Abstract
The major function of epithelial tissues is to maintain proper ion, solute, and water homeostasis. The tubule of the renal nephron has an amazingly simple structure, lined by epithelial cells, yet the segments (i.e., proximal tubule vs. collecting duct) of the nephron have unique transport functions. The functional differences are because epithelial cells are polarized and thus possess different patterns (distributions) of membrane transport proteins in the apical and basolateral membranes of the cell. K+ channels play critical roles in normal physiology. Over 90 different genes for K+ channels have been identified in the human genome. Epithelial K+ channels can be located within either or both the apical and basolateral membranes of the cell. One of the primary functions of basolateral K+ channels is to recycle K+ across the basolateral membrane for proper function of the Na+-K+-ATPase, among other functions. Mutations of these channels can cause significant disease. The focus of this review is to provide an overview of the basolateral K+ channels of the nephron, providing potential physiological functions and pathophysiology of these channels, where appropriate. We have taken a “K+ channel gene family” approach in presenting the representative basolateral K+ channels of the nephron. The basolateral K+ channels of the renal epithelia are represented by members of the KCNK, KCNJ, KCNQ, KCNE, and SLO gene families.
Keywords: renal epithelia, KCNK, KCNJ, KCNQ, KCNE, SLO
the major function of epithelial tissues is to maintain proper ion, solute, and water homeostasis. Transepithelial transport is not a simple feat; rather it is a well-orchestrated physiological process involving numerous membrane transport proteins. Epithelial cells are polarized and thus possess different patterns (distributions) of membrane transport proteins in the apical and basolateral membranes of the cell depending upon the specific function of a given epithelium (104, 108). For example, a concerted effort of various ion transport proteins must occur in a given epithelium to carry out a specific function such as Na+ reabsorption (Fig. 1) (or Na+-glucose reabsorption, K+ reabsorption, K+ secretion, or Cl− secretion). The human genome project has led to the identification of numerous additional ion transport proteins at the molecular level. Subsequently, the physiological roles of these proteins in transepithelial ion/solute transport have been elucidated, resulting in the identification of many diseases of epithelia caused by mutations of ion channels and transporters. These include various renal tubulopathies, including Bartter's syndrome (42, 64, 136), Gitelman's syndrome (37, 88), EAST syndrome (for infant Epilepsy, severe Ataxia, moderate Sensorineural deafness and renal salt wasting Tubulopathy) (2, 11), SeSAME syndrome (for Seizures, Sensorineural deafness, Ataxia, Mental retardation and Electrolyte imbalance) (107, 115, 137), Liddle's disease (14, 118), nephrogenic diabetes insipidus (10, 80), and renal tubular acidosis syndrome (134).
Fig. 1.
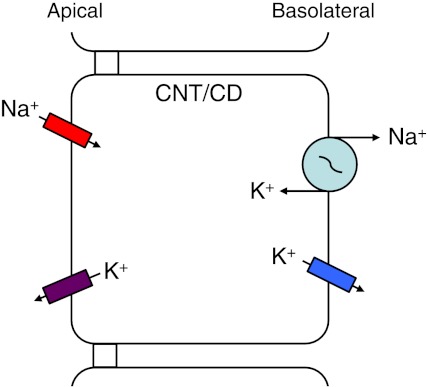
Cellular model of a polarized epithelial cell. Specific ion transport proteins are distributed within the apical and basolateral membranes of an epithelial cell for a particular epithelial transport function, Na+ reabsorption in this example, for cells of the connecting tubule (CNT) and the collecting duct (CD). The cells of the CNT and CD can also perform K+ secretion.
Potassium channels are present within all cells of the body. Over 90 different genes for K+ channel α- and β-subunits have been identified in the human genome [Hugo Genome Organization (HUGO) Nomenclature Committee; http://genenames.org/genefamilies/KCN]. K+ channels are located in both apical and/or basolateral membranes of epithelial cells and occur in a wide range of structures of various organs (systems) including kidney tubules, respiratory airways, small and large intestine, gall bladder, sweat duct, pancreatic duct, and epididymis of the male and fallopian tubes of the female reproductive tracts, for example.
The smallest functional unit of the kidney is the nephron, which is a tubular structure composed of a single layer of epithelial cells lining a series of contiguous segments: the proximal tubule (PT), thin descending limb (TDL), thin and thick ascending (TAL) limbs of the loop of Henle, distal convoluted tubule (DCT), connecting tubule (CNT), and collecting duct (CD). Each segment of the nephron has specific ion, solute, and water transport functions (discussed below), and as such, specific repertoires of transport proteins are distributed within the apical and basolateral membranes of the individual epithelial cells. Therefore, K+ channels of renal epithelial cells play critical roles in proper ion transport tubular function with the goal of overall homeostasis.
The specific function of a given K+ channel is dependent upon the overall function of the epithelial cell and the membrane within which the K+ channel resides. In particular, the cellular functions of K+ channels of renal epithelial cells include 1) generation and maintenance of the negative cell membrane potential (76); 2) hyperpolarization of cell membrane potential during a physiological response, thereby providing a driving force for electrogenic transepithelial ion/solute transport (e.g., to drive Na+-glucose reabsorption and Na+ reabsorption); 3) recycling of K+ across the basolateral membrane required for maintaining electroneutrality; for example, the basolateral K+ current is coupled with the Na+-K+-ATPase pump current to balance the charge movement across the apical membrane for Na+ reabsorption (18, 65, 109); 4) recycling of K+ across the apical membrane of the TAL cells for continuous function of the Na+-K+-2Cl−-cotransporter (NKCC2) (135); 5) providing a route for K+ secretion across the apical membrane (30); and 6) participating in tubular cell volume regulation (5).
This review does not presume to be a definitive survey of all the epithelial basolateral K+ channels but is focused on the recent past and advances in our knowledge (and molecular diseases) of basolateral membrane K+ channels of renal tubule epithelial cells. The reader is referred to a number of excellent review articles on epithelial K+ channels in both renal (28, 44, 71, 72, 136) and nonrenal tissues (1, 3, 9, 28, 38, 41, 45, 46, 56, 59, 94, 95, 105, 130, 132, 133). These articles include information pertaining to both apical and basolateral membrane K+ channels in a wide range of epithelial tissues.
There are numerous K+ channels located within the basolateral membrane of the epithelial cells of the various tubular segments of the nephron. It is our goal to provide an overview of these channels, as well as to provide potential physiological functions and pathophysiology of these channels, where appropriate. We have taken a “K+ channel gene family” approach in presenting the representative basolateral K+ channels of the nephron. However, before we present the specific K+ channels of the nephron, it is important to briefly discuss the major function(s) of the various segments of the nephron.
Overview of the Transport Function of Segments of the Nephron
PT.
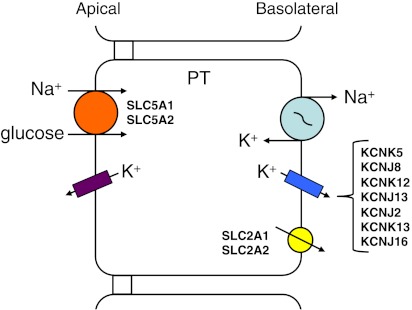
The PT is responsible for the reabsorption of approximately two-thirds of the ion, solute, and water filtered by the glomerulus. The epithelial cells of the PT are an example of a leaky absorptive epithelium in which the tight junctions have a low resistance, and thus the tight junctions of PT cells are important routes of paracellular transport (71). Ions and solutes reabsorbed by the PT cells include Na+, Cl−, K+, HCO3−, phosphate, and sulfate, along with glucose, amino acids, and some organic acids, among other molecules. Many of these molecules are reabsorbed via luminal Na+-dependent transport proteins. For example, reabsorption of Na+ and glucose from the filtrate occurs via apically located Na+-glucose cotransporters (SGLT1, SLC5A1; or SGLT2, SLC5A2 depending upon the portion of the PT) driven by the Na+ gradient (Fig. 2). Na+ is moved across the basolateral membrane by the Na+-K+-ATPase while glucose is transported by either glucose transporter 1 (GLUT1, SLC2A1) or 2 (GLUT2, SLC2A2) depending upon the portion of the PT. Additionally, a considerable amount of secretion of organic acids and bases into the tubular filtrate occurs along the length of the PT. Many different ion channels and transporters, of which basolateral K+ channels play a critical role, are responsible for the transcellular transport of ion and solute along the length of the PT. K+ channels of the KCNK and KCNJ gene families have been reported from the basolateral membrane of PT cells of various species.
Fig. 2.
Cellular model for the Na+-glucose reabsorption of the proximal tubule (PT). Entry of Na+ and glucose across the apical membrane is by either Na+-glucose cotransporter 1 or 2 (SLC5A1, SLC5A2) depending upon the section of the PT. Glucose is transported across the basolateral membrane by either glucose transporter 1 or 2 (SLC2A1, SLC2A2) depending upon the section of the PT. The reabsorbed Na+ exits the cell by the Na+-K+-ATPase, while K+ moves across the basolateral membrane via a K+ channel. There are a number of K+ channels, from 2 K+ channel gene families, that have been identified within the basolateral membrane of the PT of various animals. The basolateral K+ channels provided are ranked based on evidence of physiological function followed by evidence based on histochemistry, immunostaining, or immunogold labeling, for instance. This is true for the lists of the basolateral K+ channels presented in the legends for Figs. 3–5. Abbreviations of these K+ channels are provided in the text.
TAL.
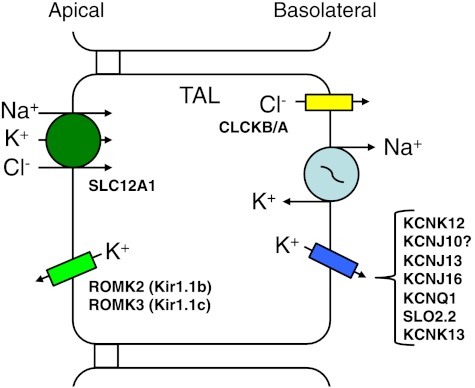
The epithelial cells of the TAL are responsible for the reabsorption of 20–25% of filtered Na+, K+, and Cl− each day. These ions are reabsorbed through both transcellular and paracellular routes. The apical step for the transcellular reabsorption of Na+, K+, and Cl− is via the apical NKCC2 (SLC12A1) while Na+ and K+ exit the cell by the Na+-K+-ATPase and basolateral K+ channels, respectively (Fig. 3). Cl− crosses the basolateral membrane via a Cl− channel (CLCKB/A). In the TAL, K+ channels play crucial roles in both the apical and basolateral membranes. The apical ROMK2 (Renal Outer Medullary K+; Kir1.1b) K+ channel aids in recycling K+ to the luminal solution to maintain the local K+ concentration for the proper function of SLC12A1 (136) while the basolateral K+ channels aid in recycling K+ back to the interstitium to provide K+ for proper Na+-K+-ATPase function. There are a number of basolateral K+ channels that have been described for TAL cells, which include members of the KCNK, KCNJ, KCNQ, and SLO gene families.
Fig. 3.
Cellular model of Na+, K+, and Cl− reabsorption by the thick ascending limb (TAL) of the loop of Henle. The apical entry step for these 3 ions is via the Na+-K+-2Cl− cotransporter (SLC12A1). Na+ is transported across the basolateral membrane by the Na+-K+-ATPase, and Cl− exits the cell by a basolateral Cl− channel (CLCKB/A). There are a number of basolateral K+ channels, from 4 K+ channel gene families, which been identified within the basolateral membrane of the TAL of various animals. The apical K+ channels are ROMK 2 (Kir1.1b) and ROMK3 (Kir1.1c) (136). Abbreviations of all K+ channels and transporters are provided in the text.
DCT.
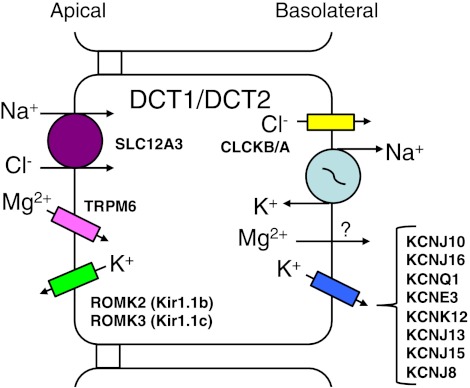
The cells of the DCT are responsible for reabsorption of nearly 5% of the Na+ and Cl− from the filtrate. There is a gradation of physiological function of the DCT cells, with respect to the reabsorption of Na+, depending upon whether one is examining the epithelial cells of the early portion of the DCT (DCT1) or the late DCT (DCT2) (16, 79). The cellular entry step, in both portions of the DCT, for Na+ and Cl− is via the apical Na+-Cl− cotransporter (NCCT; SLC12A3) across the apical membrane of the DCT cell, utilizing the Na+ gradient (Fig. 4). Additionally, the apical amiloride-sensitive sodium channel (ENaC) has been reported in the DCT2 (ENaC is not shown in Fig. 4), as well as SLC12A3 (79). The reabsorbed Na+ exits the DCT cell by the Na+-K+-ATPase, K+ exits via basolateral K+ channels, and Cl− exits through a Cl− channel (CLCKB/A). Therefore, basolateral K+ channels, again, aid in the recycling of K+ across the basolateral membrane and for reabsorption of K+ ultimately back to the blood. There are a number of basolateral K+ channels that have been described for DCT cells, which include members of the KCNK, KCNJ, KCNQ and KCNE gene families.
Fig. 4.
Cellular model for NaCl reabsorption by early (DCT1) and late (DCT2) distal convoluted tubule cells. Na+ and Cl− enter the cell via the Na+-Cl− cotransporter (SLC12A3). The reabsorbed Na+ and Cl− exit DCT1 and DCT2 as described in Fig. 3. Cellular K+ leaves the cell via a number of K+ channels that have been described from 4 different gene families. Mg2+ enters the DCT1 and DCT2 cells by the Mg2+ channel (TRPM6) and exits the cell by purported Mg2+ (?) transporters. The apical K+ channels are the same as found within the TAL. Some of the ion transport proteins within DCT1 and DCT2 are different. DCT2 cells also have apical epithelial Na+ and Ca2+ channels and basolateral Ca2+ transporters that are discussed in the text. Abbreviations of all channels and transporters are provided in the text. The figure is modified from Bandulik et al. (2).
CNT and CD.
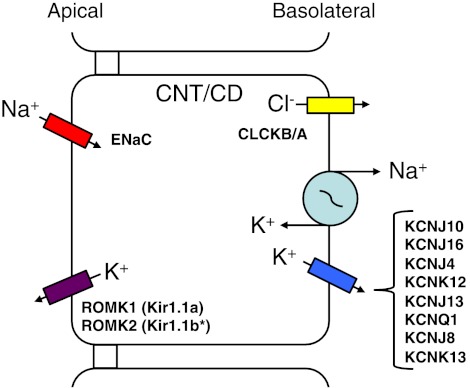
The (principal) cells of the CNT and CD are responsible for ∼10% of the reabsorption of Na+ and Cl− from the daily filtrate. The process of reabsorption of Na+ for the CNT and the CD is similar. The entry step for Na+ is via ENaC, resulting in Na+ moving down its concentration gradient into the cell (Fig. 5). The exit step for Na+ from the cell is by the Na+-K+-ATPase while K+ is recycled back out of the cell via basolateral K+ channels. Members of the KCNK, KCNJ, and KCNQ gene families have been reported in the basolateral membranes of CNT and CD cells.
Fig. 5.
Cellular model of Na+ reabsorption by the CNT and the CD. Na+ enters CNT and CD cells via the epithelial sodium channel (ENaC), going down its electrochemical gradient. The reabsorbed Na+ is transported across the basolateral membrane by the Na+-K+-ATPase. K+ is transported out across the basolateral membrane via a number of K+ channels belonging to 3 different K+ channel gene families. CNT and CD cells also are capable of K+ secretion utilizing the entry of K+ via the Na+-K+-ATPase, after which K+ exits across the apical membrane via ROMK (136). Abbreviations of all channels and transporters are provided in the text. *ROMK2 (Kir1.1b) is located in the cortical collecting duct, but not in the outer medullary collecting duct (136).
Members of the KCNK Gene Family in the Nephron
The KCNK gene family is composed of a series of 18 channels (to date) whose subunits are composed of 4 transmembrane domains and 2 pore regions. Lazdunski and colleagues (75) described KCNK1 (TWIK-1; Tandem Weak Inward Rectifying K+ Channel), the first member of the KCNK gene family. This group of channels is considered as “leak” K+ channels, which stabilize the cellular negative resting membrane potential (28), additionally, some members are sensitive to pH, temperature, volume, and/or membrane stretch (28, 34, 35).
Levy et al. (76) provided significant insight into the segmental expression the KCNK K+ channels within the human nephron. Using RT-PCR, Levy and colleagues identified KCNK1, KCNK3, KCNK5, KCNK10, and KCNK13 within the nephron and KCNK3, KCNK6, and KCNK10 within the glomerulus (76). Unfortunately, the study by Levy et al. (76) did not determine the membrane location of the KCNK channels, nor were the authors able to differentiate the three segments of the PT or to tease out the K+ channels between the DCT and the CD of the distal nephron. Nonetheless, that study provided a framework of the possible KCNK K+ channels present within the nephron.
There have been at least three members of the KCNK gene family identified in the basolateral membrane of cells from the nephron in a range of studies encompassing tubules from humans, rats, mice, and rabbits.
KCNK5 in the nephron.
KCNK5 (TASK-2; TWIK-Related Acid Sensitive K+ Channel) which is now classified as a TALK (TWIK-Related Alkaline pH-Activated K+ Channel) was identified as the first member of the TALK subfamily of KCNK channels and was cloned from the human kidney and identified in the PT, distal tubule, and CD by Lazdunski and coworkers (110). The KCNK5 K+ channel is a noninactivating, outward rectified, acid-sensitive K+ channel whose gating is modulated by extracellular pH (110). Since its initial discovery, Poujeol and coworkers, (5, 77, 78), using primary cell cultures of PT cells from control and KCNK5 knockout (KO) mice, have demonstrated that basolateral KCNK5 plays significant roles in volume regulation. Indeed, Barriere et al. (5) identified swelling-activated K+ currents attributable to KCNK5 in PT cells, and cells cultured from KO mice exhibited completely impaired volume-regulatory responses. Therefore, KCNK5 plays a critical role in cell volume regulation within PT cells.
Furthermore, Warth et al. (134) suggested, based on electrophysiological experiments coupled with pH and HCO3− measurements of PT cells from control and KO KCNK5 mice, that the basolateral KCNK5 K+ channel senses the basolateral external pH resulting from HCO3− transport. They further suggested that KCNK5 served as a “molecular switch” that modulates the K+ conductance relative to the rate of basolateral HCO3− transport (134). Recently, it has been reported that heterologously expressed KCNK5 can be modulated by intracellular pH (pHi) and that a lysine (Lys245) in the fourth transmembrane domain of KCNK5 is the specific residue that is responsible for the pHi gating of KCNK5 (92). These studies certainly provide exciting evidence for a major role of KCNK5 in pH homeostasis of PT cells and its potential role in renal proximal tubular acidosis (134).
KCNK12 and KCNK13 in the nephron.
Derst and colleagues (106) first reported the KCNK12 (THIK-2; Tandem Pore Domain Halothane Inhibited K+ Channel) and KCNK13 (THIK-1) K+ channels of the THIK subfamily of KCNK K+ channels from rat kidney and brain among other tissues. Similarly, Lazdunski and coworkers (31) identified a human homolog of KCNK12 predominantly expressed in the pancreas. However, recently, Theilig et al. (124) reported that KCNK12 and KCNK13 were abundantly expressed in the nephron of both mice and rats. They clearly identified KCNK12 in the basolateral membrane of PT, TAL, DCT, CNT and CD cells of mice by immunostaining; furthermore, they suggested that KCNK12 contributes to recycling of K+ across the basolateral membrane, aiding the function of the Na+-K+-ATPase. Additionally, Theilig et al. (124) reported expression (RT-PCR) of KCNK13 within the PT, TAL, and CD cells and KCNK12 within the CD cells of human renal tissue. Theilig et al. (124) suggested that these KCNK channels might play a role in energy efficiency by modulating Na+ and Cl− transport during hypoxic conditions.
Certainly, the numbers of and roles of basolateral KCNK K+ channels of the nephron are increasing (28). Further excitement with respect to the KCNK gene family has arisen by the implication of KCNK5 in renal proximal tubular acidosis (134). Indeed, Bayliss and Barrett (6) have suggested that two-pore channels are emerging as potential therapeutic targets for renal-related disorders.
KCNJ Gene Family Members in the Nephron
Many members of the KCNJ gene family, encoding Inward Rectifying K+ channels (Kir), have been identified in the recent molecular physiological era. The first member of the KCNJ gene family cloned was the ATP-dependent, inwardly rectified ROMK1 (KCNJ1; Kir 1.1) K+ channel isolated from the rat kidney by Hebert and coworkers (50). To date, there are 18 members of the KCNJ gene family that include epithelial K+ transport channels, classic Kir channels, G protein-gated K+ channels, and ATP-sensitive K+ channels (46). Members of this gene family are characterized by two membrane-spanning domains linked by a pore-forming region and have intracellular NH2- and COOH-terminal domains (46). KCNJ1 (3 splice variants: ROMK1, Kir1.1a; ROMK2, Kir1.1b; ROMK3, Kir1.1c) is a predominant apical membrane K+ channel in various segments of the nephron and has been elegantly reviewed by Welling and Ho (136). The basolateral KCNJ K+ channels play a role in K+ homeostasis and contribute to the resting cellular membrane potential and recycling of K+ across the basolateral membrane of renal epithelial cells.
KCNJ2 (Kir2.1, IRK1) and KCNJ16 (Kir5.1) in the nephron.
Jan and colleagues (68) originally cloned KCNJ2 (Kir2.1) from the mouse macrophage. KCNJ2 is generally found in cardiac, skeletal muscle, and nervous tissue (46). However, Derst et al. (22) identified both KCNJ2 and KCNJ16 (Kir5.1, discussed further below) by RT-PCR in human PT cells, and furthermore they demonstrated that KCNJ2 and KCNJ16 colocalized on chromosome 17q. KCNJ16 is known to form heteromeric channels with other members of the KCNJ gene family (103), and it has been identified in the basolateral membrane of DCT and CD cells (69, 98, discussed later). Indeed, Derst et al. (22) reported that KCNJ2 coassembled with KCNJ16 when expressed in Xenopus laevis oocytes. Additionally, Tucker et al. (126) and Tanemoto et al. (121) reported that heteromeric channels composed of KCNJ16 and KCNJ10 (Kir4.1) are inhibited by pHi and the pH sensitivity of the heteromeric channel is dependent upon KCNJ16. This further suggests that KCNJ16 is crucial for maintenance of pH by proximal tubular cells. Last, Derst and coworkers (22) suggested these Kir subunits form channels and reside within the basolateral membrane of PT cells; however, the basolateral localization remains to be determined.
KCNJ4 (Kir2.3, IRK3, HIR, HRK1, hIRK2) in the CD of the nephron.
The KCNJ4 K+ channel was originally described from human and murine brain, specifically from the hippocampus, but this channel was also shown to be within heart and skeletal muscle by the groups of Kurachi (89), Vanderberg (101), and Nichols (83). This channel is an inward rectifier with a single-channel conductance of ∼10 pS and inhibited by known K+ channel blockers (e.g., Ba2+, Cs+, TEA) as characterized by expressing KCNJ4 in the X. laevis oocyte expression system. These initial studies did not demonstrate KCNJ4 within kidney tissue.
Welling (135) presented the first comprehensive study of KCNJ4 (reported by Welling as CCD-IRK3) of renal epithelial cells. Welling isolated KCNJ4 from the established mouse CD cell line, M1 (119). He demonstrated the presence of KCNJ4 within the M1 cell line by Northern blotting, but KCNJ4 was not present within either Madin-Darby canine kidney (MDCK) or pig kidney (LLC-PK1) cell lines, both of distal nephron origin. Additionally, Welling (135) provided the first evidence of KCNJ4 in native mouse kidney by RT-PCR. The characteristics of this channel, expressed in X. laevis oocytes, were a strongly inwardly rectified channel, with a conductance of 14.5 pS, high open probability, and inhibition by Ba2+ (135). Based on sequence homology, Welling suggested that the channel he characterized was the channel reported by Kurachi (89), Vanderberg (101), and Nichols (83) and their colleagues. Subsequently, Welling and coworkers (73, 74) established that KCNJ4 was targeted to the basolateral membrane in a polarized epithelium and at that time suggested that KCNJ4 was the channel that encoded for the small-conductance K+ channel of CD principal cells and it played a physiological role in K+ homeostasis.
Consistent with Welling's work, Millar et al. (87) identified the presence of KCNJ4 in both whole mouse cortex and cortical CDs by RT-PCR. Additionally, Millar et al. patched the basolateral aspect of single, isolated CD tubules and demonstrated that the KCNJ4 had a similar biophysical profile (strong inward rectification, sensitivity to Ba2+, half-maximum at 0 mV for Ba2+ block, for example) to the channel reported by Welling (135). Furthermore, Millar et al. (87) implied that the dominant nature of the conductance associated with KCNJ4 suggested that this channel played an important part in K+ handling in the principal cells of the cortical CD.
KCNJ8 (Kir6.1) in the nephron.
The ATP-dependent K+ channel (KATP) was first described by Noma (93) from cardiac tissue using patch-clamp experiments. Since then, KATP channels have been reported from pancreatic β-cells, skeletal muscle, vascular smooth muscle, and renal tubule cells (46, 56). The KCNJ8 K+ channel was first cloned from rat pancreatic cells (55). KCNJ8 plays a multitude of roles in various tissues, including shortening of the action potential, cellular loss of K+ during metabolic complications of the heart, insulin secretion, regulation of smooth muscle function, regulation of skeletal muscle excitability, and release of neurotransmitters (56).
Zhou et al. (142) demonstrated robust labeling of KCNJ8 in the basolateral membrane of DCT and CD cells as well as weak expression of KCNJ8 in PT cells of the rat by immunohistochemistry. Mauerer et al. (85, 86) described, by electrophysiological experiments, an ATP-sensitive K+ channel from the basolateral membrane of PT cells of the salamander (Ambystoma) that was modulated by PKA, PKC, and pH, yet was not affected by stretch or a swelling-activated response. In contrast, Brochiero et al. (13) reported that the KCNJ8 K+ channel of rabbit PT cells was taurine sensitive and suggested that KCNJ8 played a role in osmoregulation as taurine was a known exportable osmolyte (70). Therefore, the loss of intracellular taurine and the activation of KCNJ8 would act collectively to regulate cell volume of PT cells (13). KCNJ8 certainly plays a role in volume regulation; however, further investigation is required to elucidate the role of KCNJ8 in proximal tubular physiology.
KCNJ10 (Kir4.1, Kir1.2, KAB-2) and KCNJ16 (Kir5.1, BIR9) in the nephron.
Adelman and colleagues (12) first described the inwardly rectified potassium channels KCNJ10 and KCNJ16 cloned from brain, heart, and skeletal muscle tissues. Kurachi and coworkers (57) examined the distribution of KCNJ10 within the rat nephron. They clearly identified KCNJ10 within the basolateral membrane of cells of the DCT, CNT, and CD segments of the rat nephron by immunohistochemistry and immunogold electron microscopy (57). KCNJ10 exists as a homomeric channel, but it can form a heteromeric channel with KCNJ16 (103). Subsequently, Paulais et al. (100) identified a basolateral K+ channel in the TAL of mice that had similar electrophysiological properties to that of a K+ channel described by Hurst et al. (54) from rabbit TAL cells, and strikingly similar to the heteromeric KCNJ10/KCNJ16 K+ channel reported by Pessia et al. (103). Additionally, Lourdel et al. (81) identified KCNJ10 and KCNJ16 along with KCNJ15 (Kir4.2) by mRNA expression from mouse DCT cells, and they demonstrated immuolocalization of KCNJ10 in the basolateral enfoldings of DCT cells. They characterized, by patch clamping isolated DCT tubules, the biophysical properties of a K+ channel that strongly resembled KCNJ10/KCNJ16. Futhermore, Lachheb et al. (69) reported significant mRNA expression for KCNJ10 and KCNJ16 from mouse CD cells. Also, Lachheb et al. (69) patched the basolateral membrane of CD cells, and one of the K+ channels exhibited many characteristics of KCNJ10/KCNJ16. They further suggested that the K+ channel reported by Paulais et al. (100) was actually the heteromeric KCNJ10/KCNJ16 K+ channel (69). Finally, Derst et al. (22) demonstrated the expression of KCNJ16 within the human TAL and reported that KCNJ16 formed heteromeric channels with KCNJ2 when expressed in X. laevis oocytes.
KCNJ13 (Kir7.1, Kir1.4) in the nephron.
Krapivinsky et al. (67) first described the KCNJ13 K+ channel from the kidney, small intestine, prostate, testis, and brain. This channel is quite unique with characteristics different from most Kir K+ channels such as: a very small conductance of 50 fS, not sensitive to ATP, low sensitivity to barium (a nonspecific blocker of K+ channels) and the inward rectification of the channel is independent of extracellular K+ (26, 67). KCNJ13 shares only 38% homology with its closest member (KCNJ15) of the KCNJ gene family (67).
Ookata et al. (96) reported KCNJ13 within PT cells of rat tubules by Western blotting and further demonstrated KCNJ13 within the basolateral membrane of rat DCT, CNT, and CD cells by immunostaining. Additionally, Derst et al. (21) demonstrated the presence of KCNJ13 K+ channels in isolated PTs from human and guinea pig kidneys based on RT-PCR experiments. Using immunohistochemistry, Derst et al. clearly demonstrated the presence of KCNJ13 in the basolateral membrane of guinea pig PT cells. The electrophysiological data of the guinea pig KCNJ13, expressed in X. laevis oocytes, was consistent with previously reported data on human KCNJ13 (26). Derst et al. suggested that the KCNJ13 K+ channel was well suited to mediate K+ exit across the basolateral membrane of the PT cells because the channel has a very low dependence on K+ permeation by external K+ (21). Therefore, KCNJ13 maintains a high efflux of K+ at low interstitial K+ concentrations (21). KCNJ13 warrants further study to uncover the role this channel plays in renal physiology.
KCNQ gene family member KCNQ1 (KvLQT1, Kv7.1) and KCNE family members KCNE1 (MinK) and KCNE3 (MIRP2) in the nephron.
The members of the KCNQ gene family are voltage-activated K+ channels. Wang and coworkers (131) cloned KCNQ1, the first member of this gene family indentified. This channel was found in a number of tissues, including cardiac muscle, skeletal muscle, and epithelia (59). Subsequently, two groups demonstrated that KCNQ1 coassembled with a β-subunit, KCNE1, a purported one-transmembrane domain channel first described from the rat kidney by Nakanishi and colleagues (127), to form a slow outwardly rectifying K+ channel characteristic of the cardiac IKs K+ channel that aids in the repolarization of the cardiac cells (4, 114). In addition to coassembling with KCNE1, KCNQ1 can coassemble with the β-subunit KCNE3 in a variety of epithelial tissues, including the intestine (19, 116). The KCNQ1/KCNE3 K+ channel was characterized as a cAMP-dependent channel exhibiting a linear current-voltage relationship that is different from the KCNQ1/KCNE1 K+ channel (116). Mutations within KCNQ1 give rise to a number of diseases, including long QT syndrome (LQT1) and deafness (58, 91).
The presence of KCNQ1 and KCNE1 within the kidney was verified by RT-PCR, in situ hybridization, and immunofluorescence (20, 120, 128). In fact, KCNQ1 and KCNE1 are expressed in the apical membrane of mammalian PT cells (120, 128), and it was suggested that KCNQ1 plays a role in Na+ reabsorption by the PT cells of the mouse (129). Nevertheless, Wingo and colleagues (141) determined the distribution of KCNQ1 in the mouse kidney. Using Northern blot analysis, they reported the presence of KCNQ1 from the whole kidney, after which they conducted RT-PCR on isolated tubule segments and detected KCNQ1 within the DCT, CNT, and CD and weak signals for the early PT and the cortical TAL. Using immunostaining, they demonstrated clear basolateral membrane labeling of KCNQ1 in cells from the TAL, DCT, CNT, and CD. However, Wingo and coworkers did not have any experimental evidence for KCNQ1 within the basolateral membrane of the PT cells and further suggested that the discrepancy between their data and the study by Vallon et al. (128) might result from different KCNQ1 isoforms. Recently, Cirovic et al. (17) confirmed basolateral KCNQ1 within the DCT cells of frog kidney. However, those authors did not mention a possible β-subunit that aggregates with KCNQ1 within the frog DCT.
The function(s) of basolateral KCNQ1 within the various segments of the nephron is still to be determined; however, these channels could certainly be responsible for recycling of K+ across the basolateral membrane as described above for other basolateral K+ channels. Additionally, KCNQ1 would be well positioned to play a role in K+ reabsorption during times of a low-K+ diet, for instance.
Interestingly, Duranton et al. (27) identified the β-subunit KCNE3 within cultured mouse DCT cells. Furthermore, they examined the involvement of KCNQ1-KCNE3 in the LPS-induced apoptosis of mouse DCT cells and revealed there is an activation of KCNQ1-KCNE3 conductance by LPS. Duranton et al. put forth the possibility that the KCNQ1-KCNE3 channel may play a crucial role in LPS-mediated pathogenesis of renal disease during septicemia. This report is quite exciting, as the role of basolateral K+ channels in renal epithelia is gaining more clinical relevance.
SLO gene family member SLO2.2 in the TAL of the nephron.
The SLO mammalian gene family is composed of four members (113), and the first member cloned was SLO1, better known as the maxi-K channel (also KCa1.1, BK, KCNMA1) (15). The members of this gene family have a large single-channel conductance (60–270 pS), exhibit several subconductance states, and Ca2+, Na+, Cl−, and pH can modulate most members of the SLO gene family. SLO2.2 (KCNT1, KNa, KCa4.1, SLACK, Sequence Like A Calcium-Activated K+) is a Na+- and Cl−-activated K+ channel that was first reported from cardiac myocytes (61) and cloned by Kaczmarek and colleagues (60) and Salkoff and coworkers (138, 139). Generally, these K+ channels are found in excitable tissues.
Recently, Paulais et al. (99) reported the first occurrence of the Na+-sensitive K+ channel SLO2.2 from renal epithelial cells. In fact, SLO2.2 was identified in only the TAL cells of the mouse using RT-PCR. Using single-channel patch-clamp experiments, Paulais et al. described the functional identification of the basolateral 150-pS Na+-, Cl−-activated K+ channel that was neither pH nor ATP sensitive. The physiological role of SLO2.2 is still yet to be established. However, Paulais et al. suggested, based on the Na+ sensitivity of SLO2.2, that this channel could participate in the apical/basolateral “physiological crosstalk” coupling of basolateral K+ conductance to Na+ entry across the apical membrane which has been debated for many years (8, 23, 43, 117). Interestingly, the Na+-regulatory sensor motif of SLO2.2 has been recently identified within the RAC2 (Regulators of Conductance of K+) domain, which is intriguing as RAC domains are designed to couple specific ion-sensing mechanisms to channel gating (140). Additionally, SLO2.2 can play a role in maintaining the membrane potential and recycling K+ across the basolateral membrane. This report by Paulais et al. (99) appears, to our knowledge, to be the only report of SLO2.2 in renal epithelial tissues. This channel certainly requires additional investigation to determine its physiological role in the function of ion transport physiology of the TAL.
Other basolateral K+ channels within the nephron.
In addition to the K+ channels described above, there have been many K+ channels identified within the basolateral membrane of renal epithelial cells from a number of animals (frog, salamander, rabbit, rat, X. laevis). However, the authors have been unable to ascertain to which specific K+ channel gene families these K+ channels belong. Unclassified channels are reported in the basolateral membrane of PT cells (7, 32, 33, 51–53, 62, 63, 97, 111, 125), TAL cells (24, 39, 40), DCT cells (123), and CNT/CD cells (36, 47–49, 82).
Renal Pathophysiology and Basolateral K+ Channels
The molecular physiological era has ushered in many opportunities for determining the molecular basis of diseases, and thus our understanding of renal tubular diseases has blossomed over the past two decades. We have identified, in the introduction to this paper, a number of molecular diseases that are specific to the kidney; however, those diseases do not involve basolateral K+ channels of the nephron.
Here, we will focus on two related “renal” syndromes, SeSAME and EAST, which have been recently described, involving renal basolateral K+ channels that elicit physiological complications with tubular function, but also afflict other organ systems based on the distribution of these K+ channels. As we presented earlier, KCNJ10 (Kir4.1) exists as a homomeric channel, but it can form heteromeric channels with KCNJ16 (Kir5.1) (103) and is found predominantly within the DCT, CNT, and CD cells (57, 69, 81, 84, 100). Additionally, these channels are found within a wide range of tissues, including brain, cochlea, heart, and skeletal tissues (57, 81, 84). Therefore, mutations in these channels can lead to clinical manifestations in multiple tissues as has been recently reported for SeSAME and EAST. These syndromes have similar symptoms that are described below. However, we present these syndromes separately based on their original descriptions (11, 115).
SeSAME syndrome-KCNJ10.
Lifton and colleagues (115) described a previously unreported human autosomal recessive syndrome which was characterized by seizures, sensorineural deafness, ataxia, mental retardation and electrolyte imbalance (named SeSAME) coupled with hypokalemia, metabolic alkalosis, and hypomagnesmia. This study entailed examining individuals of four kindreds from Afghan, Great Britain, and Canadian ancestries. The original patient exhibited general seizures, developmental delay, atrophy in the lower limbs, marked ataxia, progressive hearing loss, hypokalemia, metabolic alkalosis, and hypomagnesmia (115). Additionally, other patients exhibited elevated renin and aldosterone, further suggesting a salt-wasting complication within the kidney. By linkage analysis, Scholl et al. (115) localized the putative causative gene to a 2.5-Mb segment of chromosome 1q23.2–23.3. The case for the original patient showed no homozygosity to any known loci of Bartter's or Gitelman's syndromes, both well-described syndromes of the TAL and DCT, respectively (37, 64, 115).
Scholl et al. (115) suspected that the gene of interest in this syndrome would be expressed in the brain, inner ear, and kidney, at least, as reports that KCNJ10 knockout mice exhibited neurological and renal phenotypes similar to those of their patients (25, 66, 90). Thus KCNJ10 was the candidate gene to pursue. Using direct DNA sequencing of KCNJ10, Lifton's group identified six previously unidentified nonsense and missense mutations (R65P, C140R, T164I, A167V R199stop, and R297C; widely expressed in the N terminus, external loop, transmembrane domain 2, and the C terminus) on both alleles in all patients. These mutations occurred at positions that were completely conserved among all vertebrate species examined. Furthermore, Scholl et al. (115) stated that a number of those mutations had previously been shown to play a role in loss of function in related K+ channels. Additionally, recent reports have further characterized mutations of KCNJ10 reported by Lifton and colleagues (107, 112, 122, 137).
Molecular basis of renal manifestations of SeSAME- KCNJ10.
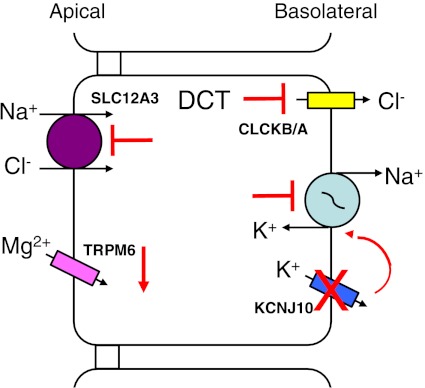
Similar to other salt-wasting diseases (Bartter's, Gitelman's syndromes), renal complications of SeSAME can be better understood by examining the ion transport cellular models of the various segments of the nephron. Based on the location of KCNJ10 within the nephron and the clinical manifestations of SeSAME, the function of DCT, CNT, and CD cells are critical in understanding the molecular basis of SeSAME. The general ion transport functions of the DCT cells (Fig. 4) include 1) Na+ and Cl− reabsorption by luminal SLC12A3, 2) luminal uptake of Mg2+ by TRPM6 (Transient Receptor Potential Melastatin subtype 6), and 3) luminal Ca2+ reabsorption by TRPV5 (Transient Receptor Potential Vanilloid member 5) (not shown in Fig. 4) in the DCT2 cells (2, 115). The basolateral Na+-K+-ATPase plays a number of crucial roles in the overall transport function of the DCT cell by transporting Na+ out of the cell for the exchange with interstitial K+. Furthermore, the continued operation of the Na+-K+-ATPase maintains the proper electrochemical gradient for the apical influx of Na+ (and thus Cl−) and maintaining the cell's negativity for enhanced ion transport function. Cellular K+ exits the basolateral membrane via KCNJ10 and maintains the cell's negative potential, contributing to electrical driving forces for transcellular ion transport. The reabsorbed Cl− exits the cell via the basolateral CLCKB/A channel aided by the electrochemical gradient. Finally, intracellular Mg2+ is transported across the basolateral membrane by putative Mg2+ transporters (shown in Fig. 4 but not Fig. 6). Loss of KCNJ10 function (Fig. 6) coupled with the potentially reduced activity of the Na+-K+-ATPase can have a number of downstream effects on ion transport, including 1) diminished Na+ and Cl− entry across the apical membrane of the DCT cell, leading to salt-wasting; 2) reduced cell negative potential, which lowers the driving force for entry of Mg2+ via TRPM6 or the exit of cellular Cl− by CLCKB/A across the basolateral membrane, resulting in reduced reabsorption of Mg2+ and Cl−; and 3) reduced Na+ and Cl− reabsorption by the DCT, leading to the increase of renin and aldosterone, as reported in some patients (115), which will ultimately lead to increased Na+ reabsorption and K+ secretion by the CNT and CD cells, resulting in hypokalemia and metabolic alkalosis, as seen in Bartter's and Gitelman's syndromes (37, 64).
Fig. 6.
Molecular model of altered ion transport in SeSAME/EAST syndromes (see the text for definitions) in DCT cells by mutations of KCNJ10. Normal ion transport function of DCT cells is shown in Fig. 4. The partial or loss of function of KCNJ10 results in the reduced electrical driving forces for ion transport; which cause the reduced function of SLC12A3 and therefore less Na+ and Cl− entry across the apical membrane of the cell. The reduced entry of Cl− leads to decreased transport of Cl− via CLCKB/A across the basolateral membrane. Additionally, the driving force for Mg2+ via TRPM6 is reduced, causing diminished overall Mg2+ reabsorption. The overall result of impaired KCNJ10 is NaCl wasting and reduced Cl− and Mg2+ reabsorption by the DCT cells. Abbreviations of all channels and transporters are provided in the text. The figure is modified from Bandulik et al. (2).
Here, we have only examined the renal complications of SeSAME. Our understanding of this syndrome is just being realized, and future studies are required to fully elucidate the large ramifications of SeSAME in nonrenal tissues.
EAST syndrome-KCNJ10.
Kleta and colleagues (11) reported another autosomal recessive syndrome involving KCNJ10 shortly after Scholl et al. (115), in which five children from two consanguineous families of Pakistan descent exhibited infant epilepsy, severe ataxia, moderate sensorineural deafness and renal salt wasting tubulopathy, which has been named EAST syndrome. These individuals exhibited seizures in infancy; also, these children experienced speech and motor delay, gate ataxia, intention tremor, and dysdiadochokinesia (inability to perform rapid, alternating movements), which are all consistent with cerebellar dysfunction (11). All children displayed renal symptoms of elevated renin, hypokalemic metabolic akalosis, hypomagnesemia, and hypocalciuria, again suggesting altered renal tubular function, in addition to the neurological symptoms. Again, KCNJ10 was the candidate gene screened by linkage analysis. Bockenhauer et al. (11) screened the complete coding region of KCNJ10, which revealed a homozygous missense mutation R65P (within the N terminus just before transmembrane domain 1) in four of the children and another homozygous missense mutation G77R (within transmembrane domain 1) in the last child. The R65 and G77 amino acids were conserved among all 21 species determined by a protein-homology analysis.
Bockenhauer et al. (11) further heterologously expressed the wild-type KCNJ10 channel and two mutant KCNJ10 channels in X. laevis oocytes and reported that the wild-type channel exhibited robust currents typical of an inward rectifier K+ channel. However, current from the mutant R65P KCNJ10 channels were reduced to 25% and current from mutant G77R KCNJ10 channels were reduced to only 5% of the current exhibited by wild-type control channels (11). Thus the decrease in function of mutant KCNJ10 channels could result in an altered electrochemical driving force for transcellular NaCl reabsorption, resulting in the similar alteration of ion transport function of the DCT cells as described above for the molecular basis of SeSAME syndrome.
Recently, Warth and colleagues (107) described a new mutation of KCNJ10 (R175Q) that could play a role in altered renal function in EAST syndrome patients. When this mutation was heterologously expressed in HEK cells, Reichold et al. (107) noted that the mutated channel only exhibited channel flickering with no clear single-channel level and had only 10–15% the open probability of the wild-type channel. Additionally, they examined the pH sensitivity of wild-type KCNJ10 and the R175Q mutant channel in excised inside-out patches. The wild-type channel had a half-maximal activity observed at pH 6.3, as previously described (102). However, the R175Q mutant channel was inactive at physiological pH, but shifted to a half-maximal activity at pH 9.35. So, the single point mutation of KCNJ10 dramatically shifted the pH sensitivity of the channel to quite alkaline conditions. In addition to the molecular channel evidence, Reichold et al. (107) also examined the distal tubular morphology of an EAST syndrome patient by using material from kidney biopsies. Electron microscopy studies revealed that the DCT cells had reduced numbers of mitochondria and diminished basolateral infoldings. With a view of these results coupled together, EAST syndrome patients would exhibit compromised reabsorptive capacity of DCT cells based on morphology and channel function (107).
Very recently, Zdebik and coworkers (29) described three new mutations of KCNJ10 (R65C, F75L, and V259stop) from patients clinically diagnosed with EAST syndrome. These mutations were heterologously expressed in X. laevis oocytes, and all mutant channels exhibited significantly impaired channel function, suggesting these mutations caused reduced basolateral membrane K+ conductance and abnormal distal nephron function.
SeSAME/EAST and KCNJ16.
Much attention has been paid to the role of KCNJ10 in the complications of SeSAME and EAST syndromes as discussed above. Of course, KCNJ10 forms both homomeric channels and can also form heteromeric channels with KCNJ16 (103). Recently, Paulais et al. (98) examined the renal phenotype of mice that lacked KCNJ16 (knockout; KCNJ16−/−), and thus in those animals the homomeric KCNJ10 channel would presumably be a primary basolateral K+ channel in the TAL, DCT, CNT, and CD (11, 81, 100, 107). Interestingly, compared with SeSAME/EAST patients, the KCNJ16−/− mice exhibited, barring hypokalemia and K+ wasting without NaCl wasting, very few symptoms of SeSAME or EAST syndromes. The KCNJ16−/− mice exhibited normal NaCl reabsorption by the TAL, metabolic acidosis, hyperchloremia, hypermagnesuria, and hypercalciuria.
Paulais et al. (98) examined the pharmacological and electrophysiological characteristics of KCNJ16+/+ and KCNJ16−/− mice to begin to understand the renal phenotypic differences between the knockout mice and SeSAME/EAST patients. Paulais et al. studied the physiological function of the tubules by determining the effects of diuretics, which inhibit specific Na+-dependent transporters or ENaC, on ion transport function of the TAL and distal nephron. The effects of furosemide (inhibitor of SLC12A1) and amiloride (inhibitor of ENaC) on ion transport function were the same for tubules from both KCNJ16+/+ and KCNJ16−/− mice. These results suggested that the loss of KCNJ16 did not alter ion transport of the TAL and distal nephron (98). Next, Paulais et al. examined the effects of hydrochlorothiazide (HCTZ), an inhibitor of SLC12A3 in the DCT, and demonstrated that HCTZ induced a significantly greater increase in Na+ in the urine of KCNJ16−/− mice compared with urine Na+ levels of KCNJ16+/+ mice. Paulais et al. concluded that the genetic loss of KCNJ16 leads to a stimulation of NaCl reabsorption by the DCT cells in the knockout mice. Additionally, using single-channel experiments with DCT cells, Paulais et al. demonstrated the single-channel properties of KCNJ16+/+ (i.e., KCNJ10+KCNJ16) had a conductance of 44 ± 1 pS and an open probability of 0.31 ± 0.05 while channel properties of KCNJ16−/− had a conductance of 23 ± 1 pS and an open probability of 0.74 ± 0.04, which supported previous findings of heteromeric and homomeric KCNJ10 channels (81, 103, 121).
Furthermore, Paulais et al. demonstrated, with single-channel studies, that the 44-pS KCNJ16+/+ channel was sensitive to pHi over the range of pH 6.8–8; as has been reported (69, 81, 100, 102, 103, 121, 126), while the KCNJ16−/− channel was not pHi sensitive over that same pH range. As Tucker et al. (126) had proposed “a novel functional role in pH-dependent regulation of renal K+ buffering and recycling” for KCNJ16, Paulais et al. (98) now provided dramatic proof, with the KCNJ16−/− mice, supporting Tucker's proposed function for KCNJ16. Therefore, the results from the pH and electrophysiological studies suggested that, for the KCNJ16−/− mouse, the K+ channels (presumably KCNJ10) responsible for basolateral K+ conductance would be highly active and have altered pH sensitivity.
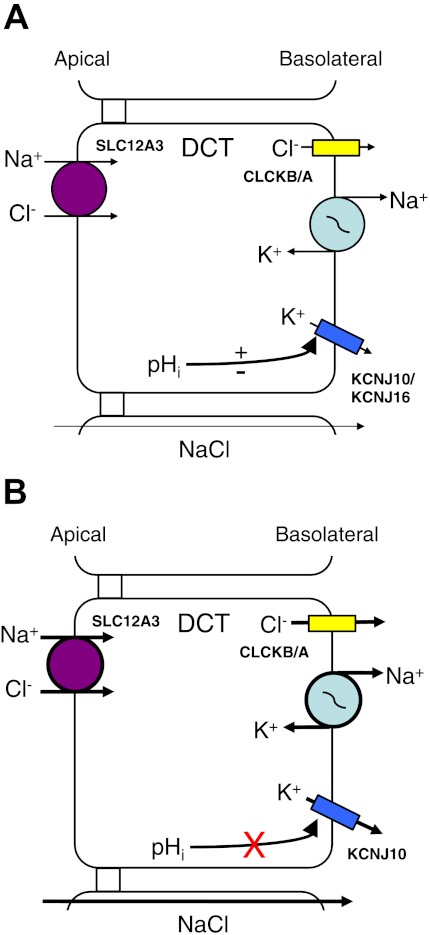
How does the loss of KCNJ16 lead to the symptoms exhibited by the KCNJ16−/− mouse? Paulais et al. (98) focused on the normal/abnormal function of the DCT as a means of explaining the symptomology of the knockout mouse. As can be seen in Fig. 7A, the normal function of the DCT is for NaCl reabsorption across the DCT epithelial cells. However, with the loss of KCNJ16, the homomeric KCNJ10 channel is thus expressed within the basolateral membrane of the DCT cells; however, the homomeric channel does not exhibit the pH sensitivity as the heteromeric KCNJ10/KCNJ16 channel does, leading to an enhanced activity. Therefore, there is an increased basolateral K+ conductance (i.e., KCNJ10), leading to increased Na+-K+-ATPase function, with increased activity of the apical SLC12A3, and an increased driving force for Cl− exit by CLCKB/A; this ultimately leads to increased NaCl reabsorption across the DCT cell (Fig. 7B) (98).
Fig. 7.
Molecular model of altered ion transport in SeSAME/EAST syndromes in DCT cells by mutations of KCNJ16. A: normal ion transport function of DCT cells. The heteromeric KCNJ10/KCNJ16 has intracellular pH (pHi) sensitivity due to KCNJ16. B: knockout of KCNJ16 leads to symptoms (described in the text) which are different from symptoms resulting from mutations of the KCNJ10 homomeric channel. With KCNJ16−/− animals, the pHi sensitivity of the homomeric KCNJ10 channels is altered. Indeed, the homomeric KCNJ10 channel has enhanced functions, leading to increased activity of the Na+-K+-ATPase, which leads to accelerated transport of Na+ and Cl− via SLC12A3 and increased Cl− movement through CLCKB/A. The overall result of loss of KCNJ16 is enhanced reabsorption of NaCl across the DCT cells. Abbreviations of all channels and transporters are provided in the text. The figure is modified from Paulais et al. (98).
At the time of this writing, we have been unable to locate any published paper(s) that describe a mutation within KCNJ16. It is very exciting to envision how our knowledge of SeSAME and EAST syndromes will blossom once specific mutations of KCNJ16 are discovered.
Concluding Remarks
Basolateral K+ channels play critical roles in the epithelial ion/solute transport physiology of the nephron and aid in overall homeostasis of the body. There is a wide variety of K+ channels from a number of gene families that have been described and are found in specific locations throughout the segments of the nephron. Our understanding of the roles these K+ channels play in overall renal homeostasis and pathophysiology is still emerging. The possible role of KCNK5 as a “molecular switch” in renal proximal tubular acidosis is an exciting development in the field of proximal tubule physiology. The SeSAME and EAST syndromes, involving KCNJ10 and KCNJ16, have only recently been described and have certainly opened new and exciting research avenues for many renal molecular physiologists. The renal research future is certainly bright, and we anticipate major strides in our understanding of the role of basolateral K+ channels in renal tubulopathies during the next decade.
GRANTS
This work was supported by National Health, Lung, and Blood Institute Grants HL-083060 and HL-092157 to D. C. Devor and an Aims grant from the Department of Physiology of the University of Otago to K. L. Hamilton.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.L.H. and D.C.D. provided conception and design of research; K.L.H. and D.C.D. performed experiments; K.L.H. and D.C.D. analyzed data; K.L.H. and D.C.D. interpreted results of experiments; K.L.H. and D.C.D. prepared figures; K.L.H. and D.C.D. drafted manuscript; K.L.H. and D.C.D. edited and revised manuscript; K.L.H. and D.C.D. approved final version of manuscript.
ACKNOWLEDGMENTS
This paper is presented in memory of our friends Dr. James K. Bubien and Dr. Dale J. Benos, both of whom contributed much to our understanding of the physiology and pathophysiology of the kidney. We want to apologize to all authors whom we were unable to reference due to space limitations. We thank Dr. Corina M. Balut for comments on this manuscript. We also thank Dr. Thomas E. Kleyman for discussions on the transport proteins of the DCT.
REFERENCES
- 1. Bachmann O, Juric M, Seidler U, Manns MP, Yu H. Basolateral ion transporters involved in colonic epithelial electrolyte absorption, anion secretion and cellular homeostasis. Acta Physiol 201: 33–46, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Bandulik S, Schmidt K, Bockenhauer D, Zbelik AA, Humberg E, Kleta R, Warth R, Reichold M. The salt-wasting phenotype of EAST syndrome, a disease with multifaceted symptoms lined to the KCNJ10 K+ channel. Pflügers Arch 461: 423–435, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Bardou O, Trinh NT, Brochiero E. Molecular diversity and function of K+ channels in airway and alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 296: L145–L155, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. KvLQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature 384: 78–80, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Barriere H, Belfodil R, Rubera I, Tauc M, Lesage F, Poujeol C, Guy N, Barhanin J, Poujeol P. Role of TASK2 potassium channels regarding volume regulation in primary cultures of mouse proximal tubules. J Gen Physiol 122: 177–190, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bayliss DA, Barrett PQ. Emerging roles for two-pore-domain potassium channels and their potential therapeutic impact. Trends Pharmacol Sci 29: 566–575, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beck JS, Hurst AM, Lapointe JY, Laprade R. Regulation of basolateral K channels in proximal tubule studied during continuous microperfusion. Am J Physiol Renal Fluid Electrolyte Physiol 264: F496–F501, 1993 [DOI] [PubMed] [Google Scholar]
- 8. Beck JS, Laprade R, Lapointe JY. Coupling between transepithelial Na transport, and basolateral K conductance in renal proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol 266: F517–F527, 1994 [DOI] [PubMed] [Google Scholar]
- 9. Berkefeld H, Fakler B, Schulte H. Ca2+-activated K+ channels: from protein complexes to function. Physiol Rev 90: 1437–1459, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Bichet DG. Vasopressin receptor mutations in nephrogenic diabetes insipidus. Semin Nephrol 28: 245–251, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Bockenhauer D, Feather S, Standescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, van't Hoff W, Al Masri O, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillion MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R. Epilepsy, ataxia, sensorineural deafness, tubulopathy and KCNJ10 mutations. N Engl J Med 360: 1960–1970, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bond CT, Pessia M, Xia XM, Lagrutta A, Kavanaugh MP, Adelman JP. Cloning and expression of a family of inward rectifier potassium channels. Receptors Channels 2: 183–191, 1994 [PubMed] [Google Scholar]
- 13. Brochiero E, Wallendorf B, Gagnon D, Laprade R, LaPointe JY. Cloning of rabbit Kir6.1, SUR2A, and SUR2B: possible candidates for a renal KATP channel. Am J Physiol Renal Physiol 282: F289–F300, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Bubien JK, Ismailov II, Berdiev BK, Cornwell T, Lifton RP, Fuller CM, Achard JM, Benos DJ, Warnock DG. Liddle's disease: abnormal regulation of amiloride-sensitive Na+ channel by β-subunit mutation. Am J Physiol Cell Physiol 270: C208–C213, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Butler A, Tsunoda S, McCobb DP, Wei A, Salkoff L. mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science 261: 221–224, 1993 [DOI] [PubMed] [Google Scholar]
- 16. Câmpean V, Kricke J, Ellison D, Luft FC, Bachmann S. Localization of thiazide-sensitive Na+-Cl− cotransport and associated gene products in mouse DCT. Am J Physiol Renal Physiol 281: F1028–F1035, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Cirovic S, Markovic-Lipkovski J, Todorovic J, Nesovic-Ostojic J, Jovic M, Ilic S, Tatic S, Cemerikic D. Differential expression of KCNQ1 K+ channel in tubular cells of frog kidney. Eur J Histochem 54: e4, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dawson DC, Richards NW. Basolateral K conductance: role in regulation of NaCl absorption and secretion. Am J Physiol Cell Physiol 259: C181–C195, 1990 [DOI] [PubMed] [Google Scholar]
- 19. Dedek K, Waldegger S. Colocalization of KCNQ1/KCNE channel subunits in the mouse gastrointestinal tract. Pflügers Arch 442: 896–902, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Demolombe S, Franco D, De Boer P, Kuperschmidt S, Roden D, Pereon Y, Jarry A, Moorman AFM, Escande D. Differential expression of KvLQT1 and its regulator IsK in mouse epithelia. Am J Physiol Cell Physiol 280: C359–C372, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Derst C, Hirsch JR, Preisig-Muller R, Wischmeyer E, Karschin A, Doring F, Thomzig A, Veh RW, Schlatter E, Kummer W, Daut J. Cellular localization of the potassium channel Kir7.1 in guinea pig and human kidney. Kidney Int 59: 2197–2205, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Derst C, Karschin C, Wischmeyer E, Hirsch JR, Preisig-Muller R, Rajan S, Engel H, Grzeschik KH, Daut J, Karschin A. Genetic and functional linkage of Kir5.1 and Kir2.1 channel subunits. FEBS Lett 491: 305–311, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Diamond JM. Transcelluar cross-talk between epithelia cells membranes. Nature 300: 683–685, 1982 [DOI] [PubMed] [Google Scholar]
- 24. Di Stefano A, Greger R, Desfleurs E, de Rouffignac C, Wittner M. A Ba2+-insensitive K+ conductance in the basolateral membrane of rabbit cortical thick ascending limb cells. Cell Physiol Biochem 8: 89–105, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD. Conditional knock-out of Kir4.1 leads to glial membrane dopolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci 27: 11354–11365, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Doring F, Derst C, Wishmeyer E, Karschin C, Schneggenburger R, Daut J, Karschin A. The epithelial inward rectifier channel Kir7.1 displays unusual K+ permeation properties. J Neurosci 18: 8625–8636, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Duranton C, Rubera I, L'Hoste S, Cougnon M, Poujeol P, Barhanin J, Tauc M. KCNQ1 K+ channels are involved in lipopolysaccharide-induced apoptosis of distal kidney cells. Cell Physiol Biochem 25: 367–378, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev 90: 559–605, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Freudenthal B, Kulaveerasingam D, Lingappa L, Shah MA, Brueton L, Wassmer E, Ognjanovic M, Dorison N, Reichold M, Bockenhauer D, Kleta R, Zdebik AA. KCNJ10 mutations disrupt function in patients with EAST syndrome. Nephron Physiol 119: 40–48, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Frindt G, Palmer LG. Ca-activated K channels in apical membrane of mammalian CCT, and their role in K secretion. Am J Physiol Renal Fluid Electrolyte Physiol 252: F458–F467, 1987 [DOI] [PubMed] [Google Scholar]
- 31. Girard C, Duprat F, Terrenoire C, Tinel N, Fosset M, Romey G, Lazdunski M, Lesage F. Genomic and functional characteristics of novel human pancreatic 2P domain K+ channels. Biochem Biophys Res Commun 282: 249–256, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Gögelein H. Ion channels in mammalian proximal renal tubules. Ren Physiol Biochem 13: 8–25, 1990 [PubMed] [Google Scholar]
- 33. Gögelein H, Greger R. Properties of single K+ channels in the basolateral membrane of rabbit proximal straight tubule. Pflügers Arch 410: 288–295, 1987 [DOI] [PubMed] [Google Scholar]
- 34. Goldstein SA, Bayliss DA, Kim D, Lesage F, Plant LD, Rajan S. International Union of Pharmacology. LV. Nomenclature and molecular relationships of two-P channels. Pharmacol Rev 57: 527–540, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Goldstein SA, Bockenhauer D, O'Kelly I, Zilberberg N. Potassium leak currents and the KCNK family of two-p-domain subunits. Nat Rev Neurosci 2: 175–184, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Gray DA, Frindt G, Zhang YY, Palmer LG. Basolateral K+ conductance in principle cells of rat CCD. Am J Physiol Renal Physiol 288: F493–F504, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Graziana G, Fedeli C, Moroni L, Cosmai L, Badlamenti S, Ponticelli C. Gitelman syndrome: pathophysiological and clinical aspects. Q J Med 103: 741–748, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Greger R. Ion channels in mammalian proximal tubules. Renal Physiol Biochem 13: 8–25, 1990 [PubMed] [Google Scholar]
- 39. Gu R, Wang WH. Arachidonic acid inhibits K channels in basolateral membrane of the thick ascending limb. Am J Physiol Renal Physiol 283: F407–F414, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Gu R, Wang J, Zhang Y, Li W, Xu Y, Shan H, Wang WH, Yang B. Adenosine stimulates the basolateral 50 pS K channels in the thick ascending limb of the rat kidney. Am J Physiol Renal Physiol 293: F299–F305, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Halm DR. Secretory control of basolateral membrane potassium and chloride channels in colonic crypt cells. Adv Exp Med Biol 559: 119–129, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Hamilton KL, Butt AG. The molecular basis of renal tubular transport disorders. Comp Biochem Physiol 126A: 305–321, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Harvey BJ. Crosstalk and epithelial ion transport. Curr Opin Nephrol Hypertens 3: 523–528, 1994 [DOI] [PubMed] [Google Scholar]
- 44. Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev 85: 319–371, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heitzmann D, Warth R. Physiology and pathophysiology of potassium channels in gastrointestinal epithelia. Physiol Rev 88: 1119–1182, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90: 291–366, 2010 [DOI] [PubMed] [Google Scholar]
- 47. Hirsch J, Schlatter E. K+ channels in the basolateral membrane of rat cortical collecting duct. Pflügers Arch 424: 470–477, 1993 [DOI] [PubMed] [Google Scholar]
- 48. Hirsch J, Schlatter E. K+ channels in the basolateral membrane of rat cortical collecting duct are regulated by a cGMP-dependent protein kinase. Pflügers Arch 429: 338–344, 1995 [DOI] [PubMed] [Google Scholar]
- 49. Hirsch J, Schlatter E. K+ channels in the basolateral membrane of rat cortical collecting duct. Kidney Int 48: 1036–1046, 1995 [DOI] [PubMed] [Google Scholar]
- 50. Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature 362: 31–38, 1993 [DOI] [PubMed] [Google Scholar]
- 51. Hunter M. Stretch-activated channels in the basolateral membrane of single proximal cells of frog kidney. Pflügers Arch 416: 448–453, 1990 [DOI] [PubMed] [Google Scholar]
- 52. Hunter M. Potassium selective channels in the basolateral membrane of single proximal tubule cells of frog kidney. Pflügers Arch 418: 26–34, 1991 [DOI] [PubMed] [Google Scholar]
- 53. Hurst AM, Beck JS, Laprade R, Lapointe JY. Na+ pump inhibition downregulates an ATP-sensitive K+ channel in rabbit proximal convoluted tubule. Am J Physiol Renal Fluid Electrolyte Physiol 264: F760–F764, 1993 [DOI] [PubMed] [Google Scholar]
- 54. Hurst AM, Duplain M, Lapointe JY. Basolateral membrane potassium channels in rabbit cortical thick ascending limb. Am J Physiol Renal Fluid Electrolyte Physiol 263: F462–F467, 1992 [DOI] [PubMed] [Google Scholar]
- 55. Inagaki N, Tsuura Y, Namba N, Masuda K, Gonoi T, Horie M, Seino Y, Mizuta M, Seino S. Cloning and functional characterization of a novel ATP-sensitive potassium channel ubiquitously expressed in rat tissues, including pancreatic islets, pituitary, skeletal muscle, and heart. J Biol Chem 270: 5691–5694, 1995 [DOI] [PubMed] [Google Scholar]
- 56. Isomoto S, Kondo C, Kurachi Y. Inwardly rectifying potassium channels: their molecular heterogeneity and function. Jpn J Physiol 47: 11–39, 1997 [DOI] [PubMed] [Google Scholar]
- 57. Ito M, Inanobe A, Horio Y, Hibino H, Isomoto S, Ito H, Mori K, Tonosaki A, Tomoike H, Kurachi Y. Immunolocalization of an inwardly rectifying K+ channel, KAB-2 (Kir4.1), in the basolateral membrane of renal distal tubular epithelia. FEBS Lett 388: 11–15, 1996 [DOI] [PubMed] [Google Scholar]
- 58. Jervell A, Lange-Nielsen F. Congenital deaf-mutism, functional heart disease with prolongation of the Q-T interval and sudden death. Am Heart J 54: 59–68, 1957 [DOI] [PubMed] [Google Scholar]
- 59. Jespersen T, Grunnet M, Olesen SP. The KCNQ1 potassium channel: from gene to physiological function. Physiology 20: 408–416, 2005 [DOI] [PubMed] [Google Scholar]
- 60. Joiner WJ, Tang MD, Wang LY, Dworetzky SI, Boissard CG, Gan L, Grobkoff VK, Kaczmarek L. Formation of intermediate-conductance calcium-activated potassium channels by interaction of Slack and Slo subunits. Nat Neurosci 1: 462–469, 1998 [DOI] [PubMed] [Google Scholar]
- 61. Kameyama M, Kakei M, Sato R, Shibasaki T, Matsuda H, Irisawa H. Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature 309: 354–356, 1984 [DOI] [PubMed] [Google Scholar]
- 62. Kawahara K. A stretch-activated K+ channel in the basolateral membrane of Xenopus kidney proximal tubule cells. Pflügers Arch 415: 624–629, 1990 [DOI] [PubMed] [Google Scholar]
- 63. Kawahara K, Hunter M, Giebisch G. Potassium channels in Necturus proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol 253: F488–F494, 1987 [DOI] [PubMed] [Google Scholar]
- 64. Kleta R, Bockenhauer D. Bartter syndromes and other salt-losing tubulopathies. Nephron Physiol 104: 73–80, 2006 [DOI] [PubMed] [Google Scholar]
- 65. Koefoed-Johnsen V, Ussing HH. The nature of the frog skin potential. Acta Physiol Scand 42: 298–308, 1958 [DOI] [PubMed] [Google Scholar]
- 66. Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina. J Neurosci 20: 5733–5740, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Krapivinsky G, Medina I, Eng L, Krapivinsky L, Yang Y, Clapham DE. A novel inward rectifier K+ channel with unique pore properties. Neuron 20: 995–1005, 1998 [DOI] [PubMed] [Google Scholar]
- 68. Kubo Y, Baldwin TJ, Jan YN, Jan LY. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature 362: 127–133, 1993 [DOI] [PubMed] [Google Scholar]
- 69. Lachheb S, Cluzeaud F, Bens M, Genete M, Hibino H, Lourdel S, Kurachi Y, Vandewalle A, Teulon J, Paulais M. Kir4.1/Kir5.1 channel forms the major K+ channel in the basolateral membrane of mouse renal collecting duct principal cells. Am J Physiol Renal Physiol 294: F1398–F1407, 2008 [DOI] [PubMed] [Google Scholar]
- 70. Lang F. Mechanisms and significance of cell volume regulation. J Am Coll Nutr 26: 613S–623S, 2007 [DOI] [PubMed] [Google Scholar]
- 71. Lang F, Rehwald W. Potassium channels in renal epithelial transport regulation. Physiol Rev 72: 1–32, 1992 [DOI] [PubMed] [Google Scholar]
- 72. Lang F, Vallon V, Knipper M, Wangemann P. Functional significance of channels and transporters expressed in the inner ear and kidney. Am J Physiol Cell Physiol 293: C1187–C1208, 2007 [DOI] [PubMed] [Google Scholar]
- 73. Le Maout S, Brejon M, Olsen O, Merot J, Welling PA. Basolateral membrane targeting of a renal-epithelial inwardly rectifying potassium channel from the cortical collecting duct, CCD-IRK3, in MDCK cells. Proc Natl Acd Sci USA 94: 13329–13334, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Le Maout S, Welling PA, Brejon M, Olsen O, Merot J. Basolateral membrane expression of a K+ channel, Kir 2.3, is directed by a cytoplasmic COOH-terminal domain. Proc Natl Acd Sci USA 98: 10475–10480, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lesage F, Guillemare E, Fink M, Duprat F, Lazdunski M, Romey G, Barhanin J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J 15: 1004–1011, 1996 [PMC free article] [PubMed] [Google Scholar]
- 76. Levy DI, Velazquez H, Goldstein SA, Bockenhauer D. Segment-specific expression of 2P domain potassium channel genes in human nephron. Kidney Int 65: 918–926, 2004 [DOI] [PubMed] [Google Scholar]
- 77. L'Hoste S, Barriere H, Belfodil R, Duranton C, Tauc M, Poujeol C, Barhanin J, Poujeol P. Extracellular pH alkalinization by Cl−/HCO3− exchanger is crucial for TASK2 activation by hypotonic shock in proximal cell lines from mouse kidney. Am J Physiol Renal Physiol 292: F628–F638, 2007 [DOI] [PubMed] [Google Scholar]
- 78. L'Hoste S, Poet M, Duranton C, Belfodil R, Barriere H, Rubera I, Tauc M, Poujeol C, Barhanin J, Poujeol P. Role of TASK2 in the control of apoptotic volume decrease in proximal kidney cells. J Biol Chem 282: 36692–36703, 2007 [DOI] [PubMed] [Google Scholar]
- 79. Loffing J, Loffing-Cueni D, Valderrabano V, Klausli L, Hebert SC, Rossier BC, Hoenderop JG, Bindels RJ, Kaissling B. Distribution of transcelluar calcium and sodium transport pathways along mouse distal nephron. Am J Physiol Renal Physiol 281: F1021–F1027, 2001 [DOI] [PubMed] [Google Scholar]
- 80. Loonen AJ, Knoers NV, van Os CH, Deen PM. Aquaporin 2 mutations in nephrogenic diabetes insipidus. Semin Nephrol 28: 252–265, 2008 [DOI] [PubMed] [Google Scholar]
- 81. Lourdel S, Paulais M, Cluzeaud F, Bens M, Tanemoto M, Kurachi Y, Vandewalle A, Teulon J. An inward rectifier K+ channel at the basolateral membrane of the mouse distal convoluted tubule: similarities with Kir4.1-Kir5.1 heteromeric channels. J Physiol 538: 391–404, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lu M, Giebisch G, Wang W. Nitric oxide links the apical Na+ transport to the basolateral K+ conductance in the rat cortical collecting duct. J Gen Physiol 110: 717–726, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Makhina E, Kelly AJ, Lopatin AN, Mercer RW, Nichols CG. Cloning and expression of a novel human brain inward rectifier potassium channel. J Biol Chem 269: 20468–20474, 1994 [PubMed] [Google Scholar]
- 84. Marcus DC, Wu T, Wangemann P, Kofuji P. KCNJ10 (Kir4.1) potassium channel knockout abolishes endocochlear potential. Am J Physiol Cell Physiol 282: C403–C407, 2002 [DOI] [PubMed] [Google Scholar]
- 85. Mauerer UR, Boulpaep EL, Segal AS. Regulation of an inwardly rectifying ATP-sensitive K+ channel in the basolateral membrane of renal proximal tubule. J Gen Physiol 111: 139–160, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mauerer UR, Boulpaep EL, Segal AS. Properties of an inwardly rectifying ATP-sensitive K+ channel in the basolateral membrane of renal proximal tubule. J Gen Physiol 111: 161–180, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Millar ID, Taylor HC, Cooper GJ, Kibble JD, Robson L. Kir2.3-like K+ conductance in mouse cortical collecting duct principal cells. J Membr Biol 211: 173–184, 2006 [DOI] [PubMed] [Google Scholar]
- 88. Miller RT. Genetic disorders of NaCl transport in the distal convoluted tubule. Nephron Physiol 118: 15–21, 2011 [DOI] [PubMed] [Google Scholar]
- 89. Morishige K, Takahashi N, Jahangir A, Yamada M, Koyama H, Zanelli JS, Kurachi Y. Molecular cloning and functional expression of a novel brain-specific inward rectifier potassium channel. FEBS Lett 346: 251–256, 1994 [DOI] [PubMed] [Google Scholar]
- 90. Neusch C, Rozengurt N, Jacobs RE, Lester HA, Kofuji P. Kir4.1 potassium channel subunit is crucial for oligodendrocyte development and in vivo myelination. J Neurosci 21: 5429–5438, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Neyroud N, Tesson F, Denjoy I, Leibovici M, Donger C, Barhanin J, Fauré S, Gary F, Coumel P, Petit C, Schwartz K, Guicheney P. A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange-Nielsen cardioauditory syndrome. Nat Genet 15: 186–189, 1997 [DOI] [PubMed] [Google Scholar]
- 92. Niemeyer MI, Cid LP, Pena-Munzenmayer G, Sepuleveda FV. Separate gating mechanisms mediate the regulation of K2P potassium channel TASK-2 by intra- and extracellular pH. J Biol Chem 285: 16467–16475, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Noma A. ATP-regulated K+ channels in cardiac muscle. Nature 305: 147–148, 1993 [DOI] [PubMed] [Google Scholar]
- 94. O'Grady SM, Lee SY. Chloride and potassium channel function in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 284: L689–L700, 2003 [DOI] [PubMed] [Google Scholar]
- 95. O'Grady SM, Lee SY. Molecular diversity and function of voltage-gated (Kv) potassium channels in epithelial cells. Int J Biochem Cell Biol 37: 1578–1594, 2005 [DOI] [PubMed] [Google Scholar]
- 96. Ookata K, Tojo A, Suzuki Y, Nakamura N, Kimura K, Wilcox CS, Hirose S. Localization of inward rectifier potassium channel Kir7.1 in the basolateral membrane of distal nephron and collecting duct. J Am Soc Neohrol 11: 1987–1994, 2000 [DOI] [PubMed] [Google Scholar]
- 97. Parent L, Cardinal J, Sauve R. Single-channel analysis of a K channel at basolateral membrane of rabbit proximal convoluted tubule. Am J Physiol Renal Fluid Electrolyte Physiol 254: F105–F113, 1988 [DOI] [PubMed] [Google Scholar]
- 98. Paulais M, Bloch-Faure M, Picard N, Jacques T, Ramakrishnan SK, Keck M, Sohet F, Eladari D, Houillier P, Lourdel S, Teulon J, Tucker SJ. Renal phenotype in mice lacking the Kir5.1 (Kcnj16) K+ channel subunit contrasts with that observed in SeSAME/EAST syndrome. Proc Natl Acad Sci USA 108: 10361–10366, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Paulais M, Lachheb S, Teulon J. A Na+- and Cl−-activated K+ channel in the thick ascending limb of mouse kidney. J Gen Physiol 127: 205–215, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Paulais M, Lourdel S, Teulon J. Properties of an inwardly rectifying K+ channel in the basolateral membrane of mouse TAL. Am J Physiol Renal Physiol 282: F866–F876, 2002 [DOI] [PubMed] [Google Scholar]
- 101. Perier F, Radeke CM, Vandenberg CA. Primary structure and characterization of a small-conductance inwardly rectifying potassium channel from human hippocampus. Proc Natl Acad Sci USA 91: 6240–6244, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pessia M, Imbrici P, D'Adamo MC, Salvatore L, Tucker SJ. Differential pH sensitivity of Kir4.1 and Kir4.2 potassium channels and their modulation by heterpolymerisation with Kir5.1. J Physiol 532: 359–367, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pessia M, Tucker SJ, Lee K, Bond CT, Adelman JP. Subunit positional effects revealed by novel heteromeric inwardly rectifying K+ channels. EMBO J 15: 2980–2987, 1996 [PMC free article] [PubMed] [Google Scholar]
- 104. Pieczynski J, Margolis B. Protein complexes that control renal epithelial polarity. Am J Physiol Renal Physiol 300: F589–F601, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Pongs O, Schwarz JR. Ancillary subunits associated with voltage-dependent K+ channels. Physiol Rev 90: 755–796, 2010 [DOI] [PubMed] [Google Scholar]
- 106. Rajan S, Wischmeyer E, Karschin C, Preisig-Muller R, Grzeschik KH, Daut J, Karschin A, Derst C. THIK-1 and THIK-2, a novel subfamily of tandem pore domain K+ channels. J Biol Chem 276: 7302–7311, 2001 [DOI] [PubMed] [Google Scholar]
- 107. Reichold M, Zdebik AA, Lieberer E, Rapedius M, Schmidt K, Bandulik S, Sterner C, Tegtmeier I, Penton D, Baukrowitz T, Hulton SA, Witzgall R, Ben-Zeev B, Howie AJ, Kleta R, Bockenhauer D, Warth R. KCNJ10 gene mutations causing EAST syndrome (epilepsy, ataxia, sensorineural deafness, and tubulopathy) disrupt channel function. Proc Natl Acd Sci USA 107: 14490–14495, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Reuss L. Isolated-polarized epithelial cells as an experimental system for cell physiology studies. J Membr Biol 179: 1–12, 2001 [DOI] [PubMed] [Google Scholar]
- 109. Reuss L. Ussing's two-membrane hypothesis: the model and half a century of progress. J Membr Biol 184: 211–217, 2001 [DOI] [PubMed] [Google Scholar]
- 110. Reyes R, Duprat F, Lesage F, Fink M, Salinas M, Farman N, Lazdunski M. Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. J Biol Chem 273: 30863–30869, 1998 [DOI] [PubMed] [Google Scholar]
- 111. Sackin H, Palmer LG. Basolateral potassium channels in renal proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol 253: F476–F487, 1987 [DOI] [PubMed] [Google Scholar]
- 112. Sala-Rabanal M, Kucheryavykh LY, Skatchkov SN, Eaton MJ, Nichols CG. Molecular mechanisms of EAST/SeSAME syndrome mutations in Kir4.1 (KCNJ10). J Biol Chem 285: 36040–36048, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci 5: 921–931, 2006 [DOI] [PubMed] [Google Scholar]
- 114. Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of K(V)LQT1 and mink (IsK) proteins to form cardiac I(Ks) potassium channel. Nature 384: 80–83, 1996 [DOI] [PubMed] [Google Scholar]
- 115. Scholl UI, Choi M, Liu T, Ramaekers VT, Hausler MG, Grimmer J, Tobe SW, Farhi A, Nelson-Williams C, Lifton RP. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci USA 106: 5842–5847, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Schroeder BC, Waldegger S, Fehr S, Bleich M, Warth R, Greger R, Jentsch TJ. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature 403: 196–199, 2000 [DOI] [PubMed] [Google Scholar]
- 117. Schultz SG. Homocellular regulatory mechanisms in sodium-transporting epithelia: avoidance of extinction by “flush-through.” Am J Physiol Renal Fluid Electrolyte Physiol 241: F579–F590, 1981 [DOI] [PubMed] [Google Scholar]
- 118. Shimkets RA, Warnock DG, Bositis CM, Nelson-Williams C, Hansson JH, Schambelan M, Gill JR, Jr, Ulick S, Milora RV, Findling JW, Canessa CM, Rossier BC, Lifton RP. Liddle's syndrome: heritable human hypertension caused by mutations in the β subunit of the epithelial sodium channel. Cell 79: 407–414, 1994 [DOI] [PubMed] [Google Scholar]
- 119. Stoos BA, Naray-Fejes-Toth A, Carretero S, Ito S, Feyes-Toth G. Characterization of a mouse cortical collecting duct cell line. Kidney Int 39: 1168–1175, 1991 [DOI] [PubMed] [Google Scholar]
- 120. Sugimoto T, Tanabe Y, Shigemoto R, Iwai M, Takumi T, Ohkubo H, Nakanishi S. Immunohistochemical study of a rat membrane protein which induces a selective potassium permeation: its localization in the apical membrane portion of epithelial cells. J Membr Biol 113: 39–47, 1990 [DOI] [PubMed] [Google Scholar]
- 121. Tanemoto M, Kittaka N, Inanobe A, Kurachi Y. In vivo formation of a proton-sensitive K+ channel by heteromeric subunit assembly of Kir5.1 with Kir4.1. J Physiol 525: 587–592, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tang X, Hang D, Sand A, Kofuji P. Variable loss of Kir4.1 channel function in SeSAME syndrome mutations. Biochem Biophys Res Commun 399: 537–541, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Taniguchi J, Yoshitomi K, Imai M. K+ channel currents in basolateral membrane of distal convoluted tubule of rabbit kidney. Am J Physiol Renal Fluid Electrolyte Physiol 256: F246–F254, 1989 [DOI] [PubMed] [Google Scholar]
- 124. Theilig F, Goranova I, Hirsch JR, Wieske M, Unsal S, Bachmann S, Veh RW, Derst C. Cellular location of THIK-1 (K2P13.1) and THIK-2 (K2P121) K+ channels in the mammalian kidney. Cell Physiol Biochem 21: 63–74, 2008 [DOI] [PubMed] [Google Scholar]
- 125. Tsuchiya K, Wang W, Giebisch G, Welling PA. ATP is a coupling modulator of parallel Na,K-ATPase-K-channel activity in the renal proximal tubule. Proc Natl Acad Sci USA 89: 6418–6422, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Tucker SJ, Imbrici P, Salvatore L, D'Adamo MC, Pessia M. pH dependence of the inwardly rectifying potassium channel, Kir 5.1, and localization in renal tubular epithelia. J Biol Chem 275: 16404–16407, 2000 [DOI] [PubMed] [Google Scholar]
- 127. Tukumi T, Ohkubo H, Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium channel. Science 242: 1042–1045, 1988 [DOI] [PubMed] [Google Scholar]
- 128. Vallon V, Grahammer F, Richter K, Bleich M, Lang F, Barhanin J, Volkl H, Warth R. Role of KCNE1-dependent K+ fluxes in mouse proximal tubule. J Am Soc Nephrol 12: 2003–2011, 2001 [DOI] [PubMed] [Google Scholar]
- 129. Vallon V, Grahammer F, Volkl H, Sandu CD, Richter K, Rexhepaj R, Gerlach U, Rong Q, Pfeifer K, Lang F. KCNQ1-dependent transport in renal and gastrointestinal epithelia. Proc Natl Acad Sci USA 102: 17864–17869, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Van Driessche W, Zeiske W. Ion channels in epithelial cell membranes. Physiol Rev 65: 833–903, 1985 [DOI] [PubMed] [Google Scholar]
- 131. Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, Shen J, Timothy KW, Vincent GM, de Jager T, Schwartz PJ, Toubin JA, Moss AJ, Atkinson DL, Landes GM, Connors TD, Keating MT. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet 12: 17–23, 1996 [DOI] [PubMed] [Google Scholar]
- 132. Warth R. Potassium channels in epithelial transport. Pflügers Arch 446: 505–513, 2003 [DOI] [PubMed] [Google Scholar]
- 133. Warth R, Barhanin J. Function of K+ channels in the intestinal epithelium. J Membr Biol 193: 67–78, 2003 [DOI] [PubMed] [Google Scholar]
- 134. Warth R, Barriere H, Meneton P, Bloch M, Thomas J, Tauc M, Heitzmann D, Romeo E, Verrey F, Mengual R, Guy N, Bendahhou S, Lesage F, Pouljeol P, Barhanin J. Proximal renal tubular acidosis in TASK2 K+ channel-deficient mice reveals a mechanism for stabilizing bicarbonate transport. Proc Natl Acad Sci USA 101: 8215–8220, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Welling PA. Primary structure and functional expression of a cortical collecting duct Kir channel. Am J Physiol Renal Physiol 273: F825–F836, 1997 [DOI] [PubMed] [Google Scholar]
- 136. Welling PA, Ho K. A comprehensive guide to the ROMK potassium channel: form and function in health and disease. Am J Physiol Renal Physiol 297: F849–F863, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Williams DM, Lopes CMB, Rosenhouse-Dantsker A, Connelly HL, Matavel A, O-Uchi J, McBeath E, Gray DA. Molecular basis of decreased Kir4.1 function in SeSAME/EAST syndrome. J Am Soc Nephrol 21: 2117–2129, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Yuan A, Dourado M, Butler A, Walton N, Wei A, Salkoff L. Slo-2, a K+ channel with an unusual Cl− dependence. Nat Neurosci 3: 771–779, 2000 [DOI] [PubMed] [Google Scholar]
- 139. Yuan A, Santi CM, Wei A, Wang ZW, Pollak K, Nonet M, Kaczmarek L, Crowder CM, Salkoff L. The sodium-activated potassium channel is encoded by a member of the Slo gene family. Neuron 37: 765–773, 2003 [DOI] [PubMed] [Google Scholar]
- 140. Zhang Z, Rosenhouse-Dantsker A, Tang QY, Noskov S, Logothetis DE. The RCK2 domain uses a coordination site present in Kir channels to confer sodium sensitivity to Slo2.2 channels. J Neurosci 30: 7554–7562, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zheng W, Verlander JW, Lynch IJ, Cash M, Shao J, Stow LR, Cain BD, Weiner ID, Wall SM, Wingo CS. Cellular distribution of the potassium channel KCNQ1 in normal mouse kidney. Am J Physiol Renal Physiol 292: F456–F466, 2007 [DOI] [PubMed] [Google Scholar]
- 142. Zhou M, He HJ, Suzuki R, Tanaka O, Sekiguchi M, Yasuoka Y, Kawahara K, Itoh H, Abe H. Expression of ATP sensitive K+ channel subunit Kir6.1 in rat kidney. Eur J Histochem 51: 43–51 2007 [PubMed] [Google Scholar]