Yoneda T, Mizushige T, Asano H, Okamura M, Matsumura S, Eguchi A, Manabe Y, Tsuzuki S, Inoue K, Fushiki T. Contribution of gustation to the palatability of linoleic acid. Physiol Behav 96: 142– 148, 2009 [DOI] [PubMed] [Google Scholar]
- 193. San Gabriel A, Nakamura E, Uneyama H, Torii K. Taste, visceral information and exocrine reflexes with glutamate through umami receptors. J Med Invest 56, Suppl: 209– 217, 2009 [DOI] [PubMed] [Google Scholar]
- 194. San Gabriel AM, Maekawa T, Uneyama H, Yoshie S, Torii K. mGluR1 in the fundic glands of rat stomach. FEBS Lett 581: 1119–1123, 2007 [DOI] [PubMed] [Google Scholar]
- 195. Schwartz GJ. Gut fat sensing in the negative feedback control of energy balance–Recent advances. Physiol Behav 104: 621– 623, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Schwartz GJ, Fu J, Astarita G, Li X, Gaetani S, Campolongo P, Cuomo V, Piomelli D. The lipid messenger OEA links dietary fat intake to satiety. Cell Metab 8: 281– 288, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Sclafani A. Carbohydrate taste appetite, obesity: An overview. Neurosci Biobehav Rev 11: 131– 153, 1987 [PubMed] [Google Scholar]
- 198. Sclafani A. Oral and postoral determinants of food reward. Physiol Behav 81: 773– 779, 2004 [DOI] [PubMed] [Google Scholar]
- 199. Sclafani A. The sixth taste. Appetite 43: 1– 3, 2004 [DOI] [PubMed] [Google Scholar]
- 200. Sclafani A, Ackroff K. The relationship between food reward and satiation revisited. Physiol Behav 82: 89– 95, 2004 [DOI] [PubMed] [Google Scholar]
- 201. Sclafani A, Ackroff K. Flavor preferences conditioned by intragastric glucose but not fructose or galactose in C57BL/6J mice. Physiol Behav. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Sclafani A, Ackroff K, Abumrad N. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am J Physiol Regul Integr Comp Physiol 293: R1823– R1832, 2007 [DOI] [PubMed] [Google Scholar]
- 203. Sclafani A, Ackroff K, Schwartz GJ. Selective effects of vagal deafferentation and celiac-superior mesenteric ganglionectomy on the reinforcing and satiating action of intestinal nutrients. Physiol Behav 78: 285– 294, 2003 [DOI] [PubMed] [Google Scholar]
- 204. Sclafani A, Azzara AV, Touzani K, Grigson PS, Norgren R. Parabrachial nucleus lesions block taste and attenuate flavor preference and aversion conditioning in rats. Behav Neurosci 115: 920– 933, 2001 [DOI] [PubMed] [Google Scholar]
- 205. Sclafani A, Cardieri C, Tucker K, Blusk D, Ackroff K. Intragastric glucose but not fructose conditions robust flavor preferences in rats. Am J Physiol Regul Integr Comp Physiol 265: R320– R325, 1993 [DOI] [PubMed] [Google Scholar]
- 206. Sclafani A, Fanizza LJ, Azzara AV. Conditioned flavor avoidance, preference and indifference produced by intragastric infusions of galactose, glucose and fructose in rats. Physiol Behav 67: 227– 234, 1999 [DOI] [PubMed] [Google Scholar]
- 207. Sclafani A, Glass DS, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol Regul Integr Comp Physiol 299: R1643– R1650, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Sclafani A, Glendinning JI. Flavor preferences conditioned in C57BL/6 mice by intragastric carbohydrate self-infusion. Physiol Behav 79: 783– 788, 2003 [DOI] [PubMed] [Google Scholar]
- 209. Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: oral and postoral interactions. Am J Physiol Regul Integr Comp Physiol 289: R712– R720, 2005 [DOI] [PubMed] [Google Scholar]
- 210. Sclafani A, Lucas F. Abdominal vagotomy does not block carbohydrate-conditioned flavor preferences in rats. Physiol Behav 60: 447– 453, 1996 [DOI] [PubMed] [Google Scholar]
- 211. Sclafani A, Lucas F, Ackroff K. The importance of taste and palatability in carbohydrate-induced overeating in rats. Am J Physiol Regul Integr Comp Physiol 270: R1197– R1202, 1996 [DOI] [PubMed] [Google Scholar]
- 212. Sclafani A, Mann S. Carbohydrate taste preferences in rats: glucose, sucrose, maltose, fructose and Polycose compared. Physiol Behav 40: 563– 568, 1987 [DOI] [PubMed] [Google Scholar]
- 213. Sclafani A, Touzani K, Bodnar RJ. Dopamine and learned food preferences. Physiol Behav 104: 64– 68, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Sclafani A, Zukerman S, Glendinning JI, Margolskee RF. Fat and carbohydrate preferences in mice: the contribution of α-gustducin and Trpm5 taste signaling proteins. Am J Physiol Regul Integr Comp Physiol 293: R1504– R1513, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Scott TR. Taste as a basis for body wisdom. Physiol Behav 104: 57– 63, 2011 [DOI] [PubMed] [Google Scholar]
- 216. Shanahan F. Nutrient tasting and signaling mechanisms in the gut. V. Mechanisms of immunologic sensation of intestinal contents. Am J Physiol Gastrointest Liver Physiol 278: G191– G196, 2000 [DOI] [PubMed] [Google Scholar]
- 217. Shen T, Kaya N, Zhao Fl Lu Sg Cao Y, Herness S. Co-expression patterns of the neuropeptides vasoactive intestinal peptide and cholecystokinin with the transduction molecules α-gustducin and T1R2 in rat taste receptor cells. Neuroscience 130: 229– 238, 2005 [DOI] [PubMed] [Google Scholar]
- 218. Shepherd GM. Neurogastronomy. New York: Columbia University, 2012 [Google Scholar]
- 219. Shibata R, Kameishi M, Kondoh T, Torii K. Bilateral dopaminergic lesions in the ventral tegmental area of rats influence sucrose intake, but not umami and amino acid intake. Physiol Behav 96: 667– 674, 2009 [DOI] [PubMed] [Google Scholar]
- 220. Shin YK, Martin B, Golden E, Dotson CD, Maudsley S, Kim W, Jang HJ, Mattson MP, Drucker DJ, Egan JM, Munger SD. Modulation of taste sensitivity by GLP-1 signaling. J Neurochem 106: 455– 463, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Shirazi-Beechey SP, Moran AW, Batchelor DJ, Daly K, Al Rammahi M. Glucose sensing and signalling: regulation of intestinal glucose transport. Proc Nutr Soc 70: 185– 193, 2011 [DOI] [PubMed] [Google Scholar]
- 222. Smeets PAM, Erkner A, de Graaf C. Cephalic phase responses and appetite. Nutr Rev 68: 643– 655, 2010 [DOI] [PubMed] [Google Scholar]
- 223. Stearns AT, Balakrishnan A, Rhoads DB, Tavakkolizadeh A. Rapid upregulation of sodium-glucose transporter SGLT1 in response to intestinal sweet taste stimulation. Ann Surg 251: 865– 871, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Steinert RE, Beglinger C. Nutrient sensing in the gut: Interactions between chemosensory cells, visceral afferents and the secretion of satiation peptides. Physiol Behav 105: 62– 70, 2011 [DOI] [PubMed] [Google Scholar]
- 225. Stewart JE, Feinle-Bisset C, Keast RSJ. Fatty acid detection during food consumption and digestion: associations with ingestive behavior and obesity. Prog Lipid Res 50: 225– 233, 2011 [DOI] [PubMed] [Google Scholar]
- 226. Stratford JM, Finger TE. Central representation of postingestive chemosensory cues in mice that lack the ability to taste. J Neurosci 31: 9101– 9110, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Takeda M, Sawano S, Imaizumi M, Fushiki T. Preference for corn oil in olfactory-blocked mice in the conditioned place preference test and the two-bottle choice test. Life Sci 69: 847– 854, 2001 [DOI] [PubMed] [Google Scholar]
- 228. Tolhurst G, Reimann F, Gribble FM. Intestinal sensing of nutrients. In: Appetite Control, Handbook of Experimental Pharmacology, edited by Joost H-G. Berlin Heidelberg: Springer, 2012, vol. 209, p. 309– 335 [DOI] [PubMed] [Google Scholar]
- 229. Tordoff MG. Metabolic basis of learned food preferences. In: Appetite and Nutrition, edited by Friedman MI, Tordoff MG, Kare MR. New York: Marcel Dekker, 1991, p. 239–260 [Google Scholar]
- 230. Tordoff MG, Friedman MI. Hepatic-portal glucose infusions decrease food intake and increase food preference. Am J Physiol Regul Integr Comp Physiol 251: R192– R196, 1986 [DOI] [PubMed] [Google Scholar]
- 231. Tordoff MG, Ulrich PM, Sandler F. Flavor preferences and fructose: evidence that the liver detects the unconditioned stimulus for calorie-based learning. Appetite 14: 29– 44, 1990 [DOI] [PubMed] [Google Scholar]
- 232. Torii K, Kondoh T, Mori M, Ono T. Hypothalamic control of amino acid appetite. Ann NY Acad Sci 855: 417– 425, 1998 [DOI] [PubMed] [Google Scholar]
- 233. Torii K, Mimura T, Yugari Y. Effects of dietary protein on the taste preference for amino acids in rats. In: Interaction of the Chemical Senses with Nutrition, edited by Kare MR, Brand JG. Orlando, FL: Academic, 1986, p. 45–69 [Google Scholar]
- 234. Touzani K, Bodnar RJ, Sclafani A. Activation of dopamine D1 receptors in the nucleus accumbens is critical for the acquisition, but not the expression, of flavor preference conditioned by intragastric glucose in rats. Eur J Neurosci 27: 1525– 1533, 2008 [DOI] [PubMed] [Google Scholar]
- 235. Touzani K, Bodnar RJ, Sclafani A. Dopamine D1-like receptor antagonism in amygdala impairs the acquisition of glucose-conditioned flavor preference in rats. Eur J Neurosci 30: 289– 298, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Touzani K, Bodnar RJ, Sclafani A. Acquisition of glucose-conditioned flavor preference requires the activation of dopamine D1-like receptors within the medial prefrontal cortex in rats. Neurobiol Learn Mem 94: 214– 219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Touzani K, Sclafani A. Conditioned flavor preference and aversion: role of the lateral hypothalamus. Behav Neurosci 115: 84– 93, 2001 [DOI] [PubMed] [Google Scholar]
- 238. Touzani K, Sclafani A. Lateral hypothalamic lesions impair flavor-nutrient and flavor-toxin trace learning in rats. Eur J Neurosci 16: 2425– 2433, 2002 [DOI] [PubMed] [Google Scholar]
- 239. Touzani K, Sclafani A. Critical role of amygdala in flavor but not taste preference learning in rats. Eur J Neurosci 22: 1767– 1774, 2005 [DOI] [PubMed] [Google Scholar]
- 240. Touzani K, Sclafani A. Insular cortex lesions fail to block flavor and taste preference learning in rats. Eur J Neurosci 26: 1521– 1529, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Touzani K, Sclafani A. Learned flavor aversions and preferences. In: Encyclopedia of Neuroscience, edited by Squire LR. Oxford: Academic, 2009, p. 395–399 [Google Scholar]
- 242. Toyono T, Seta Y, Kataoka S, Kawano S, Shigemoto R, Toyoshima K. Expression of metabotropic glutamate receptor group I in rat gustatory papillae. Cell Tissue Res 313: 29– 35, 2003 [DOI] [PubMed] [Google Scholar]
- 243. Toyono T, Seta Y, Kataoka S, Oda M, Toyoshima K. Differential expression of the glucose transporters in mouse gustatory papillae. Cell Tissue Res 345: 243– 252, 2011 [DOI] [PubMed] [Google Scholar]
- 244. Tracy AL, Phillips RJ, Chi MM, Powley TL, Davidson TL. The gastrointestinal tract “tastes” nutrients: evidence from the intestinal taste aversion paradigm. Am J Physiol Regul Integr Comp Physiol 287: R1086– R1100, 2004 [DOI] [PubMed] [Google Scholar]
- 245. Treesukosol Y, Blonde G, Spector AC. The T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to Polycose: implications for saccharide taste receptors in mice. Am J Physiol Regul Integr Comp Physiol 296: R855– R865, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Treesukosol Y, Smith KR, Spector AC. Behavioral evidence for a glucose polymer taste receptor that is independent of the T1R2+3 heterodimer in a mouse model. J Neurosci 31: 13527– 13534, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Treesukosol Y, Smith KR, Spector AC. The functional role of the T1R family of receptors in sweet taste and feeding. Physiol Behav 105: 14– 26, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Tsurugizawa T, Kondoh T, Torii K. Forebrain activation induced by postoral nutritive substances in rats. Neuroreport 19: 1111– 1115, 2008 [DOI] [PubMed] [Google Scholar]
- 249. Tsurugizawa T, Uematsu A, Nakamura E, Hasumura M, Hirota M, Uneyama H, Torii K. Mechanisms of neural response to gastrointestinal nutritive stimuli: the gut-brain axis. Gastroenterology 137: 262– 273, 2009 [DOI] [PubMed] [Google Scholar]
- 250. Tsurugizawa T, Uematsu A, Uneyama H, Torii K. Blood oxygenation level-dependent response to intragastric load of corn oil emulsion in conscious rats. Neuroreport 20: 1625– 1629, 2009 [DOI] [PubMed] [Google Scholar]
- 251. Tsuruta M, Kawada T, Fukuwatari T, Fushiki T. The orosensory recognition of long-chain fatty acids in rats. Physiol Behav 66: 285– 288, 1999 [DOI] [PubMed] [Google Scholar]
- 252. Uebayashi H, Hatanaka T, Kanemura F, Tonosaki K. Acute anosmia in the mouse: behavioral discrimination among the four basic taste substances. Physiol Behav 72: 291– 296, 2001 [DOI] [PubMed] [Google Scholar]
- 253. Uematsu A, Tsurugizawa T, Kondoh T, Torii K. Conditioned flavor preference learning by intragastric administration of l-glutamate in rats. Neurosci Lett 451: 190– 193, 2009 [DOI] [PubMed] [Google Scholar]
- 254. Uematsu A, Tsurugizawa T, Uneyama H, Torii K. Brain-gut communication via vagus nerve modulates conditioned flavor preference. Eur J Neurosci 31: 1136– 1143, 2010 [DOI] [PubMed] [Google Scholar]
- 255. Uneyama H, Niijima A, San Gabriel A, Torii K. Luminal amino acid sensing in the rat gastric mucosa. Am J Physiol Gastrointest Liver Physiol 291: G1163– G1170, 2006 [DOI] [PubMed] [Google Scholar]
- 256. Villalba JJ, Provenza FD. Nutrient-specific preferences by lambs conditioned with intraruminal infusions of starch, casein, and water. J Anim Sci 77: 378– 387, 1999 [DOI] [PubMed] [Google Scholar]
- 257. Villalba JJ, Provenza FD, Rogosic J. Preference for flavored wheat straw by lambs conditioned with intraruminal infusions of starch administered at different times after straw ingestion. J Anim Sci 77: 3185– 3190, 1999 [DOI] [PubMed] [Google Scholar]
- 258. Vincent KM, Sharp JW, Raybould HE. Intestinal glucose-induced calcium-calmodulin kinase signaling in the gut-brain axis in awake rats. Neurogastroenterol Motil 23: e282– e293, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Warwick ZS, Weingarten HP. Determinants of high-fat diet hyperphagia: experimental dissection of orosensory and postingestive effects. Am J Physiol Regul Integr Comp Physiol 269: R30– R37, 1995 [DOI] [PubMed] [Google Scholar]
- 260. Williams DL, Cummings DE, Grill HJ, Kaplan JM. Meal-related ghrelin suppression requires postgastric feedback. Endocrinology 144: 2765– 2767, 2003 [DOI] [PubMed] [Google Scholar]
- 261. Wilson DA, Stevenson RJ. Learning to Smell: Olfactory Perception from Neurobiology to Behavior. Baltimore MD: The Johns Hopkins University, 2006 [Google Scholar]
- 262. Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 91: 733– 794, 2011 [DOI] [PubMed] [Google Scholar]
- 263. Xu H, Staszewski L, Tang H, Adler E, Zoller M, Li X. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc Natl Acad Sci USA 101: 14258– 14263, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Yamaguchi S, Ninomiya K. Umami and food palatability. J Nutr 130: 921S– 926S, 2000 [DOI] [PubMed] [Google Scholar]
- 265. Yamamoto T, Shimura T. Roles of taste in feeding and reward. In: The Senses: A Comprehensive Reference, edited by Basbaum AI, Kaneko A, Shepherd GM, Westheimer G, Albright TD, Masland RH, Dallos P, Oertel D, Firestein S, Beauchamp GK, Bushnell MC, Kaas JH, Gardner E. New York: Academic, 2008, p. 437–458 [Google Scholar]
- 266. Yamamoto T, Ueji K. Brain mechanisms of flavor learning. Front Syst Neurosci 5: 76 doi: 10.3389/fnsys.2011.00076: 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Yarmolinsky DA, Zuker CS, Ryba NJP. Common sense about taste: from mammals to insects. Cell 139: 234– 244, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Yasumatsu K, Ogiwara Y, Takai S, Yoshida R, Iwatsuki K, Torii K, Margolskee RF, Ninomiya Y. Umami taste in mice uses multiple receptors and transduction pathways. J Physiol 590: 1155– 1170, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Yee KK, Sukumaran SK, Kotha R, Gilbertson TA, Margolskee RF. Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc Natl Acad Sci USA 108: 5431– 5436, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Yeomans MR. Development of human learned flavor likes and dislikes. In: Obesity Prevention: The Role of Brain and Society on Individual Behavior, edited by Dub́e L, Bechara A, Dagher A, Drewnowski A, Lebel J, James P, Yada RY. San Diego, CA: Academic, 2010, p. 161–178 [Google Scholar]
- 271. Yeomans MR, Gould NJ, Mobini S, Prescott J. Acquired flavor acceptance and intake facilitated by monosodium glutamate in humans. Physiol Behav 93: 958– 966, 2008 [DOI] [PubMed] [Google Scholar]
- 272. Yeomans MR, Leitch M, Gould NJ, Mobini S. Differential hedonic, sensory and behavioral changes associated with flavor-nutrient and flavor-flavor learning. Physiol Behav 93: 798– 806, 2008 [DOI] [PubMed] [Google Scholar]
- 273. Yiin YM, Ackroff K, Sclafani A. Flavor preferences conditioned by intragastric nutrient infusions in food restricted and unrestricted rats. Physiol Behav 84: 217– 231, 2005 [DOI] [PubMed] [Google Scholar]
- 274. Yiin YM, Ackroff K, Sclafani A. Food deprivation enhances the expression but not acquisition of flavor acceptance conditioning in rats. Appetite 45: 152– 160, 2005 [DOI] [PubMed] [Google Scholar]
- 275. Yin TH, Tsai CT. Effects of glucose on feeding in relation to routes of entry in rats. J Comp Physiol Psychol 85: 258– 264, 1973 [DOI] [PubMed] [Google Scholar]
- 276. Yoder SM, Yang Q, Kindel TL, Tso P. Stimulation of incretin secretion by dietary lipid: is it dose dependent? Am J Physiol Gastrointest Liver Physiol 297: G299– G305, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Yoneda T, Saitou K, Mizushige T, Matsumura S, Manabe Y, Tsuzuki S, Inoue K, Fushiki T. The palatability of corn oil and linoleic acid to mice as measured by short-term two-bottle choice and licking tests. Physiol Behav 91: 304– 309, 2007 [DOI] [PubMed] [Google Scholar]
- 278. Young PT. Palatability: the hedonic response to foodstuffs. In: Handbook of Physiology. Alimentary Canal. Washington DC: Am. Physiol. Soc., 1967, sect. 6, vol. I, chapt. 26, p. 353–366 [Google Scholar]
- 279. Young RL. Sensing via intestinal sweet taste pathways. Front Neurosci 5: 23 doi: 10.3389/fnins.2011.00023: 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Yox DP, Ritter RC. Capsaicin attenuates suppression of sham feeding induced by intestinal nutrients. Am J Physiol Regul Integr Comp Physiol 255: R569– R574, 1988 [DOI] [PubMed] [Google Scholar]
- 281. Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJP. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112: 293– 301, 2003 [DOI] [PubMed] [Google Scholar]
- 282. Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJP, Zuker CS. The receptors for mammalian sweet and umami taste. Cell 115: 255– 266, 2003 [DOI] [PubMed] [Google Scholar]
- 283. Zukerman S, Ackroff K, Sclafani A. Rapid post-oral stimulation of intake and flavor conditioning by glucose and fat in the mouse. Am J Physiol Regul Integr Comp Physiol 301: R1635– R1647, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. T1R3 taste receptor is critical for sucrose but not Polycose taste. Am J Physiol Regul Integr Comp Physiol 296: R866– R876, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Zukerman S, Touzani K, Margolskee RF, Sclafani A. Role of olfaction in the conditioned sucrose preference of sweet-ageusic T1R3 knockout mice. Chem Senses 34: 685– 694, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]