Abstract
This study examined whether substitution of chromosome 5 containing the CYP4A genes from Brown Norway rat onto the Dahl S salt-sensitive (SS) genetic background upregulates the renal production of 20-HETE and attenuates the development of hypertension. The expression of CYP4A protein and the production of 20-HETE were significantly higher in the renal cortex and outer medulla of SS.5BN (chromosome 5-substituted Brown Norway rat) consomic rats fed either a low-salt (LS) or high-salt (HS) diet than that seen in SS rats. The increase in the renal production of 20-HETE in SS.5BN rats was associated with elevated expression of CYP4A2 mRNA. MAP measured by telemetry rose from 117 ± 1 to 183 ± 5 mmHg in SS rats fed a HS diet for 21 days, but only increased to 151 ± 5 mmHg in SS.5BN rats. The pressure-natriuretic and diuretic responses were twofold higher in SS.5BN rats compared with SS rats. Protein excretion rose to 354 ± 17 mg/day in SS rats fed a HS diet for 21 days compared with 205 ± 13 mg/day in the SS.5BN rats, and the degree of glomerular injury was reduced. Baseline glomerular capillary pressure (Pgc) was similar in SS.5BN rats (43 ± 1 mmHg) and Dahl S (44 ± 2 mmHg) rats. However, Pgc increased to 59 ± 3 mmHg in SS rats fed a HS diet for 7 days, while it remained unaltered in SS.5BN rats (43 ± 2 mmHg). Chronic administration of an inhibitor of the synthesis of 20-HETE (HET0016, 10 mg·kg−1·day−1 iv) reversed the antihypertensive phenotype seen in the SS.5BN rats. These findings indicate that the transfer of chromosome 5 from the BN rat onto the SS genetic background increases the renal expression of CYP4A protein and the production of 20-HETE and that 20-HETE contributes to the antihypertensive and renoprotective effects seen in the SS.5BN consomic strain.
Keywords: hypertension, glomerulosclerosis, chromosome 5, Dahl S rats, pressure natriuresis, renal hemodynamics, kidney
the dahl salt-sensitive (ss) rat is an inbred genetic model that rapidly develops severe hypertension, proteinuria, glomerulosclerosis, and renal interstitial fibrosis when fed a high salt (HS) diet (4, 7–8, 23, 25, 27, 30, 38, 43). However, the genes and pathways that contribute to the development of hypertension and renal disease have yet to be identified. Previous studies from our laboratory have demonstrated that the pressure natriuretic relationship is impaired in SS rats and that this is associated with increased Cl− transport in the thick ascending limb of Henle (TALH) (13, 15–16, 29, 44). They also exhibit a deficiency in the renal production of 20-HETE that contributes to the increase in loop Cl− transport (13, 35, 44).
More recently, Mattson et al. (20) demonstrated that substitution of chromosome 5 from the Brown Norway (BN) rat onto the SS genetic background (SS.5BN strain) attenuates the development of hypertension and proteinuria in SS.5BN rats fed a high-salt (HS) diet for 21 days, but the mechanism is unknown. Because the CYP4A genes that produce 20-HETE are located on chromosome 5 and this region has been found to cosegregate with the development of hypertension in a cross of SS and normotensive Lewis rats (34), the present study examined whether the antihypertensive and renoprotective effect of transfer of chromosome 5 from BN rats onto the SS background in the SS.5BN consomic strain is associated with upregulation of the renal expression of CYP4A protein and the formation of 20-HETE.
METHODS
General.
Experiments were performed on 163 male Dahl SS/mcw (SS) and SS.5BN consomic rats that were obtained from inbred colonies maintained at the Medical College of Wisconsin and the University of Mississippi Medical Center. The SS.5BN rats were derived as previously described using a speed congenic breeding approach (5–6) by intercrossing SS and BN rats and then backcrossing the animals with SS rats for 5 or 6 generations, while selecting founders in each generation that remained heterozygous for BN chromosome 5. The colonies were maintained in a laboratory animal care facility at both institutions, which were both approved by the American Association for the Accreditation of Laboratory Animal Care. The rats had free access to food and water throughout the study. All protocols received approval by the Animal Care Committee of both institutions.
Protocol 1. Comparison of the expression of CYP4A protein and the metabolism of arachidonic acid in SS and SS.5BN rats.
The expression of CYP4A protein and renal metabolism of arachidonic acid (AA) was compared in microsomes prepared from the renal cortex and outer medulla of 10-wk-old SS and SS.5BN rats that were maintained from weaning on a purified AIN-76 diet (Dyets, Bethlehem, PA) containing 0.4% NaCl (low salt, LS) or were switched to a AIN-76 diet containing 8.0% NaCl (high salt, HS) for 7 days prior to the experiment. We chose to examine the kidneys of these rats after only 7 days on a HS diet to avoid the effects that hypertension-induced renal injury may have on the expression of CYP4A protein and the renal metabolism of AA in the SS rats. At the end of experiments, the rats were euthanized and kidneys were collected. Microsomes were prepared from both the renal cortex and outer medulla, as previously described (2). Briefly, the renal cortex (0.5 g) or outer medulla (0.3 g) was homogenized in 3 ml of a 10 mM potassium buffer (pH 7.7) containing 250 mM sucrose, 1 mM EDTA, and 0.1 mM PMSF. The homogenates were centrifuged at 3,000 g for 5 min and 9,000 g for 15 min. The supernatant was centrifuged at 100,000 g for 1 h to obtain the microsomal fraction. The pellets were resuspended in the 100 mM potassium buffer (pH 7.25) containing 20% glycerol, 1 mM dithiothreitol, and 0.1 mM PMSF, frozen in liquid N2, and stored at −80°C until assayed.
Expression of CYP4A protein.
The expression of CYP4A protein was assessed in microsomes prepared from the cortex and outer medulla of SS and SS.5BN rats maintained on a LS diet from weaning or challenged with a HS diet for 7 days. The samples were separated by electrophoresis on 7.5% SDS-PAGE, transferred to nitrocellulose membranes, and blocked overnight in a buffer containing 10% nonfat dry milk. The membranes were incubated for 2 h with a 1:4,000 dilution of CYP4A primary antibody (cat. no. 299230, Daiichi Pure Chemicals, Tokyo, Japan) followed by a 1:4,000 dilution of a horseradish peroxidase coupled secondary antibody (sc2020, Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h. The blots were developed using an enhanced chemiluminescent kit, exposed to X-ray film, and the relative intensities of the bands in the 50- to 52-kDa range for CYP4A were determined using an Eagle eye imaging system (Stratagene, La Jolla, CA). Microsomes prepared from the liver of a fenofibrate-treated rat were used as a CYP4A standard (1 μg loaded). The blots were reprobed for β-actin for determination of equal protein loading.
Metabolism of AA.
The microsomes (0.5 mg) were incubated with a saturating concentration of AA (42 μM; Amersham Biosciences, Piscataway, NJ) and 1 mM NADPH for 30 min at 37°C. The incubations were stopped by acidification to pH 3.5 with formic acid and extracted twice with 3 ml of ethyl acetate after the addition of 2 ng of an internal standard, d6-20-hydroxyeicosatetraneoic acid (Cayman Chemicals, Ann Arbor, MI). The organic phase was collected and dried under N2. The samples were reconstituted with 50% methanol and water, and the metabolites were separated by HPLC on a Betabasic C18 column (150 × 2.1 mm, 3 μm; Thermo Hypersil-Keystone, Belletonte, PA) at a flow rate of 0.2 ml/min using an isocratic elution starting from a 51:9:40:0.01 mixture of acetonitrile:methanol:water:acetic acid for 30 min followed by a step change to 68:13:19:0.01 acetonitrile:methanol:water:acetic acid and water for 15 min. The effluent was ionized using a negative ion electrospray, and the peaks eluting with a mass/charge ratio (m/z) of 319 > 245 (20-HETE), 325 > 251 (d6-20-HETE), 319 > 301 (HETEs and EETs), 337 > 319 (DiHETEs), or 325 > 251 (internal standard) were monitored using an Applied Biosystems 3000 triple quadrupole mass spectrometer (Foster City, CA). The ratios of ion abundances in the peaks of interest vs. those seen with the internal standard were determined and compared with standard curves generated over a range from 0.5 to 10 ng for the various eicosanoids. Values are expressed as picomoles of product formed per minute per milligram of protein.
Similar experiments were performed on glomeruli and renal microvessels isolated from the kidneys of SS and SS.5BN rats fed either a LS or HS diet for 7 days by differential sieving, as previously described (7, 31–32, 42). Homogenates of the isolated glomeruli were prepared, and the metabolism of AA was determined as described above. Intact renal microvessels were incubated in 1 ml of a physiological saline solution containing 1 mM NADPH for 60 min. The reactions were stopped with formic acid and extracted with ethyl acetate after the addition of the internal standard as described above.
Protocol 2. RT-PCR for assessment of the expression of CYP4A isoforms.
RNA was extracted from the renal cortex and outer medulla of SS and SS.5BN rats using TRIzol (Sigma, St. Louis, MO). RNA (3 μg) was added to a 20-μl RT reaction using the AffinityScript quantitative PCR cDNA synthesis kit (Stratagene, La Jolla, CA). The reactions were incubated at 42°C for 15 min and terminated by heating at 95°C for 5 min. Real-time PCR analysis for CYP4A1, 4A2, 4A3, and 4A8 isoforms was carried out, as previously described using isoform-specific primers (9). β-actin was amplified in parallel as an internal control. Each sample was assayed in duplicate and CT numbers (the cycle numbers at which reporter signals reach a threshold) were used to calculate relative mRNA expression normalized to the β-actin expression.
Protocol 3. Time course of the development of hypertension and proteinuria in SS and SS.5BN rats.
These experiments were performed on 9-wk-old SS and SS.5BN rats maintained from weaning on the AIN76 diet containing 0.4% NaCl (LS). Telemetry transmitters (model TA11PA-C40; Data Sciences International, St. Paul, MN) were implanted in the right femoral artery with the base of the transmitter placed under the skin on the right side of the rat. After a 5-day recovery period, mean arterial pressure (MAP) was recorded between 1 and 4 PM during a 3-day control period. The rats were then switched to a diet containing 8% NaCl (HS), and MAP was recorded on days 7, 14, and 21 of the HS diet. At each time point, urine was collected overnight to determine protein excretion. At the end of the study, the kidneys were weighed and collected to assess the degree of hypertrophy and fixed in a 10% buffered formalin solution. Paraffin sections (3 μm) were prepared and stained with Masson's trichrome stain to assess the degree of glomerular injury on ∼30 glomeruli per section. The percentage of the glomerular capillary area filled with matrix material was scored according to the method of Raij et al. (26) on a 0–4 scale with 0 representing no injury, 2 indicating loss of 50% of glomerular capillary area, and 4 representing the complete loss of capillaries.
Protocol 4. Comparison of the pressure-natriuretic response in SS and SS.5BN rats.
These experiments were performed on 9-wk-old SS and SS.5BN rats. The rats were anesthetized with ketamine (30 mg/kg im; Phoenix Pharmaceuticals, St. Joseph, MO) and Inactin (50 mg/kg ip; Sigma, St. Louis, MO), and catheters were placed in the femoral artery for the measurement of MAP and in the femoral vein for an intravenous infusion of 2% BSA in a 0.9% NaCl solution at a rate of 100 μl/min. An adjustable aorta clamp was placed on the aorta above the left renal artery to regulate renal perfusion pressure (RPP). An ultrasound flow probe (Transonic Systems, Ithaca, NY) was placed on the left renal artery to measure renal blood flow (RBF), and a catheter was inserted into the left ureter for the collection of urine. FITC-labeled inulin (2 mg/ml; Sigma, St. Louis, MO) was added to the infusion solution for the measurement of GFR. After surgery, RPP was lowered to 100 mmHg by tightening the clamp on the aorta. After a 15-min equilibration period, urine and plasma samples were collected during a 20-min collection period. RPP was then increased to ∼150 mmHg by releasing the aortic clamp and tying of the celiac and mesenteric arteries. After a 10-min equilibration period, urine and plasma samples were recollected during a 20-min experimental period. At the end of the experiment, the left kidney was removed and weighed, and the concentrations of Na+ and inulin in the urine and plasma samples were determined.
Protocol 5. Measurement of the glomerular capillary pressure.
Glomerular capillary pressure (Pgc) was directly measured by micropuncture in 12-wk-old SS and SS.5BN rats fed either a LS or HS diet for 14 days. The measurements of Pgc were possible because the SS and SS.5BN rats used in this study have surface glomeruli. On the day of the acute experiment, the rats were anesthetized with ketamine (30 mg/kg im) and Inactin (50 mg/kg ip), and catheters were placed in the femoral artery for the measurement of arterial pressure. The rats received an intravenous infusion of 1% BSA in a 0.9% NaCl solution via the jugular vein at a rate of 1.2 ml/h. Pgc was directly measured by micropuncture using a servonull micropressure device (model 900, World Precision Instruments, Sarasota, FL), as previously described (39).
Protocol 6. Measurement of the reflection coefficient of albumin (dσalb) in isolated glomeruli.
The reflection coefficient of albumin (dσalb) was measured in 10–12-wk-old SS and SS.5BN rats fed either a LS or HS diet for 7 and 21 days by using modifications of the Savin technique (31). The rats were anesthetized with isoflurane, and a catheter was inserted into the femoral vein for injection of 75 mg/kg of a high molecular weight (250 kDa) FITC-labeled dextran that is not filtered and remains in the glomerular capillaries. After 5 min, the kidneys were harvested, and glomeruli were isolated using the sieving method in Hank's buffer solution containing 6% BSA and then transferred to 80 μl fast-exchange perfusion chamber mounted on the stage of an inverted microscope (TS-100, Nikon, Melville, NY). The FITC-labeled dextran in the glomeruli was imaged with the InCyt IM1 imaging system (Intracellular Imaging, Cincinnati, OH) using an excitation filter of 475 nm and an emission filter of 530 nm. DσAlb was determined by measuring the changes in fluorescence in each glomerulus after rapidly (<2 s) lowering the concentration of BSA in the bath from 6 to 4%. Dσalb was calculated as the measured percentage change of fluorescent intensity divided by the expected change in glomerular volume in response to a 33% decrease in oncotic pressure. A minimum of 10 glomeruli were studied from each rat, and these experiments were performed using a minimum of four rats per strain.
Protocol 7. Effects of chronic blockade of the synthesis of 20-HETE with HET0016 on the development of hypertension and renal injury in SS.5BN rats.
These experiments were performed on 9-wk-old SS.5BN rats maintained from weaning on the AIN76 diet containing 0.4% NaCl. Telemetry transmitters were implanted, as previously described above. A catheter also was inserted into the right femoral vein and routed subcutaneously to the scapular region and exteriorized through a Dacron-covered plastic button sutured subcutaneously over the scapulae. The catheter was connected to a syringe pump via hydraulic swivel for intravenous infusions. After a 7-day recovery period, MAP was recorded as described above, and an overnight urine sample was collected to determine baseline protein excretion. The rats were then switched to a diet containing 8% NaCl (HS) and separated into two groups: One group received an intravenous infusion of vehicle (11% sulfobutyl ether β-cyclodextrin; CyDex, Lenexa, KS) in a 300-mM mannitol solution at a rate of 3 ml/day, while the other received an intravenous infusion of N-hydroxy-N′-(-4-butyl-2methylphenyl) formamidine (HET0016) at a dose of 10 mg·kg−1·day−1, which is selective for inhibiting the formation of 20-HETE (41). MAP was recorded on days 7 and 14 of the HS diet. At each time point, urine was collected overnight to determine protein excretion.
Statistical analysis.
Mean values ± SE are presented. The significance of differences in control and experimental values within the same animal were determined by a paired t-test (two samples) or using an ANOVA for repeated measures and a Holm-Sidak post hoc test (multiple samples). The significance of differences in the mean values between groups was determined by one-way ANOVA followed by Holm-Sidak test. A P < 0.05 was considered to be significant.
RESULTS
Protocol 1: CYP4A expression and CYP450-dependent metabolism of AA in SS and SS.5BN rats.
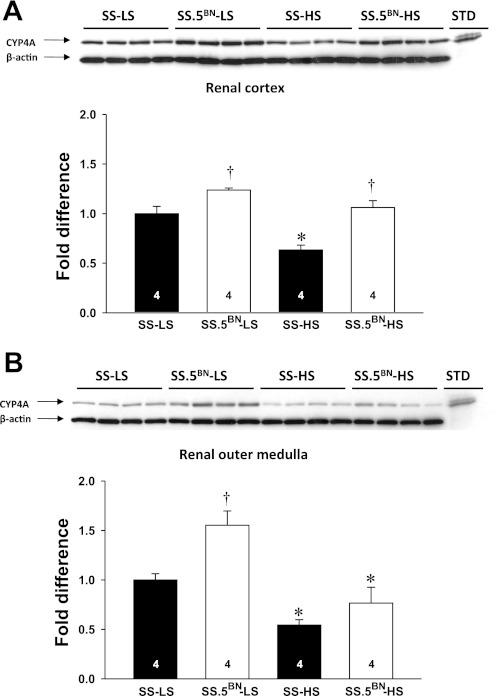
Comparisons of the expression of CYP4A protein in microsomes prepared from the renal cortex and outer medulla in SS and SS.5BN rats are presented in Fig. 1. The expression of CYP4A protein was significantly higher in the renal cortex in SS.5BN rats compared with the corresponding values observed in SS rats fed a LS or HS diet (Fig. 1A). The expression of CYP4A protein fell by 40% in the renal cortex of SS rats fed a HS diet for 7 days, while the expression of CYP4A protein remained unchanged in the renal cortex of SS.5BN rats. The expression of CYP4A protein was significantly greater in the outer medulla of SS.5BN rats fed a LS diet than that seen in SS rats (Fig. 1B). The expression of CYP4A protein in the outer medulla was reduced in both strains when fed a HS diet for 7 days, but it remained significantly higher in SS.5BN rats than in SS rats.
Fig. 1.
Comparison of the relative expression of CYP4A protein in the renal cortex (A) and outer medulla (B) in Dahl salt-sensitive (SS) and chromosome 5-substituted Brown Norway (SS.5BN) rats fed either a low-salt (LS) or high-salt (HS) diet for 7 days. Numbers in the bars indicate the number of rats studied per group. Values are expressed as means ± SE. *Significant difference from the LS value within the same strain. †Significant difference from the corresponding value in SS rats.
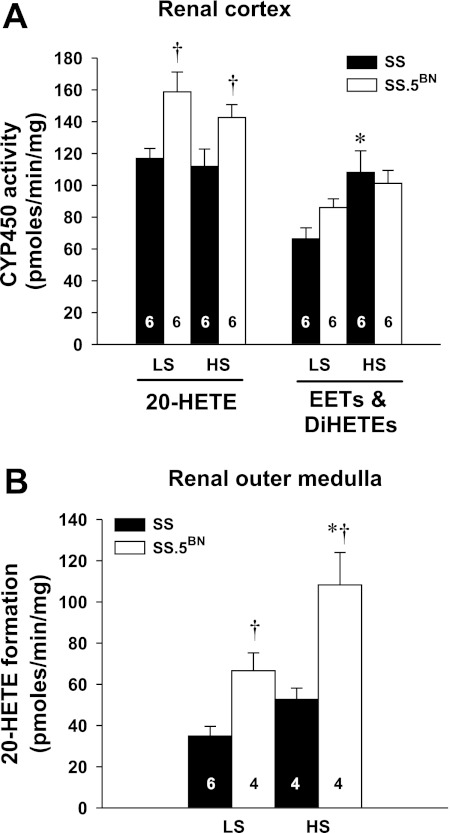
A comparison of the metabolism of arachidonic acid by renal cortical and outer medullary microsomes prepared from the kidneys of SS.5BN and SS rats is presented in Fig. 2. The formation of 20-HETE was significantly higher in the renal cortex of SS.5BN rats compared with the corresponding values seen in SS rats fed either a LS or HS diet (Fig. 2A). There was no significant difference in epoxygenase activity between the strains. The production of EETs and DiHETEs increased by 35% in the renal cortex in SS rats fed a HS diet for 7 days, but it did not increase in SS.5BN rats. The production of 20-HETE in the outer medulla of the kidney was twofold higher in SS.5BN rats than in SS rats on either a LS or HS diet for 7 days (Fig. 2B). We did not detect any epoxygenase activity in the outer medulla in either strain.
Fig. 2.
Comparison of the metabolism of arachidonic acid (AA) in the renal cortex (A) and outer medulla (B) in Dahl SS and SS.5BN rats fed either a low-salt diet (LS) or a high-salt diet (HS) for 7 days. Numbers in the bars indicate the number of rats studied per group. Values are expressed as means ± SE. *Significant difference from the corresponding value in rats fed a LS diet within the same strain. †Significant difference from the corresponding value in SS rats.
CYP450-dependent metabolism of AA in isolated glomeruli and renal microvessels.
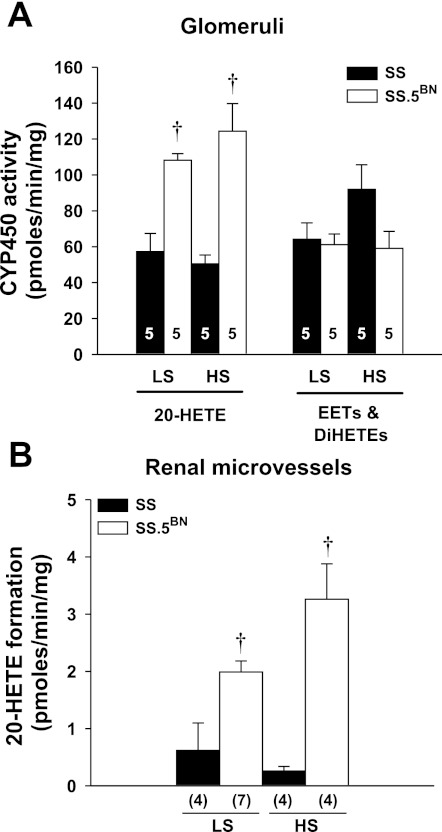
A comparison of the metabolism of AA by glomeruli isolated from SS and SS.5BN rats is presented in Fig. 3A. The glomerular formation of 20-HETE was significantly higher in SS.5BN rats compared with SS rats fed either a LS or HS diet. No significant difference in epoxygenase activity was observed between the strains.
Fig. 3.
Comparisons of the metabolism of AA in glomeruli isolated from Dahl SS and SS.5BN rats fed either a LS or a HS diet for 7 days (A) and the formation of 20-HETE in renal microvessels isolated from Dahl salt-sensitive (SS) and SS.5BN rats fed either a LS or a HS diet for 7 days (B). Numbers in the bars indicate the number of rats studied per group. Values are expressed as means ± SE. †Significant difference from the corresponding values in the SS rats.
A comparison of the formation of 20-HETE in renal microvessels isolated from SS and SS.5BN rats fed either a LS or HS diet is presented in Fig. 3B. The formation of 20-HETE was significantly higher in renal microvessels of SS.5BN rats compared with the levels observed in SS rats fed either a LS or HS diet. Increasing salt intake also had no significant effect on 20-HETE production in the glomeruli or renal microvessels isolated from either strain.
Protocol 2. Real-time PCR of CYP4A isoforms.
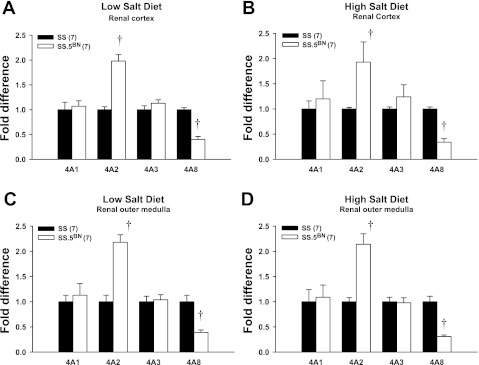
A comparison of the expression of CYP4A1, 2, 3, and 8 mRNA in the renal cortex and outer medulla of SS and SS.5BN rats is presented Fig. 4. There were no differences in the expression of CYP4A1 and CYP4A3 in the cortex (Fig. 4, A and B) or outer medulla (Fig. 4, C and D) in SS and SS.5BN rats fed either a LS or HS diet for 7 days. However, the expression of CYP4A2 was elevated, and the expression of CYP4A8 was lower in SS.5BN rats than SS in both the renal cortex and outer medulla whether the rats were fed either a LS or HS diet.
Fig. 4.
Comparison of the expression of CYP4A mRNA in the renal cortex (A and B) and outer medulla (C and D) in Dahl SS and SS.5BN rats fed either a LS diet or a HS diet for 7 days. Numbers in parentheses indicate the number of rats studied per group. Values are expressed as means ± SE. †Significant difference from the corresponding value in SS rats.
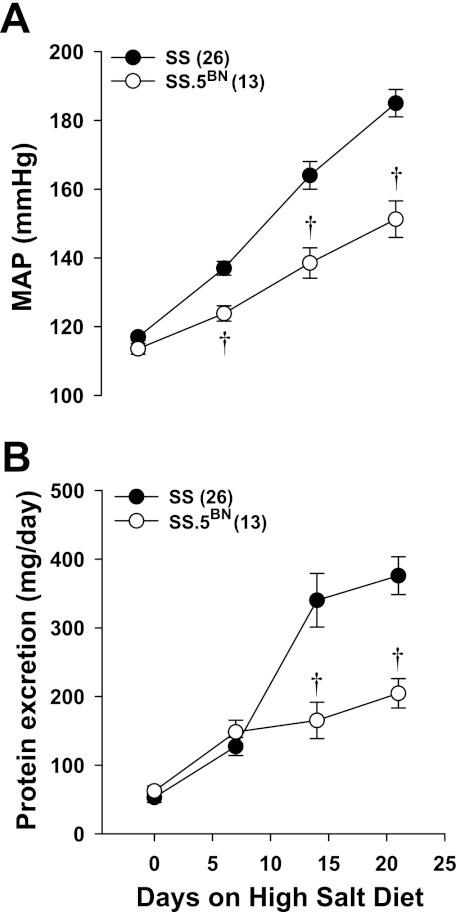
Protocol 3. Time course of the development of hypertension and proteinuria in SS and SS.5BN rats.
A comparison of the time course of the development of hypertension and proteinuria in SS and SS.5BN rats fed a HS diet for 21 days are presented in Fig. 5. On a LS diet (day 0), MAP was similar in both strains (≈116 mmHg) (Fig. 5A). However, after 21 days on a HS diet, MAP rose to 183 ± 5 mmHg in SS rats but only increased to 151 ± 5 mmHg in SS.5BN rats. Protein excretion rose from 54 ± 11 to 354 ± 17 mg/day in SS rats fed a HS diet for 21 days compared with 62 ± 7 to 205 ± 13 mg/day in the SS.5BN rats over the same period (Fig. 5B).
Fig. 5.
Time course of the development of hypertension (A) and proteinuria (B) in Dahl SS and SS.5BN rats fed a HS diet for 21 days. Numbers in parentheses indicate the number of rats studied per group. Values are expressed as means ± SE. †Significant difference from the corresponding value in the SS rats.
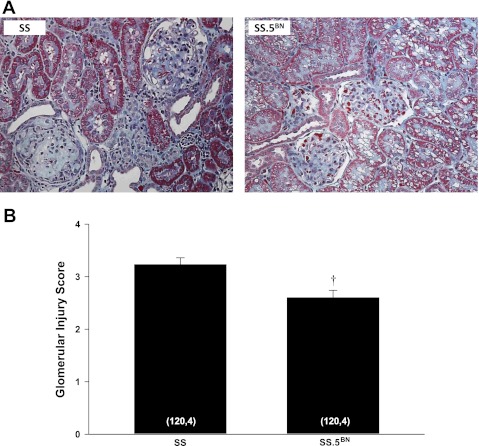
A comparison of the degree of renal injury in SS and SS.5BN rats is presented in Fig. 6. The kidneys from SS rats exhibited severe glomerular injury with mesangial expansion, glomerulosclerosis, renal interstitial fibrosis (blue), and the formation of protein casts (red). The degree of renal injury was reduced in SS.5BN rats, and these animals only exhibited focal segmental glomerulosclerosis and mesangial matrix expansion (Fig. 6A). The glomerular injury score (Fig. 6B) was significantly higher in SS rats compared with the values seen in SS.5BN rats fed a HS diet for 21 days. The degree of renal hypertrophy as indicated by kidney weights was similar in SS and SS.5BN rats fed a HS diet for 21 days.
Fig. 6.
Comparison of the degree of glomerular injury in Dahl salt-sensitive (SS) and SS.5BN rats fed a HS for 21 days. Numbers in the bars indicate the number of rats studied per group. Values are expressed as means ± SE. †Significant difference from the corresponding value in the SS rats.
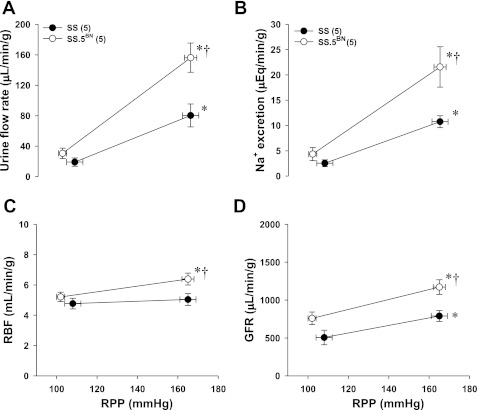
Protocol 4. Comparison of the pressure-natriuretic response in SS and SS.5BN rats.
A comparison of the pressure-natriuretic response in SS and SS.5BN rats is presented in Fig. 7. Urine flow and Na+ excretion increased from 19 ± 5 to 80 ± 15 μl·min−1·g−1 and 3 ± 1 to 11 ± 1 μEq·min−1·g−1, respectively, in SS rats when RPP was increased from 100 to ∼160 mmHg in both strains (Fig. 7, A and B). The pressure-natriuretic and diuretic responses were twofold higher in SS.5BN rats compared with SS rats. RBF was similar in SS and SS.5BN rats at 100 mmHg (Figs. 7C). However, RBF rose 20% in SS.5BN rats when RPP was increased and was significantly higher than that seen in SS rats. Baseline GFR was not significantly different in SS and SS.5BN rats at 100 mmHg (Fig. 7D). GFR increased significantly in both strains when RPP was increased to ∼160 mmHg and was significantly higher in SS.5BN rats as compared in SS rats.
Fig. 7.
Comparison of the changes in urine flow rate (A), Na+ excretion (B), renal blood flow (RBF) (C), and glomerular filtration rate (GFR) (D) in response to elevations in renal perfusion pressure (RPP) in Dahl SS and SS.5BN rats. Values are expressed as means ± SE. *Significant difference from corresponding values in the kidneys perfused at a low RPP (100 mmHg) within the same strain. †Significant difference from corresponding values measured in the SS rats.
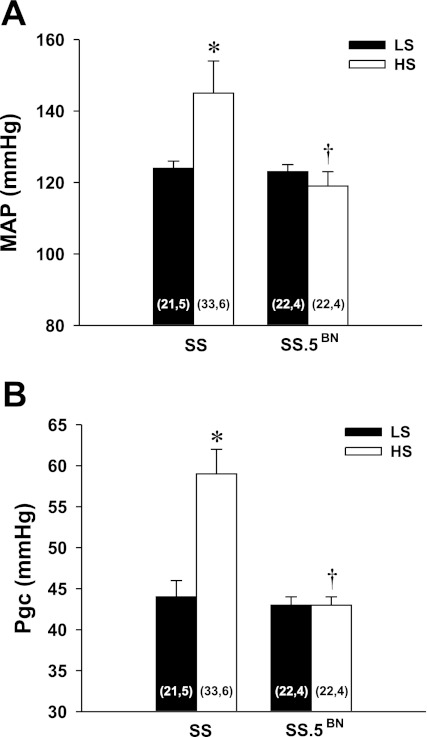
Protocol 5. Measurement of glomerular capillary pressure.
The relationship between MAP and Pgc in SS and SS.5BN rats fed either a LS or HS diet for 7 days is presented in Fig. 8. MAP measured under Inactin anesthesia during the micropuncture experiments were similar in SS and SS.5BN rats maintained on a LS diet (Fig. 8A). However, switching rats to a HS diet for 7 days increased MAP in SS rats, while it remained unaltered in SS.5BN rats. Directly measured Pgc was similar in both strains fed a LS diet (Fig. 8B). Pgc rose by 15 mmHg in SS rats fed a HS diet, whereas it did not increase in SS.5BN rats. Thus, Pgc was significantly higher in SS rats fed a HS diet than in SS.5BN rats.
Fig. 8.
Comparison of the measurement of mean arterial pressure (MAP) (A) and glomerular capillary pressure (Pgc) (B) in Dahl SS and SS.5BN rats fed a LS or a HS diet for 14 days. Numbers in the bars indicate the number of glomeruli and rats studied per group. Values are expressed as means ± SE. *Significant difference from the corresponding value in rats fed a LS diet within a strain. †Significant difference from the corresponding value in the SS rats.
Protocol 6. Glomerular permeability to albumin.
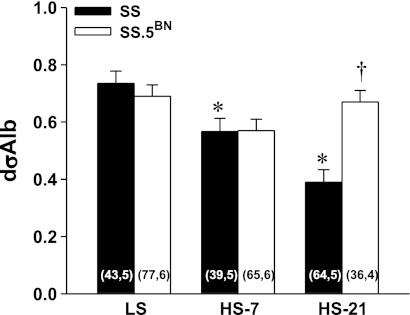
A comparison of dσalb in glomeruli of SS and SS.5BN rats fed a LS or HS diet for 7 and 21 days is presented in Fig. 9. Baseline dσalb was similar in SS and SS.5BN rats fed a LS diet. SS rats exhibited a marked decrease in dσalb values when fed a HS diet for 7 and 14 days, while we did not observe any changes in dσalb in SS.5BN rats.
Fig. 9.
Comparison of the measurement of the dilutional glomerular albumin reflection coefficient (dσAlb) in Dahl SS and SS.5BN rats fed a LS or a HS diet for 7 and 21 days. Numbers in the bars indicate the number of glomeruli and rats studied per group. Values are expressed as means ± SE. *Significant difference from the corresponding value in rats fed a LS diet within a strain. †Significant difference from the corresponding value in the SS rats.
Protocol 7. Effects of chronic blockade of the synthesis of 20-HETE on the development of hypertension and renal injury in SS.5BN rats.
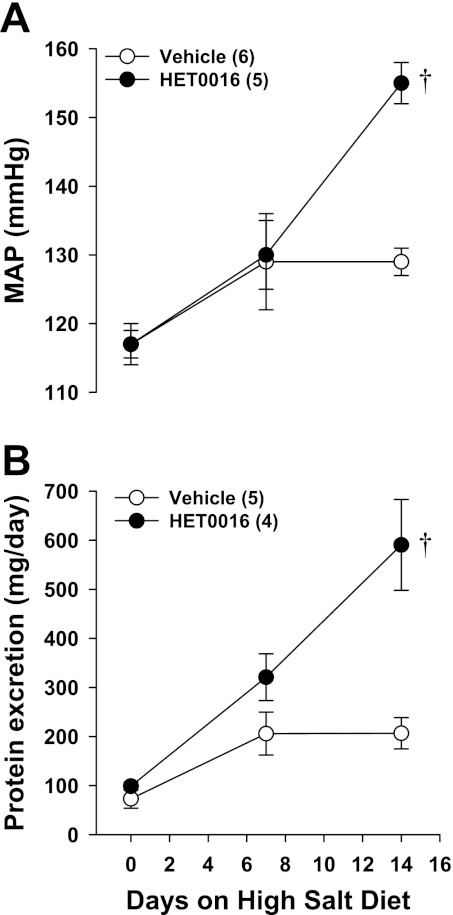
The effects of chronic treatment with an inhibitor of the synthesis of 20-HETE, HET0016, on the development of hypertension and proteinuria in SS.5BN rats are presented in Fig. 10. MAP rose from 117 ± 2 to 129 ± 2 mmHg in control SS.5BN rats fed a HS diet for 14 days (Fig. 10A). In contrast, MAP rose from 117 ± 3 to 155 ± 3 mmHg in SS.5BN rats treated with HET0016, which is similar to the increase in MAP seen in SS rats fed a HS diet for 14 days (Fig. 5A). Moreover, SS.5BN rats treated with HET0016 displayed progressive proteinuria that was significantly greater than that seen in vehicle-treated rats (591 ± 92 vs. 207 ± 32 mg/day, respectively) (Fig. 10B).
Fig. 10.
Effects of the chronic administration of an inhibitor of the formation of 20-HETE, HET0016 (10 mg·kg−1·day−1 iv), on mean arterial pressure (MAP) (A) and protein excretion (B) measured in SS.5BN rats fed a HS diet for 14 days. Numbers in the bars indicate the number of rats studied per group. Values are expressed as means ± SE. †Significant difference from the corresponding value in SS.5BN rats treated with vehicle.
DISCUSSION
Previous studies have indicated that the renal formation of 20-HETE is reduced in the outer medulla of SS rats and that this contributes to enhanced Cl− transport in the TALH, resetting of the pressure natriuretic relationship and the development of hypertension. However, it remains to be determined whether the changes in the renal formation of 20-HETE are due to unique sequence variants in the CYP4A isoforms in SS rats or secondary changes in neural and hormonal factors that influence the expression of these genes. Recently, Mattson et al. (20) reported that substitution of chromosome 5 from the BN rat onto the SS genetic background markedly inhibited the development of hypertension and albuminuria via an unknown mechanism. Because all of the genes of the CYP4A family responsible for the formation of 20-HETE are located on chromosome 5, the present study examined whether the antihypertensive effect in the SS.5BN strain is associated with upregulation of the renal expression of CYP4A protein and formation of 20-HETE. Substitution of chromosome 5 from the BN rat increased the expression of CYP4A protein and production of 20-HETE in the kidneys of SS.5BN rats compared with SS rats fed either a LS or HS diet. Real-time PCR studies indicate that this is associated with an increase in the renal expression of the CYP4A2 mRNA. The pressure-natriuretic response was markedly improved, and the increases in arterial pressure and proteinuria in response to a HS diet were significantly reduced in SS.5BN rats than the values observed in SS rats. Moreover, the progression of hypertension-induced renal injury in the SS rats fed a HS diet was associated with elevations in Pgc and glomerular permeability to albumin, both of which were significantly lower in SS.5BN rats. Finally, chronic blockade of the formation of 20-HETE with HET0016 reversed the antihypertensive and renoprotective effects seen in SS.5BN rats, and MAP rose to a similar extent as that seen in SS rats fed a high-salt diet for 14 days (Fig. 5A). Moreover, in previous studies, we reported that blockade of the synthesis of 20-HETE has no additional effect on MAP in SS rats (28, 41).
The observation that substitution of chromosome 5 from BN into the SS genetic background increases the renal formation of 20-HETE and reduces arterial pressure in response to a HS diet supports the hypothesis that a deficiency in the renal formation of 20-HETE contributes to the development of hypertension in SS rats. Interestingly, we observed a decrease in the expression of CYP4A protein and an increase in the formation of 20-HETE in the outer medulla of SS.5BN rats when fed a HS diet for 7 days. It is possible that a greater proportion of the CYP4A protein is inactive in the SS.5BN strain on a LS diet in the outer medulla. Indeed, there is evidence that nitric oxide (24) and carbon monoxide (1, 3) that is present in the outer medulla binds to the heme of CYP4A proteins and inhibits the formation of 20-HETE. Nonetheless, our finding that increasing the renal formation of 20-HETE in this case by transfer of chromosome 5 reduces salt sensitivity of blood pressure in SS.5BN rats is consistent with previous data, indicating that induction of the synthesis of 20-HETE in the kidney with fibrates opposes the development of hypertension in SS rats and other models of hypertension (30, 33, 43). In contrast, the increase in 20-HETE formation in renal microvessels in the SS.5BN strain would be expected to increase vascular tone and promote the development of hypertension. However, we believe that the upregulation of 20-HETE in the renal outer medulla to inhibit Cl− transport in the TALH predominates and overcomes any influence that increase of 20-HETE in the renal microvessels might have to reduce renal blood flow and GFR and promote the development of hypertension in the SS.5BN strain. Indeed, one might speculate that the increase in 20-HETE production in the vasculature might only serve to enhance renal myogenic and tubuloglomerular feedback responsiveness to prevent the development of glomerular injury and be renoprotective rather than prohypertensive. A recent study from our laboratory demonstrated that Pgc increases in SS rats fed a HS diet and that this is associated with the development of proteinuria and glomerular disease (41). In the current study, we examined whether the transfer of chromosome 5 from the BN rat into the SS genetic background would prevent the rise in Pgc when fed a HS diet. We found that after 7 days on a HS diet when both SS and SS.5BN rats exhibited a similar 15 mmHg increase in arterial pressure, Pgc increased markedly in SS rats, but it remained unaltered in the SS.5BN strain. This was associated with an increase in glomerular permeability to albumin and development of more severe proteinuria and renal injury in SS rats than was seen in SS.5BN rats. Chronic blockade of the formation of 20-HETE with HET0016 reversed the antihypertensive and renoprotective effects seen in the SS.5BN consomic strain. In contrast, we have previously demonstrated the chronic inhibition of 20-HETE has no effect on either arterial pressure (28, 41) or the degree of proteinuria and renal injury in SS rats (41). The mechanism for an increase in Pgc in SS rats fed a HS diet remains to be determined. However, from the current study, we observed the formation of 20-HETE in the renal microvessels was reduced in SS rats compared with that seen in SS.5BN rats when fed either a LS or HS diet. Thus, a deficiency in the formation of 20-HETE in the renal microvessels may impair both tubuloglomerular feedback (TGF) and/or myogenic responsiveness of the afferent arteriole in SS rats to elevations in arterial pressure making the kidney more susceptible to hypertension-induced renal injury. 20-HETE is expressed in afferent arterioles and has been reported to play an important role in the myogenic response by regulating vascular reactivity secondary to inhibition of Ca2+-activated K+ (KCa) channels. It also modulates tubuloglomerular feedback responsiveness, probably by affecting vascular reactivity to adenosine through modulation of KCa channel activity (45). Indeed, this hypothesis is consistent with previous observations that the myogenic response (37) and dynamic autoregulation of RBF is impaired in Dahl S rats (14) and that TGF responsiveness is decreased when these rats are fed a HS diet (40).
Besides a potential effect on renal hemodynamics, we recently observed that acute blockade of the formation of 20-HETE markedly increased glomerular permeability to albumin and that this response can be reversed by exogenous administration of a 20-HETE mimetic (7, 42). In the present study, the glomerular formation of 20-HETE was twofold higher in SS.5BN rats compared with values observed in SS rats fed either a LS or HS diet. Moreover, we observed that the albumin reflection coefficient (dσAlb), an indicator glomerular permeability, decreased in SS rats fed a HS diet, while it remained unchanged in SS.5BN rats. These data are consistent with the view that the relative deficiency in the glomerular formation of 20-HETE contributes to the loss of barrier function of the glomerulus of SS rats and the development of proteinuria.
Previous studies have indicated that the CYP4A region of chromosome 5 cosegregates with the development of hypertension in a F2 cross of SS and Lewis rats (34). The present findings that transfer of chromosome 5 from BN rats to the SS genomic background increases the expression of CYP4A protein, and the formation of 20-HETE suggests there may be a sequence variant in one of the CYP4A isoforms that contributes to the strain difference in the formation of 20-HETE. The present finding that the expression of the CYP4A2 was elevated in SS.5BN rats fed either a LS or HS diet than in SS rats suggests that this may be the isoform of interest.
In summary, the results from the current study suggest that substitution of chromosome 5 from the BN rat onto the SS genetic background attenuated the development of hypertension and the rise in Pgc and glomerular permeability to albumin in SS.5BN consomic rats fed a HS diet. The antihypertensive and renoprotective effects seen in the SS.5BN rat was associated with increases in the renal formation of 20-HETE and the expression of CYP4A protein and CYP4A2 mRNA. Moreover, chronic blockade of the formation of 20-HETE rescued the hypertensive phenotype in the SS.5BN rat. Overall, these findings further support the view that a deficiency in the renal formation of 20-HETE contributes to the development of salt-dependent hypertension, and they are important, given the results of recent studies indicating that mutations in CYP4A11 and CYP4A22 are associated with the development of hypertension and cardiovascular disease in human population studies (10–12, 17–19, 21–22, 36). These results also suggest that studies in the SS rat, along with the SS.5BN consomic rats, can serve as a useful model to better determine how alterations in formation of 20-HETE contributes to the development of hypertension, proteinuria, and renal injury.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.M.W., F.F., S.R.M., C.S., J.L., and H.J.J. performed experiments; J.M.W., F.F., S.R.M., C.S., and R.J.R. analyzed data; J.M.W., F.F., S.R.M., C.S., and R.J.R. interpreted results of experiments; J.M.W. and S.R.M. prepared figures; J.M.W. drafted manuscript; J.M.W. and R.J.R. edited and revised manuscript; J.M.W., F.F., S.R.M., C.S., J.L., H.J.J., and R.J.R. approved final version of manuscript; J.L., H.J.J., and R.J.R. conception and design of research.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants HL-29587, HL-36279, and a United Negro College Fund/Merck Postdoctoral Science Research Fellowship awarded to J. M. Williams.
REFERENCES
- 1. Abraham NG, Botros FT, Rezzani R, Rodella L, Bianchi R, Goodman AI. Differential effect of cobalt protoporphyrin on distributions of heme oxygenase in renal structure and on blood pressure in SHR. Cell Mol Biol (Noisy-le-grand) 48: 895–902, 2002 [PubMed] [Google Scholar]
- 2. Alonso-Galicia M, Maier KG, Greene AS, Cowley AW, Jr, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid in the renal and vasoconstrictor actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol 283: R60–R68, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Botros FT, Laniado-Schwartzman M, Abraham NG. Regulation of cyclooxygenase- and cytochrome p450-derived eicosanoids by heme oxygenase in the rat kidney. Hypertension 39: 639–644, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Chen PY, John PL, Kirk KA, Abrahamson DR, Sanders PW. Hypertensive nephrosclerosis in the Dahl/Rapp rat. Initial sites of injury and effect of dietary l-arginine supplementation. Lab Invest 68: 174–184, 1993 [PubMed] [Google Scholar]
- 5. Cowley AW, Liang M, Roman RJ, Greene AS, Jacob HJ. Consomic rat model systems for physiological genomics. Acta Physiol Scand 181: 585–592, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Cowley AW, Jr, Roman RJ, Jacob HJ. Application of chromosomal substitution techniques in gene-function discovery. J Physiol 554: 46–55, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dahly-Vernon AJ, Sharma M, McCarthy ET, Savin VJ, Ledbetter SR, Roman RJ. Transforming growth factor-beta, 20-HETE interaction, and glomerular injury in Dahl salt-sensitive rats. Hypertension 45: 643–648, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Dahly AJ, Hoagland KM, Flasch AK, Jha S, Ledbetter SR, Roman RJ. Antihypertensive effects of chronic anti-TGF-beta antibody therapy in Dahl S rats. Am J Physiol Regul Integr Comp Physiol 283: R757–R767, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Dunn KM, Renic M, Flasch AK, Harder DR, Falck J, Roman RJ. Elevated production of 20-HETE in the cerebral vasculature contributes to severity of ischemic stroke and oxidative stress in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 295: H2455–H2465, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elijovich F, Laffer CL. The relationship between CYP4A11 and human hypertension. J Hypertens 26: 1712–1714, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Gainer JV, Bellamine A, Dawson EP, Womble KE, Grant SW, Wang Y, Cupples LA, Guo CY, Demissie S, O'Donnell CJ, Brown NJ, Waterman MR, Capdevila JH. Functional variant of CYP4A11 20-hydroxyeicosatetraenoic acid synthase is associated with essential hypertension. Circulation 111: 63–69, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Gainer JV, Lipkowitz MS, Yu C, Waterman MR, Dawson EP, Capdevila JH, Brown NJ. Association of a CYP4A11 variant and blood pressure in black men. J Am Soc Nephrol 19: 1606–1612, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ito O, Roman RJ. Role of 20-HETE in elevating chloride transport in the thick ascending limb of Dahl SS/Jr rats. Hypertension 33: 419–423, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Karlsen FM, Andersen CB, Leyssac PP, Holstein-Rathlou NH. Dynamic autoregulation and renal injury in Dahl rats. Hypertension 30: 975–983, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Kirchner KA. Greater loop chloride uptake contributes to blunted pressure natriuresis in Dahl salt sensitive rats. J Am Soc Nephrol 1: 180–186, 1990 [DOI] [PubMed] [Google Scholar]
- 16. Kirchner KA. Increased loop chloride uptake precedes hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 262: R263–R268, 1992 [DOI] [PubMed] [Google Scholar]
- 17. Laffer CL, Gainer JV, Waterman MR, Capdevila JH, Laniado-Schwartzman M, Nasjletti A, Brown NJ, Elijovich F. The T8590C polymorphism of CYP4A11 and 20-hydroxyeicosatetraenoic acid in essential hypertension. Hypertension 51: 767–772, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lino Cardenas CL, Devos A, Toure A, Cardenas Garcia J, Kenani A, Migot-Nabias F, Broly F, Chevalier D. Arachidonic acid omega-hydroxylase CYP4A11: inter-ethnic variations in the 8590T>C loss-of-function variant. Mol Biol Rep 39: 1503–1508, 2012 [DOI] [PubMed] [Google Scholar]
- 19. Lino Cardenas CL, Renault N, Farce A, Cauffiez C, Allorge D, Lo-Guidice JM, Lhermitte M, Chavatte P, Broly F, Chevalier D. Genetic polymorphism of CYP4A11 and CYP4A22 genes and in silico insights from comparative 3D modelling in a French population. Gene 487: 10–20, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Mattson DL, Dwinell MR, Greene AS, Kwitek AE, Roman RJ, Jacob HJ, Cowley AW., Jr Chromosome substitution reveals the genetic basis of Dahl salt-sensitive hypertension and renal disease. Am J Physiol Renal Physiol 295: F837–F842, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mayer B, Lieb W, Gotz A, Konig IR, Aherrahrou Z, Thiemig A, Holmer S, Hengstenberg C, Doering A, Loewel H, Hense HW, Schunkert H, Erdmann J. Association of the T8590C polymorphism of CYP4A11 with hypertension in the MONICA Augsburg echocardiographic substudy. Hypertension 46: 766–771, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Mayer B, Lieb W, Gotz A, Konig IR, Kauschen LF, Linsel-Nitschke P, Pomarino A, Holmer S, Hengstenberg C, Doering A, Loewel H, Hense HW, Ziegler A, Erdmann J, Schunkert H. Association of a functional polymorphism in the CYP4A11 gene with systolic blood pressure in survivors of myocardial infarction. J Hypertens 24: 1965–1970, 2006 [DOI] [PubMed] [Google Scholar]
- 23. O'Donnell MP, Kasiske BL, Katz SA, Schmitz PG, Keane WF. Lovastatin but not enalapril reduces glomerular injury in Dahl salt-sensitive rats. Hypertension 20: 651–658, 1992 [DOI] [PubMed] [Google Scholar]
- 24. Oyekan AO, Youseff T, Fulton D, Quilley J, McGiff JC. Renal cytochrome P450 omega-hydroxylase and epoxygenase activity are differentially modified by nitric oxide and sodium chloride. J Clin Invest 104: 1131–1137, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int 26: 137–143, 1984 [DOI] [PubMed] [Google Scholar]
- 26. Raij L, Chiou XC, Owens R, Wrigley B. Therapeutic implications of hypertension-induced glomerular injury. Comparison of enalapril and a combination of hydralazine, reserpine, and hydrochlorothiazide in an experimental model. Am J Med 79: 37–41, 1985 [DOI] [PubMed] [Google Scholar]
- 27. Roman RJ, Alonso-Galicia M, Wilson TW. Renal P450 metabolites of arachidonic acid and the development of hypertension in Dahl salt-sensitive rats. Am J Hypertens 10: 63S–67S, 1997 [PubMed] [Google Scholar]
- 28. Roman RJ, Hoagland KM, Lopez B, Kwitek AE, Garrett MR, Rapp JP, Lazar J, Jacob HJ, Sarkis A. Characterization of blood pressure and renal function in chromosome 5 congenic strains of Dahl S rats. Am J Physiol Renal Physiol 290: F1463–F1471, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Roman RJ, Kaldunski ML. Enhanced chloride reabsorption in the loop of Henle in Dahl salt-sensitive rats. Hypertension 17: 1018–1024, 1991 [DOI] [PubMed] [Google Scholar]
- 30. Roman RJ, Ma YH, Frohlich B, Markham B. Clofibrate prevents the development of hypertension in Dahl salt-sensitive rats. Hypertension 21: 985–988, 1993 [DOI] [PubMed] [Google Scholar]
- 31. Savin VJ, Sharma R, Lovell HB, Welling DJ. Measurement of albumin reflection coefficient with isolated rat glomeruli. J Am Soc Nephrol 3: 1260–1269, 1992 [DOI] [PubMed] [Google Scholar]
- 32. Sharma R, Khanna A, Sharma M, Savin VJ. Transforming growth factor-beta1 increases albumin permeability of isolated rat glomeruli via hydroxyl radicals. Kidney Int 58: 131–136, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Shatara RK, Quest DW, Wilson TW. Fenofibrate lowers blood pressure in two genetic models of hypertension. Can J Physiol Pharmacol 78: 367–371, 2000 [PubMed] [Google Scholar]
- 34. Stec DE, Deng AY, Rapp JP, Roman RJ. Cytochrome P4504A genotype cosegregates with hypertension in Dahl S rats. Hypertension 27: 564–568, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Stec DE, Mattson DL, Roman RJ. Inhibition of renal outer medullary 20-HETE production produces hypertension in Lewis rats. Hypertension 29: 315–319, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Sugimoto K, Akasaka H, Katsuya T, Node K, Fujisawa T, Shimaoka I, Yasuda O, Ohishi M, Ogihara T, Shimamoto K, Rakugi H. A polymorphism regulates CYP4A11 transcriptional activity and is associated with hypertension in a Japanese population. Hypertension 52: 1142–1148, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Takenaka T, Forster H, De Micheli A, Epstein M. Impaired myogenic responsiveness of renal microvessels in Dahl salt-sensitive rats. Circ Res 71: 471–480, 1992 [DOI] [PubMed] [Google Scholar]
- 38. Tolins JP, Raij L. Comparison of converting enzyme inhibitor and calcium channel blocker in hypertensive glomerular injury. Hypertension 16: 452–461, 1990 [DOI] [PubMed] [Google Scholar]
- 39. van Dokkum RP, Sun CW, Provoost AP, Jacob HJ, Roman RJ. Altered renal hemodynamics and impaired myogenic responses in the fawn-hooded rat. Am J Physiol Regul Integr Comp Physiol 276: R855–R863, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Wilcox CS, Welch WJ. TGF and nitric oxide: effects of salt intake and salt-sensitive hypertension. Kidney Int Suppl 55: S9–S13, 1996 [PubMed] [Google Scholar]
- 41. Williams JM, Sarkis A, Hoagland KM, Fredrich K, Ryan RP, Moreno C, Lopez B, Lazar J, Fenoy FJ, Sharma M, Garrett MR, Jacob HJ, Roman RJ. Transfer of the CYP4A region of chromosome 5 from Lewis to Dahl S rats attenuates renal injury. Am J Physiol Renal Physiol 295: F1764–F1777, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Williams JM, Sharma M, Anjaiahh S, Falck JR, Roman RJ. Role of endogenous CYP450 metabolites of arachidonic acid in maintaining the glomerular protein permeability barrier. Am J Physiol Renal Physiol 293: F501–F505, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wilson TW, Alonso-Galicia M, Roman RJ. Effects of lipid-lowering agents in the Dahl salt-sensitive rat. Hypertension 31: 225–231, 1998 [DOI] [PubMed] [Google Scholar]
- 44. Zou AP, Drummond HA, Roman RJ. Role of 20-HETE in elevating loop chloride reabsorption in Dahl SS/Jr rats. Hypertension 27: 631–635, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. 20-HETE is an endogenous inhibitor of the large-conductance Ca2+-activated K+ channel in renal arterioles. Am J Physiol Regul Integr Comp Physiol 270: R228–R237, 1996 [DOI] [PubMed] [Google Scholar]