Abstract
Pulmonary hypertension (PH) is characterized by pulmonary arteriolar remodeling with excessive pulmonary vascular smooth muscle cell (VSMC) proliferation. This results in decreased responsiveness of pulmonary circulation to vasodilator therapies. We have shown that extracellular acidosis inhibits VSMC proliferation and migration in vitro. Here we tested whether induction of nonhypercapnic acidosis in vivo ameliorates PH and the underlying pulmonary vascular remodeling and dysfunction. Adult male Sprague-Dawley rats were exposed to hypoxia (8.5% O2) for 2 wk, or injected subcutaneously with monocrotaline (MCT, 60 mg/kg) to develop PH. Acidosis was induced with NH4Cl (1.5%) in the drinking water 5 days prior to and during the 2 wk of hypoxic exposure (prevention protocol), or after MCT injection from day 21 to 28 (reversal protocol). Right ventricular systolic pressure (RVSP) and Fulton's index were measured, and pulmonary arteriolar remodeling was analyzed. Pulmonary and mesenteric artery contraction to phenylephrine (Phe) and high KCl, and relaxation to acetylcholine (ACh) and sodium nitroprusside (SNP) were examined ex vivo. Hypoxic and MCT-treated rats demonstrated increased RVSP, Fulton's index, and pulmonary arteriolar thickening. In pulmonary arteries of hypoxic and MCT rats there was reduced contraction to Phe and KCl and reduced vasodilation to ACh and SNP. Acidosis prevented hypoxia-induced PH, reversed MCT-induced PH, and resulted in reduction in all indexes of PH including RVSP, Fulton's index, and pulmonary arteriolar remodeling. Pulmonary artery contraction to Phe and KCl was preserved or improved, and relaxation to ACh and SNP was enhanced in NH4Cl-treated PH animals. Acidosis alone did not affect the hemodynamics or pulmonary vascular function. Phe and KCl contraction and ACh and SNP relaxation were not different in mesenteric arteries of all groups. Thus nonhypercapnic acidosis ameliorates experimental PH, attenuates pulmonary arteriolar thickening, and enhances pulmonary vascular responsiveness to vasoconstrictor and vasodilator stimuli. Together with our finding that acidosis decreases VSMC proliferation, the results are consistent with the possibility that nonhypercapnic acidosis promotes differentiation of pulmonary VSMCs to a more contractile phenotype, which may enhance the effectiveness of vasodilator therapies in PH.
Keywords: pulmonary artery, pulmonary circulation, nitric oxide, vascular smooth muscle
pulmonary hypertension (PH) is a serious disease and a major and expanding public health problem with ∼1,000 new patients diagnosed every year in the United States (6, 20). PH is characterized by increased pulmonary arterial pressure and right ventricular hypertrophy (RVH). Increased pulmonary vascular resistance and pulmonary vascular remodeling lead to progressive right ventricular failure and significantly compromise the quality of life and life expectancy in affected individuals (20, 21, 51).
Although the etiology of PH is diverse and multifactorial, the underlying pathology and pathophysiology are common among its various forms. Endothelial dysfunction, excessive pulmonary vascular smooth muscle cell (VSMC) proliferation, hypertrophy, and migration, as well as various degrees of pulmonary vasoconstriction and inflammation, are major components of the pathobiology of PH and therefore represent important targets for current and emerging therapies (9, 13). Vasodilator therapies such as prostacyclin (PGI2), endothelin (ET-1) receptor antagonists, and phosphodiesterase-5 (PDE-5) inhibitors, either separate or in combination, are variably successful in slowing PH progression and prolonging survival (10, 47, 51, 71, 80). Also, it is increasingly appreciated that alternative approaches such as antiproliferative, proapoptotic, or immunomodulatory therapies hold promise in further improving the outcome of PH (5, 18, 36, 52, 54, 64, 77). Importantly, accumulating evidence supports that a phenotypic switch of pulmonary VSMCs from a contractile to a proliferative phenotype may contribute to the pathogenesis of PH (4, 24, 45, 48, 53), and reversal of this pathology could enhance the effectiveness of vasodilator therapies.
Rodent models of experimental PH recapitulate to a certain extent the histological features of human PH and provide a useful tool to study the efficacy of novel therapeutic approaches (17, 31, 46, 65). The hypoxic and monocrotaline (MCT) rat models are particularly useful in the evaluation of antiproliferative and proapoptotic therapies since medial hypertrophy of the pulmonary arterioles is a key feature in these PH models (36, 52, 54, 64). We have previously shown that chronic hypoxia and MCT treatment in rats are associated with decreased pulmonary artery reactivity and significant pulmonary arteriolar remodeling (50). We have also reported that extracellular acidosis inhibits proliferation and migration of cultured rat and mouse VSMCs (8, 11, 30). Although some studies have raised the possibility that hypercapnic acidosis may be protective in the setting of PH (42, 58), the role of acidosis per se has not been examined, and the pulmonary vascular mechanisms involved have not been clearly identified. The present study was designed to test the hypothesis that nonhypercapnic acidosis is protective in experimental PH by improving pulmonary artery reactivity and by decreasing hypertrophic remodeling. We measured the hemodynamics and examined the pulmonary arterial function in hypoxia and MCT-induced rat models of PH chronically treated or nontreated with NH4Cl to determine whether 1) nonhypercapnic acidosis improves hemodynamic parameters and ameliorates RVH in experimental PH, 2) mild acidosis improves pulmonary arterial relaxation via the endothelium-dependent NO-cGMP pathway in PH, 3) the pulmonary VSMC responsiveness to vasodilators is enhanced during nonhypercapnic acidosis in experimental PH, and 4) the beneficial effects of acidosis in experimental PH could be related to decreased pulmonary arterial remodeling.
METHODS
Animals.
Adult (12 wk) male Sprague-Dawley rats (250–300 g; Charles River Laboratories, Wilmington, MA) were housed in the animal facility in 12:12-h light-dark cycle, at 22 ± 1°C ambient temperature, and maintained on ad libitum normal Purina Rodent Chow (Purina, St. Louis, MO) and tap water. All experiments were approved by the Children's Hospital Animal Care and Use Committee and the Harvard Medical Area Standard Committee on Animals.
Hypoxic exposure.
Rats were exposed to chronic hypoxia at 8.5% O2 inside a chamber, where O2 is controlled to within a 0.2% range by an OxyCycler controller (BioSpherix, Redfield, NY) (76). Electronic controllers injected nitrogen into the hypoxic chamber to maintain the appropriate inspired O2 fraction, and ventilation was adjusted to remove CO2 so that it did not exceed 5,000 ppm (0.5%). Ammonia was removed by ventilation and activated charcoal filtration using an electric air purifier. The hypoxic chamber was opened twice a week to replenish food and water and to change the bedding. The duration of hypoxic exposure was 2 wk. Normoxic control rats were kept in the same animal room outside the hypoxic chamber.
MCT injection.
For the MCT model of PH, age-matched rats were given a single subcutaneous injection of 60 mg/kg MCT (Sigma, St. Louis, MO). Control rats were injected with the same volume of vehicle (normal saline). The rats were assessed for development of PH 28 days after injection.
Induction of nonhypercapnic acidosis.
Nonhypercapnic acidosis was induced to examine 1) whether nonhypercapnic acidosis can prevent experimental PH before the onset of the disease (tested in the hypoxic model), and 2) whether nonhypercapnic acidosis can reverse experimental PH after the disease is already established (tested in the MCT model). To induce nonhypercapnic acidosis in the hypoxic animals, ammonium chloride (NH4Cl, 1.5%) was added to the drinking water for 5 days prior to and continued during the 2 wk of hypoxic exposure (prevention protocol). Sucrose (5%) was added to increase palatability of the drinking water for both hypoxic and control normoxic rats. Water consumption was monitored and was estimated to be ∼20 ml per rat per day. For induction of nonhypercapnic acidosis in the MCT rats, NH4Cl and sucrose were added to the drinking water starting on day 21 after MCT injection and continued until day 28 (late reversal protocol).
NH4Cl treatment for 3–5 days has been used to induce metabolic acidosis in experimental animals (22, 49). Our initial experiments showed that animals pretreated with NH4Cl for 5 days then subjected to hypoxia for 1 wk with continuous NH4Cl treatment had significantly lower Fulton's index (FI) (less RVH) than animals subjected to 1 wk hypoxia without NH4Cl treatment. Because prolonged hypoxia could cause a more severe form of PH, we tested whether induction of acidosis would ameliorate hypoxic PH after prolonged hypoxic exposure. Prolonged 2 wk hypoxia caused further increase in FI, and animal pretreatment with NH4Cl for 5 days and continuous NH4Cl treatment for the 2 wk of hypoxia caused significant reduction in FI (see results).
Because acidosis was effective as a preventive strategy, we also tested whether it would be effective as a reversal strategy. Although there are no established reversal protocols for the hypoxic rat model of PH, the MCT model has been utilized in established reversal protocols: treatments aimed at “early reversal” last from day 14 to day 28, and treatments aimed at “late reversal” last from day 21 to day 28. We chose the late reversal protocol because we reasoned that if our intervention was successful this would be the most clinically relevant model. Also, given that MCT requires conversion to the toxic metabolite to cause the disease, we wanted to space the acidosis intervention at a sufficiently remote time from MCT metabolism so that the results would not be attributed to possible effects of NH4Cl on MCT metabolism.
RVSP and LVSP measurements.
At the end of the experimental exposure, rats were anesthetized with 2% isoflurane inhalation and remained spontaneously breathing. A small, transverse incision was made in the abdominal wall, and the transparent diaphragm exposed. A 23-gauge butterfly needle, with tubing attached to a pressure transducer, was inserted through the diaphragm first into the right ventricle and then into the left ventricle, and pressure measurements were recorded in spontaneously breathing animals with heart rates over 300/min by use of PowerLab monitoring hardware and software (ADInstruments, Colorado Springs, CO). Mean right and left ventricular systolic pressures (RVSP and LVSP, respectively) over the first 10 stable heart beats were recorded.
Arterial blood gas and hematocrit analyses.
After hemodynamic measurements, a 0.2-ml blood sample was collected from the cardiac chambers for determination of hematocrit, pH, and Pco2 by use of a blood gas analyzer (Roche Diagnostics Indianapolis, IN). Overanesthetized animals with respiratory depression (Pco2 >55 mmHg) were excluded from the analysis.
Right ventricular weight and FI.
Hearts and pulmonary vasculature were perfused in situ with cold 1× phosphate-buffered saline (PBS) injection into the right ventricle. The heart was excised, and both ventricles were weighed. The right ventricular free wall was then dissected and the remaining left ventricular wall and ventricular septum were weighed. RVH was assessed as FI (ratio of right ventricular weight to the left ventricular+septum weight) or as the ratio of right ventricular weight to total body weight.
Tissue preparation for ex vivo vascular function studies.
In euthanized rats, the thoracic cavity was opened, and the lung and pulmonary arteries were rapidly excised. The abdominal cavity was then opened and the mesentery and mesenteric arterial arcade were excised and placed in oxygenated Krebs solution. The right and left pulmonary artery and second order mesenteric arteries were carefully dissected and cleaned of connective tissue under microscopic visualization and cut into 3-mm-wide rings.
Isometric contraction.
Vascular segments were suspended between two tungsten wire hooks, with one hook fixed at the bottom of a tissue bath and the other hook connected to a Grass force transducer (FT03, Astro-Med, West Warwick, RI). Pulmonary artery and mesenteric artery segments from the same rat were stretched under 1 or 0.5 g of resting tension, respectively (as determined by preliminary tension-contraction curves to KCl) and allowed to equilibrate for 45 min in a temperature controlled, water-jacketed tissue bath, filled with 50 ml Krebs solution continuously bubbled with 95% O2-5% CO2 at 37°C. The changes in isometric contraction were recorded on a Grass polygraph (model 7D, Astro-Med).
After tissue equilibration, a control contraction in response to 96 mM KCl was elicited. Once maximum KCl contraction was reached, the tissue was rinsed with Krebs three times for 10 min each. The control KCl-induced contraction followed by rinsing in Krebs was repeated twice. Vascular segments were stimulated with increasing concentrations of phenylephrine (Phe, 10−9 to 10−5 M), concentration-contraction curves were constructed, and the maximal Phe contraction and the pED50 (−log M) were calculated. In other experiments, the tissues were precontracted with Phe (10−5 M), increasing concentrations (10−9 to 10−5 M) of acetylcholine (ACh) were added, and the % relaxation of Phe contraction was measured. Parallel contraction and relaxation experiments were performed in endothelium-intact vascular rings pretreated with the NO synthase (NOS) inhibitor Nω-nitro-l-arginine methyl ester (l-NAME, 3 × 10−4 M) or the guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ, 10−5 M) for 10 min. In other experiments the relaxation to increasing concentrations (10−9 to 10−5 M) of the exogenous NO donor sodium nitroprusside (SNP) was measured in vascular rings precontracted with Phe.
Lung histology and morphometric analysis.
In a subset of experimental animals, the lungs were perfused with PBS through the right ventricle to remove the blood from the pulmonary vessels, fixed with cold 4% paraformaldehyde through the trachea, then excised and fixed in 4% paraformaldehyde overnight at 4°C followed by paraffin embedding. Lung sections (6 μm) were stained with hematoxylin and eosin and examined with light microscopy by two independent investigators (E. Arons and M. Touma) in a blinded fashion. Images of the arterioles were captured with a microscope digital camera system (Nikon) and analyzed via an image analysis program (NIH Image). At least 15 arterioles of comparable size (50–100 μm diameter) per rat, from the lungs of five different rats from each experimental group were evaluated. The % wall thickness was determined by dividing the area occupied by the vessel wall by the total cross-sectional area of the arteriole as previously reported (12). This method accounts for uneven vessel wall thickness and areas that have obliquely sectioned pulmonary arterioles.
Solutions and drugs.
Krebs solution contained (in mM) 120 NaCl, 5.9 KCl, 25 NaHCO3, 1.2 NaH2PO4, 11.5 dextrose, 2.5 CaCl2, 1.2 MgCl2, at pH 7.4, and bubbled with 95% O2 and 5% CO2. KCl (96 mM) was prepared as Krebs solution with equimolar substitution of NaCl with KCl. Stock solutions of Phe, ACh, and l-NAME (10−1 M, Sigma) were prepared in distilled water. Stock solution of ODQ (10−1 M, EMD Biosciences) was prepared in DMSO. Final concentration of DMSO in experimental solution was <0.1%. All other chemicals were of reagent grade or better.
Statistical analysis.
Cumulative data from 6 to 12 rats per experimental group were analyzed and presented as means ± SE, with the n value representing the number of rats. For the hemodynamic and histology data, group comparisons were done with a one-way ANOVA and Tukey-Kramer posttest for multiple comparisons. Correlation was done with a nonparametric test (Spearman correlation, GraphPad Prism). For the ex vivo studies in vascular rings, contraction and relaxation experiments were performed on two to four rings of pulmonary artery and two rings of mesenteric artery from each rat, and the data from different vascular rings from each vascular bed were averaged for each rat. Cumulative data from six to eight different rats per experimental group were presented as means ± SE with the n value representing the number of rats. Data were first analyzed using one-way ANOVA with Scheffé's F test, where F = variance between groups/variance within groups. When a statistical difference was observed, the data were further analyzed by Student-Newman-Keuls post hoc test for multiple comparisons. Concentration-contraction curves and Phe ED50 were determined by nonlinear regression best-fit sigmoidal curve (Sigmaplot). Differences were considered statistically significant if P < 0.05.
RESULTS
Effect of NH4Cl treatment on body weight, acid-base status, and hematocrit.
Initial body weight in all rats was 250–300 g. Treatment of rats under normoxia with NH4Cl for 5 days did not significantly affect body weight (Table 1). Hypoxic animals did not gain weight over a 2-wk period. Also, following 2 wk of hypoxic exposure, NH4Cl-treated rats had significantly lower body weight (243 ± 11 g) compared with nontreated rats (289 ± 10 g, P = 0.004). In comparison, MCT-treated rats gained weight, and NH4Cl treatment did not significantly change their body weight (Table 1).
Table 1.
Body weight, arterial pH, blood gas, and hematocrit in control normoxic, hypoxic, and MCT-treated rats with or without treatment with NH4Cl (acidosis)
| Parameter | Normoxia | Normoxia+Acidosis | Hypoxia | Hypoxia+Acidosis | MCT | MCT+Acidosis |
|---|---|---|---|---|---|---|
| Body weight, g | 293 ± 12.5 | 291 ± 16 | 289 ± 10 | 243 ± 11† | 373 ± 12 | 363 ± 5 |
| Arterial pH | 7.37 ± 0.01 | 7.32 ± 0.03 | 7.29 ± 0.01* | 7.08 ± 0.02† | 7.36 ± 0.01 | 7.26 ± 0.04† |
| Pco2, mmHg | 52 ± 0.9 | 48 ± 1.8 | 45 ± 2 | 40 ± 1.8 | 49.4 ± 1.3 | 44.8 ± 2.1 |
| Plasma HCO3− | 28.8 ± 0.6 | 24.6 ± 1.9 | 21.2 ± 0.6* | 11.8 ± 0.6† | 27.5 ± 0.7 | 19.5 ± 1.7† |
| Hematocrit, % | 38.5 ± 1.2 | 36.5 ± 0.8 | 63.3 ± 2.3* | 66.1 ± 2.4* | 42 ± 1 | 43.2 ± 1.3 |
Data represent means ± SE of cumulative data from 6–12 rats.
Measurements in hypoxia or monocrotaline (MCT)-treated rats are significantly different (P < 0.05) from corresponding measurements in normoxic rats.
Measurements in NH4Cl-treated (acidosis) rats are significantly different (P < 0.05) from corresponding measurements in normoxic, hypoxic, or MCT rats without NH4Cl treatment.
To address the effectiveness of NH4Cl treatment in inducing nonhypercapnic acidosis we performed arterial blood gas analysis. As shown in Table 1, in rats under hypoxic exposure for 2-wk treatment with NH4Cl resulted in significantly lower pH values compared with hypoxic rats without NH4Cl treatment. The mean pH of hypoxic rats after a 2-wk hypoxic exposure (7.29 ± 0.01) was significantly lower than that of normoxic animals (7.37 ± 0.01, P = 0.0001), and treatment with NH4Cl led to a significantly lower pH (7.08 ± 0.02, P < 0.0001) compared with nontreated hypoxic rats in the same hypoxic chamber. These changes were not due to hypercapnia because mean Pco2 in the NH4Cl-treated hypoxic rats (40 ± 1.8 mmHg) was not significantly different from that in hypoxic rats in the same chamber and without NH4Cl treatment (45 ± 2 mmHg, P = 0.08). Of note, hypoxic rats that received NH4Cl had significantly lower HCO3− levels compared with hypoxic rats without NH4Cl treatment. In MCT-induced PH, treatment with NH4Cl for 1 wk was associated with significantly lower systemic pH (7.26 ± 0.04) and HCO3− (19.5 ± 1.7) compared with MCT-treated rats without NH4Cl treatment (mean pH 7.36 ± 0.01 and mean HCO3− 27.5 ± 0.7, P < 0.05) (Table 1).
Hematocrit, a sensitive indicator of hypoxia, was significantly greater in hypoxic animals (63.3 ± 2.3% after 2 wk hypoxic exposure) than normoxic animals (38.5 ± 1.2%, P < 0.001). Among hypoxic rats, there were no significant differences in hematocrits between NH4Cl-treated (66.1 ± 2.4%) and nontreated rats (63.3 ± 2.3% at 2 wk). This indicates that treatment with NH4Cl did not interfere with the polycythemic response to hypoxia in our experimental animals. Hematocrits were not significantly different between MCT-treated and normoxic rats with or without treatment with NH4Cl (Table 1).
Treatment with NH4Cl decreases RVSP in experimental PH.
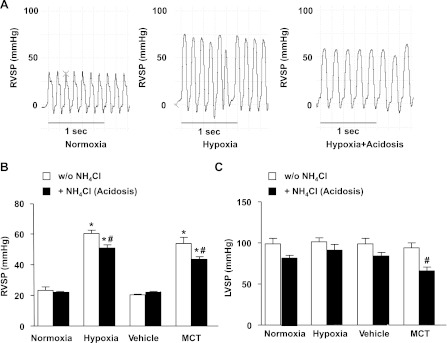
Hemodynamic measurements were performed under isoflurane anesthesia in spontaneously breathing animals using direct right ventricular puncture. Measurements of hemodynamics revealed signs of PH in hypoxic rats. The RVSP, an indicator of blood pressure in the pulmonary circulation, was significantly increased after hypoxic exposure (57.1 ± 2.8 mmHg) compared with normoxia (26.3 ± 4.9 mmHg, P < 0.05) (Fig. 1, A and B). In hypoxic rats, treatment with NH4Cl was associated with a small but significant reduction of RVSP (48.2 ± 2.2 mmHg), although these rats still had significantly higher RVSP than normoxic controls (Fig. 1). LVSP, an indicator of blood pressure in the systemic circulation, was not significantly different in hypoxic compared with normoxic rats, and treatment with NH4Cl did not significantly alter LVSP (Fig. 1C).
Fig. 1.
Effect of treatment with NH4Cl on right ventricular systolic pressure (RVSP) and left ventricular systolic pressure (LVSP) in hypoxic and monocrotaline (MCT)-treated rat models of pulmonary hypertension (PH). A: representative RVSP tracings from individual rats. B: RVSP cumulative data. C: LVSP cumulative data. Data represent means ± SE from 6–12 rats per experimental group. *Measurements in hypoxic or MCT-treated rats are significantly different (P < 0.05) from the corresponding measurements in control normoxic or vehicle-treated rats. #Measurements in NH4Cl-treated hypoxic or MCT rats are significantly different (P < 0.05) from the corresponding measurements in hypoxic or MCT rats without NH4Cl treatment. w/o, Without.
We also examined whether NH4Cl treatment in established PH (21 days after MCT injection) would be effective in reversing MCT-induced PH. Hemodynamic measurements revealed significantly greater RVSP in MCT-treated compared with vehicle-treated rats (Fig. 1B). RVSP was not significantly different in the MCT- vs. hypoxia-induced model of PH. Treatment with NH4Cl resulted in a significant reduction in RVSP in MCT-treated rats, although the RVSP in these animals remained elevated compared with vehicle-treated controls (Fig. 1B). MCT-treated rats had slightly but not significantly lower LVSP compared with vehicle-treated controls, and treatment with NH4Cl was associated with significant decrease in LVSP (Fig. 1C).
Induction of mild acidosis in experimental PH leads to amelioration of RVH.
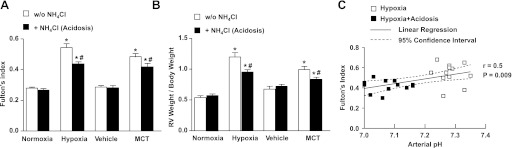
To define the effect of NH4Cl treatment on the hypoxic response of the right ventricle, we next assessed RVH using FI and the ratio of right ventricular weight to total body weight. FI was 0.28 ± 0.007 in normoxic rats and increased significantly after 2 wk of hypoxic exposure (0.54 ± 0.02, P < 0.001) (Fig. 2A). Treatment with NH4Cl for 5 days prior to and during the 2-wk hypoxic exposure resulted in significant amelioration of RVH and reduction of FI (0.44 ± 0.01, P < 0.0001). Similar findings were observed when RVH was assessed as the ratio of right ventricular weight to total body weight (Fig. 2B). Importantly, among the rats exposed to hypoxia for 2 wk with or without treatment with NH4Cl, there was a linear relationship between plasma pH and FI (Fig. 2C). It thus appears that the degree of protection by NH4Cl treatment against RVH is proportional to the degree of metabolic acidosis induced. Similarly, FI was significantly increased in MCT-treated rats compared with vehicle-injected controls, and treatment with NH4Cl significantly decreased FI in MCT-treated animals (Fig. 2A). Also, the right ventricular weight to total body weight ratio was significantly increased in MCT-treated rats compared with vehicle-injected controls and was significantly reduced during treatment with NH4Cl (Fig. 2B).
Fig. 2.
Effect of treatment with NH4Cl on right ventricular hypertrophy (RVH) in hypoxic and MCT-treated rat models of PH. RVH was assessed by Fulton's index (ratio of right ventricular weight to left ventricular+septal weight) (A) and as the ratio of right ventricular weight to total body weight (B). Data represent means ± SE from 6 to 12 rats per experimental group. *Measurements in hypoxic or MCT-treated rats are significantly different (P < 0.05) from the corresponding measurements in control normoxic or vehicle-treated rats. #Measurements in NH4Cl-treated hypoxic or MCT-treated rats are significantly different (P < 0.05) from the corresponding measurements in hypoxic or MCT rats without NH4Cl treatment. C: linear regression and 95% confidence intervals of the relationship between arterial pH and Fulton's index among rats exposed to hypoxia for 2 wk with or without treatment with NH4Cl (acidosis). Each individual rat is represented by an individual data point.
Treatment with NH4Cl preserves pulmonary artery contraction in experimental PH.
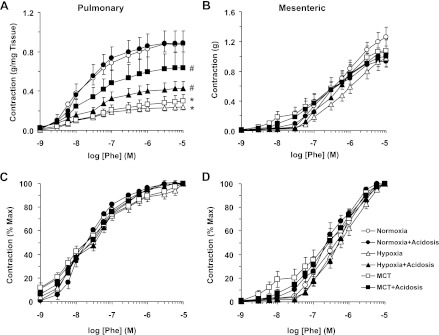
In pulmonary artery rings of normoxic rats, the α-adrenergic agonist Phe caused concentration-dependent contraction that reached a maximum at 10−5 M (Fig. 3A). The Phe-induced contraction was significantly reduced in pulmonary artery rings from hypoxic and MCT-treated rats compared with normoxic rats (Fig. 3A and Table 2), suggesting reduced pulmonary vascular reactivity in PH. In hypoxic and MCT-treated rats that received NH4Cl, Phe-induced contraction was significantly improved to levels approaching, yet still less than, those observed in control normoxic rats (Fig. 3A and Table 2). When the Phe contraction was presented as % of maximum and the ED50 was calculated, Phe appeared to be equally potent in inducing contraction in the pulmonary arteries of the various groups of rats (Fig. 3C and Table 2). In comparison, parallel experiments on mesenteric arteries from the same animals demonstrated that Phe-induced maximum contraction and the Phe ED50 did not differ among the various experimental groups (Fig. 3, B and D, and Table 2).
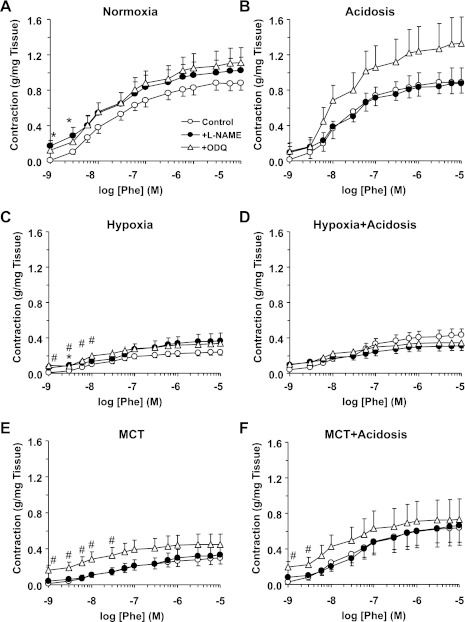
Fig. 3.
Phenylephrine (Phe)-induced contraction in pulmonary artery (A and C) and mesenteric artery (B and D) of normoxic, hypoxic, and MCT-treated rats with or without treatment with NH4Cl (acidosis). Pulmonary artery and mesenteric artery rings were stimulated with increasing concentrations of Phe. The contractile response was measured and presented in g/mg tissue weight (A) or in g (B) or as % of maximum Phe contraction (C and D). Data represent means ± SE (n = 6–8) *Measurements in hypoxia or MCT-treated rats are significantly different (P < 0.05) from corresponding measurements in control normoxic rats. #Measurements in NH4Cl-treated (acidosis) rats are significantly different (P < 0.05) from corresponding measurements in hypoxia or MCT-treated rats without NH4Cl treatment. Max, maximum.
Table 2.
Phe contraction, and ACh and SNP relaxation in pulmonary and mesenteric arteries of control normoxic, hypoxic and MCT-treated rats with or without treatment with NH4Cl (acidosis)
| Parameter | Normoxia | Normoxia+Acidosis | Hypoxia | Hypoxia+Acidosis | MCT | MCT+Acidosis |
|---|---|---|---|---|---|---|
| Pulmonary artery | ||||||
| Phe Max, 10−5 M contraction, g/mg | 0.88 ± 0.08 | 0.89 ± 0.12 | 0.24 ± 0.03* | 0.43 ± 0.07*† | 0.30 ± 0.07* | 0.63 ± 0.16† |
| +l-NAME, 3×10−4 M | 1.02 ± 0.15 | 0.88 ± 0.17 | 0.37 ± 0.09* | 0.31 ± 0.05* | 0.33 ± 0.11* | 0.66 ± 0.22† |
| +ODQ, 10−5 M | 1.11 ± 0.17 | 1.33 ± 0.30 | 0.34 ± 0.06* | 0.35 ± 0.06* | 0.45 ± 0.11* | 0.73 ± 0.23 |
| Phe pED50, −log M | 7.71 ± 0.08 | 7.68 ± 0.09 | 7.67 ± 0.09 | 7.46 ± 0.09 | 7.58 ± 0.16 | 7.53 ± 0.08 |
| +l-NAME | 7.56 ± 0.13 | 7.82 ± 0.13 | 7.47 ± 0.17 | 7.32 ± 0.14 | 7.20 ± 0.18 | 7.42 ± 0.11 |
| +ODQ | 7.81 ± 0.10 | 7.66 ± 0.11 | 7.67 ± 0.14 | 7.59 ± 0.15 | 7.76 ± 0.10 | 7.61 ± 0.15 |
| Phe contraction % 96 mM KCl | 94.97 ± 2.23 | 96.46 ± 4.09 | 94.04 ± 5.90 | 93.64 ± 5.10 | 113.51 ± 10.23 | 104.96 ± 5.53 |
| +l-NAME | 106.17 ± 4.67‡ | 113.93 ± 11.72 | 185.65 ± 66.34 | 113.51 ± 11.15 | 158.80 ± 19.57*‡ | 142.65 ± 11.66‡ |
| +ODQ | 116.72 ± 4.53‡ | 114.25 ± 7.18‡ | 133.74 ± 13.06‡ | 135.85 ± 15.50‡ | 194.34 ± 24.66*‡ | 155.33 ± 10.28*‡ |
| ACh, 10−5 M % relaxation | 48.56 ± 2.88 | 45.33 ± 4.81 | 14.35 ± 5.14* | 31.92 ± 5.53*† | 7.98 ± 3.00* | 18.54 ± 2.42*† |
| SNP, 10−5 M % relaxation | 91.86 ± 2.78 | 95.30 ± 1.56 | 48.18 ± 10.72* | 78.28 ± 6.21† | 58.45 ± 6.07* | 82.22 ± 4.70† |
| Mesenteric artery | ||||||
| Phe Max, 10−5 M contraction, g | 1.26 ± 0.14 | 0.93 ± 0.07 | 0.97 ± 0.12 | 1.06 ± 0.16 | 1.08 ± 0.16 | 1.00 ± 0.14 |
| pED50, −log M | 6.28 ± 0.12 | 6.57 ± 0.15 | 6.15 ± 0.12 | 6.26 ± 0.09 | 6.63 ± 0.25 | 6.55 ± 0.16 |
| ACh, 10−5 M % relaxation | 93.26 ± 3.19 | 77.43 ± 12.21 | 94.18 ± 4.10 14 | 88.68 ± 7.35 12 | 87.45 ± 6.68 | 95.53 ± 2.74 |
| SNP, 10−5 M % relaxation | 96.20 ± 2.02 | 98.85 ± 1.15 | 92.86 ± 7.14 | 100.0 ± 0.00 | 90.48 ± 7.14 | 97.74 ± 1.45 |
Data represent means ± SE of cumulative data from 6–8 rats.
Measurements in hypoxia or MCT-treated rats are significantly different (P < 0.05) from corresponding measurements in normoxic rats.
Measurements in NH4Cl-treated (acidosis) rats are significantly different (P < 0.05) from corresponding measurements in normoxic, hypoxic, or MCT rats without NH4Cl treatment.
Measurements in Nω-nitro-l-arginine methyl ester (l-NAME) or guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ)-treated arteries are significantly different from corresponding measurement in nontreated arteries. Phe, phenylephrine; Max, maximum; SNP, sodium nitroprusside.
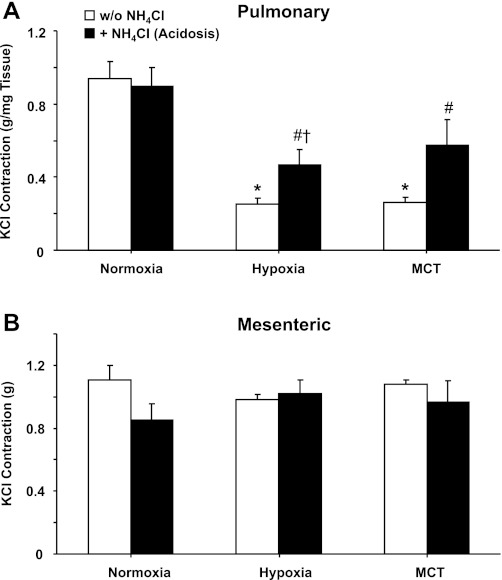
To examine the effect of acidosis on another vasoconstrictor stimulus, the contractile response to membrane depolarization by KCl was examined. Membrane depolarization by 96 mM KCl caused significant contraction in pulmonary and mesenteric artery of normoxic rats (Fig. 4). KCl contraction was significantly reduced in pulmonary arteries of hypoxic and MCT-treated rats compared with normoxic rats, and treatment with NH4Cl significantly improved KCl contraction in the hypoxic and MCT-treated groups (Fig. 4A). KCl-induced contraction remained significantly lower in pulmonary arteries of hypoxia+acidosis rats compared with normoxia+acidosis rats but was enhanced in MCT rats treated with NH4Cl to levels not significantly different from those in normoxia+acidosis rats. In contrast, KCl contraction was not significantly different in mesenteric arteries of normoxic, hypoxic, and MCT-treated animals irrespective of NH4Cl treatment (Fig. 4B).
Fig. 4.
KCl-induced contraction in pulmonary artery (A) and mesenteric artery (B) of normoxic, hypoxic, and MCT-treated rats with or without treatment with NH4Cl (acidosis). Pulmonary and mesenteric artery rings were stimulated with 96 mM KCl, and the contractile response was measured and presented in g/mg tissue (A) or in g (B). Data represent means ± SE (n = 6–8). *Measurements in hypoxia or MCT-treated rats are significantly different (P < 0.05) from corresponding measurements in control normoxic rats. #Measurements in NH4Cl-treated (acidosis) rats are significantly different (P < 0.05) from corresponding measurements in hypoxia or MCT-treated rats without NH4Cl treatment. †Measurements in hypoxia+acidosis rats are significantly different (P < 0.05) from corresponding measurements in normoxia+acidosis rats.
Pretreatment of pulmonary artery with the NOS inhibitor l-NAME (3 × 10−4 M) or the guanylate cyclase inhibitor ODQ (10−5 M) for 10 min caused an increase in basal tension and slightly enhanced the magnitude of Phe-induced contraction in normoxic rats (Fig. 5A and Table 2). In pulmonary artery of normoxic rats treated with NH4Cl, l-NAME did not cause any change in Phe contraction, and ODQ caused an apparent yet insignificant enhancement of Phe contraction (Fig. 5A and Table 2). Treatment of pulmonary artery rings with l-NAME or ODQ caused a small increase in basal tension in hypoxic and MCT-treated rats and minimally enhanced the magnitude of Phe contraction (Fig. 5, C and E, and Table 2), and the Phe responses were still less than those of control normoxic rats. Also, in pulmonary artery rings of hypoxic and MCT-treated rats that received NH4Cl, l-NAME and ODQ caused little change in basal tension and Phe contraction (Fig. 5, D and F, and Table 2). The Phe contractile response as % of maximal Phe-induced contraction and the Phe ED50 were similar in l-NAME- and ODQ-treated pulmonary artery from normoxic rats with or without NH4Cl treatment (Fig. 6, A and B, and Table 2). Although the Phe ED50 was not significantly different in the pulmonary artery of the different experimental groups (Table 2), the Phe contractile response as % of maximum was significantly enhanced with ODQ in pulmonary artery segments of hypoxic and MCT-treated rats with (Fig. 6, D and F) or without treatment with NH4Cl (Fig. 6, C and E). Also, presenting the Phe contraction as % of Ca2+-dependent KCl contraction demonstrated that the Phe contraction was slightly, yet significantly enhanced by ODQ in pulmonary arteries from normoxic rats irrespective of NH4Cl treatment (Fig. 7, A and B). In comparison, in the presence of l-NAME or ODQ, the Phe contraction as % of KCl was dramatically enhanced in hypoxic and MCT-treated rats with (Fig. 7, D and F) or without NH4Cl treatment (Fig. 7, C and E and Table 2). Combined, these findings suggest that a potential compensatory mechanism involving the NO-cGMP signaling pathway may be activated in experimental PH, and NH4Cl treatment did not significantly alter or interfere with this potential rescue mechanism.
Fig. 5.
Effect of blockade of the NO-cGMP pathway on Phe-induced contraction in pulmonary artery of normoxic, hypoxic, and MCT-treated rats with or without treatment with NH4Cl (acidosis). Pulmonary artery rings of control normoxic (A), normoxic+acidosis (B), hypoxic (C), hypoxic+acidosis (D), MCT-treated (E), and MCT+acidosis rats (F) were either nontreated (○) or pretreated with the NO synthase (NOS) inhibitor Nω-nitro-l-arginine methyl ester (l-NAME; 3 × 10−4 M; ●) or the guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; 10−5 M; ▵) for 10 min. The tissues were stimulated with increasing concentrations of Phe, and the contractile response was measured and presented as g/mg tissue weight. Data represent means ± SE (n = 6–8). *Measurements in l-NAME-treated pulmonary artery segments are significantly different (P < 0.05) from corresponding measurements in nontreated segments. #Measurements in ODQ-treated pulmonary artery segments are significantly different (P < 0.05) from corresponding measurements in nontreated segments.
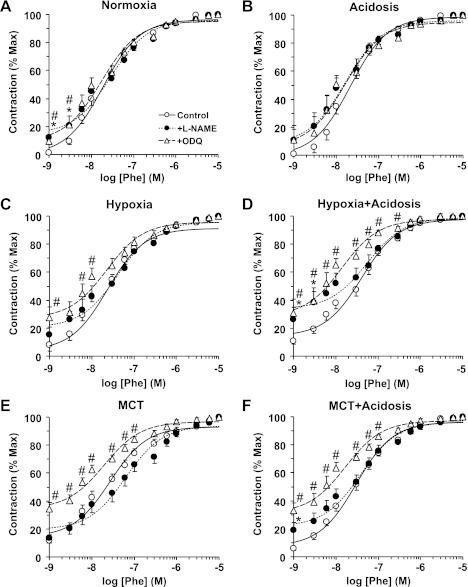
Fig. 6.
Sensitivity to Phe-induced contraction during blockade of the NO-cGMP pathway in pulmonary artery of normoxic, hypoxic, and MCT-treated rats with or without treatment with NH4Cl (acidosis). Pulmonary artery rings of control normoxic (A), normoxic+acidosis (B), hypoxic (C), hypoxic+acidosis (D), MCT-treated (E), and MCT+acidosis rats (F) were either nontreated (○) or pretreated with the NOS inhibitor l-NAME (3 × 10−4 M; ●) or the guanylate cyclase inhibitor ODQ 10−5 M (▵) for 10 min. The tissues were stimulated with increasing concentrations of Phe, and the contractile response was measured and presented as % of maximum Phe contraction. Data represent means ± SE (n = 6–8). *Measurements in l-NAME-treated pulmonary artery segments are significantly different (P < 0.05) from corresponding measurements in nontreated segments. #Measurements in ODQ-treated pulmonary artery segments are significantly different (P < 0.05) from corresponding measurements in nontreated segments.
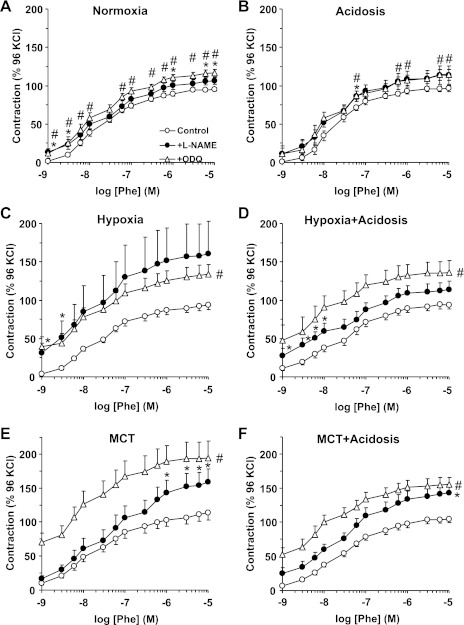
Fig. 7.
Phe-induced contraction as % of Ca2+-dependent 96 mM KCl-induced contraction during blockade of the NO-cGMP pathway in pulmonary artery of normoxic, hypoxic, and MCT-treated rats with or without treatment with NH4Cl (acidosis). Pulmonary artery segments of control normoxic (A), normoxic+acidosis (B), hypoxic (C), hypoxic+acidosis (D), MCT-treated (E), and MCT+acidosis rats (F) were either nontreated (○) or pretreated with the NOS inhibitor l-NAME (3 × 10−4 M; ●) or the guanylate cyclase inhibitor ODQ (10−5 M; ▵) for 10 min. The tissues were stimulated with increasing concentrations of Phe, and the contractile response was measured and presented as % of the control Ca2+-dependent 96 mM KCl-induced contraction. Data represent means ± SE (n = 6–8). *Measurements in l-NAME-treated pulmonary artery segments are significantly different (P < 0.05) from corresponding measurements in nontreated segments. #Measurements in ODQ-treated pulmonary artery segments are significantly different (P < 0.05) from corresponding measurements in nontreated segments.
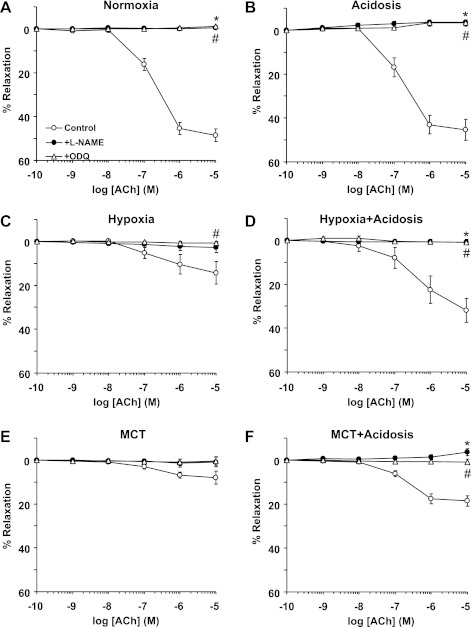
Treatment with NH4Cl improves pulmonary artery relaxation in experimental PH.
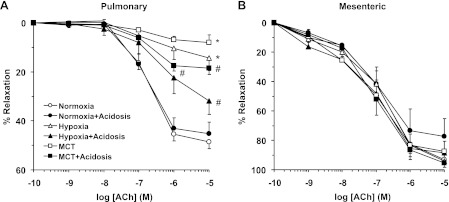
ACh caused concentration-dependent relaxation in Phe-precontracted pulmonary artery rings of normoxic rats that reached a maximum of 48.56 ± 2.88% at 10−5 M (Fig. 8A). ACh relaxation was not significantly different in pulmonary arteries of normoxic rats with or without NH4Cl treatment (Fig. 8A). ACh-induced pulmonary artery relaxation was significantly reduced in hypoxic and MCT-treated rats compared with normoxic rats (Fig. 8A and Table 2), suggesting either reduced production of, or decreased responsiveness to, endothelium-derived vasodilators such as NO in experimental PH. ACh-induced relaxation was enhanced in hypoxic and MCT-treated rats that received NH4Cl to levels approaching, but still less than, those observed in normoxic rats (Fig. 8A and Table 2). In contrast, ACh relaxation was not significantly different in Phe-precontracted mesenteric artery rings of normoxic, hypoxic, and MCT-treated rats with or without NH4Cl treatment (Fig. 8B and Table 2).
Fig. 8.
ACh-induced relaxation in pulmonary and mesenteric artery rings of control normoxic, hypoxic, and MCT-treated rats with or without treatment with NH4Cl (acidosis). Pulmonary artery (A) and mesenteric artery segments (B) were precontracted with Phe (10−5 M), increasing concentrations of ACh were added and the % relaxation of Phe contraction was measured. Data represent means ± SE (n = 6–8). *Measurements in hypoxic and MCT-treated rats are significantly different (P < 0.05) from corresponding measurements in control normoxic rats. #Measurements in NH4Cl-treated (acidosis) rats are significantly different (P < 0.05) from corresponding measurements in hypoxia or MCT-treated rats without NH4Cl treatment.
In pulmonary artery rings of normoxic rats with or without treatment with NH4Cl, the NOS inhibitor l-NAME and the guanylate cyclase inhibitor ODQ abolished ACh relaxation, suggesting the involvement of the NO-cGMP pathway (Fig. 9, A and B). Pretreatment with l-NAME or ODQ also abolished the remaining small ACh-induced relaxation in pulmonary artery of hypoxic and MCT-treated rats (Fig. 9, C and E), suggesting that the residual vasorelaxation response to ACh in experimental PH is mediated by the NO-cGMP pathway. l-NAME and ODQ also inhibited the improved ACh relaxation in hypoxic and MCT-treated rats that received NH4Cl (Fig. 9, D and F), suggesting that the enhanced ACh relaxation induced by NH4Cl involves enhanced production of, or responsiveness to, the NO-cGMP signaling pathway.
Fig. 9.
Effect of blockade of the NO-cGMP pathway on ACh-induced relaxation in pulmonary artery of normoxic, hypoxic, and MCT-treated rats with or without treatment with NH4Cl (acidosis). Pulmonary artery segments of control normoxic (A), normoxic+acidosis (B), hypoxic (C), hypoxic+acidosis (D), MCT-treated (E), and MCT+acidosis rats (F) were either nontreated (○) or pretreated with the NOS inhibitor l-NAME (3 × 10−4 M; ●), or the guanylate cyclase inhibitor ODQ (10−5 M; ▵) for 10 min. The tissues were precontracted with Phe (10−5 M), increasing concentrations of ACh were added and the % relaxation of Phe contraction was measured. Data represent means ± SE (n = 6–8). *Measurements in l-NAME-treated pulmonary artery segments are significantly different (P < 0.05) from corresponding measurements in nontreated segments. #Measurements in ODQ-treated pulmonary artery segments are significantly different (P < 0.05) from corresponding measurements in nontreated segments.
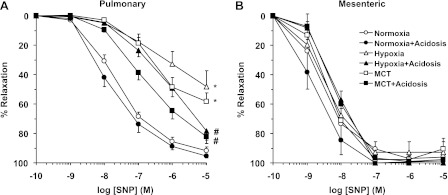
In pulmonary artery segments precontracted with Phe (10−5 M), the exogenous NO donor SNP caused concentration-dependent relaxation that was significantly reduced in hypoxic and MCT-treated rats compared with normoxic rats (Fig. 10A and Table 2), suggesting decreased responsiveness of VSMCs to vasodilators. SNP-induced relaxation was significantly improved in hypoxic and MCT-treated rats that received NH4Cl to levels approaching those observed in normoxic rats (Fig. 10A and Table 2). In contrast, SNP-induced relaxation was not significantly different in mesenteric arteries of normoxic, hypoxic, and MCT-treated rats with or without NH4Cl treatment (Fig. 10B and Table 2).
Fig. 10.
Sodium nitroprusside (SNP)-induced relaxation in pulmonary and mesenteric artery of normoxic, hypoxic, and MCT-treated rats with or without treatment with NH4Cl (acidosis). Pulmonary artery (A) and mesenteric artery segments (B) were precontracted with Phe (10−5 M), increasing concentrations of SNP were added and the % relaxation of Phe contraction was measured. Data represent means ± SE (n = 6–8). *Measurements in hypoxic and MCT-treated rats are significantly different (P < 0.05) from corresponding measurements in normoxic rats. #Measurements in NH4Cl-treated (acidosis) rats are significantly different (P < 0.05) from corresponding measurements in hypoxia or MCT-treated rats without NH4Cl treatment.
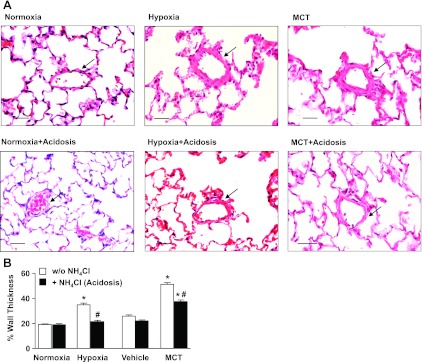
Treatment with NH4Cl ameliorates pulmonary vascular remodeling in experimental PH.
To test for possible relation between the changes in vascular function in the pulmonary vessels and structural remodeling of the pulmonary arterioles, lung histology, and morphometric analysis were performed on lung tissue sections from all experimental groups. In lung tissue sections stained with hematoxylin and eosin, the % wall thickness of the pulmonary arterioles was significantly greater in hypoxic rats compared with control normoxic rats (Fig. 11). In contrast, in hypoxic rats treated with NH4Cl the pulmonary vascular remodeling was markedly reduced, and the thickness of the pulmonary arterioles was comparable to that of the normoxic controls (Fig. 11, A and B). The medial and adventitial thickness was also increased in pulmonary arterioles from MCT-treated rats (day 28) compared with vehicle-treated controls. In MCT rats treated with NH4Cl from day 21 to day 28 after MCT injection (late reversal), a significant reduction in pulmonary arteriolar wall thickness was observed compared with MCT-injected animals without NH4Cl treatment (Fig. 11B).
Fig. 11.
Effect of treatment with NH4Cl on pulmonary arteriolar remodeling in hypoxic and MCT models of PH. A: representative hematoxylin and eosin-stained lung sections from control normoxic, hypoxic (2 wk), and MCT-treated rats with or without treatment with NH4Cl (acidosis). Pulmonary arterioles 50–100 μm in diameter are indicated with arrows. Total magnification ×20. Scale bar = 50 μm. B: quantitative morphometric analysis of % wall thickness of pulmonary arterioles defined as the area occupied by the vessel wall divided by the total cross-sectional area of the arteriole. Fifteen pulmonary arterioles (50–100 μM diameter) from 5 rats per experimental group were analyzed by 2 independent investigators. Percent wall thickness was measured and presented as means ± SE. *Measurements in hypoxic or MCT-treated rats are significantly different (P < 0.05) from corresponding measurements in control normoxic or vehicle-treated rats. #Measurements in NH4Cl-treated hypoxic or MCT-treated rats are significantly different (P < 0.05) from corresponding measurements in hypoxic or MCT-treated rats without NH4Cl treatment.
DISCUSSION
Extracellular pH has an important role in the regulation of systemic and pulmonary vascular tone. Although hypercapnia and acidosis are clinically associated with pulmonary vasoconstriction, studies in adult and newborn rats showed that hypercapnia was protective in hypoxia-induced PH (42, 58). Ooi and colleagues (58) reported that chronic hypercapnia inhibited hypoxic pulmonary vascular remodeling in adult rats but did not report the arterial pH of the hypercapnic rats. The authors found improved ACh-induced relaxation in pulmonary arteries of hypercapnic rats in the setting of hypoxia, which is in agreement with our vascular function findings. However, they found that hypercapnia interfered with the polycythemic response in hypoxic rats, whereas in our study nonhypercapnic acidosis did not interfere with the hypoxic sensing and signaling leading to polycythemia. Kantores and coworkers (42) reported that hypercapnia attenuated oxidant stress and ameliorated PH in a hypoxic neonatal rat model, possibly through prevention of hypoxic upregulation of ET-1 in distal airway epithelium and pulmonary arteriolar wall. Although this study reported mean arterial pH of 7.10 in the hypercapnic/hypoxic rats compared with 7.33 in hypoxic controls, the study did not address whether the protection seen in the hypercapnic rats was mediated by acidosis. Thus, although acidosis was likely present in these studies (42, 58), neither study assessed the role of acidosis in mediating the protective effects of hypercapnia. Interestingly, Hales and colleagues (60–62, 67) suggested that pulmonary artery VSMC Na+/H+ exchanger and intracellular alkalinization may play a pathogenetic role in experimental PH. Together, these studies provide indirect evidence that acidosis may be protective in experimental PH.
To test for a more direct evidence that acidosis is protective in experimental PH, the present study demonstrates that induction of mild nonhypercapnic acidosis in hypoxic and MCT-treated rats is associated with 1) improved pulmonary hemodynamics and reduced RVSP and RVH, 2) improved pulmonary vascular function and relaxation via the endothelium-dependent NO-cGMP pathway, 3) enhanced pulmonary VSMC responsiveness to vasodilators, and 4) decreased pulmonary arteriolar hypertrophic remodeling.
Chronic treatment with NH4Cl has been used to induce metabolic acidosis in experimental animals (22, 28, 41, 49, 55). In the present study, treatment with 1.5% NH4Cl for 19 days in the hypoxic group and 7 days in the MCT group was associated with a decrease in arterial pH, without any significant changes in arterial Pco2 levels, and was well tolerated. Induction of nonhypercapnic acidosis in hypoxic rats was associated with improved indexes of PH including RVSP, RVH, and FI, suggesting efficiency of acidosis in preventing the progression of PH. Also, induction of acidosis in MCT-treated rats was associated with reduced RVSP, RVH, and FI, supporting the efficiency of acidosis intervention in reversing the pathology of PH.
To identify the vascular mechanisms involved in the improved pulmonary hemodynamics during nonhypercapnic acidosis in experimental PH, we examined vascular function in pulmonary arteries from hypoxic and MCT rats with and without NH4Cl treatment. Consistent with our previous report (50), pulmonary artery contraction to the α-adrenergic receptor agonist Phe was reduced in hypoxic and MCT-treated rats. This is in agreement with a report that ET-1-induced constriction of pulmonary artery is reduced in the hypoxic rat model of PH (37). Other studies have shown that chronic hypoxia is associated with increased vasomotor tone and enhanced production/activity of ET-1 and angiotensin II (AngII) in the lung (56, 66). The difference in the results could be due to the vasoconstrictive agonist (Phe vs. ET-1 or AngII) or the vascular preparation used (pulmonary artery vs. isolated perfused lung). Importantly, Phe-induced contraction was improved in hypoxic and MCT rats treated with NH4Cl. This is unlikely due to changes in the sensitivity of α-adrenergic receptors because the Phe ED50 was not significantly different between normoxic, hypoxic, and MCT-treated rats with or without NH4Cl treatment. This is also unlikely due to increased expression of α-adrenoreceptors because contraction to high KCl, a receptor-independent response, was improved in hypoxic and MCT rats treated with NH4Cl, suggesting that nonhypercapnic acidosis may improve a common postreceptor signaling pathway in pulmonary vessels.
To investigate whether the reduced pulmonary artery contraction in hypoxic and MCT rats and its improvement during nonhypercapnic acidosis involve changes in endothelium-dependent NO-cGMP pathway (23, 35), we tested the effects of blockade of NO production by l-NAME or inhibition of guanylate cyclase and cGMP production by ODQ. Even with NOS or guanylate cyclase inhibition, Phe contraction remained significantly less in hypoxic and MCT rats than normoxic rats, suggesting that the α-adrenergic postreceptor signaling mechanisms or the pulmonary artery contractile machinery are less responsive to Phe. Although the Phe ED50 was not significantly different in the pulmonary artery of different experimental groups, the Phe contractile response as % of maximum was markedly enhanced by ODQ in hypoxic and MCT-treated rats with and without acidosis. Also, Phe contraction as % of KCl contraction was enhanced by l-NAME or ODQ in pulmonary arteries from hypoxic and MCT rats with or without treatment with NH4Cl. These findings can be explained by possible activation of the NO-cGMP pathway as a compensatory rescue mechanism in experimental PH, and suggest that NH4Cl treatment does not interfere with this compensatory mechanism. Also, assuming that KCl contraction is mainly due to Ca2+ influx (43), then the enhancing effects of blockers of NO-cGMP on Phe contraction in pulmonary arteries of hypoxic and MCT rats with NH4Cl treatment could be due to increased Ca2+-sensitization pathways of VSMC contraction such as protein kinase C and Rho kinase. These Ca2+-sensitization pathways are likely obscured by compensatory activation of NO-cGMP in experimental PH but uncovered during treatment of pulmonary arteries with blockers of NO-cGMP. This is supported by reports that the RhoA/Rho kinase system plays a key role in PH (15) and that treatment with Rho-kinase inhibitors reduces RVH and reverses pulmonary arterial remodeling in the hypoxic rat model of PH (79).
In concordance with previous reports (2, 50), ACh-induced relaxation was reduced in pulmonary artery of hypoxic and MCT rats. ACh relaxation was improved during induction of nonhypercapnic acidosis in rat models of PH. The enhanced ACh relaxation induced by NH4Cl treatment is less likely due to changes in endothelial cholinergic receptors because the relaxation to the exogenous NO donor SNP was also reduced in PH rats and improved during induction of nonhypercapnic acidosis. Because ACh-induced relaxation was blocked by l-NAME or ODQ, the enhanced ACh relaxation during NH4Cl treatment may be explained by enhanced NO synthesis. This is unlikely the only mechanism, because blockers of NO-cGMP enhanced Phe contraction in pulmonary arteries of hypoxic and MCT rats with or without acidosis, suggesting possible compensatory activation of the NO-cGMP pathway in experimental PH. The reduced ACh relaxation in the PH rats and its improvement with acidosis is also unlikely due to decreased NO bioavailability due to increased oxidative stress in the setting of hypoxia (16), because ACh-induced relaxation was also reduced in the MCT model and improved during induction of acidosis in MCT rats. A plausible explanation for the reduced ACh relaxation in hypoxic and MCT-treated rats is possible structural changes in the pulmonary vascular wall and decreased responsiveness of pulmonary VSMCs to vasodilators. This is supported by the reduced pulmonary artery relaxation to the NO donor SNP in hypoxic and MCT-treated rats, and consistent with the report that both endothelium-dependent and -independent relaxation are reduced in the rat model of MCT-induced progressive lung injury (25). Consequently, the enhanced SNP-induced relaxation in pulmonary arteries of hypoxic and MCT rats treated with NH4Cl can be explained by prevention or reversal of structural changes in the pulmonary vasculature and improved responsiveness of pulmonary VSMCs to vasodilators. However, other factors contributing to the vascular responsiveness to SNP may include PDE-5 and protein kinase G activity and should be examined in future studies.
Increased thickness of pulmonary arterioles is a key structural feature of hypoxic PH, as evidenced by remodeling of the small pulmonary arteries, vascular cell proliferation, and obliteration of the pulmonary microvasculature (9, 21, 53, 59). We and others have shown that PH in hypoxic and MCT-treated rats is associated with reduced pulmonary responsiveness to vasoconstrictors and endogenous and exogenous nitrovasodilators (2, 25, 27, 50, 67) and extensive pulmonary arteriolar thickening and remodeling (9, 12, 44, 50, 53, 59). Multiple mechanisms may contribute to pulmonary vascular remodeling in PH including resident medial pulmonary VSMC hypertrophy and hyperplasia and a phenotypic switch from a contractile to a synthetic phenotype; transdifferentiation of circulating and resident progenitor, adventitial, or endothelial cells to a VSMC-like phenotype; and intimal and adventitial changes.
Although evidence supports a role of pulmonary VSMC phenotypic switch in the pathogenesis PH, an imbalance between pulmonary vasoconstrictors such as ET-1 and vasodilators such as NO and PGI2 has also been implicated in PH (19, 26, 33, 34, 38, 64), and vasodilators are a major component of the current therapy for PH (10, 47, 71, 80). However, a large number of patients do not respond to vasodilators, possibly because of excessive pulmonary vascular remodeling. Interventions to improve the responsiveness of the remodeled pulmonary arteries to vasodilators could be a useful approach in PH. We found that acidosis prevented and reversed established remodeling and improved pulmonary vascular responsiveness to vasoactive mediators. Also, we previously reported that extracellular acidosis inhibited proliferation and migration of cultured rat and mouse VSMCs (8, 11, 30). These observations support that the reduced wall thickness in pulmonary arterioles and improved responsiveness to vasoconstrictor and vasodilator stimuli in pulmonary arteries of PH rats treated with NH4Cl may be related to restoration of VSMC phenotype from proliferative to contractile with enhanced contraction mechanisms and increased plasticity and responsiveness to vasodilator signaling.
A potential confounding factor is the body weight during hypoxia and NH4Cl treatment. Hypoxic animals did not gain weight, and hypoxic animals treated with NH4Cl actually lost weight. The protective effects of NH4Cl on hypoxia-induced PH are unlikely due to changes in body weight because NH4Cl treatment was also protective in MCT-induced PH despite no effect on animal weight. It is also unlikely that weight changes could have altered the hemodynamic and structural indexes of PH because the hemodynamic measurements did not show a correlation with body weight and the analysis of RVH took animal weight into consideration. A potential limitation of the vascular function studies is that they were performed on extralobar pulmonary arteries instead of intralobar resistance vessels, which are thought to play a more important role in the regulation of pulmonary vascular resistance; future studies should compare the effects of nonhypercapnic acidosis in extralobar and intralobar vessels. Another potential limitation is whether the present study directly addressed the contribution of endothelial cells vs. VSMCs to the vascular responses to acidosis. Several pieces of ex vivo and in vitro evidence support that acidosis elicits effects on VSMCs that may be, at least in part, responsible for the protective pulmonary vascular effects observed. Although we did not physically remove the endothelium, we tested the effects of chemical blockade of endothelium-derived NO-cGMP and found that in pulmonary arteries treated with l-NAME or ODQ Phe contraction was still reduced in hypoxia and MCT rat models of PH compared with normoxia rats, and improved in hypoxia+acidosis and MCT+acidosis rats (Table 2). Also, the improved response to SNP, an exogenous NO donor and endothelium-independent vasodilator, in pulmonary arteries from hypoxia+acidosis and MCT+acidosis animals provides indirect evidence that NH4Cl treatment improves VSMC sensitivity to exogenous NO independent of the endothelium. Additionally, the histology data demonstrated thickening of the pulmonary arteriolar wall in hypoxia and MCT rats, and amelioration of the vascular wall hypertrophy in hypoxia+acidosis and MCT+acidosis rats. Furthermore, our previously published in vitro data demonstrated that acidosis decreased VSMCs proliferation and migration and enhanced their susceptibility to apoptosis, which may underlie the ameliorating effects of acidosis on pulmonary vascular remodeling in experimental PH (8). Although these data point to an effect of acidosis on VSMCs, possible contribution of the endothelium to the effects of acidosis on the hemodynamics and vascular responses cannot be ruled out and should be examined in future studies. It is also important to define the effects of nonhypercapnic acidosis on other tissues and organs such as the heart and kidney. In our models of PH, a 2-wk hypoxic exposure or 4-wk after MCT treatment were not associated with significant changes in LVSP or the responsiveness of mesenteric vessels to vasoconstrictor or vasodilator stimuli, indicating specific changes in the pulmonary but not the systemic circulation in experimental PH. Similarly, NH4Cl treatment in the hypoxic model of PH was not associated with significant changes in LVSP or the responsiveness of mesenteric vessels to vasoconstrictor or vasodilator stimuli, supporting specific changes in the pulmonary but not systemic vasculature. In MCT-induced PH, treatment with NH4Cl did not alter the responsiveness of mesenteric vessels to vasoactive mediators but caused a significant reduction in LVSP, possibly due to direct cardiac effects of the combination of MCT (3) and NH4Cl.
The rodent models of hypoxia- and MCT-induced PH share some hemodynamic and histological features with human PH, including increased pulmonary arterial pressure, RVH, and pulmonary vascular remodeling. Although these “classic” animal models may not fully recapitulate the pathological changes in human PH (7, 73) and may lack some of the hallmarks of the disease such as the plexogenic lesions, they have been useful especially in intervention studies. All current therapies (including PGI2, ET-1 receptor antagonists, and PDE-5 inhibitors) for all categories of human PH have been tested and proven successful in these animal models. Future work should test the effectiveness of the acidosis intervention in new models of PH in which neointimal arteriopathy and plexogenic lesions more closely resemble the human condition. These models include the S100A4/Mts1 protein-overexpressing mouse infected with M1-γ-herpesvirus 68, left pneumonectomized MCT-injected rat, chronic hypoxic Sugen 5416 (VEGF receptor blocker)-injected rat or mouse, Sugen 5416-injected athymic nude rat, chronic hypoxic athymic nude rat, MCT-injected endothelin-B receptor-deficient rat, and IL-6-overexpressing hypoxic mouse (1, 14, 29, 38–40, 57, 70, 72, 74, 75, 78).
Perspectives
Human PH is characterized by progressive fibroproliferative obliteration of pulmonary arterioles and various degrees of pulmonary vasoconstriction, inflammation, and thrombosis, leading to progressive increase in pulmonary vascular resistance and RVH and failure (9, 21, 44, 59). Current vasodilator therapies for PH include PDE-5 inhibitors, PGI2 analogs, ET-1 type A receptor antagonists, and Ca2+ channel blockers (63, 68). To enhance their effectiveness, these therapeutic approaches are often used in combination (32, 51). Although many vasodilators also have antiproliferative effects on VSMCs, there is no definitive evidence that pulmonary vascular remodeling in human PH is reversible. Also, current vasodilator therapies are not universally successful in altering PH progression and increasing survival. Therefore, novel approaches that directly target pulmonary vessel wall pathology are needed to reverse the established pulmonary vascular pathology in PH patients.
Careful evaluation of the findings in animal models could spearhead studies in humans to determine the effects of acidosis on the course of PH. Ample experimental evidence supports that specific antiproliferative, proapoptotic, immunomodulatory, and cell-based therapies could be effective in PH (5, 18, 36, 52, 54, 64, 69, 77); however, translation to clinical application is lagging behind these new discoveries. Because vasodilators such as PGI2, nitrates, and PDE-5 inhibitors are commonly used as a first line of treatment in severe PH (10, 47, 71, 80), it is important to find interventions that could enhance the responsiveness of the pulmonary circulation to these vasodilators. The present data suggest that induction of nonhypercapnic acidosis could improve the pulmonary hemodynamics and pulmonary vascular function and could reduce pulmonary artery remodeling and therefore may provide a complementary approach to enhance the effectiveness of vasodilator therapy in PH. Further studies using suitable experimental models of PH and additional methods of inducing acidosis in vivo are needed to define the underlying mechanisms and translational potential of this approach.
GRANTS
This work was supported by grants from The National Heart, Lung, and Blood Institute (HL-65998, HL-98724, HL-55454, HL-85446, and K08HL-77344) and The Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD-60702 and T32HD-007466). H. Christou was supported by the Peabody Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation 121: 2747–2754, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Adnot S, Raffestin B, Eddahibi S, Braquet P, Chabrier PE. Loss of endothelium-dependent relaxant activity in the pulmonary circulation of rats exposed to chronic hypoxia. J Clin Invest 87: 155–162, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akhavein F, St-Michel EJ, Seifert E, Rohlicek CV. Decreased left ventricular function, myocarditis, and coronary arteriolar medial thickening following monocrotaline administration in adult rats. J Appl Physiol 103: 287–295, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Aoshima D, Murata T, Hori M, Ozaki H. Time-dependent phenotypic and contractile changes of pulmonary artery in chronic hypoxia-induced pulmonary hypertension. J Pharm Sci 110: 182–190, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Baber SR, Deng W, Master RG, Bunnell BA, Taylor BK, Murthy SN, Hyman AL, Kadowitz PJ. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol 292: H1120–H1128, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 137: 376–387, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Bauer NR, Moore TM, McMurtry IF. Rodent models of PAH: are we there yet? Am J Physiol Lung Cell Mol Physiol 293: L580–L582, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Brenninkmeijer L, Kuehl C, Miele Geldart A, Arons E, Christou H. Heme Oxygenase-1 does not mediate the effects of extracellular acidosis on vascular smooth muscle cell proliferation, migration, and susceptibility to apoptosis. J Vasc Res 48: 285–296, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation 109: 159–165, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Burger CD. Pulmonary hypertension in COPD: a review and consideration of the role of arterial vasodilators. COPD 6: 137–144, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Christou H, Bailey N, Kluger MS, Mitsialis SA, Kourembanas S. Extracellular acidosis induces heme oxygenase-1 expression in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 288: H2647–H2652, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Christou H, Morita T, Hsieh CM, Koike H, Arkonac B, Perrella MA, Kourembanas S. Prevention of hypoxia-induced pulmonary hypertension by enhancement of endogenous heme oxygenase-1 in the rat. Circ Res 86: 1224–1229, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Christou HA, Khalil RA. Sex hormones and vascular protection in pulmonary arterial hypertension. J Cardiovasc Pharmacol 56: 471–474, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ciuclan L, Bonneau O, Hussey M, Duggan N, Holmes AM, Good R, Stringer R, Jones P, Morrell NW, Jarai G, Walker C, Westwick J, Thomas M. A novel murine model of severe pulmonary arterial hypertension. Am J Respir Crit Care Med 184: 1171–1182, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Connolly MJ, Aaronson PI. Key role of the RhoA/Rho kinase system in pulmonary hypertension. Pulm Pharmacol Ther 24: 1–14, 2011 [DOI] [PubMed] [Google Scholar]
- 16. Cowan DB, Jones M, Garcia LM, Noria S, del Nido PJ, McGowan FX., Jr Hypoxia and stretch regulate intercellular communication in vascular smooth muscle cells through reactive oxygen species formation. Arterioscler Thromb Vasc Biol 23: 1754–1760, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Crossno JT, Jr, Garat CV, Reusch JE, Morris KG, Dempsey EC, McMurtry IF, Stenmark KR, Klemm DJ. Rosiglitazone attenuates hypoxia-induced pulmonary arterial remodeling. Am J Physiol Lung Cell Mol Physiol 292: L885–L897, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Daley E, Emson C, Guignabert C, de Waal Malefyt R, Louten J, Kurup VP, Hogaboam C, Taraseviciene-Stewart L, Voelkel NF, Rabinovitch M, Grunig E, Grunig G. Pulmonary arterial remodeling induced by a Th2 immune response. J Exp Med 205: 361–372, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fagan KA, Oka M, Bauer NR, Gebb SA, Ivy DD, Morris KG, McMurtry IF. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol 287: L656–L664, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Farber HW. The status of pulmonary arterial hypertension in 2008. Circulation 117: 2966–2968, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med 351: 1655–1665, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Fawcett J, Hsu FW, Tsao T, Rabkin R. Effect of metabolic acidosis on the insulin-like growth factor-I system and cathepsins B and L gene expression in the kidney. J Lab Clin Med 136: 468–475, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Fleming I, Busse R. NO: the primary EDRF. J Mol Cell Cardiol 31: 5–14, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Frid MG, Li M, Gnanasekharan M, Burke DL, Fragoso M, Strassheim D, Sylman JL, Stenmark KR. Sustained hypoxia leads to the emergence of cells with enhanced growth, migratory, and promitogenic potentials within the distal pulmonary artery wall. Am J Physiol Lung Cell Mol Physiol 297: L1059–L1072, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fullerton DA, Hahn AR, McIntyre RC., Jr Mechanistic imbalance of pulmonary vasomotor control in progressive lung injury. Surgery 119: 98–103, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Geraci MW, Gao B, Shepherd DC, Moore MD, Westcott JY, Fagan KA, Alger LA, Tuder RM, Voelkel NF. Pulmonary prostacyclin synthase overexpression in transgenic mice protects against development of hypoxic pulmonary hypertension. J Clin Invest 103: 1509–1515, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gillespie MN, Olson JW, Reinsel CN, O'Connor WN, Altiere RJ. Vascular hyperresponsiveness in perfused lungs from monocrotaline-treated rats. Am J Physiol Heart Circ Physiol 251: H109–H114, 1986 [DOI] [PubMed] [Google Scholar]
- 28. Green ML, Hatch M, Freel RW. Ethylene glycol induces hyperoxaluria without metabolic acidosis in rats. Am J Physiol Renal Physiol 289: F536–F543, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Greenway S, van Suylen RJ, Du Marchie Sarvaas G, Kwan E, Ambartsumian N, Lukanidin E, Rabinovitch M. S100A4/Mts1 produces murine pulmonary artery changes resembling plexogenic arteriopathy and is increased in human plexogenic arteriopathy. Am J Pathol 164: 253–262, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guan J, Wu X, Arons E, Christou H. The p38 mitogen-activated protein kinase pathway is involved in the regulation of heme oxygenase-1 by acidic extracellular pH in aortic smooth muscle cells. J Cell Biochem 105: 1298–1306, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guilluy C, Sauzeau V, Rolli-Derkinderen M, Guerin P, Sagan C, Pacaud P, Loirand G. Inhibition of RhoA/Rho kinase pathway is involved in the beneficial effect of sildenafil on pulmonary hypertension. Br J Pharmacol 146: 1010–1018, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoeper MM, Markevych I, Spiekerkoetter E, Welte T, Niedermeyer J. Goal-oriented treatment and combination therapy for pulmonary arterial hypertension. Eur Respir J 26: 858–863, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Homma N, Nagaoka T, Morio Y, Ota H, Gebb SA, Karoor V, McMurtry IF, Oka M. Endothelin-1 and serotonin are involved in activation of RhoA/Rho kinase signaling in the chronically hypoxic hypertensive rat pulmonary circulation. J Cardiovasc Pharmacol 50: 697–702, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Hoshikawa Y, Voelkel NF, Gesell TL, Moore MD, Morris KG, Alger LA, Narumiya S, Geraci MW. Prostacyclin receptor-dependent modulation of pulmonary vascular remodeling. Am J Respir Crit Care Med 164: 314–318, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Ignarro LJ. Nitric oxide. A novel signal transduction mechanism for transcellular communication. Hypertension 16: 477–483, 1990 [DOI] [PubMed] [Google Scholar]
- 36. Ito T, Okada T, Miyashita H, Nomoto T, Nonaka-Sarukawa M, Uchibori R, Maeda Y, Urabe M, Mizukami H, Kume A, Takahashi M, Ikeda U, Shimada K, Ozawa K. Interleukin-10 expression mediated by an adeno-associated virus vector prevents monocrotaline-induced pulmonary arterial hypertension in rats. Circ Res 101: 734–741, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Itoh H, Yokochi A, Yamauchi-Kohno R, Maruyama K. Effects of the endothelin ET(A) receptor antagonist, TA-0201, on pulmonary arteries isolated from hypoxic rats. Eur J Pharmacol 376: 233–238, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Ivy D, McMurtry IF, Yanagisawa M, Gariepy CE, Le Cras TD, Gebb SA, Morris KG, Wiseman RC, Abman SH. Endothelin B receptor deficiency potentiates ET-1 and hypoxic pulmonary vasoconstriction. Am J Physiol Lung Cell Mol Physiol 280: L1040–L1048, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Ivy DD, McMurtry IF, Colvin K, Imamura M, Oka M, Lee DS, Gebb S, Jones PL. Development of occlusive neointimal lesions in distal pulmonary arteries of endothelin B receptor-deficient rats: a new model of severe pulmonary arterial hypertension. Circulation 111: 2988–2996, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ivy DD, Yanagisawa M, Gariepy CE, Gebb SA, Colvin KL, McMurtry IF. Exaggerated hypoxic pulmonary hypertension in endothelin B receptor-deficient rats. Am J Physiol Lung Cell Mol Physiol 282: L703–L712, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Jara A, Felsenfeld AJ, Bover J, Kleeman CR. Chronic metabolic acidosis in azotemic rats on a high-phosphate diet halts the progression of renal disease. Kidney Int 58: 1023–1032, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Kantores C, McNamara PJ, Teixeira L, Engelberts D, Murthy P, Kavanagh BP, Jankov RP. Therapeutic hypercapnia prevents chronic hypoxia-induced pulmonary hypertension in the newborn rat. Am J Physiol Lung Cell Mol Physiol 291: L912–L922, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Khalil RA, van Breemen C. Sustained contraction of vascular smooth muscle: calcium influx or C-kinase activation? J Pharmacol Exp Ther 244: 537–542, 1988 [PubMed] [Google Scholar]
- 44. Klings ES, Farber HW. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med 350: 2521–2522; author reply 2521–2522, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Lagna G, Ku MM, Nguyen PH, Neuman NA, Davis BN, Hata A. Control of phenotypic plasticity of smooth muscle cells by bone morphogenetic protein signaling through the myocardin-related transcription factors. J Biol Chem 282: 37244–37255, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Laudi S, Trump S, Schmitz V, West J, McMurtry IF, Mutlak H, Christians U, Weimann J, Kaisers U, Steudel W. Serotonin transporter protein in pulmonary hypertensive rats treated with atorvastatin. Am J Physiol Lung Cell Mol Physiol 293: L630–L638, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Lee JE, Hillier SC, Knoderer CA. Use of sildenafil to facilitate weaning from inhaled nitric oxide in children with pulmonary hypertension following surgery for congenital heart disease. J Intensive Care Med 23: 329–334, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Li X, Zhang X, Leathers R, Makino A, Huang C, Parsa P, Macias J, Yuan JX, Jamieson SW, Thistlethwaite PA. Notch3 signaling promotes the development of pulmonary arterial hypertension. Nat Med 15: 1289–1297, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lucioni A, Womack C, Musch MW, Rocha FL, Bookstein C, Chang EB. Metabolic acidosis in rats increases intestinal NHE2 and NHE3 expression and function. Am J Physiol Gastrointest Liver Physiol 283: G51–G56, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Mam V, Tanbe AF, Vitali SH, Arons E, Christou HA, Khalil RA. Impaired vasoconstriction and nitric oxide-mediated relaxation in pulmonary arteries of hypoxia- and monocrotaline-induced pulmonary hypertensive rats. J Pharmacol Exp Ther 332: 455–462, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McLaughlin VV, Benza RL, Rubin LJ, Channick RN, Voswinckel R, Tapson VF, Robbins IM, Olschewski H, Rubenfire M, Seeger W. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol 55: 1915–1922, 2010 [DOI] [PubMed] [Google Scholar]
- 52. McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, Puttagunta L, Michelakis ED. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest 115: 1479–1491, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mitani Y, Ueda M, Komatsu R, Maruyama K, Nagai R, Matsumura M, Sakurai M. Vascular smooth muscle cell phenotypes in primary pulmonary hypertension. Eur Respir J 17: 316–320, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Nishimura T, Vaszar LT, Faul JL, Zhao G, Berry GJ, Shi L, Qiu D, Benson G, Pearl RG, Kao PN. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation 108: 1640–1645, 2003 [DOI] [PubMed] [Google Scholar]
- 55. Nowik M, Kampik NB, Mihailova M, Eladari D, Wagner CA. Induction of metabolic acidosis with ammonium chloride (NH4Cl) in mice and rats—species differences and technical considerations. Cell Physiol Biochem 26: 1059–1072, 2010 [DOI] [PubMed] [Google Scholar]
- 56. Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 100: 923–929, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Okada K, Tanaka Y, Bernstein M, Zhang W, Patterson GA, Botney MD. Pulmonary hemodynamics modify the rat pulmonary artery response to injury. A neointimal model of pulmonary hypertension. Am J Pathol 151: 1019–1025, 1997 [PMC free article] [PubMed] [Google Scholar]
- 58. Ooi H, Cadogan E, Sweeney M, Howell K, O'Regan RG, McLoughlin P. Chronic hypercapnia inhibits hypoxic pulmonary vascular remodeling. Am J Physiol Heart Circ Physiol 278: H331–H338, 2000 [DOI] [PubMed] [Google Scholar]
- 59. Pietra GG, Edwards WD, Kay JM, Rich S, Kernis J, Schloo B, Ayres SM, Bergofsky EH, Brundage BH, Detre KM. Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary Pulmonary Hypertension Registry. Circulation 80: 1198–1206, 1989 [DOI] [PubMed] [Google Scholar]
- 60. Quinn DA, Dahlberg CG, Bonventre JP, Scheid CR, Honeyman T, Joseph PM, Thompson BT, Hales CA. The role of Na+/H+ exchange and growth factors in pulmonary artery smooth muscle cell proliferation. Am J Respir Cell Mol Biol 14: 139–145, 1996 [DOI] [PubMed] [Google Scholar]
- 61. Quinn DA, Du HK, Thompson BT, Hales CA. Amiloride analogs inhibit chronic hypoxic pulmonary hypertension. Am J Respir Crit Care Med 157: 1263–1268, 1998 [DOI] [PubMed] [Google Scholar]
- 62. Quinn DA, Honeyman TW, Joseph PM, Thompson BT, Hales CA, Scheid CR. Contribution of Na+/H+ exchange to pH regulation in pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol 5: 586–591, 1991 [DOI] [PubMed] [Google Scholar]
- 63. Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med 327: 76–81, 1992 [DOI] [PubMed] [Google Scholar]
- 64. Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, Grimminger F. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest 115: 2811–2821, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schermuly RT, Kreisselmeier KP, Ghofrani HA, Yilmaz H, Butrous G, Ermert L, Ermert M, Weissmann N, Rose F, Guenther A, Walmrath D, Seeger W, Grimminger F. Chronic sildenafil treatment inhibits monocrotaline-induced pulmonary hypertension in rats. Am J Respir Crit Care Med 169: 39–45, 2004 [DOI] [PubMed] [Google Scholar]
- 66. Shimoda LA, Sham JS, Sylvester JT. Altered pulmonary vasoreactivity in the chronically hypoxic lung. Physiol Res 49: 549–560, 2000 [PubMed] [Google Scholar]
- 67. Silverman ES, Thompson BT, Quinn DA, Kinane TB, Bonventre JV, Hales CA. Na+/H+ exchange in pulmonary artery smooth muscle from spontaneously hypertensive and Wistar-Kyoto rats. Am J Physiol Lung Cell Mol Physiol 269: L673–L680, 1995 [DOI] [PubMed] [Google Scholar]
- 68. Sitbon O, Humbert M, Jais X, Ioos V, Hamid AM, Provencher S, Garcia G, Parent F, Herve P, Simonneau G. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation 111: 3105–3111, 2005 [DOI] [PubMed] [Google Scholar]
- 69. Smith AM, Jones RD, Channer KS. The influence of sex hormones on pulmonary vascular reactivity: possible vasodilator therapies for the treatment of pulmonary hypertension. Curr Vasc Pharmacol 4: 9–15, 2006 [DOI] [PubMed] [Google Scholar]
- 70. Spiekerkoetter E, Alvira CM, Kim YM, Bruneau A, Pricola KL, Wang L, Ambartsumian N, Rabinovitch M. Reactivation of gammaHV68 induces neointimal lesions in pulmonary arteries of S100A4/Mts1-overexpressing mice in association with degradation of elastin. Am J Physiol Lung Cell Mol Physiol 294: L276–L289, 2008 [DOI] [PubMed] [Google Scholar]
- 71. Stehlik J, Movsesian MA. Combined use of PDE5 inhibitors and nitrates in the treatment of pulmonary arterial hypertension in patients with heart failure. J Card Fail 15: 31–34, 2009 [DOI] [PubMed] [Google Scholar]
- 72. Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res 104: 236–244, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 297: L1013–L1032, 2009 [DOI] [PubMed] [Google Scholar]
- 74. Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 15: 427–438, 2001 [DOI] [PubMed] [Google Scholar]
- 75. Taraseviciene-Stewart L, Nicolls MR, Kraskauskas D, Scerbavicius R, Burns N, Cool C, Wood K, Parr JE, Boackle SA, Voelkel NF. Absence of T cells confers increased pulmonary arterial hypertension and vascular remodeling. Am J Respir Crit Care Med 175: 1280–1289, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vitali SH, Mitsialis SA, Liang OD, Liu X, Fernandez-Gonzalez A, Christou H, Wu X, McGowan FX, Kourembanas S. Divergent cardiopulmonary actions of heme oxygenase enzymatic products in chronic hypoxia. PLoS One 4: e5978, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang XX, Zhang FR, Shang YP, Zhu JH, Xie XD, Tao QM, Chen JZ. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J Am Coll Cardiol 49: 1566–1571, 2007 [DOI] [PubMed] [Google Scholar]
- 78. White RJ, Meoli DF, Swarthout RF, Kallop DY, Galaria II, Harvey JL, Miller CM, Blaxall BC, Hall CM, Pierce RA, Cool CD, Taubman MB. Plexiform-like lesions and increased tissue factor expression in a rat model of severe pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 293: L583–L590, 2007 [DOI] [PubMed] [Google Scholar]
- 79. Xu EZ, Kantores C, Ivanovska J, Engelberts D, Kavanagh BP, McNamara PJ, Jankov RP. Rescue treatment with a Rho-kinase inhibitor normalizes right ventricular function and reverses remodeling in juvenile rats with chronic pulmonary hypertension. Am J Physiol Heart Circ Physiol 299: H1854–H1864, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yin N, Kaestle S, Yin J, Hentschel T, Pries AR, Kuppe H, Kuebler WM. Inhaled nitric oxide versus aerosolized iloprost for the treatment of pulmonary hypertension with left heart disease. Crit Care Med 37: 980–986, 2009 [DOI] [PubMed] [Google Scholar]