Abstract
Restoration of the epithelial barrier following acute lung injury is critical for recovery of lung homeostasis. After injury, alveolar type II epithelial (ATII) cells spread and migrate to cover the denuded surface and, eventually, proliferate and differentiate into type I cells. The chemokine CXCL12, also known as stromal cell-derived factor 1α, has well-recognized roles in organogenesis, hematopoiesis, and immune responses through its binding to the chemokine receptor CXCR4. While CXCL12/CXCR4 signaling is known to be important in immune cell migration, the role of this chemokine-receptor interaction has not been studied in alveolar epithelial repair mechanisms. In this study, we demonstrated that secretion of CXCL12 was increased in the bronchoalveolar lavage of rats ventilated with an injurious tidal volume (25 ml/kg). We also found that CXCL12 secretion was increased by primary rat ATII cells and a mouse alveolar epithelial (MLE12) cell line following scratch wounding and that both types of cells express CXCR4. CXCL12 significantly increased ATII cell migration in a scratch-wound assay. When we treated cells with a specific antagonist for CXCR4, AMD-3100, cell migration was significantly inhibited. Knockdown of CXCR4 by short hairpin RNA (shRNA) caused decreased cell migration compared with cells expressing a nonspecific shRNA. Treatment with AMD-3100 decreased matrix metalloproteinase-14 expression, increased tissue inhibitor of metalloproteinase-3 expression, decreased matrix metalloproteinase-2 activity, and prevented CXCL12-induced Rac1 activation. Similar results were obtained with shRNA knockdown of CXCR4. These findings may help identify a therapeutic target for augmenting epithelial repair following acute lung injury.
Keywords: acute respiratory distress syndrome, epithelial repair, acute lung injury
acute respiratory distress syndrome is a severe form of lung injury with high mortality and morbidity (10, 58, 62). A prominent feature of acute respiratory distress syndrome is injury to the endothelial and epithelial barrier. The specific mechanisms of repair and the restoration of lung barrier function are incompletely understood. Migration of local epithelial stem cells and recruitment of other progenitor cells to repair the damaged tissue are crucial for recovery of the injured lung (2, 48). These cells are stimulated and recruited by growth factors, cytokines, and chemokines released at the site of injury (14). Alveolar type II (ATII) cells are thought to be the predominant local stem cell that repairs the alveolar epithelial surface (54). Accumulated evidence indicates that ATII cells express growth factor and cytokine receptors that are involved in repair, including receptors for hepatocyte growth factor, keratinocyte growth factor, EGF, FGF-10, IL-2, and IL-1β (24, 33, 34, 43, 44, 56).
Chemokines are low-molecular-weight proteins that have been shown to involved in cell migration, proliferation, differentiation, and inflammation in asthma, chronic obstructive pulmonary disease, and acute lung injury (7, 8, 12, 28, 37, 42). However, their role in ATII cell-mediated repair has not been extensively studied. Chemokines have also been shown to control the expression of adhesion receptors on cells and, thereby, influence interactions with the extracellular matrix (ECM) (47). ATII cells have been shown to express the chemokine receptors CCR2, CCR3, CXCR2, and CXCR3 (7, 12, 46, 57), and stimulation of CCR2 by its ligand monocyte chemoattractant protein 1 has been shown to stimulate repair of wounded cell monolayers. Mice lacking the CXCR4 gene or the gene for its ligand CXCL12 (stromal cell-derived factor 1α) do not survive (40, 55). Expression of CXCR4 has been shown in alveolar epithelial cells (39), but the role of CXCR4 in epithelial repair has not been investigated.
In the present study, we report that primary cultures of rat ATII cells and a mouse lung epithelial (MLE12) cell line express CXCR4, and this receptor is activated by binding with its ligand CXCL12. Our data indicate a novel role for CXCR4 in the repair of wounded ATII cells involving Rac1, matrix metalloproteinase (MMP)-14, and MMP-2.
MATERIALS AND METHODS
Reagents.
DMEM-penicillin-streptomycin-trypsin-EDTA solution and PBS were purchased from GIBCO Life Technologies (Grand Island, NY); FBS from Hyclone (Logan, UT); HEPES, KCl, MgCl2, Triton X-100, sodium vanadate, PMSF, aprotinin, and leupeptin from Sigma (St. Louis, MO); Tween 20 from Bio-Rad (Hercules, CA); rhodamine-phalloidin and DRAQ-5 (a nuclear stain) from Molecular Probes (Eugene, OR); AMD-3100 [a highly specific antagonist of CXCR4 (20)] and the Rac1 inhibitor W56 from Sigma Aldrich (St. Louis, MO); the Rac1 and CDC42 activation assay kit from Millipore (Billerica, MA); gradient gels (4–12%) for Western blot and 10% gelatin gel, renaturation buffer, and developing buffer for zymography from Invitrogen (Carlsbad, CA); antibodies of CXCR4 (catalog nos. ab13854, sc-9046, and LS-C250) from Abcam (Cambridge, MA), Santa Cruz Biotechnology (Santa Cruz, CA), and LS Biosciences (Seattle, WA), respectively; CXCR4 blocking antibody, control isotype IgG for CXCR4, and an antibody for MMP-2 from Santa Cruz Biotechnology; and monoclonal antibodies for MMPs specific for MMP-1, MMP-3, MMP-9, MMP-11, MMP-12, MMP-13, MMP-14, and MMP-21 from Epitomics (Burlingame, CA).
Cell culture.
Primary rat ATII cells were isolated according to the methods described previously (16, 19). The animal use protocol was approved by the Institutional Animal Care and Use Committee at the University of Tennessee Health Science Center. Briefly, ATII cells were isolated from male Sprague-Dawley rats by elastase digestion and differential adherence on IgG-coated dishes. ATII cells were identified using Nile Red (Sigma) staining of lamellar bodies, and >90% of the cells were Nile Red-positive on day 2. We used plastic six-well plates or chamber slides that were coated with rat lung fibroblast (RLF) matrix deposited by RLF-6 cells (American Type Culture Collection) for wound-healing assays with ATII cells (16). Freshly isolated cells were seeded to confluence at 3.5 × 106 cells/well in ATII culture medium (DMEM with 10% FBS, 4 mM glutamine, 1% penicillin-streptomycin, and 0.25 μM amphotericin B), and experiments were performed on day 2 after isolation. MLE12 cells, an alveolar epithelial cell line established from pulmonary tumors in transgenic mice (30), were obtained from the American Type Culture Collection and cultured using medium containing DMEM with 10% FBS, 4 mM glutamine, and 1% penicillin-streptomycin.
Measurement of CXCL12.
CXCL12 in the conditioned media of ATII and MLE12 cells and in the bronchoalveolar lavage fluid (BAL) from rats was measured using an ELISA according to the manufacturer's protocol (NovaTeinBio, Cambridge, MA). Samples were collected from control cells or cells subjected to multiple scratch wounding 24 h earlier. Multiple scratch wounds were made as previously described (18). BAL was collected from control rats or from rats following 4 h of mechanical ventilation with an injurious tidal volume of 25 ml/kg body wt using the FlexiVent (SCIREQ, Montreal, PQ, Canada) with room air, as previously described (35).
Development of the CXCR4-shRNA cell line.
A stable cell line with decreased expression of CXCR4 was developed in MLE12 cells using a short-hairpin RNA (shRNA; catalog no. SC-35422-V, Santa Cruz Biotechnology) according to the manufacturer's protocol. A cell line with control shRNA was developed in the same cell line using a scrambled vector (catalog no. SC-108080, Santa Cruz Biotechnology). The antibiotic puromycin was used in the media to select the positive clone. The knockdown status of CXCR4 was assessed by Western blotting for CXCR4 protein expression.
Zymography.
MMP-2 activity was determined using gelatin zymography (45). Cells were seeded at equal densities and grown to confluence. An equivalent volume of medium (2 ml) was conditioned by the cells for 24 h. Cell lysates and conditioned media containing equal amounts of protein were run in native condition (nondenatured) in 10% gelatin gels. Gels were incubated first in renaturation buffer and then in developing buffer according to the manufacturer's protocol (Invitrogen). The final zymogram was developed by exposure of the gel to staining solution (1% Coomassie blue in 40% methanol, 10% acetic acid, and 50% water) and then to destaining solution without Coomassie blue (40% methanol, 10% acetic acid, and 50% water). A white band on the gel indicated the activated MMP-2 that cleaved the gelatin substrate on the gel. MMP-2 appeared as a ∼66-kDa band. The specificity of MMP-2 was confirmed by immunoblot by screening for MMPs using specific antibodies.
Small interfering RNA knockdown of MMP-14.
MMP-14 was transiently knocked down in primary rat ATII cells using small interfering RNA (siRNA; Dharmacon). siRNA was transfected into semiconfluent ATII cells on day 1 after isolation using an siRNA transfection kit (Santa Cruz Biotechnology), and experiments were begun on day 3 after isolation.
Wound-healing assay.
Cell migration was measured according to our previous methods (15). Confluent monolayers of ATII, MLE12, or CXCR4-shRNA cells were wounded by scraping a pipette tip across the monolayer to produce initial 1,000- to 1,200-μm wounds. Images were collected with a Cool Snap charge-coupled device camera (Roper Scientific, Trenton, NJ) mounted on an Eclipse TE300 inverted microscope with a ×4 objective (Nikon, Melville, NY). Images were obtained at the initial time of wounding and then 16–24 h after wounding. Metamorph software was used to record the coordinates for each wound location using a computer-controlled stage, so that the same location was used for postwounding measurement, and data were analyzed by Metamorph imaging software. The mean wound width at 24 h was calculated and normalized to the original wound width as a percentage. All results are from at least three independent wells from at least two separate experiments (n = 6).
SDS-PAGE.
Protein concentration was determined by the Bradford method (9). Equal amounts of protein were resolved by 4–12% SDS-PAGE and electrophoretically transferred onto PVDF membranes. Membranes were blocked for 1 h in 5% nonfat milk (Bio-Rad) in Tris-buffered saline containing 0.01% Tween 20 (TBST) and incubated overnight at 4°C with the appropriate primary antibody. Membranes were washed with TBST and then incubated with the secondary antibody for 1 h at room temperature. Finally, blots were developed on X-ray film using the enhanced chemiluminescence method (Amersham Biosciences, Piscataway, NJ).
Activated Rac1 assay.
Rac1 activity assays were performed according to the manufacturer's instructions (Millipore). ATII, CXCR4-shRNA, and scrambled shRNA cells were pretreated with or without AMD-3100 (2 μg/ml) for 1 h. Pretreated cells were exposed to rat or mouse CXCL12 (100 ng/ml) for 2 min. Ice-cold PBS was added to the wells, which were washed twice. Finally, cells were harvested by addition of 100 μl of ice-cold lysis buffer in each well (supplied in the kit) mixed with a cocktail of protease inhibitors (10 μl/ml; catalog no. 78425, Thermo Scientific, Rockford, IL). Cell lysates were centrifuged at 14,000 g at 4°C for 10 min, and an equal amount of protein in an equal volume of lysis buffer (500 μl) was incubated with p21-activated kinase, which was chemically coupled with agarose beads at 4°C for 1 h. The beads were pelleted by brief centrifugation (10,000 g at 4°C for 10 s) and washed three times with the lysis buffer. GTP-bound Rac1 was solubilized in Laemmli sample buffer and separated using a 4–12% Bis-Tris gel for Western blotting. Anti-Rac1 (1:500 dilution) and horseradish peroxidase-conjugated anti-mouse secondary (1:5,000 dilution; Cell Signaling Technology, Boston, MA) antibodies were used to probe the Rac1 in Western blots.
Confocal microscopy.
Expression of CXCR4 in ATII and MLE12 cells was assessed by confocal microscopy. ATII or MLE12 cells were grown to confluency on precleaned coverslips and then fixed with 4% paraformaldehyde. Cells were permeabilized with 0.1% Triton X-100 and then blocked with 2% BSA. CXCR4 primary antibody was incubated at 1:100 dilution overnight at 4°C. The secondary antibody labeled with Alexa Fluor 488 (Molecular Probes) was used at 1:500 dilution at room temperature for 30 min. The nucleus was stained with DRAQ5 (Molecular Probes) at 1:1,000 dilution for 15 min at room temperature. Images were obtained using a confocal microscope (Olympus) with ×20 and ×40 objectives. For examination of F-actin distribution in migrating cells, control and CXCR4-shRNA cells were fixed and stained with rhodamine-phalloidin. Images were obtained using a ×100 objective.
Densitometry of immunoblot.
Densitometry of bands obtained by immunoblot was performed using the software ImageJ developed by the National Institutes of Health. Each band was normalized against the corresponding β-actin, GAPDH, or total Rac1 loading control. These values were normalized to the control condition. For zymography, the image was inverted, so that the white band became black, and densitometry was assessed as described above.
Statistical analysis.
Values are means ± SE. Measurements were made on at least three independent wells from at least two different isolations (ATII cells) or experiments (MLE12). A t-test was used when only two groups were compared, and one-way ANOVA with Bonferroni's method was used for comparisons of multiple treatments to determine significant differences between individual conditions. Significant differences were determined based on a threshold of P < 0.05. Statistical comparisons were made using Sigmastat 3.5 (Jandel Scientific, San Rafael, CA).
RESULTS
CXCL12 was secreted following injury.
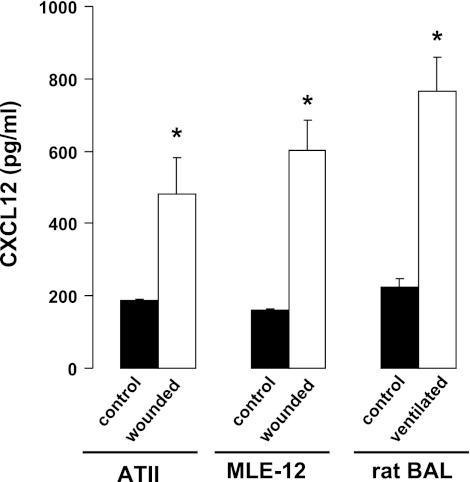
To determine whether CXCL12 secretion was increased following injury, we measured CXCL12 in the BAL of rats ventilated with an injurious tidal volume for 4 h. As shown in Fig. 1, CXCL12 in the BAL was significantly increased in ventilated rats compared with control (nonventilated) rats. We then measured the secretion of CXCL12 from primary rat ATII cells and from MLE12 cells. When cells were injured by multiple scratch wounds, the concentration of CXCL12 was significantly higher in the conditioned medium than in medium from unwounded control cells.
Fig. 1.
CXCL12 secretion was increased following injury in rats and in cultured cells. CXCL12 was measured by ELISA in the conditioned media of rat alveolar type II (ATII) or mouse lung epithelial (MLE12) cells 24 h after multiple scratch wounds were applied to the confluent cells. Control cells were unwounded. CXCL12 was also measured in bronchoalveolar lavage fluid (BAL) from control rats and rats ventilated with a tidal volume of 25 ml/kg. Values are means ± SE (n = 3). *P < 0.05 vs. control.
ATII and MLE12 cells express the chemokine receptor CXCR4.
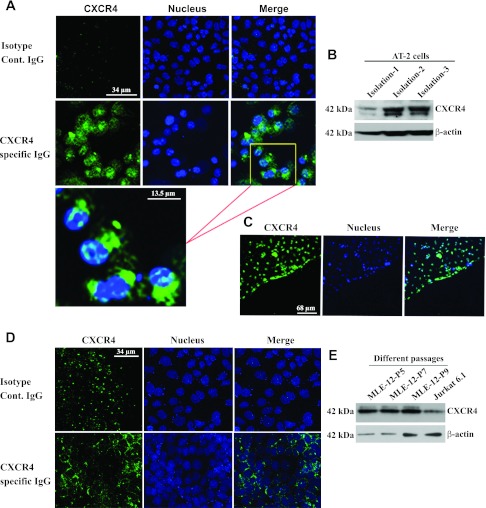
To demonstrate the expression of CXCR4 in ATII cells and MLE12 cells, we used immunofluorescence (Fig. 2, A, C, and D). These images show clear expression of CXCR4 in freshly isolated ATII cells (Fig. 2, A and C) and in confluent cultures of MLE12 cells (Fig. 2D). Figure 2C shows CXCR4 expression at the leading edge of wounded ATII cells. To further support these findings, Western blots from total cell lysates demonstrate CXCR4 expression in ATII cells from three different isolations (Fig. 2B) and in MLE12 cells from different passages (Fig. 2E).
Fig. 2.
ATII and MLE12 cells express CXCR4. Immunofluorescence shows expression of CXCR4 (green) in ATII (A and C) and MLE12 (D) cells, with nuclei indicated by DRAQ5 staining (blue). C: cells migrating at a wound edge. B and E: Western blots of CXCR4 from 3 different isolations of ATII cells (B) and 3 different passages of MLE12 cells (E). Lysate from Jurkat 6.1 cells was used as a positive control, and β-actin was used as loading control.
Blocking CXCR4 impeded wound healing in vitro.
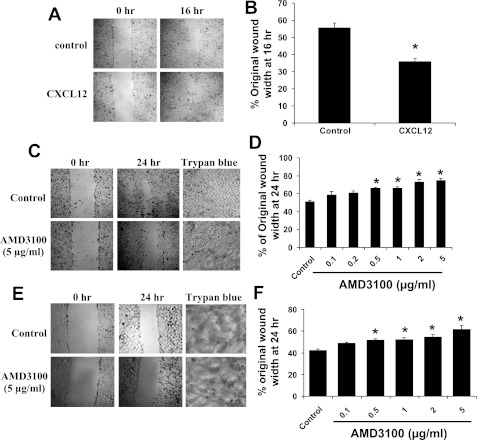
To determine whether CXCR4 has a functional role in alveolar epithelial repair mechanisms, we measured cell migration in response to CXCL12 (100 ng/ml) in a scratch-wound model. CXCL12 significantly increased wound closure by 16 h in ATII cells (Figs. 3, A and B). Next, we examined the effect of treatment with a specific blocker of CXCR4, AMD-3100, on wound closure. AMD-3100 inhibited wound closure of ATII (Fig. 3, C and D) and MLE12 (Fig. 3, E and F) cells in a dose-dependent manner. AMD-3100 at >0.5 μg/ml caused significant inhibition in both cell types. To confirm that the inhibition was not due to cell toxicity, we examined cell viability by Trypan blue exclusion. We found that cell viability was 93.8 and 94.2% in control ATII and MLE12 cells, respectively, while cell viability following 24 h of treatment with 5 μg/ml AMD-3100 was 94.5 and 93.9% (Fig. 3, C and E).
Fig. 3.
CXCL12 stimulated wound healing, and blocking of CXCR4 impeded wound healing. Scratch wounds were made on confluent monolayers of ATII (A–D) and MLE12 (E and F) cells, and cells were treated with CXCL12 (A and B) or AMD-3100 (C–F). Wound widths were measured after 16 or 24 h on 3 fields per well and averaged. A, C, and E: images of initial and final wounds; B, D, and F: data expressed as percentage of original wound width at 16 or 24 h. CXCL12 (100 ng/ml) stimulated wound closure in ATII cells. Treatment with AMD-3100 caused a dose-dependent inhibition of wound closure in ATII and MLE12 cells. Values are means ± SE (n = 6). *P < 0.05 vs. untreated control. Images of untreated cells and cells treated with 5 μg/ml AMD-3100 for 24 h and stained with Trypan blue (C and E) indicate no effect on cell viability.
Knockdown of CXCR4 with shRNA attenuated wound healing.
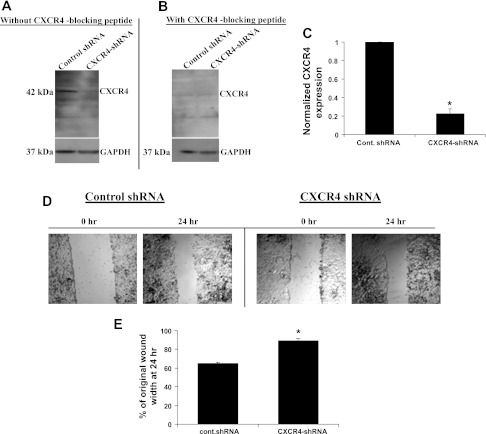
To further investigate the role of CXCR4 in cell migration, we developed a stable MLE12 cell line expressing shRNA against CXCR4 (CXCR4-shRNA cells). Figure 4 shows that expression of CXCR4 was significantly decreased in these cells by ∼80% compared with cells expressing a scrambled shRNA. Figure 4A is a representative immunoblot of CXCR4 from control and CXCR4-shRNA cells, and Fig. 4B demonstrates the specificity of the CXCR4 immunoblot with a CXCR4 blocking peptide. The knockdown shown in Fig. 4C remained consistent in consecutive passages. Figure 4, D and E, shows that wound closure was significantly inhibited in CXCR4-shRNA cells compared with control cells.
Fig. 4.
Knockdown of CXCR4 with short hairpin RNA (shRNA) attenuated wound healing. CXCR4 was knocked down in MLE12 cells using shRNA. A and C: decreased CXCR4 expression in CXCR4-shRNA compared with control shRNA MLE12 cell line and densitometry summarized from 5 sets of blots. GAPDH was used as a loading control. B: specificity of immunoblot for CXCR4 with addition of a CXCR4 blocking peptide. D: initial and final images of wounds in control shRNA and CXCR4-shRNA cells. E: wound closure at 24 h. Values are means ± SE (n = 6). *P < 0.05 vs. control shRNA.
CXCR4 regulates MMP-2, MMP-14, and tissue inhibitor of metalloproteinase-3.
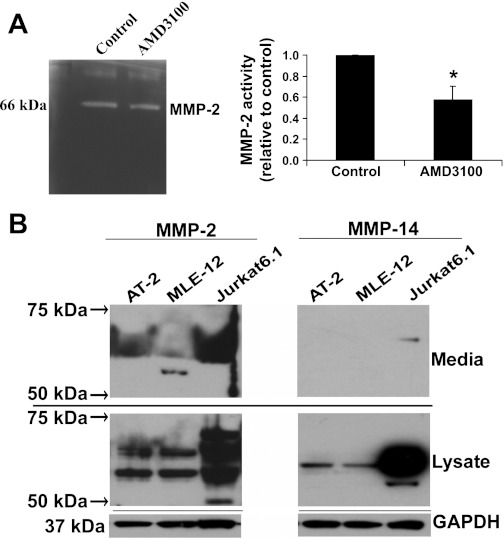
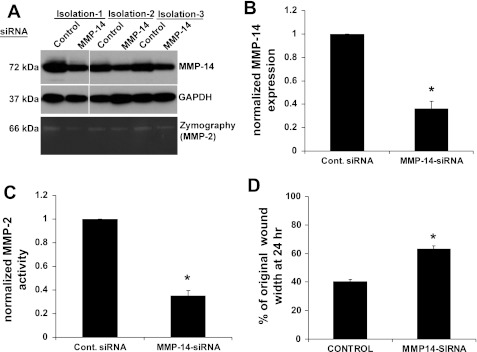
MMPs are important regulators of the ECM and are typically activated during epithelial repair processes (59). CXCR4 activation has been previously linked to the regulation of MMPs (49). To determine whether inhibition of CXCR4 affected activity of MMPs, we performed zymography experiments using ATII cells and shRNA cell lines. Figure 5A shows that blocking of CXCR4 with AMD-3100 reduced the activity of an MMP at 66 kDa in the conditioned medium from ATII cells. Using Western blotting, we screened cell lysate and supernatant samples for MMP-1, MMP-2, MMP-3, MMP-9, MMP-11, MMP-12, MMP-13, MMP-14, and MMP-21. We found immunoreactivity to MMP-2 in the conditioned media and cell lysates of ATII and MLE12 cells at ∼60–72 kDa, which encompasses the active (∼62–66 kDa) and pro (∼72 kDa) forms of MMP-2 (Fig. 5B). We also found immunoreactivity to MMP-14 in the lysate of cells at ∼60–72 kDa, which would include pro-MMP-14, but little immunoreactivity was found in the conditioned medium in the expected range for pro-MMP-14 (∼66 kDa) or active MMP-14 (∼54 kDa). MMP-14 is known to cleave pro-MMP-2 to active MMP-2. To determine whether decreased expression of MMP-14 would cause reduced MMP-2 activity, we used siRNA against MMP-14 in rat ATII cells. As shown in Fig. 6, A and B, expression of MMP-14 was decreased by ∼60%, and this led to a significant decrease in MMP-2 activity in the conditioned medium (Fig. 6, A and C). Furthermore, decreased expression of MMP-14 caused a significant inhibition of wound closure (Fig. 6D).
Fig. 5.
CXCR4 regulates matrix metalloproteinase (MMP)-2 and MMP-14 in ATII and MLE12 cells. A: zymography from conditioned media collected from untreated ATII cells and cells treated with 2 μg/ml AMD-3100 for 24 h. Densitometry from 3 experiments was normalized to untreated control. B: conditioned media and cell lysates from ATII and MLE12 cells were run for PAGE in 4–12% gradient Bis-Tris gels, and immunoblot was performed using different antibodies of MMPs. Of the MMPs screened (MMP-1, MMP-2, MMP-3, MMP-9, MMP-11, MMP-12, MMP-13, MMP-14, and MMP-21), only MMP-2 and MMP-14 showed strong reactivity. GAPDH was used as a loading control for cell lysates. Medium was conditioned by cells seeded at equal densities and with an equivalent volume. Jurkat 6.1 cells were used as a positive control for MMP-2 and MMP-14.
Fig. 6.
Knockdown of MMP-14 caused decreased MMP-2 activity and inhibited wound closure. MMP-14 was knocked down in rat ATII cells using small interfering RNA (siRNA). A and B: siRNA caused significantly decreased expression of MMP-14 in 3 different isolations. A and C: MMP-2 activity was significantly decreased by siRNA against MMP-14. D: MMP-14 knockdown caused a significant decrease in wound closure. Values are means ± SE (n = 3). A scrambled siRNA sequence was used as a control for all studies. *P < 0.05 vs. control siRNA.
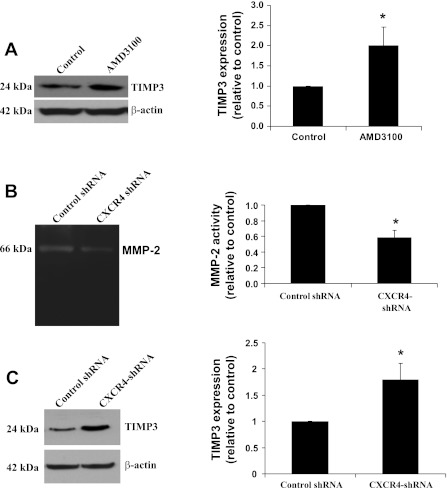
Previous reports suggest that MMP-2 and MMP-14 activation can be regulated by tissue inhibitor of metalloproteinase-3 (TIMP-3) (5, 22, 25). Therefore, we assayed the expression of TIMP-3 in control and AMD-3100-treated ATII cells. Inhibition of CXCR4 caused a significant increase in TIMP-3 expression (Fig. 7A). When we examined the basal activity of MMP-2 in control MLE12 and CXCR4-shRNA cell lines, we found a significant reduction in MMP-2 activity in the CXCR4-shRNA cells (Fig. 7B). Similarly, TIMP-3 expression was significantly increased in CXCR4-shRNA cells compared with control cells (Fig. 7C).
Fig. 7.
CXCR4 regulates tissue inhibitor of metalloproteinase (TIMP)-3 expression and MMP-2 activity in ATII and MLE12 cells. A: expression of TIMP-3 in ATII cells in the presence and absence of AMD-3100 and densitometric analysis. β-Actin was used as loading control. B: zymography and densitometric analysis from control shRNA and CXCR4-shRNA MLE12 cells. C: TIMP-3 expression in control shRNA and CXCR4-shRNA MLE12 cells. Values are means ± SE (n = 3). *P < 0.05 vs. control.
CXCL12 activates the small GTPase Rac1.
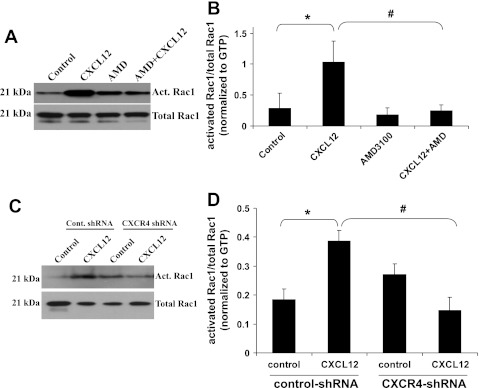
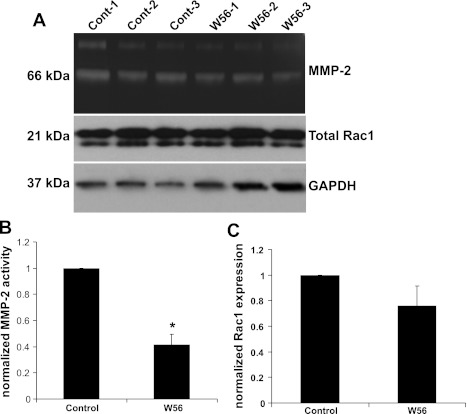
Rac1 was previously reported to regulate actin polymerization and cell migration in response to CXCL12 (23). After 2 min of treatment with CXCL12, we found that Rac1 activity was significantly increased in ATII cells, and AMD-3100 blocked this increase (Fig. 8, A and B). Treatment with CXCL12 also increased Rac1 activity in control shRNA cells, but this did not occur in CXCR4-shRNA (Fig. 8, C and D). To determine whether changes in Rac1 activity would affect MMP-2 activity, we treated MLE12 cells with the Rac1 inhibitor W56 and performed zymography using the conditioned medium. MMP-2 activity was significantly decreased in the cells treated with Rac1 inhibitor (Fig. 9B), but there was no change in total Rac1 levels (Fig. 9C).
Fig. 8.
CXCL12 activates the small GTPase Rac1. ATII (A and B) and MLE12 (C and D) cells were treated with or without CXCL12 (100 ng/ml) for 2 min in the presence or absence of AMD-3100 (AMD, 2 μg/ml for 2 h prior to CXCL12). Cell lysates were collected, and Rac1 activity was assayed. Densitometry was performed and values were normalized by dividing the level of activated (Act) Rac1 by the total Rac1 level and then normalizing to GTP control level. Values are means ± SE; n = 3 (A) and 4 (B). *P < 0.05 vs. control cells. #P < 0.05 vs. CXCL12-treated cells.
Fig. 9.
Inhibition of Rac1 activity decreased MMP-2 activity. MLE12 cells were treated with or without W56 (50 μM) for 24 h, and conditioned media were collected for zymography (A and B) and cell lysates were collected for measurement of total Rac1 (C). Densitometric analysis shows that inhibition of Rac1 caused decreased activity of MMP-2, but total Rac1 levels were unchanged. Values are means ± SE; n = 3. *P < 0.05 vs. control.
CXCR4 regulates actin structure at the wound edge.
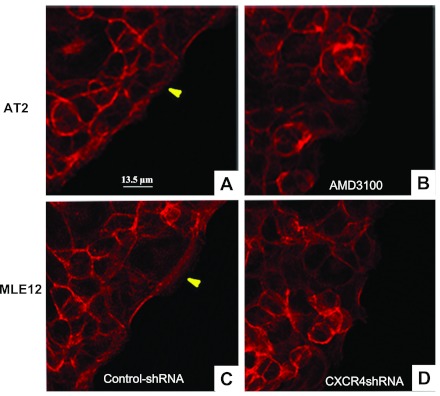
To investigate the role of CXCR4 on actin structure in migrating cells at the leading edge of a wound, we visualized actin using Alexa Fluor 594-labeled phalloidin on a confocal microscope. As we showed previously, a prominent band of actin forms at the leading edge of migrating cells (Fig. 10, A and C). When ATII cells were treated with AMD-3100, this band was lost (Fig. 10B). This band was also less prominent in CXCR4-shRNA cells (Fig. 10D).
Fig. 10.
CXCR4 regulates actin structure at the wound edge. ATII (A and B) and control shRNA MLE12 (C) and CXCR4-shRNA (D) cells were stained with Alexa Fluor 594-labeled phalloidin 12 h after wounding. A and C: prominent actin bands (arrowheads) in untreated ATII cells and control shRNA MLE12 cells, respectively. B: CXCR4 inhibition with AMD-3100 (2 μg/ml) caused reduced organization of the actin band at the leading edge. D: a similar effect by reduced expression of CXCR4 in CXCR4-shRNA cells.
DISCUSSION
ATII cells have stem cell-like characteristics that facilitate the repair of the alveolar epithelium following injury (1, 14, 36, 51). One of the most important events in the early stages of epithelial repair is the migration of ATII cells to cover the denuded area, which is followed by later proliferation and differentiation. Bone marrow-derived hematopoietic stem cells also contribute to the repair of injured lungs, and these cells are recruited to the site of injury by chemokines such as CXCL12 (11, 27, 52, 53, 60). While the role of chemokine signaling is well recognized in the recruitment of stem cells and immune cells, the role of CXCL12/CXCR4 signaling in the migration of ATII cells has not been previously shown. We showed for the first time that CXCL12 secretion was increased in wounded cells in culture and in the BAL of rats ventilated with a high tidal volume (Fig. 1). We then confirmed that primary cultures of rat ATII cells and MLE12 cells express CXCR4 (Fig. 2). We showed that CXCL12 stimulated ATII cell migration and that inhibition or knockdown of CXCR4 caused decreased migration of ATII and MLE12 cells, respectively (Figs. 3 and 4). Our shRNA data demonstrated that the knockdown level of CXCR4 was ∼80%, but wound healing was inhibited by ∼30%. Thus the magnitude of downmodulation of CXCR4 by shRNA did not translate functionally to loss of cell migration, suggesting that other CXCR4-independent pathways are involved in wound repair. It is also possible that proliferation accounted for a significant portion of wound closure, which may not have been affected by CXCR4.
To investigate the mechanisms by which CXCR4 stimulated migration, we used zymography to determine whether MMP activity was altered by CXCR4 inhibition or knockdown. Secretion and activation of MMPs are important components of cell migration, as MMPs digest the ECM and promote a path for migrating cells. Interestingly, our data revealed that inhibition or knockdown of CXCR4 caused decreased expression of MMP-14 and decreased activity of MMP-2 (Figs. 5 and 7). MMP-2 and MMP-14 (also known as MT1-MMP) have important roles in lung repair and development, as activation of MMP-2 by MMP-14 has been shown in lung cells, and knockout mice exhibit abnormal airway and alveolar structures (3, 29, 31, 41). MMP-14 function was recently linked to CXCR4 activity during metastasis of melanoma cells to the lung (6), and independent and coordinated roles for these molecules were identified. Our results suggest that loss of function of CXCR4 in ATII cells decreased the secretion of active MMP-2, but we did not determine whether inhibition or downmodulation of MMP-2 would affect CXCR4. We did show that knockdown of MMP-14 caused decreased MMP-2 activity and inhibited wound closure (Fig. 6).
In conjunction with decreased CXCR4 activity, we also observed an increase in TIMP-3 expression (Fig. 7). TIMP-3 is a strong regulator of several MMPs, including MMP-14, MMP-2, and MMP-9 (5, 22, 25). Unlike TIMP-1 and TIMP-2, TIMP-3 remains adherent to the ECM and accelerates cellular transformation and proliferation (61). TIMP-3 plays an important role in the regulation of airway branching (26). Our data demonstrated a significant increase of TIMP-3 when CXCR4 was inhibited or downregulated, suggesting that CXCR4 caused loss of MMP-14 expression and MMP-2 activity through increased TIMP-3 expression.
A limitation of our study is that we did not evaluate the effect of CXCL12/CXCR4 on proliferation during wound closure. We previously demonstrated that ∼13% of the cells near the wound edge of cultured ATII cells had incorporated ethidium deoxyuridine (as an index of proliferation) by 24 h compared with ∼4% of unwounded cells (13). Thus we cannot rule out the possibility that CXCL12/CXCR4 may also affect cell proliferation. Since ATII cells spread, migrate, proliferate, and differentiate into type I cells in vivo, our model is limited, and the effects of CXCL12/CXCR4 on alveolar repair should be assessed in vivo. Our model also does not incorporate the complex architecture of the alveolus, in which ATII cells do not exist as a flat monolayer of cells.
Epithelial cell migration is associated with dramatic actin remodeling and cytoskeletal rearrangement. We previously identified that RhoA and Rac1 are important regulators of cell migration in bronchial and alveolar epithelial cells (15–17). Since CXCR4 activation has been shown to cause induction of Rac1 activity in lymphocytes (4, 23), we showed that Rac1 was stimulated by CXCL12 and that this could be blocked by AMD-3100 or CXCR4 knockdown (Fig. 8). As discussed above, Bartolome et al. (6) showed that migration of melanoma cells was dependent on functional interactions between CXCR4 and MMP-14, and they also showed that this involved Rac1. We found that inhibition of Rac1 caused decreased MMP-2 activity without affecting the overall expression of Rac1 (Fig. 9). CXCL12-mediated stimulation of intestinal epithelial cell migration was found to involve ERK1/2 MAP kinase and phosphatidylinositol 3-kinase signaling and increased F-actin accumulation at the leading edge of wounded monolayers (38, 50). We showed that inhibition or knockdown of CXCR4 inhibited the formation of a thick actin band at the leading edge of migrating cells (Fig. 10). Our results suggest that CXCR4 activation following wounding causes a Rac1-dependent increase in MMP-2 activity. Mice deficient in Rac2 expression exhibit decreased levels of MMP-2 and MMP-9 (21), and treatment of mice with a Rac1/Rac2 inactivator (8-oxo-2′-deoxyguanosine) caused diminished activity of MMP-2 and MMP-9 in lungs (32). Our results elucidate a novel role of the chemokine receptor CXCR4 in ATII cell migration and show that CXCR4 regulates TIMP-3 and MMP-14 expression, Rac1-dependent MMP-2 activation, and actin remodeling.
GRANTS
These studies were supported by National Heart, Lung, and Blood Institute Grant HL-094366 (C. M. Waters).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
M.C.G. and C.M.W. are responsible for conception and design of the research; M.C.G., P.S.M., and V.K.G. performed the experiments; M.C.G. and C.M.W. analyzed the data; M.C.G., P.S.M., V.K.G., S.E.S., and C.M.W. interpreted the results of the experiments; M.C.G. and C.M.W. prepared the figures; M.C.G. and C.M.W. drafted the manuscript; M.C.G., P.S.M., S.E.S., and C.M.W. edited and revised the manuscript; M.C.G. and C.M.W. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We are grateful to Charlean Luellen for technical assistance.
REFERENCES
- 1. Adamson IY, Hedgecock C, Bowden DH. Epithelial cell-fibroblast interactions in lung injury and repair. Am J Pathol 137: 385–392, 1990 [PMC free article] [PubMed] [Google Scholar]
- 2. Atabai K, Ishigaki M, Geiser T, Ueki I, Matthay MA, Ware LB. Keratinocyte growth factor can enhance alveolar epithelial repair by nonmitogenic mechanisms. Am J Physiol Lung Cell Mol Physiol 283: L163–L169, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Atkinson JJ, Holmbeck K, Yamada S, Birkedal-Hansen H, Parks WC, Senior RM. Membrane-type 1 matrix metalloproteinase is required for normal alveolar development. Dev Dyn 232: 1079–1090, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Azab AK, Azab F, Blotta S, Pitsillides CM, Thompson B, Runnels JM, Roccaro AM, Ngo HT, Melhem MR, Sacco A, Jia X, Anderson KC, Lin CP, Rollins BJ, Ghobrial IM. RhoA and Rac1 GTPases play major and differential roles in stromal cell-derived factor-1-induced cell adhesion and chemotaxis in multiple myeloma. Blood 114: 619–629, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci 115: 3719–3727, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Bartolome RA, Ferreiro S, Miquilena-Colina ME, Martinez-Prats L, Soto-Montenegro ML, Garcia-Bernal D, Vaquero JJ, Agami R, Delgado R, Desco M, Sanchez-Mateos P, Teixido J. The chemokine receptor CXCR4 and the metalloproteinase MT1-MMP are mutually required during melanoma metastasis to lungs. Am J Pathol 174: 602–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beck LA, Tancowny B, Brummet ME, Asaki SY, Curry SL, Penno MB, Foster M, Bahl A, Stellato C. Functional analysis of the chemokine receptor CCR3 on airway epithelial cells. J Immunol 177: 3344–3354, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Bisset LR, Schmid-Grendelmeier P. Chemokines and their receptors in the pathogenesis of allergic asthma: progress and perspective. Curr Opin Pulm Med 11: 35–42, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 10. Brun-Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, Bion J, Romand JA, Villar J, Thorsteinsson A, Damas P, Armaganidis A, Lemaire F. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med 30: 51–61, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Chen WC, Tzeng YS, Li H, Tien WS, Tsai YC. Lung defects in neonatal and adult stromal-derived factor-1 conditional knockout mice. Cell Tissue Res 342: 75–85, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Christensen PJ, Du M, Moore B, Morris S, Toews GB, Paine R., 3rd Expression and functional implications of CCR2 expression on murine alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 286: L68–L72, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Crosby LM, Luellen CL, Zhang Z, Tague LL, Sinclair SE, Waters CM. The balance of life and death in alveolar epithelial type II cells: proliferation, apoptosis and the effects of cyclic stretch on wound healing. Am J Physiol Lung Cell Mol Physiol 301: L536–L546, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol 298: L715–L731, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Desai LP, Aryal AM, Ceacareanu B, Hassid A, Waters CM. RhoA and Rac1 are both required for efficient wound closure of airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 287: L1134–L1144, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Desai LP, Chapman KE, Waters CM. Mechanical stretch decreases migration of alveolar epithelial cells through mechanisms involving Rac1 and Tiam1. Am J Physiol Lung Cell Mol Physiol 295: L958–L965, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Desai LP, Sinclair SE, Chapman KE, Hassid A, Waters CM. High tidal volume mechanical ventilation with hyperoxia alters alveolar type II cell adhesion. Am J Physiol Lung Cell Mol Physiol 293: L769–L778, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Desai LP, White SR, Waters CM. Mechanical stretch decreases FAK phosphorylation and reduces cell migration through loss of JIP3-induced JNK phosphorylation in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 297: L520–L529, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dobbs LG. Isolation and culture of alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 258: L134–L147, 1990 [DOI] [PubMed] [Google Scholar]
- 20. Donzella GA, Schols D, Lin SW, Este JA, Nagashima KA, Maddon PJ, Allaway GP, Sakmar TP, Henson G, De Clercq E, Moore JP. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med 4: 72–77, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Dooley JL, Abdel-Latif D, St Laurent CD, Puttagunta L, Befus D, Lacy P. Regulation of inflammation by Rac2 in immune complex-mediated acute lung injury. Am J Physiol Lung Cell Mol Physiol 297: L1091–L1102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. English JL, Kassiri Z, Koskivirta I, Atkinson SJ, Di Grappa M, Soloway PD, Nagase H, Vuorio E, Murphy G, Khokha R. Individual Timp deficiencies differentially impact pro-MMP-2 activation. J Biol Chem 281: 10337–10346, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Freret M, Gouel F, Buquet C, Legrand E, Vannier JP, Vasse M, Dubus I. Rac-1 GTPase controls the capacity of human leukaemic lymphoblasts to migrate on fibronectin in response to SDF-1α (CXCL12). Leuk Res 35: 971–973, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Geiser T, Jarreau PH, Atabai K, Matthay MA. Interleukin-1β augments in vitro alveolar epithelial repair. Am J Physiol Lung Cell Mol Physiol 279: L1184–L1190, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Gill SE, Pape MC, Leco KJ. Absence of tissue inhibitor of metalloproteinases 3 disrupts alveologenesis in the mouse. Dev Growth Differ 51: 17–24, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Gill SE, Pape MC, Leco KJ. Tissue inhibitor of metalloproteinases 3 regulates extracellular matrix-cell signaling during bronchiole branching morphogenesis. Dev Biol 298: 540–554, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Gomperts BN, Strieter RM. Fibrocytes in lung disease. J Leukoc Biol 82: 449–456, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Gropper MA, Wiener-Kronish J. The epithelium in acute lung injury/acute respiratory distress syndrome. Curr Opin Crit Care 14: 11–15, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Henderson N, Markwick LJ, Elshaw SR, Freyer AM, Knox AJ, Johnson SR. Collagen I and thrombin activate MMP-2 by MMP-14-dependent and -independent pathways: implications for airway smooth muscle migration. Am J Physiol Lung Cell Mol Physiol 292: L1030–L1038, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Ikeda K, Clark JC, Bachurski CJ, Wikenheiser KA, Cuppoletti J, Mohanti S, Morris RE, Whitsett JA. Immortalization of subpopulations of respiratory epithelial cells from transgenic mice bearing SV40 large T antigen. Am J Physiol Lung Cell Mol Physiol 267: L309–L317, 1994 [DOI] [PubMed] [Google Scholar]
- 31. Kheradmand F, Rishi K, Werb Z. Signaling through the EGF receptor controls lung morphogenesis in part by regulating MT1-MMP-mediated activation of gelatinase A/MMP2. J Cell Sci 115: 839–848, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim JS, Kim DY, Lee JK, Ro JY, Chung MH. 8-Oxo-2′-deoxyguanosine suppresses allergy-induced lung tissue remodeling in mice. Eur J Pharmacol 651: 218–226, 2011 [DOI] [PubMed] [Google Scholar]
- 33. Leslie CC, McCormick-Shannon K, Shannon JM, Garrick B, Damm D, Abraham JA, Mason RJ. Heparin-binding EGF-like growth factor is a mitogen for rat alveolar type II cells. Am J Respir Cell Mol Biol 16: 379–387, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Lesur O, Brisebois M, Thibodeau A, Chagnon F, Lane D, Fullop T. Role of IFN-γ and IL-2 in rat lung epithelial cell migration and apoptosis after oxidant injury. Am J Physiol Lung Cell Mol Physiol 286: L4–L14, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Makena PS, Luellen CL, Balazs L, Ghosh MC, Parthasarathi K, Waters CM, Sinclair SE. Preexposure to hyperoxia causes increased lung injury and epithelial apoptosis in mice ventilated with high tidal volumes. Am J Physiol Lung Cell Mol Physiol 299: L711–L719, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mason RJ. Biology of alveolar type II cells. Respirology 11 Suppl: S12–S15, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Mehrad B, Keane MP, Gomperts BN, Strieter RM. Circulating progenitor cells in chronic lung disease. Expert Rev Respir Med 1: 157–165, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moyer RA, Wendt MK, Johanesen PA, Turner JR, Dwinell MB. Rho activation regulates CXCL12 chemokine stimulated actin rearrangement and restitution in model intestinal epithelia. Lab Invest 87: 807–817, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Murdoch C, Monk PN, Finn A. Functional expression of chemokine receptor CXCR4 on human epithelial cells. Immunology 98: 36–41, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 382: 635–638, 1996 [DOI] [PubMed] [Google Scholar]
- 41. Oblander SA, Zhou Z, Galvez BG, Starcher B, Shannon JM, Durbeej M, Arroyo AG, Tryggvason K, Apte SS. Distinctive functions of membrane type 1 matrix-metalloprotease (MT1-MMP or MMP-14) in lung and submandibular gland development are independent of its role in pro-MMP-2 activation. Dev Biol 277: 255–269, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Panina P, Mariani M, D'Ambrosio D. Chemokine receptors in chronic obstructive pulmonary disease (COPD). Curr Drug Targets 7: 669–674, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Panos RJ, Bak PM, Simonet WS, Rubin JS, Smith LJ. Intratracheal instillation of keratinocyte growth factor decreases hyperoxia-induced mortality in rats. J Clin Invest 96: 2026–2033, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Panos RJ, Patel R, Bak PM. Intratracheal administration of hepatocyte growth factor/scatter factor stimulates rat alveolar type II cell proliferation in vivo. Am J Respir Cell Mol Biol 15: 574–581, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Pardo A, Ridge K, Uhal B, Sznajder JI, Selman M. Lung alveolar epithelial cells synthesize interstitial collagenase and gelatinases A and B in vitro. Int J Biochem Cell Biol 29: 901–910, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Pechkovsky DV, Goldmann T, Ludwig C, Prasse A, Vollmer E, Muller-Quernheim J, Zissel G. CCR2 and CXCR3 agonistic chemokines are differently expressed and regulated in human alveolar epithelial cells type II. Respir Res 6: 75, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Razzaque MS, Taguchi T. Pulmonary fibrosis: cellular and molecular events. Pathol Int 53: 133–145, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc 3: 364–372, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Singh S, Singh UP, Grizzle WE, Lillard JW., Jr CXCL12-CXCR4 interactions modulate prostate cancer cell migration, metalloproteinase expression and invasion. Lab Invest 84: 1666–1676, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Smith JM, Johanesen PA, Wendt MK, Binion DG, Dwinell MB. CXCL12 activation of CXCR4 regulates mucosal host defense through stimulation of epithelial cell migration and promotion of intestinal barrier integrity. Am J Physiol Gastrointest Liver Physiol 288: G316–G326, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Smith LJ, Kaplan NB, Brody J. Response of normal and beige mouse alveolar type 2 cells to lung injury. Am Rev Respir Dis 122: 947–957, 1980 [DOI] [PubMed] [Google Scholar]
- 52. Song JS, Kang CM, Kang HH, Yoon HK, Kim YK, Kim KH, Moon HS, Park SH. Inhibitory effect of CXC chemokine receptor 4 antagonist AMD3100 on bleomycin induced murine pulmonary fibrosis. Exp Mol Med 42: 465–472, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Strieter RM, Keeley EC, Hughes MA, Burdick MD, Mehrad B. The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of pulmonary fibrosis. J Leukoc Biol 86: 1111–1118, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sugahara K, Tokumine J, Teruya K, Oshiro T. Alveolar epithelial cells: differentiation and lung injury. Respirology 11 Suppl: S28–S31, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 393: 591–594, 1998 [DOI] [PubMed] [Google Scholar]
- 56. Upadhyay D, Correa-Meyer E, Sznajder JI, Kamp DW. FGF-10 prevents mechanical stretch-induced alveolar epithelial cell DNA damage via MAPK activation. Am J Physiol Lung Cell Mol Physiol 284: L350–L359, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Vanderbilt JN, Mager EM, Allen L, Sawa T, Wiener-Kronish J, Gonzalez R, Dobbs LG. CXC chemokines and their receptors are expressed in type II cells and upregulated following lung injury. Am J Respir Cell Mol Biol 29: 661–668, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Vincent JL, Sakr Y, Ranieri VM. Epidemiology and outcome of acute respiratory failure in intensive care unit patients. Crit Care Med 31: S296–S299, 2003 [DOI] [PubMed] [Google Scholar]
- 59. Werb Z, Chin JR. Extracellular matrix remodeling during morphogenesis. Ann NY Acad Sci 857: 110–118, 1998 [DOI] [PubMed] [Google Scholar]
- 60. Xu J, Mora A, Shim H, Stecenko A, Brigham KL, Rojas M. Role of the SDF-1/CXCR4 axis in the pathogenesis of lung injury and fibrosis. Am J Respir Cell Mol Biol 37: 291–299, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yang TT, Hawkes SP. Role of the 21-kDa protein TIMP-3 in oncogenic transformation of cultured chicken embryo fibroblasts. Proc Natl Acad Sci USA 89: 10676–10680, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest 133: 1120–1127, 2008 [DOI] [PubMed] [Google Scholar]