Abstract
Cyclin-dependent kinase (Cdk)-5 is activated by both cyclin I and the noncyclin activator p35 in terminally differentiated cells such as kidney podocytes and neurons. Cyclin I and p35 are restricted to podocytes in the kidney, and each limit podocyte apoptosis by activating Cdk5. To determine whether both activators are necessary, or whether they serve backup roles, a double cyclin I-p35 null mouse was generated. Experimental glomerular disease characterized by podocyte apoptosis was then induced by administering an anti-podocyte antibody. The results showed that under nonstressed conditions double mutants had normal kidney structure and function and were indistinguishable from wild-type, cyclin I−/−, or p35−/− mice. In contrast, when stressed with disease, podocyte apoptosis increased fourfold compared with diseased cyclin I−/− or p35−/− mice. This resulted in a more pronounced decrease in podocyte number, proteinuria, and glomerulosclerosis. Under normal states and nephritic states, levels for the prosurvival protein Bcl-2 were lower in double cyclin I−/− p35−/− mice than the other mice. Similarly, levels of Bcl-xL, another prosurvival member, were lower in normal and nephritic double cyclin I−/− p35−/− mice but similar to single-cyclin I−/− mice. Moreover, levels of ERK1/2 and MEK1/2 activation were lower in nephritic double cyclin I−/− p35−/− mice but similar to single-cyclin I−/− mice. The results demonstrate that the activators of Cdk5, p35, and cyclin I are not required for normal kidney function. However, they play pivotal coordinated roles in maintaining podocyte survival during stress states in disease.
Keywords: Cdk5; podocyte, glomerulonephritis
podocytes are terminally differentiated glomerular epithelial cells, which represent a highly specialized component of the glomerular filtration barrier. Podocyte survival is critical to maintain a normal cell number. Apoptosis in diabetic and nondiabetic kidney disease leads to reduced podocyte number, which then underlies the development of proteinuria and glomerulosclerosis (24).
Recent studies have shown that specific cell-cycle proteins regulate cell survival, beyond their role in the regulation of proliferation (4, 13, 14, 20). Cyclin-dependent kinases (Cdks), small serine/threonine protein kinases that regulate cell-cycle progression, have been shown to play a critical role in the survival of certain kidney and nonkidney cells (8, 22). In the kidney, the glomerular expression of Cdk5 is limited to podocytes. For Cdk5 to have a normal biological function, it is activated by cyclin I and differs from other Cdks in that it is also activated by the noncyclin proteins p35 and p25 (8, 9, 17, 18, 23). Recent studies have shown that the absence of either cyclin I or p35 confers increased susceptibility of podocytes to apoptosis in disease (5, 7, 10). However, the dual role of these two Cdk5 activators in governing podocyte apoptosis is not known.
Accordingly, the current studies used a genetic approach to test the hypothesis that the ability of Cdk5 to maximally protect podocytes from apoptosis requires the presence of both cyclin I and p35, and that their roles are not redundant, but rather are both essential for normal function.
MATERIALS AND METHODS
Generation of Mutant Mice
We have previously reported on the generation of the cyclin I−/− mouse (10). The p35 mice were obtained from Inez Vincent (11), Both lines (p35 and cyclin I) were then intercrossed. Heterozygous offspring were again intercrossed until the wild-type allele of both p35 and cyclin I were eliminated. Genotyping of cyclin I −/− was identified by PCR amplification of a 1,900-bp fragment with a primers to the β-gal gene (forward 1BR+, 5′-TAGGACATGGGTCTCA-3′, reverse, 5′-CATCAAGGAAACAATGGACTACTG-3′) and amplification of a 1,300-bp fragment with primers to the cyclin I genomic DNA just 5′ to the SpeI site (forward 1BR+, 5′-TAGGACATGGGTCTCA-3′, reverse, 5′-GGTGTGACTCTATGGTATTTC-3′). Genotyping of p35−/− was identified by PCR amplification of a 316-bp fragment with primers to the Neo gene (Neo forward 5′-GATCTGGACGAAGAGCTCAGGG-3′, Neo reverse 5′-CGTCAAGAAGGCGATAGAAGGCG-3′) and amplification of a 440-bp fragment with primers to p35 genomic DNA (p35 forward 5′-ACCTCTGCAGGGACACCCAAACG-3′, p35 reverse 5′-GTGGGTCGGCATTGATCTGCAGC-3′). PCR for cyclin I and p35 genotyping was amplified for 36 cycles at the following temperatures and times: 93°C for 30 s, 57°C for 30 s, and 65°C for 2 min and 94°C for 45 s, 60°C for 45 s, and 72°C for 1 min, respectively.
Experimental Glomerular Disease
Podocyte apoptosis was induced in vivo by inducing experimental glomerular disease in 8- to 12-wk-old mice by intraperitoneal injection of a sheep anti-rabbit glomerular antibody (20 mg/g body wt) on 2 consecutive days as described previously (7, 10). This model is characterized by podocyte injury leading to apoptosis, and the subsequent development of proteinuria and glomerulosclerosis. The following mice strains received disease-inducing antibody: cyclin I+/+, p35+/+, cyclin I−/−, p35−/−, and double cyclin I−/− p35−/−. Mice were housed under standardized pathogen-free conditions in the University of Washington animal facility. The experimental protocol was approved by the Animal Care Committee of the University of Washington, Seattle. Normal mice (not injected, n = 4–8/strain) and diseased mice (n = 4–8 per strain for each time point) were killed 7 days after the second injection of anti-glomerular antibody. Urine was collected overnight from each animal before disease induction and before euthanasia to measure proteinuria using the sulfosalicyclic assay as we have previously reported (7, 10). At euthanasia, kidney tissue was fixed in 10% neutral buffered formalin and embedded in paraffin for immunostaining.
Immunostaining
Tissue was deparaffinized in Histo-Clear (National Diagnostics, Atlanta, GA) and rehydrated in graded ethanol. Endogenous peroxidase activity was blocked with 3% hydrogen peroxidase. Apoptosis was measured by quantitating cleaved caspase-3 (CC3) staining. Caspase-3 is activated, or cleaved, to CC3, the main apoptosis effector, in response to a variety of preapoptotic stimuli. CC3 constitutes a final common pathway in the cell death process and is a surrogate marker of apoptosis (7). Podocyte number was quantitated by measuring WT-1, p57, and green fluorescence protein (GFP) staining (see below); Bcl-2 and Bcl-xL are prosurvival proteins. ERK1/2 and MEK1/2 activation was also assessed to determine the effects of the MAPK pathway on apoptosis.
Single staining.
The kidney sections were incubated overnight at 4°C with primary antibodies directed against WT-1 (sc192, 1:15,000 dilution, Santa Cruz Biotechnology, Santa Cruz, CA), p57 (sc8298, 1:700 dilution, Santa Cruz Biotechnology), GFP (AB3080, 1:400 dilution, Chemicon International, Temecula, MA), cleaved caspase-3 (CST-9661, 1:200 dilution, Cell Signaling Technology, Danvers, MA), Bcl-2 N-19 (sc-492, 1:200 dilution, Santa Cruz Biotechnology), Bcl-xL (CST-2762, 1:150 dilution, Cell Signaling Technology), phospho-ERK1/2 Thr202/Tyr204 (CST-9102, 1:150 dilution, Cell Signaling Technology), and phospho-MEK1/2 Ser217/221 (CST-9154, 1:150 dilution, Cell Signaling Technology), all diluted in 1% BSA/PBS. The sections were washed repeatedly in PBS before incubation with biotin-conjugated mouse anti-rabbit secondary antibody (Jackson ImmunoResearch, West Grove, PA) diluted in 1% BSA/PBS for 1 h at room temperature. An ABC kit (Vector Laboratories, Burlingame, CA) was used for signal amplification, and 3,3′-diaminobenzamidine (DAB; Sigma, St. Louis, MO) was used as a chromogen. Slides were counterstained with hematoxylin (Sigma-Aldrich), dehydrated, and mounted in Histomount (National Diagnostics).
Double immunostaining methods.
To identify and quantitate the number of podocytes that express phospho-ERK1/2 (pERK1/2), double staining was performed for pERK1/2 with antibody to p57. p57 specifically stains podocytes in glomeruli. Staining with an antibody to p57 was performed first. The methods for p57 staining were identical to the single staining described earlier and was visualized with the Vector SG substrate kit, with positive cells being blue gray in color (Vector). Following SG substrate color development, blocking steps were performed. Because the antibodies being used were developed in the rabbit, an anti-rabbit IgG antibody Fab fragment (Jackson ImmunoResearch) was used to saturate all the binding sites created during the first set of staining. Next, the second set of staining was performed for pERK1/2. For pERK1/2 staining, an ABC kit (Vector Laboratories) was used for signal amplification. Staining was visualized by DAB, with positive cells having a brown color.
Assessment of Podocyte Number
Recent data from our group and others showed that WT-1 staining may not be specific for podocytes only as has traditionally been thought (1, 3, 15, 21), Accordingly, pilot studies were performed to determine the utility of p57 staining as a measure of podocyte number. As we and others have shown, p57 expression is restricted to podocytes within the kidney. We have also shown there is a marked decrease in p57 expression in animal models of podocyte injury (2, 12, 19). This suggested the utility of using p57 as an indicator to measure a decrease in healthy podocyte number (2, 12, 19). Accordingly, pilot studies were conducted in 8- to 12-wk-old nephrin-Cre GFP to test this possibility. The rationale for this is as follows: the nephrin promoter, which is specific to podocytes, was ligated to Cre gene expression. Cre excises lox-p sites flanking a stop codon, which in turn induces expression of a GFP reporter specifically in podocytes, enabling them to be counted. Formalin-fixed tissue (n = 4–6/time point) from normal and diseased mice (days 14 and 17) was stained by indirect immunohistochemistry for GFP, WT-1, and p57. Podocyte numbers per glomerular tuft, as assessed by GFP, WT-1, and p57 staining, were compared.
Before disease induction, the number of GFP-positive, p57-positive, and WT-1 positive podocytes were similar (10.02 ± 1.10, 9.53 ± 1.21, and 10.37 ± 1.87, respectively). At days 14 and 17 after disease induction, podocyte numbers, as estimated for all three stains, were not statistically different (day 14: GFP, 5.02 ± 0.37, WT-1, 5.40 ± 0.51, and p57, 5.26 ± 0.52; day 17: GFP, 4.93 ± 0.62, WT-1, 5.36 ± 0.39, and p57, 4.95 ± 0.42, respectively). Histological preparations show WT-1 stained some parietal epithelial cells (PECs) and cellular crescents, but GFP and p57 stains were specific to podocytes. These data showed that p57 staining correlates with GFP staining and can therefore be used as an accurate podocyte marker to assess podocyte number.
Assessment of Glomerulosclerosis
To measure the degree of glomerular scarring, formalin-fixed sections were stained with periodic acid-Schiff, and a minimum 50 glomeruli from individual animals were examined by microscopy. Glomerulosclerosis was determined and graded quantitatively by the percentage of glomerular tuft area involvement as follows as previously reported (7, 10): grade 1, <25%; grade 2, <25–50%; grade 3, <50–75%; and grade-4, <75–100%. The percentage of affected glomeruli in all kidney cross sections was evaluated for each normal and diseased mouse strain.
Statistical Analysis
All results are expressed as mean ± S.D. calculated with GraphPad Prism version 4.00c for Macintosh (GraphPad Software, San Diego, CA, USA). Analysis of variance (ANOVA) with Tukey-Kramer adjustment for multiple comparisons was applied. A p-value below 0.05 was considered significant.
RESULTS
Double Cyclin I−/− p35−/− Mice are Normal Under Stress-Free Conditions
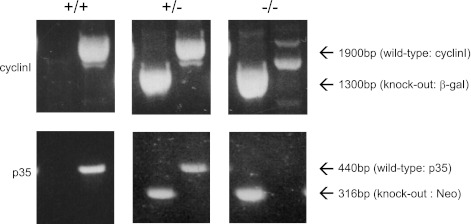
Genotyping was performed to confirm that the crossing of cyclin I−/− and p35−/− mice generated double mutant mice, and the results are shown in Fig. 1. Cyclin I+/+ p35+/+ mice show a single 1,900-bp band for the cyclin I wild-type fragment and 440 bp for the p35 wild-type fragment following PCR amplification of genomic DNA. Similarly, cyclin I−/− p35−/− mice show a single 1,300-bp band for the β-gal fragment and 316 bp for the p35 neo fragment following PCR amplification of genomic DNA. As expected, mice heterozygous for cyclin I or p35 show the presence of all four bands.
Fig. 1.
Generation of cyclin I−/− p35−/− mice. Genotyping was done by PCR. Top: bands corresponding to cyclin I wild-type (1,900 base pairs)- and β-galactosidase (cyclin I knockout, 1,300 base pairs)-amplified products identifying cyclin I +/+, +/−, and −/− genotypes. Bottom: bands corresponding to p35 wild-type (440 base pairs) and neomycin (p35 knockout, 316 base pairs)-amplified products identifying p35 +/+, +/−, and −/− genotypes.
All double mutant mice were viable at birth and showed no gross abnormalities by 10 mo of age. Double cyclin I−/− p35−/− mice grown under nonstressed conditions had normal kidney histology by light microscopy (results not shown). Urine measurements showed no differences in the protein/creatinine ratio compared with wild-type: cyclin I−/− and p35−/− mice (wild-type, 8.26 ± 4.60; cyclin I−/−, 8.32 ± 7.75; p35−/−, 6.03 ± 6.40; cyclin I−/− p35−/−, 7.00 ± 7.39 mg/mg, P > 0.05 vs. wild-type, cyclin I−/− and p35−/−). These data show that although Cdk5 is activated by both cyclin I and p35, the absence of both of these proteins in double null mice has no impact on kidney development, morphology, or function, when mice are maintained under normal (nonstressed) conditions.
Apoptosis was Most Severe in Diseased Cyclin I−/− p35−/− Mice
We have previously described podocyte apoptosis in this animal model (7, 10). Staining for CC3 was used to assess apoptosis in the current study, and the results are shown in Fig. 2A. Before disease induction, no CC3 staining was detected in glomeruli from wild-type, either single null and double null mice. Representative images shown in Fig. 2, B–E. However, CC3 staining was increased 7 days after disease induction, and quantitated as follows: wild-type 0.049 ± 0.011, cyclin I−/−, 0.093 ± 0.011 (P < 0.001 vs. wild-type); p35−/−, 0.094 ± 0.008 (P < 0.001 vs. wild-type; P > 0.05 vs. cyclin I −/−); cyclin I−/− p35−/−, 0.180 ± 0.052 (P < 0.001 vs. cyclin I−/− and p35−/−). Representative images shown in Fig. 2, F–I. These data show that apoptosis was increased in diseased cyclin I−/− and p35−/− mice and that this increase was substantially greater when both cyclin I and p35 were absent in the double null mice.
Fig. 2.
Apoptosis measured by immunohistochemistry for cleaved caspase 3 (CC3). A: quantification of CC3-positive cells per glomerular cross section in nephritic mice. Seven days after disease induction, podocyte apoptosis in double cyclin I−/− p35−/− mice increased more than single cyclin I−/− and p35−/− mice. *P < 0.05 vs. wild-type. **P < 0.05 vs. cyclin I−/− or p35−/−. C, control; D, disease (day 7). Also shown are representative images of immunostaining for CC3. B and F: wild-type. C and G: cyclin I−/−. D and H: p35−/−. E and I: cyclin I−/− p35−/−. B–E: unmanipulated. F–I: glomerulonephritis. Arrows indicate CC3-positive apoptotic nuclei. Original magnification ×400.
Decrease in Podocyte Number was Most Severe in Diseased Cyclin I−/− p35−/− Mice
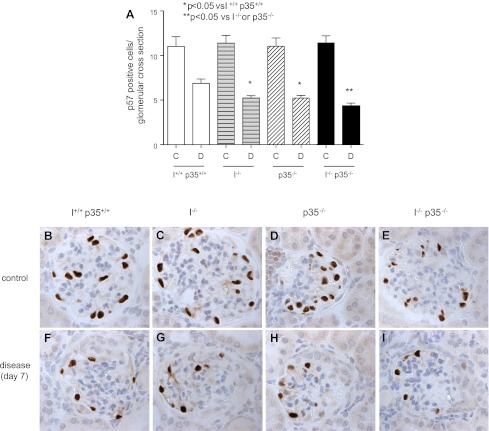
Before disease induction, the numbers of positively staining p57 cells (podocytes) were similar and not statistically different in all four mice strains (Fig. 3A; wild-type, 11.02 ± 1.08; cyclin I−/−, 11.37 ± 0.87; p35−/−, 11.03 ± 0.93; cyclin I−/− p35−/−, 11.28 ± 0.92). Representative images are shown in Fig. 3, B–E. Seven days after disease induction, the number of p57-positive cells per glomerular tuft was lower in single cyclin I−/− mice (P < 0.05 vs. diseased wild-type) and p35−/− mice (P < 0.05 vs. diseased wild-type). Podocyte number was further significantly reduced in double cyclin I−/− p35−/− mice compared with single cyclin I−/− and p35−/−mice (Fig. 3A; wild-type, 6.87 ± 0.48; cyclin I−/−, 5.22 ± 0.28; p35−/−, 5.22 ± 0.29; cyclin I−/− p35−/−, 4.28 ± 0.36). Representative images are shown in Fig. 3, F–I. These findings show that both cyclin I and p35 are necessary to prevent a decrease in podocyte number in disease and that a decrease in one of these Cdk5 activators did not compensate for the other as we originally thought.
Fig. 3.
Podocyte number measured by immunohistochemistry for p57. A: quantification of p57-positive cells per glomerular cross section in nephritic mouse models. At baseline, a similar complement of podocytes was seen in wild-type, single cyclin I−/−, p35−/−, and double cyclin I−/− p35−/− mice. Seven days after disease induction, podocyte number was lower in double cyclin I−/− p35−/− than single cyclin I−/− and single p35−/− mice. *P < 0.05 vs. wild-type. **P < 0.05 vs. cyclin I−/− or p35−/−. C, control; D, disease (day 7). Also shown are representative images of immunostaining for p57. B and F: wild-type. C and G: cyclin I−/−. D and H: p35−/−. E and I: cyclin I−/− p35−/−. B–E: unmanipulated. F–I: glomerulonephritis. Original magnification ×400.
Double Null Mice are More Vulnerable to Kidney Disease Severity
As mentioned earlier, before disease induction, urinary protein (mg/24 h) was similar in all mouse strains (Fig. 4A; wild-type, 0.64 ± 0.78; cyclin I−/−, 0.77 ± 0.65; p35−/−, 1.32 ± 0.79; cyclin I−/− p35−/−, 1.37 ± 0.99 mg/24 h, P > 0.05). Urinary protein/creatinine ratios (mg/mg) were also similar in all mouse strains (Fig. 4B; wild-type, 8.26 ± 4.60; cyclin I−/−, 8.32 ± 7.75; p35−/−, 6.03 ± 6.40; cyclin I−/− p35−/−, 7.00 ± 7.39 mg/mg, P > 0.05). At day 7 of disease, proteinuria levels (mg/24 h) were significantly higher in single cyclin I and p35 null mice compared with wild-type mice (Fig. 4A; wild-type, 3.47 ± 0.50; cyclin I−/−, 6.05 ± 1.29; p35−/−, 5.97 ± 1.34; cyclin I−/− p35−/−, 17.11 ± 6.22; P < 0.05). Proteinuria was four times greater in diseased double cyclin I−/− p35−/−mice compared with diseased wild-type and three times greater compared with diseased single cyclin I−/− and p35−/− mice (P < 0.001). Similar results were obtained in diseased mice when the urinary protein/creatinine ratio (mg/mg) was measured (Fig. 4B; wild-type, 24.06 ± 10.92; cyclin I−/−, 62.47 ± 24.28; p35−/−, 62.93 ± 23.15; cyclin I−/− p35−/−, 91.49 ± 22.93 mg/mg). These data show that the absence of either cyclin I or p35 was accompanied by increased proteinuria in disease and that if both were absent, proteinuria was significantly further increased. Thus, in contrast to states when animals are not stressed, both cyclin I and p35 are required and essential for normal function when the animals are undergoing stress such as experimental glomerular disease.
Fig. 4.
Renal function at baseline and 7 days after disease induction. Urinary protein concentration (mg/24 h; A) and urinary protein:creatinine ratio (mg/mg; B) in all mouse models were similar at baseline. At day 7, the amount of proteinuria was significantly higher in double cyclin I−/− p35−/− mice than in wild-type, single cyclin I−/−, and single p35−/− mice.*P < 0.05 vs. wild-type. **P < 0.05 vs. cyclin I−/− or p35−/−. C, control; D, disease (day 7).
Glomerulosclerosis was Most Severe in Diseased Double Null Mice
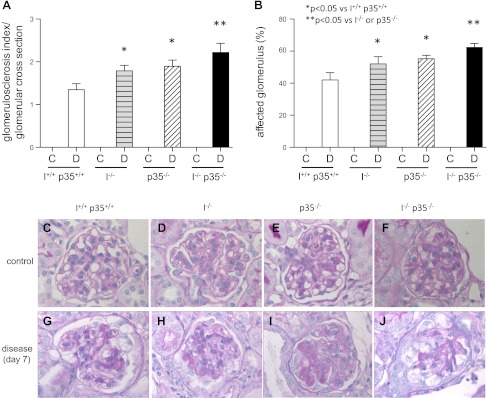
Studies have shown a direct correlation between a decrease in podocyte number and the magnitude of glomerulosclerosis (24). Glomerulosclerosis was therefore quantitated in all mice. Glomerulosclerosis was not detected in any of the mice before disease, regardless of strain (P > 0.05 between strains) (Fig. 5A). As expected, at day 7 of disease glomerulosclerosis increased in wild-type mice (Fig. 5A; mean score of 1.51 ± 0.16 vs. 0 in normal, P < 0.001). Representative images are shown in Fig. 5, C–F. Glomerulosclerosis was more severe at day 7 in diseased p35−/− mice (Fig. 5A; mean score of 1.91 ± 0.14; P < 0.001 vs. diseased p35+/+ mice) and in diseased cyclin I−/− mice (Fig. 5A; mean score of 1.82 ± 0.12; P < 0.001 vs. diseased cyclin I+/+ mice). There was no statistical difference in the magnitude of glomerulosclerosis between diseased single p35−/− and cyclin I−/− mice (P > 0.05). Interestingly, glomerulosclerosis was significantly more in diseased double cyclin I−/− p35−/− mice (Fig. 5A; mean score 2.23 ± 0.20) compared with diseased p35−/− (P < 0.001) and diseased cyclin I−/− (P < 0.001) mice. Representative images are shown in Fig. 5, G–J.
Fig. 5.
Glomerulosclerosis at baseline and 7 days after disease induction. A: glomerulosclerosis index per glomerular cross section. B: affected glomeruli (%) in nephritic mouse models. At baseline, all groups showed normal glomeruli. Seven days after disease induction, glomerulosclerosis index and affected percentage of all glomerular cross sections increased. *P < 0.05 vs. wild-type. **P < 0.05 vs. cyclin I or p35−/−. C, control; D, disease (day 7). Also shown are Representative images of periodic acid-Schiff (PAS) staining used for scoring. C and G: wild-type. D and H: cyclin I−/−. E and I: p35−/−. F and J: cyclin I−/− p35−/−. C–F: unmanipulated. G–J: glomerulonephritis. Original magnification ×400.
The percentage of glomeruli with glomerulosclerosis in each kidney biopsy from all strains was also quantitated, and the results are shown in Fig. 5B. The proportion of affected glomeruli per glomerular cross section (%) was the lowest in wild-type mice and highest in double cyclin I−/− p35−/− mice (wild-type, 42.00 ± 4.58; cyclin I−/−, 52.00 ± 4.54; p35−/−, 55.33 ± 2.08; cyclin I−/− p35−/−, 62.33 ± 2.51; P < 0.05). These data show that the total number of glomeruli with sclerosis and the magnitude of this sclerosis within individual glomeruli were significantly increased in diseased mice in the absence of both cyclin I and p35 compared with the absence of either alone.
Differential Expression of Bcl-2 and Bcl-xL Depends on p35 and Cyclin I Levels
To better understand potential reasons underlying the increase in apoptosis in double mutant mice, levels of the prosurvival proteins Bcl-2 and Bcl-xL were measured by immunostaining. Before disease induction, the number of cells staining positive for Bcl-2 was substantially reduced in double cyclin I−/− p35−/− mice compared with wild-type (Fig. 6A; 0.102 ± 0.054 vs. 0.276 ± 0.030, P < 0.05), cyclin I−/− (Fig. 6A; 0.102 ± 0.054 vs. 0.189 ± 0.031, P < 0.05), and p35−/− mice (Fig. 6A; 0.102 ± 0.054 vs. 0.192 ± 0.034, P < 0.05). Staining for Bcl-2 increased in diseased wild-type mice. However, the increase was not as robust in diseased cyclin I−/− and p35−/− mice. Furthermore, the increase in Bcl-2 in double cyclin I−/− p35−/− mice was significantly less than single cyclin I−/− or p35−/− mice (Fig. 6A; wild-type, 0.843 ± 0.121; cyclin I−/−, 0.657 ± 0.173; p35−/−, 0.667 ± 0.121; cyclin I−/− p35−/−, 0.331 ± 0.144; P < 0.001). Representative images are shown in Fig. 6, C–J.
Fig. 6.
Immunohistochemistry for prosurvival Bcl-2 family proteins Bcl-2 and Bcl-xL. Shown is quantification of Bcl-2- and Bcl-xL-positive cells in tuft corrected with podocyte number in nephritic mice. A: both at baseline and 7 days after disease induction, there were significantly fewer Bcl-2-positive podocytes in double cyclin I−/− p35−/− than in wild-type, single cyclin I−/−, and p35−/− mice. B: the number of Bcl-xL-positive podocytes was significantly lower in genetically modified mice than in wild-type mice except for single p35−/− mice. NS, no significant differences. C, control; D, disease (day 7). *P < 0.05 vs. wild-type. **P < 0.05 vs. cyclin I or p35−/−. Also shown are representative micrographs of immunostaining for Bcl-2 and Bcl-xL. C, G, K, and O: wild-type. D, H, L, and P: cyclin I−/−. E, I, M, and Q: p35−/−. F, J, N, and R: cyclin I−/− p35−/−. C–F and K–N: unmanipulated. G–J and O–R: glomerulonephritis. Original magnification ×400.
Similarly, the number of cells positive for Bcl-xL was significantly lower in normal cyclin I−/− p35−/− mice compared with wild-type (Fig. 6B; 0.030 ± 0.029 vs. 0.121 ± 0.053, P < 0.05); and p35−/−mice (Fig. 6B; 0.030 ± 0.029 vs. 0.089 ± 0.019, P < 0.05) but not cyclin I−/−mice (Fig. 6B; 0.030 ± 0.029 vs. 0.030 ± 0.032, P not significant). Seven days after disease induction, there was also an increase in cells staining for Bcl-xL; however, there was less of an increase in cyclin I−/− and cyclin I−/− p35−/− mice than p35−/− mice, which was not statistically different from wild-type mice (Fig. 6B; wild-type, 0.446 ± 0.116; cyclin I−/−, 0.251 ± 0.061; p35−/−, 0.468 ± 0.098; cyclin I−/− p35−/−, 0.225 ± 0.058, positive cells in tuft corrected with podocyte number). Representative images are shown in Fig. 6, K–R.
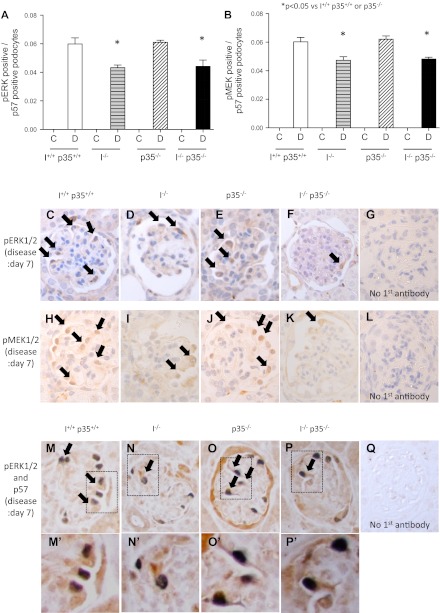
Phospho-ERK Levels Require p35 and Cyclin I for Maximal Expression
We have previously reported that certain MAPK proteins are required for normal podocyte survival (5). Immunohistochemistry for active ERK1/2 and MEK1/2 was performed as indicators of the MAPK pathway activity. No staining in the glomerular tuft was observed in any of the mouse strains before disease. Seven days after disease induction, the results were similar to Bcl-xL in that both ERK1/2 and MEK1/2 increased in disease in all mice, but there was less of an increase in single cyclin I−/− and double cyclin I−/− p35−/− mice than wild-type or single p35−/− mice (Fig. 7A; wild-type, 0.060 ± 0.004; cyclin I−/−, 0.043 ± 0.002; p35−/−, 0.061 ± 0.001; cyclin I−/− p35−/−, 0.044 ± 0.004) (Fig. 7B; wild-type, 0.060 ± 0.003; cyclin I−/−, 0.047 ± 0.002; p35−/−, 0.062 ± 0.002; p35−/− cyclin I−/−, 0.048 ± 0.001) (P < 0.05). Representative images are shown in Fig. 7, C–Q.
Fig. 7.
Immunohistochemistry for ERK1/2 and MEK1/2 activation.. Shown is quantification of pERK1/2- and pMEK1/2-positive cells in tuft corrected with podocyte number in nephritic mice. There were significantly fewer pERK1/2 (A)- and pMEK1/2 (B)-positive podocytes in double cyclin I−/− p35−/− mice compared with those in wild-type, single cyclin I−/−, and p35−/− mice. *P < 0.05 vs. wild-type. **P < 0.05 vs. cyclin I or p35−/−. No pERK1/2- or pMEK1/2-positive podocytes were present at baseline. C, control; D, disease (day 7). Also shown are representative micrographs of immunostaining for pERK1/2 and pMEK1/2. C and H: wild-type. D and I: cyclin I−/−. E and J: p35−/−. F and K: cyclin I−/− p35−/−. C–Q, glomerulonephritis. G, L, and Q: no primary antibody. C–F and H–K: single staining. M–P: double staining for p57 (nuclear, blue/gray) and pERK (cytoplasmic, brown). M′, N′, O′, and P′: light microscopic view of the insets shown in M, N, O, and P, respectively. Original magnification ×400.
DISCUSSION
Podocytes and other terminally differentiated cells have a limited capacity to proliferate, and thus when they undergo apoptosis, overall cell number tends to decrease. This typically leads to undesirable consequences such as proteinuria and glomerular scarring. Nature has clearly set up many survival mechanisms for most cells, and these mechanisms may be unique to terminally differentiated cells. The results of these studies show that to exert maximal protection of kidney podocytes, two activators of Cdk5, cyclin I and p35, must be present.
To determine whether cyclin I and p35 are redundant in terms of cell survival, we used a genetic approach, where mice were generated that were null for both cyclin I and p35. To our knowledge, this is the first report of such a mouse. Recall that single cyclin I null mice and single p35 null mice are normal (5, 7, 10). The first major finding in this study was that when both cyclin I and p35 were deleted, mice had normal kidneys structurally and functionally when maintained under nonstressed conditions. This was indeed a surprise as both cyclin I and p35 are constitutively expressed in normal podocytes and both activate Cdk5. Thus, under normal conditions, the activation of Cdk5 by other activators beyond cyclin I and Cdk5 is likely sufficient to maintain normal kidney function.
A second major finding in this study was that in contrast to nonstressed states, mice lacking both cyclin I and p35 were significantly more vulnerable to experimental glomerular disease severity compared with wild-type mice and mice that lacked either cyclin I or p35 alone. The data showed that although podocyte apoptosis increases in wild-type mice with disease, and single cyclin I and p35 null mice as we have previously reported (7, 10), double mutants had significantly more podocyte apoptosis during disease than the other strains tested. Moreover, this lead to a more marked reduction in podocyte number in these mice compared with diseased single mutants. This finding likely explains why double mutants had more proteinuria and glomerulosclerosis in experimental disease compared with wild-types and single mutants with the same disease model.
One might have concerns regarding the potential effects of background mouse strain differences on disease susceptibility. Therefore, we compared several indicators of disease severity between cyclin I+/+ mice on the C57BL/6 background and p35+/+ on the Balb/C background. First, we compared deposition of sheep IgG (inducing antibody) in all animals by immunofluorescence. There were no differences between animals (data not shown), similar to what we have described for the single cyclin I−/− and p35−/− mice (7, 10). Second, proteinuria levels (mg/24 h) showed no statistical differences between diseased cyclin I+/+ and p35+/+ mice (cyclin I+/+, 3.72 ± 0.35; p35+/+, 3.28 ± 0.56; P = 0.2123). Third, podocyte number, another indicator of disease severity, also showed no statistical differences between diseased cyclin I+/+ and p35+/+ mice (cyclin I+/+, 6.87 ± 0.20; p35+/+, 6.87 ± 0.81; P = 0.9911). Finally, this animal model is characterized by podocyte apoptosis, in the absence of any infiltrating leukocytes. These factors suggest that the impact of the loss of cyclin I and p35 on systemic factors, such as inflammatory mediators, was unlikely in this model (16). Similarly, the potential effect of strain alone for the changes observed in these studies was unlikely.
Taken together, the data support a paradigm where cyclin I and p35 are not required for kidney structure and function under normal (nondiseased) states. In contrast, they are critical for maximal cell survival in disease. Moreover, while the magnitude of apoptosis is comparable in diseased cyclin I and diseased p35 mice, apoptosis was substantially greater when both activators were absent. This strongly suggests that cyclin I and p35 do not compensate for one another and that both are needed to maximize cell survival.
One might ask why double mutant mice are more susceptible to apoptosis in disease and not under normal states. We recently reported, using single cyclin I−/− and single p35−/− mice, that cyclin I and p35 act via independent mechanisms to regulate levels of Bcl-2 family prosurvival proteins (5). Cdk5 sets a threshold for survival in postmitotic cells, and active Cdk5 forms two independent lines of defense to promote survival of postmitotic cells. Cyclin I-Cdk5 activates the MEK1/2-ERK1/2 pathway independently of Raf. leading to increased transcriptional activity of Bcl-2 and Bcl-xL. In addition, p35-Cdk5 phosphorylates Bcl-2 directly and thereby stabilizes Bcl-2 protein levels (5). Levels of the proapoptotic protein Bax were not influenced by cyclin I or p35 (5).
In the present study, Bcl-2 levels were much lower in nephritic double p35−/− cyclin I−/− mice than other diseased mice, and the decreased level of Bcl-xL in nephritic double p35−/− cyclin I−/− mice was similar to single cyclin I−/− mice. Moreover, the decrease in ERK1/2 and MEK1/2 activation in nephritic double p35−/− cyclin I−/− mice was similar to single cyclin I−/− mice. These findings suggest that cyclin I-Cdk5 regulates Bcl-2 and Bcl-xL by activating the MEK/ERK pathway and increasing transcriptional activity. The effects of p35-Cdk5 occur at the posttranslational level, and Cdk5-p35-cyclin I jointly regulate apoptosis, as found in our previous in vitro and in vivo studies using single cyclin I−/− and p35−/− mice (5, 7, 10).
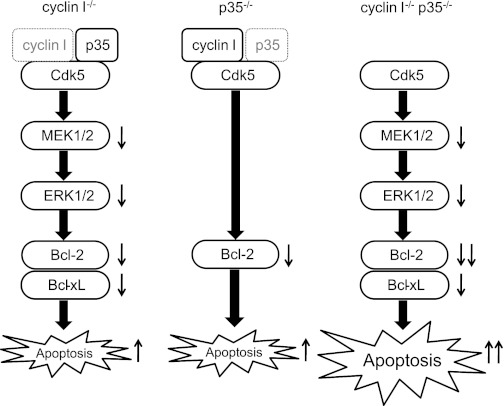
In summary, active cyclin I-Cdk5 and p35-Cdk5 are both required for podocyte survival and protect against apoptosis in vitro and in vivo under conditions of stress (5–7). Although the mechanism is not clear, it is possible that cyclin I and p35 jointly activate Cdk5 to regulate apoptosis (Fig. 8). This regulation is very important as podocyte apoptosis reduces overall cell number, resulting in proteinuria and glomerulosclerosis.
Fig. 8.
Active cyclin I-Cdk5 and p35-Cdk5 are both required for podocyte survival. Cyclin I-Cdk5 leads to phosphorylation of MEK1/2 and ERK1/2, which leads to increased levels of the prosurvival proteins Bcl-2 and Bcl-xL. In contrast, p35-Cdk5 increases Bcl-2 protein levels and has no effect on Bcl-xL. Both cyclin I and p35 play pivotal coordinated roles in maintaining podocyte survival during disease.
A limitation of our study warrants mention. The present study examined in vivo responses and was unable to resolve details including protein-protein interaction of the mechanism of apoptosis. An understanding of these processes might require an in vitro investigation of double cyclin I−/− p35−/− mice. Such an understanding might identify new therapeutic approaches to prevention of apoptosis and to protect postmitotic cells.
In conclusion, these results demonstrated that podocyte apoptosis in a diseased kidney model was much higher in double cyclin I−/− p35−/− than single p35−/− and cyclin I−/− mice. This suggests p35 and cyclin I play pivotal, coordinated roles in apoptosis in glomeruli. The identification of critical survival proteins that reduce apoptosis might likely provide new targets for intervention in renal dysfunction. Ongoing studies are intended to further define the role of the p35/cyclin I/Cdk5-axis in glomerular epithelial cell differentiation, apoptosis, and survival.
GRANTS
This work was supported by National Institutes of Diabetes and Digestive and Kidney Diseases Grants DK60525, DK56799, and DK51096 (to S. J. Shankland), the Uehara Memorial Foundation (to Y. Taniguchi), and by the Kochi Organization for Medical Reformation and Renewal (to Y. Taniguchi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Y. Taniguchi, J.W.P., R.D.K., P.B., and S.J.S. provided conception and design of research; Y. Taniguchi performed experiments; Y. Taniguchi, J.W.P., and S.J.S. analyzed data; Y. Taniguchi, J.W.P., H.H., R.D.K., A.M.C., J.Z., Y. Terada, P.B., and S.J.S. interpreted results of experiments; Y. Taniguchi prepared figures; Y. Taniguchi drafted manuscript; Y. Taniguchi, J.W.P., P.B., and S.J.S. edited and revised manuscript; Y. Taniguchi approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Inez Vincent for providing the p35−/− mice used in this study.
REFERENCES
- 1. Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ. Recruitment of podocytes from glomerular parietal cells. J Am Soc Nephrol 20: 333– 343, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bariety J, Bruneval P, Meyrier A, Mandet C, Hill G, Jacquot C. Podocyte involvement in human immune crescentic glomerulonephritis. Kidney Int 68: 1109– 1119, 2005. [DOI] [PubMed] [Google Scholar]
- 3. Bariety J, Mandet C, Hill GS, Bruneval P. Parietal podocytes in normal human glomeruli. J Am Soc Nephrol 17: 2770– 2780, 2006. [DOI] [PubMed] [Google Scholar]
- 4. Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell 14: 159– 169, 2008. [DOI] [PubMed] [Google Scholar]
- 5. Brinkkoetter PT, Oliver P, Wu JS, Henderson S, Krofft RD, Pippin JW, Hockenbery D, Roberts JM, Shankland SJ. Cyclin I activates Cdk5 and regulates expression of Bcl-2 and Bcl-xL in postmitotic mouse cells. J Clin Invest 119: 3089– 3101, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brinkkoetter PT, Pippin JW, Shankand SJ. Cyclin I-Cdk5 governs survival in post-mitotic cells. Cell Cycle 9: 1729– 1731, 2010. [DOI] [PubMed] [Google Scholar]
- 7. Brinkkoetter PT, Wu JS, Ohse T, Krofft RD, Schermer B, Benzing T, Pippin JW, Shankland SJ. p35, the non-cyclin activator of Cdk5, protects podocytes against apoptosis in vitro and in vivo. Kidney Int 77: 690– 699, 2010. [DOI] [PubMed] [Google Scholar]
- 8. Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol 2: 749– 759, 2001. [DOI] [PubMed] [Google Scholar]
- 9. Griffin SV, Hiromura K, Pippin JW, Petermann AT, Blonski MJ, Krofft R, Takahashi S, Kulkarni AB, Shankland SJ. Cyclin-dependent kinase 5 is a regulator of podocyte differentiation, proliferation, and morphology. Am J Pathol 165: 1175– 1185, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Griffin SV, Oliver JP, Pippin JW, Roberts JM, Shankland SJ. Cyclin I protects podocytes from apoptosis. J Biol Chem 281: 28048– 28057, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Hallows JL, Chen K, Depinho RA, Vincent I. Decreased cyclin-dependent kinase 5 (cdk5) activity is accompanied by redistribution of cdk5 and cytoskeletal proteins and increased cytoskeletal protein phosphorylation in p35 null mice. J Neurosci 23: 10633– 10644, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hiromura K, Haseley LA, Zhang P, Monkawa T, Durvasula R, Petermann AT, Alpers CE, Mundel P, Shankland SJ. Podocyte expression of the CDK-inhibitor p57 during development and disease. Kidney Int 60: 2235– 2246, 2001. [DOI] [PubMed] [Google Scholar]
- 13. Hiromura K, Pippin JW, Blonski MJ, Roberts JM, Shankland SJ. The subcellular localization of cyclin dependent kinase 2 determines the fate of mesangial cells: role in apoptosis and proliferation. Oncogene 21: 1750– 1758, 2002. [DOI] [PubMed] [Google Scholar]
- 14. Hiromura K, Pippin JW, Fero ML, Roberts JM, Shankland SJ. Modulation of apoptosis by the cyclin-dependent kinase inhibitor p27 (Kip1). J Clin Invest 103: 597– 604, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohse T, Vaughan MR, Kopp JB, Krofft RD, Marshall CB, Chang AM, Hudkins KL, Alpers CE, Pippin JW, Shankland SJ. De novo expression of podocyte proteins in parietal epithelial cells during experimental glomerular disease. Am J Physiol Renal Physiol 298: F702– F711, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ophascharoensuk V, Pippin JW, Gordon KL, Shankland SJ, Couser WG, Johnson RJ. Role of intrinsic renal cells versus infiltrating cells in glomerular crescent formation. Kidney Int 54: 416– 425, 1998. [DOI] [PubMed] [Google Scholar]
- 17. Paglini G, Caceres A. The role of the Cdk5-p35 kinase in neuronal development. Eur J Biochem 268: 1528– 1533, 2001. [PubMed] [Google Scholar]
- 18. Rosales JL, Lee KY. Extraneuronal roles of cyclin-dependent kinase 5. Bioessays 28: 1023– 1034, 2006. [DOI] [PubMed] [Google Scholar]
- 19. Shankland SJ, Eitner F, Hudkins KL, Goodpaster T, D'Agati V, Alpers CE. Differential expression of cyclin-dependent kinase inhibitors in human glomerular disease: role in podocyte proliferation and maturation. Kidney Int 58: 674– 683, 2000. [DOI] [PubMed] [Google Scholar]
- 20. Shankland SJ, Floege J, Thomas SE, Nangaku M, Hugo C, Pippin J, Henne K, Hockenberry DM, Johnson RJ, Couser WG. Cyclin kinase inhibitors are increased during experimental membranous nephropathy: potential role in limiting glomerular epithelial cell proliferation in vivo. Kidney Int 52: 404– 413, 1997. [DOI] [PubMed] [Google Scholar]
- 21. Smeets B, Angelotti ML, Rizzo P, Dijkman H, Lazzeri E, Mooren F, Ballerini L, Parente E, Sangrinati C, Mazzinghi B, Ronconi E, Becherucci F, Benigni A, Steenbergen E, Lasagni L, Remuzzi G, Wetzels J, Romagnani P. Renal progenitor cells contribute to hyperplastic lesions of podocytepathies and crescentic glomerulonephritis. J Am Soc Nephrol 20: 2593– 2603, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Su SC, Tsai LH. Cyclin-dependent kinases in brain development and disease. Annu Rev Cell Dev Biol 27: 13.1– 13.27, 2011. [DOI] [PubMed] [Google Scholar]
- 23. Weishaupt JH, Neusch C, Bahr M. Cyclin-dependent kinase 5 (CDK5) and neuronal cell death. Cell Tissue Res 312: 1– 8, 2003. [DOI] [PubMed] [Google Scholar]
- 24. Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941– 2952, 2005. [DOI] [PubMed] [Google Scholar]