Abstract
CC chemokine ligand-2 (CCL2)/monocyte chemoattractant protein (MCP)-1 expression is upregulated during pulmonary inflammation, and the CCL2-CCR2 axis plays a critical role in leukocyte recruitment and promotion of host defense against infection. The role of CCL2 in mediating macrophage subpopulations in the pathobiology of noninfectious lung injury is unknown. The goal of this study was to examine the role of CCL2 in noninfectious acute lung injury. Our results show that lung-specific overexpression of CCL2 protected mice from bleomycin-induced lung injury, characterized by significantly reduced mortality, reduced neutrophil accumulation, and decreased accumulation of the inflammatory mediators IL-6, CXCL2 (macrophage inflammatory protein-2), and CXCL1 (keratinocyte-derived chemokine). There were dramatic increases in the recruitment of myosin heavy chain (MHC) II IA/IEintCD11cint cells, exudative macrophages, and dendritic cells in Ccl2 transgenic mouse lungs both at baseline and after bleomycin treatment compared with levels in wild-type mice. We further demonstrated that MHCII IA/IEintCD11cint cells engulfed apoptotic cells during acute lung injury. Our data suggested a previously undiscovered role for MHCII IA/IEintCD11cint cells in apoptotic cell clearance and inflammation resolution.
Keywords: inflammation resolution, apoptosis, acute lung injury
accumulation of leukocytes at sites of inflammation is essential for host defense during inflammation. CC chemokine ligand-2 (CCL2)/monocyte chemoattractant protein-1 (MCP-1) and its receptor CCR2 have been well documented for their ability to induce leukocyte infiltration during inflammation (8, 19, 38, 39, 43). Studies have shown that CCL2 is elevated in various pulmonary diseases, including chronic obstructive pulmonary disease (10), asthma (47), and interstitial lung diseases such as idiopathic pulmonary fibrosis and systemic sclerosis (30, 31, 44). Reports (5, 41, 52) also suggested that CCL2 is induced after lung injury in animal models. CCR2 is the primary receptor for CCL2 and plays an important role in monocyte recruitment, T-cell polarization, and development of type 1 immune responses to pathogen insults (4, 7, 36, 38, 48).
CCL2-CCR2 interactions induce leukocyte recruitment and cytokine production. There are two major roles of CCL2-CCR2 in this context. The proper activation of the immune system combats infections and tissue injury. For example, overexpression of CCL2 protected mice from bacterial acute lung injury by promoting monocyte infiltration (39, 43, 51). Blockade of CCR2 by administration of anti-CCR2 antibody to Streptococcus pneumoniae-infected mice led to progressive lung inflammation with impaired leukocyte recruitment (51). On the other hand, the exaggerated immune response may lead to fatal outcomes. High chemokine and cytokine levels were found in the patients with H5N1-infection, resulting in intense inflammatory responses (11). A previous study (27) showed that CCL2-overexpressing mice as well as influenza-challenged wild-type (WT) mice recruited CCR2+ monocytes, monocyte-derived dendritic cells, and exudative macrophages (exMACs) in the lung, resulting in the induction of immune pathology and mortality associated with Influenza infection.
A second aspect of the role of CCL2-CCR2 interaction is the clearance of inflammatory cells, infectious particles, and injured tissue debrides. Overexpression of CCL2 improves bacterium clearance in mice (39, 43, 51). Deficiency in CCL2 in mice led to an increased bacterial burden or viral load following Pseudomonas aeruginosa (25) or influenza (12). Blockade of CCR2 by administration of anti-CCR2 antibody to S. pneumoniae-infected mice led to progressive lung inflammation with impaired leukocyte recruitment and bacterium clearance (51). The role of CCL2 in the clearance of apoptotic cells in noninfectious injury is not clear.
During lung inflammation, neutrophil infiltration is followed by apoptosis (40). Resolution of inflammation relies on successful clearance of apoptotic cells (9, 26, 46, 49). Failure of efficient clearance of apoptotic cells (efferocytosis) leads to secondary necrosis resulting in induction of proinflammatory responses (16, 20, 21, 50). Moreover, ingestion of apoptotic cells by macrophages induces the release of anti-inflammatory mediators and suppresses proinflammatory mediator production (13, 15, 29). Administration of CCL2 protein to mice with acute bacterial pneumonia enhanced apoptotic neutrophil clearance and attenuated lung injury, whereas administration of anti-CCL2 antibodies reduced apoptotic neutrophil clearance and worsened lung inflammation (1).
The role of CCL2 in clearance of apoptotic cells in noninfectious injury is not clear. Our current study showed that overexpression of CCL2 significantly protected the mice from bleomycin-induced lung injury. Bleomycin-treated Ccl2 transgenic mice had increased monocyte-macrophages and reduced apoptotic neutrophils in the lung compared with WT mice. We identified a monocyte-macrophage subpopulation, myosin heavy chain (MHC) II IA/IEintCD11cint cells, recruited by CCL2 after lung injury as the major cell type that engulfs apoptotic cells following bleomycin-induced lung injury.
MATERIALS AND METHODS
Mice.
Ccl2 transgenic mice harboring an endogenous human CCL2 transgene under the control of the surfactant protein-C promoter selectively expressed by type II alveolar epithelial cells (SPC-Ccl2) were previously described (19). The mice were backcrossed onto C57Bl/6 background more than eight generations before use. Eight- to ten-week-old Ccl2 transgenic mice and age- and gender-matched littermates were used for experiments. CCR2-deficient mice (CCR2−/−) were purchased from the Jackson Laboratory (Bar Harbor, ME). Ccl2 transgenic mice (Ccl2+) were cross-bred with CCR2-deficient mice to generate CCL2-overexpressing mice on CCR2-deficient background (Ccl2+/CCR2−/−). Mice were housed and cared for in a pathogen-free facility at Duke University, and all animal experiments were approved by the Institutional Animal Care and Use Committee at Duke University.
Bleomycin administration and bronchoalveolar lavage.
Bleomycin was administered to Ccl2 transgenic mice on C57Bl/6 background and age- and gender-matched littermate WT controls as described previously (24). While the animals were under anesthesia, 5 U/kg Blenoxane (Mayne Pharma, Paramus, NJ) in 25 μl PBS were instilled into the mouse trachea with a 26-G needle inserted between the cartilaginous rings of the trachea. To lavage, mice were anesthetized and lungs and heart were surgically exposed. The trachea was cannulated, and the lungs were lavaged three times with 0.8-ml aliquots of cold PBS. The first 0.8-ml bronchoalveolar lavage (BAL) was used for cytokine protein measurement. The live cells from all three 0.8-ml aliquots of BAL were recovered and counted using a hemocytometer. Cytospin preparations of BAL cells were stained conventionally, and differential cell counts were performed.
Flow cytometric analysis of BAL cells.
BAL cells were resuspended in PBS containing 0.5% BSA and 0.02% sodium azide. Nonspecific binding was blocked by incubation with 25 μg/ml Fc Block (BD Biosciences, San Diego, CA) for 15 min at 4°C. Samples were incubated with antibodies to MHCII IA/IE, Gr-1, CD11b, CD11c, Ly-6G (all antibodies were obtained from BD Biosciences), and anti-human CD3 antibody (eBioscience, San Diego, CA) at 4°C for 30 min. Samples were washed twice with PBS and then subjected to flow analysis. For apoptotic staining, annexin V and DNA intercalating agent propidium iodide were used to identify apoptotic cells with fresh samples without fixation. Flow cytometry was performed using a FACSCanto II flow cytometer (BD Immunocytometry Systems, San Jose, CA) and analyzed using FlowJo 8.7 software (Tree Star, Ashland, OR).
ELISA.
BAL fluid (from the first 0.8 ml BAL) was used for ELISA assays. Mouse CXCL2/macrophage inflammatory protein-2 (MIP-2), CXCL1/keratinocyte-derived chemokine (KC), IL-6, and human and mouse CCL2 proteins in mouse BAL were measured immunologically using commercial ELISA kits per the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Apoptotic cell clearance.
Jurkat cells were ultraviolet radiated for 25 min and tumbled at 37°C for 4 h. This routinely yields a population containing ∼70% apoptotic cells. One and a half million apoptotic Jurkat cells were given to bleomycin challenged Ccl2 transgenic mice and littermate WT controls intranasally. Mice were killed and lavaged 2 h later. BAL cells were subjected to antibody staining and flow cytometry analysis.
Statistical analysis.
Data are expressed as the means ± SE where applicable. Differences in measured variables between genetically altered mice and control groups were assessed using the two-sided Student t-test, and the differences between survival curves were determined by the Log-rank test. Statistical difference was accepted at P < 0.05.
RESULTS
CCL2 was upregulated in bleomycin-induced lung injury.
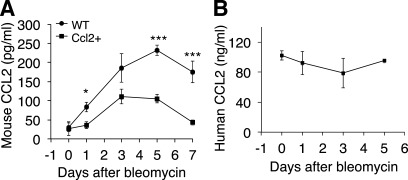
To elucidate the role of CCL2 in noninfectious lung injury, we examined CCL2 protein expression in the BAL of WT C57Bl/6 mice following bleomycin treatment. CCL2 protein rose to a detectable level at day 1 and peaked at day 5 after bleomycin challenge in WT mice (Fig. 1A). The expression pattern of CCL2 protein level is correlated with the amplitude of the bleomycin-induced lung inflammation. These data are consistent with previous reports (41, 52). Mouse CCL2 concentrations in Ccl2 transgenic mice increased following bleomycin lung injury with a similar pattern in WT mice but at a lower level (Fig. 1A). High concentrations of human CCL2 protein were expressed in Ccl2 transgenic mouse lungs under static states (Fig. 1B) at ∼500 times higher than that of mouse CCL2 protein (Fig. 1, A and B). Human CCL2 protein concentrations were largely unchanged following bleomycin injury (Fig. 1B).
Fig. 1.
CC chemokine ligand-2 (CCL2) protein was upregulated after bleomycin-induced lung injury. A: 8- to 10-wk old C57Bl/6 wild-type (WT) mice and Ccl2 transgenic mice were treated with bleomycin at 5 U/kg intratracheally. Mice were killed at indicated time points and mouse CCL2 proteins in bronchoalveolar lavage (BAL) were measured using ELISA (n = 4–5 mice; *P < 0.05, ***P < 0.001). B: high levels of human CCL2 were expressed in Ccl2 transgenic mouse BAL with and without bleomycin challenge (n = 3–5 mice).
Ccl2 transgenic mice were markedly resistant to mortality from bleomycin-induced lung injury.
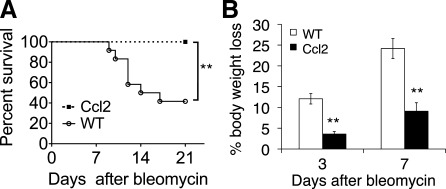
Age- and gender-matched Ccl2 transgenic mice and littermate control mice were subjected to bleomycin treatment. While WT littermates died after bleomycin treatment at a rate similar to previous reports (45), all Ccl2 transgenic mice survived bleomycin challenges at 5 U/kg (Fig. 2A). This unexpected protection of Ccl2 mice was accompanied by significant protection from body weight loss at day 3 and day 7 after bleomycin treatment compared with that of WT littermates (Fig. 2B). Importantly, there were no differences in baseline body weight between unchallenged Ccl2 transgenic mice and littermate controls. The average body weight of 8- to 10-wk-old male CCL2 mice was 24.9 g and wild type was 25.0 g (n = 12–13; P = 0.766). The average body weight of female CCL2 mice was 19.2 g and wild type was 18.3 g (n = 11–13; P = 0.088).
Fig. 2.
Ccl2 transgenic mice were resistant to bleomycin challenge. A: Ccl2 transgenic mice and WT littermate controls were treated with bleomycin. Percentages of mice surviving were recorded through 21 days after bleomycin treatment at 5 U/kg (n = 12; **P < 0.01). This represents 4 similar experiments. B: reduced body weight loss in Ccl2 transgenic mice after bleomycin injury. Mice were treated with bleomycin at 5 U/kg and weighed before and after treatment. Percentage of body weight loss on day 3 and day 7 after treatment compared with body weight at day 0 was recorded (n = 5, **P < 0.01 between genotypes). This represents 3 similar experiments.
Dysregulated inflammatory cell accumulation in Ccl2 transgenic mouse lungs after bleomycin injury.
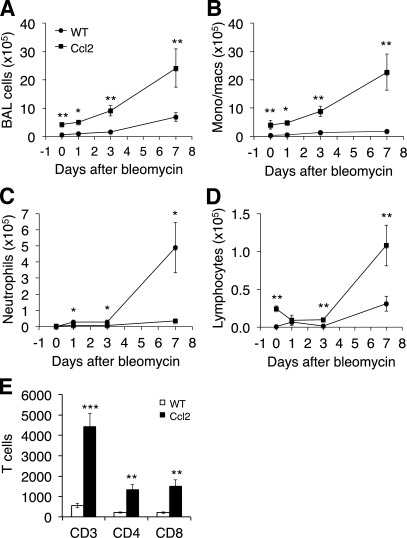
To explore the mechanisms by which overexpression of CCL2 protects mice from bleomycin-induced lung injury, we examined the inflammatory response in the lungs of Ccl2 transgenic mice and littermate controls after bleomycin treatment. BAL cells were collected from Ccl2 transgenic and WT mice treated with bleomycin 5 U/kg at indicated days. Total live cells from BAL fluid were counted, and significant increases in total BAL cells in Ccl2 transgenic mouse lung following bleomycin treatment were observed (Fig. 3A). BAL differential cell counts showed a significant increase in monocytes/macrophages in Ccl2 transgenic mouse lungs compared with WT mice both at baseline and after bleomycin treatment (Fig. 3B). Interestingly, a significant reduction in neutrophil accumulation was observed in bleomycin-treated Ccl2 transgenic mice compared with that of WT mice (Fig. 3C). There was also an increase in lymphocytes in Ccl2 transgenic mouse lungs (Fig. 3, D and E). Flow cytometry analysis showed the increased lymphocytes in CCL2 transgenic mouse lungs were mainly CD3 positive T cells, and both CD4+ and CD8+ cells were increased (Fig. 3E).
Fig. 3.
Profile of inflammatory cells in the lung. BAL cells were collected from Ccl2 transgenic and WT mice before (day 0) and after bleomycin treatment (5 U/kg) at indicated days. A: total live cells recovered from BAL fluid were counted. B–D: cell differential counts were performed after cytospin and staining. B: monocytes/macrophages. C: neutrophils. D: lymphocytes (day 0, n = 3; day 1, n = 4–5; day 3, n = 7; day 7, n = 8 – 11; *P < 0.05, **P < 0.01 between genotypes). Similar results were obtained from three separate experiments. E: BAL cells isolated 3 days after bleomycin were stained with T-cell markers and flow cytometry analysis was used to determine the percentage of T cells in BAL (n = 4; **P < 0.01, ***P < 0.001 between genotypes).
CCR2 deficiency abolished the protective effect of CCL2 against bleomycin-induced lung injury.
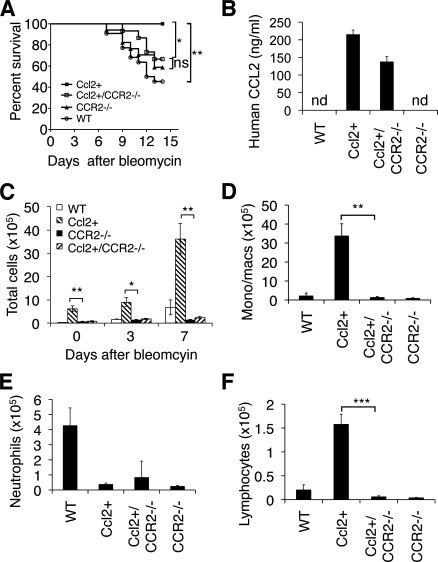
CCL2 binds and activates CCR2 (6, 14) and other chemokine receptors (3). We therefore wanted to determine whether the protective effect of CCL2 was dependent on CCR2. Ccl2 transgenic mice were crossed with CCR2 null mice to generate mice overexpressing CCL2 on a CCR2 null background (Ccl2+/CCR2−/−). Although Ccl2+/CCR2−/− mice produced high levels of human CCL2 protein (Fig. 4B), the survival advantage after bleomycin treatment was lost (Fig. 4A). We then compared the inflammatory cell influx between Ccl2+/CCR2−/− and Ccl2+ mice after bleomycin treatment and found that there was a significantly reduction of total BAL cells in Ccl2+/CCR2−/− mice compared with Ccl2+ mice (Fig. 4C). Cell differential count of BAL cells from day 7 bleomycin-treated mice showed that monocytes/macrophages and lymphocytes in BAL fluid of Ccl2+/CCR2−/− mice were significantly reduced compared with that in the BAL fluid of Ccl2+ mice (Fig. 4, D and F). A significant reduction in neutrophils was observed in Ccl2 transgenic mice as well as in CCR2−/− mice compared with that of WT mice (Fig. 4E). Crossing Ccl2+ mice with CCR2−/− mice did not restore neutrophils (Fig. 4E). These data demonstrated that the protective effect of CCL2 depends on the presence of CCR2.
Fig. 4.
CCR2 deficiency abolished the protective effect of CCL2 to lung injury and leukocyte recruitment. A: Ccl2 transgenic, CCR2−/−, Ccl2+/CCR2−/−, and WT mice were treated with bleomycin at 5 U/kg, and the percentage of mice survived was recorded through 14 days after treatment (n = 12–15 each group; **P = 0.0025 between WT and Ccl2+; *P = 0.031 between Ccl2+ and Ccl2+/CCR2−/−; ns, not significant). B: human CCL2 protein in BAL of Ccl2 transgenic, CCR2−/−, Ccl2+/CCR2−/−, and WT mice was measured with ELISA (n = 3–5; nd, not detectable). C: total BAL cell count of untreated (day 0) and bleomycin-treated (day 3 and day 7) Ccl2 transgenic, CCR2−/−, Ccl2+/CCR2−/−, and WT mice (n = 4–5; P values indicate differences between Ccl2+ mice and Ccl2+/CCR2−/− mice; **P < 0.01, *P < 0.05 between genotypes). Similar results were obtained in 2 separate experiments. D–F: cell differential counts were performed with BAL cells from day 7 bleomycin-treated mice. D: monocytes/macrophages. E: neutrophils. F: lymphocytes (n = 4–8; **P < 0.01, ***P < 0.001).
Ccl2 transgenic mice had reduced accumulation of inflammatory mediators in BAL after bleomycin-induced lung injury.
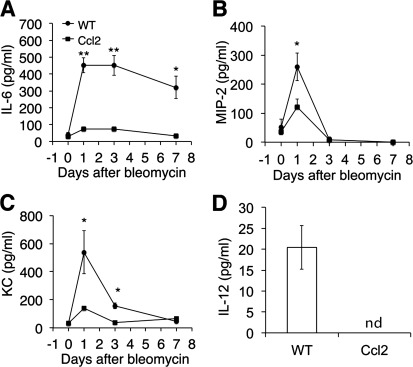
Next, we wanted to determine if CCL2 expression influences cytokine release during bleomycin injury. We measured the inflammatory mediators IL-6, CXCL1/KC, and CXCL2/MIP-2 in the BAL of Ccl2 transgenic mice and WT controls at different time points from day 1 to day 7 after bleomycin treatment and found a significant reduction in all three inflammatory cytokines in the BAL of Ccl2 transgenic mice compared with that of WT mice (Fig. 5, A–C).
Fig. 5.
Reduced inflammatory mediators in Ccl2 transgenic mouse lungs. BAL of Ccl2 transgenic and WT control mice were collected at indicated days after bleomycin treatment (5 U/kg). IL-6 (A), CXCL2/macrophage inflammatory protein-2 (MIP-2; B), and CXCL1/keratinocyte-derived chemokine (KC; C) proteins in BAL were measured (n = 4–6; *P < 0.05, **P < 0.01 between genotypes). D: IL-12 proteins in BAL from 1 day after bleomycin were measured. Similar results were obtained from three separate experiments.
IL-12 was also reduced in the BAL of bleomycin-treated Ccl-2 transgenic mice (Fig. 5D).
Overexpression of CCL2 led to fewer apoptotic neutrophils in the lung following bleomycin-induced injury.
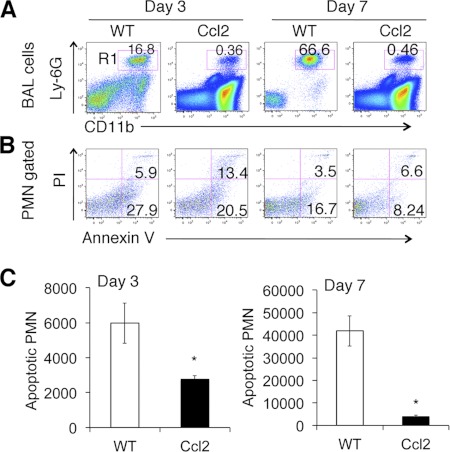
The observation of increased mononuclear cell recruitment (Fig. 3B) coupled with a significant decrease in neutrophils in bleomycin-treated Ccl2 transgenic mouse lungs (Fig. 3C) led us to anticipate that there might be enhanced clearance of apoptotic cells in the context of CCL2 overexpression. We analyzed apoptotic neutrophils at day 3 and day 7 after bleomycin treatment by staining BAL neutrophils with annexin V and propidium iodide. We found that the percentage of neutrophils (CD11b+Ly-6G+) was significantly lower in bleomycin-treated Ccl2+ mouse BAL compared with that in the BAL of WT mice (Fig. 6A), consistent with the observation of fewer neutrophils within the BAL of Ccl2+ mice by cell differential count. The percentage of apoptotic cells within total neutrophil population of Ccl2+ mice was not lower than that of WT mice (Fig. 6B). However, the total number of apoptotic neutrophils in the BAL of Ccl2+ mice was markedly lower compared with that of WT mice (Fig. 6, B and C).
Fig. 6.
Reduced apoptotic neutrophils in Ccl2 transgenic mouse lung. A: CD11b vs. neutrophil marker Ly-6G identified much less neutrophils (R1, CD11b+Ly-6G+) in Ccl2 transgenic mice than in WT. B: quadrant analysis of PI and annexin V staining of (R1) gated BAL neutrophils [polymorphonuclear neutrophils (PMN); n = 4–5]. C: total number of apoptotic neutrophils (PI, propidium iodide; annexin V+PI− and annexin V+PI+) in BAL of day 3 and day 7 bleomycin-treated Ccl2 and WT mice (n = 3–4; *P < 0.05). Similar results were obtained from 3 separate experiments.
Overexpression of CCL2 led to the recruitment of unique inflammatory cell subpopulations in the lung.
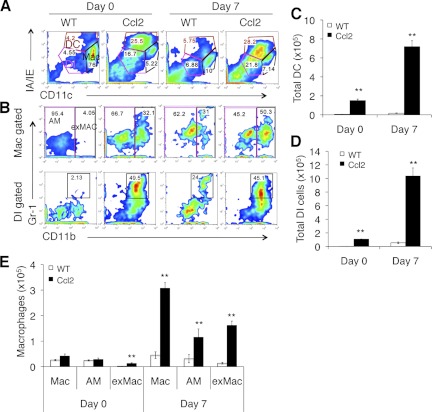
We utilized the approach published previously (27) to analyze the inflammatory cell populations in Ccl2 transgenic mice after noninfectious lung injury. We found that the previously described double intermediate population (DI; IA/IEintCD11cint) was markedly increased in the BAL of unchallenged Ccl2 transgenic mice compared with that of WT mice. This increase was further enhanced after bleomycin injury (Fig. 7A). We also found that the percentage of dendritic cells (IA/IEhiCD11chi) was increased in the BAL of Ccl2 transgenic mice compared with that of WT mice, either with or without bleomycin injury (Fig. 7A).
Fig. 7.
Flow cytometry analysis of BAL cells. BAL cells from untreated (day 0) and bleomycin-treated (day 7) Ccl2 transgenic and WT mice were stained with monocyte/macrophage markers and were subjected to flow cytometric analysis on gated total live cells. A: IA/IE vs. CD11c expression separated dendritic cells (DC; IA/IEhiCD11chi), macrophages (Mac; IA/IEintCD11chi), and double intermediate (DI; IA/IEintCD11cint). B: CD11b vs. Gr-1 expression within macrophage gate distinguishes CD11b− alveolar macrophages (AM) and CD11b+ exudate macrophages (exMAC). Gr-1+CD11b+ cells in the DI cells were increased by both injury and expression of CCL2. C-E: total cell number of gated dendritic cells (C), DI cells (D), macrophages, and exMACs AMs (E) in BAL of Ccl2 transgenic mice and WT mice at day 0 and day 7 after bleomycin (n = 4–5; **P < 0.01 between genotypes). Similar results were achieved from 3 separate experiments.
In unchallenged WT mice, resident alveolar macrophages (IA/IEintCD11chi) are the major cell type in BAL (Fig. 7A). Percentages of alveolar macrophages in bleomycin-treated Ccl2 transgenic mice were similar to bleomycin-treated WT mice (Fig. 7A). CD11b expression within the macrophage gate distinguishes CD11b− resident alveolar macrophages (IA/IEintCD11chiCD11b−) and CD11b+ exMACs (IA/IEintCD11chiCD11b+; Fig. 7B). BAL of naïve WT mice contained mainly CD11b− alveolar macrophages, and CD11b+ exMACs increased upon bleomycin treatment in WT mice (Fig. 7B). There was a high percentage of CD11b+ exMACs in BAL of both unchallenged and bleomycin-treated Ccl2 transgenic mice.
Within gated DI cells, there was a higher percentage of Gr-1+CD11b+ cells in the BAL of both unchanged and bleomycin-treated Ccl2 mice compared with that of WT mice (Fig. 7B). In WT mice, very few Gr-1+CD11b+ DI cells in BAL were observed in unchanged state, and the percentage of Gr-1+CD11b+ cells was increased upon bleomycin treatment at day 7 (Fig. 7B).
The total cell numbers of dendritic cells (Fig. 7C), DI cells (Fig. 7D), and macrophages (alveolar macrophages and exMACs; Fig. 7E) in the BAL of Ccl2+ mice were significantly higher both at baseline and after bleomycin challenge. These data demonstrate that CCL2 overexpression augments bleomycin injury-induced recruitment of exMACs, DI cells, and dendritic cells.
Enhanced apoptotic cell clearance in Ccl2 transgenic mice after bleomycin-induced lung injury.
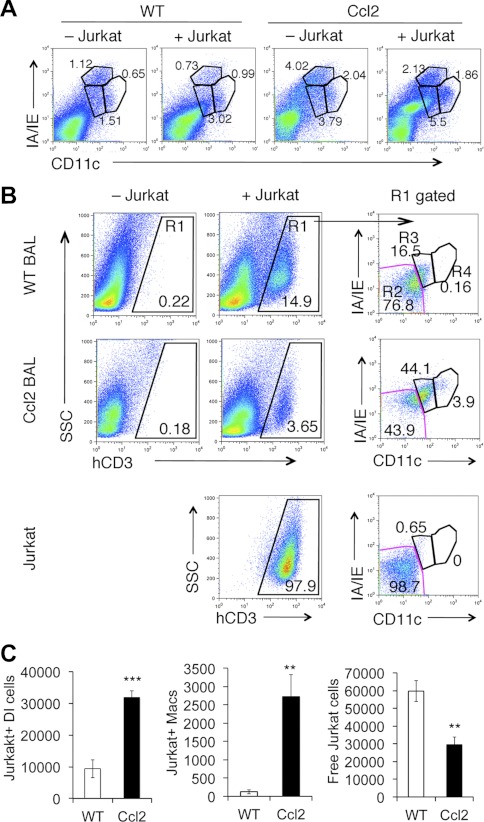
To further define the enhanced apoptotic cell clearance of Ccl2 transgenic mice and to identify the subpopulations of mononuclear cells that are responsible for uptake of apoptotic cells in lungs, apoptotic Jurkat cells were instilled into the lungs of bleomycin-treated Ccl2 transgenic and WT mice. Mice were killed 2 h after Jurkat cell instillation, and total BAL cells were subjected to flow cytometry analysis to both identify recovered Jurkat cells in BAL and the monocyte/macrophage subpopulations that engulf Jurkat cells. We first observed an increase of IA/IEintCD11cint DI cell population in both Ccl2 transgenic and WT control mice 2 h after Jurkat cell instillation (Fig. 8A). In Fig. 8B, Jurkat cells were gated (R1) from total BAL cells based on anti-human CD3 antibody staining. The gated Jurkat cells were then separated into IA/IE−CD11c− free Jurkat cells (R2), IA/IEintCD11cint cells that are Jurkat cells engulfed in DI cells or Jurkat+ DI cells (R3), and IA/IEintCD11chigh Jurkat+ macrophages (R4). The percentage of Jurkat+ DI cells and Jurkat+ macrophages was higher in BAL of Ccl2 transgenic mice compared with that in WT mice (Fig. 8B). The total number of Jurkat+ DI cells and Jurkat+ macrophages in Ccl2 transgenic mouse BAL was much higher compared with that of WT mice 2 h after Jurkat cell instillation (Fig. 8C). Jurkat+ DI cells are the predominant cell type in both Ccl2 transgenic mice and WT mice (Fig. 8, B and C). Total free Jurkat cells were significantly reduced in BAL of Ccl2 transgenic mice compared with WT mice (Fig. 8C). However, we did not observe Jurkat+ dendritic cells in BAL of either bleomycin-treated Ccl2+ or WT mice 2 h after apoptotic Jurkat cell instillation with this analysis setting. These data demonstrate that DI cells take up apoptotic cells, suggesting a previously undetected novel role for DI cells in removing apoptotic cells from injured lung. Taken together, CCL2 plays a key role in recruiting mononuclear cells including DI cells, and the latter in turn promote the removal of apoptotic inflammatory cells, leading to the resolution of lung inflammation.
Fig. 8.
Enhanced apoptotic cell clearance in Ccl2 transgenic mice. One and a half million apoptotic Jurkat cells were instilled into day 3 bleomycin-treated Ccl2 transgenic and WT control mouse lungs. Two hours later the mice were killed and lungs were lavaged. BAL cells were stained and subjected to flow cytometry analysis. A: increased IA/IEintCD11cint DI cells after Jurkat cells instillation. B: R1 gated Jurkat cells (human CD3 positive) from total BAL cells were separated with myosin heavy chain (MHC) II IA/IE and CD11c into 3 populations: R2 were free Jurkat cells (IA/IE−CD11c−), R3 were Jurkat cells associated with DI cells (IA/IEintCD11cint), and R4 were Jurkat cells associated with macrophages. Bottom: apoptotic Jurkat cells used as control. C: total number of Jurkat expressing DI cells, Jurkat expressing of macrophages, and number of free Jurkat cells recovered from BAL of Ccl2 transgenic and WT mice (n = 4; **P < 0.01, ***P < 0.001 between genotypes).
DISCUSSION
In the current study, we tested the hypothesis that CCL2 recruits specific monocyte populations that engulf apoptotic cells, promoting the resolution of noninfectious lung injury. We found that Ccl2 transgenic mice were resistant to bleomycin-induced mortality. Ccl2 transgenic mice showed a significant increase in mononuclear cell infiltration both at baseline and after bleomycin challenge and produced significantly less proinflammatory chemokines and cytokines after bleomycin treatment. Most importantly, we identified that DI cells recruited by overexpression of Ccl2 play a role in clearance of apoptotic cells and promote resolution of inflammation after noninfectious lung injury.
Previous reports demonstrated that CCL2 was upregulated during infectious (42) and noninfectious lung injury (5, 10, 41, 52). CCL2 has been showed to induce leukocyte infiltration during inflammation (8, 38, 39, 43). Overexpression of human CCL2 in alveolar type II epithelial cells elicited a substantial recruitment of monocytic cells into both parenchymal and alveolar compartments of mouse lungs in the absence of lung inflammation (19). Ccl2 transgenic mice were protected from acute lung injury caused by bacterial and influenza viral infections through a mechanism of improving pathogen clearance by mononuclear phagocytes in the lungs of Ccl2 transgenic mice (27, 43, 51). Administering CCL2 protein into mouse lungs enhanced the ability of alveolar macrophages to ingest neutrophils, while CCL2 neutralizing antibody impaired phagocytosis of alveolar macrophages and neutrophil clearance in acute bacterial pneumonia (1, 25). Our data further demonstrated that overexpressing CCL2 protected mice from bleomycin-induced noninfectious lung injury with increased recruitment of DI cells, exMACs, and dendritic cells into mouse lungs following injury. We further identified a previously undiscovered function of DI cells; they ingest apoptotic cells during lung inflammation.
We demonstrate that Ccl2 transgenic mice crossed with CCR2 null mice had impaired leukocyte recruitment and reduced survival after bleomycin lung injury compared with Ccl2 transgenic mice, suggesting that the protective effect of CCL2 in noninfectious lung injury requires CCR2. This finding was consistent with previous reports (51) in an infectious lung injury model that interfering with the CCL2/CCR2 axis by administration of CCR2 antibody to Ccl2 transgenic mice reduced mononuclear phagocyte recruitment, reduced bacterium clearance, and impaired the resolution/repair process of S. pneumoniae infections.
A primary finding of our work was that Ccl2 transgenic mice were protected from bleomycin-induced lung inflammation, exhibiting a significant reduction in the accumulation of neutrophils after injury and a reduction in chemokines important for neutrophil recruitment. There are conflicting reports whether CCL2 alone recruits neutrophils into lungs using direct CCL2 protein intratracheal instillation to the lungs of mice with differing gene background (2, 28). We did not observe an increase in neutrophil recruitment in the lungs of unchallenged Ccl2 transgenic mice on the C57Bl/6 background. This observation was consistent with previous reports on Ccl2 transgenic mice (19, 43). The potential cause for the reduced neutrophil accumulation in the BAL of Ccl2 transgenic mice after injury may be due to the reduction in chemokines such as CXCL2/MIP2 and CXCL1/KC. CXCL1/KC is one of the major chemokines that recruit neutrophils during inflammation (35). Enhanced apoptotic cell clearance in Ccl2 transgenic mice is another factor that contributed to the reduction of neutrophils in the mouse lungs after bleomycin injury.
We hypothesized that the reduced inflammation resulted from the ability of CCL2 to promote the resolution of inflammation. Resolution of inflammation is a coordinately regulated process. Following injury, apoptotic epithelial and inflammatory cells must be removed (20). Without effective clearance of apoptotic cells, inflammation will progress unabated with severe consequences to the host. The primary mechanism of apoptotic neutrophil clearance in the lung is by various macrophages (9, 18, 22). Here we show that like well-defined macrophages, DI cells play a role in removal of apoptotic inflammatory cells. DI cells (MHCIIintCD11cint) were recently identified in influenza-infected mouse lungs (27). We provide data that DI cells accumulate in naïve Ccl2 transgenic mice and are further increased upon bleomycin lung injury. Although DI cells were also increased in the lungs of bleomycin-treated WT mice, the total number of DI cells was much higher in Ccl2 transgenic mice than in WT mice. The elevated numbers of DI cells in Ccl2 transgenic mice were associated with enhanced clearance of apoptotic cells. These data suggest that CCL2 expression recruits phagocytes to sites of injury and promotes apoptotic cell clearance by a recently described population of DI cells. Further studies are needed to explore the function, recruitment, cytokine release, and surface marker expression of DI cells after lung injury.
The CCL2-CCR2 axis has been shown to regulate lung injury and fibrosis in animal models (17, 23, 34, 37). CCR2-deficient mice were protected from bleomycin-induced lung injury (17, 32, 37), and anti-CCL2 gene therapy attenuated bleomycin-induced lung fibrosis (23). However, Ccl2-deficient mice were not protected from FITC-induced lung fibrosis (33). Furthermore, CCL2-overexpressing mice showed protection from infectious animal models, for example, Mycobacterium bovis bacille Calmette-Guérin (43), Streptococcus pneumonia (51), and influenza (27). Multiple factors and signaling pathways such as T-cell recruitment (36), IFNγ production (24), and fibrocyte recruitment (32, 33) might be involved in regulating the development of fibrosis in the context of the overexpression of CCL2. We were surprised to find that CCL2 overexpression did not demonstrate an impact on lung fibrosis after bleomycin treatment, but the data suggest that the protean functions of CCL2 have competing effects on the pathogenic mechanisms of fibrosis. Further studies are needed to dissect the role of CCL2 in fibrogenesis.
CCL2 is upregulated in multiple lung diseases in human and plays an important role in attenuating infectious lung injury (38, 39, 43, 51). In the current study, we demonstrate that overexpressing CCL2 protects mice from bleomycin-induced noninfectious lung injury by enhancing apoptotic cell clearance and inhibiting the release of proinflammatory mediators. This study provides evidence for CCL2 as a potential therapeutic target for treating noninfectious lung injury.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.L., M.D.G., D.J., and P.W.N. conception and design of research; J.L., Y.J., R.M.T., T.X., N.L., and M.L. performed experiments; J.L., Y.J., R.M.T., T.X., N.L., M.L., M.D.G., and D.J. analyzed data; J.L., R.M.T., T.X., M.D.G., D.J., and P.W.N. interpreted results of experiments; J.L. and D.J. prepared figures; J.L. and D.J. drafted manuscript; J.L., M.D.G., D.J., and P.W.N. edited and revised manuscript; J.L., Y.J., R.M.T., T.X., N.L., M.L., M.D.G., D.J., and P.W.N. approved final version of manuscript.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health Grants R01-HL-060539, R01-AI-052201, R01-HL-077291, and P50-HL-084917.
REFERENCES
- 1. Amano H, Morimoto K, Senba M, Wang H, Ishida Y, Kumatori A, Yoshimine H, Oishi K, Mukaida N, Nagatake T. Essential contribution of monocyte chemoattractant protein-1/C-C chemokine ligand-2 to resolution and repair processes in acute bacterial pneumonia. J Immunol 172: 398–409, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Balamayooran G, Batra S, Balamayooran T, Cai S, Jeyaseelan S. Monocyte chemoattractant protein 1 regulates pulmonary host defense via neutrophil recruitment during escherichia coli infection. Infect Immun 79: 2567–2577, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Balkwill F. Cancer and the chemokine network. Nat Rev Cancer 4: 540–550, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr, Broxmeyer HE, Charo IF. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest 100: 2552–2561, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brieland JK, Jones ML, Flory CM, Miller GR, Warren JS, Phan SH, Fantone JC. Expression of monocyte chemoattractant protein-1 (MCP-1) by rat alveolar macrophages during chronic lung injury. Am J Respir Cell Mol Biol 9: 300–305, 1993 [DOI] [PubMed] [Google Scholar]
- 6. Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci USA 91: 2752–2756, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Charo IF, Peters W. Chemokine receptor 2 (CCR2) in atherosclerosis, infectious diseases, and regulation of T-cell polarization. Microcirculation 10: 259–264, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Conti P, DiGioacchino M. MCP-1 and RANTES are mediators of acute and chronic inflammation. Allergy Asthma Proc 22: 133–137, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Cox G, Crossley J, Xing Z. Macrophage engulfment of apoptotic neutrophils contributes to the resolution of acute pulmonary inflammation in vivo. Am J Respir Cell Mol Biol 12: 232–237, 1995 [DOI] [PubMed] [Google Scholar]
- 10. de Boer WI, Sont JK, van Schadewijk A, Stolk J, van Krieken JH, Hiemstra PS. Monocyte chemoattractant protein 1, interleukin 8, and chronic airways inflammation in COPD. J Pathol 190: 619–626, 2000 [DOI] [PubMed] [Google Scholar]
- 11. de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, Qui PT, Cam BV, Ha do Q, Guan Y, Peiris JS, Chinh NT, Hien TT, Farrar J. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 12: 1203–1207, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dessing MC, van der Sluijs KF, Florquin S, van der Poll T. Monocyte chemoattractant protein 1 contributes to an adequate immune response in influenza pneumonia. Clin Immunol 125: 328–336, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 101: 890–898, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fantuzzi L, Borghi P, Ciolli V, Pavlakis G, Belardelli F, Gessani S. Loss of CCR2 expression and functional response to monocyte chemotactic protein (MCP-1) during the differentiation of human monocytes: role of secreted MCP-1 in the regulation of the chemotactic response. Blood 94: 875–883, 1999 [PubMed] [Google Scholar]
- 15. Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, Henson PM. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress proinflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem 281: 38376–38384, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123: 321–334, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Gharaee-Kermani M, McCullumsmith RE, Charo IF, Kunkel SL, Phan SH. CC-chemokine receptor 2 required for bleomycin-induced pulmonary fibrosis. Cytokine 24: 266–276, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Grigg JM, Savill JS, Sarraf C, Haslett C, Silverman M. Neutrophil apoptosis and clearance from neonatal lungs. Lancet 338: 720–722, 1991 [DOI] [PubMed] [Google Scholar]
- 19. Gunn MD, Nelken NA, Liao X, Williams LT. Monocyte chemoattractant protein-1 is sufficient for the chemotaxis of monocytes and lymphocytes in transgenic mice but requires an additional stimulus for inflammatory activation. J Immunol 158: 376–383, 1997 [PubMed] [Google Scholar]
- 20. Henson PM, Tuder RM. Apoptosis in the lung: induction, clearance and detection. Am J Physiol Lung Cell Mol Physiol 294: L601–L611, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Henson PM, Vandivier RW, Douglas IS. Cell death, remodeling, and repair in chronic obstructive pulmonary disease? Proc Am Thorac Soc 3: 713–717, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hussain N, Wu F, Zhu L, Thrall RS, Kresch MJ. Neutrophil apoptosis during the development and resolution of oleic acid-induced acute lung injury in the rat. Am J Respir Cell Mol Biol 19: 867–874, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Inoshima I, Kuwano K, Hamada N, Hagimoto N, Yoshimi M, Maeyama T, Takeshita A, Kitamoto S, Egashira K, Hara N. Anti-monocyte chemoattractant protein-1 gene therapy attenuates pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 286: L1038–L1044, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Jiang D, Liang J, Hodge J, Lu B, Zhu Z, Yu S, Fan J, Gao Y, Yin Z, Homer R, Gerard C, Noble PW. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J Clin Invest 114: 291–299, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kannan S, Huang H, Seeger D, Audet A, Chen Y, Huang C, Gao H, Li S, Wu M. Alveolar epithelial type II cells activate alveolar macrophages and mitigate P. Aeruginosa infection. PLos One 4: e4891, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leitch AE, Duffin R, Haslett C, Rossi AG. Relevance of granulocyte apoptosis to resolution of inflammation at the respiratory mucosa. Mucosal Immunol 1: 350–363, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin KL, Suzuki Y, Nakano H, Ramsburg E, Gunn MD. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol 180: 2562–2572, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Maus U, Huwe J, Maus R, Seeger W, Lohmeyer J. Alveolar JE/MCP-1 and endotoxin synergize to provoke lung cytokine upregulation, sequential neutrophil and monocyte influx, and vascular leakage in mice. Am J Respir Crit Care Med 164: 406–411, 2001 [DOI] [PubMed] [Google Scholar]
- 29. McDonald PP, Fadok VA, Bratton D, Henson PM. Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-beta in macrophages that have ingested apoptotic cells. J Immunol 163: 6164–6172, 1999 [PubMed] [Google Scholar]
- 30. Meloni F, Caporali R, Marone Bianco A, Paschetto E, Morosini M, Fietta AM, Patrizio V, Bobbio-Pallavicini F, Pozzi E, Montecucco C. BAL cytokine profile in different interstitial lung diseases: a focus on systemic sclerosis. Sarcoidosis Vasc Diffuse Lung Dis 21: 111–118, 2004 [PubMed] [Google Scholar]
- 31. Mercer PF, Johns RH, Scotton CJ, Krupiczojc MA, Konigshoff M, Howell DC, McAnulty RJ, Das A, Thorley AJ, Tetley TD, Eickelberg O, Chambers RC. Pulmonary epithelium is a prominent source of proteinase-activated receptor-1-inducible CCL2 in pulmonary fibrosis. Am J Respir Crit Care Med 179: 414–425, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol 166: 675–684, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol 35: 175–181, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moore BB, Paine R, 3rd, Christensen PJ, Moore TA, Sitterding S, Ngan R, Wilke CA, Kuziel WA, Toews GB. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol 167: 4368–4377, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Moser B, Clark-Lewis I, Zwahlen R, Baggiolini M. Neutrophil-activating properties of the melanoma growth-stimulatory activity. J Exp Med 171: 1797–1802, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T, Gunn MD. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol 10: 394–402, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Okuma T, Terasaki Y, Kaikita K, Kobayashi H, Kuziel WA, Kawasuji M, Takeya M. C-C chemokine receptor 2 (CCR2) deficiency improves bleomycin-induced pulmonary fibrosis by attenuation of both macrophage infiltration and production of macrophage-derived matrix metalloproteinases. J Pathol 204: 594–604, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Osterholzer JJ, Curtis JL, Polak T, Ames T, Chen GH, McDonald R, Huffnagle GB, Toews GB. CCR2 mediates conventional dendritic cell recruitment and the formation of bronchovascular mononuclear cell infiltrates in the lungs of mice infected with Cryptococcus neoformans. J Immunol 181: 610–620, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peters W, Cyster JG, Mack M, Schlondorff D, Wolf AJ, Ernst JD, Charo IF. CCR2-dependent trafficking of F4/80dim macrophages and CD11cdim/intermediate dendritic cells is crucial for T cell recruitment to lungs infected with Mycobacterium tuberculosis. J Immunol 172: 7647–7653, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Rydell-Tormanen K, Uller L, Erjefalt JS. Neutrophil cannibalism–a back up when the macrophage clearance system is insufficient. Respir Res 7: 143, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sakanashi Y, Takeya M, Yoshimura T, Feng L, Morioka T, Takahashi K. Kinetics of macrophage subpopulations and expression of monocyte chemoattractant protein-1 (MCP-1) in bleomycin-induced lung injury of rats studied by a novel monoclonal antibody against rat MCP-1. J Leukoc Biol 56: 741–750, 1994 [DOI] [PubMed] [Google Scholar]
- 42. Salentin R, Gemsa D, Sprenger H, Kaufmann A. Chemokine receptor expression and chemotactic responsiveness of human monocytes after influenza A virus infection. J Leukoc Biol 74: 252–259, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Schreiber O, Steinwede K, Ding N, Srivastava M, Maus R, Langer F, Prokein J, Ehlers S, Welte T, Gunn MD, Maus UA. Mice that overexpress CC chemokine ligand 2 in their lungs show increased protective immunity to infection with Mycobacterium bovis bacille Calmette-Guerin. J Infect Dis 198: 1044–1054, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Suga M, Iyonaga K, Ichiyasu H, Saita N, Yamasaki H, Ando M. Clinical significance of MCP-1 levels in BALF and serum in patients with interstitial lung diseases. Eur Respir J 14: 376–382, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, Hart WK, Pardo A, Blackwell TS, Xu Y, Chun J, Luster AD. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med 14: 45–54, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science 296: 155–158, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Tillie-Leblond I, Hammad H, Desurmont S, Pugin J, Wallaert B, Tonnel AB, Gosset P. CC chemokines and interleukin-5 in bronchial lavage fluid from patients with status asthmaticus. Potential implication in eosinophil recruitment. Am J Respir Crit Care Med 162: 586–592, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Traynor TR, Kuziel WA, Toews GB, Huffnagle GB. CCR2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection. J Immunol 164: 2021–2027, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Wang L, Medan D, Mercer R, Shi X, Huang C, Castranova V, Ding M, Rojanasakul Y. Role of neutrophil apoptosis in vanadium-induced pulmonary inflammation in mice. J Environ Pathol Toxicol Oncol 21: 343–350, 2002 [PubMed] [Google Scholar]
- 50. Wang L, Scabilloni JF, Antonini JM, Rojanasakul Y, Castranova V, Mercer RR. Induction of secondary apoptosis, inflammation, and lung fibrosis after intratracheal instillation of apoptotic cells in rats. Am J Physiol Lung Cell Mol Physiol 290: L695–L702, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Winter C, Taut K, Srivastava M, Langer F, Mack M, Briles DE, Paton JC, Maus R, Welte T, Gunn MD, Maus UA. Lung-specific overexpression of CC chemokine ligand (CCL) 2 enhances the host defense to Streptococcus pneumoniae infection in mice: role of the CCL2-CCR2 axis. J Immunol 178: 5828–5838, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Zhang K, Gharaee-Kermani M, Jones ML, Warren JS, Phan SH. Lung monocyte chemoattractant protein-1 gene expression in bleomycin-induced pulmonary fibrosis. J Immunol 153: 4733–4741, 1994 [PubMed] [Google Scholar]