Abstract
Most patients with acute lung injury (ALI) and acute respiratory distress syndrome of septic and nonseptic nature require assisted ventilation with positive pressure, which at suboptimal range may further exacerbate lung dysfunction. Previous studies described enhancement of agonist-induced Rho GTPase signaling and endothelial cell (EC) permeability in EC cultures exposed to pathologically relevant cyclic stretch (CS) magnitudes. This study examined a role of pathologic CS in modulation of pulmonary EC permeability caused by IL-6, a cytokine increased in sepsis and acting in a Rho-independent manner. IL-6 increased EC permeability, which was associated with activation of Jak/signal transducers and activators of transcription, p38 MAP kinase, and NF-κB signaling and was augmented by EC exposure to 18% CS. Rho kinase inhibitor Y-27632 suppressed the synergistic effect of 18% CS on IL-6-induced EC monolayer disruption but did not alter the IL-6 effects on static EC culture. 18% CS also increased IL-6-induced ICAM-1 expression by pulmonary EC and neutrophil adhesion, which was attenuated by Y-27632. Intratracheal IL-6 administration in C57BL/6J mice increased protein content and cell count in bronchoalveolar lavage fluid. These changes were augmented by high tidal volume mechanical ventilation (HTV; 30 ml/kg, 4 h). Intravenous injection of Y-27632 suppressed IL6/HTV-induced lung injury. In conclusion, this study proposes a novel mechanism contributing to two-hit model of ALI: in addition to synergistic effects on Rho-dependent endothelial hyper-permeability triggered by thrombin, TNFα, LPS, or other agonists, ventilator-induced lung injury-relevant CS may also exacerbate Rho-independent mechanisms of EC permeability induced by other inflammatory mediators such as IL-6 via mechanisms involving Rho activity.
Keywords: cyclic stretch, actin, interleukin-6, vascular permeability, endothelium
ventilator support is an indispensable treatment for critically ill patients. However, suboptimal regimen of mechanical ventilation leading to ventilator-induced lung injury (VILI), multiorgan dysfunction, and high rates of morbidity and mortality remain one of the most important problems in the management of patients with preexisting respiratory complications in the intensive care unit (31, 34).
Pathologic mechanical forces leading to VILI trigger several signaling mechanisms including activation of signaling kinases, ion channels, small GTPases, second messengers, inflammatory signaling, and gene expression (14, 16, 39). Increased vascular endothelial permeability (2), inflammatory cytokine production (41), and apoptosis (18) induced by pathological mechanical stimulation causes alveolar flooding, leukocyte infiltration, and hypoxemia leading to morbidity and mortality (26, 34). Because edemagenic and inflammatory mediators appear in pulmonary circulation, both in the course of septic inflammation (12) and suboptimal mechanical ventilation (11), two-hit animal models, which combine administration of proinflammatory agents and mechanical ventilation at high tidal volume (HTV), more appropriately reflect the common comorbidities and risk factors present in patients with acute lung injury (ALIU; Ref. 26). Also, the study of a combination of animal models and pulmonary cells exposed to pathologic mechanical stimulation and inflammatory agonists may provide vital information about molecular mechanisms regulating lung endothelial or epithelial permeability in VILI patients.
We (2) have previously described enhancement of agonist-induced endothelial cell (EC) barrier disruption by VILI-relevant magnitude of cyclic stretch (CS). Further studies identified a Rho-dependent mechanism of synergistic effects by pathologic CS and thrombin on the lung EC barrier disruption (4, 8) and demonstrated a beneficial effect of inhibition of Rho signaling on HTV-induced pulmonary vascular permeability and barrier recovery in the in vitro and in vivo models of VILI (8, 29).
Interleukin-6 (IL-6) is a well-recognized inflammatory mediator upregulated in ALI/acute respiratory distress syndrome patients and in the animal models of septic and ventilator-induced lung injury (35, 36). The IL-6 receptor system is distinct from the Toll-like receptor family and consists of two polypeptide chains: an 80-kDa IL-6 receptor (IL-6R) and a 130-kDa signal transducer (gp130). IL-6R also exists in the transmembrane and a soluble form (23). The soluble IL-6R can form a stimulatory complex with IL-6, which associates with the gp130 coreceptor on the EC membrane surface and triggers activation of JAK-Jak/signal transducers and activators of transcription (Stat)-mediated transcription, phosphatidylinositol 3-kinase (PI3-kinase)/AKT cascade, and MAP kinase pathways (19). The signaling pathways activated in pulmonary endothelium by IL-6 are not clearly understood, and the precise mechanisms of IL-6-mediated EC barrier dysfunction remain to be elucidated. Unlike other mediators of ALI such as lipopolysaccharide (LPS), thrombin, and TNFα, IL-6 has not been shown to directly activate Rho signaling. In the current study, we utilized in vitro and in vivo models of VILI to test the interplay between Rho-dependent and Rho-independent mechanisms of EC permeability and lung barrier dysfunction induced by IL-6 and pathologic mechanical stimulation.
MATERIALS AND METHODS
Cell culture and reagents.
Human pulmonary artery endothelial cells (HPAEC) and cell culture basal medium with growth supplements were obtained from Lonza (Allendale, NJ), cultured according to the manufacturer's protocol, and used at passages 5–8. Mouse and human IL-6 and human IL-6 soluble receptor were obtained from R&D Systems (Minneapolis, MN). Di-phospho-myosin light chain (MLC), phospho-heat shock protein (HSP)27, phospho-Stat3, and IκBα antibodies were from Cell Signaling (Beverly, MA); ICAM-1 antibody was from Santa Cruz Biotechnology (Santa Cruz, CA); vascular endothelin (VE)-cadherin antibody was from BD Transduction Laboratories (San Diego, CA); phospho-myosin-associated phosphatase type 1 (MYPT1) antibody was purchased from Millipore (Billerica, MA); and Jak I inhibitor and Y-27632 were from EDM (La Jolla, CA). All reagents for immunofluorescence were purchased from Molecular Probes (Eugene, OR). Unless specified, biochemical reagents were obtained from Sigma (St. Louis, MO).
Cell culture under CS.
CS experiments were performed using FX-4000T Flexcell Tension Plus system (Flexcell International, McKeesport, PA) equipped with a 25-mm BioFlex loading station, as previously described (2). In brief, untreated EC or cells after siRNA transfection were exposed to high magnitude CS (18% distension, sinusoidal wave, and 25 cycles/min) to recapitulate a HTV mechanical ventilation regimen. At 2 h, plates were treated with vehicle or IL-6 followed with continuous exposure to CS. Control BioFlex plates with static EC culture were placed in the same cell culture incubator and processed similarly to CS-preconditioned cells. At the end of experiment, cell lysates were collected for Western blot analysis, or CS-exposed endothelial monolayers were fixed with 3.7% formaldehyde and subjected to immunofluorescence staining as previously described (7).
Small interfering RNA transfection.
To reduce the content of endogenous RhoA, cells were treated with gene-specific small interfering (si)RNA duplexes. Predesigned standard purity siRNA sets (Homo sapiens) were ordered from Dharmacon (Lafayette, CO), and transfection of EC with siRNA was performed as previously described (30). After 48 h of transfection, cells were used for experiments or harvested for Western blot verification of specific protein depletion. Nonspecific, nontargeting siRNA (Dharmacon, Lafayette, CO) was used as a control treatment.
Immunofluorescence.
Endothelial monolayers plated on glass coverslips were subjected to double immunofluorescence staining with appropriate antibody, as described previously (7). Texas Red phalloidin was used to visualize F-actin. After being immunostained, slides were analyzed using a Nikon video imaging system (Nikon Instech, Tokyo, Japan). Images were processed with Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA) software.
Immunoblotting.
After stimulation, cells were lysed, and protein extracts were separated by SDS-PAGE, transferred to nitrocellulose membrane, and probed with specific antibodies as previously described (2).
Measurement of transendothelial electrical resistance.
The endothelial monolayer barrier properties were evaluated by the highly sensitive biophysical assay with an electrical cell-substrate impedance sensing system (Applied Biophysics, Troy, NY) that allows measurements of transendothelial electrical resistance (TER) in real time, which reflects agonist-induced EC permeability changes (8).
Neutrophil migration and adhesion assays.
Neutrophil chemotaxis was measured in a 96-well chemotaxis chamber (Neuroprobe, Gaithersburg, MD) as described previously (27). Briefly, freshly isolated neutrophils were placed in a 96-well chemotaxis chamber and incubated with 200 μl of preconditioned culture media that were collected from static EC cultures or from pulmonary EC grown on BioFlex and exposed to 4-h CS at 18% amplitude with or without 2-h pretreatment with IL-6/soluble receptor (SR; 40 ng/ml/10 ng/ml). Preliminary experiments have established the number of cells (4 × 104 cells) used allow the optimal cell migration without clogging the pores of Transwell filter of the upper chamber. Data were expressed as percentage of cell migration. Polymorphonuclear leukocyte (PMN) adhesion to the CS-preconditioned EC was assessed at the end of 2-h CS session by addition of the neutrophils freshly isolated from healthy donors to the EC monolayers grown in the sixwell BioFlex plates right after CS experiment. Neutrophil adhesion on HPAEC was assessed as described previously (28). Neutrophil adhesion data were expressed as a percentage of adhesion for all treated groups.
Cytokine analysis.
Concentrations of IL-8, keratinocyte-derived chemokine, and macrophage inflammatory protein-1α in control and treated bronchoalveolar lavage (BAL) fluid or cell conditioned medium samples were measured using an ELISA kit available from R&D Systems according to manufacturer's instructions.
Mechanical ventilation protocol.
All animal care and treatment procedures were approved by the University of Chicago Institutional Animal Care and Use Committee and were handled according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were randomized to concurrently receive sterile saline solution or IL-6 (5 mg/kg it, 16 h) followed by 4 h of mechanical ventilation with HTV (30 ml/kg) as previously described (6). Spontaneously breathing animals served as controls. In experiments with Rho inhibitor, mice were injected with Y-27632 (2 mg/kg iv) before IL-6 instillation. Bacterial LPS (0.63 mg/kg; Escherichia coli O55:B5) was injected in mice intratracheally, and parameters of lung injury were measured at 16 h after LPS challenge. BAL was performed using 1 ml of sterile Hank's balanced salt buffer and measurements of cell count and protein concentration were conducted as previously described (15). Measurements of Evans blue were performed as described elsewhere (6).
Statistical analysis.
Results are expressed as means ± SD. Experimental samples were compared with controls by unpaired Student's t-test. For multiple-group comparisons, a one-way ANOVA and post hoc multiple comparison tests were used. P < 0.05 was considered statistically significant.
RESULTS
IL6 increases permeability and activates inflammatory signaling in human pulmonary EC.
Effects of IL-6 on pulmonary EC permeability were monitored by measurements of TER. Treatment with either IL-6 or its SR alone did not significantly change basal resistance, while a combination of IL-6 and SR caused TER decline in a dose-dependent manner (data not shown), which reached maximal levels after 5 h of treatment (Fig. 1A). All following in vitro experiments were performed using IL-6 and SR at concentrations of 40 and 10 ng/ml, respectively.
Fig. 1.
Effects of IL-6 on endothelial permeability and inflammatory signaling. A: at the time indicated by arrow, pulmonary endothelial cell (EC) plated on microelectrodes were treated with either vehicle or a mixture of IL-6 and its soluble receptor (SR; 15 ng/ml/3.75 ng/ml; 30 ng/ml/7.5 ng/ml; 40 ng/ml/10 ng/ml; 60 ng/ml/15 ng/ml; 100 ng/ml/25 ng/ml) and used for measurements of transendothelial electrical resistance (TER). B: cells were treated with IL-6 and SR (40 ng/ml/10 ng/ml) for indicated periods of time. Levels of phosphorylated proteins or total IκBα content in the cell lysates were determined by Western blot analysis using specific antibodies. Equal protein loading was confirmed by determination of β-tubulin content in total cell lysates. Rearranged lanes from the same blot are outlined by vertical dotted line. Shown are representative results of 3–5 independent experiments. Stat, signal transducers and activators of transcription; HSP27, heat shock protein 27.
IL-6-induced EC permeability changes were associated with time-dependent phosphorylation of the regulatory molecule Stat3, which reflects activation of the canonical IL-6-mediated Jak/Stat pathway. IL-6 also induced degradation of the NF-κB complex inhibitory subunit IκBα and phosphorylation of p38 MAPK downstream target HSP27 (Fig. 1B). EC treatment with IL-6 over the duration of experiment did not change total Stat, p38 MAPK, and HSP27 protein levels (data not shown). These events indicate an activation of an inflammatory NF-κB cascade and p38 stress MAP kinase signaling by IL-6. To evaluate the role of Jak/Stat cascade in IL-6-induced EC barrier dysfunction, HPAEC were pretreated with a pharmacologic Jak inhibitor followed by analysis of EC barrier properties after IL-6 challenge. TER measurements demonstrate that Jak inhibition attenuated IL-6-induced hyperpermeability (Fig. 2A). Morphologic analysis of pulmonary endothelium revealed disruption of the EC monolayer after 5 h of IL-6 challenge, the time point corresponding to pronounced increase in EC monolayer permeability. IL-6 induced formation of paracellular gaps, reflecting EC monolayer barrier compromise. In contrast to EC barrier disruption induced by other inflammatory agonists such as TNFα, LPS, or transforming growth factor-β, the IL-6-induced gap formation was accompanied by only modest increase in a number of actin stress fibers. Analysis of adherens junction remodeling visualized by VE-cadherin staining revealed dramatic disruption of cell-cell contacts in response to IL-6 challenge, manifested by the disappearance of a continuous adherens junction pattern. The Jak inhibitor markedly attenuated IL-6-induced EC monolayer disruption (Fig. 2B). Biochemical studies showed that Jak inhibition suppressed IL-6-induced phosphorylation of Stat3 and attenuated IL-6-induced IκBα degradation and HSP27 phosphorylation, which suggests activation of NF-κB- and p38 MAPK signaling downstream of Jak/Stat (Fig. 2C).
Fig. 2.
Effects of Jak/Stat pathway inhibition on IL-6 effects in pulmonary EC. EC were pretreated with Jak I inhibitor (5 μM, 30 min) followed by IL-6 and SR (40 ng/ml/10 ng/ml) stimulation. A: TER measurements were performed over the time indicated. B: actin cytoskeletal remodeling (IL-6/SR, 40 ng/ml/10 ng/ml; 5 h) was examined by immunofluorescence staining with Texas red-conjugated phalloidin. Paracellular gaps are marked by arrows. Immunofluorescence staining for vascular endothelial (VE)-cadherin was performed to visualize adherens junctions. C: phosphorylation profile of proteins or IκBα content were analyzed by Western blotting (IL-6/SR, 40 ng/ml/10 ng/ml, 1 h). Equal protein loading was confirmed by determination of β-tubulin content in total cell lysates. Shown are representative results of 3–5 independent experiments. Veh, vehicle.
IL6-induced EC barrier compromise is Rho independent.
Recent reports, including our works, show the role of the Rho pathway in the increased lung vascular endothelial permeability induced by inflammatory agonists LPS (33), transforming growth factor-β (3, 9), and TNFα (21). In the following studies, we investigated the role of Rho signaling in IL-6-induced EC permeability. Activation of Rho signaling was evaluated by analysis of site-specific phosphorylation of Rho-kinase substrate, myosin-binding subunit of MYPT1, and MLC. In contrast to Rho-activating agonist thrombin, IL-6 treatment induced barely detectable increases in MYPT1 and MLC phosphorylation (Fig. 3A). Total MYPT1 and MLC protein levels in IL-6 treated EC did not change (data not shown).
Fig. 3.
Effects of Rho inhibition on IL-6-induced EC barrier compromise. Human pulmonary artery endothelial cells (HPAEC) were pretreated with vehicle or Y-27632 (2 μM, 30 min) followed by stimulation with IL-6/SR (40 ng/ml/10 ng/ml). A: phosphorylation of myosin-binding subunit of myosin-associated phosphatase type 1 (MYPT1) and myosin light chain (MLC) was detected by Western blot with corresponding phospho-specific antibodies. Thrombin treatment was used as positive control for Rho activation. Equal protein loading was confirmed by determination of β-tubulin content in total cell lysates. B: TER was measured over the 15-h period. In control experiments cells were stimulated with thrombin (0.3 U/ml) with or without Y-27632 pretreatment. C: actin cytoskeleton remodeling was examined by immunofluorescence staining with Texas red-conjugated phalloidin. Y-27632 pretreatment was 2 μM, 30 min; IL-6/SR stimulation was with 40 ng/ml/10 ng/ml for 5 h. Paracellular gaps are marked by arrows. Shown are representative results of 3–5 independent experiments.
Potential involvement of the Rho pathway in EC barrier dysfunction induced by IL-6 was further examined in experiments with HPAEC pretreatment with pharmacological Rho inhibitor Y-27632. Inhibition of Rho did not significantly affect IL-6-induced permeability (Fig. 3B, left). In contrast, Y-27632 was effective against thrombin-induced Rho-dependent permeability (Fig. 3B, right). The effects of Rho inhibition on IL-6-induced EC cytoskeletal remodeling were examined by immunofluorescence staining and visualization of actin cytoskeleton in pulmonary EC after 5 h of IL-6 treatment. Pretreatment of HPAEC with Rho inhibitor Y-27632 did not affect IL-6-induced formation of paracellular gaps, which suggests a Rho-independent mechanism of IL-6-induced permeability (Fig. 3C).
Pathologic mechanical stimulation promotes IL-6-induced barrier disruption in lung endothelium.
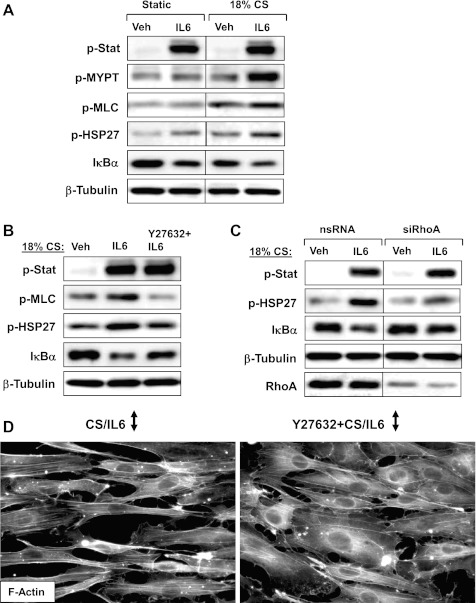
To assess the effects of mechanical stimulation on development of IL-6-induced EC barrier dysfunction, we utilized a previously characterized model of ECs subjected to pathologically relevant levels of CS (4). Human pulmonary EC grown to confluence on Flexcell plates and preconditioned at 18% CS for 2 h were treated with vehicle or IL-6 for 1 h with continuous CS. Activation of intracellular signaling by IL-6 was evaluated by increased phosphorylation of signaling proteins. CS preconditioning further increased activation of p38 MAPK and NF-κB pathways induced by IL-6, as detected by increased phosphorylation of HSP27 and enhanced IκBα degradation, respectively. Of note, Stat phosphorylation was not significantly affected by CS preconditioning. In contrast to static conditions, MLC phosphorylation was significantly elevated in CS-preconditioned EC upon stimulation with IL-6 (Fig. 4A). CS exposure over the duration of experiment did not change total protein levels of Jak/Stat, p38 MAPK, HSP27, MLC, and MYPT (data not shown).
Fig. 4.
Effects of stretch preconditioning on IL-6-induced EC barrier dysfunction: role of Rho. A and B: HPAEC grown on Flexcell plates were subjected to 18% cyclic stretch (CS) for 2 h followed by IL-6/SR (40–10 ng/ml, 1 h) stimulation (A) with or without Y-27632 (2 μM, 30 min) pretreatment (B). Levels of HSP27, Stat3, MLC, MYPT1 phosphorylation, and IκBα degradation in the total cell lysates were determined by Western blot analysis. C: human pulmonary EC were transfected with Rho-specific or nonspecific siRNA. After 48 h of transfection cells were subjected to 18% CS for 2 h followed by combined IL-6 and SR treatment for 1 h and analysis of protein content and phosphorylation with specific antibodies. Rho protein depletion was confirmed by Western blot. D: immunofluorescence staining of CS-preconditioned (2 h), IL-6/SR challenged (5 h, with continuing CS) EC with or without prior Y-27632 pretreatment (30 min) was performed with Texas Red-conjugated phalloidin to detect actin filaments. Black arrows indicate the main direction of CS vector. Shown are representative results of 3–5 independent experiments.
Recent reports (5, 13) suggest involvement of Rho signaling in the development of lung injury induced by mechanical ventilation at HTV. We next tested involvement of the Rho pathway in exacerbation of IL-6-induced EC barrier disruption by mechanical forces. HPAEC preconditioned at 18% CS (1.5 h) were pretreated with Rho kinase inhibitor Y-27632 for 30 min and then stimulated with IL-6 under continuous CS. Y-27632 completely inhibited CS/IL-6-induced MLC phosphorylation in EC exposed to IL-6 and 18% CS and markedly attenuated HSP27 phosphorylation and IκBα degradation. In contrast, Stat phosphorylation was not altered by Y-27632 (Fig. 4B). In additional experiments, we performed siRNA-based Rho knockdown. HPAEC were transiently transfected with Rho-specific or nonspecific siRNA for 48 h followed by 2-h CS preconditioning and IL-6 stimulation under continuing CS. Rho knockdown did not significantly affect basal activity of Jak/Stat, HSP-27 phosphorylation and IκBα levels but attenuated HSP-27 phosphorylation, and IκBα degradation induced by combined treatment with IL-6 and 18% CS. In contrast, IL-6- and CS-induced Stat phosphorylation was not inhibited by Rho knockdown (Fig. 4C).
The role of Rho signaling on EC barrier disruption and cytoskeletal remodeling induced by IL-6 and 18% CS was examined in the next experiments. Pulmonary EC were preconditioned at 18% CS for 1.5 h followed by Y-27632 pretreatment for 30 min and stimulation with IL-6 for 5 h under continuing CS. CS further promoted IL-6-induced gap formation (Fig. 4D), compared with static EC culture (Fig. 2B). Addition of Y-27632 after 1.5 h of CS did not affect orientation of CS-preconditioned EC (data not shown) but markedly attenuated gap formation induced by 18% CS and IL-6 (Fig. 4D).
The next experiments attempted to recapitulate more a clinically relevant scenario of EC exposure to 18% CS after IL-6 challenge, which relates to mechanical ventilation of diseased lungs. Cell monolayers pretreated with IL-6 for 1 h were next exposed to 18% CS exposure for 2 h. Similarly to the CS preconditioning model described above, application of CS after IL-6 exposure did not affect activation of Jak/Stat but did increase IL-6-induced activation of p38 MAPK and NF-κB pathways and stimulated Rho signaling and MLC phosphorylation (Fig. 5A). Rho kinase inhibition by Y-27632 (30-min pretreatment before CS exposure) suppressed HSP-27 phosphorylation and IκBα degradation and completely blocked MLC phosphorylation in response to IL-6 and 18% CS but was without effect on Stat3 phosphorylation (Fig. 5B). Next, we performed analysis of cytoskeletal remodeling in agonist-stimulated stretched EC. In agreement with our previous findings (2, 6), pretreatment with Y-27632 before CS exposure inhibited CS-induced cell orientation (data not shown). Importantly, Y-27632 attenuated the potentiating effect of 18% CS on IL-6-induced paracellular gap formation (Fig. 5C).
Fig. 5.
Effects of stretch postconditioning on IL-6-induced EC barrier dysfunction: role of Rho. A and B: HPAEC grown on Flexcell plates were subjected to IL-6/SR (40 ng/ml/10 ng/ml, 1 h) stimulation followed by 18% CS (2 h; A) with or without Y-27632 (2 μM, 30 min) pretreatment (B). Levels of HSP27, Stat3, MLC phosphorylation, and IκBα degradation in the total cell lysates were determined by Western blot analysis. Equal protein loading was confirmed by determination of β-tubulin content in total cell lysates. C: HPAEC were treated with IL-6/SR for 2.5 h followed by addition of vehicle or Y-27632 for 30 min and 18% CS exposure for additional 2 h. Cytoskeletal remodeling in control and stretched EC monolayers was analyzed by immunofluorescence staining for F-actin. Arrows indicate the main direction of CS vector. Shown are representative results of 3–5 independent experiments.
Eighteen percent of CS promotes vascular inflammatory responses induced by IL-6.
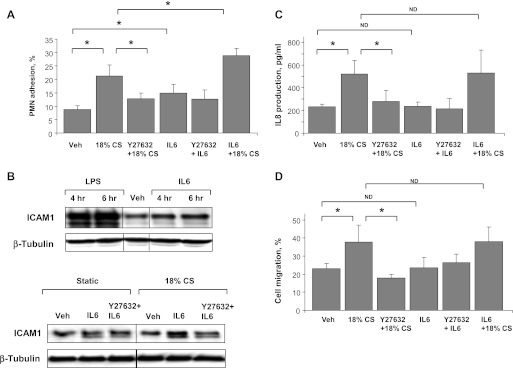
To further assess the role of 18% CS in the modulation of pulmonary EC inflammatory effects induced by IL-6 treatment, confluent EC monolayers were pretreated with IL-6 (2 h) followed by 18% CS exposure (4 h) and analysis of PMN adhesion. The optimal time (4 h of CS exposure) for detection of PMN adhesion was determined in preliminary studies. In static conditions, IL-6 modestly increased PMN adhesion (by 40%), which was further elevated by EC exposure to 18% CS. Interestingly, CS alone caused twofold increase in PMN adhesion to CS-preconditioned EC. Combination of IL-6 and 18% CS further increased PMN adhesion by 45% compared with 18% CS treatment alone (Fig. 6A). Other experiments examined effects of IL-6, 18% CS and their combination on activation of ICAM-1 surface expression in EC. In contrast to LPS stimulation, IL-6 treatment of static EC cultures (5 h) caused only modest increase in ICAM-1 expression detected by Western blot (Fig. 6B, top). Similarly, 18% CS exposure alone (4 h) did not induce significant elevation of ICAM-1 expression, whereas combined treatment with IL-6 and 18% CS caused a synergistic effect on the increase in ICAM-1 expression, which was partially suppressed by Rho kinase inhibition (Fig. 6B, bottom).
Fig. 6.
Role of Rho in the development of inflammation in IL-6/HTV two-hit in vitro model. Human pulmonary EC were stimulated with IL-6/SR (40 ng/ml/10 ng/ml, 2 h) followed by exposure to 18% CS for 4 h with or without Y-27632 (2 μM, 30 min) pretreatment before CS. A: polymorphonuclear leukocyte (PMN) adhesion was determined in control and treated samples after IL-6/SR or IL-6/SR + CS exposure with or without Y-27632 pretreatment. B: ICAM-1 expression was analyzed by Western blot in control and treated samples after 4 and 6 h of IL-6/SR or LPS (300 ng/ml) challenge (top); and after IL-6/SR or IL-6/SR + CS exposure with or without Y-27632 pretreatment (bottom). Equal protein loading was confirmed by determination of β-tubulin content in total cell lysates. C: IL-8 production was determined in control and treated samples after IL-6/SR or IL-6/SR + CS exposure using ELISA. D: analysis of PMN migration was performed in control and IL-6/SR + CS treated samples with or without Y-27632 pretreatment. Data are expressed as means ± SD of 3–6 independent experiments; *P < 0.05.
To further test the role of the Rho pathway in CS-mediated enhancement of IL-6 inflammatory response, HPAEC were stimulated with IL-6 (1.5 h), treated with Y-27632 or vehicle (30 min), and exposed to 18% CS (4 h). Interestingly, IL-8 production measured by ELISA assay (Fig. 6C) and neutrophil migration in the presence of culture media from control and treated EC (Fig. 6D) was not significantly affected by IL-6 treatment, and Y-27632 pretreatment did not alter IL-6 effects. In contrast, 18% CS preconditioning caused a 2.4-fold increase in IL-8 production and enhanced neutrophil migration, compared with static EC culture. Inhibition of Rho kinase significantly attenuated effects of 18% CS. These data suggest that in our model IL-6-induced inflammatory response is mediated at least in part via ICAM-1-mediated neutrophil adhesion and does not depend on release of chemoattractant IL-8, whereas pathologic CS affects both pathways of endothelial activation leading to development of inflammation.
Pathological mechanical ventilation enhances IL-6-induced lung injury in vivo.
We next evaluated the results from pulmonary EC models in the murine two-hit model of lung injury induced by HTV mechanical ventilation and IL-6. Optimal IL-6 concentration for in vivo use was determined in the initial set of experiments (data not shown). Intratracheal instillation of 1.5, 5, or 15 mg/kg of mouse IL-6 with or without IL-6 soluble receptor (ratio 4:1) was performed for 4, 8, and 16 h, and the conditions using mice treatment with IL-6 (16 h, 5 mg/kg IL-6, without IL-6 soluble receptor) were used for all subsequent in vivo studies.
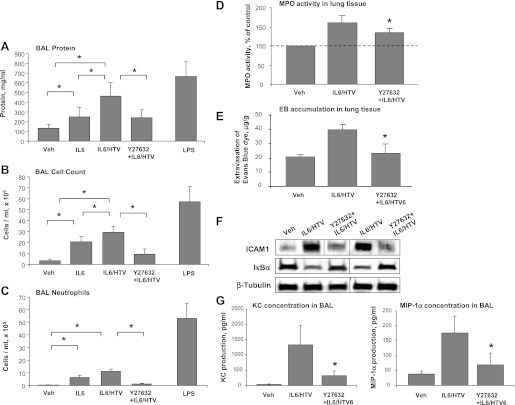
Intratracheal administration of IL-6 at the dose used in this study caused noticeable increase in BAL protein concentration (Fig. 7A), elevation of BAL total cell count (Fig. 7B), and increased number of PMNs (Fig. 7C). Concurrent HTV mechanical ventilation (30 ml/kg, 4 h HTV) further increased parameters of lung injury induced by IL-6 instillation (Fig. 7, A–C).
Fig. 7.
Role of Rho in the development of lung injury in IL-6/HTV two-hit in vivo model. Mice were subjected to intravenous injection with vehicle or IL-6 (5 mg/kg it) with or without Y-27632 (2 mg/kg iv) instillation followed by mechanical ventilation at high tidal volume (HTV; 30 ml/kg, 4 h). Control animals were allowed to breathe spontaneously. LPS-treated mice (0.63 mg/kg it) served as positive controls. A–C: measurements of protein concentration (A), total cell count (B), and differential PMN count (C) were performed in bronchoalveolar lavage (BAL) fluid taken from control and experimental animals. Data are expressed as means ± SD of 4 independent experiments; n = 6–10 per condition; *P < 0.05. D: myeloperoxidase (MPO) activity was determined in control and treated lung tissue samples. MPO data are expressed as %control ± SD of 4 independent experiments; n = 6–10 per condition; *P < 0.05. E: Evans blue dye (30 ml/kg iv) was injected 2 h before termination of the experiment. Lung vascular permeability was assessed by Evans blue accumulation in the lung tissue. Quantitative analysis of Evans blue labeled albumin extravasation was performed by spectrophotometric analysis of Evans blue extracted from the lung tissue samples; n = 4 per condition; *P < 0.05. F: expression levels of IκBα and ICAM-1 were determined in control and treated lung tissue samples. Equal protein loading in Western blot experiments was confirmed by determination of β-tubulin content in tissue homogenates. Rearranged lanes from the same blot are outlined by vertical dotted line. G: analysis of keratinocyte-derived chemokine (KC) and macrophage inflammatory protein-1α (MIP)-1α levels was performed in BAL samples from control and treated mice by ELISA. Data are expressed as means ± SD of 4 independent experiments; *P < 0.05.
Intratracheal LPS instillation was used in these experiments as a “septic” control to scale lung injury induced by HTV and IL-6. The data show that IL-6 alone is a less potent activator of lung inflammation and injury, and the combined effects of IL-6 and HTV did not overcome lung injury induced by LPS. Furthermore, the increase in BAL protein content and cell counts induced by HTV alone was significantly lower than effects caused by combination of IL-6 and HTV, suggesting further enhancement of IL-6 induced lung injury and barrier dysfunction by pathologic mechanical ventilation.
A role of Rho signaling in the development of IL-6/HTV-induced lung injury was evaluated in experiments with concurrent administration of Y-27632 and IL-6. In this two-hit model of ALI, Y-27632 dramatically attenuated elevation of protein concentration, total cell count and PMN count in BAL fluid caused by IL-6 and HTV treatment (Fig. 7, A–C). Pretreatment with Y-27632 also significantly reduced lung tissue myeloperoxidase activity (Fig. 7D) and lung vascular leakage detected by Evans blue accumulation in the lung parenchyma (Fig. 7E) in HTV + IL-6 stimulated mice. Western blot analysis of lung tissue samples revealed attenuation of IL-6- and HTV-induced IκBα degradation and upregulated ICAM-1 expression in Y-27632-treated animals (Fig. 7F).
Finally, analysis of inflammatory cytokines in BAL fluid in this two-hit model of ALI demonstrated that combined treatment with HTV and IL-6 induced production of keratinocyte-derived chemokine and macrophage inflammatory protein-1α, while Rho kinase inhibition by Y-27632 suppressed production of these cytokines (Fig. 7G).
DISCUSSION
This study characterized for the first time the two-hit model of ALI induced by IL-6 and HTV mechanical ventilation. The results demonstrate that inflammatory and barrier disruptive pathways triggered by pathologic stretch and IL-6 are initiated independently but employ both common and independent mechanisms. Precise signaling mechanisms activated by IL-6 remain to be elucidated. Rapid ERK, p38 MAPK, and JNK phosphorylation induced within 5 min has been demonstrated after IL-6 injection and led to PMN infiltration and cytokine production in the muscle (24). Our results extend these findings and demonstrate that treatment of static pulmonary EC cultures with IL-6 caused activation of Stat3 but also stimulated p38 MAPK-HSP27 signaling and IκB degradation leading to activation of the NF-κB pathway, increased ICAM-1 expression and PMN adhesion, and increased EC permeability.
Our data also show that in addition to activation of barrier-disruptive inflammatory signaling, IL-6 increased EC permeability via stimulation of VE-cadherin dissociation from adherens junctions. Because VE-cadherin internalization is induced by its serine/threonine or tyrosine phosphorylation (10, 20), and ligation of IL-6 with its receptor gp130 has been shown to activate PI3-kinase and Src kinase family member, Fer (40, 42), it is possible that IL-6-induced VE-cadherin dissociation from adherens junctions may be mediated by gp130-Fer/PI3-kinase mechanism, although analysis of this mechanism was not the focus of this study. Both the IL-6-induced EC permeability and Stat3, NF-κB, and p38 signaling was abolished by Jak inhibition suggesting that potential IL-6-induced activation of Src is downstream of Jak kinase.
The results of this study show synergistic effects of pathologic CS on IL-6-induced inflammatory signaling in pulmonary EC and suggest a role for Rho signaling in CS-induced enhancement of IL-6 barrier disruptive and proinflammatory effects. Activation of Rho leading to increased MLC phosphorylation and actomyosin contraction is well-recognized mechanism of increased vascular endothelial permeability. In agreement with previous reports, 18% CS increased MLC phosphorylation in vehicle-treated and IL-6 stimulated EC. However, in addition to MLC phosphorylation, 18% CS also increased activation of p38, NF-κB, but not Stat pathways in the IL-6-stimulated EC monolayers. This synergistic effect was dependent on Rho activity, inhibited by Y-27632, and resulted in exacerbation of IL-6-induced EC monolayer disruption, lung barrier dysfunction, and inflammation. Although cross talk between p38 MAPK, NF-κB, and Rho signaling has been reported (17, 25), the precise mechanisms and the hierarchy of these interactions remain to be investigated. Altogether, these signaling events contribute to IL-6- and CS-induced EC barrier disruption. Thus convergence of CS and IL-6 signaling pathways on downstream targets that can be additionally regulated by Rho provides a mechanistic basis for inflammatory and barrier disruptive signal amplification observed in two-hit models of ALI.
With the use of two protocols of pulmonary EC challenge with IL-6 and CS in vitro, one with 18% CS preconditioning before IL-6 treatment, and another with IL-6 pretreatment followed by 18% CS, similar synergistic effects on gap formation and barrier disruptive signaling were observed. In both protocols, inhibition of Rho or Rho kinase significantly attenuated EC barrier dysfunction in the two-hit ALI model. These results support the notion that Rho signaling is a synergistic mechanism of IL-6 and CS barrier disruptive and inflammatory effects and that it is only effective when both stimuli are present in the system.
IL-6 is clearly elevated in acute respiratory distress syndrome patients and correlates with severity of outcomes (1). However, despite well-documented involvement of IL-6 in the mechanisms of VILI-associated inflammation and EC barrier dysfunction, also shown in this study, the idea of IL-6 blockage as a therapeutic option for VILI treatment should be considered with caution. Injection of IL-6 blocking antibodies in mice after ventilator induced lung injury significantly increased rates of BAL albumin flux (38). Surprisingly, IL-6 overexpression even protected mice against hyperoxic lung injury by regulating Bax and reducing apoptosis through a PI-3 kinase pathway (22). In turn, IL-6 generated by mast cells improved survival during sepsis by enhancing neutrophil killing of bacteria (32) and reduced DNA damage during hypoxia (37). IL-6 from a hematopoietic cell source limited alveolar barrier disruption potentially by reducing neutrophil contact with the endothelium (38). These data suggest that such paradoxical effects of IL-6 on modulation of ALI appear to be dependent on the particular cell type producing IL-6 and etiology of ALI.
In conclusion, this study demonstrates a synergistic mechanism of IL-6 and pathological mechanical stimulation of pulmonary vascular endothelium leading to activation of inflammatory signaling and EC barrier dysfunction. Our results show that parallel activation of Rho-independent mechanisms by circulating IL-6 and Rho-dependent mechanisms by suboptimal mechanical ventilation lead to further exacerbation of ALI. Thus, in addition to reduction of ALI induced by Rho-activating mediators such as thrombin, the beneficial effect of Rho downregulation shown in the IL-6/HTV model suggests that inhibition of Rho may be a promising strategy for general treatment of VILI conditions.
GRANTS
Support for this work was provided by National Heart, Lung, and Blood Institute Grants HL-87823, HL-076259, and HL-058064 (to K. G. Birukov) and HL-089257 and HL-107920 and American Heart Association Midwest Affiliate Grant-in-Aid (to A. A. Birukova).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.A.B. and K.G.B. conception and design of research; A.A.B., T.W., and K.G.B. analyzed data; A.A.B., A.Y.M., A.R.L., and K.G.B. interpreted results of experiments; A.A.B. prepared Figs.; A.A.B. and K.G.B. drafted manuscript; A.A.B. and K.G.B. edited and revised manuscript; A.A.B. and K.G.B. approved final version of manuscript; Y.T., A.Y.M., and T.W. performed experiments.
ACKNOWLEDGMENTS
We thank Katherine Higginbotham for proofreading the manuscript.
REFERENCES
- 1. Belperio JA, Keane MP, Lynch JP, III, Strieter RM. The role of cytokines during the pathogenesis of ventilator-associated and ventilator-induced lung injury. Semin Respir Crit Care Med 27: 350–364, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, Garcia JG. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol 285: L785–L797, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Birukova AA, Birukov KG, Adyshev D, Usatyuk P, Natarajan V, Garcia JG, Verin AD. Involvement of microtubules and Rho pathway in TGF-beta1-induced lung vascular barrier dysfunction. J Cell Physiol 204: 934–947, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Birukova AA, Chatchavalvanich S, Rios A, Kawkitinarong K, Garcia JG, Birukov KG. Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am J Pathol 168: 1749–1761, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Birukova AA, Fu P, Xing J, Cokic I, Birukov KG. Lung endothelial barrier protection by iloprost in the 2-hit models of ventilator-induced lung injury (VILI) involves inhibition of Rho signaling. Transl Res 155: 44–54, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Birukova AA, Fu P, Xing J, Yakubov B, Cokic I, Birukov KG. Mechanotransduction by GEF-H1 as a novel mechanism of ventilator-induced vascular endothelial permeability. Am J Physiol Lung Cell Mol Physiol 298: L837–L848, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Birukova AA, Malyukova I, Poroyko V, Birukov KG. Paxillin-β-catenin interactions are involved in Rac/Cdc42-mediated endothelial barrier-protective response to oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol 293: L199–L211, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Birukova AA, Moldobaeva N, Xing J, Birukov KG. Magnitude-dependent effects of cyclic stretch on HGF- and VEGF-induced pulmonary endothelial remodeling and barrier regulation. Am J Physiol Lung Cell Mol Physiol 295: L612–L623, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clements RT, Minnear FL, Singer HA, Keller RS, Vincent PA. RhoA and Rho-kinase dependent and independent signals mediate TGF-beta-induced pulmonary endothelial cytoskeletal reorganization and permeability. Am J Physiol Lung Cell Mol Physiol 288: L294–L306, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell 16: 209–221, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Dos Santos CC, Slutsky AS. Invited review: mechanisms of ventilator-induced lung injury: a perspective. J Appl Physiol 89: 1645–1655, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Dreyfuss D, Ricard JD. Acute lung injury and bacterial infection. Clin Chest Med 26: 105–112, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Finigan JH, Boueiz A, Wilkinson E, Damico R, Skirball J, Pae HH, Damarla M, Hasan E, Pearse DB, Reddy SP, Grigoryev DN, Cheadle C, Esmon CT, Garcia JG, Hassoun PM. Activated protein C protects against ventilator-induced pulmonary capillary leak. Am J Physiol Lung Cell Mol Physiol 296: L1002–L1011, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frank JA, Matthay MA. Science review: mechanisms of ventilator-induced injury. Crit Care 7: 233–241, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fu P, Birukova AA, Xing J, Sammani S, Murley JS, Garcia JG, Grdina DJ, Birukov KG. Amifostine reduces lung vascular permeability via suppression of inflammatory signalling. Eur Respir J 33: 612–624, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia CS, Prota LF, Morales MM, Romero PV, Zin WA, Rocco PR. Understanding the mechanisms of lung mechanical stress. Braz J Med Biol Res 39: 697–706, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Garcia MC, Ray DM, Lackford B, Rubino M, Olden K, Roberts JD. Arachidonic acid stimulates cell adhesion through a novel p38 MAPK-RhoA signaling pathway that involves heat shock protein 27. J Biol Chem 284: 20936–20945, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hammerschmidt S, Kuhn H, Grasenack T, Gessner C, Wirtz H. Apoptosis and necrosis induced by cyclic mechanical stretching in alveolar type II cells. Am J Respir Cell Mol Biol 30: 396–402, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer 41: 2502–2512, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Hou WH, Liu IH, Tsai CC, Johnson FE, Huang SS, Huang JS. CRSBP-1/LYVE-1 ligands disrupt lymphatic intercellular adhesion by inducing tyrosine phosphorylation and internalization of VE-cadherin. J Cell Sci 124: 1231–1244, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kakiashvili E, Speight P, Waheed F, Seth R, Lodyga M, Tanimura S, Kohno M, Rotstein OD, Kapus A, Szaszi K. GEF-H1 mediates tumor necrosis factor-alpha-induced Rho activation and myosin phosphorylation: role in the regulation of tubular paracellular permeability. J Biol Chem 284: 11454–11466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim HA, Park JH, Lee S, Choi JS, Rhim T, Lee M. Combined delivery of dexamethasone and plasmid DNA in an animal model of LPS-induced acute lung injury. J Control Release 156: 60–69, 2011 [DOI] [PubMed] [Google Scholar]
- 23. Kishimoto T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther 8, Suppl 2: S2, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manjavachi MN, Motta EM, Marotta DM, Leite DF, Calixto JB. Mechanisms involved in IL-6-induced muscular mechanical hyperalgesia in mice. Pain 151: 345–355, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Matoba K, Kawanami D, Ishizawa S, Kanazawa Y, Yokota T, Utsunomiya K. Rho-kinase mediates TNF-alpha-induced MCP-1 expression via p38 MAPK signaling pathway in mesangial cells. Biochem Biophys Res Commun 402: 725–730, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA, Jr, Hoffman E, Hubmayr RD, Leppert M, Matalon S, Munford R, Parsons P, Slutsky AS, Tracey KJ, Ward P, Gail DB, Harabin AL. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 167: 1027–1035, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Meliton AY, Munoz NM, Meliton LN, Binder DC, Osan CM, Zhu X, Dudek SM, Leff AR. Cytosolic group IVa phospholipase A2 mediates IL-8/CXCL8-induced transmigration of human polymorphonuclear leukocytes in vitro. J Inflamm 7: 14, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meliton AY, Munoz NM, Zhu X, Leff AR. Attenuated translocation of group IVa phospholipase A2 and up-regulated annexin-1 synthesis by glucocorticoid blocks beta 2-integrin adhesion in neutrophils. J Leukoc Biol 83: 344–351, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Nonas S, Birukova AA, Fu P, Xing J, Chatchavalvanich S, Bochkov VN, Leitinger N, Garcia JG, Birukov KG. Oxidized phospholipids reduce ventilator-induced vascular leak and inflammation in vivo. Crit Care 12: R27, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Singleton PA, Chatchavalvanich S, Fu P, Xing J, Birukova AA, Fortune JA, Klibanov AM, Garcia JG, Birukov KG. Akt-mediated transactivation of the S1P1 receptor in caveolin-enriched microdomains regulates endothelial barrier enhancement by oxidized phospholipids. Circ Res 104: 978–986, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Slutsky AS, Tremblay LN. Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med 157: 1721–1725, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Sutherland RE, Olsen JS, McKinstry A, Villalta SA, Wolters PJ. Mast cell IL-6 improves survival from Klebsiella pneumonia and sepsis by enhancing neutrophil killing. J Immunol 181: 5598–5605, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tasaka S, Koh H, Yamada W, Shimizu M, Ogawa Y, Hasegawa N, Yamaguchi K, Ishii Y, Richer SE, Doerschuk CM, Ishizaka A. Attenuation of endotoxin-induced acute lung injury by the Rho-associated kinase inhibitor, Y-27632. Am J Respir Cell Mol Biol 32: 504–510, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Uhlig S. Ventilation-induced lung injury and mechanotransduction: stretching it too far? Am J Physiol Lung Cell Mol Physiol 282: L892–L896, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Villar J, Cabrera NE, Casula M, Flores C, Valladares F, Diaz-Flores L, Muros M, Slutsky AS, Kacmarek RM. Mechanical ventilation modulates TLR4 and IRAK-3 in a non-infectious, ventilator-induced lung injury model. Respir Res 11: 27, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang YL, Malik AB, Sun Y, Hu S, Reynolds AB, Minshall RD, Hu G. Innate immune function of the adherens junction protein p120-catenin in endothelial response to endotoxin. J Immunol 186: 3180–3187, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Waxman AB, Kolliputi N. IL-6 protects against hyperoxia-induced mitochondrial damage via Bcl-2-induced Bak interactions with mitofusins. Am J Respir Cell Mol Biol 41: 385–396, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wolters PJ, Wray C, Sutherland RE, Kim SS, Koff J, Mao Y, Frank JA. Neutrophil-derived IL-6 limits alveolar barrier disruption in experimental ventilator-induced lung injury. J Immunol 182: 8056–8062, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wurfel MM. Microarray-based analysis of ventilator-induced lung injury. Proc Am Thorac Soc 4: 77–84, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamauchi-Takihara K. gp130-mediated pathway and heart failure. Future Cardiol 4: 427–437, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Yang G, Im HJ, Wang JH. Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene 363: 166–172, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zoubeidi A, Rocha J, Zouanat FZ, Hamel L, Scarlata E, Aprikian AG, Chevalier S. The Fer tyrosine kinase cooperates with interleukin-6 to activate signal transducer and activator of transcription 3 and promote human prostate cancer cell growth. Mol Cancer Res 7: 142–155, 2009 [DOI] [PubMed] [Google Scholar]