Abstract
We have previously shown that inhibition of transforming growth factor-β (TGF-β) signaling attenuates hypoxia-induced inhibition of alveolar development and abnormal pulmonary vascular remodeling in the newborn mice and that endothelin-A receptor (ETAR) antagonists prevent and reverse the vascular remodeling. The current study tested the hypothesis that inhibition of TGF-β signaling attenuates endothelin-1 (ET-1) expression and thereby reduces effects of hypoxia on the newborn lung. C57BL/6 mice were exposed from birth to 2 wk of age to either air or hypoxia (12% O2) while being given either BQ610 (ETAR antagonist), BQ788 (ETBR antagonist), 1D11 (TGF-β neutralizing antibody), or vehicle. Lung function and development and TGF-β and ET-1 synthesis were assessed. Hypoxia inhibited alveolar development, decreased lung compliance, and increased lung resistance. These effects were associated with increased TGF-β synthesis and signaling and increased ET-1 synthesis. BQ610 (but not BQ788) improved lung function, without altering alveolar development or increased TGF-β signaling in hypoxia-exposed animals. Inhibition of TGF-β signaling reduced ET-1 in vivo, which was confirmed in vitro in mouse pulmonary endothelial, fibroblast, and epithelial cells. ETAR blockade improves function but not development of the hypoxic newborn lung. Reduction of ET-1 via inhibition of TGF-β signaling indicates that TGF-β is upstream of ET-1 during hypoxia-induced signaling in the newborn lung.
Keywords: lung development, infant, persistent pulmonary hypertension
chronic hypoxia exposure in neonatal mice inhibits lung alveolar septation and the normal postnatal reduction in thickness of the pulmonary arteries. The resulting inhibition of alveolarization and abnormal vascular remodeling creates a reproducible animal model that mimics the histology of human bronchopulmonary dysplasia. We (3) have previously shown that hypoxia attenuates the normal postnatal decrease in endothelin-1 (ET-1) and leads to abnormal pulmonary vascular remodeling (HPVR) in newborn mice. These effects can be prevented and partially reversed by endothelin-A receptor (ETAR) antagonism (2). We (4) have recently demonstrated that inhibition of transforming growth factor-β (TGF-β) signaling attenuates chronic hypoxia-induced inhibition of alveolar development and HPVR in newborn mice. While increased signaling by both TGF-β and ET-1 mediates the effects of hypoxia, the functional relationship between ET-1 and TGF-β signaling in the hypoxic newborn lung has not been determined. It is known that ET-1 and TGF-β have similar profibrogenic effects that converge on common pathways, such as collagen synthesis (10). In isolated perfused adult rat lungs, TGF-β increases ET-1 synthesis (13), while in isolated alveolar epithelial cells, ET-1 increases TGF-β1 synthesis and signaling via ETAR and induces epithelial-mesenchymal transition (11). Based on our previous studies and the existing literature, we hypothesized that inhibition of TGF-β signaling would attenuate ET-1 signaling and thereby reduce effects of hypoxia on the newborn lung (i.e., that ET-1 is downstream of TGF-β), with the alternate hypothesis that ETAR antagonism would attenuate TGF-β signaling (i.e., that TGF-β is downstream of ET-1 signaling via ETAR).
METHODS
All protocols were approved by the Institutional Animal Care and Use Committee of University of Alabama at Birmingham and were consistent with the Public Health Service policy on Humane Care and Use of Laboratory Animals (Office of Laboratory Animal Welfare, August 2002) and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 96–01, revised 2002). All experiments, unless otherwise specified, were done with a minimum of six mice from at least two litters for each experimental condition.
In Vivo Studies
Animal model.
Newborn C57BL/6 mouse pups and their dams were exposed to normobaric hypoxia or room air from birth in a plexiglas chamber as previously described (2, 4, 16), while being administered either ETAR antagonist (BQ610; Ref. 2), ETBR antagonist (BQ788), TGF-β neutralizing antibody (1D11; Ref. 16), or PBS of similar volume (vehicle). Briefly, a Pro-Ox 110 oxygen controller (Bio-Spherix, Redfield, NY) servo-controlled the oxygen concentration to the set level (12% for hypoxia group or 21% for air group) by controlling the inflow of a mixture of nitrogen and O2 gases. O2 concentration (OM-100 oxygen analyzer; Newport Medical Instruments, Newport Beach, CA), humidity, temperature, and barometric pressure (Fisherbrand Digital Barometer; Fisher Scientific, Pittsburgh, PA) within the chamber were monitored continuously. Daily animal maintenance was carried out, with exposure of the animals to room air for <10 min per day. A standard mouse pellet diet and water were provided ad libitum.
For ETAR antagonism, BQ610 (Peptides International, Louisville, KY) at 20 μg·g−1·day body wt−1 (20 mg·kg−1·day−1) was given intraperitoneal daily by microsyringe. This dose was chosen based on the effective dosage in our previous studies (2, 3). For ETBR antagonism, BQ788 (Peptides International; Ref. 17) was similarly given intraperitoneal daily at 20 μg·g−1·day−1. For inhibition of TGF-β signaling, we used TGF-β neutralizing antibody (Clone 1D11, MAB1835; R&D Systems, Minneapolis, MN), which neutralizes all three isoforms of TGF-β (-β1, -β2, and -β3), at a dose of 20 μg by intraperitoneal injection on postnatal days 1, 5, and 10 (20 μg/g body wt on postnatal day 1, 6 μg/g on day 5, and 4 μg/g on day 10). In our previous study (16), this dosing resulted in a marked reduction (50–75%) of TGF-β signaling.
For additional studies of inhibition of TGF-β signaling, we used the DNTGFβRII mouse (on C57BL/6 background) as previously described (4). The DNTGFβRII mouse expresses an inducible cytoplasmically truncated TGFβRII receptor that competes with endogenous receptors for heterodimeric complex (TGFβRI and RII) formation and is thus an inducible dominant-negative mutant (21). The DNTGFβRII lacks the cytoplasmic kinase domain and has no intrinsic activity. The DNTGFβRII is under the control of a metallothionein-derived promoter induced by administering ZnSO4 (20 μg/g ip) daily from birth to DNTGFβRII pups maintained in air (DT-zinc-air group) or hypoxia (DT-zinc-hypoxia group). DNTGFβRII mice given saline (vehicle control) and maintained in air (DT-saline-air group) or hypoxia (DT-saline-hypoxia group) served as controls. This dose of Zn2+ did not affect lung development, and administration of ZnSO4 and induction of the expression of the mutated receptor in the pups were well tolerated (4).
Analysis of lung function.
After completion of hypoxia or air exposure, the mice were sedated with ketamine/xylazine and pulmonary function was evaluated on a flexiVent as previously described (16). Briefly, a 24-G Angiocath was inserted into the trachea and fixed with a ligature of 3–0 silk. The flexiVent apparatus (SCIREQ, Montreal, Canada) equipped with a module 1 was used to perform measurement maneuvers including perturbations (predefined pressure of volume waveforms) such as forced oscillations, using room air in the closed-chest animal. The tidal volume was set at 6 ml/kg, similar to that clinically used, with a respiratory rate of 150/min. Measurements made included total resistance (R; which encompasses Rn, G, and chest wall resistance. Chest wall resistance in the mouse is essentially zero), compliance (C), airway resistance (Rn or Raw; Newtonian resistance, which is primarily the resistance of the central or upper airways), and tissue elastance (H). Calibration of the flexiVent was done using the tracheal cannula to be used, before each experiment. Lung volumes were measured by volume displacement after completion of the flexiVent measurements.
Mice were then euthanized and the lungs were inflation fixed for histology, or lung homogenates were prepared for RNA and protein analysis (4, 5, 16).
Lung morphometry.
For lung morphometry, the chest was opened and the lungs were inflation fixed via the trachea with 10% formalin at 20 cmH2O pressure, and the right ventricle was perfused with formalin at the same pressure until the effluent was clear to flush out the blood in the pulmonary vessels. After formalin fixation for 24 h, samples were changed to 70% ethanol to avoid overfixation. Five-micromolar sections were stained by hematoxylin-eosin for pulmonary morphometry. Each section consisted of a coronal section from apex to base of both lungs from a newborn mouse.
Lung alveolar morphometry was performed as previously described (4, 5, 16) using MetaMorph software (v.6.2r4, Universal Imaging) interfaced with a Nikon TE2000U microscope equipped with a QiCam Fast Cooled high-resolution CCD camera. Alveolar development was evaluated by mean linear intercepts (MLI; an estimate of alveolar size as increased development and septation is associated with smaller alveoli; Ref. 14) and radial alveolar counts (RAC; an estimate of the number of alveolar septae from the terminal bronchiole to the nearest connective tissue septum; Ref. 6). Images from six random ×100 lung fields were taken from each animal, with one image from the apex, middle, and base of each lung for MLI measurement, and six RAC measurements were performed on each animal.
Analysis of mRNA.
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA) from homogenized lung from mice at 14 days of age, treated with DNase I, quantified, and then reverse transcribed using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen). Quantitative real-time PCR (qPCR) for ET-1, ETAR, ETBR, collagens, and TGF-β isoforms (Table 1 showing primer sequences) was performed using the Bio-Rad iCycler System as described previously (4, 5, 16).
Table 1.
Mouse primer sequences (3′-5′) for real-time quantitative RT-PCR
| Primer Name | Sequence |
|---|---|
| 18S forward | GTC TGC CCT ATC AAC TTT CG |
| 18S reverse | ATG TGG TAG CCG TTT CTC A |
| Collagen 1a1 forward | CCA AGG GTA ACA GCG GTG AA |
| Collagen 1a1 reverse | CCT CGT TTT CCT TCT TCT CCG |
| Collagen 1a2 forward | TGT TGG CCC ATC TGG TAA AGA |
| Collagen 1a2 reverse | CAG GGA ATC CGA TGT TGC C |
| Collagen 3a1 forward | TCA AGT CTG GAG TGG GAG G |
| Collagen 3a1 reverse | TCC AGG ATG TCC AGA AGA ACC A |
| ET-1 forward | CCT-GGA-CAT-CAT-CTG-GGT-C |
| ET-1 reverse | TGT-GGC-CTT-ATT-GGG-AAG |
| ETAR forward | ATC-GGG-ATC-CCC-TTG-ATT-AC |
| ETAR reverse | ACA-GCA-ACA-GAG-GCA-GGA-CT |
| ETBR forward | TGT-TCG-TGC-TAG-GCA-TCA-TC |
| ETBR reverse | AGC-AAT-CTG-CAT-ACC-GCT-CT |
| TGF-β1 forward | GCC-CTG-GAT-ACC-AAC-TAT-TGC-TT |
| TGF-β1 reverse | AGT-TGG-CAT-GGT-AGC-CCT-TG |
| TGF-β2 forward | CTT-CAC-CAC-AAA-GAC-AGG-AAC-CT |
| TGF-β2 reverse | TGC-CAT-CAA-TAC-CTG-CAA-ATC-T- |
| TGF-β3 forward | GGA-AAT-CAA-ATT-CAA-AGG-AGT-GG |
| TGF-β3 reverse | AGT-TGG-CAT-AGT-AAC-CCT-TAG-G |
ETAR and ETBR, endothelin-A and -B receptors; TGF-β, transforming growth factor-β.
Western blot analysis.
Newborn mouse lungs were homogenized in 1 ml of a tissue protein extraction reagent (T-PER; Pierce Biotechnology, Rockford, IL) containing complete proteinase inhibitor cocktail (Roche Diagnostics, Basel, Switzerland) and centrifuged at 7,000 g for 5 min, and the supernatant was frozen at −80°C until analysis as described previously (4, 16). Protein concentrations were measured using the Bio-Rad Bradford protein assay (Bio-Rad, Hercules, CA). Ten micrograms of protein per lane were fractionated by 10% Tris-Glycine SDS-PAGE electrophoresis, followed by transfer to a PVDF membrane (Millipore, Billerica, MA). Western Blot analysis was done using specific primary antibodies (developed in rabbit, reactive against mouse) for pSmad2 (1:500; Cell Signaling Technology, Danvers, MA), ETAR (1:1,000; Thermo Scientific, Rockford, IL), ETBR (1:1,000; Thermo Scientific), and β-tubulin (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. The secondary antibody was a goat anti-rabbit secondary antibody (Sigma, St. Louis, MO) used at 1:10,000 dilution for 1 h at room temperature. Immunoreactive bands were visualized by treatment with Immun-Star Western blotting detection reagents (Bio-Rad) according to the manufacturer's instructions. Densitometry was done, normalizing for β-tubulin, a protein that did not change significantly with hypoxia in this model.
ELISA.
All undiluted lung homogenates were analyzed as a single batch for ET-1 by ELISA (R&D Systems) as described in the manufacturer's protocol. ET-1 concentrations were normalized by protein concentration.
Immunohistochemistry.
Antigen retrieval was performed on paraffin-embedded sections by heating in pH 6.0 citrate buffer (LabVision, Fremont, CA) for 20 min. The primary antibody for ET-1 (Phoenix Pharmaceuticals, Burlingame, CA) was used at 1:100 dilution for 30 min, and the secondary antibody and DAB staining kit were used as described in the product manual (DAKO Envision+HRP-DAB; DakoCytomation, Carpineteria, CA).
In Vitro Studies
Endothelial cells.
Temperature-sensitive, conditionally immortalized mouse pulmonary microvascular endothelial cells (mPMVEC) isolated from H-2Kb-tsA58 SV40 large T Ag transgenic mice were kindly provided by Dr. Mark de Caestecker (9). The mPMVEC were used to confirm ET-1 regulation by TGF-β noted by the in vivo studies. Cells were maintained in EGM-2 media (Lonza, Walkersville, MD) with IFN-γ (10 ng/ml; ProSpec, East Brunswick, NJ) at 33°C. To ensure degradation of the SV40 T antigen, cells were changed to 37°C in the absence of IFN-γ for 72 h and passaged once before study.
Cells were seeded at 70–80% confluence in EGM-2 media and made quiescent in serum-free EGM-2 overnight. The cells were exposed to air (21% oxygen) or hypoxia (1% oxygen) with or without TGF-β1 (2 ng/ml; R&D Systems) in the presence or absence of SB431542 [10 μM; a potent inhibitor of TGF-β signaling by inhibition of activin receptor-like kinase receptors; Cayman Chemical, Ann Arbor, MI] or TGF-β neutralizing antibody (1D11; 50 ng/ml) for 8 h. The cells were washed with PBS and total RNA was extracted with TRIzol (Invitrogen), followed by qPCR analysis as described earlier. Conditioned media were evaluated by ELISA for ET-1 (R&D Systems).
Epithelial cells.
Murine lung epithelial (MLE-12; ATCC CRL-2110) cells were a kind gift from Dr. Jeffrey Whitsett (Cincinnati Children's Hospital Medical Center and University of Cincinnati College of Medicine; Ref. 26). They were grown in ATCC complete growth medium and used from passages 18–22. Similar to mPMVEC, the MLE-12 were exposed to air or hypoxia with or without TGF-β1, SB431542, or 1D11 for 8 h followed by RNA isolation and qPCR analysis as described earlier.
Newborn mouse lung fibroblasts.
Newborn mouse lung fibroblasts (NMLF) were isolated by explant culture from the periphery of lung from 1-day-old newborn mice (18). NMLF were maintained in MEM with 10% FBS and penicillin-streptomycin and used from passages 3–6. Similar to mPMVEC, the NMLF were exposed to air or hypoxia with or without TGF-β1, SB431542, or 1D11 for 8 h followed by RNA isolation and qPCR analysis as described earlier.
Additionally, to determine if ET-1 exposure or ETAR antagonism increased TGF-β1,-β2, or -β3 mRNA or TGF-β protein, MLE-12 and NMLF were exposed to ET-1 (100 nM) in combination with either vehicle (PBS) or BQ610 (10 μM) for 8 h, and TGF-β1,-β2, or -β3 mRNA was measured by qPCR analysis as described earlier. mPMVEC were not used as they do not have ETAR. Active and total TGF-β (all isoforms) were measured by the PAI-1 luciferase assay (1). Cell lysates were prepared using reporter lysis buffer (Promega, Madison, WI), and luciferase activity was measured as relative light units using a luminometer (Orion Microplate Luminometer; Berthold, Pforzheim, Germany).
Statistical analysis.
Results were expressed as means ± SE. The data were analyzed by two-way ANOVA to test for separate and combined effects of treatment (ETAR antagonist or vehicle) and hypoxia on measurements. Multiple comparison testing (Student-Newman-Keuls) was performed if statistical significance (P < 0.05) was noted by ANOVA.
RESULTS
No change in mortality was noted in the hypoxia-exposed or BQ610, BQ788, or 1D11-supplemented mouse pups, and the mortality rate was ∼10% in all groups. Hypoxia led to growth retardation in mice (20–30% smaller at 14 days than air-exposed pups; 6–6.5 vs. 7–8 g), and BQ610, BQ788, or 1D11 administration did not alter body weight either in air or hypoxia compared with vehicle-exposed mice.
In Vivo Studies
Hypoxia-induced inhibition of alveolar development was not attenuated by ETAR or ETBR antagonism.
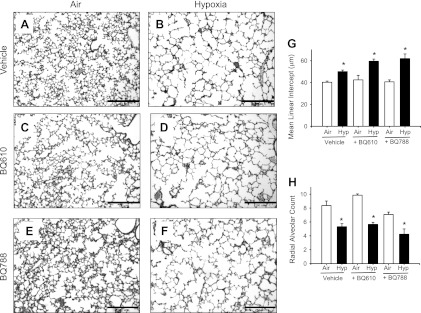
Hypoxia inhibited alveolar development, as evidenced by an increase in the MLI and a reduction in RAC in hypoxia-vehicle compared with air-vehicle pups (Fig. 1, A–H). Air-BQ610 and air-BQ788 had MLI and RAC similar to air-vehicle pups. Hypoxia-BQ610 and hypoxia-BQ788 pups had MLI and RAC similar to hypoxia-vehicle pups, indicating that inhibition of ET-1 signaling via ETAR or ETBR did not change hypoxia-induced inhibition of alveolarization (Fig. 1, A–H).
Fig. 1.
Alveolar development in mouse pups at 14 days of age. A–F: representative photomicrographs of hematoxylin-eosin stained sections of lungs from mouse pups given either vehicle (A and B), BQ610 [endothelin-A receptor (ETAR) antagonist; C and D], or BQ788 (ETBR antagonist; E and F) during 14 days of air (A, C, and E) or hypoxia (B, D, and F) exposure (×100; calibration bars = 250 μm). In mouse pups given vehicle, alveolar size is larger in hypoxia-exposed (B) compared with air-exposed mice (A), indicating delay in septation. Administration of BQ610 or BQ788 did not attenuate the hypoxia-induced increase in alveolar size and did not change alveoli of air-exposed animals. Mean linear intercept (G) and radial alveolar count (H) at 14 days of age in mouse pups given either vehicle, BQ610, or BQ788, while being exposed to air or hypoxia (Hyp; data are means ± SE; n = 6 mice/ group; *P < 0.05 vs. corresponding air; #P < 0.05 vs. corresponding vehicle).
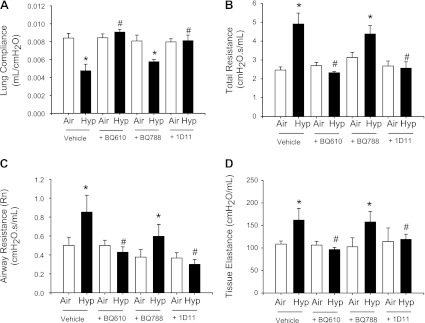
Hypoxia-induced alterations in lung function are prevented by ETAR antagonism but not ETBR antagonism.
Exposure to hypoxia did not alter lung volume (data not shown) but reduced lung compliance and increased total lung resistance in the hypoxia-vehicle mice (Fig. 2, A and B). Increases in both airway resistance and tissue elastance were observed in the hypoxia-vehicle mice. These alterations in lung function were prevented by administration of BQ610 during hypoxia, with the result that lung compliance, total resistance, airway resistance, and tissue elastance in hypoxia-BQ610 pups were similar to those of air-vehicle mice (Fig. 2, A–D). Air-exposed mice given BQ610 had lung function similar to air-vehicle mice (Fig. 2, A–D). The changes in lung function seen in mice given BQ610 were similar to those in mice given 1D11 (inhibition of TGF-β signaling). Air-BQ788 mice had lung function similar to air-vehicle mice, and hypoxia-BQ788 mice had lung function similar to hypoxia-vehicle mice.
Fig. 2.
BQ610 (ETAR antagonist) and 1D11 [transforming growth factor-β (TGF-β) neutralizing antibody], but not BQ788 (ETBR antagonist), prevent hypoxia-induced alterations in lung function. Lung compliance (A), total lung resistance (B), airway resistance (Rn; C), and tissue elastance (D) in 14-day mouse pups exposed from birth to air or hypoxia, in combination with either vehicle, BQ610, BQ788, or 1D11 (data are means ± SE, n = 6 mice/group; *P < 0.05 corresponding air; #P < 0.05 vs. corresponding vehicle).
As ETAR antagonism, but not ETBR antagonism, was noted to mimic the effects of inhibition of TGF-β signaling on lung function and on vascular remodeling in our prior studies (2, 4), subsequent experiments evaluated the interaction of ETAR antagonism with TGF-β signaling.
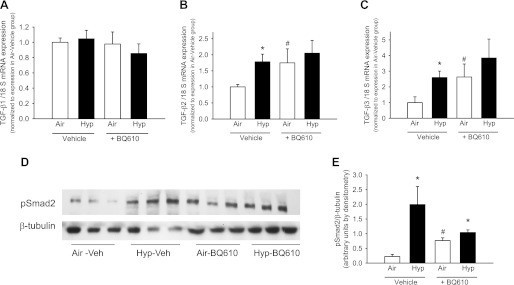
ETAR antagonism increases TGF-β2 and -β3 synthesis and TGF-β signaling.
Exposure to hypoxia increased TGF-β2 and -β3 (but not TGF-β1) mRNA in the hypoxia-vehicle group, associated with an increase in the pSmad2/β-tubulin ratio on the Western blot (Fig. 3, A–E), indicating increased TGF-β signaling. Air-exposed mice given BQ610 had increased TGF-β2 and -β3 mRNA compared with air-vehicle group, similar in magnitude to the hypoxia-vehicle group. The air-BQ610 group also had an increase in the pSmad2/β-tubulin ratio consistent with an increase in TGF-β signaling (Fig. 3, D and E). Hypoxia-BQ610 mice had TGF-β2 and TGF-β3 mRNA expression similar to the hypoxia-vehicle and air-BQ610 mice, i.e, elevated compared with air-vehicle mice. pSmad2/β-tubulin in the hypoxia-BQ610 mice was elevated compared with air-vehicle mice and was not statistically different from the hypoxia-vehicle or air-BQ610 groups (Fig. 3, D and E).
Fig. 3.
BQ610 increases TGF-β2 and -β3 mRNA and pSmad2 protein by Western blot in lung homogenates at 14 days of age. A–C: mRNA levels of TGF-β1 (A), TGF-β2 (B), and TGF-β3 (C) measured by real-time quantitative RT-PCR (qPCR) in homogenized lungs from mice exposed to air or hypoxia in combination with either vehicle or BQ610 from birth to 14 days. D: representative Western blot of lung homogenates for pSmad2 and β-tubulin from lung homogenates of mouse pups given either vehicle or BQ610 during 14 days of air or hypoxia exposure. E: pSmad2/β-tubulin ratio in Western blots quantitated by densitometry (n = 4–6 mice/group; data are means ± SE; *P < 0.05 vs. corresponding air at same time point; #P < 0.05 vs. corresponding vehicle).
Inhibition of TGF-β signaling using 1D11 or DNTGFβRII attenuates ET-1 synthesis.
Exposure to hypoxia increased ET-1 mRNA and protein in vehicle treated wild-type mice (Fig. 4). Immunohistochemical analysis indicated that endothelial cells (primarily) and epithelial cells (alveolar and airway) stained for ET-1, and inhibition of TGF-β signaling was associated with a qualitative reduction of ET-1 at these locations (Fig. 4, A–D). Administration of 1D11 reduced ET-1 mRNA in air-exposed (air-1D11) and hypoxia-exposed (hypoxia-1D11) mice compared with corresponding vehicle controls (air-vehicle and hypoxia-vehicle, respectively; Fig. 4E). ET-1 protein in the hypoxia-1D11 group was reduced compared with hypoxia-vehicle and was statistically not different from air-vehicle or air-1D11 (Fig. 4G). DT-saline-hypoxia mice had an increase in ET-1 mRNA (Fig. 4F) compared with DT-saline-air group. DT-zinc-air and DT-zinc-hypoxia mice with inhibition of TGF-β signaling had reduced ET-1 mRNA compared with the corresponding saline exposed mice (Fig. 4F).
Fig. 4.
Inhibition of TGF-β signaling reduces endothelin-1 (ET-1) immunohistochemical staining and ET-1 synthesis in vivo. A–D: representative photomicrographs of lung sections stained for ET-1 from mouse pups given either vehicle (A and B) or 1D11 (TGF-β neutralizing antibody; C and D) during 14 days of air (A and C) or hypoxia (B and D) exposure (PA, pulmonary artery; Br, bronchiole; ×400; calibration bars = 50 μm). ET-1 (brown staining), primarily in pulmonary artery endothelium, is increased in hypoxia-vehicle mice and reduced in hypoxia-1D11 mice. Homogenized lungs from mouse pups exposed to air or hypoxia in combination with either vehicle or 1D11 from birth to 14 days were used for measurement of mRNA levels of ET-1 by real-time qPCR (E) and ET-1 protein by ELISA (G). F: mRNA levels of ET-1 were measured by qPCR in DNTGFβRII mouse pups exposed to air or hypoxia from birth to 14 days of age in combination with either vehicle or zinc (inducer of DNTGFβRII) (n = 6 mice/group; data are means ± SE; *P < 0.05 vs. corresponding air at same time point; #P < 0.05 vs. corresponding vehicle).
Effects of hypoxia exposure and inhibition of TGF-β signaling on ETAR and ETBR.
No significant changes were noted in ETAR mRNA or protein with either hypoxia exposure or inhibition of TGF-β signaling, or the combination (hypoxia + inhibition of TGF-β signaling; Table 2). ETBR mRNA was increased by hypoxia and inhibition of TGF-β signaling, with no additional increase by the combination (hypoxia + inhibition of TGF-β signaling), but no change in ETBR protein was noted by Western blot (Table 2).
Table 2.
ETAR and ETBR mRNA and protein during hypoxia exposure and inhibition of TGF-β signaling
| DT-Saline-Air (Control) | DT-Saline-Hypoxia | DT-Zinc-Air | DT-Zinc-Hypoxia | |
|---|---|---|---|---|
| ETAR | ||||
| mRNA | 1.0 ± 0.16 | 1.7 ± 0.43 | 1.4 ± 0.18 | 1.7 ± 0.4 |
| Protein | 1.0 ± 0.15 | 1.2 ± 0.03 | 1.2 ± 0.06 | 1.3 ± 0.05 |
| ETBR | ||||
| mRNA | 1.0 ± 0.16 | 3.3 ± 0.03* | 2.4 ± 0.35† | 2.7 ± 0.33 |
| Protein | 1.0 ± 0.09 | 1.3 + 0.05 | 1.4 + 0.1 | 1.2 + 0.08 |
Values are normalized to expression in DNTGFβRI (DT)-Saline-Air (Control) and are means ± SE; n = 6/group. ETAR and ETBR mRNA is by quantitative PCR, and protein is by densitometry of Western blots expressed in arbitrary units.
P < 0.05 vs. corresponding air;
P < 0.05 vs. corresponding vehicle.
Effects of hypoxia exposure and inhibition of ETAR or of TGF-β signaling on collagen.
Hypoxia exposure increased collagen 1a1, 1a2, and 3a1 mRNA by ∼1.50 ± 0.25-fold compared with air-vehicle animals, and both BQ610 (ETAR antagonism) and 1D11 (inhibition of TGF-β signaling) decreased mRNA of all three collagens to air-vehicle levels (0.8–1.2-fold change compared with air-vehicle) during hypoxia exposure, without altering mRNA expression during air exposure (data not shown).
In Vitro Studies
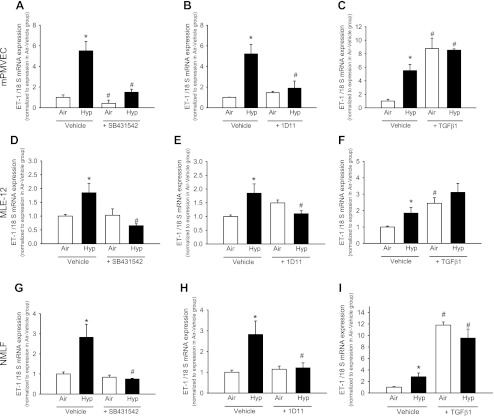
Hypoxia increased ET-1 mRNA in mPMVEC (Fig. 5). Exposure of mPMVEC to SB431542 reduced ET-1 during both air and hypoxia exposure compared with the corresponding vehicle exposed cells (Fig. 5A). 1D11 did not alter ET-1 mRNA in mPMVEC during air exposure, but hypoxia-1D11 cells had ET-1 mRNA lower than hypoxia-vehicle cells and similar to that of air-vehicle cells (Fig. 5B).
Fig. 5.
TGF-β signaling regulates ET-1 synthesis in vitro in mouse pulmonary microvascular endothelial cells (mPMVEC), murine lung epithelial cells (MLE-12), and newborn mouse lung fibroblasts (NMLF). mPMVEC were exposed to air or hypoxia (1% oxygen) in combination with either vehicle or SB431542 (activin receptor-like kinase inhibitor; A), 1D11 (TGF-β neutralizing antibody; B), or TGF-β1 (2 ng/ml; C), and ET-1 mRNA was measured by qPCR. Similar exposures were done for MLE-12 (D–F) and NMLF (G–I) (n = 6 wells/group; data are means ± SE; *P < 0.05 vs. corresponding air at same time point; #P < 0.05 vs. corresponding vehicle).
Addition of TGF-β1 increased ET-1 mRNA in air-exposed and hypoxia-exposed mPMVEC (Fig. 5C). TGF-β1-exposed mPMVEC in hypoxia had ET-1 mRNA expression comparable with TGF-β1-exposed mPMVEC in air (Fig. 5C). The ET-1 concentrations measured by ELISA (in pg/mg of total protein) in conditioned media were as follows: air-vehicle: 2.3 ± 0.5; hypoxia-vehicle: 6.5 ± 0.1; air + TGF-β1: 8.8 ± 0.3; hypoxia + TGF-β1: 7 ± 0.3; air + SB431542: 2.3 ± 0.1; and hypoxia + SB431542: 5.1 ± 0.3. These results indicate that hypoxia and TGF-β1 increase ET-1 release (with no additional increase when combined) and TGF-β inhibition reduces hypoxia-induced increases in ET-1.
Similar results were observed for MLE-12 and NMLF for ET-1 synthesis in response to TGF-β1 (Fig. 5, D–I), although the magnitude of ET-1 mRNA increase with hypoxia was lower in these cells. TGF-β mRNA or protein on exposure to ET-1 or BQ610 did not significantly change (<0.5-fold change) in MLE-12 and NMLF (data not shown).
DISCUSSION
The present study is the first to determine the interaction of the TGF-β signaling pathway with ET-1 signaling in vivo in the hypoxia exposed newborn lung. We confirmed our hypothesis that inhibition of TGF-β signaling attenuated ET-1 synthesis and determined that ETAR antagonism did not diminish and in fact enhanced TGF-β signaling in vivo. Our data indicate that TGF-β is upstream of ET-1 during chronic hypoxia-induced signaling in the newborn lung.
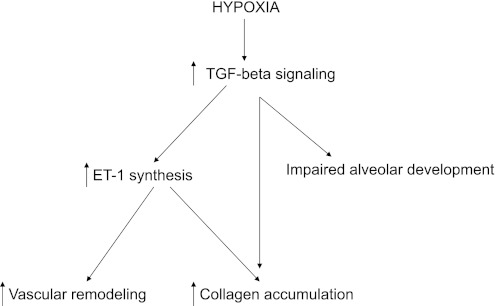
We (4) have shown earlier that blocking TGF-β signaling using inducible DNTGFβRII mice attenuated both neonatal hypoxia-induced abnormal vascular remodeling and hypoxia-induced inhibition of alveolar development, indicating that TGF-β plays a role in mediating both of these processes in the developing lung. This study demonstrates that TGF-β signaling is a regulator of ET-1 synthesis. We (2) have previously demonstrated that ET-1, acting via ETAR, plays a role in the pathophysiology of neonatal HPVR, as administration of BQ610 completely prevented and partially reversed hypoxia-induced increase in pulmonary arterial wall thickness and right ventricular hypertrophy. In this study, we observed no attenuation of hypoxia-induced inhibition of lung development with ETAR or ETBR antagonism, indicating that ET-1 signaling via ETAR or ETBR is not involved in alveolar septation even though it is downstream of TGF-β and involved in vascular remodeling. Together, these findings indicate that TGF-β signaling modulates vascular remodeling via ET-1 acting on ETAR and regulates alveolar development via pathways other than ET-1 (Fig. 6 shows a possible schematic of this interaction). We (15) have recently demonstrated that hypoxia attenuates peroxisome proliferator-activated receptor-γ (PPAR-γ) signaling, and that rosiglitazone (a PPAR-γ agonist) supplementation attenuated hypoxia-induced inhibition of alveolar development and hypoxia-associated enhanced TGF-β signaling, indicating that TGF-β-PPAR-γ interactions may be one of these other mechanisms. Excessive TGF-β signaling is also known to increase lysyl oxidase, a regulator of elastin and collagen cross-linking, another mechanism important in alveolarization (12).
Fig. 6.
Possible schematic of interactions between TGF-β and ET-1 signaling in the newborn lung during hypoxia. During hypoxia, an increase in TGF-β signaling increases ET-1, which contributes to abnormal vascular remodeling and collagen accumulation. Hypoxia-induced increases in TGF-β signaling also inhibit alveolar development through non-ET-1-dependent mechanisms.
Excessive TGF-β signaling associated with Thy-1 deficiency (as noted during hypoxia) is associated with increased lung collagen, decreased lung compliance, and increased lung resistance, and these abnormalities can be prevented by inhibition of TGF-β signaling with 1D11 (16). In the current study, ETAR antagonism prevented the hypoxia-induced decreases in lung compliance and increases in resistance, similar to inhibition of TGF-β signaling with 1D11. We also found that the magnitude of reduction in collagen synthesis with ETAR antagonism and with 1D11 is similar. We (3) have previously observed that hypoxia attenuates the normal postnatal decrease in ET-1 and total collagen content in the newborn murine lung, and ETAR blockade reduced total collagen accumulation independent of oxygen exposure. Other investigators (23, 27) have shown that ET-1 induces a program of matrix synthesis in lung fibroblasts. It is known that matrix proteins such as collagen and elastin are major determinants of lung compliance and elastance (7, 8). Thus we conclude that the reduction with ETAR blockade of the hypoxia-induced accumulation of collagen and possibly other extracellular matrix proteins may contribute to the improvement in lung function.
Interestingly, we found in the current study that ETAR antagonism with BQ610 increased TGF-β signaling and increased TGF-β2 and TGF-β3 mRNA synthesis in vivo, even in normoxia. This novel observation is contrary to our initial assumption that ETAR antagonism would reduce TGF-β signaling. Jain et al. (11) have shown that ET-1 increases TGF-β1 production via ETAR in alveolar epithelial cells in vitro. Our discordant in vivo observations suggest that there may be compensatory mechanisms that increase TGF-β signaling when ET-1 signaling is blocked. The exact mechanisms underlying this effect need to be determined and could not be evaluated further, as this phenomenon was not demonstrated in vitro in our experiments. Regardless, although TGF-β signaling was increased with ETAR blockade, the improvement in lung function and vascular remodeling and reduction in collagen observed with the intervention indicates that the overall effect of ETAR blockade is beneficial. Also, as no inhibition of alveolarization or abnormalities in lung function were noted with elevated TGF-β signaling in the air-BQ610 mice, it is possible that this phenomenon of increased TGF-β signaling with ETAR antagonism is not clinically important.
Inhibition of TGF-β signaling reduced ET-1 in vivo as well as in vitro. This is a novel finding in this model but is not unexpected, as TGF-β increases ET-1 synthesis in isolated perfused adult rat lungs (13) and in isolated human pulmonary microvascular endothelial cells (25) and fibroblasts (22). Shi-wen et al. (22, 24) have shown that ET-1 contributes to the ability of TGF-β to promote a profibrotic phenotype in lung fibroblasts, via induction of ET-1 by TGF-β through a Smad-independent activin receptor-like kinase 5/c-Jun NH2-terminal kinase/activator protein-1 (AP-1) dependent mechanism. The exact mechanism of regulation of ET-1 by TGF-β is controversial. While Shi-wen et al. (24) suggest that TGF-β increases ET-1 via a Smad-independent mechanism through an AP-1 site on the ET-1 promoter, other reports indicate that functional cooperation between Smad proteins and AP-1 is needed (19, 20) and that cAMP-dependent mechanisms may also be contributory (13). Inhibition of TGF-β signaling using 1D11 or SB431542 or DNTGFβRII in the in vitro and in vivo models was associated with roughly comparable effects but with some differences in the magnitude of ET-1 mRNA expression, which may be due to differences in the magnitude of inhibition or precise mechanism of inhibition of TGF-β signaling. It is important to note that while TGF-β2 and -β3 synthesis (and not that of TGF- β1) were increased by hypoxia, TGF-β1 activation is increased during hypoxia by multiple mechanisms [e.g., by reduction in Thy-1 as we have shown earlier (16)] and all three isoforms may contribute to increased downstream signaling during hypoxic exposure. Unfortunately, the current techniques of TGF-β inhibition [e.g., neutralizing antibodies (16) and inducible dominant negative receptor transgenic mice (4)] are not isoform specific, and it is not possible to reliably determine the relative effects of the different isoforms.
Our study has multiple strengths. We evaluated the effects of inhibiting ET-1 and TGF-β signaling during a critical phase of postnatal lung development, during the process of alveolar septation. Both lung structure and function were examined following exposure to normoxia or hypoxia, in combination with either vehicle or BQ610. In addition to assessing the effect of an ETAR antagonist on lung development and function, we also determined the effect of TGF-β signaling on ET-1 synthesis and showed that TGF-β regulates ET-1 using both in vivo and in vitro methods and utilizing multiple methods of inhibition of TGF-β signaling (1D11 neutralizing antibody, SB431542 receptor kinase inhibitor, and DNTGFβRII transgenic mice). We acknowledge that our study also has limitations. Mouse models may not closely simulate human disorders due to interspecies differences. The model of chronic hypoxia exposure, while reproducible and useful, may more closely simulate impaired lung development noted with chronic intrauterine hypoxia and growth restriction rather than that noted with bronchopulmonary dysplasia. TGF-β and ET-1 signaling are ubiquitous in the lung, involving many cell types and with multiple possible interactions, and it is difficult to determine which cell types are primarily responsible for resulting pathophysiology.
In conclusion, chronic hypoxia is associated with impairment of lung development and lung function, associated with abnormal vascular remodeling and increases in ET-1 and TGF-β signaling. We have previously shown that ETAR antagonism prevents and partially reverses hypoxia-induced vascular remodeling (2) and collagen accumulation (3), while inhibition of TGF-β signaling attenuates both vascular remodeling and inhibited alveolar development (4). In this study, we have demonstrated that ETAR antagonism does not attenuate hypoxia-induced inhibition of alveolar development although it improves lung function and showed that TGF-β is upstream of ET-1 during hypoxia-induced signaling in the newborn lung. Our results suggest that endothelin receptor antagonists being increasingly used in infants with bronchopulmonary dysplasia and pulmonary hypertension may improve vascular remodeling but not alveolar development.
GRANTS
This study is supported in part by National Institutes of Health Grants R01-HL-092906, K08-HD-046513, HL-44195, HL-50147, HL-45990, HL-07457, HL-56046, HL-86706, and C06-RR-15490, a Children's Center for Research and Innovation grant, American Thoracic Society/Pulmonary Hypertension Association Grant ATS-PH-06-006, Research Facilities Improvement Program Grant C06-RR-15490, and an American Heart Association Grant 0455197B.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.O. and N.A. conception and design of research; N.O., T.N., W.Z., A.B., M.L.J., and N.A. performed experiments; N.O. and N.A. interpreted results of experiments; N.O., S.O., Y.-F.C., and N.A. edited and revised manuscript; N.O., T.N., W.Z., A.B., M.L.J., S.O., Y.-F.C., and N.A. approved final version of manuscript; N.A. analyzed data; N.A. prepared figures; N.A. drafted manuscript.
ACKNOWLEDGMENTS
This work was presented in part at the American Thoracic Society's Meeting, New Orleans, LA, May 14-19, 2010.
REFERENCES
- 1. Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem 216: 276–284, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Ambalavanan N, Bulger A, Murphy-Ullrich J, Oparil S, Chen YF. Endothelin-A receptor blockade prevents and partially reverses neonatal hypoxic pulmonary vascular remodeling. Pediatr Res 57: 631–636, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ambalavanan N, Li P, Bulger A, Murphy-Ullrich J, Oparil S, Chen YF. Endothelin-1 mediates hypoxia-induced increases in vascular collagen in the newborn mouse lung. Pediatr Res 61: 559–564, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ambalavanan N, Nicola T, Hagood J, Bulger A, Serra R, Murphy-Ullrich J, Oparil S, Chen YF. Transforming growth factor-beta signaling mediates hypoxia-induced pulmonary arterial remodeling and inhibition of alveolar development in newborn mouse lung. Am J Physiol Lung Cell Mol Physiol 295: L86–L95, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ambalavanan N, Nicola T, Li P, Bulger A, Murphy-Ullrich J, Oparil S, Chen YF. Role of matrix metalloproteinase-2 in newborn mouse lungs under hypoxic conditions. Pediatr Res 63: 26–32, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 1–postnatal lung growth. Thorax 37: 572–579, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Faffe DS, D'Alessandro ES, Xisto DG, Antunes MA, Romero PV, Negri EM, Rodrigues NR, Capelozzi VL, Zin WA, Rocco PR. Mouse strain dependence of lung tissue mechanics: role of specific extracellular matrix composition. Respir Physiol Neurobiol 152: 186–196, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Faffe DS, Zin WA. Lung parenchymal mechanics in health and disease. Physiol Rev 89: 759–775, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frank DB, Abtahi A, Yamaguchi DJ, Manning S, Shyr Y, Pozzi A, Baldwin HS, Johnson JE, de Caestecker MP. Bone morphogenetic protein 4 promotes pulmonary vascular remodeling in hypoxic pulmonary hypertension. Circ Res 97: 496–504, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Horstmeyer A, Licht C, Scherr G, Eckes B, Krieg T. Signalling and regulation of collagen I synthesis by ET-1 and TGF-beta1. FEBS J 272: 6297–6309, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Jain R, Shaul PW, Borok Z, Willis BC. Endothelin-1 induces alveolar epithelial-mesenchymal transition through endothelin type A receptor-mediated production of TGF-beta1. Am J Respir Cell Mol Biol 37: 38–47, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumarasamy A, Schmitt I, Nave AH, Reiss I, van der Horst I, Dony E, Roberts JD, Jr, de Krijger RR, Tibboel D, Seeger W, Schermuly RT, Eickelberg O, Morty RE. Lysyl oxidase activity is dysregulated during impaired alveolarization of mouse and human lungs. Am J Respir Crit Care Med 180: 1239–1252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee SD, Lee DS, Chun YG, Paik SH, Kim WS, Kim DS, Kim WD, Tuder RM, Voelkel NF. Transforming growth factor-beta1 induces endothelin-1 in a bovine pulmonary artery endothelial cell line and rat lungs via cAMP. Pulm Pharmacol Ther 13: 257–265, 2000 [DOI] [PubMed] [Google Scholar]
- 14. McGowan S, Jackson SK, Jenkins-Moore M, Dai HH, Chambon P, Snyder JM. Mice bearing deletions of retinoic acid receptors demonstrate reduced lung elastin and alveolar numbers. Am J Respir Cell Mol Biol 23: 162–167, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Nicola T, Ambalavanan N, Zhang W, James ML, Rehan V, Halloran B, Olave N, Bulger A, Oparil S, Chen YF. Hypoxia-induced inhibition of lung development is attenuated by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Am J Physiol Lung Cell Mol Physiol 301: L125–L134, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nicola T, Hagood JS, James ML, Macewen MW, Williams TA, Hewitt MM, Schwiebert L, Bulger A, Oparil S, Chen YF, Ambalavanan N. Loss of Thy-1 inhibits alveolar development in the newborn mouse lung. Am J Physiol Lung Cell Mol Physiol 296: L738–L750, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Okada M, Nishikibe M. BQ-788, a selective endothelin ET(B) receptor antagonist. Cardiovasc Drug Rev 20: 53–66, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Phan SH, Varani J, Smith D. Rat lung fibroblast collagen metabolism in bleomycin-induced pulmonary fibrosis. J Clin Invest 76: 241–247, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodriguez-Pascual F, Redondo-Horcajo M, Lamas S. Functional cooperation between Smad proteins and activator protein-1 regulates transforming growth factor-beta-mediated induction of endothelin-1 expression. Circ Res 92: 1288–1295, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Rodriguez-Pascual F, Reimunde FM, Redondo-Horcajo M, Lamas S. Transforming growth factor-beta induces endothelin-1 expression through activation of the Smad signaling pathway. J Cardiovasc Pharmacol 44, Suppl 1: S39–42, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Serra R, Johnson M, Filvaroff EH, LaBorde J, Sheehan DM, Derynck R, Moses HL. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol 139: 541–552, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shi-wen X, Kennedy L, Renzoni EA, Bou-Gharios G, du Bois RM, Black CM, Denton CP, Abraham DJ, Leask A. Endothelin is a downstream mediator of profibrotic responses to transforming growth factor beta in human lung fibroblasts. Arthritis Rheum 56: 4189–4194, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Shi-Wen X, Renzoni EA, Kennedy L, Howat S, Chen Y, Pearson JD, Bou-Gharios G, Dashwood MR, du Bois RM, Black CM, Denton CP, Abraham DJ, Leask A. Endogenous endothelin-1 signaling contributes to type I collagen and CCN2 overexpression in fibrotic fibroblasts. Matrix Biol 26: 625–632, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Shi-Wen X, Rodriguez-Pascual F, Lamas S, Holmes A, Howat S, Pearson JD, Dashwood MR, du Bois RM, Denton CP, Black CM, Abraham DJ, Leask A. Constitutive ALK5-independent c-Jun N-terminal kinase activation contributes to endothelin-1 overexpression in pulmonary fibrosis: evidence of an autocrine endothelin loop operating through the endothelin A and B receptors. Mol Cell Biol 26: 5518–5527, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Star GP, Giovinazzo M, Langleben D. Effects of bone morphogenic proteins and transforming growth factor-beta on In-vitro production of endothelin-1 by human pulmonary microvascular endothelial cells. Vascul Pharmacol 50: 45–50, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Wikenheiser KA, Vorbroker DK, Rice WR, Clark JC, Bachurski CJ, Oie HK, Whitsett JA. Production of immortalized distal respiratory epithelial cell lines from surfactant protein C/simian virus 40 large tumor antigen transgenic mice. Proc Natl Acad Sci USA 90: 11029–11033, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu SW, Howat SL, Renzoni EA, Holmes A, Pearson JD, Dashwood MR, Bou-Gharios G, Denton CP, du Bois RM, Black CM, Leask A, Abraham DJ. Endothelin-1 induces expression of matrix-associated genes in lung fibroblasts through MEK/ERK. J Biol Chem 279: 23098–23103, 2004 [DOI] [PubMed] [Google Scholar]