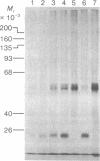
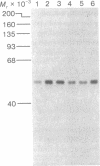
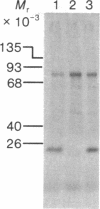
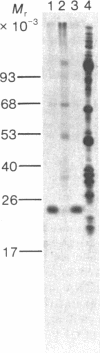
Abstract
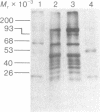
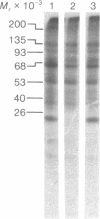
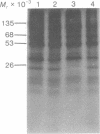
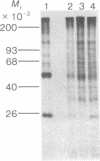
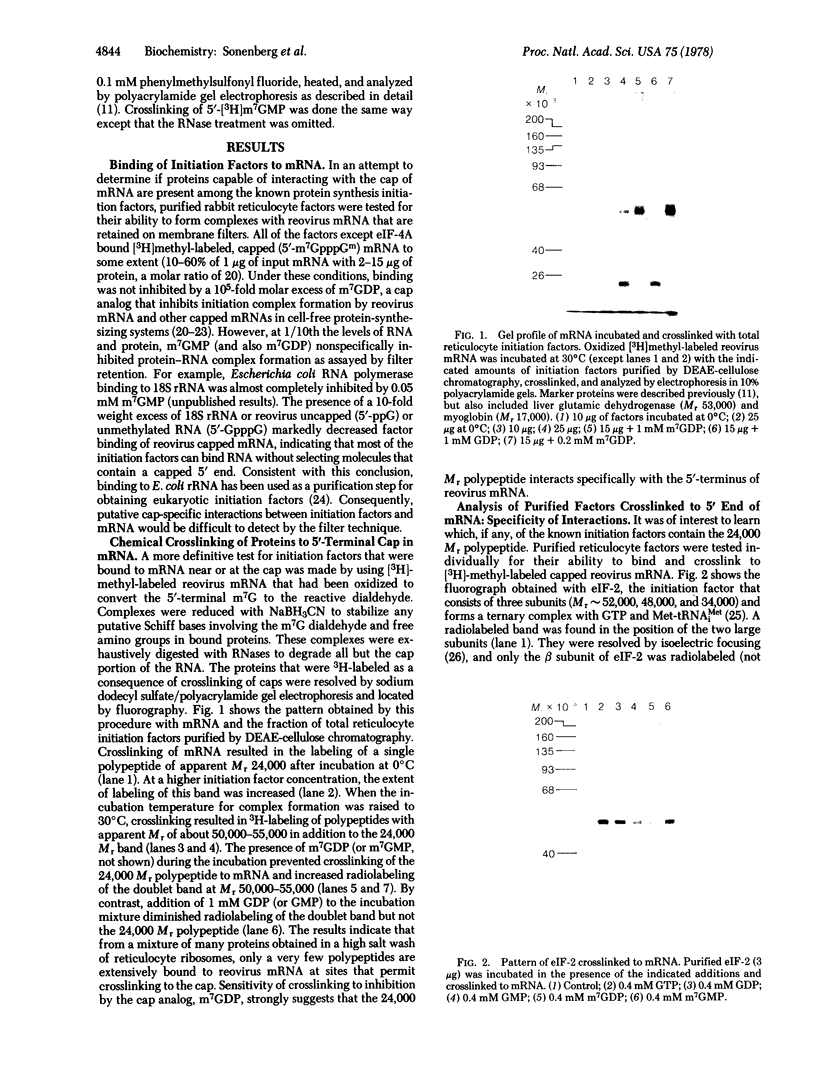
Protein synthesis initiation factors prepared from rabbit reticulocyte and mouse ascites ribosomes were tested for the ability to crosslink to the 5' cap of mRNA. Crosslinking of one polypeptide of apparent molecular weight 24,000 was inhibited by the cap analogs, m7GMP and m7GDP, indicating a specific interaction with the cap. Although specific crosslinking of the 24,000 molecular weight polypeptide was found with eukaryotic initiation factor 3 and to a lesser extent with initiation factor 4B, both of these factors contained less than stoichiometric amounts of this polypeptide. The crosslinking method provides a highly sensitive and specific assay for cap-binding proteins and should facilitate their purification for functional studies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F., Bosch L., Cohn W. E., Lodish H., Merrick W. C., Weissbach H., Wittmann H. G., Wool I. G. International symposium on protein synthesis. Summary of Fogarty Center-NIH Workshop held in Bethesda, Maryland on 18-20 October, 1976. FEBS Lett. 1977 Apr 1;76(1):1–10. doi: 10.1016/0014-5793(77)80109-9. [DOI] [PubMed] [Google Scholar]
- Barrieux A., Rosenfeld M. G. Characterization of GTP-dependent Met-tRNAf binding protein. J Biol Chem. 1977 Jun 10;252(11):3843–3847. [PubMed] [Google Scholar]
- Barrieux A., Rosenfeld M. G. Comparison of mRNA binding by Met-tRNAf binding protein and mRNA-associated proteins. J Biol Chem. 1977 Jan 10;252(1):392–398. [PubMed] [Google Scholar]
- Benne R., Hershey J. W. Purification and characterization of initiation factor IF-E3 from rabbit reticulocytes. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3005–3009. doi: 10.1073/pnas.73.9.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne R., Hershey J. W. The mechanism of action of protein synthesis initiation factors from rabbit reticulocytes. J Biol Chem. 1978 May 10;253(9):3078–3087. [PubMed] [Google Scholar]
- Benne R., Luedi M., Hershey J. W. Purification and characterization of initiation factors IF-E4 and IF-E6 from rabbit reticulocytes. J Biol Chem. 1977 Aug 25;252(16):5798–5803. [PubMed] [Google Scholar]
- Both G. W., Furuichi Y., Muthukrishnan S., Shatkin A. J. Effect of 5'-terminal structure and base composition on polyribonucleotide binding to ribosomes. J Mol Biol. 1976 Jul 5;104(3):637–658. doi: 10.1016/0022-2836(76)90126-1. [DOI] [PubMed] [Google Scholar]
- Canaani D., Revel M., Groner Y. Translational discrimination of 'capped' and 'non-capped' mRNAS: inhibition of a series of chemical analogs of m7GpppX. FEBS Lett. 1976 May 1;64(2):326–331. doi: 10.1016/0014-5793(76)80321-3. [DOI] [PubMed] [Google Scholar]
- Filipowicz W., Furuichi Y., Sierra J. M., Muthukrishnan S., Shatkin A. J., Ochoa S. A protein binding the methylated 5'-terminal sequence, m7GpppN, of eukaryotic messenger RNA. Proc Natl Acad Sci U S A. 1976 May;73(5):1559–1563. doi: 10.1073/pnas.73.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisby D., Eaton M., Fellner P. Absence of 5' terminal capping in encephalomyocarditis virus RNA. Nucleic Acids Res. 1976 Oct;3(10):2771–2787. doi: 10.1093/nar/3.10.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., LaFiandra A., Shatkin A. J. 5'-Terminal structure and mRNA stability. Nature. 1977 Mar 17;266(5599):235–239. doi: 10.1038/266235a0. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Shatkin A. J. Differential synthesis of blocked and unblocked 5'-termini in reovirus mRNA: effect of pyrophosphate and pyrophosphatase. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3448–3452. doi: 10.1073/pnas.73.10.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerman J. G., Shafritz D. A. Interaction of poly(A) and mRNA with eukaryotic initiator met-tRNA-f binding factor: identification of this activity on reticulocyte ribonucleic acid protein particles. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1021–1025. doi: 10.1073/pnas.72.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey E. D., Weber L. A., Baglioni C. Inhibition of initiation of protein synthesis by 7-methylguanosine-5'-monophosphate. Proc Natl Acad Sci U S A. 1976 Jan;73(1):19–23. doi: 10.1073/pnas.73.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaempfer R., Rosen H., Israeli R. Translational control: recognition of the methylated 5' end and an internal sequence in eukaryotic mRNA by the initiation factor that binds methionyl-tRNAfMet. Proc Natl Acad Sci U S A. 1978 Feb;75(2):650–654. doi: 10.1073/pnas.75.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper W. M., Berry K. W., Merrick W. C. Purification and properties of rabbit reticulocyte protein synthesis initiation factors M2Balpha and M2Bbeta. J Biol Chem. 1976 Sep 25;251(18):5551–5557. [PubMed] [Google Scholar]
- Merrick W. C., Kemper W. M., Anderson W. F. Purification and characterization of homogeneous initiation factor M2A from rabbit reticulocytes. J Biol Chem. 1975 Jul 25;250(14):5556–5562. [PubMed] [Google Scholar]
- Muthukrishnan S., Morgan M., Banerjee A. K., Shatkin A. J. Influence of 5'-terminal m7G and 2'--O-methylated residues on messenger ribonucleic acid binding to ribosomes. Biochemistry. 1976 Dec 28;15(26):5761–5768. doi: 10.1021/bi00671a012. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov L. P., Spirin A. S., Erni B., Staehelin T. RNA-binding proteins of rabbit reticulocytes contain the two elongation factors and some of the initiation factors of translation. FEBS Lett. 1978 Apr 1;88(1):21–26. doi: 10.1016/0014-5793(78)80598-5. [DOI] [PubMed] [Google Scholar]
- Prichard P. M., Anderson W. F. Preparation of rabbit reticulocyte initiation factor IF-M3. Methods Enzymol. 1974;30:136–141. doi: 10.1016/0076-6879(74)30016-x. [DOI] [PubMed] [Google Scholar]
- Roman R., Brooker J. D., Seal S. N., Marcus A. Inhibition of the transition of a 40 S ribosome-Met-tRNA-i-Met complex to an 80 S ribosome-Met-tRNA-i-Met- complex by 7-Methylguanosine-5'-phosphate. Nature. 1976 Mar 25;260(5549):359–360. doi: 10.1038/260359a0. [DOI] [PubMed] [Google Scholar]
- Safer B., Adams S. L., Kemper W. M., Berry K. W., Lloyd M., Merrick W. C. Purification and characterization of two initiation factors required for maximal activity of a highly fractionated globin mRNA translation system. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2584–2588. doi: 10.1073/pnas.73.8.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer B., Anderson W. F., Merrick W. C. Purification and physical properties of homogeneous initiation factor MP from rabbit reticulocytes. J Biol Chem. 1975 Dec 10;250(23):9067–9075. [PubMed] [Google Scholar]
- Schreier M. H., Erni B., Staehelin T. Initiation of mammalian protein synthesis. I. Purification and characterization of seven initiation factors. J Mol Biol. 1977 Nov;116(4):727–753. doi: 10.1016/0022-2836(77)90268-6. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Weinstein J. A., Safer B., Merrick W. C., Weber L. A., Hickey E. D., Baglioni C. Evidence for role of m7G5'-phosphate group in recognition of eukaryotic mRNA by initiation factor IF-M3. Nature. 1976 May 27;261(5558):291–294. doi: 10.1038/261291a0. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Shimotohno K., Kodama Y., Hashimoto J., Miura K. I. Importance of 5'-terminal blocking structure to stabilize mRNA in eukaryotic protein synthesis. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2734–2738. doi: 10.1073/pnas.74.7.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Shatkin A. J. Reovirus mRNA can be covalently crosslinked via the 5' cap to proteins in initiation complexes. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4288–4292. doi: 10.1073/pnas.74.10.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara S. M., Traugh J. A., Sharp S. B., Lundak T. S., Safer B., Merrick W. C. Effect of hemin on site-specific phosphorylation of eukaryotic initiation factor 2. Proc Natl Acad Sci U S A. 1978 Feb;75(2):789–793. doi: 10.1073/pnas.75.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel H., Erni B., Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis. II. The assembly of the initiation complex with purified initiation factors. J Mol Biol. 1977 Nov;116(4):755–767. doi: 10.1016/0022-2836(77)90269-8. [DOI] [PubMed] [Google Scholar]
- Zan-Kowalczewska M., Bretner M., Sierakowska H., Szczesna E., Filipowicz W., Shatkin A. J. Removal of 5'-terminal m7G from eukaryotic mRNAs by potato nucleotide pyrophosphatase and its effect on translation. Nucleic Acids Res. 1977 Sep;4(9):3065–3081. doi: 10.1093/nar/4.9.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]