Abstract
Nicorandil is an orally available drug that can act as a nitric oxide donor, an antioxidant, and an ATP-dependent K channel activator. We hypothesized that it may have a beneficial role in treating diabetic nephropathy. We administered nicorandil to a model of advanced diabetic nephropathy (the streptozotocin-induced diabetes in mice lacking endothelial nitric oxide synthase, eNOSKO); controls included diabetic eNOS KO mice without nicorandil and nondiabetic eNOS KO mice treated with either nicorandil or vehicle. Mice were treated for 8 wk. Histology, blood pressure, and renal function were determined. Additional studies involved examining the effects of nicorandil on cultured human podocytes. Here, we found that nicorandil did not affect blood glucose levels, blood pressure, or systemic endothelial function, but significantly reduced proteinuria and glomerular injury (mesangiolysis and glomerulosclerosis). Nicorandil protected against podocyte loss and podocyte oxidative stress. Studies in cultured podocytes showed that nicorandil likely protects against glucose-mediated oxidant stress via the ATP-dependent K channel as opposed to its NO-stimulating effects. In conclusion, nicorandil may be beneficial in diabetic nephropathy by preserving podocyte function. We recommend clinical trials to determine whether nicorandil may benefit diabetic nephropathy or other conditions associated with podocyte dysfunction.
Keywords: podocyte, endothelial dysfunction, ATP-dependent K channel, sulfonylurea receptor
diabetic nephropathy develops in 30–40% of diabetic subjects and is a major cause of morbidity and mortality. Current treatment consists of tight blood glucose control, lowering of blood pressure to 130/80 mmHg, and the use of inhibitors of the renin-ngiotensin system (RAS) (7, 36). However, treatment remains imperfect (4). In particular, it is likely that the presence of endothelial dysfunction requires a special therapeutic strategy since several studies documented refractoriness of a RAS inhibitor is associated with endothelial dysfunction in diabetic patients (9).
Our group and others have developed an animal model of advanced diabetic nephropathy using endothelial nitric oxide synthase-deficient (eNOSKO) mice (10, 22). Interestingly, this animal model receives less benefit from RAS blockades compared with diabetic wild-type mice (12). Thus another therapeutic option is needed in diabetes with endothelial dysfunction.
Organic nitrates are used to provide nitric oxide (NO), which is expected to improve endothelial function and cardiovascular disease. However, long-term effects are limited due to the development of tolerance (35). In addition, organic nitrates are found to rather exacerbate endothelial dysfunction due to inducing oxidative stress (35). Hence organic nitrates as previously tested are unlikely to provide a benefit in diabetic nephropathy.
Nicorandil, or 2-[(pyridin-3-ylcarbonyl)amino]ethyl nitrate, is a clinically proven antianginal agent that causes vasodilatation by dual action: one is releasing NO and the other is a binding to and opening of the ATP-dependent K channel (3), the latter of which is associated with the reduction of reactive oxygen species (ROS) in endothelial cells (6). These actions likely result in an decrease in cardiovascular events in subjects with coronary artery diseases (2, 26). Similarly, a recent study documented that nicorandil decreased proteinuria in hypertensive patients receiving low doses of angtiotensin receptor blockers (ARBs) (14). These reports led us to hypothesize that nicorandil could be beneficial in the treatment of diabetic nephropathy even under conditions of severe endothelial dysfunction.
In this study, we tested the effect of nicorandil in the diabetic mice in which eNOS production is permanently disturbed. Data demonstrate that nicorandil does not result in an improvement in systemic endothelial dysfunction in contrast to expectation. However, we found that nicorandil directly reduced oxidative stress in podocytes via the ATP-dependent K channel, independently of NO.
METHODS
Experimental Protocols
All animal experiments were performed in accordance with the Animal Care and Use Committee of the University of Colorado. Male C57BL/6J-Nos3tm1nc mice (eNOSKO mice) were purchased from Jackson Laboratory (Bar Harbor, ME) at 8 wk of age. Mice were fed a standard laboratory chow ad libitum. Diabetic nephropathy was induced by intraperitoneal injections of streptozotocin (50 mg·kg−1·day−1 for 5 consecutive days) dissolved in 10 mM citrate buffer, pH 4.5 (5). Diabetes was defined as nonfasting blood glucose >250 mg/dl using a blood glucose meter (One Touch Ultra; Life Scan, Milpitas, CA). While no mice developed diabetes at day 7 after streptozotocin administration, 33, 72, and 85% of mice became diabetic at 2, 3, and 4 wk, respectively. Only mice which developed hyperglycemia at 4 wk were included in the study. Mice were divided into four subgroups: 1) a nondiabetic group, 2) a nicorandil-treated nondiabetic group, 3) a diabetic group, and 4) a nicorandil-treated diabetic group (n = 8/group). At 4 wk when the onset of diabetes was confirmed in all animals, 30 mg/kg of nicorandil (Chugai Pharmaceutical, Tokyo, Japan) was started. To constantly administer the same amount of nicorandil (30 mg·kg−1·day−1), the concentration of nicorandil in the drinking water was adjusted every 4 days along with as per the water intake volume. Water bottles were monitored daily throughout the study to ensure no leakage occurred. Systolic blood pressure was measured every other week using a tail-cuff sphygmomanometer (Visitech BP-2000; Visitech Systems, Apex, NC). Urine was collected overnight using metabolic cages (Techniplast, Exton, PA). All the mice were euthanized 8 wk after starting nicorandil treatment to obtain blood samples and kidney tissues.
Laboratory Studies
Urine albumin, urine 8-hydroxy-2-deoxyguanosine (8-OHdG), and urine creatinine were measured with Albuwell M (Exocell, Philadelphia, PA), an OxiSelect Oxidative DNA Damage ELISA Kit (Cell Biolabs, San Diego, CA), and Creatinine LiquiColor Test (Enzymatic Methodology; Stanbio, Boerne, TX), respectively. Serum creatinine concentration was analyzed with HPLC-tandem mass spectrometry (MS/MS; Applied Biosystems 3200 Qtrap). Creatinine and [2H3]creatinine (CDN isotopes) were detected in the multiple reaction monitoring mode, monitoring the transitions of the m/z from 114 to 44.2 and m/z from 117 to 47.2, respectively. Serum levels of P-selectin and ICAM-1 were measured with a Mouse P-selectin/CD62 Quantikine ELISA kit and Mouse ICAM-1/CD54 Quantikine ELISA kit, respectively (R&D Systems, Minneapolis, MN).
Histological Analysis
Formalin-fixed, paraffin-embedded sections (2.5-μm) were stained with the periodic acid-Schiff reagent (PAS) for light microscopy. On coronal sections of the kidney, all glomeruli (50–100 glomeruli) were examined for evaluation of mesangiolysis and glomerulosclerosis. Glomerulosclerosis was defined as obstruction of the capillary lumen caused by mesangial expansion or collapsed capillaries, whereas the degree of mesangiolysis was calculated as the number of glomeruli with mesangiolysis (dissolution of the mesangial matrix) divided by that of total glomeruli (22). Kidney sections were observed by two investigators in a blinded manner.
Immunohistochemistry
Either formalin or methyl Carnoy's solution-fixed, paraffin-embedded sections were used for immunohistochemistry as previously described (11). The following antibodies were used as primary antibodies: 1) rabbit anti-type IV collagen antibody (Chemicon International, Temecula, CA); 2) rat anti-mouse F4/80 antibody (Serotec, Raleigh, NC); 3) rabbit anti-WT-1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA); 4) goat anti-8-OHdG antibody (Abcam, Cambridge, MA); 5) rabbit anti-nitrotyrosine antibody (Chemicon); and 6) rabbit anti-NPHS2 (podocin) antibody (Abcam). Briefly, after deparaffinization, the sections were treated with 3% H2O2 for 10 min to inactivate endogenous peroxidase activity. For F4/80, 8-OHdG, and nitrotyrosine, the sections were treated with 10 mM citrate buffer (pH 6.0) for 30 min in a steamer for antigen retrieval. After incubation with a background sniper (Biocare Medical, Concord, CA) for 15 min, sections were incubated with primary antibodies overnight at 4°C. The sections were also incubated with rabbit anti-IgG secondary antibodies for 30 min before immunoperoxidase staining was conducted using the Mach2 rabbit HRP polymer (Biocare Medical). Slides were counterstained with methyl green.
The number of positive cells for F4/80 was counted in all glomeruli at ×400 magnification in each section. After immunohistochemistry for WT-1 was performed, podocyte number per glomerulus was calculated using the Weibel-Gomez method as previously reported (25). Ten representative glomeruli were analyzed on each section for this calculation. To assess the type IV collagen- and podocin-positive area, the digital images at ×400 magnification were analyzed using Image scope software (Aperio Technologies, Vista, CA). The percent positive area was determined as the 3,3-diaminobenzidine-positive pixel values per examined interest area from all glomeruli in each section.
Immunofluorescence in the Mouse Kidney
Double immunofluorescence staining was performed as previously described (24). Briefly, frozen sections (4-μm) were fixed in acetone for 10 min. The sections were blocked with 5% animal serum complex and then incubated overnight with primary antibodies, rabbit anti-ABCC9 (sulfonylurea receptor 2; SUR-2) antibody (Abcam), rabbit anti-cGMP antibody (Chemicon), or mouse monoclonal anti-synaptopodin antibody (Novus Biologicals, Littleton, CO), at 4°C. After incubation with either Alexa Fluor 488-labeled goat anti-rabbit IgG (Invitrogen, Carlsbad, CA) or Alexa Fluor 546-labeled goat anti-mouse IgG (Invitrogen) for 2 h at room temperature, sections were mounted with vectashield anti-fade mounting medium (Vector Labs, Burlingame, CA). A laser-scanning confocal microscope LSM 510 META (Carl Zeiss Microimaging, Thornwood, NY) was used to obtain images.
Western Blotting
Kidney tissues were homogenized in cell lysis buffer (Cell Signaling, Danvers, MA) at 4°C. Briefly, samples were processed for SDS-PAGE and electrotransferred onto a nitrocellulose membranes. A rabbit anti-nitrotyrosine (Chemicon) antibody, rabbit anti-β-actin antibodies (Sigma-Aldrich, St. Louis, MO), an HRP-labeled anti-rabbit IgG antibody (Cell Signaling), and Immun Star HRP (Bio-Rad, Hercules, CA) were used. The density of each band was determined using National Institutes of Health Image software and expressed as a value relative to the density of the corresponding band of β-actin.
Cell Culture
Conditionally immortalized human podocytes were cultured in RPMI 1640 medium (Mediatech, Manassas, VA) supplemented with 10% FBS, penicillin (100 U/l), streptomycin (100 μg/l), and Insulin-Transferrin-Selenium A supplements (Invitrogen). Cells were cultured at 33°C to enhance the expression of large T antigen and propagate podocytes, followed by incubation for 10 days at 37°C to induce differentiation into mature podocytes before initiation of experiments. For the experimental studies, podocytes were cultured in DMEM with 5.5 mM normal glucose (NG), NG+19.5 mM mannitol (NG+Man), 25mM high glucose (HG), and HG+10−5 M nicorandil (HG+Nico) for 72 h.
PCR
Total RNA was extracted from cultured podocytes using an RNeasy Mini Kit (Qiagen, Chatsworth, CA). The first-strand cDNA was synthesized from 1 μg of total RNA using an iScript cDNA Synthesis Kit (Bio-Rad). For the detection of SUR2A and SUR2B mRNA, the following oligonucleotide primers were used: SUR2A, 5′-TGAGGGTATTTTAGTGGAGTGTG-3′ (forward) and 5′-CAAAGTGGAAAAGAGGCCATTC-3′ (reverse); SUR2B, 5′-TGGTGACAATAGCTCATCGAG-3′ (forward) and 5′-TCCATTTTCCTGAGCCAAGAG-3′ (reverse); and β-actin, 5′-TGAGATGCGTTGTTACAGGAAG-3′ (forward) and 5′-GTGGACTTGGGAGAGGACTG-3′ (reverse). The PCR program was optimized and performed as denaturation at 95°C for 3 min followed by 50 cycles of amplification (SUR2A and SUR2B, 95°C for 30 s, 54°C for 30 s, 72°C for 1 min; β-actin, 95°C for 30 s, 56°C for 30 s, 72°C for 1 min, respectively) using the MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad). The amount of PCR products was normalized with β-actin mRNA to determine the relative expression ratio for SUR2A and SUR2B mRNA.
ROS Detection Assay in Cultured Podocytes
An Image-iT LIVE Green Reactive Oxygen Species Detection Kit (Invitrogen) was used to measure the generation of ROS. Briefly, podocytes were incubated in PBS containing 25 μM 5-(and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA) for 30 min at 37°C in the dark. This probe is converted by intracellular esterase and ROS to carboxy-DCF, which emits a bright green fluorescence. After incubation, the cells were washed with PBS three times and observed by a laser-scanning confocal microscope LSM 510 META (Carl Zeiss) to obtain images. The intensity in podocytes was measured by Zen 2009 software (Carl Zeiss). A total of 10 fields (at ×400) were examined to determine the averaged value of cells in each image.
Cell Number
A conventional MTT assay was used to assess cultured podocyte number (23). Podocytes were seeded in 96-well plates at 10,000 cells/well and allowed to differentiate at 37°C for 10 days. Seventy-two hours after stimulation by NG or HG medium with or without nicorandil, 20 μl of thiazolyl blue tetrazolium bromide (Sigma-Aldrich) dissolved in PBS at 5 mg/ml was added into each well containing 100 μl of medium, and the plate was incubated for 3 h at 37°C. MTT solvent (4 mM HCl and 0.1% P-40 in isopropanol) was used before absorbance was read at 590 nm with a reference filter of 620 nm. Data were expressed as values relative to NG.
Statistical Analysis
All values are expressed as means ± SD. Statistical analysis was performed with ANOVA using Tukey's (for in vivo experiments) or Bonferroni's (in vitro experiments) method to compare four groups. A level of P < 0.05 was considered statistically significant.
RESULTS
General Characteristics
The injections of streptozotocin resulted in marked hyperglycemia and an increased kidney size (a ratio of kidney/body weight) accompanied by a significant loss of body weight in the eNOSKO mice. Urinary albumin excretion and creatinine clearance were also increased, consistent with early hyperfiltration. Nicorandil significantly reduced albuminuria by 70% in diabetic eNOSKO mice while no effects were observed on renal function (Table 1).
Table 1.
General characteristics of nondiabetic and diabetic mice
| NonDM | NonDM+Nicorandil | DM | DM+Nicorandil | |
|---|---|---|---|---|
| Body wt, g | 26.9 ± 2.6 | 27.3 ± 1.4 | 22.6 ± 1.9* | 22.5 ± 1.7* |
| Blood glucose, mg/dl | 119.5 ± 7.0 | 118.1 ± 10.2 | 376.3 ± 85.4* | 353.0 ± 24.9* |
| Kidney wt/body wt ratio (×10−3) | 4.5 ± 0.4 | 4.4 ± 0.7 | 7.4 ± 1.7* | 7.3 ± 0.7* |
| Urine albumin/creatinine ratio (×10−1) | 10.6 ± 6.7 | 9.2 ± 4.6 | 57.2 ± 12.4* | 17.2 ± 10.4*† |
| Ccr, ml/min | 0.22 ± 0.19 | 0.21 ± 0.14 | 0.34 ± 0.10 | 0.39 ± 0.27 |
Values are means ± SD; n = 8/group. NonDM, nondiabetic; DM, diabetic; Ccr, creatinine clearance.
P < 0.01 vs. NonDM.
P < 0.05 vs. DM.
Glomerular Histology
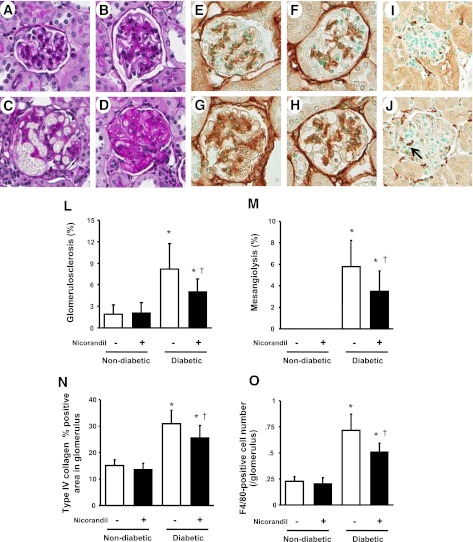
Compatible with previous reports (10, 22), diabetic conditions caused glomerular hypertrophy and mesangial expansion accompanied by more severe glomerular lesions such as mesangiolysis or glomerulosclerosis in eNOSKO mice compared with nondiabetic glomeruli (Fig. 1, A–D). Nicorandil treatment significantly reduced the development of mesangiolysis and glomerulosclerosis (Fig. 1, L and M, respectively). Similarly, type IV collagen deposition, a marker of mesangial matrix expansion, was markedly increased in diabetic eNOSKO mice (Fig. 1G) compared with nondiabetic groups (Fig. 1, E and F) while it was reduced by nicorandil treatment (Fig. 1, H and N). Similarly, diabetic conditions induced F4/80-positive cell infiltration in the glomeruli of diabetic eNOSKO mice whereas nicorandil significantly reduced the number of infiltrating cells (Fig. 1, I, J, and O).
Fig. 1.
Glomerular disease in diabetic mice lacking endothelial nitric oxide synthase (eNOSKO). Representative light microscopic appearance of glomerular lesions is shown (periodic acid-Schiff staining, ×400 original magnification). Compared with nondiabetic (A), diabetic eNOSKO mice show mesangial expansion (B), mesangiolysis (C), and glomerulosclesosis (D). Nicorandil treatment significantly reduces the incidences of mesangiolysis (L) and glomerulosclerosis (M) in diabetic eNOSKO mice. Immunohistochemistry for mesangial accumulation of type IV collagen for nondiabetes (E), nondiabetes with nicorandil (F), diabetes (G), and diabetes with nicorandil (H) in eNOSKO mice (×400 original magnification) is shown. Type IV collagen-positive area in the glomerulus is significantly reduced by nicorandil treatment in diabetic eNOSKO mice (N). Induction of diabetes also results in an increase in the number of F4/80-positive cells in the glomerulus (J, arrow) compared with nondiabetic mice (I). Nicorandil exhibits an inhibitory effect on the glomerular infiltration of monocytes/macrophages in diabetic eNOSKO mice (O). Values are means ± SD; n = 8/group. *P < 0.01 vs. nondiabetes. †P < 0.05 vs. diabetes.
Investigations of Mechanisms Whereby Nicorandil is Protective
Effect on blood pressure.
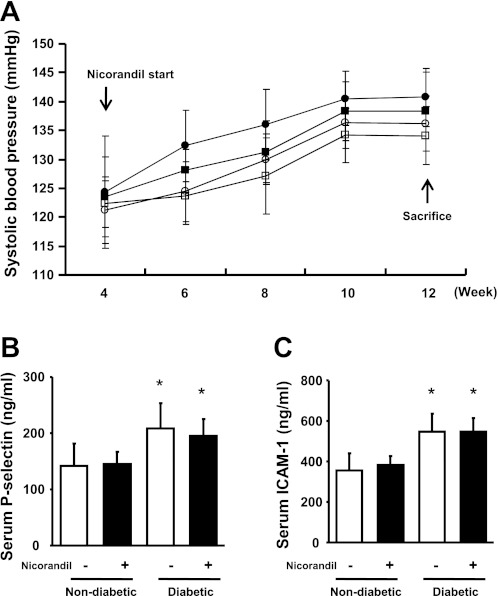
Blood pressure tended to be higher in diabetic eNOSKO mice after 2 wk compared with nondiabetic mice, but the difference did not reach statistical significance (Fig. 2A). Nicorandil did not significantly lower blood pressure in either diabetic or nondiabetic groups during the 8-wk treatment period.
Fig. 2.
Blood pressure and markers for endothelial injury in diabetic eNOSKO mice. Time course of blood pressure is shown (A). Nicorandil treatment was initiated at week 4. ○, Nondiabetes; ●, diabetes; □, nondiabetes with nicorandil treatment; ■, diabetes with nicorandil treatment. There are no significant differences in blood pressure among the 4 groups at each time point. Representative markers for endothelial injury, P-selectin (B) and ICAM-1 (C), at week 12 are shown. Both of these markers are significantly high in diabetic eNOSKO mice. Nicorandil treatment is not effective in altering the serum levels of these factors. *P < 0.01 vs. nondiabetes; n = 8/group.
Effects on endothelial function.
To evaluate whether nicorandil improves endothelial function, serum markers for endothelial dysfunction, P-selectin and ICAM-1, were measured. Serum P-selectin and serum ICAM-1 levels were significantly elevated in diabetic eNOSKO mice; however, nicorandil did not improve either marker (Fig. 2, B and C). These data suggest that nicorandil likely failed to improve systemic endothelial function in eNOSKO mice.
Effects on podocytes.
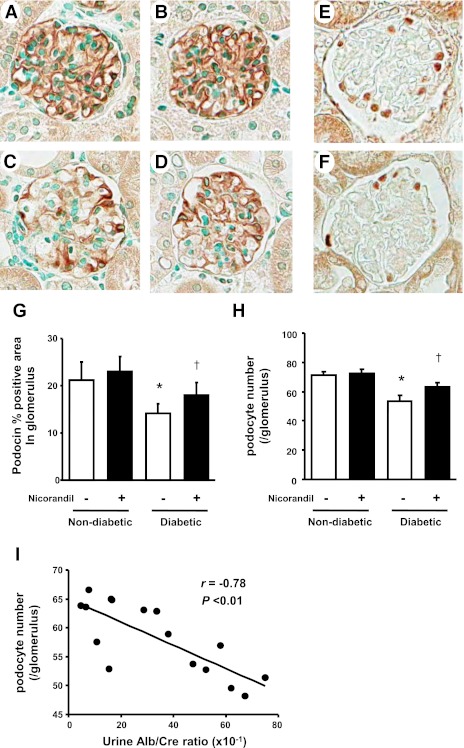
One of the key findings in diabetic nephropathy is a loss of podocytes (27), which has been hypothesized to increase the risk for both proteinuria as well as glomerulosclerosis (13). Thus the marked benefit of nicroandil on proteinuria in the absence of blood pressure control raised the hypothesis that nicroandil might be having a specific effect on the podocytes. To assess the effect on podocytes, we performed immunohistochemistry with the tissues for WT-1 (which marks podocyte nuclei) and podocin (a podocyte specific marker). It was shown that diabetes markedly decreased the expression of podocin (Fig. 3C) compared with nondiabetic groups (Fig. 3, A and B). However, nicorandil partially restored its expression (Fig. 3, D and G). Similarly, the number of WT-1-positive podocytes per glomelurus was decreased in diabetic eNOSKO mice whereas nicorandil also significantly prevented the decrease in the number of podocytes (Fig. 3, E, F, and H). As shown in Fig. 3I, urinary albumin excretion in diabetic animals with/without nicorandil was negatively correlated with podocyte number in this study.
Fig. 3.
Expression of podocin and WT-1 in diabetic eNOSKO mice. The expression of podocin, a podocyte-specific marker, in glomeruli was determined by immunohistochemistry for nondiabetes (A), nondiabetes with nicorandil (B), diabetes (C), and diabetes with nicorandil (D) in eNOSKO mice (×400 original magnification). The reduction of the podocin-positive area in glomeruli as detected by image analysis is significantly inhibited by nicorandil treatment in diabetic eNOSKO mice (G). Immunohistochemistry for WT-1, another marker of podocytes, is also shown in nondiabetic mice (E) and diabetic mice (F). Diabetes induces a decrease in the number of WT-1-positive podocytes in glomeruli (F) compared with nondiabetic mice (E). The number of WT-1-positive podocytes in glomeruli, which is determined by the Weibel-Gomez method, is shown (H). Nicorandil treatment significantly prevents the decrease in podocyte number in diabetic eNOSKO mice (H). Albuminuria is negatively correlated with WT-1-positive podocytes (I). Values are means ± SD; n = 8/group. *P < 0.01 vs. nondiabetes. †P < 0.05 vs. diabetes.
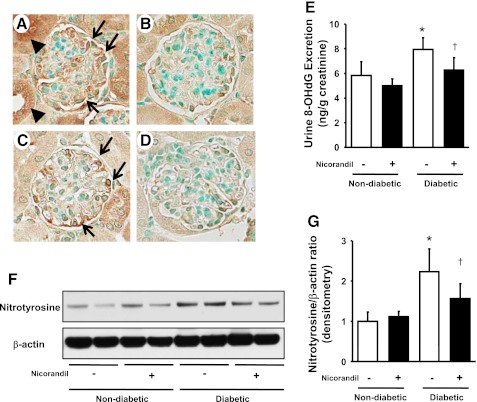
The loss of podocytes was associated with increased oxidative stress in podocytes. Indeed, 8-OHdG (Fig. 4A) and nitrotyrosine (Fig. 4C) were increased in podocytes of diabetic mice, and it appeared to be inhibited in nicorandil-treated mice (Fig. 4, B and D). Consistent with these data, urinary levels of 8-OHdG (Fig. 4E) as well as renal cortical levels of nitrotyrosine (Fig. 4, F and G) were also elevated in diabetic eNOSKO mice and were reversed by nicorandil treatment (Fig. 4, F and G). Perhaps the suppression of oxidative stress by nicorandil could account for the reduction in nitrotyrosine.
Fig. 4.
Levels of 8-hydroxy-2-deoxyguanosine (8-OHdG) and nitrotyrosine in diabetic eNOSKO mice. Immunohistochemistry reveals that 8-OHdG-positive cells (arrows) are mainly located in podocytes in glomeruli (A) as well as injured tubular epithelial cells (arrowheads) in diabetic eNOSKO mice. Nitrotyrosine (arrows) is also mainly located in podocytes (C) in diabetic eNOSKO mice in a pattern similar to 8-OHdG. These increased immunoreactivities appear to be suppressed by nicorandil (B and D). ELISA shows that urinary excretion of 8-OHdG, one of the representative oxidative stress markers, is elevated in diabetic mice compared with the nondiabetic group. Nicorandil treatment significantly inhibits the production in 8-OHdG (E). Immunoblots with renal cortex for nitrotyrosine and β-actin are shown (F). Densitometric analysis shows that an increase in the intensity of nitrotyrosine in diabetic eNOSKO mice is significantly inhibited by nicorandil treatment (G). Values are means ± SD; n = 8/group. *P < 0.01 vs. nondiabetes. †P < 0.05 vs. diabetes.
Effects of nicorandil on podocytes in vivo.
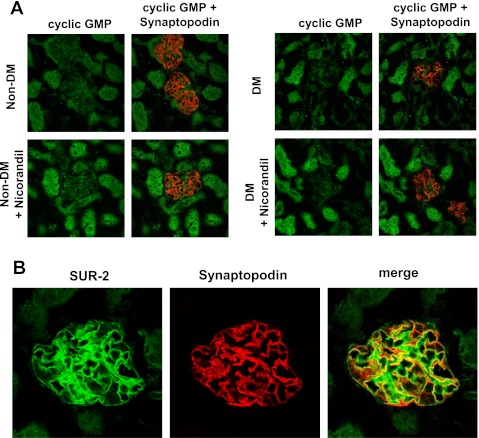
The remarkable protective effect of nicorandil on podocytes in diabetic eNOSKO mice led to the hypothesis that nicorandil might have direct effects on the podocyte. Since nicorandil is capable of donating NO, we initially assumed that its protective effects on podocytes could be due to biological actions of NO. Hence, we examined the distribution of cGMP, as a second messenger in NO signaling. However, in both nondiabetic (Fig. 5A, left) and diabetic eNOSKO mice (Fig. 5A, right), cGMP was not predominantly detected in podocyte, but it was positive in tubular epithelial cells.
Fig. 5.
Sulfonylurea receptor 2 (SUR-2) expression and cGMP in eNOSKO mouse kidneys. Double immunofluorescent stainings for cGMP (green) and synaptopodin (red) are shown (A). The immunoreactivity for cGMP on tubular cells is increased by nicorandil treatment in both the nondiabetic and diabetic (DM) groups, but such a signal is confined to tubular epithelial cells and not the glomerulus. Double immunofluorescent staining for SUR-2 (green) and synaptopodin (red, a podocyte marker) is shown in B. Strong expression of SUR-2 shows both a linear and mesangial pattern. Double immunofluorescent staining (merge) indicates that SUR-2 is colocalized to podocytes. In this merged image, the green color indicates probable mesangial expression of SUR-2.
Given these facts, we then hypothesized that the effect of nicorandil on podocytes might be via effects on SUR-2 of the ATP-dependent K channel (28, 31). As shown in Fig. 5B, SUR-2 expression is likely expressed by both mesangial cells and podocytes in vivo in the normal mouse, which was not altered in diabetic eNOSKO mice. Double staining of SUR-2 with synaptopodin (a marker of podocytes) confirmed the expression of the ATP-dependent K channel in podocytes. In contrast, SUR-2 expression was barely detected in tubules. Thus it is likely that nicorandil directly interacts with podocytes via the ATP-dependent K channel rather than NO-cGMP signaling.
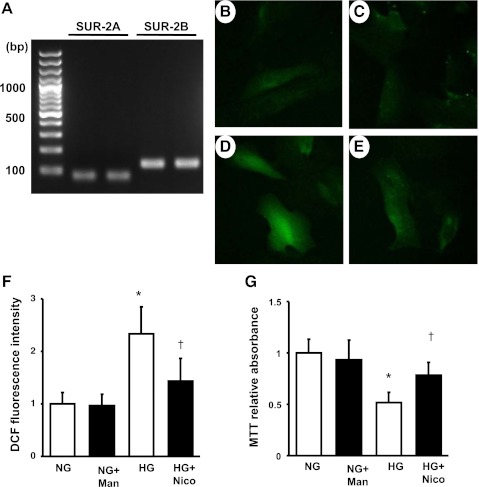
By using human cultured podocytes, we verified the mRNA expression of SUR-2 in differentiated immortalized podocytes. RT-PCR detected the expression of both subtypes, SUR-2A and SUR-2B in the podocytes (Fig. 6A).
Fig. 6.
Expression of SUR-2 and production of oxidative stress in the cultured podocytes. RT-PCR shows the mRNA expression of both subtypes of SUR-2, SUR-2A and SUR-2B, in differentiated immortalized podocytes (A). A DCF signal is measured as reactive oxygen species (ROS) in differentiated immortalized podocytes (B–E). Podocytes were incubated with with normal glusose (NG), NG+mannnitol (NG+Man), high glucose (HG), or HG+nicorandil (HG+Nico) for 72 h. Compared with NG (B) or NG+Man (C), immnofluorescent intensity for DCF is markedly higher in HG (D). Nicorandil treatment significantly inhibited the ROS signal in response to HG (E). Quantification of DCF signals is shown in F. An MTT assay indicates that HG reduces podocyte number whereas nicorandil prevents its reaction (G). Values are means ± SD; n = 4 for DCF assay and n = 6 for MTT assay in each group. *P < 0.01 vs. nondiabetes. †P < 0.05 vs. diabetes.
Reduced production of ROS by nicorandil in cultured podocytes.
Finally, we examined the direct effect of nicorandil on the intracellular production of ROS in cultured podocytes. Using the DCF detection assay, we assessed the amount of ROS as the conversion from H2DCFDA to DCF. The intensity of DCF fluorescence was markedly increased in podocytes with HG (Fig. 6D) compared with NG or NG+M (Fig. 6, B and C). However, nicorandil treatment significantly reduced the DCF intensity in HG (Fig. 6, E and F). Given that excess production of ROS led to podocyte loss in vivo, we finally examined the cultured podocyte number in NG or HG condition using an MTT assay. A reduction in podocyte number, which was defined as a decrease in the formation of reduced MTT, was observed in HG whereas nicorandil significantly preserved its reaction (Fig. 6G). These results indicate that podocyte protection by nicorandil observed in the diabetic mice may be attributed to a reduction in the intracellular production of ROS.
DISCUSSION
In the present study, we examined the effects of nicorandil in diabetic eNOSKO mice in which endothelial NO production is genetically and therefore permanently blocked. Here, we assumed that NO released from nicorandil might compensate for a deficiency of endothelial NO and ameliorate the progression of advanced diabetic nephropathy. While nicorandil exhibited a protective effect on proteinuria and glomerular histology, such protection was unlikely due to NO donation or improvement of endothelial function but rather to podocyte protection. In particular, podocytes are found to express an ATP-dependent K channel, which therefore is able to be stimulated by nicorandil. Our in vitro study suggested that nicorandil could reduce oxidative stress through its binding to the ATP-dependent K channel.
Podocytes play a pivotal role in maintaining the integrity of the glomerular filtration barrier, and therefore podocyte injury is thought to lead to the development of albuminuria. While podocyte damages can be caused by diabetes, it might be due to the ability of glucose to increase oxidative stress (33, 34). We also previously reported that a lack of endothelial NO results in podocyte injury in the mouse (24), and therefore endothelial dysfunction could contribute to the impairment of podocyte function in diabetes. Given these facts, targeting oxidative stress in the podocytes could be a therapeutic option to block diabetic nephropathy.
Nicorandil, a nicotinamide nitrate, exerts vasodilatory effects and therefore has been used clinically for the treatment of ischemic heart diseases (3). Such vasodilatory effects are due to the ability of nicorandil to donate NO and consequently to activate the soluble guanylate cyclase (sGS)-cGMP pathway. This compound is also known to stimulate the ATP-dependent K channel, predominantly exists in the mitochondria, to increase transmembrane potassium conductance and induce vasodilatation. In addition, an opening of the ATP-dependent K channel also likely contributes to cardiac protection owing to the development of ischemic preconditioning, a phenomenon whereby intermittent bouts of transient ischemia render the heart more resistant to future ischemic insults (20). Recently, it has been reported that nicorandil exhibits some protections in kidney disease, including anti-Thy.1 antibody-induced mesangial proliferating glomerulonephritis (32) and ischemic-reperfusion injury in the rat (30). While the mechanism of renoprotective efficacy of nicorandil has not yet been elucidated, mechanisms likely involve the protection of mitochondrial function and prevention of cell apoptosis (8).
SUR-2, a composing subunit of the ATP-dependent K channel, is found to be expressed in many tissues, including pancreatic islet cells, heart, skeletal muscle, vascular smooth muscle, and brain (29). The current study is, to our knowledge, the first documentation showing that SUR-2 is expressed in podocytes. While nicorandil has a dual function in which it is able to donate NO and stimulate the ATP-dependent K channel, the protective effect on podocytes is likely through opening of ATP-dependent K channels (37) because a cGMP signal, as a second messenger of NO, was not detected in the glomerulus. With respect to the localization of SUR-2 in the kidney, Zhou et al. (38) found that SUR-2 is expressed in tubular epithelial cells, yet their finding is distinct from our results. However, such discrepancy could be explained by the difference in species or antibody used.
Over the past decade, both inorganic and organic nitrates, as NO donors, have been tested for cardioprotection in subjects with coronary artery disease. However, unexpected outcomes have been documented in many clinical trials. In fact, many of these compounds paradoxically produce oxidative stress, induce endothelial dysfunction, and result in nitrate tolerance (17–19). Precise mechanisms for nitrate tolerance are being uncovered (16), but it is likely that oxidative stress derived from other nitrate compounds impairs aldehyde dehydrogenase to cause nitrate tolerance in mitochondria (15, 21). In contrast, clinical studies, albeit a limited number of investigations, documented (1) that unlike other nitrates, nicorandil does not seem to induce tolerance in its ability to donate NO (26). A precise mechanism for this unique and favorable effect of nicorandil remains to be determined. The antioxidative function of nicorandil could account for this benefit.
A major finding in this study is our demonstration that advanced diabetic nephropathy in which endothelial dysfunction is not reversible could be prevented by nicorandil treatment in mice. Although it did not seem to improve systemic endothelial function, chronic administration of nicorandil reduced oxidative stress by stimulating the the ATP-dependent K channel and consequently protected podocytes independently of donating NO. However, we also have to mention that this benefit of nicorandil was mild, and therefore further studies are required to determine how to completely block the progression of advanced diabetic nephropathy.
The authors note several liminations in this study, including a lack of groups with wild-type mice. Perhaps it might be better to evaluate the effect of nicorandil in diabetic wild-type mice as such a benefit might be expected. Another point is that glomerular endothelial function has not yet been elucidated in this model. While nicorandil failed to reduce serum P-selectin and ICAM1 levels, we documented the favorable effect on mesangiolysis, which is believed to be caused by glomerular endothelial dysfunction. Hence it might be conceivable that nicorandil could protect glomerular endothelial cells from diabetic insults. Further study is needed to clarify these issues, in paricular by use of diabetic wild-type mice.
In conclusion, we demonstrate that nicorandil ameliorated glomerular disease in streptozotocin-induced diabetic eNOSKO mice. The therapeutic efficacy of nicorandil observed in the present study suggests the potential of this drug as an additional option in treating diabetic nephropathy in patients with endothelial dysfunction.
GRANTS
This study was supported by a Japan Heart Foundation/Bayer Yakuhin Research Grant (K. Tanabe), National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-5212 (R. J. Johnson), and a grant from Cardero Therapeutics (T. Nakagawa).
DISCLOSURES
No conflicts of interest, financial or otherwise, are delcared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.T., M.A.L., W.K., C.J.R., M.M., J.K., M.A.S., and P.W.M. performed experiments; K.T. and T.N. analyzed data; K.T. and T.N. interpreted results of experiments; K.T. and T.N. prepared figures; K.T. and T.N. drafted manuscript; K.T., M.A.L., W.K., C.J.R., M.M., J.K., G.F.S., M.A.S., P.W.M., H.M., R.J.J., and T.N. approved final version of manuscript; C.J.R., G.F.S., H.M., R.J.J., and T.N. edited and revised manuscript; G.F.S., H.M., R.J.J., and T.N. provided conception and design of research.
REFERENCES
- 1. Anonymous. The effect of intensive treatment of diabetes on the development, and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 329: 977– 986, 1993. [DOI] [PubMed] [Google Scholar]
- 2. Anonymous. Effect of nicorandil on coronary events in patients with stable angina: the Impact Of Nicorandil in Angina (I.O.N.A) randomised trial. Lancet 359: 1269– 1275, 2002. [DOI] [PubMed] [Google Scholar]
- 3. Barbato JC. Nicorandil: the drug that keeps on giving. Hypertension 46: 647– 648, 2005. [DOI] [PubMed] [Google Scholar]
- 4. Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, Investigators RS. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861– 869, 2001. [DOI] [PubMed] [Google Scholar]
- 5. Brosius FC, 3rd, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter EH, Levi M, McIndoe RA, Sharma K, Smithies O, Susztak K, Takahashi N, Takahashi T, Animal Models of Diabetic Complications Consortium Mouse models of diabetic nephropathy. J Am Soc Nephrol 20: 2503– 2512, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eguchi Y, Takahari Y, Higashijima N, Ishizuka N, Tamura N, Kawamura Y, Ishida H. Nicorandil attenuates FeCl3-induced thrombus formation through the inhibition of reactive oxygen species production. Circ J 73: 554– 561, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 348: 383– 393, 2003. [DOI] [PubMed] [Google Scholar]
- 8. Garlid KD, Dos Santos P, Xie ZJ, Costa AD, Paucek P. Mitochondrial potassium transport: the role of the mitochondrial ATP-sensitive K+ channel in cardiac function and cardioprotection. Biochim Biophys Acta 1606: 1– 21, 2003. [DOI] [PubMed] [Google Scholar]
- 9. Jawa A, Nachimuthu S, Pendergrass M, Asnani S, Fonseca V. Impaired vascular reactivity in African-American patients with type 2 diabetes mellitus and microalbuminuria or proteinuria despite angiotensin-converting enzyme inhibitor therapy. J Clin Endocrinol Metab 91: 31– 35, 2006. [DOI] [PubMed] [Google Scholar]
- 10. Kanetsuna Y, Takahashi K, Nagata M, Gannon MA, Breyer MD, Harris RC, Takahashi T. Deficiency of endothelial nitric-oxide synthase confers susceptibility to diabetic nephropathy in nephropathy-resistant inbred mice. Am J Pathol 170: 1473– 1484, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kosugi T, Heinig M, Nakayama T, Connor T, Yuzawa Y, Li Q, Hauswirth WW, Grant MB, Croker BP, Campbell-Thompson M, Zhang L, Atkinson MA, Segal MS, Nakagawa T. Lowering blood pressure blocks mesangiolysis and mesangial nodules, but not tubulointerstitial injury, in diabetic eNOS knockout mice. Am J Pathol 174: 1221– 1229, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kosugi T, Heinig M, Nakayama T, Matsuo S, Nakagawa T. eNOS knockout mice with advanced diabetic nephropathy have less benefit from renin-angiotensin blockade than from aldosterone receptor antagonists. Am J Pathol 49: 51– 54, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kriz W, Hosser H, Hahnel B, Gretz N, Provoost AP. From segmental glomerulosclerosis to total nephron degeneration and interstitial fibrosis: a histopathological study in rat models and human glomerulopathies. Nephrol Dial Transplant 13: 2781– 2798, 1998. [DOI] [PubMed] [Google Scholar]
- 14. Lee TM, Chang NC. Effect of nicorandil on proteinuria in well controlled hypertensive patients. J Hypertens 27: 618– 625, 2009. [DOI] [PubMed] [Google Scholar]
- 15. Mano T, Shinohara R, Nagasaka A, Nakagawa H, Uchimura K, Hayashi R, Nakano I, Tsugawa T, Watanabe F, Kobayashi T, Fujiwara K, Nakai A, Itoh M. Scavenging effect of nicorandil on free radicals and lipid peroxide in streptozotocin-induced diabetic rats. Metabolism 49: 427– 431, 2000. [DOI] [PubMed] [Google Scholar]
- 16. Munzel T, Daiber A, Mulsch A. Explaining the phenomenon of nitrate tolerance. Circ Res 97: 618– 628, 2005. [DOI] [PubMed] [Google Scholar]
- 17. Munzel T, Harrison DG. Evidence for a role of oxygen-derived free radicals and protein kinase C in nitrate tolerance. J Mol Med 75: 891– 900, 1997. [DOI] [PubMed] [Google Scholar]
- 18. Munzel T, Kurz S, Rajagopalan S, Thoenes M, Berrington WR, Thompson JA, Freeman BA, Harrison DG. Hydralazine prevents nitroglycerin tolerance by inhibiting activation of a membrane-bound NADH oxidase. A new action for an old drug. J Clin Invest 98: 1465– 1470, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Munzel T, Li H, Mollnau H, Hink U, Matheis E, Hartmann M, Oelze M, Skatchkov M, Warnholtz A, Duncker L, Meinertz T, Forstermann U. Effects of long-term nitroglycerin treatment on endothelial nitric oxide synthase (NOS III) gene expression, NOS III-mediated superoxide production, and vascular NO bioavailability. Circ Res 86: E7– E12, 2000. [DOI] [PubMed] [Google Scholar]
- 20. Murry CE, Richard VJ, Reimer KA, Jennings RB. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res 66: 913– 931, 1990. [DOI] [PubMed] [Google Scholar]
- 21. Naito A, Aniya Y, Sakanashi M. Antioxidative action of the nitrovasodilator nicorandil: inhibition of oxidative activation of liver microsomal glutathione S-transferase and lipid peroxidation. Jpn J Pharmacol 65: 209– 213, 1994. [DOI] [PubMed] [Google Scholar]
- 22. Nakagawa T, Sato W, Glushakova O, Heinig M, Clarke T, Campbell-Thompson M, Yuzawa Y, Atkinson MA, Johnson RJ, Croker B. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J Am Soc Nephrol 18: 539– 550, 2007. [DOI] [PubMed] [Google Scholar]
- 23. Nakagawa T, Sato W, Sautin YY, Glushakova O, Croker B, Atkinson MA, Tisher CC, Johnson RJ. Uncoupling of vascular endothelial growth factor with nitric oxide as a mechanism for diabetic vasculopathy. J Am Soc Nephrol 17: 736– 745, 2006. [DOI] [PubMed] [Google Scholar]
- 24. Nakayama T, Sato W, Yoshimura A, Zhang L, Kosugi T, Campbell-Thompson M, Kojima H, Croker BP, Nakagawa T. Endothelial von Willebrand factor release due to eNOS deficiency predisposes to thrombotic microangiopathy in mouse aging kidney. Am J Pathol 176: 2198– 2208, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nicholas SB, Basgen JM, Sinha S. Using stereologic techniques for podocyte counting in the mouse: shifting the paradigm. Am J Nephrol 33, Suppl 1: 1– 7, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nishimura M, Tokoro T, Nishida M, Hashimoto T, Kobayashi H, Imai R, Yamazaki S, Okino K, Iwamoto N, Takahashi H, Ono T. Oral nicorandil to reduce cardiac death after coronary revascularization in hemodialysis patients: a randomized trial. Am J Kidney Dis 54: 307– 317, 2009. [DOI] [PubMed] [Google Scholar]
- 27. Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342– 348, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reimann F, Ashcroft FM, Gribble FM. Structural basis for the interference between nicorandil and sulfonylurea action. Diabetes 50: 2253– 2259, 2001. [DOI] [PubMed] [Google Scholar]
- 29. Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol 81: 133– 176, 2003. [DOI] [PubMed] [Google Scholar]
- 30. Shimizu S, Saito M, Kinoshita Y, Ohmasa F, Dimitriadis F, Shomori K, Hayashi A, Satoh K. Nicorandil ameliorates ischaemia-reperfusion injury in the rat kidney. Br J Pharmacol 163: 272– 282, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shindo T, Yamada M, Isomoto S, Horio Y, Kurachi Y. SUR2 subtype (A and B)-dependent differential activation of the cloned ATP-sensitive K+ channels by pinacidil and nicorandil. Br J Pharmacol 124: 985– 991, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sudo H, Hirata M, Kanada H, Yorozu K, Tashiro Y, Serizawa K, Yogo K, Kataoka M, Moriguchi Y, Ishizuka N. Nicorandil improves glomerular injury in rats with mesangioproliferative glomerulonephritis via inhibition of proproliferative and profibrotic growth factors. J Pharmacol Sci 111: 53– 59, 2009. [DOI] [PubMed] [Google Scholar]
- 33. Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55: 225– 233, 2006. [PubMed] [Google Scholar]
- 34. Thallas-Bonke V, Thorpe SR, Coughlan MT, Fukami K, Yap FY, Sourris KC, Penfold SA, Bach LA, Cooper ME, Forbes JM. Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-alpha-dependent pathway. Diabetes 57: 460– 469, 2008. [DOI] [PubMed] [Google Scholar]
- 35. Thomas GR, DiFabio JM, Gori T, Parker JD. Once daily therapy with isosorbide-5-mononitrate causes endothelial dysfunction in humans: evidence of a free-radical-mediated mechanism. J Am Coll Cardiol 49: 1289– 1295, 2007. [DOI] [PubMed] [Google Scholar]
- 36. Wilmer WA, Rovin BH, Hebert CJ, Rao SV, Kumor K, Hebert LA. Management of glomerular proteinuria: a commentary. J Am Soc Nephrol 14: 3217– 3232, 2003. [DOI] [PubMed] [Google Scholar]
- 37. Yamada M, Kurachi Y. A functional role of the C-terminal 42 amino acids of SUR2A and SUR2B in the physiology and pharmacology of cardiovascular ATP-sensitive K+ channels. J Mol Cell Cardiol 39: 1– 6, 2005. [DOI] [PubMed] [Google Scholar]
- 38. Zhou M, He HJ, Tanaka O, Suzuki R, Sekiguchi M, Yasuoka Y, Kawahara K, Itoh H, Abe H. Localization of the sulphonylurea receptor subunits, SUR2A and SUR2B, in rat renal tubular epithelium. Tohoku J Exp Med 214: 247– 256, 2008. [DOI] [PubMed] [Google Scholar]