Abstract
Previous studies showed that coenzyme Q1 (CoQ1) reduction on passage through the rat pulmonary circulation was catalyzed by NAD(P)H:quinone oxidoreductase 1 (NQO1) and mitochondrial complex I, but that NQO1 genotype was not a factor in CoQ1 reduction on passage through the mouse lung. The aim of the present study was to evaluate the complex I contribution to CoQ1 reduction in the isolated perfused wild-type (NQO1+/+) and Nqo1-null (NQO1−/−) mouse lung. CoQ1 reduction was measured as the steady-state pulmonary venous CoQ1 hydroquinone (CoQ1H2) efflux rate during infusion of CoQ1 into the pulmonary arterial inflow. CoQ1H2 efflux rates during infusion of 50 μM CoQ1 were not significantly different for NQO1+/+ and NQO1−/− lungs (0.80 ± 0.03 and 0.68 ± 0.07 μmol·min−1·g lung dry wt−1, respectively, P > 0.05). The mitochondrial complex I inhibitor rotenone depressed CoQ1H2 efflux rates for both genotypes (0.19 ± 0.08 and 0.08 ± 0.04 μmol·min−1·g lung dry wt−1 for NQO1+/+ and NQO1−/−, respectively, P < 0.05). Exposure of mice to 100% O2 for 48 h also depressed CoQ1H2 efflux rates in NQO1+/+ and NQO1−/− lungs (0.43 ± 0.03 and 0.11 ± 0.04 μmol·min−1·g lung dry wt−1, respectively, P < 0.05 by ANOVA). The impact of rotenone or hyperoxia on CoQ1 redox metabolism could not be attributed to effects on lung wet-to-dry weight ratios, perfusion pressures, perfused surface areas, or total venous effluent CoQ1 recoveries, the latter measured by spectrophotometry or mass spectrometry. Complex I activity in mitochondria-enriched lung fractions was depressed in hyperoxia-exposed lungs for both genotypes. This study provides new evidence for the potential utility of CoQ1 as a nondestructive indicator of the impact of pharmacological or pathological exposures on complex I activity in the intact perfused mouse lung.
Keywords: pulmonary circulation, quinone, knockout mice, isolated perfused mouse lung, NAD(P)H:quinone oxidoreductase 1, mass spectrometry
changes in redox enzyme activity and/or cofactor redox status have been associated with promotion of and protection from lung injury in humans and rodents, including in aging, with exposure to ozone, sulfur mustard, and hyperoxia, and in other conditions (1, 10, 18, 31, 35, 51, 52). The vast majority of such studies, comprising measurements in lung cells, tissue homogenates, or subcellular fractions, involve destruction of lung tissue.
As a step toward developing nondestructive probes for lung redox function, we have relied on the observation that the pulmonary endothelium and lung tissue participate in alteration of the redox status and disposition of redox-active compounds as they pass through the pulmonary circulation (2–6, 36). Such compounds include certain amphipathic quinones, which are reduced to their two-electron reduction products, or hydroquinones, on passage through the lung. Furthermore, quinones with different physical and chemical properties have different propensities to be reduced via specific target quinone reductases within the lung tissue (2, 4, 6, 36). Identification of the quinone reductases involved in this metabolism was initially based on the use of quinone reductase inhibitors. However, using Nqo1-null (NQO1−/−), NQO1+/−, and wild-type (NQO1+/+) mice, we recently acquired genetic evidence for the specificity of the quinone duroquinone (DQ) as an NAD(P)H:quinone oxidoreductase 1 (NQO1) electron acceptor on passage through the mouse lung (36).
Coenzyme Q1 (CoQ1) is another amphipathic quinone that has solubility characteristics similar to those of DQ (21, 25). In the isolated perfused rat lung, CoQ1 reduction to its hydroquinone (CoQ1H2) is catalyzed by NQO1 and mitochondrial complex I (4). Initial studies in the mouse lung suggest that CoQ1 reduction capacity was nearly the same on passage through the NQO1+/+ or NQO1−/− mouse lung (36). Given that rat NQO1 is structurally and catalytically different from the mouse (and human) enzyme, this distinction between the two species may not be surprising (24). In fact, intact bovine pulmonary arterial endothelial cells in culture reduce CoQ1 preferentially via mitochondrial complex I, rather than NQO1 (39). Thus the first objective of the present study was to evaluate the contribution of complex I to CoQ1 reduction on passage through the mouse pulmonary circulation.
If CoQ1 could be identified to be reduced predominantly via complex I, a second objective was to investigate the impact of hyperoxia (100% O2 for 48 h) on CoQ1 reduction in the isolated perfused mouse lung. Hyperoxia was selected because it is a common model of acute lung injury and oxidative stress. Furthermore, our previous studies demonstrate that complex I activity and other mitochondrial functions are affected in rat lung exposed to hyperoxia (85% O2 for 48 h) and in pulmonary arterial endothelial cells in culture (95% O2 for 48 h) (4, 27, 38, 39). We also evaluated the impact of the hyperoxic exposure on lung NQO1 activity because of the possibility that NQO1 induction could result in competition between NQO1 and complex I for CoQ1 reduction. NQO1−/− mouse lung studies were included to eliminate any possible contribution of lung NQO1 to CoQ1 reduction and to provide further genetic evidence for the contribution, or lack thereof, of NQO1 and complex I to CoQ1 reduction in the intact perfused control or hyperoxia-exposed mouse lung.
MATERIALS AND METHODS
Materials.
2,3-Dimethoxy-5-methyl-6-(3-methyl-2-butenyl)-1,4-benzoquinone (CoQ1), N-[3-(2-furyl)acryloyl]-Phe-Gly-Gly (FAPGG), FITC-dextran, and other chemicals, unless otherwise specified, were purchased from Sigma-Aldrich. Protease inhibitor cocktail set III was obtained from Calbiochem; primers for genotyping from MWG Oberon; and the dNTPs and Taq polymerase (TaKaRa Ex Taq kit) from Clontech Laboratories.
Animals.
Nqo1-null (NQO1−/−) breeder mice were obtained from the colony at the National Cancer Institute, and animals were genotyped as previously described (36). The generation of the original Nqo1-null mice is described elsewhere (45). Wild-type (NQO1+/+) mice were purchased from Jackson Laboratory (129P3/J, strain 000690). Hyperoxic exposures were carried out in a 26 × 18 × 22 cm sealed chamber (total volume 10.3 liters) with a port for delivering 100% O2 at a flow rate of 1.1 l/min. Animals had free access to food and water. The animal breeding, housing, and experimental protocols were approved by the Institutional Animal Care and Use Committee of the Zabocki Veterans Affairs Medical Center.
Preparation of isolated perfused mouse lung.
The intact perfused mouse lung preparation is described elsewhere (2, 36). Mice were anesthetized with an intraperitoneal injection of Beuthanasia-D Special (0.3 mg/g pentobarbital sodium and 0.04 mg/g phenytoin sodium). The trachea was clamped, the chest was opened, and heparin (0.7 IU/g body wt) was injected into the right ventricle. The pulmonary artery and trachea were cannulated (0.86-mm-ID, 1.27-mm-OD polyethylene tubing). The heart was cut away, allowing the venous effluent to drain directly from the severed pulmonary vein. The lungs were removed from the chest and attached to a ventilation-perfusion system. The single-pass perfusion system was primed with the perfusate (4.7 mM KCl, 2.51 mM CaCl2, 1.19 mM MgSO4, 2.5 mM KH2PO4, 118 mM NaCl, 25 mM NaHCO3, 5.5 mM glucose, and 5% bovine serum albumin) maintained at 37°C and equilibrated with a gas mixture of 15% O2 and 6% CO2 in N2, resulting in perfusate Po2, Pco2, and pH of 105 Torr, 40 Torr, and 7.4, respectively. Initially, perfusate was pumped (model 7523-90 Cole-Parmer Masterflex L/S Pump) from a reservoir through the lungs at a flow rate of 0.3 ml/min that was gradually increased until the lungs and venous effluent were clear of blood. The flow rate was then set at 2 ml/min, the flow rate at which experiments were performed. The lungs were ventilated with 8 mmHg end-inspiratory pressure and 3 mmHg end-expiratory pressure at 80 breaths/min with the same gas mixture used to gas the perfusate. The pulmonary arterial, or perfusion, pressure was referenced to atmospheric pressure at the level of the left atrium and monitored continuously during the course of the experiments. The venous effluent pressure was atmospheric pressure.
Experimental protocols.
To determine the infusion time needed for the venous effluent CoQ1 and CoQ1H2 to reach steady state, pulse infusions (4-min duration) containing CoQ1 (50 μM) or the intravascular indicator FITC-dextran (average molecular wt ∼43,200) were made into the pulmonary arterial inflow of one room air-exposed and one hyperoxia-exposed NQO1+/+ lung. Venous effluent samples were collected every 10 s for 1 min and then every 30 s for 3 min from the beginning of the infusion into the pulmonary arterial inflow.
For experiments carried out at steady state, an initial venous effluent sample (∼1 ml) was collected from the venous outflow of the lung perfused with control perfusate as a blank for the spectrophotometric absorbance measurements. The reservoir was emptied and refilled with 13 ml of perfusate containing the angiotensin-converting enzyme substrate FAPGG (145 μM), the fractional hydrolysis of which provides a measure of lung perfused surface area. At 90–150 s from the start of the FAPGG perfusion, two 1-ml venous effluent samples were collected, and at 150 s, a reservoir sample was also collected. The reservoir was then emptied and refilled with fresh control perfusate, and the lung and perfusion system were washed free of residual FAPGG by perfusion for 5 min. A single venous effluent sample was collected as a blank, and the reservoir was emptied and refilled with 14 ml of CoQ1 or CoQ1H2 (50 or 200 μM) containing perfusate. Where specifically noted, KCN (2 mM), rotenone (20 μM), or rotenone (20 μM) + dicumarol (0.35 mM) was also present in the perfusate. At 210–240 s from the start of the CoQ1 perfusion, a single 1-ml venous effluent sample was collected, and at 240 s, a reservoir sample was also collected. The lung and perfusion system were washed again with the complex I inhibitor rotenone (20 μM) in the perfusate, and the 50 μM CoQ1 perfusion study was carried out as described above. Then the lung and perfusion system were washed a final time with control perfusate, and the FAPGG infusion was repeated as described above. At the end of each experiment, the lungs were weighed, dried, and reweighed to obtain dry weights and wet-to-dry weight ratios.
To quantify loss of CoQ1 attributable to nonspecific binding to the perfusion system tubing, experiments were carried out as described above, except the lung was omitted from the setup.
Sample processing and quinone concentration calculations.
The permeability-surface area product (PS, ml/min), a measure of the rate constant of angiotensin-converting enzyme-catalyzed FAPGG hydrolysis on passage through the lung and an index of perfused capillary surface area, was calculated from the infused arterial FAPGG concentration ([FAPGG]i) and steady-state venous effluent FAPGG concentration ([FAPGG]o) as follows: PS = −F ln(1 − E), where E = 1 − [FAPGG]o/[FAPGG]i and F is the perfusate flow rate (36). The angiotensin-converting enzyme inhibitor captopril (30 μM) blocks >95% of the FAPGG hydrolysis on passage through the lung, as evidence of the specificity of the substrate for angiotensin-converting enzyme (36).
For determination of venous effluent CoQ1 and CoQ1H2 concentrations, samples were centrifuged at 4°C for 1 min at 10,000 g (AccuSpin Micro centrifuge, Fisher Scientific), and 100 μl of each sample were added to each of two microcentrifuge tubes, one prefilled with 10 μl of potassium ferricyanide (1.8 mM) to oxidize any hydroquinone to quinone and the other with 10 μl of 1 mM EDTA in H2O. Ice-cold absolute ethanol (0.8 ml) was added, and the samples were mixed and centrifuged at 10,000 g for 5 min at 10°C.
The concentration of total CoQ1 + CoQ1H2 in each venous effluent sample was calculated from the absorbance at 265 nm of the fully oxidized (with ferricyanide added) sample using the extinction coefficient for CoQ1 (0.0143 μM−1·cm−1). The concentration of CoQ1H2 was calculated from the difference in absorbance (at 265 nm) between the oxidized (with ferricyanide) and the unoxidized (without ferricyanide) sample using the extinction coefficient 0.0121 μM−1·cm−1. The CoQ1 concentration was calculated from the difference between the CoQ1 + CoQ1H2 and CoQ1H2 concentrations (36, 50). Perfusate samples that had passed through the lungs but contained no quinone were treated in the same manner as the rest of the samples and used as the blanks for absorbance measurements. CoQ1H2 auto-oxidation rate was negligible within the experimental time frame <1.1%/min (39). Where FITC-dextran was used as an intravascular marker, concentrations were quantified from the absorbance at 495 nm using the extinction coefficient 0.0935 μM−1·cm−1 (36).
Studies with DQ were carried out essentially as described for CoQ1, except DQ (50 μM) was substituted for CoQ1 and the hydroquinone measured was DQH2 (36). Procedures for quantifying DQ and DQH2 are described elsewhere (36).
Liquid chromatography-electrospray ionization-mass spectrometry.
For each lung studied, effluent samples were collected following paired CoQ1 infusions into the perfusion system, first without and then with the lung in place. The paired samples were treated with potassium ferricyanide to oxidize any CoQ1H2 to CoQ1, as described above, and analyzed for CoQ1 spectrophotometrically, also as described above, and by mass spectrometry. For mass spectrometry, 5-μl samples were injected onto the HPLC system, which consisted of a reverse-phase C18 column (Kromasil, 250 × 2 mm) with water-acetonitrile-0.1% formic acid as a mobile phase and the flow rate set at 0.200 ml/min. The acetonitrile concentration increases linearly from 50% to 100% over 20 min. The retention times are 12.871 min for CoQ1H2 and 16.186 min for CoQ1. Drying gas flow rate is 12 l/min, drying gas temperature is 350°C, nebulizer pressure is 35 psig, capillary voltage is 3,000 V, and fragmenter voltage is 90 V. Detection is made in the positive mode with mass-to-charge ratios of 251 and 253 used for determination of CoQ1 and CoQ1H2, respectively.
Preparation of lung mitochondria-enriched fractions.
Lungs were washed free of residual blood with 10 mM HEPES (pH 7.4) containing 5.5 mM glucose and 5% dextran. The lungs were removed from the perfusion system, weighed, and minced in 15 volumes (wt/vol) of ice-cold homogenization buffer (pH 7.4) containing 10 mM HEPES, 200 mM mannitol, 70 mM sucrose, 1 mM EGTA, 2% fatty acid-free BSA, and protease inhibitor cocktail (50 μl/g lung tissue; set III, Calbiochem). The minced lung suspension was homogenized in a Dounce homogenizer (7 ml total volume, unground glass mortar, ID = 0.435 ± 0.001 in.) on ice with 10 strokes with pestle A (diameter = 0.4305 ± 0.0005 in., nominal clearance = 0.0045 in.) followed by 20 strokes with pestle B (diameter = 0.4325 ± 0.0005 in., nominal clearance = 0.0025 in.). The homogenate was centrifuged (1,100 g at 4°C for 2 min), the pellet was discarded, and the supernatant was centrifuged (18,500 g at 4°C for 10 min). The supernatant was discarded, and the pellet was washed twice by resuspension in 8 ml of homogenization buffer without BSA and centrifuged (18,500 g at 4°C for 10 min). The final pellet was resuspended in 0.6 ml of homogenization buffer without BSA and with protease inhibitor cocktail and stored at −80°C in 100-μl aliquots.
Complex I and IV activity assays in mitochondria-enriched lung fractions.
Complex I activity was determined by measurement of the oxidation rate of NADH to NAD+ spectrophotometrically at 340 nm with 432 nm as the reference wavelength at 35°C in 25 mM potassium phosphate buffer (pH 7.4) containing 5.0 mM MgCl2, 0.25% fatty acid-free BSA, 2.0 mM KCN, 3 μM antimycin A, 85 μM decylubiquinone, and 150 μM NADH with 5 μM rotenone or DMSO vehicle. The reaction was started with the addition of ∼50 μg of lung mitochondrial fraction protein. NADH absorbance was converted to concentration using the extinction coefficient 6.22 mM−1·cm−1. The rotenone-sensitive component was considered the complex I contribution to NADH oxidation. Activity is reported as nanomoles of NADH oxidized per minute per milligram of mitochondrial fraction protein. The assay was adapted from that described elsewhere (20).
Complex IV activity was determined by measurement of the oxidation of ferricytochrome c spectrophotometrically at 550 nm in 40 mM potassium phosphate buffer (pH 6.2, 25°C) containing 0.1% Triton X-100 and 25 μM ferricytochrome c. The reaction was started with the addition of ∼20 μg of lung mitochondrial fraction protein. Absorbance was converted to concentration using the extinction coefficient 19.6 mM−1·cm−1. Activity is reported as nanomoles of ferricytochrome c oxidized per minute per milligram of mitochondrial fraction protein. The assay was adapted from that described elsewhere (13). Protein was measured by the Bio-Rad protein assay.
NQO1 and glucose-6-phosphate dehydrogenase in lung homogenate cytosol fractions.
A portion of the lung tissue was minced and homogenized in ice-cold homogenization buffer (10 ml buffer/g lung tissue, pH 7.4) using a Polytron tissue homogenizer. The homogenate was centrifuged (12,100 g) at 4°C for 30 min, and the supernatant (cytosol fraction) was stored at −80°C. Lung cytosol fraction NQO1 activity was measured as previously described (36). Cytosol fraction (∼10 μg) was added to a semimicrocuvette containing 1 ml of reaction buffer consisting of 25 mM Tris·HCl (pH 7.4), BSA (0.23 mg/ml), 0.01% (vol/vol) Tween 20, 50 μM 2,6-dichlorophenolindophenol (DCIP), and 5 μM flavin adenine dinucleotide with or without 20 μM dicumarol. The reaction was initiated by the addition of NADPH (final concentration 200 μM), and DCIP reduction was measured spectrophotometrically at 600 nm (25°C). NQO1 activity was calculated as the difference in the initial DCIP reduction rates in the absence and presence of dicumarol, using an extinction coefficient of 21.0 mM−1·cm−1. Glucose-6-phosphate dehydrogenase activity was determined by measuring the rate of NADPH generation from NADP+ spectrophotometrically at 340 nm (extinction coefficient 6.22 mM−1·cm−1) at 25°C following the addition of lung cytosol fraction (∼75 μg protein) to a reaction mixture containing 55 mM Tris·HCl (pH 7.4), 3.3 mM MgCl2·6H2O, 3.3 mM glucose 6-phosphate, and 2.0 mM NADP+ (29). The lung cytosol fraction protein was measured using the Bio-Rad protein assay, and reaction rates were normalized to protein (36).
Statistics.
Statistical comparisons between hydroquinone efflux rates and other experimental parameters were determined by ANOVA followed by the Tukey's honestly significant difference test for multiple comparisons or paired or unpaired t-tests where noted; P < 0.05 was the criterion for statistical significance. Box-Cox normalizing transforms were used if the data were nonnormal or had heterogenous variability.
RESULTS
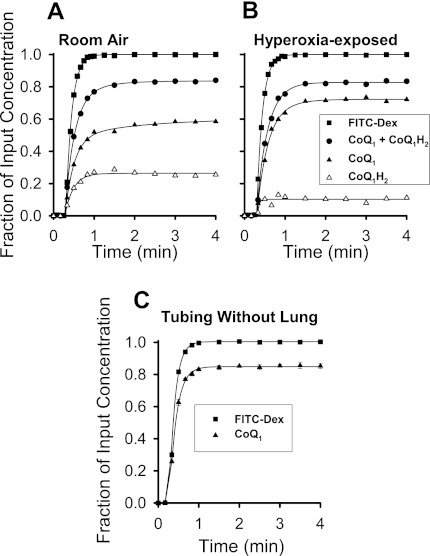
To determine the time for CoQ1 redox metabolism on passage through the lung to reach steady state, continuous infusions (4-min duration) of 50 μM CoQ1 or FITC-dextran were made into the pulmonary arterial inflow of a room air- or hyperoxia-exposed NQO1+/+ mouse lung. The pulmonary venous effluent CoQ1, CoQ1H2, and CoQ1 + CoQ1H2 concentration vs. time curves are shown in Fig. 1, A and B. The concentrations are expressed as fractions of CoQ1 concentration (50 μM) in the reservoir perfusing the lung. Also shown is the FITC-dextran venous outflow concentration curve, with concentration normalized to the reservoir FITC-dextran concentration. FITC-dextran is an intravascular marker, and thus its concentration curve represents what the CoQ1 curve would have looked like if the infused CoQ1 had remained in the perfusate and had been convected through the pulmonary circulation without undergoing redox or other reactions within the lung tissue. In this context, the FITC-dextran curve emphasizes the impact of passage through the lung on the disposition and redox status of CoQ1 collected in the venous effluent.
Fig. 1.
Coenzyme Q1 (CoQ1) and CoQ1 hydroquinone (CoQ1H2) venous effluent fractional concentration vs. time curves following CoQ1 (50 μM) infusion into room air- or hyperoxia-exposed NQO1+/+ mouse lungs. A and B: CoQ1, CoQ1H2, and CoQ1 + CoQ1H2 concentrations [expressed as fractions of infused pulmonary arterial CoQ1 concentration (50 μM)] and FITC-dextran concentrations (expressed as fraction of infused FITC-dextran concentration) in NQO1+/+ lung from animals exposed to room air or hyperoxia (100% O2 for 48 h). C: CoQ1 and FITC-dextran infusion into the perfusion system tubing without the lung in place. NQO1, NAD(P)H:quinone oxidoreductase 1; NQO1−/−, Nqo1-null; NQO1+/+, wild type.
CoQ1H2 first appears in the pulmonary venous effluent at ∼0.5 min after the start of perfusion with CoQ1, and by ∼1 min, the fractional concentrations of CoQ1 and CoQ1H2 in the venous effluent reach a quasi-steady state (Fig. 1, A and B). By this time, for the room air-exposed lung, ∼25% of the CoQ1 infused into the pulmonary arterial inflow appears in the venous effluent as CoQ1H2 and ∼55% as CoQ1 (Fig. 1A). For the hyperoxia-exposed lung, a lower proportion of the infused CoQ1 is converted to venous effluent CoQ1H2. All subsequent lung studies were performed at steady state.
Figure 1C shows the impact of the perfusion system (i.e., tubing) on the tubing outflow concentration vs. time curves for a FITC-dextran and a CoQ1 infusion when the lung is not in place. There were no statistically significant differences (by ANOVA) between the total infused CoQ1 recovered (as CoQ1 + CoQ1H2) from the perfusion system with (Fig. 1, A and B) and without (Fig. 1C) the lung in place. The interpretation is that the majority of total infused CoQ1 missing in the pulmonary venous effluent can be attributed to nonspecific binding to the tubing, consistent with our previous observations of quinone binding to tubing or experimental plastic ware (4, 6, 39, 40). In addition, there was no detectable difference between the extent of CoQ1 reduction for two successive infusions of 50 μM CoQ1 into the same lung preparation, ruling out a cumulative effect of multiple infusions in the subsequent studies (n = 4, P > 0.05 by paired t-test).
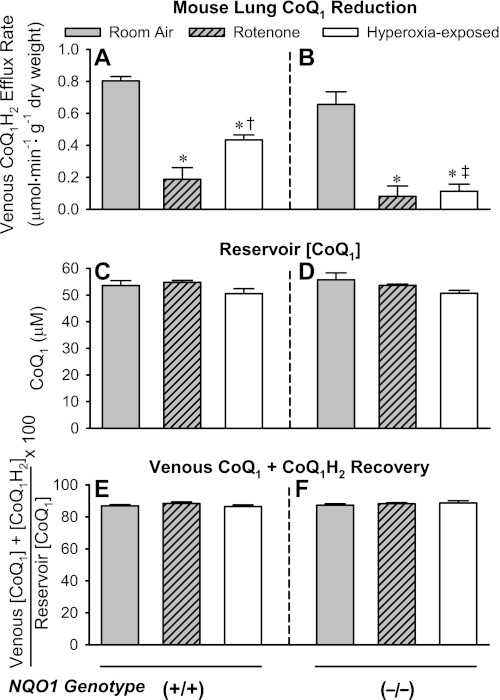
Previous studies in intact perfused rat lung implicated mitochondrial complex I and NQO1 in CoQ1 reduction and then used the complex I inhibitor-sensitive (i.e., rotenone-sensitive) CoQ1 reduction fraction as a means to detect a hyperoxia-induced decrease in complex I activity. To evaluate these observations in the intact perfused mouse lung, CoQ1 was infused into the pulmonary arterial inflow of NQO1+/+ and NQO1−/− lungs. There were three experimental groups for each genotype: lungs from room air-exposed mice with control lung perfusate (room air), lungs from room air-exposed mice with rotenone added to the lung perfusate (rotenone), and lungs from hyperoxia-exposed mice. Figure 2, A and B, shows that there was no detectable difference in the steady-state pulmonary venous effluent CoQ1H2 efflux rates for the room air NQO1+/+ and NQO1−/− lungs during CoQ1 infusion, consistent with previous observations (36). However, the CoQ1H2 efflux rates were depressed for both genotypes when the complex I inhibitor rotenone was present in the perfusate or when the lungs were from hyperoxia-exposed mice. In addition, the impact of hyperoxia on CoQ1H2 efflux rate was greater in NQO1−/− than NQO1+/+ lungs. Figure 2, C–F, shows that any differences between steady-state CoQ1H2 efflux rates shown in Fig. 2, A and B, cannot be attributed to differences in the amount of CoQ1 in the perfusion reservoir (Fig. 2, C and D) or the total recovery of CoQ1, calculated from the venous effluent CoQ1 + CoQ1H2 (Fig. 2, E and F). The total CoQ1 recoveries in Fig. 2, E and F, are also not significantly different from the CoQ1 recovery in the tubing-only study in Fig. 1C (ANOVA), again implying that the loss of CoQ1 in the study in Fig. 2, E and F, can be largely attributed to binding to the perfusion system tubing rather than to, e.g., further metabolism in, or binding to, lung tissue.
Fig. 2.
Pulmonary venous CoQ1H2 efflux rates when CoQ1 is infused into the pulmonary arterial inflow of NQO1+/+ or NQO1−/− lung and effects of rotenone in the lung perfusate or exposure of the mice to hyperoxia. A and B: CoQ1H2 efflux rates in NQO1+/+ and NQO1−/− lungs. C and D: CoQ1 concentration ([CoQ1]) in the perfusion reservoir (infusion concentration) for NQO1+/+ and NQO1−/− lungs. E and F: percentage of infused CoQ1 that appears as [CoQ1] + CoQ1H2 concentration ([CoQ1H2]) in the venous effluent for NQO1+/+ and NQO1−/− lungs. Values are means ± SE; n = 10 room air-exposed lungs, 4 room air-exposed lungs with rotenone added to the lung perfusate, and 6 hyperoxia-exposed lungs (A, C, and E) and 8 room air-exposed lungs, 4 room air-exposed lungs with rotenone added to the lung perfusate, and 5 hyperoxia-exposed lungs (B, D, and F). Significantly different (by ANOVA and Tukey's honestly significant difference test) within A or within B: *P < 0.05 vs. genotype-matched control room air-exposed lungs; †P < 0.05 vs. genotype-matched rotenone-treated lungs; ‡P < 0.05 vs. hyperoxia-exposed NQO1+/+ lungs. There were no significant differences (ANOVA) between values within any group (C–F) or between groups (C and D or E and F).
The next step was to further evaluate recovery of total CoQ1 (as CoQ1 + CoQ1H2) in the pulmonary venous effluent following the CoQ1 infusions into the pulmonary arterial inflow. For each of a series of room air- and hyperoxia-exposed lungs, paired infusions were carried out, the first into the perfusion system without the lung in place (lung-bypass) and the second with the lung added into the system. The lung-bypass perfusion system effluent and the through-lung venous effluent samples from each pair were analyzed for total CoQ1 by spectrophotometry and mass spectrometry. The analysis was carried out after the addition of an oxidizing agent (potassium ferricyanide) to all samples, so that the total CoQ1 recovery included the CoQ1 + CoQ1H2 in the venous effluent. Full oxidation of any venous effluent CoQ1H2 by the latter procedure was confirmed by mass spectrometry, which showed that there was no detectable CoQ1H2 in the samples (data not shown). Table 1 shows that the mean recovery for the through-lung vs. lung-bypass conditions was >95.8% for all conditions. There were no significant differences between recoveries for the room air- vs. hyperoxia-exposed lungs and no significant differences between recoveries determined by spectrophotometry and mass spectrometry.
Table 1.
Spectrophotometry and mass spectrometry analysis of venous effluent CoQ1 recoveries in CoQ1 infused room-air and hyperoxia-exposed lungs
| Total Venous CoQ1 + CoQ1H2 Recovery, % |
|||
|---|---|---|---|
| n | Spectrophotometry | Mass spectrometry | |
| Room air | |||
| NQO1−/− | 3 | 98.7 ± 1.6 | 95.8 ± 0.7 |
| Hyperoxia | |||
| NQO1−/− | 3 | 99.1 ± 1.3 | 98.5 ± 2.3 |
| NQO1+/+ | 1 | 99.9 | 96.4 |
Coenzyme Q1 (CoQ1) total recoveries were determined for each lung using paired samples from the perfusion system effluent without (lung bypass) and with (pulmonary venous effluent) a lung in place and calculated as follows: [pulmonary venous effluent total CoQ1 (as CoQ1 + CoQ1H2) ÷ perfusion system effluent (lung-bypass) CoQ1] × 100. Values are means ± SE. There were no significant differences between recoveries for room air- and recoveries for hyperoxia-exposed lungs and no significant differences between recoveries determined by spectrophotometry and mass spectrometry (2-way ANOVA). NQO1, NAD(P)H:quinone oxidoreductase 1; NQO1−/−, Nqo1-null; NQO1+/+, wild type.
Table 2 shows that there were no significant differences between any of the study groups in Fig. 2 with respect to mean mouse age, body weight, or hematocrit. Although there was a small but significant increase in the dry weight of hyperoxia-exposed NQO1−/− compared with room air-exposed NQO1+/+ lungs, there were no significant differences between the corresponding lung wet-to-dry weight ratios. Furthermore, there were no detectable differences between the experimental groups with respect to PS for the angiotensin-converting enzyme substrate FAPGG or the perfusion pressures at the beginning or end of the perfusion studies (Table 3). The PS for FAPGG is interpreted as reflective of the perfused lung vascular surface area. Thus the differences in CoQ1 redox metabolism observed in the Fig. 2 studies cannot be attributed to differences between the study groups with respect to any of these physiological parameters.
Table 2.
Ages, body weights, hematocrits, lung dry weights, and lung wet-to-dry weight ratios in Fig. 2 study
| NQO1+/+ |
NQO1−/− |
|||||
|---|---|---|---|---|---|---|
| Room air (n = 10) | Rotenone (n = 4) | Hyperoxia (n = 6) | Room air (n = 8) | Rotenone (n = 4) | Hyperoxia (n = 5) | |
| Age, days | 85 ± 8 | 99 ± 3 | 90 ± 12 | 71 ± 7 | 74 ± 5 | 81 ± 6 |
| Body weight, g | 25.4 ± 0.8 | 24.9 ± 0.7 | 24.6 ± 0.6 | 25.5 ± 1.2 | 25.3 ± 1.7 | 26.8 ± 1.1 |
| Hematocrit, % | 43.0 ± 1.1 | 46.6 ± 2.5 | 48.3 ± 1.7 | 45.0 ± 0.8 | 40.9 ± 2.1 | 47.0 ± 2.9 |
| Lung dry weight, mg | 27.1 ± 0.7 | 27.8 ± 1.6 | 29.6 ± 1.2 | 27.3 ± 0.7 | 29.6 ± 0.9 | 31.4 ± 1.0* |
| Lung wet-to-dry weight ratio | 5.4 ± 0.1 | 5.5 ± 0.1 | 5.6 ± 0.2 | 5.4 ± 0.1 | 6.0 ± 0.2 | 5.7 ± 0.1 |
Values are means ± SE.
Significantly different from NQO1+/+ room air (1-way ANOVA and Tukey's post hoc test for multiple pairwise comparisons).
Table 3.
Lung permeability-surface area products for the angiotensin- converting enzyme substrate FAPGG and perfusion pressures at the start and end of the experimental protocols
| PS, ml/min |
Perfusion Pressure, Torr |
|||
|---|---|---|---|---|
| Start | End | Start | End | |
| NQO1+/+ | ||||
| Room air | 1.90 ± 0.10 | 1.88 ± 0.11 | 5.2 ± 0.2 | 5.2 ± 0.3 |
| Rotenone | 2.11 ± 0.22 | 1.95 ± 0.15 | 4.9 ± 0.3 | 5.6 ± 0.9 |
| Hyperoxia | 1.80 ± 0.18 | 1.68 ± 0.17 | 5.7 ± 0.4 | 6.1 ± 0.4 |
| NQO1−/− | ||||
| Room air | 1.61 ± 0.17 | 1.60 ± 0.15 | 5.8 ± 0.4 | 5.8 ± 0.2 |
| Rotenone | 1.94 ± 0.31 | 1.86 ± 0.27 | 4.8 ± 0.2 | 5.0 ± 0.4 |
| Hyperoxia | 1.79 ± 0.31 | 1.67 ± 0.30 | 5.6 ± 0.3 | 5.6 ± 0.3 |
Values are means ± SE for lungs in Fig. 2 study. Permeability-surface area product (PS) is a reflection of perfused lung vascular surface area. FAPGG, N-[3-(2-furyl)acryloyl]-Phe-Gly-Gly. There were no statistical differences for PS or perfusion pressure between room air-exposed, rotenone-treated, and hyperoxia-exposed lungs at the start or the end of the experiment, and there were no statistical differences for any given lung condition between values for either parameter at the start or end of the experiment (2-way ANOVA).
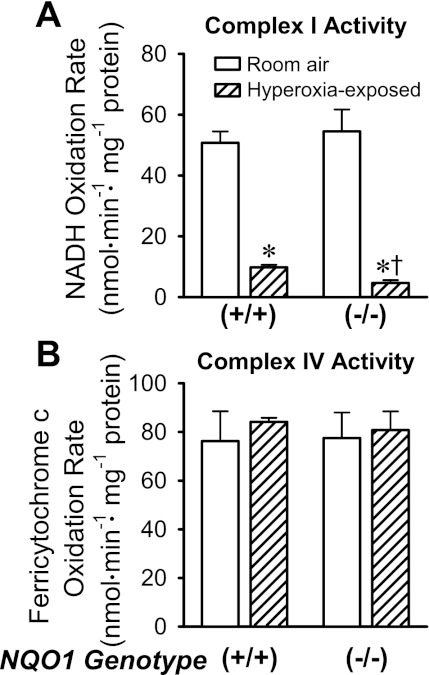
Figure 3A shows complex I activities measured in lung mitochondria-enriched fractions. There was no significant differences between complex I activities in NQO1+/+ and NQO1−/− lungs under room air conditions (Fig. 3A). However, after hyperoxic exposures, complex I activity was lower in NQO1−/− than NQO1+/+ lungs. Complex IV activities were not significantly affected by genotype or hyperoxia (Fig. 3B). The implication is that the loss of complex I activity in the hyperoxia-exposed lungs is not attributable to a general loss of mitochondria or a nonspecific effect on mitochondrial function.
Fig. 3.
Complex I (A) and complex IV (B) activities in mitochondria-enriched lung fractions. Values are means ± SE; n = 4 room air-exposed NQO1+/+, 4 hyperoxia-exposed NQO1+/+, 5 room air-exposed NQO1−/−, and 4 hyperoxia-exposed NQO1−/− lung mitochondria-enriched fractions. Significantly different (by ANOVA and Tukey's honestly significant difference test): *P < 0.05 vs. genotype-matched control (room air); †P < 0.05 vs. hyperoxia-exposed NQO1+/+.
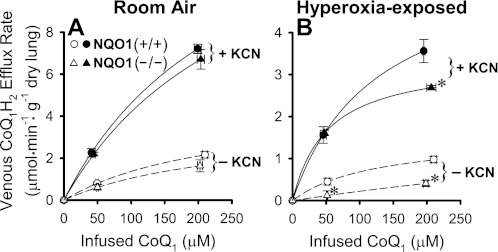
In the perfused rat lung and in pulmonary arterial endothelial cells in culture, CoQ1H2 efflux rates reflect the net effects of CoQ1 reduction and CoQ1H2 oxidation and, thus, represent only a lower bound on the CoQ1 reduction rates (4, 39). Mitochondrial electron transport complex III is a common enzymatic hydroquinone oxidation route in cells and tissues. Elimination of complex III-catalyzed CoQ1H2 oxidation would be anticipated to minimize competition for pulmonary venous efflux of complex I-generated CoQ1H2, thereby providing CoQ1H2 efflux rates that more closely approximate actual CoQ1 reduction rates. In the Fig. 4 study, an inhibitor of cytochrome oxidase, KCN (2 mM), was added to the perfusate to block mitochondrial electron transport and close complex III as a site of CoQ1H2 oxidation. Figure 4, A and B, shows the effects of KCN on venous CoQ1H2 efflux rate vs. reservoir (infused) CoQ1 concentration curves for room air- and hyperoxia-exposed lungs for both genotypes. First, for the room air-exposed lungs, KCN increases the CoQ1H2 efflux rates for both genotypes at both CoQ1 concentrations (closed vs. open symbols in Fig. 4A). There is no significant difference between the CoQ1H2 efflux rates for the NQO1+/+ and NQO1−/− lungs at either CoQ1 concentration in the absence of KCN (open symbols in Fig. 4A), nor is there a difference in the presence of KCN (closed symbols in Fig. 4A). With the change in y-axis scaling for Fig. 4A vs. Fig. 4B taken into account, it can be seen that hyperoxic exposure decreases the CoQ1H2 efflux rates for both genotypes at both CoQ1 concentrations without or with KCN (open symbols in Fig. 4B vs. 4A or closed symbols in Fig. 4B vs. 4A, respectively).
Fig. 4.
Effect of KCN on CoQ1H2 venous efflux rates vs. infused CoQ1 concentrations for room air- and hyperoxia-exposed NQO1+/+ and NQO1−/− lungs. Values are means ± SE; n = 4 for all conditions in A and n = 4 for NQO1+/+ and 3 for NQO1−/− in B. Each value in B is significantly lower than that for the paired condition-CoQ1 concentration in A. Significantly different (by ANOVA and Tukey's honestly significant difference test): *P < 0.05 vs. paired NQO1+/+ value within the hyperoxia-exposed group, i.e., within B.
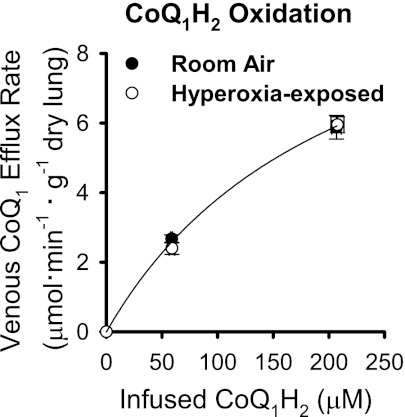
To account for the net effects of CoQ1H2 oxidation attributable to complex III, ROS, or other mechanisms, CoQ1H2 was infused into the pulmonary arterial inflow, and CoQ1 efflux rate was measured. The study was carried out in NQO1−/− lungs in the presence of rotenone and dicumarol to emphasize the net effects of passage through the lung on CoQ1H2 oxidation. Figure 5 shows that the CoQ1 efflux rates were greater for infusions of 200 μM than 50 μM CoQ1H2 for both sets of lungs but that there was no significant difference between the rates for the room air- and hyperoxia-exposed lungs at 200 or 50 μM CoQ1H2. The key point is that whatever CoQ1H2 oxidation mechanism(s) may be present, their net effects are the same for the room air- and hyperoxia-exposed lungs and, therefore, cannot account for the differences in CoQ1 redox metabolism in these two conditions.
Fig. 5.
CoQ1 efflux rates vs. infused CoQ1H2 concentrations for room air- and hyperoxia-exposed NQO1−/− lungs. Values are means ± SE; n = 4 room air- and 4 hyperoxia-exposed lungs at each CoQ1H2 concentration. There were no significant differences between values for room air- and hyperoxia-exposed lungs at 50 or 200 μM CoQ1H2 (P > 0.05, by t-test). There were no statistically significant differences between any of the physiological parameters reported in Tables 2 and 3 (which pertain to the Fig. 2 study) for the animals or lungs in the Fig. 5 study and also no statistically significant differences in the recoveries of infused CoQ1 as venous effluent CoQ1 + CoQ1H2 in the Fig. 5 study (statistical analysis as described in Table 2 and 3 footnotes and Fig. 2 legend).
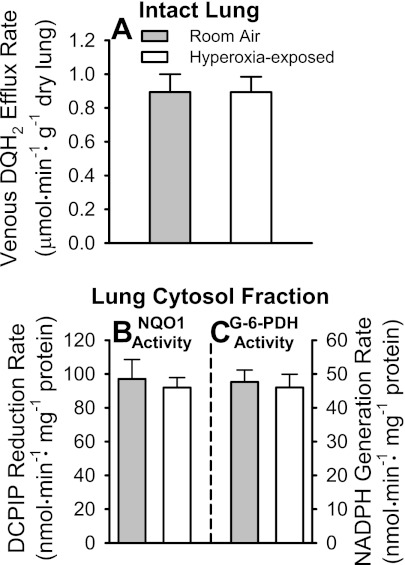
Using NQO1+/+, NQO1+/−, and NQO1−/− mice, we previously demonstrated that DQ acts as a specific probe for NQO1 in the isolated perfused mouse lung (36). Figure 6A shows the pulmonary venous DQH2 efflux rate during infusion of 50 μM DQ into the NQO1+/+ mouse pulmonary circulation. There was no detectable effect of hyperoxia on the efflux rates, implying that the hyperoxic exposure did not affect NQO1 activity. Figure 6B shows that NQO1 activity in cytosolic fractions of lung homogenates is also the same in room air- and hyperoxia-exposed lungs. The cytosolic activity of the rate-limiting enzyme of the pentose phosphate pathway, glucose-6-phosphate dehydrogenase, is also not affected by the hyperoxic exposure (Fig. 6C).
Fig. 6.
Pulmonary venous duroquinone (DQ) hydroquinone (DQH2) efflux rates during DQ infusion into pulmonary arterial inflow of room air- and hyperoxia-exposed NQO1+/+ mouse lungs, and lung cytosol fraction NQO1 and glucose-6-phosphate dehydrogenase (G-6-PDH) activities. A: steady-state pulmonary venous DQH2 efflux rates during 50 μM DQ infusion into pulmonary arterial inflow. B: lung cytosol fraction NQO1 activities for lungs in A. DCIP, 2,6-dichlorophenolindophenol. C: lung cytosol fraction G-6-PDH activities for lungs in A. Values are means ± SE; n = 5 room air- and 6 hyperoxia-exposed lungs. There were no statistically significant differences (by t-test) between values within A, B, or C.
DISCUSSION
The present study demonstrates that alterations in CoQ1 redox metabolism on passage through the intact perfused mouse lung can reveal a decrease in mitochondrial complex I activity caused by two independent treatments: addition of the complex I inhibitor rotenone to the lung perfusate and exposure of the animal to hyperoxia. The results of a series of experiments were required to reach this conclusion, including infusion of CoQ1 or CoQ1H2 into the pulmonary arterial inflow of NQO1+/+ and NQO1−/− mice, without and with metabolic inhibitors, and analysis of the CoQ1 redox forms in the pulmonary venous effluent. First, when CoQ1 was infused into the lung, the pulmonary venous CoQ1H2 efflux rates were decreased in the presence of the mitochondrial complex I inhibitor rotenone. There was no statistically significant difference between the total or rotenone-sensitive CoQ1H2 efflux rates in NQO1+/+ and NQO1−/− lungs, suggesting that NQO1 is not a dominant CoQ1 reductase accessible via the pulmonary circulation in the mouse. The redox mechanism(s) underlying the rotenone-insensitive CoQ1 redox metabolism was not identified. However, when rotenone is present, a contribution of NQO1 to CoQ1 reduction in the wild-type lung may be unmasked due to decreased competition for CoQ1 with the normally dominating complex I.
The decrease in pulmonary venous CoQ1H2 efflux rate in CoQ1-infused hyperoxia-exposed compared with room air-exposed lungs was consistent with the depressed complex I activity measured in the mitochondria-enriched fractions from the hyperoxia-exposed animals. That is, the CoQ1H2 efflux rates and the complex I activity measurements were more depressed in the NQO1−/− compared with the NQO1+/+ hyperoxia-exposed lungs. One implication is that the NQO1−/− mice are more sensitive to the pulmonary effects of the hyperoxic exposure. It is also possible that at least a portion of the difference in CoQ1H2 efflux rates in hyperoxia-exposed NQO1+/+ vs. NQO1−/− lungs may be explained by an NQO1 contribution in the former when the dominant competing CoQ1 reductase, complex I, is rendered less active by the hyperoxia. However, any such contribution cannot be attributed to a hyperoxia-induced increase in NQO1 activity in the wild-type mouse lungs, because there was no evidence that this occurred (Fig. 6). Overall, the observations emphasize the point that the likelihood that any given quinone will act as an electron acceptor for any given quinone reductase within a tissue containing a complex mixture of quinone reductases depends on the physical and chemical properties of the quinone, its concentration, and its propensity to react with the given quinone reductase vs. others, as well as the kinetics of any competing reactions.
While the NQO1−/− lung studies may be viewed as providing only indirect evidence for a role of complex I in CoQ1 redox metabolism in the lung, they are important nonetheless, because NQO1 is broadly implicated in the reduction of many quinones and other redox-active compounds in various tissues and cells. CoQ1 reduction via NQO1 has been observed in primary cultures of rat hepatocytes and astrocytes and the isolated intact perfused rat lung (4, 16, 22). The relatively selective reduction of CoQ1 by complex I within the mouse lung is impressive, given that NQO1 is a predominantly cytosolic (>90%) enzyme, wherein CoQ1 must traverse the cytosol before it reaches the mitochondria (49). In the isolated perfused rat lung, CoQ1 acts as an electron acceptor for complex I and NQO1 (4). The distinction between the behavior of CoQ1 in the mouse and rat lung is consistent with structural differences between mouse and rat NQO1 that influence catalytic activity and electron acceptor specificity and, for many electron acceptors, tend to render the rat enzyme more active (24). Since rat NQO1 competes strongly with complex I for CoQ1, an NQO1 inhibitor was needed to discern the complex I contribution to CoQ1 reduction in the previous rat studies. The depression in rotenone-sensitive CoQ1 reduction previously observed in the hyperoxia-exposed (85% O2 for 48 h) rat lung in the presence of the NQO1 inhibitor was interpreted to reflect decreased complex I activity (4). However, there was some question regarding this interpretation, since off-target effects of dicumarol may include uncoupling of electron transport and other mitochondrial perturbations (23, 28, 30, 34). Thus the present study validates the rat lung studies and implies that whatever the off-target effects of dicumarol, they did not obscure the impact of hyperoxia on intact lung complex I activity, as detected with CoQ1 in the rat lung model (4).
It is important to note that CoQ1H2 efflux rates represent the net effect of lung redox processes on CoQ1 and CoQ1H2, including CoQ1 reduction at complex I, a minor contribution of rotenone-insensitive reductases, and CoQ1H2 reoxidation. As for the reoxidation mechanisms, we previously observed that when KCN was in the perfusate during DQ infusions into the pulmonary arterial inflow of rat or mouse lungs, the DQH2 appearance rate in the pulmonary venous outflow was increased; the same was observed for the CoQ1-CoQ1H2 redox pair in rat lung studies (2, 4). The same effect was observed in the present study in Fig. 4. The data can be explained by KCN inhibition of complex IV, which closes complex III as a site of CoQ1H2 reoxidation (2, 4). Blockade of complex III-catalyzed CoQ1H2 reoxidation minimizes competition for lung efflux of the complex I-generated CoQ1H2. Thus, CoQ1H2 efflux rates obtained in the presence of KCN more closely approximate the CoQ1 reduction rates on passage through the lung than those obtained in the absence of KCN, the latter of which provide only a lower bound on the CoQ1 reduction rate. That the CoQ1H2 efflux rates remain decreased in the hyperoxia-exposed lungs even when KCN is present eliminates an effect of hyperoxia on complex III-catalyzed CoQ1H2 oxidation activity as an explanation for the decrease in CoQ1 reduction.
Complex III apparently represents a dominant pathway of CoQ1H2 oxidation on passage through the lung, as revealed by the large increases in CoQ1H2 lung efflux rates in CoQ1-infused lungs when KCN is present. However, this is not the only possible hydroquinone oxidation mechanism taking place on passage through the lung. For example, hydroquinone scavenging of ROS results in hydroquinone oxidation (12). This, or any other oxidation mechanism, could diminish the CoQ1H2 available for venous efflux and be interpreted as a net decrease in CoQ1 reduction in hyperoxia-exposed (or rotenone-treated) lungs. However, the Fig. 5 study shows that there is no detectable effect of hyperoxia on the net CoQ1H2 oxidation capacity, whatever the mechanism, as exemplified in the NQO1−/− lung. This observation rules out differences in CoQ1H2 oxidation activity as an explanation for the differences in CoQ1 redox metabolism on passage through room air- and hyperoxia-exposed lungs.
Another mechanism that could cause an apparent decrease in CoQ1 reduction capacity would be an unidentified hyperoxia-induced metabolizing system(s) that competes for CoQ1 or CoQ1H2, making them less available to undergo reactions with complex I or III, respectively. CoQ1 and CoQ1H2 have been reported to undergo phase I and II reactions, respectively, and cytochrome P-450 1A expression is increased in the hyperoxia-exposed mouse lung (8, 15, 33). However, the present study ruled out such irreversible hydroxylation or conjugation reactions as explanations for the difference between CoQ1 redox metabolism in room air- and hyperoxia-exposed lungs. Greater than 95% of the CoQ1 infused into room air- or hyperoxia-exposed lungs was recovered as CoQ1 + CoQ1H2 in the pulmonary venous effluent. This was the case whether the measurements were made by spectrophotometry or liquid chromatography-electrospray ionization-mass spectrometry techniques. Phase I or II reactions would generate compounds with spectrophotometrically distinct absorbance spectra, different HPLC retention times, and/or different mass-to-charge ratios, wherein they would not appear as recovered CoQ1 + CoQ1H2. This is not to say that such metabolites are not present, only that, if they are, they can account for no more than 5% of the total infused CoQ1 and that this minor amount of CoQ1 and/or CoQ1H2 that is not recovered in the pulmonary venous effluent is not significantly different for room air- and hyperoxia-exposed lungs. The lack of substantial phase I or II metabolism (other than that catalyzed by NQO1) on passage through the lung may be related to the short time that the quinone is actually in the pulmonary circulation under the single-pass constant-infusion conditions in the present study. The transit time through the pulmonary circulation under these conditions is on the order of several seconds.
Taken together, the data imply that the decreased pulmonary venous CoQ1H2 efflux rate in the CoQ1-infused hyperoxia-exposed compared with room air-exposed lungs can be explained by a decrease in CoQ1 reduction capacity on passage through lungs of both NQO1 genotypes. That the decreased reduction capacity reflects a complex I deficiency is supported by the results of the complex I activity assays in lung mitochondrial fractions. Among the measurements made, the depression in complex I activity was the only effect of hyperoxia on the lung. That is, the lung wet-to-dry weight ratios (postperfusion), perfusion pressures, and perfused surface areas (as reflected by the PS for the angiotensin-converting enzyme substrate FAPGG) were not detectably different for the room air- and hyperoxia-exposed lungs for NQO1+/+ or NQO1−/− mice. The lack of overt lung injury was consistent with the selection of the length of the hyperoxic exposure, which was anticipated to initiate effects of hyperoxia in the lung, but to limit morbidity and mortality, wherein most mouse strains survive this level of O2 for ≥72 h (37). Our observations are also consistent with the considerable variation in mice with regard to the time course of the various manifestations of hyperoxia-induced lung injury and time to death, depending on strain, age, sex, and other factors (26, 32, 37, 44). It is of note that the wild-type and background strain for our NQO1−/− mouse colony, 129P3/J, was identified as among the more susceptible strains with respect to survival in >95% O2 (mean survival time of 93 h) compared with 16 other strains of female mice (males were used in the present study) (43). Thus the sensitivity of complex I activity in the present study is not that surprising, given previous reports of hyperoxic effects on various lung tissue and lung endothelial and epithelial cell mitochondrial functions, including complex I activity (4, 7, 11, 14, 41, 46, 53). We previously observed a hyperoxia-induced depression of complex I activity in the rat lung and bovine pulmonary arterial endothelial cells in culture (4, 38). Functional or temporal relationships, if any, of the changes in complex I activity in hyperoxic lung injury on survival times in different mouse strains remain to be seen.
There was no evidence of NQO1 induction in the hyperoxia-exposed NQO1+/+ mice. Linkage analysis studies have identified nuclear factor erythroid-derived 2 (Nrf2) as a gene candidate for susceptibility to hyperoxic lung injury, with Nqo1 as one of the genes whose expression is under the control of the Nrf2-encoded transcription factor (Nrf2) (17, 47, 48). The lack of detectable NQO1 induction in the present studies is again consistent with the general concept of strain-, age-, and/or sex-dependent temporal variations in the appearance of other manifestations of hyperoxia-induced lung injury, morbidity, and mortality in mice (26, 32, 37, 44). For example, an array of lung Nrf2-dependent antioxidant and phase II enzymes, including NQO1, were induced following 48–72 h of hyperoxic (>95% O2) exposure in ICR/Sv129 mice, but, consistent with our study, no change in lung NQO1 gene expression was seen during the first 48 h of exposure to >95% O2 in C57Bl/6 mice (17, 42). The similarity of the C57Bl/6 and 129P3/J (wild-type in the present study) strains in this regard, wherein both have been reported as relatively hyperoxia-sensitive, is consistent with the concept of a weaker protective response in these mice (32). Whether NQO1 is protective against the effects of hyperoxia on lung complex I activity may be implied by, but was not further explored in, the present study. The double NQO1/NQO2 knockout mouse was reported to be more susceptible than the wild-type mouse to hyperoxic lung injury (100% O2 for 72 h), and NQO1 polymorphisms resulting in depressed NQO1 activity have been associated with acute lung injury in humans (19, 47). On the other hand, elevation of NQO1 activity via chemical inducers does not prolong survival of rats exposed to hyperoxia (54).
Regardless of any contributions, or lack thereof, of complex I or NQO1 activities to lung injury or protection following the hyperoxic exposure, the depression in lung complex I activity appears as a relatively early effect under the experimental conditions described. In addition, our study provides inhibitor and genetic-based evidence supporting further exploration of the concept of CoQ1 as a probe for complex I activity in the intact perfused mouse lung and demonstrates its utility for detecting changes in lung complex I activity in the hyperoxic model. It also makes use of DQ as an indicator for intact lung NQO1 activity, providing deepening support for its utility as a probe for this hallmark phase II enzyme. Together, the two quinone probes provide signals for distinct redox enzyme systems within the intact tissue, one mitochondrial and the other cytosolic, which are differentially sensitive to the effects of oxidative stress (e.g., hyperoxia). We have also shown that quinone redox metabolism is sensitive to the impact of electrophilic stress in intact pulmonary arterial endothelial cells in culture (9). The implication is that DQ and CoQ1 can be important tools to obtain information regarding lung redox metabolism phenotypes following pharmacological or toxicological exposures, wherein the phenotypic signature emanating from the intact organ is accessible in the perfusate, which is analogous to the blood. Since there are no means to assess complex I activity in vivo, support for the utility of CoQ1 as an indicator of complex I activity in the intact lung would represent a technical advance for studying mitochondria as a target of oxidative stress or genetic abnormalities in the intact lung and perhaps other organs. One concept is that such information would provide indications of lung mitochondrial stress relatively early in the time course of lung injury, as in the present study, wherein complex I injury detected at 48 h of breathing 100% O2 precedes any overt, nonspecific lung injury or mortality that is well documented by others to occur in mice after 72 h of such exposure.
GRANTS
This work was supported by the Department of Veterans Affairs (VA Medical Research Funds) and the National Cancer Institute Intramural Research Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.D.B. and M.P.M. are responsible for conception and design of the research; R.D.B., B.J.L., and S.B. performed the experiments; R.D.B. and M.P.M. analyzed the data; R.D.B., C.R.M., and M.P.M. interpreted the results of the experiments; R.D.B. and M.P.M. prepared the figures; R.D.B., C.R.M., B.J.L., F.J.G., and M.P.M. edited and revised the manuscript; R.D.B., C.R.M., and M.P.M. approved the final version of the manuscript; M.P.M. drafted the manuscript.
ACKNOWLEDGMENTS
The authors thank R. Babygirija and T. Takahashi (Department of Surgery, Medical College of Wisconsin and Zablocki Veterans Affairs Medical Center) for generous help in carrying out the genotyping and K. Nithipatakom and M. Isbell (Mass Spectrometry Facility, Department of Pharmacology, Medical College of Wisconsin) for undertaking the mass spectrometry analysis.
REFERENCES
- 1. Allen CB, Guo XL, White CW. Changes in pulmonary expression of hexokinase and glucose transporter mRNAs in rats adapted to hyperoxia. Am J Physiol Lung Cell Mol Physiol 274: L320–L329, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Audi SH, Bongard RD, Dawson CA, Siegel D, Roerig DL, Merker MP. Duroquinone reduction during passage through the pulmonary circulation. Am J Physiol Lung Cell Mol Physiol 285: L1116–L1131, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Audi SH, Bongard RD, Okamoto Y, Merker MP, Roerig DL, Dawson CA. Pulmonary reduction of an intravascular redox polymer. Am J Physiol Lung Cell Mol Physiol 280: L1290–L1299, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Audi SH, Merker MP, Krenz GS, Ahuja T, Roerig DL, Bongard RD. Coenzyme Q1 redox metabolism during passage through the rat pulmonary circulation and the effect of hyperoxia. J Appl Physiol 105: 1114–1126, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Audi SH, Olson LE, Bongard RD, Roerig DL, Schulte ML, Dawson CA. Toluidine blue O and methylene blue as endothelial redox probes in the intact lung. Am J Physiol Heart Circ Physiol 278: H137–H150, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Audi SH, Bongard RD, Krenz GS, Rickaby DA, Haworth ST, Eisenhauer J, Roerig DL, Merker MP. Effect of chronic hyperoxic exposure on duroquinone reduction in adult rat lungs. Am J Physiol Lung Cell Mol Physiol 289: L788–L797, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Bassett DJ, Reichenbaugh SS. Lung mitochondrial function following oxygen exposure and diethyl maleate-induced depletion of glutathione. Toxicol Appl Pharmacol 115: 161–167, 1992 [DOI] [PubMed] [Google Scholar]
- 8. Bogeski I, Gulaboski R, Kappl R, Mirceski V, Stefova M, Petreska J, Hoth M. Calcium binding and transport by coenzyme Q. J Am Chem Soc 133: 9293–9303, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Bongard RD, Krenz GS, Gastonguay AJ, Williams CL, Lindemer BJ, Merker MP. Characterization of the threshold for NAD(P)H:quinone oxidoreductase (NQO1) activity in intact sulforaphane treated pulmonary arterial endothelial cells. Free Radic Biol Med 50: 953–962, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Poljak A, Grant R. Age related changes in NAD+ metabolism oxidative stress and sirt1 activity in Wistar rats. PLos One 6: e19194, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11. Buccellato LJ, Tso M, Akinci OI, Chandel NS, Budinger GRS. Reactive oxygen species are required for hyperoxia-induced Bax activation and cell death in alveolar epithelial cells. J Biol Chem 279: 6753–6760, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Cadenas E, Hochstein P, Ernster L. Pro- and antioxidant functions of quinones and quinone reductases in mammalian cells. Adv Enzymol Relat Areas Mol Biol 65: 97–146, 1992 [DOI] [PubMed] [Google Scholar]
- 13. Capaldi RA, Marusich MF, Taanman JW. Mammalian cytochrome c oxidase: characterization of enzyme and immunological detection of subunits in tissue extracts and whole cells. Methods Enzymol 260: 117–132, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Carraway MS, Suliman HB, Kliment C, Welty-Wolf KE, Oury TD, Piantadosi CA. Mitochondrial biogenesis in the pulmonary vasculature during inhalational lung injury and fibrosis. Antioxidants Redox Signal 10: 269–276, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan TS, O'Brien PJ. Hepatocyte metabolism of coenzyme Q1 (ubiquinone-5) to its sulfate conjugate decreases its antioxidant activity. Biofactors 18: 207–218, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Chan TS, Teng S, Wilson JX, Galati G, Khan S, O'Brien PJ. Coenzyme Q cytoprotective mechanisms for mitochondrial complex I cytopathies involves NAD(P)H:quinone oxidoreductase 1 (NQO1). Free Radic Res 36: 421–427, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol 26: 175–182, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Cho HY, Reddy SP, Debiase A, Yamamoto M, Kleeberger SR. Gene expression profiling of NRF2-mediated protection against oxidative injury. Free Radic Biol Med 38: 325–343, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Das A, Kole L, Wang L, Barrios R, Moorthy B, Jaiswal AK. BALT development and augmentation of hyperoxic lung injury in mice deficient in NQO1 and NQO2. Free Radic Biol Med 40: 1843–1856, 2006 [DOI] [PubMed] [Google Scholar]
- 20. de Wit LE, Sluiter W. Reliable assay for measuring complex I activity in human blood lymphocytes and skin fibroblasts. Methods Enzymol 456: 169–181, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Di Virgilio F, Azzone GF. Activation of site I redox-driven H+ pump by exogenous quinones in intact mitochondria. J Biol Chem 257: 4106–4113, 1982 [PubMed] [Google Scholar]
- 22. Dragan M, Dixon SJ, Jaworski E, Chan TS, O'Brien PJ, Wilson JX. Coenzyme Q1 depletes NAD(P)H and impairs recycling of ascorbate in astrocytes. Brain Res 1078: 9–18, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Du J, Daniels DH, Asbury C, Venkataraman S, Liu J, Spitz DR, Oberley LW, Cullen JJ. Mitochondrial production of reactive oxygen species mediate dicumarol-induced cytotoxicity in cancer cells. J Biol Chem 281: 37416–37426, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Faig M, Bianchet MA, Talalay P, Chen S, Winski S, Ross D, Amzel LM. Structures of recombinant human and mouse NAD(P)H:quinone oxidoreductases: species comparison and structural changes with substrate binding and release. Proc Natl Acad Sci USA 97: 3177–3182, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fato R, Estornell E, di Bernardo S, Pallotti F, Parenti CG, Lenaz G. Steady-state kinetics of the reduction of coenzyme Q analogs by complex I (NADH:ubiquinone oxidoreductase) in bovine heart mitochondria and submitochondrial particles. Biochemistry 35: 2705–2716, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Frank L, Bucher JR, Roberts RJ. Oxygen toxicity in neonatal and adult animals of various species. J Appl Physiol 45: 699–704, 1978 [DOI] [PubMed] [Google Scholar]
- 27. Gan Z, Audi SH, Bongard RD, Gauthier KM, Merker MP. Quantifying mitochondrial and plasma membrane potentials in intact pulmonary endothelial cells based on extracellular disposition of rhodamine dyes. Am J Physiol Lung Cell Mol Physiol 300: L762–L772, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gonzalez-Aragon D, Ariza J, Villalba JM. Dicoumarol impairs mitochondrial electron transport and pyrimidine biosynthesis in human myeloid leukemia HL-60 cells. Biochem Pharmacol 73: 427–439, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Hartwick AT, Sivak JG. Epithelial activity of hexokinase and glucose-6-phosphate dehydrogenase in cultured bovine lenses recovering from pharmaceutical-induced optical damage. Mol Vis 9: 594–600, 2003 [PubMed] [Google Scholar]
- 30. Heiskanen A, Spegel C, Kostesha N, Lindahl S, Ruzgas T, Emnéus J. Mediator-assisted simultaneous probing of cytosolic and mitochondrial redox activity in living cells. Anal Biochem 384: 11–19, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Ho YS. Transgenic and knockout models for studying the role of lung antioxidant enzymes in defense against hyperoxia. Am J Respir Crit Care Med 166: S51–S56, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Hudak BB, Zhang LY, Kleeberger SR. Inter-strain variation in susceptibility to hyperoxic injury of murine airways. Pharmacogenetics 3: 135–143, 1993 [DOI] [PubMed] [Google Scholar]
- 33. Jiang W, Couroucli XI, Wang L, Barrios R, Moorthy B. Augmented oxygen-mediated transcriptional activation of cytochrome P450 (CYP) 1A expression and increased susceptibilities to hyperoxic lung injury in transgenic mice carrying the human CYP1A1 or mouse 1A2 promoter in vivo. Biochem Biophys Res Commun 407: 79–85, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Laruelle C, Godfroid JJ. Quantitative structure-activity relationships for dicoumarol antivitamins K in the uncoupling of oxidative phosphorylation. J Med Chem 18: 85–90, 1975 [DOI] [PubMed] [Google Scholar]
- 35. Laskin JD, Black AT, Jan YH, Sinko PJ, Heindel ND, Sunil V, Heck DE, Laskin DL. Oxidants and antioxidants in sulfur mustard induced injury. Ann NY Acad Sci 1203: 92–100, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lindemer BJ, Bongard RD, Hoffmann R, Baumgardt S, Gonzalez FJ, Merker MP. Genetic evidence for NAD(P)H:quinone oxidoreductase 1 catalyzed quinone reduction on passage through the mouse pulmonary circulation. Am J Physiol Lung Cell Mol Physiol 300: L773–L780, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L379–L399, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Merker MP, Audi SH, Bongard RD, Lindemer BJ, Krenz GS. Influence of pulmonary arterial endothelial cells on quinone redox status: effect of hyperoxia induced increase in NAD(P)H quinone oxidoreductase 1 (NQO1). Am J Physiol Lung Cell Mol Physiol 289: L788–L797, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Merker MP, Audi SH, Lindemer BJ, Krenz GS, Bongard RD. Role of mitochondrial electron transport complex I in coenzyme Q1 reduction by intact pulmonary arterial endothelial cells and the effect of hyperoxia. Am J Physiol Lung Cell Mol Physiol 293: L809–L819, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Merker MP, Bongard RD, Krenz GS, Zhao H, Fernandes V, Kalyanaraman B, Hogg N, Audi SH. Impact of pulmonary arterial endothelial cells on duroquinone redox status. Free Radic Biol Med 37: 86–103, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Morton RL, Ikle D, White CW. Loss of lung mitochondrial aconitase activity due to hyperoxia in bronchopulmonary dysplasia in primates. Am J Physiol Lung Cell Mol Physiol 274: L127–L133, 1998 [DOI] [PubMed] [Google Scholar]
- 42. Perkowski S, Sun J, Singhal S, Santiago J, Leikauf GD, Albelda SM. Gene expression profiling of the early pulmonary response to hyperoxia in mice. Am J Respir Cell Mol Biol 28: 682–696, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Prows DR, Hafertepen AP, Gibbons WJ, Winterberg AV, Nick TG. A genetic mouse model to investigate hyperoxic acute lung injury survival. Physiol Genomics 30: 262–270, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Prows DR, Winterberg AV, Gibbons WJ, Burzynski BB, Liu C, Nick TG. Reciprocal backcross mice confirm major loci linked to hyperoxic acute lung injury survival time. Physiol Genomics 38: 158–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Radjendirane V, Joseph P, Lee YH, Kimura S, Klein-Szanto AJ, Gonzalez FJ, Jaiswal AK. Disruption of the DT diaphorase (NQO1) gene in mice leads to increased menadione toxicity. J Biol Chem 273: 7382–7389, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Ratner V, Starkov A, Matsiukevich D, Polin RA, Ten VS. Mitochondrial dysfunction contributes to alveolar developmental arrest in hyperoxia-exposed mice. Am J Respir Cell Mol Biol 40: 511–518, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reddy AJ, Christie JD, Aplenc R, Fuchs B, Lanken PN, Kleeberger SR. Association of human NAD(P)H:quinone oxidoreductase 1 (NQO1) polymorphism with development of acute lung injury. J Cell Mol Med 13: 1784–1791, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reddy NM, Kleeberger SR, Kensler TW, Yamamoto M, Hassoun PM, Reddy SP. Disruption of Nrf2 impairs the resolution of hyperoxia-induced acute lung injury and inflammation in mice. J Immunol 182: 7264–7271, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ross D, Kepa JK, Winski SL, Beall HD, Anwar A, Siegel D. NAD(P)H:quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem Biol Interact 129: 77–97, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Schatz G, Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. VII. Oxidative phosphorylation in the diphosphopyridine nucleotide-cytochrome b segment of the respiratory chain: assay and properties in submitochondrial particles. J Biol Chem 241: 1429–1438, 1966 [PubMed] [Google Scholar]
- 51. Tipple TE, Welty SE, Nelin LD, Hansen JM, Rogers LK. Alterations of the thioredoxin system by hyperoxia: implications for alveolar development. Am J Respir Cell Mol Biol 41: 612–619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Voynow JA, Fischer BM, Zheng S, Potts EN, Grover AR, Jaiswal AK, Ghio AJ, Foster WM. NAD(P)H quinone oxidoreductase 1 is essential for ozone-induced oxidative stress in mice and humans. Am J Respir Cell Mol Biol 41: 107–113, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 53. Waxman AB, Kolliputi N. IL-6 protects against hyperoxia-induced mitochondrial damage via Bcl-2-induced Bak interactions with mitofusions. Am J Respir Cell Mol Biol 41: 385–396, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Whitney PL, Frank L. Does lung NAD(P)H:quinone reductase (DT-diaphorase) play an antioxidant enzyme role in protection from hyperoxia? Biochim Biophys Acta 1156: 275–282, 1993 [DOI] [PubMed] [Google Scholar]