Abstract
Our previous studies showed that streptozotocin (STZ)-induced diabetic male rats have increased estradiol and decreased testosterone levels that correlate with renal injury (Xu Q, Wells CC, Garman GH, Asico L, Escano CS, Maric C. Hypertension 51: 1218–1224, 2008). We further showed that either supplementing dihydrotestosterone (DHT) or inhibiting estradiol biosynthesis in these diabetic rats was only partially renoprotective (Manigrasso MB, Sawyer RT, Marbury DC, Flynn ER, Maric C. Am J Physiol Renal Physiol 301: F634–F640, 2011; Xu Q, Prabhu A, Xu S, Manigrassso MB, Maric C. Am J Physiol 297: F307–F315, 2009). The aim of this study was to test the hypothesis that the combined therapy of DHT supplementation and inhibition of estradiol synthesis would afford better renoprotection than either treatment alone. The study was performed in 12-wk-old male nondiabetic (ND), STZ-induced diabetic (D), and STZ-induced diabetic rats that received the combined therapy of 0.75 mg/day of DHT along with 0.15 mg·kg−1·day−1 of an aromatase inhibitor, anastrozole (Dta), for 12 wk. Treatment with the combined therapy resulted in attenuation of albuminuria by 84%, glomerulosclerosis by 55%, and tubulointerstitial fibrosis by 62%. In addition, the combined treatment decreased the density of renal cortical CD68-positive cells by 70% and decreased protein expression of transforming growth factor-β protein expression by 60%, collagen type IV by 65%, TNF-α by 55%, and IL-6 by 60%. We conclude that the combined treatment of DHT and blocking aromatase activity in diabetic male STZ-induced diabetic rats provides superior treatment than either treatment alone in the prevention of diabetic renal disease.
Keywords: diabetes, estrogen, testosterone
studies in humans have shown that diabetes is associated with an imbalance in sex hormone levels. Namely, males have low testosterone and high estradiol levels (12–13, 29, 42) in contrast to females that exhibit low estradiol and higher testosterone levels (30, 38). Furthermore, studies in both clinical and experimental models have shown that this imbalance correlates with renal injury. Specifically, in men with type 1 diabetes, the decrease in testosterone/increase in estradiol correlates with the progression from microalbuminuria to end-stage renal disease and the decline in estimated glomerular filtration rate (29–30, 38, 44). In experimental models, this imbalance also correlates with the progression of albuminuria, systemic inflammation, and fibrosis (44). These observations suggest that sex hormones may play an important role in the progression and pathogenesis of diabetic renal disease and that possibly restoring the balance of these hormones to those observed in nondiabetic subjects may attenuate or abolish the progression of renal injury. Our previous studies in the female STZ-induced diabetic rat have shown that restoring circulating estradiol to physiological levels resulted in partial attenuation of diabetes-associated renal injury by reducing albuminuria, creatinine clearance, glomerulosclerosis, tubulointerstitial fibrosis, and transforming growth factor-β (TGF-β) protein expression (27). In the male STZ-induced diabetic rat, supplementing dihydrotestosterone (DHT), the non-aromatizable androgen (43), or blocking estradiol synthesis (26) partially attenuated albuminuria, markers of inflammation, and tubulointerstitial fibrosis. These studies indicate that individually restoring the levels of either androgens or estrogens is not sufficient to provide full renoprotection. Specifically, since STZ-induced diabetic male rats exhibit both reductions in testosterone and increases in estradiol levels, it is conceivable that restoring either testosterone or estradiol levels on their own would only be partially renoprotective. Thus the aim of the present study was to examine whether the combined dihydrotestosterone supplementation along with inhibition of aromatase activity would provide more complete protection from STZ-induced diabetic renal injury in male rats.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats (12 wk of age) were purchased from Harlan (Madison, WI) and were maintained on regular rat chow and tap water ad libitum (Harlan Teklan 8640, Harlan Laboratories,). Animals were randomly divided into three treatment groups: nondiabetic (ND; n = 6), STZ-induced diabetic (D; n = 6), and STZ-induced diabetic treated with DHT and anastrozole (Dta; n = 6). Diabetes was induced as previously described (27), and all diabetic rats received 2–4 U of insulin every 3 days (Lantus, Aventis Pharmaceuticals, Kansas City, MO) by subcutaneous (sc) injection to maintain blood glucose levels between 300 and 450 mg/dl, to promote weight gain and to prevent mortality. All experiments were approved by the University of Mississippi Medical Center Animal Care and Use Committee.
DHT and anastrozole treatment.
Seven days after the induction of diabetes, all animals were anesthetized with 2% isoflurane and implanted with a placebo or pellets continuously delivering sc 0.75 mg/day DHT (Innovative Research of America, Sarasota, FL). After pellet insertion, all animals were orally gavaged with either 0.9% saline or 0.15 mg·kg−1·day−1 anastrozole (AstraZeneca Pharmaceuticals, Wilmington, DE). The doses of DHT and anastrozole were based on our previously published studies (26, 43).
Urine albumin excretion.
Urine albumin concentration was measured after 4, 8, and 12 wk of diabetes using a Nephrat II albumin kit (Exocel, Philadelphia, PA) according to the manufacturer's protocol and as previously described (26).
Measurement of plasma hormone levels.
Plasma estradiol levels were measured by radioimmunoassay (catalog no. DSL-4800; Diagnostic System Labs, Webster, TX), and plasma DHT levels were measured by ELISA (Alpha Diagnostic International, San Antonio, TX) according to the manufacturer's protocol.
Glomerulosclerosis and tubulointerstitial fibrosis.
To assess markers of renal pathology, indices of glomerulosclerosis (GSI) and tubulointerstitial fibrosis (TIFI) were evaluated using a semiquantitative scoring method as previously described (27).
Immunohistochemistry.
Paraffin-embedded sections (4 μm) were incubated with 10% nonimmune goat or 0.1% bovine serum to block nonspecific immunolabeling. Sections were then incubated with antisera against CD68 (1:200; mouse monoclonal; catalog no. MCA341R; Serotec, Oxford, UK), collagen IV (1:500; goat polyclonal; catalog no. 1340-01; Southern Biotech, Birmingham, AL), or TGF-β (1:200; rabbit polyclonal, catalog no. sc-146; Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight as described previously for these particular antibodies (19–20, 44). After washing with phosphate buffered saline, sections were incubated with appropriate secondary antibodies followed by incubation with the avidin-biotin complex (Vector, Burlingame, CA). Positive immunoreaction was detected as described previously described (44).
The density of CD68-positive cells was quantified as we previously described (44). Briefly, CD68-positive cells in 40 different fields per animal from each group were quantified and expressed per millimeter squared.
Western blotting.
Homogenized renal cortical samples (30–50 μg; n = 6/group) were denatured at 95°C for 5–15 min, except for collagen IV which was not denatured, loaded onto SDS-PAGE precast gels (Bio-Rad, Hercules, CA), and transferred to nitrocellulose membranes as described previously (26). Membranes were incubated with antisera against TGF-β (1:500; rabbit polyclonal, catalog no. sc-146; Santa Cruz Biotechnology), collagen IV (1:500; mouse monoclonal; catalog no. MAB1910; Millipore, Temecula, CA), IL-6 (1:500; goat polyclonal; catalog no. sc-1265; Santa Cruz Biotechnology), or TNF-α (1:500; mouse monoclonal; catalog no. sc-52746; Santa Cruz Biotechnology) as previously described for these particular antibodies (5, 6, 20, 44, 46) followed by appropriate secondary antibodies conjugated to horseradish peroxidase. Proteins were visualized by enhanced chemiluminescence (Thermo Scientific, Rockford, IL), and the densities of specific bands were quantified by densitometry using Scion Image (version α 4.0.3.2) software. The membranes were then striped and reprobed with an antibody against β-actin (1:1,000; mouse monoclonal; catalog no. 4970; Cell Signaling, Danvers, MA). The densities of specific bands were then normalized to the densities of bands probed for β-actin.
Statistical analysis.
All values are expressed as mean ± SE and were analyzed using one-way ANOVA (Prism 5, Graph Pad Software, San Diego, CA). Post hoc comparisons were performed using a Newman-Keuls multiple comparison test. Differences were considered statistically significant at P < 0.05.
RESULTS
Metabolic parameters.
Compared with ND animals, D animals had higher blood glucose and HbA1C levels (Table 1). D animals also had increased food intake and kidney/body weight ratio and decreased body weight. No differences in any of these parameters were observed between D and Dta animals (Table 1).
Table 1.
Metabolic parameters after 12 wk of diabetes
| Parameters | ND | D | Dta |
|---|---|---|---|
| Blood glucose, mg/dl | 78 ± 2 | 370 ± 18c | 338 ± 20c |
| HbA1C | 4.3 ± 0.1 | 9.3 ± 0.4c | 10.3 ± 0.5c |
| Food intake, g | 25 ± 2.4 | 48.2 ± 5.5b | 41.0 ± 4.4a |
| Body wt, g | 430 ± 10 | 319 ± 11c | 333 ± 10c |
| Kidney wt/body wt, g/kg | 3.0 ± 0.5 | 5.3 ± 0.4c | 5.0 ± 0.2c |
| Plasma DHT/testis wt, ng·ml−1·g−1 | 404.5 ± 15.9 | 190.9 ± 28.2c | 470.0 ± 35.6d |
| Plasma estradiol, pg/ml | 3.5 ± 0.7 | 45.1 ± 7.3c | 16.7 ± 4.0d |
Values are means ± SE. ND, nondiabetic; D, diabetic; Dta, diabetic treated with dihydrotestosterone (DHT) and anastrozole.
P < 0.05 vs. ND.
P < 0.01 vs. ND.
P < 0.001 vs. ND.
P < 0.001 vs. D.
Urine albumin excretion.
After 4 wk of diabetes, D animals already had a 577% increase in urine albumin excretion (UAE) compared with ND animals. UAE in D animals was further increased by 1,058% after 8 wk of diabetes and by 1,543% after 12 wk of diabetes compared with ND animals at the same time point. In contrast, Dta animals had comparable UAE levels to ND throughout the entire 12 wk of the study (Fig. 1).
Fig. 1.
Urine albumin excretion (UAE) at 4, 8, and 12 wk. ND, nondiabetic; D, STZ-induced diabetic; Dta, STZ-induced diabetic rats that received the combined therapy of DHT along with an aromatase inhibitor, anastrozole (Dta). *ND vs. D, P < 0.01. #Dta vs. D, P < 0.05.
Sex hormone levels.
Confirming our previous report (43), diabetes was associated with a 53% reduction in circulating DHT levels compared with the ND group. The Dta group had similar levels of DHT as the ND group, and a 146% increase in plasma DHT levels compared with D animals (Table 1). Note that circulating DHT levels are expressed per testicular weight since exogenous androgen supplementation is known to decrease testicular weight (1).
Also confirming our previous report (26), diabetes was associated with an 1,186% increase in plasma estradiol levels (Table 1). While the Dta animals had a 63% decrease in plasma estradiol levels compared with D animals, no differences were observed between the Dta and ND animals (Table 1).
Glomerulosclerosis and tubulointerstitial fibrosis.
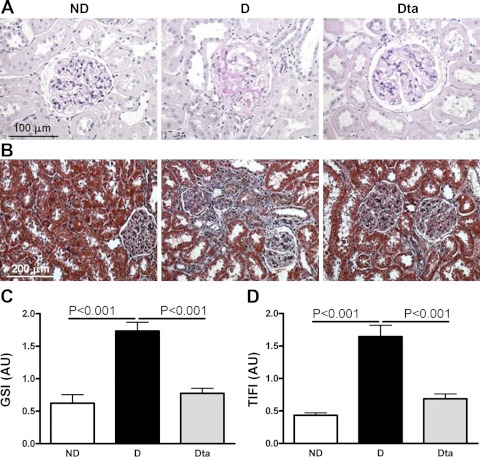
Diabetes was associated with moderate glomerular injury typical of 12 wk of STZ-induced diabetes (Fig. 2A). Compared with D animals, the GSI was reduced by 55% in Dta animals (Fig. 1C).Similarly, diabetes was associated with moderate tubulointerstitial fibrosis (Fig. 1B). Compared with D animals, the TIFI was reduced by 62% in the Dta compared with D animals (Fig. 1D).
Fig. 2.
Renal pathology. A: periodic acid-Schiff-stained section of the renal cortex. B: Masson's trichrome-stained sections of the renal cortex. C: glomerulosclerosis index (GSI). D: tubulointerstitial fibrosis index (TIFI). Original magnification ×400 for GSI and ×200 for TIFI. Values are means ± SE.
Androgen receptor and estrogen receptor-α protein expression.
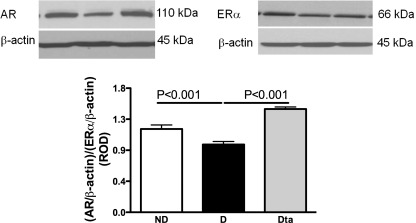
Diabetes was associated with a 19% decrease in androgen receptor (AR)/estrogen receptor (ER) α protein expression compared with ND animals. Dta animals had a 52% increase in AR/ERα protein expression compared with D and similar protein expression compared with those observed in the ND group (Fig. 3).
Fig. 3.
Renal androgen receptor (AR) and estrogen receptor (ER) α protein expression. Top: representative immunoblot of renal AR and ERα protein expression. Bottom: densitometric scans in relative optical density (ROD) expressed as the renal AR/ERα/β-actin ratio; n = 6.
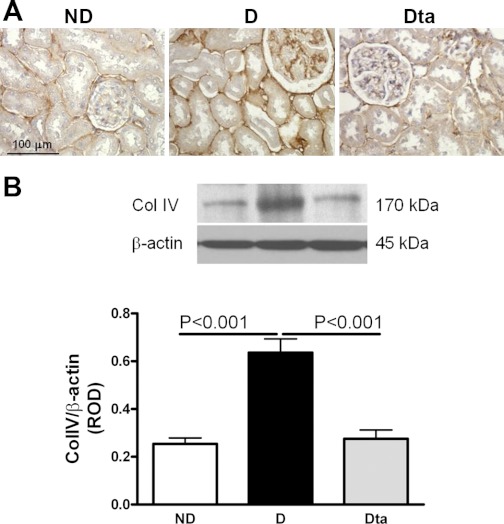
Collagen type IV protein expression.
In D animals, collagen type IV was immunolocalized to basement membranes of all epithelial elements, as well as in the expanded mesangial areas in the glomerulus and the surrounding tubulointerstitium (Fig. 4A). Similar to ND animals, collagen type IV immunostaining in the Dta animals was only evident in the basement membranes, as expected for a healthy animal. Quantitative analysis by Western blotting confirmed the immunohistochemical observations in that collagen type IV protein expression was increased in the D compared with ND group by 151% and decreased by 57% in the Dta group compared with the D animals (Fig. 4B).
Fig. 4.
Collagen type IV protein (ColIV) expression. A: collagen type IV immunolocalization. Original magnification ×400. B: collagen type IV protein expression. Top: representative immunoblot of collagen type IV protein expression. Bottom: densitometric scans in ROD expressed as the renal collagen type IV/β-actin ratio; n = 6. Values are means ± SE.
Inflammatory markers.
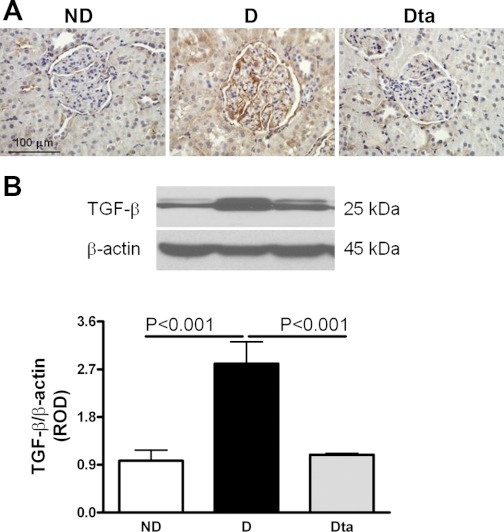
In the D animals, TGF-β was immunolocalized to podocytes and proximal tubules. While prominent staining was observed in the D animals, TGF-β immunolocalization was virtually abolished in the Dta animals and was comparable to ND animals (Fig. 5A). These data were confirmed by Western blotting, where D animals had a 187% increase in TGF-β protein expression compared with ND animals and which was reduced by 60% in the Dta animals compared with D animals (Fig. 5B).
Fig. 5.
TGF-β protein expression. A: TGF-β immunolocalization. Original magnification ×400. B: TGF-β protein expression. Top: representative immunoblot of TGF-β protein expression. Bottom: densitometric scans of the 25-kDa band in ROD expressed as the renal TGF-β/β-actin ratio; n = 6. Values are means ± SE.
CD68-positive cells, indicating the presence of activated macrophages, were abundant in both the glomerulus and tubulointerstitium of D animals (Fig. 6A). D animals had a 282% increase in CD68-positive cell staining compared with ND, while Dta animals had a 70% decrease in the abundance of CD68-positive cells compared with the D group (Fig. 6B).
Fig. 6.
CD68-positive cell abundance. A: CD68 immunolocalization. Original magnification ×400. B: quantitative analysis of CD68-positive cell abundance. Values are means ± SE. Outlined areas are glomeruli; G, glomerulus.
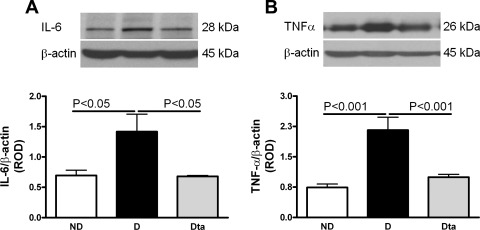
IL-6 is secreted by multiple cell types, including fibroblasts, monocytes, and endothelial cells, as a 23- to 30-kDa phosphorylated and variably glycosylated molecule (31). We analyzed the 28-kDa product of IL-6 by Western blotting which found that D animals had a 104% increase in IL-6 protein expression compared with ND. Compared with D animals, Dta animals had a 60% reduction in IL-6 protein expression (Fig. 7A). Similarly, TNF-α protein expression was increased by 192% in D animals compared with ND. TNF-α protein expression was reduced by 55% in the Dta animals compared with D (Fig. 7B).
Fig. 7.
Renal IL-6 and TNF-α protein expression. A: renal IL-6 protein expression. Top: representative immunoblot of renal IL-6 protein expression. Bottom: densitometric scans in ROD expressed as the renal IL-6/β-actin ratio; n = 6. B: renal TNF-α protein expression. Top: representative immunoblot of renal TNF-α protein expression. Bottom: densitometric scans in ROD expressed as the renal TNF-a/β-actin ratio; n = 6. Values are means ± SE.
DISCUSSION
The findings of the present study confirm our previous reports that diabetes is associated with an imbalance in sex hormone levels (32, 43). In particular, male STZ-induced diabetic rats exhibit decreased testosterone and increased estradiol levels, and this is associated with increases in UAE, GSI, and TIFI, along with increased protein expression of markers of inflammation and fibrosis. We have shown that raising testosterone (i.e., DHT, the non-aromatizable and more biologically active metabolite) to physiological levels partially attenuated diabetic kidney disease (43). Similarly, inhibiting estradiol synthesis by preventing aromatization of androgens to estrogens was also shown to partially attenuate diabetic renal disease (26). The fact that these studies individually showed only partial renoprotection provided the rationale for the present study, which examined the renoprotective effects of combined DHT supplementation along with aromatase inhibition in the male STZ-induced diabetic rat.
One of the hallmarks of diabetic renal disease is increased UAE (3). In the present study, increased UAE was observed as early as 4 wk of diabetes and continued to increase throughout the length of the study (12 wk). However, animals receiving the combined treatment of DHT and aromatase inhibition were protected from this increase and had UAE values similar to those observed in ND animals. Similarly, the diabetes-associated increase in glomerulosclerosis, tubulointerstitial fibrosis, and collagen type IV protein expression was largely prevented with concomitant DHT supplementation and inhibition of estradiol synthesis. Several studies have reported profibrotic effects of androgens in a unilateral nephrectomy model and STZ-induced diabetes in males (32, 36, 40), and these effects of androgens appear to be dose dependent. Indeed our studies have shown that DHT can have both pro- and antifibrotic effects depending on the dose used (32, 36). While estrogens are commonly thought to exert antifibrotic effects in experimental models of renal injury in females (7, 17), much less is known about their effects in males. A study in TGF-β transgenic mice has shown that 17-β estradiol reduces glomerulopathy in males, but to a lesser extent than it does in females (2). While the present study did not examine the direct effects of estrogens in male STZ-induced diabetic rats, it indicates that inhibiting its actions is beneficial with respect to diabetes-associated glomerulosclerosis and tubulointerstitial fibrosis. Further studies are warranted to examine the direct effects of estrogens in diabetic male rats.
Diabetic renal disease is characterized by renal inflammation, and our study shows that DHT supplementation along with aromatase inhibition largely attenuates the expression of markers of inflammation, including TGF-β, TNF-α, IL-6, and macrophage infiltration. Indeed, it has long been recognized that testosterone has immune-modulating actions, and the larger incidence of immune-mediated diseases observed in women and androgen-deficient men are attributed to the immunosuppressive effects of androgens compared with estrogens (8). Clinical observations have shown an association between low testosterone and higher levels of multiple inflammatory markers, including IL-1β, IL-6, sIL-6r, TNF-α, and C-reactive protein in chronic conditions of older men (22). Furthermore, supplementing hypogonadal men with and without diabetes with androgens has been shown to reduce inflammatory cytokine levels (25). In addition, low testosterone, as well as a reduced androgen/estrogen ratio, have been detected in both genders in systemic inflammatory diseases, such as systemic lupus erythematosus and rheumatoid arthritis (21). This lack of anti-inflammatory androgens coupled with the higher levels of estrogens may lead to proinflammatory conditions, as we have also observed in the STZ-induced diabetic male rat.
Men with type 1 and type 2 diabetes, as well as experimental models of diabetes, exhibit decreased circulating testosterone (9, 12–13, 18, 39, 42, 44) and increased circulating estradiol levels (42, 44). Furthermore, low testosterone and high estradiol levels in type 1 diabetic men are associated with a decline in renal function (29). This imbalance in the physiological levels of both androgens and estrogens in diabetic men may be a predictor of the severity of renal injury with progression of diabetes. While no clinical study to date has directly examined the role of restoring either androgens or estrogen levels to physiological range in diabetic men to prevent or reverse renal damage, lack of testosterone in male patients with diabetes has been shown to increase mortality among dialysis patients (4) and is associated with endothelial dysfunction, increased risk of heart failure, and cardiovascular disease in both diabetic and nondiabetic subjects (14, 23–24, 41, 45). Furthermore, testosterone supplementation has been shown to be beneficial in the setting of diabetes. In one case report, testosterone replacement therapy was shown to attenuate insulin resistance and improve several cardiovascular risk factors in hypogonadal men with type 2 diabetes (16).One of the underlying mechanisms for this observation may be the antiapoptotic effect of testosterone, as observed in STZ-induced diabetic, castrated rats (33, 35).
We have previously shown that inhibition of estradiol synthesis in diabetic male rats is beneficial in preventing the progression of renal disease (33, 35), suggesting that elevated estradiol levels may be one of the contributing factors in the development of renal disease. This is in contrast to the beneficial effects of estradiol observed in experimental models of both nondiabetic and diabetic renal disease in females, where the majority of studies indicate that estradiol is renoprotective (10, 27–28, 34). However, it is conceivable that estradiol, rather than exerting beneficial effects as it does in females, may produce deleterious effects in males. Supporting this notion are studies showing that estradiol supplementation accelerates the development of renal disease in the analbuminemic rat (15)and delays wound healing in castrated male mice (11). Therefore, it appears that the effects of androgens and estrogens may be sex specific. While the mechanisms for these sex-specific effects remain unclear, it is conceivable that differential expression of sex hormone receptors may be one of the reasons. Confirming our previous reports, diabetic male rats have a reduction in AR/ERα protein expression in the renal cortex compared with nondiabetic animals (26, 37), and the combined treatment with DHT and anastrozole restored this protein expression to that seen in ND. These observations suggest that the changes in the ratio of AR/ERα protein expression parallel the change in the relative balance of testosterone/estradiol levels. Furthermore, this relative balance in the expression of AR/ERα receptors may lead to differential effects of sex hormones not just in the opposite sex, but also in different disease models.
In summary, the present study demonstrates that restoring the balance of sex hormones by supplementing DHT and inhibiting estradiol synthesis prevents the progression of renal disease in the male STZ-induced diabetic rat. These data underscore the importance of sex hormones in the pathophysiology of diabetic renal disease and warrant further studies to elucidate the mechanisms by which sex hormones exert their actions in the diabetic kidney.
GRANTS
This work was supported by an RO1 grant (DK075832) from the National Institutes of Health/National Institute of Diabetes, Digestive and Kidney Diseases to C Maric-Bilkan, a Juvenile Diabetes Research Foundation (JDRF) postdoctoral fellowship grant (3–2010-459) to M. B. Manigrasso, and PO1 grant (HL51971) to J. E. Hall for the support of core facilities used for part of this study.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.B.M., R.T.S., Z.M.H.J., and E.R.F. performed experiments; M.B.M. analyzed data; M.B.M. and C.M.-B. interpreted results of experiments; M.B.M. prepared figures; M.B.M. drafted manuscript; M.B.M. and C.M.-B. edited and revised manuscript; C.M.-B. conception and design of research; C.M.-B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Stephanie Evans for technical assistance with paraffin sectioning.
REFERENCES
- 1. Bauman DH, Richerson JT, Britt AL. A comparison of body and organ weights, physiologic parameters, and pathologic changes in target organs of rats given combinations of exercise, anabolic hormone, and protein supplementation. Am J Sports Med 16: 397–402, 1988 [DOI] [PubMed] [Google Scholar]
- 2. Birch Nielsen C, Krag S, Osterby R, Flyvbjerg A, Nyengaard J, Forman A, Wogensen L. Transforming growth factor beta1-induced glomerulopathy is prevented by 17beta-estradiol supplementation. Virchows Arch 444: 561–566, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Caramori ML, Fioretto P, Mauer M. Enhancing the predictive value of urinary albumin for diabetic nephropathy. J Am Soc Nephrol 17: 339–352, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Carrero JJ, Qureshi AR, Parini P, Arver S, Lindholm B, Barany P, Heimburger O, Stenvinkel P. Low serum testosterone increases mortality risk among male dialysis patients. J Am Soc Nephrol 20: 613–620, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castello L, Froio T, Maina M, Cavallini G, Biasi F, Leonarduzzi G, Donati A, Bergamini E, Poli G, Chiarpotto E. Alternate-day fasting protects the rat heart against age-induced inflammation and fibrosis by inhibiting oxidative damage and NF-kB activation. Free Radic Biol Med 48: 47–54, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Chen HY, Huang XR, Wang W, Li JH, Heuchel RL, Chung AC, Lan HY. The protective role of Smad7 in diabetic kidney disease: mechanism and therapeutic potential. Diabetes 60: 590–601, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chin M, Isono M, Isshiki K, Araki S, Sugimoto T, Guo B, Sato H, Haneda M, Kashiwagi A, Koya D. Estrogen and raloxifene, a selective estrogen receptor modulator, ameliorate renal damage in db/db mice. Am J Pathol 166: 1629–1636, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cutolo M, Wilder RL. Different roles for androgens and estrogens in the susceptibility to autoimmune rheumatic diseases. Rheum Dis Clin North Am 26: 825–839, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab 89: 5462–5468, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Doublier S, Lupia E, Catanuto P, Periera-Simon S, Xia X, Korach K, Berho M, Elliot SJ, Karl M. Testosterone and 17beta-estradiol have opposite effects on podocyte apoptosis that precedes glomerulosclerosis in female estrogen receptor knockout mice. Kidney Int 79: 404–413, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gilliver SC, Emmerson E, Campbell L, Chambon P, Hardman MJ, Ashcroft GS. 17beta-Estradiol inhibits wound healing in male mice via estrogen receptor-alpha. Am J Pathol 176: 2707–2721, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grossmann M, Panagiotopolous S, Sharpe K, MacIsaac RJ, Clarke S, Zajac JD, Jerums G, Thomas MC. Low testosterone and anaemia in men with type 2 diabetes. Clin Endocrinol (Oxf) 70: 547–553, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Grossmann M, Thomas MC, Panagiotopoulos S, Sharpe K, Macisaac RJ, Clarke S, Zajac JD, Jerums G. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 93: 1834–1840, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Iglesias P, Carrero JJ, Diez JJ. Gonadal dysfunction in men with chronic kidney disease: clinical features, prognostic implications and therapeutic options. J Nephrol 25: 31–42, 2012 [DOI] [PubMed] [Google Scholar]
- 15. Joles JA, van Goor H, Koomans HA. Estrogen induces glomerulosclerosis in analbuminemic rats. Kidney Int 53: 862–868, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Jones TH, Arver S, Behre HM, Buvat J, Meuleman E, Moncada I, Morales AM, Volterrani M, Yellowlees A, Howell JD, Channer KS. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care 34: 828–837, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Keck M, Romero-Aleshire MJ, Cai Q, Hoyer PB, Brooks HL. Hormonal status affects the progression of STZ-induced diabetes and diabetic renal damage in the VCD mouse model of menopause. Am J Physiol Renal Physiol 293: F193–F199, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, Valkonen VP, Salonen R, Salonen JT. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care 27: 1036–1041, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Li Y, Chen Q, Liu FY, Peng YM, Hou T, Duan SB, Li J, Luo JH, Sun L, Ling GH. Norcantharidin attenuates tubulointerstitial fibrosis in rat models with diabetic nephropathy. Ren Fail 33: 233–241, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Lian M, Hewitson TD, Wigg B, Samuel CS, Chow F, Becker GJ. Long-term mineralocorticoid receptor blockade ameliorates progression of experimental diabetic renal disease. Nephrol Dial Transplant 27: 906–912, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Maestroni GJ, Sulli A, Pizzorni C, Villaggio B, Cutolo M. Melatonin in rheumatoid arthritis: a disease-promoting and modulating hormone? Clin Exp Rheumatol 20: 872–873, 2002 [PubMed] [Google Scholar]
- 22. Maggio M, Basaria S, Ble A, Lauretani F, Bandinelli S, Ceda GP, Valenti G, Ling SM, Ferrucci L. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab 91: 345–347, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Malkin CJ, Channer KS, Jones TH. Testosterone and heart failure. Curr Opin Endocrinol Diabetes Obes 17: 262–268, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Malkin CJ, Jones TH, Channer KS. Testosterone in chronic heart failure. Front Horm Res 37: 183–196, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab 89: 3313–3318, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Manigrasso MB, Sawyer RT, Marbury DC, Flynn ER, Maric C. Inhibition of estradiol synthesis attenuates renal injury in male streptozotocin (STZ)-induced diabetic rats. Am J Physiol Renal Physiol 301: F634–F640, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mankhey RW, Bhatti F, Maric C. 17β-Estradiol replacement improves renal function and pathology associated with diabetic nephropathy. Am J Physiol Renal Physiol 288: F399–F405, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Mankhey RW, Wells CC, Bhatti F, Maric C. 17β-Estradiol supplementation reduces tubulointerstitial fibrosis by increasing MMP activity in the diabetic kidney. Am J Physiol Regul Integr Comp Physiol 292: R769–R777, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Maric C, Forsblom C, Thorn L, Waden J, Groop PH. Association between testosterone, estradiol and sex hormone binding globulin levels in men with type 1 diabetes with nephropathy. Steroids 75: 772–778, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matsushita M, Tamura K, Osada S, Kogo H. Effect of troglitazone on the excess testosterone and LH secretion in thyroidectomized, insulin-resistant, type 2 diabetic Goto-Kakizaki rats. Endocrine 27: 301–305, 2005 [DOI] [PubMed] [Google Scholar]
- 31. May LT, Shaw JE, Khanna AK, Zabriskie JB, Sehgal PB. Marked cell-type-specific differences in glycosylation of human interleukin-6. Cytokine 3: 204–211, 1991 [DOI] [PubMed] [Google Scholar]
- 32. Metcalfe PD, Leslie JA, Campbell MT, Meldrum DR, Hile KL, Meldrum KK. Testosterone exacerbates obstructive renal injury by stimulating TNF-alpha production and increasing proapoptotic and profibrotic signaling. Am J Physiol Endocrinol Metab 294: E435–E443, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Morimoto S, Mendoza-Rodriguez CA, Hiriart M, Larrieta ME, Vital P, Cerbon MA. Protective effect of testosterone on early apoptotic damage induced by streptozotocin in rat pancreas. J Endocrinol 187: 217–224, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Negulescu O, Bognar I, Lei J, Devarajan P, Silbiger S, Neugarten J. Estradiol reverses TGF-beta1-induced mesangial cell apoptosis by a casein kinase 2-dependent mechanism. Kidney Int 62: 1989–1998, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Palomar-Morales M, Morimoto S, Mendoza-Rodriguez CA, Cerbon MA. The protective effect of testosterone on streptozotocin-induced apoptosis in beta cells is sex specific. Pancreas 39: 193–200, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Pawluczyk IZ, Tan EK, Harris KP. Rat mesangial cells exhibit sex-specific profibrotic and proinflammatory phenotypes. Nephrol Dial Transplant 24: 1753–1758, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Prabhu A, Xu Q, Manigrasso MB, Biswas M, Flynn E, Iliescu R, Lephart ED, Maric C. Expression of aromatase, androgen and estrogen receptors in peripheral target tissues in diabetes. Steroids 75: 779–787, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Salonia A, Lanzi R, Scavini M, Pontillo M, Gatti E, Petrella G, Licata G, Nappi RE, Bosi E, Briganti A, Rigatti P, Montorsi F. Sexual function and endocrine profile in fertile women with type 1 diabetes. Diabetes Care 29: 312–316, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Selvin E, Feinleib M, Zhang L, Rohrmann S, Rifai N, Nelson WG, Dobs A, Basaria S, Golden SH, Platz EA. Androgens and diabetes in men: results from the Third National Health and Nutrition Examination Survey (NHANES III). Diabetes Care 30: 234–238, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Sun J, Devish K, Langer WJ, Carmines PK, Lane PH. Testosterone treatment promotes tubular damage in experimental diabetes in prepubertal rats. Am J Physiol Renal Physiol 292: F1681–F1690, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Traish AM, Saad F, Feeley RJ, Guay A. The dark side of testosterone deficiency. III. Cardiovascular disease. J Androl 30: 477–494, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Vikan T, Schirmer H, Njolstad I, Svartberg J. Low testosterone and sex hormone-binding globulin levels and high estradiol levels are independent predictors of type 2 diabetes in men. Eur J Endocrinol 162: 747–754, 2010 [DOI] [PubMed] [Google Scholar]
- 43. Xu Q, Prabhu A, Xu S, Manigrasso MB, Maric C. Dose-dependent effects of dihydrotestosterone in the streptozotocin-induced diabetic rat kidney. Am J Physiol Renal Physiol 297: F307–F315, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu Q, Wells CC, Garman JH, Asico L, Escano CS, Maric C. Imbalance in sex hormone levels exacerbates diabetic renal disease. Hypertension 51: 1218–1224, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yilmaz MI, Sonmez A, Qureshi AR, Saglam M, Stenvinkel P, Yaman H, Eyileten T, Caglar K, Oguz Y, Taslipinar A, Vural A, Gok M, Unal HU, Yenicesu M, Carrero JJ. Endogenous testosterone, endothelial dysfunction, and cardiovascular events in men with nondialysis chronic kidney disease. Clin J Am Soc Nephrol 6: 1617–1625, 2011 [DOI] [PubMed] [Google Scholar]
- 46. Yim HE, Ha KS, Bae IS, Yoo KH, Hong YS, Lee JW. Postnatal early overnutrition dysregulates the intrarenal renin-angiotensin system and extracellular matrix-linked molecules in juvenile male rats. J Nutr Biochem [Epub ahead of print] [DOI] [PubMed] [Google Scholar]