Abstract
We investigated the signaling basis for tubule pathology during fibrosis after renal injury. Numerous signaling pathways are activated physiologically to direct tubule regeneration after acute kidney injury (AKI) but several persist pathologically after repair. Among these, transforming growth factor (TGF)-β is particularly important because it controls epithelial differentiation and profibrotic cytokine production. We found that increased TGF-β signaling after AKI is accompanied by PTEN loss from proximal tubules (PT). With time, subpopulations of regenerating PT with persistent loss of PTEN (phosphate and tension homolog) failed to differentiate, became growth arrested, expressed vimentin, displayed profibrotic JNK activation, and produced PDGF-B. These tubules were surrounded by fibrosis. In contrast, PTEN recovery was associated with epithelial differentiation, normal tubule repair, and less fibrosis. This beneficial outcome was promoted by TGF-β antagonism. Tubule-specific induction of TGF-β led to PTEN loss, JNK activation, and fibrosis even without prior AKI. In PT culture, high TGF-β depleted PTEN, inhibited differentiation, and activated JNK. Conversely, TGF-β antagonism increased PTEN, promoted differentiation, and decreased JNK activity. Cre-Lox PTEN deletion suppressed differentiation, induced growth arrest, and activated JNK. The low-PTEN state with JNK signaling and fibrosis was ameliorated by contralateral nephrectomy done 2 wk after unilateral ischemia, suggesting reversibility of the low-PTEN dysfunctional tubule phenotype. Vimentin-expressing tubules with low-PTEN and JNK activation were associated with fibrosis also after tubule-selective AKI, and with human chronic kidney diseases of diverse etiology. By preventing tubule differentiation, the low-PTEN state may provide a platform for signals initiated physiologically to persist pathologically and cause fibrosis after injury.
Keywords: tubule atrophy, acute kidney injury
tubulointerstitial fibrosis (TIF) is the main pathology that drives progression of kidney disease to the end stage (9, 16, 29). TIF comprises two components: tubule “atrophy” and interstitial fibrosis. The premise of progression often imputes a degree of autonomy to fibrosis that neglects the tubule component, whereas epithelial defects likely precede and evoke fibrosis in most renal disease (4, 9, 16, 23, 29). In glomerular disease, tubule atrophy precedes fibrosis, and fibroblasts proliferate only around atrophic tubules (23). On the other hand, defective repair of primary tubule damage during repetitive or single episodes of acute kidney injury (AKI) may also cause TIF (12, 30, 45).
Tubule defects that cause TIF after AKI include increased activity of transforming growth factor (TGF)-β (14, 37), Notch (3), c-Jun N-terminal kinase (JNK) (10, 17, 27, 45), platelet-derived growth factor (PDGF)-B (39), and connective tissue growth factor (CTGF) (45). These abnormalities may be interconnected; and TGF-β may be a proximal trigger. TGF-β, its receptors, and Smad signaling are increased in tubules proliferating after ischemia, suggesting an autocrine signaling loop (14, 31, 37). TGF-β antibodies ameliorated the TIF that developed after AKI consistent with a role for the cytokine in fibroblast activation (37). We studied another aspect of TGF-β in tubules—how autocrine signaling by this cytokine produces tubule pathology.
We found that TGF-β signaling is high in proliferating proximal tubule (PT) cells, suppressed in confluent differentiated cultures, and high again when confluent cultures dedifferentiate and proliferate after wounding (14). Remarkably, TGF-β antagonism accelerated the differentiation of subconfluent PT cultures even as they proliferated, promoted redifferentiation of tubules regenerating after ischemic AKI, and decreased fibrosis (14). We proposed that tubules recovering from AKI develop defects of “failed differentiation” caused by persistent regenerative signaling (41). According to this theory, some tubules are repaired normally after AKI, but others fail to redifferentiate. Tubules with “failed differentiation” continue to signal vicariously by pathways triggered earlier to mediate dedifferentiation, migration, and proliferation. Signaling hyperactivity and cytokine secretion after AKI are regenerative responses that should subside after epithelial repair. Failure to suppress physiologically initiated signaling not only prevents redifferentiation but also drives fibrosis through persistent cytokine activity (41).
Because our previous data suggested that persistently increased TGF-β signaling prevents redifferentiation of tubules being repaired after injury, we investigated possible mechanisms. There are proven TGF-β pathways that compromise epithelial integrity by disrupting cell junctions. Regardless, we used an unbiased approach to screen for other TGF-β-dependent mechanisms that might alter the epithelial phenotype. Among proteins modified by TGF-β was PTEN, an antagonist of PI3K signaling. PTEN has lipid/tyrosine phosphatase-dependent and -independent activities that if not physiologically regulated could fundamentally alter cell behavior (34). We found that tubule PTEN is suppressed when TGF-β signaling is persistently elevated and investigated its role in tubule repair. Our findings suggest that PTEN deficiency interferes with physiological recovery of tubules regenerating after injury and gives rise to an abnormal epithelial phenotype. The defective tubules that result are growth arrested, but they remain undifferentiated and retain proliferative signaling that is dysfunctionally profibrotic because it is persistent.
METHODS
Animals.
Male Sprague-Dawley rats were subjected to 45-min ischemia-reperfusion injury (IRI) of left kidneys and right nephrectomy (14). After 4 h, 60 mg/kg of SD208 in 1% methylcellulose or vehicle were given orally, twice daily for 4 days. SD208 (SCIOS) is a TGF-β receptor antagonist (18). Unilateral IRI (UIRI) was produced in 250- to 300-g male Sprague-Dawley rats under pentobarbital sodium anesthesia at 37°C. Left renal artery was clamped (aneurysm clips, Roboz instruments) for 40 min. Right kidney was untouched. Controls received sham surgery. Rats were killed after 2 and 4 wk of reperfusion (2 or 4 wk of UIRI). In another group, abdomen was opened 2 wk after UIRI and right kidney was removed. After 2 more wk, these rats were killed (4 wk left kidney UIRI with right nephrectomy at 2 wk).
Maleate AKI (48) was produced in ∼250-g male Sprague-Dawley rats. A 110-mg/ml solution of maleic acid disodium salt (Sigma) in saline neutralized to pH 7.4 was injected subcutaneously at 1,100 mg/kg body wt. Controls received saline. A dose of 600 mg/kg produced AKI with PT necrosis in the outer stripe of outer medulla (OSOM) as reported for mice (48), but this damage was fully repaired. To study injury with incomplete repair, we used more maleate. Serum creatinine was measured by HPLC (47).
Mice with Pax8-rtTA/tet-o-TGF-β1 and Pax8-rtTA/Ptet-TGF-β1 transgenes were described (22). Briefly, double transgenes to inducibly express TGF-β in kidney tubules were made by mating Pax8-rtTA mice expressing reverse tetracycline-dependent transactivator (rtTA) controlled by a mouse Pax8 promoter with tet-o-TGF-β1 or Ptet-TGF-β1 mice expressing constitutively active TGF-β1. Inclusion of 0.2 mg/ml of doxycycline (DOX) with 5% sucrose in drinking water resulted in tubule-specific TGF-β induction (22). We obtained tissue from mice with DOX induction for 2, 3, or 4 days or over 6 cycles of DOX for 2 and 5 days without DOX. Tissues from mice with tubule-specific TGF-β1 induction were compared with DOX-treated nonresponsive controls and with responsive mice without DOX. TGF-β production by tubules resulted acutely in epithelial dedifferentiation and degeneration and early proliferation of peritubular fibroblasts, and autophagic tubule death and fibrosis after multiple cycles of induction (22).
Human biopsies.
Research on formalin-fixed tissues in paraffin blocks received IRB approval (HSC20110282H). Pathology reports during 2009–2010 were reviewed and biopsies were chosen for diagnoses of moderate or severe TIF and either normal kidney or normal kidney with minimal fibrosis. Hematoxylin and eosin (H&E)- and periodic acid Schiff-stained slides were examined and cases were screened for tissue adequacy and preservation. Biopsies with TIF included diagnoses of lupus nephritis, focal and segmental glomerulosclerosis, diabetic nephropathy, IgA nephropathy, and C-ANCA-related glomerulonephritis. Biopsies with normal tissue or minimal fibrosis included cases of minimal change disease.
Antibodies and reagents.
Antibody sources were PTEN (clones 138G6 and D4.3), Akt, p-Akt S473, p-Smad2 (S465/467), phospho-JNK (Thr183/Tyr185), c-Jun (60A8), phospho-c-Jun (Ser63) (54B3), S6 ribosomal protein, phospho-S6 (S235/236) ribosomal protein (Cell Signaling, Danvers, MA); JNK (R&D Systems, Minneapolis, MN); PTEN clone 6H2.1 (Cascade Bioscience, Winchester, MA); Smad2/3, (BD, San Diego, CA); Na+-K+-ATPase α-subunit (DSHB, Iowa City, IA); α-smooth muscle actin (SMA; Thermo-Fisher, Waltham, MA); CD10/neprilysin/neutral endopeptidase (NEP; NovoCastra, Newcastle upon Tyne, UK); Ksp cadherin, keratin clones AE1/AE3, secondary antibodies conjugated with Alexa dyes 488, 568, and 647 (Invitrogen, Carlsbad, CA); vimentin clone V9, Ki67 clone SP6 (Thermo-Fisher, Waltham, MA); villin (Millipore, Billerica, MA); PDGF-B (PGF007) (Mochida Pharmaceuticals); PDGFR-β, CTGF (Santa Cruz Biotechnology, Santa Cruz, CA); collagen type I (Millipore); collagen type III (Abcam, Cambridge, MA); GAPDH (Rockland Immunochemicals, Gilbertsville, PA); ImmPRESS horseradish peroxidase (HRP) polymer-conjugated secondary antibodies and ImmPACT DAB reagent (Vector Labs, Burlingame, CA), and HRP-labeled secondary antibodies (Jackson ImmunoResearch, West Grove, PA). Meprin HMC14 antibody was from Judith Bond and John Bylander.
Sources of other reagents were PHAE-HRP and PHAE-AP lectin conjugates (EY Laboratories, San Mateo, CA); MACH 2 mouse and rabbit AP-Polymer Detection systems and Warp Red Chromogen (Biocare Medical, Concord, CA); SD-208 (2-[5-chloro-2-fluorophenyl]pteridin-4-yl)pyridin-4-yl amine (SCIOS, now subsidiary of Johnson and Johnson, New Brunswick, NJ); adenoviral vectors AdCreM2 and AdfloxLacZ1 (Microbix Biosystems, Mississauga, Ontario, Canada); recombinant human TGF-β1 (R&D Systems); SB431542, maleic acid disodium salt (Sigma, St. Louis, MO).
Morphology and immunohistochemistry.
Rat kidneys were perfusion fixed with periodic acid-lysine-paraformaldehyde (PLP) (14). Mouse kidneys were perfusion fixed with 3% paraformaldehyde (PFA) in PBS or 3% PFA, 0.05% picric acid in cacodylate buffer/sucrose with 10% hydroxyethylstarch in saline (22). After antigen retrieval in 99°C 1 mM Tris-EDTA for 20–30 min, deparaffinized sections were blocked with 2.5% horse serum before immunohistochemistry (IHC) with primary antibodies followed by ImmPRESS HRP polymer-conjugated secondary antibodies (14). For AP polymer-conjugated PHAE lectin and secondary antibodies, Mach2 system with Warp Red chromogen was used. PTEN staining intensities were semiquanitatively assessed by stereology. For immunofluorescence (IF), deparaffinized sections with antigen retrieval or cryosections of fixed tissue permeabilized with 0.5% SDS were used. Sections were examined by confocal fluorescence microscopy. TIF was graded 0–5 after Masson Trichrome staining. PLP-fixed tissue was postfixed in 2% glutaraldehyde in 0.1 M Na cacodylate buffer pH 7.2 and 1% OsO4 and processed for electron microscopy (EM) (14).
Western blotting.
Cortex and OSOM were dissected, frozen, and ground in liquid nitrogen and extracted with 4× SDS Laemmli buffer. Protein loading was assessed by densitometry of Coomassie Blue-stained gels and loading controls. Cultured cells were extracted with 1× SDS buffer. SDS-PAGE and Western blotting were described (14). PTEN was quantitated using IRDye 800-conjugated secondary antibodies (Rockland) and the Odyssey system (LI-COR).
Growth, wounding, and PTEN knockout of cultured PT cells.
BUMPT cells (36) were grown (14) and wounded as described (24). Female PTENloxP/loxP mice (38) were bred with a male “Immortomouse” (Charles River) to get transgenes for T-antigen and either heterozygous PTEN+/loxP or homozygous PTENloxP/loxP. PT primary cultures from these mice were generated as described (14) and clonal lines were developed. We used primary cultures from kidneys of PTENloxP/loxP mice without T-antigen or PTEN floxed mice with T-antigen and a PTEN+/loxP cell line expressing T-antigen minimally at 37°C. For PTEN knockout studies, PT primary cultures from Floxed mice or clonal PTEN+/loxP cells were infected with adenoviruses with control LacZ or Cre recombinase (Ad-LacZ; Ad-CreM2; Microbix Inc) at 2 or 4 MOI.
Statistics.
Tests were performed using paired Student's t-tests and by ANOVA. Comparisons were considered significant for P values <0.05.
RESULTS
EM identifies a distinctive PT cell phenotype that fails to differentiate during recovery from IRI.
Left kidneys of rats were subjected to 45-min IRI with right nephrectomy. Treatment with TGF-β receptor antagonist SD208 did not affect AKI severity (14). After 7 days of IRI, the OSOM of kidneys was similarly affected in rats without or with SD208-dilated tubules lined by flat undifferentiated epithelium with Ki67 proliferation indexes marginally above those seen in rats without IRI (not shown). After 14 days of IRI without SD208, the OSOM and to a lesser degree the cortex showed focal TIF. Tubules associated with fibrosis continued to exhibit flat epithelium without brush border (Figs. 9 and 10 of Ref. 14). Such abnormal tubules were fewer than expected from widespread damage seen by 1–3 days of ischemia and the proliferative response thereafter (not shown). We surmised that one set of previously damaged tubules had recovered normal structure even without SD208, whereas another population had been defectively repaired. In contrast, most tubules in the SD208 group at 14 days were normal and fibrosis was mild (illustrated in Figs. 9 and 10 of Ref. 14).
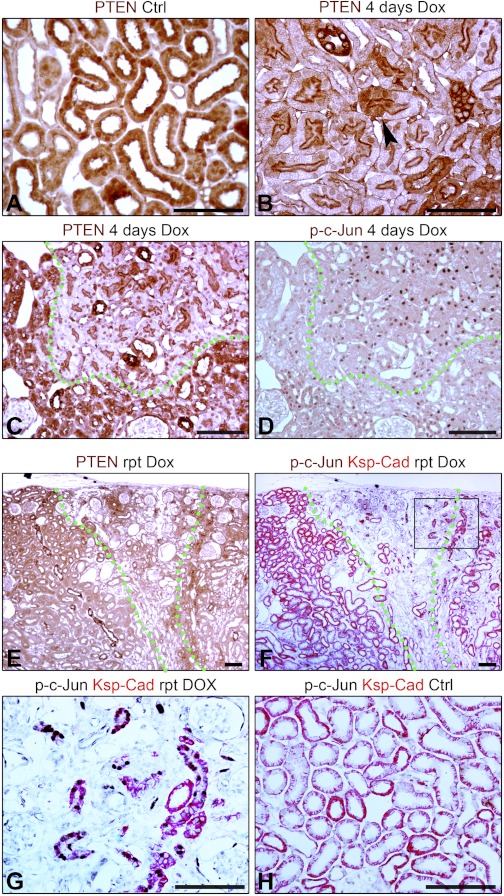
Fig. 9.
A and B: IHC for PTEN in kidneys of control mouse (Ctrl) and Pax8-rtTA/tet-o-TGF-β1 mouse with doxycycline (Dox) for 4 days. Arrowhead: sharp transition between cells with high and low PTEN. C and D: serial sections of kidney from Pax8-rtTA/tet-o-TGF-β1 mouse with Dox for 4 days showing IHC for PTEN or phospho-c-Jun. E and F: serial sections of kidney from Pax8-rtTA/tet-o-TGF-β1 mouse with Dox treatment over 6 repeated cycles (rpt) showing IHC for PTEN (E) or 2-color IHC for ksp-cadherin and phospho-c-Jun (F). PTEN-positive tubules show differentiation marker ksp-cadherin. Undifferentiated, atrophic tubules with less ksp-cadherin have p-c-Jun in nuclei. G: higher magnification of F. Atrophic tubules show phospho-c-Jun in nuclei. H: two-color IHC for ksp-cadherin and phospho-c-Jun in kidney from control mouse. Scale bars = 100 μm.
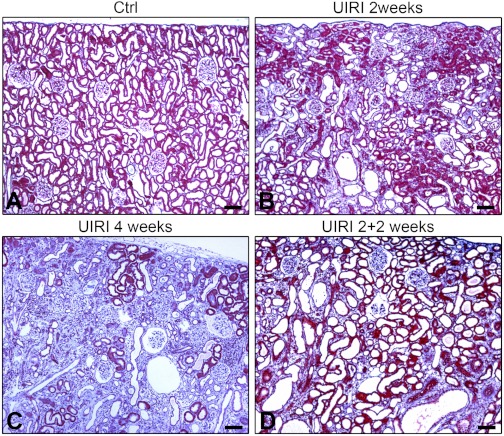
Fig. 10.
A, B, C, and D: Masson Trichrome stain of sections from control right kidney after 2 wk (A), and unilateral IRI (UIRI) left kidneys after 2 wk (B), 4 wk (C), or 4 wk with contralateral nephrectomy that was done 2 wk after ischemia (D). Scale bars = 100 μm.
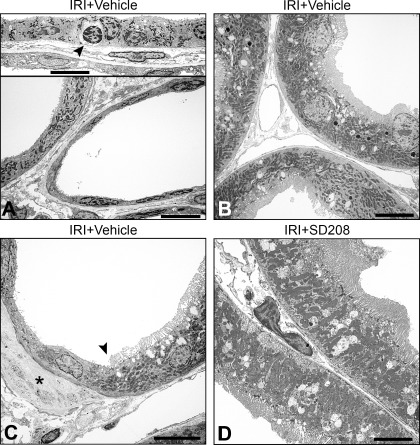
By EM, tubules in fibrotic foci at 14 days were variably dilated and exhibited undifferentiated epithelium with occasional apoptotic nuclei (Fig. 1A). In contrast, tubules close to but outside the TIF foci showed differentiated epithelium (Fig. 1B). We also noted “mosaic” profiles of juxtaposed undifferentiated and differentiated PT epithelium within the same tubule (arrowhead, Fig. 1C). Patches of undifferentiated epithelium but not normal cells in such mosaic tubules were associated with fibrosis (Fig. 1C, *). Thus, there was dichotomy of fate with respect to differentiation not only between populations of tubules but also between cells in the same tubule. Location, ultrastructural features, and IHC markers distinguished these mosaic tubules from transitions of PT to descending thin limbs. Similar criteria showed that the majority of “atrophic” tubules associated with fibrosis were of PT origin. In kidneys of SD208-treated rats, most tubules were normal (Fig. 1D).
Fig. 1.
Electron micrographs of 14-day ischemia-reperfusion injury (IRI) kidneys from rats with vehicle only (A, B, C) or SD208 (D). A: tubules showing flat epithelium with few microvilli and expanded interstitium containing fibroblasts. One cell is apoptotic (inset, arrowhead). B: normal proximal tubule (PT) architecture in tubules adjacent to an area of tubulointerstitial fibrosis (TIF). C: sharp transition (arrowhead) of nearly normal PT cell to undifferentiated epithelium. Interstitial fibrosis (*) abuts “atrophic” but not normal epithelium. D: normal PT architecture in SD208-treated rat. Scale bars = 10 μm.
Regenerating PT that fail to redifferentiate during repair of IRI have low PTEN, high-PI3K signaling downstream of PTEN, and express vimentin.
Consistent with previous work (14, 37), we found increased Smad2 phosphorylation (S465/467) in kidney extracts 2 days after IRI as well as increase of Smad2 protein. Then, instead of decreasing during recovery, signals for phospho-Smad2 and Smad2 continued to increase, as TIF developed (Fig. 2A). By IF, the increased Smad2 localized to cytoplasm and nuclei of undifferentiated tubules in areas of TIF, but not of adjacent differentiated PT (not shown). We screened for events that might mediate the TGF-β-induced tubule phenotype. By Western blotting, PTEN decreased early after IRI but rebounded by 2 days and increased to supernormal levels through 7 and 14 days (Fig. 2A). Phosphorylated Akt increased simultaneously (Fig. 2A), an unexpected finding in cells with high PTEN. Suspecting that the signals were averages of different cell populations, we examined PTEN by IHC.
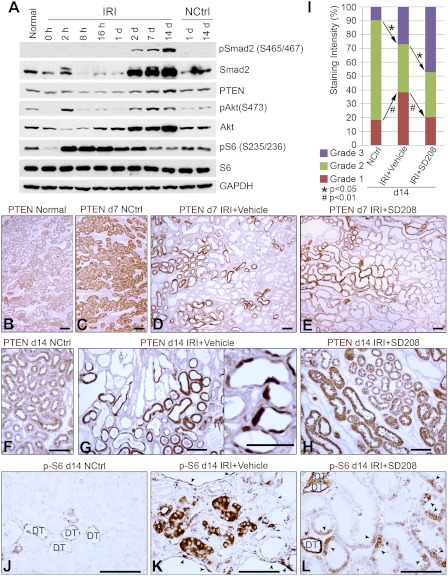
Fig. 2.
A: Western blots of IRI kidneys and nonischemic controls probed for Smad2 and phospho-Smad2 (pSmad2), PTEN, Akt and phospho-Akt (pAkt), S6 protein and phospho-S6 protein (pS6). B, C, D, and E: immunohistochemistry (IHC) for PTEN in normal (B), 7-day nephrectomized control (C), vehicle-treated 7-day IRI (D), and SD208-treated 7-day IRI (E) kidneys. Assessed after 7 days of IRI, PTEN staining patterns were similar, as were the histopathological appearances by hematoxylin and eosin (H&E) stain (not shown), despite vehicle or SD208 treatment. In the PTEN-positive population of tubules from vehicle (D)- or SD208 (E)-treated kidneys, the staining intensity of most tubules is greater than that of tubules in normal (B) or nephrectomized control (C) groups. F: PTEN IHC, 14-day control kidney. G: PTEN IHC, 14-day IRI kidney (vehicle-treated) showing PTEN-positive and -negative tubules. Inset: sharp transitions of PTEN-positive and -negative cells within individual tubules. H: PTEN IHC, 14-day IRI kidney (SD208-treated). Tubules that recovered normal structure in IRI groups showed more intense PTEN reactivity than controls. I: proportions of tubule cell mass with PTEN staining intensities seen most commonly in controls (grade 2), little or no staining (grade 1), or more intense staining (grade 3), in groups (n = 7) of control and vehicle or SD208-treated IRI kidneys. J: phospho-S6 (pS6) IHC, 14-day control kidney. Distal nephron segments (DT) show normal baseline staining. K: pS6 IHC, 14-day IRI (vehicle treatment). pS6 reactivity present in tubules as well as individual cells (arrowheads) of dilated tubules in area of fibrosis. L: pS6 IHC, 14-day IRI (SD208 treatment). In addition to baseline distal nephron (DT) staining for pS6, there is pS6 staining in PT also (arrowheads), but decreased compared with K. Scale bars = 50 μm.
PTEN localized to PT cytoplasm and nuclei before IRI and declined in most tubules by 1–3 days of IRI (not shown). By 7 days, there were two populations of tubules—one, lined by regenerating epithelium with low or absent PTEN in both vehicle-treated and SD208-treated rats—and the other with normal structure but increased PTEN staining relative to controls; the relative numbers of PTEN-deficient tubules and PTEN-replete tubules were similar between vehicle-treated and SD208 groups (Fig. 2, D and E). By 14 days, IRI kidneys from vehicle-treated rats continued to show two populations of PT (Fig. 2G). One set—tubules in foci of TIF—had little or no PTEN. The other set—between TIF foci—showed normal or more intense PTEN staining compared with tubules of control kidneys. There were sharp transitions of PT cells with intense PTEN staining to epithelium with little PTEN (Fig. 2G, inset), corresponding to “mosaic” tubules seen by EM (Fig. 1C).
We used stereology to measure tubule mass with low, normal, or high PTEN in the OSOM of kidneys. In normal kidneys, the distal nephron stained faintly for PTEN. Therefore, in controls, ∼18% tubule mass corresponding to thick limbs, distal tubules, and collecting ducts received low scores of 1, reflecting staining intensities below that seen in normal PT (Fig. 2I). In contrast, the proportion with little PTEN and a score of 1 was ∼38% in 2-wk IRI kidneys treated with vehicle only, accounting for additional PT mass with little PTEN. Notably, the proportion with normal PTEN (score of 2) declined, and the fraction with increased staining (score of 3) was elevated, in IRI kidneys (Fig. 2I). In IRI kidneys protected by SD208, the fraction with low-PTEN staining reverted back to control values, while that with high PTEN increased (Fig. 2I).
Two weeks after IRI, tubules in foci of TIF showed increased staining for phospho-Akt (S473; not shown), reflecting the persistently increased signal seen by Western blotting (Fig. 2A). The IHC signal for phospho-S6 (S235/236), a reporter for Akt-mTOR activation, also remained increased in tubules associated with fibrosis 2 wk after IRI (Fig. 2K), although the averaged signal by Western blotting had reverted back to control levels (Fig. 2A). Increased phospho-S6 in “atrophic” PT with low PTEN is a surprising finding, because Akt-mTOR signaling is associated with hyperplasia and hypertrophy and not with “atrophy” that better describes the PTEN-depleted epithelium. SD208 largely reversed the PTEN depletion and phospho-S6 increase seen 2 wk after IRI (Fig. 2L).
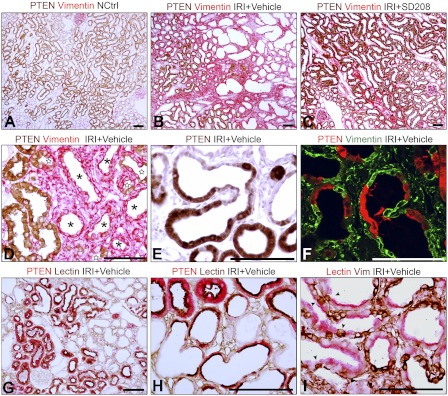
Because PTEN-deficient tubules failed to recover normal structure after AKI, we surmised that they would express vimentin, a marker for dedifferentiation in regenerating PT, that becomes suppressed again as tubules redifferentiate (15, 39, 43, 44). Atrophic tubules in chronic kidney disease (CKD) also express vimentin (15). Tubules with PTEN lacked vimentin, and tubules associated with fibrosis showed vimentin but not PTEN (Fig. 3, B, D, F). Vimentin-positive tubules coexpressed the epithelial marker keratin (not shown) as reported by Grone et al. (15). Tubules with PTEN expressed differentiation markers: for the brush border, PHA-lectin (Fig. 3, G and H), meprin and villin (not shown) and for basolateral membranes, ksp-cadherin and Na+-K+-ATPase (not shown). The association of PTEN and brush-border staining and reciprocal expression of PTEN and vimentin in differentiated and undifferentiated cells were observed in mosaic tubules as well (Fig. 3, F, G, H, I). Indeed, single vimentin-positive undifferentiated cells without PTEN were often found within PTEN-positive, vimentin-negative differentiated epithelium, and vice versa (Fig. 4). These mutually exclusive patterns of PTEN or vimentin expression and association of PHA-lectin binding with PTEN but not vimentin suggested that epithelial fate during repair had been decided at the single cell level. PTEN-deficient vimentin staining tubules were surrounded by fibroblasts expressing vimentin (Fig. 3, B, D, F), type I and type III collagen (not shown), and (myo)fibroblast markers α-SMA and PDGFR-β (not shown). These tubular and interstitial repair responses were ameliorated by SD208 (Fig. 3C). Undifferentiated “atrophic” tubules also expressed PDGF-B and CTGF, fibrogenic peptides that are vicariously produced by tubules in other models of fibrosis (32, 39) (data not shown; but illustrated for PDGF-B in Fig. 7 in Ref. 41).
Fig. 3.
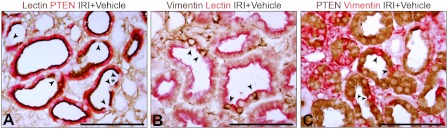
A–D: PTEN and vimentin 2-color IHC in kidneys of 14-day control (A), 14-day IRI (vehicle-treated; B, D), and 14-day IRI (SD208-treated) rats (C). D: epithelium of PTEN-negative tubules (*), some epithelial cells of “mosaic” tubules (stars), and fibroblasts stain for vimentin. E: PTEN IHC in 14-day IRI kidney (vehicle treatment). F: by 2-color confocal immunofluorescence (IF; 0.1-μm optical section) in 14-day IRI kidney (vehicle treatment) staining was “either or” for PTEN or vimentin. Interstitial fibroblasts stain for vimentin (also in B, C, D). G and H: PTEN and PHA lectin 2-color IHC in 14-day IRI kidney (vehicle treatment). Presence of PTEN coincides with PHA lectin staining in brush border; cells without PTEN lack brush border. I: PHA lectin and vimentin 2-color IHC in 14-day IRI kidney (vehicle treatment). Arrowheads mark transitions of lectin-positive differentiated cells without vimentin and lectin-negative cells with vimentin (predicted to lack PTEN, as per D and F). Scale bars = 100 μm.
Fig. 4.
Two-color IHC of 14-day IRI kidneys (vehicle treatment) showing PHA lectin and PTEN (A), vimentin and PHA lectin (B), and PTEN and vimentin (C). Phenotype preservation or transformation to PTEN deficiency and failed differentiation occur in individual cells (arrowheads; *). Scale bars = 100 μm.
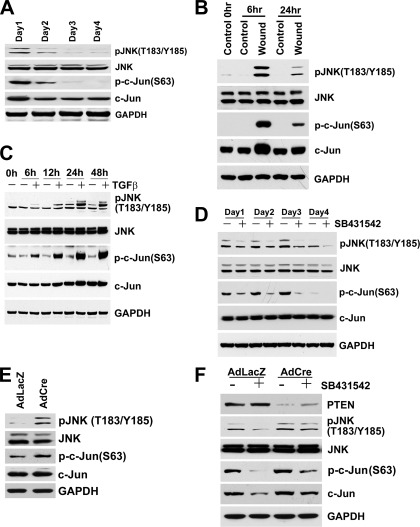
Fig. 7.
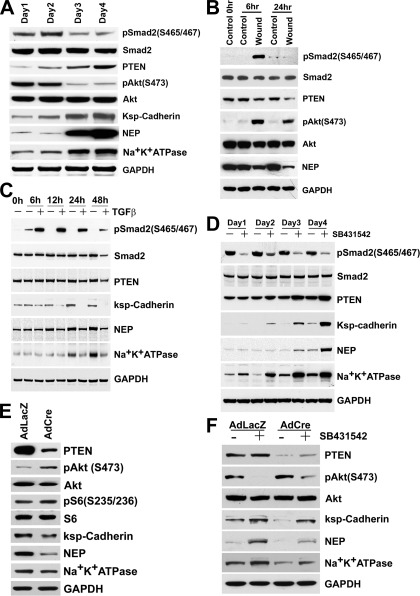
Western blots from cultured cells. A: BUMPT cells grown to confluence over 4 days. In this model, as contact inhibition occurs (3–4 days), autocrine TGF-β becomes suppressed and cells differentiate (14). As phospho-Smad2 (p-Smad2) decreases and ksp-cadherin, neutral endopeptidase (NEP), and Na+-K+-ATPase increase, PTEN increases ∼3-fold and phospho-Akt is diminished. Cell extracts used for Western blotting are from the experiment in Fig. 1B of Geng et al. (14). (PTEN was quantitated using IRDye 800-labeled secondary antibodies in the LI-COR Odyssey system.) B: confluent BUMPT cultures were wounded to remove ∼90% cells and studied 6 and 24 h later. Regeneration is accompanied by increased TGF-β signaling and dedifferentiation (14). PTEN declines and phospho-Akt increases during this period; concurrently, differentiation marker NEP is decreased. C: BUMPT cells were cultured with 2 ng/ml TGF-β for 48 h. D: BUMPT cells at very low density were grown with 1 μM SB431542 or vehicle. With or without TGF-β antagonist, cells were proliferating and remained subconfluent over 4 days, but PTEN increased and cells differentiated. Cell extracts used for Western blotting are from the experiment shown in Fig. 5 of Geng et al. (14). E: confluent PTEN+/loxP PT cell cultures were infected with 2 MOI AdfloxLacZ or AdCreM2 and studied after 3 days. F: subconfluent PTEN+/loxP PT cell cultures were infected with 2 MOI AdfloxLacZ or AdCreM2 and concurrently grown with 2 μM SB431542 or vehicle for 2 days.
PTEN-deficient tubules exhibit persistently increased JNK signaling during fibrosis after ischemic AKI.
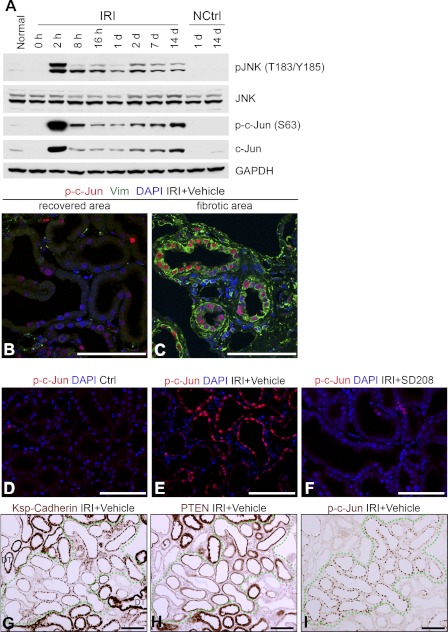
Repair of kidney injury is associated with phlogistic and profibrotic signaling by c-Jun N-terminal kinase (JNK) (10, 26, 27, 45) and JNK inhibition decreases TIF after ischemia (10, 45). JNK and c-Jun were activated by 2 h of IRI (Fig. 5A) consistent with earlier work (33). These early increases of JNK and c-Jun activation subsided over 24 h but did not return to basal levels, and they remained persistently increased over 2 wk (Fig. 5A). Because repair of IRI would require not only the reconstitution of tubule structure and function but also the return to normalcy of regenerative signaling, we suspected that undifferentiated “atrophic” tubules were the seat of persistent JNK signaling. Accordingly, nuclear phospho-c-Jun was present in vimentin-positive tubules associated with fibrosis but not in vimentin-negative tubules 2 wk after IRI (Fig. 5, B and C), and significantly decreased in tubules from kidneys protected by SD208 (Fig. 5, E and F). Furthermore, PTEN-depleted undifferentiated tubules but not PTEN-replete differentiated tubules exhibited nuclear phospho-c-Jun (Fig. 5, G, H, I).
Fig. 5.
A: Western blots of IRI kidneys and controls probed for phospho-JNK (pJNK), JNK, phospho-c-Jun (p-c-Jun), and c-Jun. B and C: two-color IF for phospho-c-Jun and vimentin in area with tubules that recovered normal structure (B) and fibrotic area with defective tubules (C). Nuclear phospho-c-Jun associates with epithelial vimentin staining. Interstitial fibroblasts also express vimentin. D, E, and F: nuclear phospho-c-Jun in 14-day control (D), 14-day IRI kidney treated with vehicle (E), and 14-day IRI kidney treated with SD208 (F). G, H, and I: IHC for ksp-cadherin, PTEN, and phospho-c-Jun in serial sections of 14-day IRI kidney (vehicle-treated). Nuclear phospho-c-Jun is present only in tubules with diminished PTEN and ksp-cadherin. Scale bars = 100 μm.
Selective tubule damage is sufficient to produce the PTEN-depleted JNK signaling epithelial phenotype associated with fibrosis.
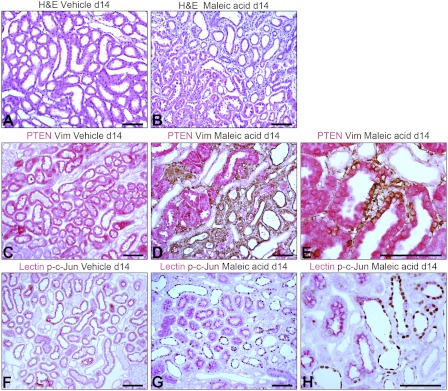
Ischemic injury of nontubule cells could produce fibrosis that secondarily causes tubule atrophy. Therefore, we investigated whether selective epithelial damage leads to the PTEN-depleted tubule phenotype and TIF. To this end, we used the maleate AKI model. Owing to transporter abundance, maleate enters PT cells in large amounts relative to other cells, compromises mitochondrial metabolism, and produces selective PT injury (48). Injection of maleate produced AKI. Serum creatinine before maleate (mg/dl, means ± SE) was 0.26 ± 0.04, rising to 1.54 ± 0.33 after 2 days and subsiding to 0.38 ± 0.10 by day 14 (n = 6, P < 0.005 for days 0 and 14 vs. day 2). Corresponding values in saline controls were 0.19 ± 0.05, 0.19 ± 0.03, and 0.17 ± 0.02 (n = 5). Two days after maleate, we found PT cell death, extensive in the OSOM and focal in the cortex (not shown). After 7 days, tubules showed regenerative changes with interstitial hypercellularity. After 14 days, most tubules had recovered normal structure but there was focal TIF, indistinguishable from that seen after IRI (Fig. 6, A and B). By IHC, PTEN was depleted or undetectable in vimentin-positive tubules of TIF foci, in contrast to adjacent PTEN-positive tubules with normal structure (Fig. 6, D and E). Such undifferentiated tubules displayed nuclear phospho-c-Jun (Fig. 6, G and H).
Fig. 6.
A and B: H&E-stained sections of kidneys from rats 14 days after control saline (A) or disodium maleate (B) injections. TIF after maleate AKI is indistinguishable from that which develops after ischemic AKI. C, D, and E: two-color IHC for PTEN and vimentin (Vim) in control (C) and 14-day maleate AKI kidneys (D and E). Entire tubules (D) and portions of tubules or single cells (E) show phenotype conversion. F, G, and H: PHA lectin and phospho-c-Jun 2-color IHC in control (F) and maleate AKI kidneys (G and H). Alterations exactly parallel those seen after IRI (Figs. 3–5). Scale bars = 100 μm.
PTEN expression and epithelial differentiation are coordinately regulated by TGF-β in PT cell cultures.
The maleate model emphasized a primary role for epithelial processes rather than interstitial or vascular factors in defective tubule repair after AKI. However, it remained unclear how this pathology occurs. The close association of PTEN depletion and failed differentiation in tubules with persistently increased signaling by TGF-β and JNK pathways suggested that the phenomena are interconnected. We investigated these connections using cultured PT cells.
Autocrine TGF-β signaling is inhibited by cell density in cultured PT cells—high in growing cells but low in growth-arrested differentiated cultures (14). Revisiting this paradigm (14), we found that PTEN is low in growing undifferentiated cells but increased about threefold in differentiated cultures (Fig. 7A). Conversely, removal of ∼90% confluent cell mass by wounding (24) induced TGF-β signaling, dedifferentiation, and proliferation (14), while PTEN decreased (Fig. 7B). TGF-β signaling in wounded cultures was inhibited by neutralizing antibodies, indicating the nascent production of active TGF-β (14). As wounded cultures regained cell mass and differentiated, TGF-β signaling became suppressed once more while PTEN increased again (not shown). Therefore, PTEN correlated directly with differentiation status and inversely with TGF-β signaling intensity. Together, the data suggested that TGF-β signaling, PTEN, and differentiation are controlled by cell density in an autoregulated loop. We speculated that this homeostatic control is overcome by increased TGF-β. Accordingly, exposure to exogenous TGF-β decreased PTEN and inhibited the expression of differentiation markers Na+-K+-ATPase, ksp-cadherin, and NEP (Fig. 7C). These effects were blocked by TGF-β receptor antagonist SB431542 (not shown). We knew that TGF-β receptor antagonism promotes PT differentiation after IRI and in culture by blocking autocrine effects (14). Therefore, we reexamined the effects of SB431542 on PTEN expression in the absence of exogenous TGF-β. SB431542 increased PTEN, while promoting differentiation (Fig. 7D), effects reproduced by adenoviral expression of Alk5KR, a dominant negative TGF-β type I receptor, and Smad7 (not shown).
Cre-Lox PTEN ablation compromises differentiation; differentiation promoting actions of TGF-β antagonism involve PTEN.
Suspecting that PT differentiation is PTEN dependent, we examined the effects of Cre-Lox PTEN ablation. Ablation of one PTEN allele in PTEN+/loxP cultures by adenoviral Cre vector (Ad-Cre) induced PTEN depletion and activation of Akt and Akt-mTOR reporter ribosomal S6; Ad-LacZ was ineffective (Fig. 7E). At confluence, Ad-LacZ cells showed high expression of differentiation markers, whereas Ad-Cre cells showed diminished expression (Fig. 7E). When PTEN+/loxP cells were infected with Ad-Cre or Ad-LacZ and exposed to SB431542 or vehicle, PTEN increased in Ad-LacZ cells as a result of treatment with SB431542. Cells with Ad-Cre and SB431542 had slightly higher PTEN levels than Ad-Cre cells without the inhibitor, suggesting that PTEN derived from one allele had responded to the drug. Nevertheless, PTEN was markedly depleted in Ad-Cre cells regardless of TGF-β inhibition (Fig. 7F). Increases and decreases of PTEN caused reciprocal changes of Akt activation (Fig. 7F). The opposing actions of Ad-Cre and SB431542 on cells with single floxed or intact PTEN alleles were reflected by corresponding effects on differentiation. Accordingly, Ad-Cre cells with SB431542 showed modestly increased differentiation compared with cells without the inhibitor. However, compared with Ad-Lac-Z cells, PTEN depletion by Ad-Cre inhibited differentiation, both in the presence and absence of SB431542 (Fig. 7F).
Low PTEN is associated with growth arrest of PT cells in culture and in vivo.
Because it is a PIP3 phosphatase, low PTEN should stimulate proliferative signaling by Akt. However, after infection of 0.22 × 106 PTEN+/loxP cells with Ad-LacZ or Ad-Cre (2 MOI each on days 1 and 3), cell numbers on days 3 and 5 were (×106, means ± SD) 0.87 ± 0.13 and 1.13 ± 0.06 for LacZ and 0.70 ± 0.15 and 0.55 ± 0.18 for Cre (n = 3, P < 0.05 on day 5). Therefore, despite Akt activation (Fig. 7E), PTEN-depleted cells became growth arrested. In view of these results, we assessed Ki67 proliferation indexes in tubules after IRI. As expected for a proliferative response to injury, Ki67 labeling increased 3 days after IRI. PTEN was uniformly low by IHC at this time in regenerating PT (not shown). This is analogous to the decrease of PTEN that occurred in cultured PT cells that proliferated in response to loss of cell mass by wounding (Fig. 7B). However, during late stages of repair, when some populations of regenerating tubules differentiated but others did not, proliferation indexes returned to control values. Two weeks after IRI, Ki67-positive nuclei per tubule profile in the OSOM (means ± SE) were IRI −0.13 ± 0.0, n = 8; IRI plus SD208 −0.17 ± 0.0, n = 6; controls −0.24 ± 0.1, n = 5; NS. These values reflected the proliferation status of undifferentiated tubules associated with fibrosis as well as tubules with normal morphology. Therefore, in culture and during tubule repair after IRI, PTEN depletion was associated with growth arrest, and not proliferation. PTEN depletion could induce cellular senescence (1). However, in culture and in vivo, PTEN-depleted PT cells were devoid of senescence markers β-galactosidase and Ink family cdk inhibitors (not shown).
PTEN depletion mediates TGF-β-induced JNK signaling in cultured PT cells.
We found that autocrine JNK signaling activity is regulated by cell density in cultured PT cells, coordinately with TGF-β signaling and PTEN. That is, low-density cells displayed high levels of phosphorylated JNK and c-Jun (Fig. 8A), which corresponded to high autocrine TGF-β signaling (14) and low PTEN (Fig. 7A), whereas JNK and c-Jun phosphorylation became suppressed in high-density growth-arrested cells (Fig. 8A) at the same time that TGF-β signaling was also suppressed (14) and PTEN had increased (Fig. 7A). Moreover, wounding of confluent growth-arrested PT cells resulted not only in cell proliferation, activation of TGF-β signaling (14), and decrease of PTEN (Fig. 7B), but also in large increases of JNK and c-Jun phosphorylation (Fig. 8B). Thus, TGF-β signaling, PTEN, epithelial differentiation, and JNK signaling appeared to be controlled coordinately by cell density-dependent autoregulation. We asked whether TGF-β would deregulate the effects of cell density on JNK activity. JNK and c-Jun phosphorylation increased progressively during prolonged TGF-β treatment (Fig. 8C). Furthermore, antagonism of autocrine TGF-β signaling by SB431542 produced the opposite effect, i.e., suppression of JNK and c-Jun signals (Fig. 8D). Therefore, we asked whether PTEN plays a role in TGF-β-mediated JNK signaling. PTEN depletion by Ad-Cre caused increased JNK and c-Jun phosphorylation (Fig. 8E). Furthermore, SB431542 decreased the phosphorylation of JNK and c-Jun in Ad-LacZ cells with intact PTEN, but this suppressive effect of TGF-β antagonism was blunted in PTEN-depleted Ad-Cre cells (Fig. 8F). We examined whether the effects of PTEN depletion on JNK were mediated through TGF-β production. JNK activation may involve TAK1, a TGF-β-activated kinase (28). However, TGF-β expression was not increased in PTEN-depleted cells (not shown). Therefore, low PTEN was sufficient, in itself, to cause JNK activation.
Fig. 8.
Western blots from cultured cells. A: BUMPT cells grown to confluence over 4 days. With contact inhibition (3–4 days), autocrine TGF-β decreases and cells differentiate (14) (see Fig. 7A). Concomitantly, phosphorylation of JNK and c-Jun is suppressed. B: confluent BUMPT cultures wounded to remove ∼90% cells and studied 6 and 24 h later. Regeneration is accompanied by increased TGF-β signaling and dedifferentiation (14) (see Fig. 7B), and it increased phosphorylation of JNK and c-Jun. C: BUMPT cells were cultured with 2 ng/ml TGF-β for 48 h; TGF-β increases JNK and c-Jun phosphorylation. D: BUMPT cells were grown at very low density with 1 μM TGF-β receptor antagonist SB431542 or vehicle for 4 days. Increase of PTEN and accelerated differentiation caused by SB431542 (Fig. 7D) are accompanied by diminished phosphorylation of JNK and c-Jun. E: confluent PTEN+/loxP PT cell cultures were infected with 2 MOI AdfloxLacZ or AdCreM2 and studied after 3 days. Decreased PTEN (Fig. 7E) is accompanied by increased JNK and c-Jun phosphorylation. F: subconfluent PTEN+/loxP PT cell cultures were infected with 2 MOI AdfloxLacZ or AdCreM2 and concurrently grown with 2 μM SB431542 or vehicle for 2 days. PTEN is decreased by Ad-Cre regardless of SB431542 treatment, and this is accompanied by enhanced JNK and c-Jun phosphorylation.
Fibrosis after AKI may be related to TGF-β and CTGF secretion stimulated by JNK signaling in tubules undergoing pathological growth arrest during repair (45). In our study, undifferentiated tubules located in fibrotic areas showed increased expression of PDGF-B and CTGF by IHC (not shown; but illustrated for PDGF-B in Fig. 7 of Ref. 41). To investigate whether PTEN loss leads to TGF-β, PDGF-B, or CTGF expression, we measured mRNA by real-time PCR in cultured PT cells. mRNA for the peptides did not increase in cells with Cre-Lox PTEN depletion (not shown). Thus, PTEN deficiency did cause growth arrest–and could trigger JNK signaling in cultured PT cells, but this alteration was insufficient, in itself, to augment TGF-β, PDGF-B, or CTGF expression. Therefore, it seems unlikely that the expression of fibrogenic peptides in the dysfunctional tubules that develop after AKI can be explained by PTEN depletion alone.
Tubule-specific induction of TGF-β reproduces the PTEN-depleted high-JNK signaling epithelial phenotype.
We used double transgenic mice (Pax8-rtTA/tetO-TGF-β1) in which the Pax8-promoter directs expression of the rtTA specifically to renal tubular epithelial cells. Upon doxycycline induction, rtTA binds and transactivates a tetracycline-dependent promoter and triggers expression of the effector gene TGF-β1, producing epithelial degeneration and TIF (22). We examined tissue by IHC. By 4 days of TGF-β induction, PTEN decreased in PT epithelium (Fig. 9B). Tubules with PTEN loss (Fig. 9C) displayed increased JNK/c-Jun signaling (Fig. 9D). TIF develops during the chronic phase of TGF-β induction in these kidneys (22). Tubules in fibrotic foci displayed undifferentiated epithelium contrasting with adjacent normal PT. PTEN was decreased in undifferentiated tubules of fibrotic foci (Fig. 9, E and F). Tubules with low PTEN in fibrotic areas showed nuclear phospho-c-Jun (Fig. 9G). These data show that sustained TGF-β signaling decreases tubule PTEN and activates JNK signaling in vivo and that these alterations precede tubule dedifferentiation, tubule degeneration, and fibrosis.
PTEN-depleted tubule phenotype associated with fibrosis is potentially reversible.
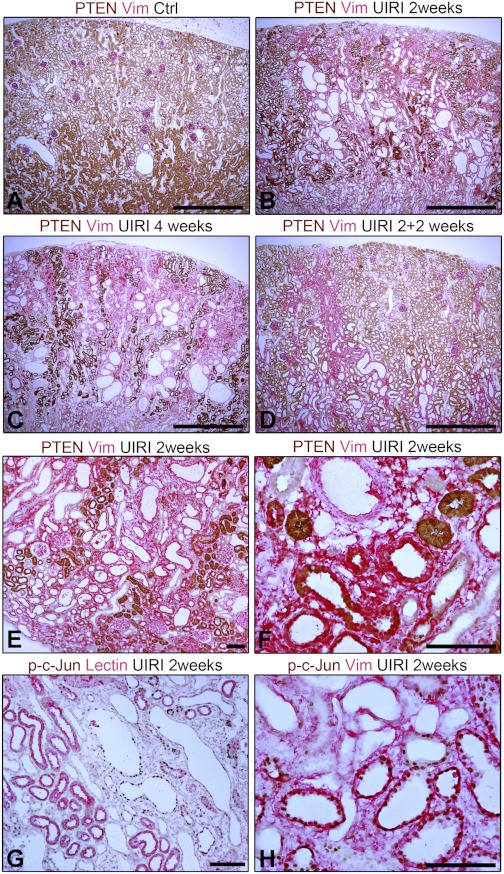
The presence of an intact kidney compromises recovery from UIRI. The ischemic kidney undergoes atrophy over time (12) and the TIF that develops is more severe than in other ischemic models (45). Removal of the intact kidney after prolonged reperfusion decreases chronic damage by recruiting functional nephrons (12, 45). This effect of an intact kidney on chronic injury after UIRI is termed “renal counterbalance” (13). We used the counterbalance model to ask whether failed tubule differentiation with low-PTEN and high-JNK signaling is reversible. Two weeks after UIRI, there was tubule atrophy and TIF in left ischemic kidneys; after 2 more wk, TIF tended to progress further. However, if the right kidney was removed 2 wk after UIRI, TIF after 2 more wk was milder than in UIRI rats with intact right kidneys for either 2 wk or the entire 4-wk period (Fig. 10). Accordingly, TIF grades of 4-wk UIRI kidneys 2 wk after right nephrectomy (means ± SE −1.6 ± 0.3, n = 8) were lower than 2 or 4 wk after UIRI with right kidney intact (means ± SE −3.0 ± 0.4, n = 6 and 3.2 ± 0.5, n = 8; P < 0.05).
PTEN was lost from large numbers of PT 2 wk after UIRI; such tubules showed “atrophic” morphology (Fig. 11B). In contrast to PTEN-replete tubules, PTEN-depleted tubules expressed vimentin, which was present in fibroblasts also (Fig. 11, E and F). These alterations became worse in UIRI rats with intact right kidneys for the entire 4 wk (Fig. 11C), but were ameliorated by right nephrectomy 2 wk after ischemia (Fig. 11D). Tubules devoid of brush border that expressed vimentin but not normally differentiated tubules showed nuclear phospho-c-Jun (Fig. 11, G and H). Although nephrectomy is not therapeutically significant, these results do suggest that PTEN loss and tubule dedifferentiation are potentially correctable. Therefore, the UIRI model may be useful to screen other clinically applicable measures to similarly ameliorate postischemic tubule atrophy.
Fig. 11.
A, B, C, and D: two-color IHC for PTEN and vimentin in control right kidney (A), and UIRI left kidneys after 2 wk (B), 4 wk (C), or 4 wk with contralateral nephrectomy that was done 2 wk after ischemia (D). E and F: two-color IHC for PTEN and vimentin in UIRI kidney after 2 wk. Large numbers of PTEN-deficient tubules stain for vimentin, as also interstitial fibroblasts. G and H: two-color IHC for phospho-c-Jun and differentiation marker PHA lectin (G) or phospho-c-Jun and vimentin in UIRI kidney after 2 wk (H). Scale bars: A–D = 1 mm; E–H = 100 μm.
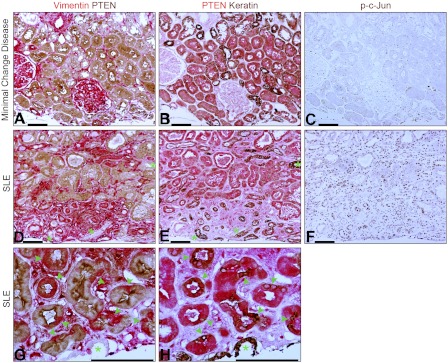
PTEN depletion, failed differentiation, and JNK signaling in tubules are features of TIF in human CKD.
We examined kidney tissue from human patients by IHC (Fig. 12). In biopsies reported to be normal (including minimal change disease), PTEN staining was moderate to strong in PT but weak in the distal nephron (Fig. 12, A and B), similar to findings in normal rats. Vimentin was present in vascular structures and interstitial cells, but not tubules. However, biopsies from diverse chronic diseases showed PTEN loss from tubules associated with fibrosis. PTEN-negative tubules stained for vimentin as well as keratin (Fig. 12, D and E). As in rats after IRI, there were abrupt transitions in PT between cells with PTEN but no vimentin, and cells with vimentin but no PTEN; such transitions involved single cells or small clusters (Fig. 12, G and H). In contrast, distal nephron segments expressed keratin but not vimentin in both normal and diseased kidneys (Fig. 12, A, B, D, E, G, and H; indicated by green * in G and H). Interstitial cells stained for vimentin but not keratin (Fig. 12 D,E). Distinction of nuclear phospho-c-Jun staining between PTEN-positive and PTEN-negative tubules was not as clear in human biopsies as in animal models, perhaps caused by the delay of fixation that biopsies undergo before fixation. Nevertheless, there was a trend for increased phospho-c-Jun staining of tubules in biopsies with TIF compared with those without (Fig. 12, C and F).
Fig. 12.
A, B, and C: serial sections of biopsy from kidney with minimal change disease showing 2-color IHC for PTEN and vimentin (A), PTEN and keratin (B), and phospho-c-Jun (C). Vimentin-positive structures are glomeruli, arteriole, and interstitial cells. Distal nephron segments show more intense reactivity for keratin than PTs. D, E, and F: serial sections of biopsy from kidney with lupus nephritis and TIF showing 2-color IHC for PTEN and vimentin (D), PTEN and keratin (E), or phospho-c-Jun (F). Foci of TIF show vimentin-positive tubules and fibroblasts (D). Most of the vimentin-positive tubules (D) also stain for keratin (E). However, some tubules (distal nephron segments) do not stain for vimentin, but react intensely for keratin (*). G and H: serial sections of kidney with lupus nephritis and TIF showing 2-color IHC for vimentin and PTEN (G) or PTEN and keratin (H); vimentin-negative epithelial staining for keratin is in a collecting duct (*). Note also several abrupt transitions between PTEN-positive, vimentin-negative PT epithelium to vimentin-positive cells staining intensely for keratin (arrowheads). Scale bars = 100 μm.
DISCUSSION
As dead epithelium is shed from injured tubules, surviving cells dedifferentiate, migrate, and proliferate (4). Normal repair requires redifferentiation of epithelium after tubule mass is reconstituted. We show that failure of a subpopulation of regenerating tubules to differentiate is associated with fibrosis after IRI. Tubules with failed differentiation were derived from PT, the most injury-prone nephron segment. We reported previously that wounding of confluent PT cultures triggers TGF-β signaling. The autocrine trigger is provided by wound-induced production of active TGF-β, shown by the effects of neutralizing antibodies (14). Wounding induces dedifferentiation, migration, and proliferation, mimicking tubule regeneration in vivo. When cells reached quiescence at high density, autocrine TGF-β signaling became suppressed, and cells differentiated. Remarkably, when autocrine TGF-β signaling was inhibited in undifferentiated primary cultures of PT, cells grew faster, but concurrently underwent differentiation (14). These beneficial effects of TGF-β receptor antagonism are consistent with known homeostatic functions of the cytokine in epithelia, and they suggested that culture conditions induce autocrine TGF-β signaling at intensities that exceed physiological needs. Analogously, TGF-β signaling activated by IRI in tubules (37) may exceed the threshold for physiological regeneration; and persistently high autocrine TGF-β after IRI could prevent redifferentiation, a notion supported by better epithelial differentiation in vivo produced by a TGF-β antagonist (14). In culture, TGF-β disrupts PT cell junctions (49). However, cell junction integrity is but one aspect of the differentiation repertoire. As shown by data obtained in cultured mouse PT cells and after IRI in rats, TGF-β antagonism promoted the restoration of a broad spectrum of differentiated features—structural, functional, and biochemical—in regenerating PT cells (14). The signaling underpinnings of these effects remained unclear. We now introduce PTEN as one important link between TGF-β signaling and differentiation.
TGF-β regulation of PTEN in the kidney has received little attention. TGF-β decreased the translation of PTEN in mesangial cells through microRNA species 216a and 217 (20). How TGF-β regulates PTEN in PT cells is unexplored. During physiological growth, we found that TGF-β signaling and PTEN were coordinately but inversely regulated. PTEN was low when TGF-β signaling was high during proliferation, but it increased when TGF-β signaling was low in differentiated cultures. Shown by Cre-Lox deletion, PTEN was required to maintain differentiation in PT cultures and was involved in the promotion of differentiation by TGF-β antagonist SB431542. Variations of PTEN in cultured cells were mirrored in vivo. PTEN declined after IRI as surviving cells dedifferentiated and proliferated, and it increased when recovering PT differentiated once more. In contrast, tubules that failed to redifferentiate—the population associated with TIF—did not recover PTEN, became defective, and exhibited phlogistic JNK signaling. Remarkably, adjacent cells of single tubules also exhibited abrupt contrasts of PTEN expression, differentiation, and JNK signaling. Thus, signaling decisions that determined cell fate during repair appeared to have been made at the level of individual cells. This would suggest that defective differentiation was caused by an autocrine signaling disorder. Paracrine modulation may have played a broader role but the images of single normal cells surrounded by defective epithelium and vice versa suggest that autocrine defects were dominant. Paracrine factors secreted by interstitial cells, by themselves, are unlikely to have spared single cells in an epithelial population with such precision. Our experiments with maleate suggest further that epithelial injury dominates in determining tubule fate and subsequent fibrosis. Interstitial damage is not required a priori.
The effects of PTEN deficiency were counterintuitive since Akt signaling caused by PTEN loss should increase, not inhibit, cell proliferation or should induce hypertrophy and not atrophy. Indeed, tissue-specific PTEN knockout induces hypertrophy, hyperplasia, and neoplasia in several tissues (5, 7, 21). Unlike AKI, these models describe effects over long periods that allow for adaptations to genomic instability caused by PTEN loss (35) that may perturb cell growth. Of note, senescent epithelium was found to coexist with hyperplasia or neoplasia in PTEN-null prostate (7) and acute PTEN ablation caused growth arrest and senescence in fibroblasts (1). In our study, heterozygous PTEN ablation produced growth arrest and dedifferentiation of PT cells but did not induce the expression of senescence markers.
The impact of PTEN loss and JNK activation on fibrosis needs consideration in context of other signaling disorders that may be involved. ERK-MAPK is one example. Following rapid activation, phospho-ERK (Thr202/Tyr204) subsided by 2 days of IRI, but subsequently increased during fibrosis (not shown). Phospho-ERK localized to undifferentiated tubules of the PTEN-depleted phenotype in fibrotic tissue but not to normal tubules (not shown). Such tubules also expressed fibrogenic peptides: PDGF-B and CTGF (not shown; but illustrated for PDGF-B in Fig. 7 of Ref. 41). On the other hand, in cultured PT cells, selective PTEN depletion increased JNK activity (Fig. 8E), but not TGF-β, PDGF-B, or CTGF expression (not shown). Nevertheless, JNK inhibition ameliorated fibrosis after IRI (10, 17, 45). By itself, PTEN depletion likely cannot explain TIF and requires cooperation with other TGF-β intermediates. Our data suggest that TGF-β signaling depletes PTEN in tubules. After normal repair, TGF-β signaling is suppressed, PTEN recovers, and tubules differentiate. However, unknown pathological events mitigate PTEN recovery in some tubules and prevent differentiation, allowing for unabated signaling through multiple pathways, including TGF-β. This results in a vicious cycle of signaling and dedifferentiation that perpetuates tubule dysfunction. In this setting, JNK activity alone may be insufficient for fibrosis; cooperation with other signaling intermediates may be necessary. The actions of JNK-MAPK and other MAPKs including ERK form AP-1 complexes of Jun, Fos, and related proteins (19), and transcription by AP-1 is required for TGF-β-mediated fibrosis in other systems (2). However, TGF-β-dependent transcription typically involves interdependent activity of AP-1 and Smads (11, 25, 42, 50), suggesting that AP-1 formed downstream of JNK needs to interact with Smads to cause fibrosis. Induction of PDGF-B and CTGF by TGF-β as well as TGF-β autoinduction are Smad mediated (8, 40, 46), and both CTGF induction and TGF-β autoinduction involve interdependent signaling by JNK/ERK and Smads (8, 46). Moreover, MAPK signaling initiated by epidermal growth factor receptor activity is required for sustained TGF-β expression during angiotensin II-induced renal fibrosis (6). Therefore, we suggest that JNK signaling in PTEN-depleted tubules cooperates with Smad signaling to increase fibrogenic peptide expression. Figure 13 summarizes the interactions of TGF-β, PTEN, and MAPK signaling that we propose may be involved in renal fibrosis after AKI.
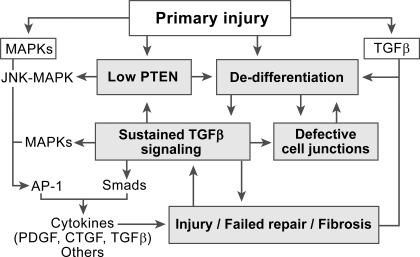
Fig. 13.
Proposed schema of the signaling basis for failed tubule repair and associated fibrosis after acute kidney injury. Following primary injury, there is a loss of PTEN and activation of signaling by MAPKs and TGF-β in surviving tubule epithelium that is transformed to a dedifferentiated, migratory, and proliferating phenotype. If tubules are repaired normally, cessation of proliferation is accompanied by suppression of signaling initiated earlier, recovery of PTEN, and by redifferentiation of epithelium (not shown). However, as depicted here, some tubules fail to redifferentiate despite growth arrest. Cells in these tubules have severe PTEN loss and unabated signaling by TGF-β and MAPKs. We suggest that persistently high TGF-β signaling, sustained PTEN loss, and ensuing failed epithelial differentiation constitute a vicious cycle of abnormalities that maintain a state of defective tubule repair. In this setting of persistent signaling, AP-1 complexes formed by the actions of MAPK (JNK, ERK, others) and Smads activated by TGF-β cooperate to increase the production of cytokines that induce fibrosis in the immediately adjacent interstitial microenvironment.
How PTEN relates to fibrosis after IRI needs to be examined using genetic models of tubule-specific PTEN deletion or gain-of-function inducibly following the initiation of AKI. We do not know how PTEN deficiency prevents tubule redifferentiation in a tubule subpopulation after IRI. However, we suggest that PTEN-depleted epithelium provides a platform for the persistence of signaling that needs to be suppressed after tubule repair. TIF develops as a consequence of this dysfunction. Thus, we may predict that fibrosis after AKI in a model of tubule-specific PTEN deletion will be more severe and that inducible gain of PTEN might “rescue” the kidney from TIF. Such experiments will be technically demanding but feasible.
In summary, we describe findings that explain several aspects of tubule pathology during fibrosis after IRI. Notwithstanding other TGF-β effectors and signaling mediators that operate in parallel, the PTEN step is likely to be a critical part of the mechanism that produces defective tubules. PTEN deficiency possibly relates to TIF development after kidney damage. Similar themes—PTEN loss, dedifferentiation, JNK signaling, and fibrosis—in animal models of AKI and in human CKD—suggest that defective repair of tubules with persistent PTEN loss underlies TIF in diverse contexts. Epithelial PTEN deficiency is closely linked temporally and spatially to interstitial fibrosis. At least in early stages, PTEN loss, tubule pathology, and surrounding fibroblast proliferation are potentially reversible. We believe that PTEN is an important target for TIF research. The growth arrest response to PTEN loss in cultured cells and the paradoxical finding of PTEN deficiency in growth-arrested, dedifferentiated tubules with persistent regenerative signaling after IRI raise fundamental questions regarding the role played by this tumor suppressor in renal disease.
GRANTS
This work was supported by research grants DK37139 (to M. A. Venkatachalam), DK54472 (to P. Saikumar), DK61653 (to K. A. Griffin), DK40426 (to A. K. Bidani) from the National Institutes of Health (NIH), Veterans Affairs Administration (to K. A. Griffin), Deutsche Forschungsgemeinschaft (FOR406 to W. Kriz and R. Koesters) and funds from the Department of Pathology, University of Texas Health Science Center San Antario (UTHSCSA). Anyonya Guntur and Michael Naski kindly provided PTENloxP/loxP mice, originally derived from the laboratory of Tak W. Mak. Confocal images were generated in the Core Optical Imaging Facility, which is supported by UTHSCSA, NIH-National Cancer Institute P30 CA54174 (Cancer Therapy and Research Center at UTHSCSA), and NIH-National Institute of Aging P01AG19316.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.L., H.G., R.K., J.M.W., W.K., and M.A.V. conception and design of research; R.L., H.G., A.J.P., P.K.S., D.G.M., and R.K. performed experiments; R.L., H.G., A.J.P., P.S., K.A.G., R.K., J.M.W., A.K.B., W.K., and M.A.V. analyzed data; R.L., H.G., A.J.P., P.K.S., P.S., K.A.G., R.K., A.K.B., W.K., and M.A.V. interpreted results of experiments; R.L., H.G., and D.G.M. prepared figures; R.L., P.K.S., K.A.G., J.M.W., A.K.B., and M.A.V. edited and revised manuscript; R.L., H.G., A.J.P., P.K.S., P.S., K.A.G., R.K., J.M.W., A.K.B., W.K., and M.A.V. approved final version of manuscript; M.A.V. drafted manuscript.
ACKNOWLEDGMENTS
We thank Hanna Abboud for advice, encouragement, and review of the manuscript.
REFERENCES
- 1. Alimonti A, Nardella C, Chen Z, Clohessy JG, Carracedo A, Trotman LC, Cheng K, Varmeh S, Kozma SC, Thomas G, Rosivatz E, Woscholski R, Cognetti F, Scher HI, Pandolfi PP. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J Clin Invest 120: 681– 693, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Avouac J, Palumbo K, Tomcik M, Zerr P, Dees C, Horn A, Maurer B, Akhmetshina A, Beyer C, Sadowski A, Schneider H, Shiozawa S, Distler O, Schett G, Allanore Y, Distler JHW. Inhibition of AP-1 signaling abrogates TGF-β mediated activation of fibroblasts and prevents experimental fibrosis. Arthritis & Rheumatism [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3. Bielesz B, Sirin Y, Si H, Niranjan T, Gruenwald A, Ahn S, Kato H, Pullman J, Gessler M, Haase VH, Susztak K. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest 120: 4040– 4054, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonventre JV. Pathophysiology of AKI: injury and normal and abnormal repair. Contrib Nephrol 165: 9– 17, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Chen JK, Nagai K, Chen J, Plieth D, Neilson EG, Harris RC. Renal proximal tubule-specific PTEN deletion induces renal hypertrophy by activation of Akt-mTOR signaling pathway (Abstract). J Am Soc Nephrol 18: 28A, 2007 [Google Scholar]
- 6. Chen J, Chen JK, Nagai K, Plieth D, Tan M, Lee TC, Threadgill DW, Neilson EG, Harris RC. EGFR signaling promotes TGFβ-dependent renal fibrosis. J Am Soc Nephrol 23: 215– 224, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, Cordon-Cardo C, Pandolfi PP. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436: 725– 730, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cicha I, Goppelt-Struebe M. Connective tissue growth factor: context-dependent functions and mechanisms of regulation. Biofactors 35: 200– 208, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Cook HT. The origin of renal fibroblasts and progression of kidney disease. Am J Pathol 176: 22– 24, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Borst MH, Prakash J, Sandovici M, Klok PA, Hamming I, Kok RJ, Navis G, van Goor H. c-Jun NH2-terminal kinase is crucially involved in renal tubulo-interstitial inflammation. J Pharmacol Exp Ther 331: 896– 905, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Engel ME, McDonnell MA, Law BK, Moses HL. Interdependent SMAD and JNK signaling in transforming growth factor-beta-mediated transcription. J Biol Chem 274: 37413– 37420, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Finn WF. Enhanced recovery from postischemic acute renal failure. Micropuncture studies in the rat. Circ Res 46: 440– 448, 1980 [DOI] [PubMed] [Google Scholar]
- 13. Finn WF. Renal counterbalance. J Lab Clin Med 105: 523– 530, 1985 [PubMed] [Google Scholar]
- 14. Geng H, Lan R, Wang G, Siddiqi AR, Naski MC, Brooks AI, Barnes JL, Saikumar P, Weinberg JM, Venkatachalam MA. Inhibition of autoregulated TGFbeta signaling simultaneously enhances proliferation and differentiation of kidney epithelium and promotes repair following renal ischemia. Am J Pathol 174: 1291– 1308, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grone HJ, Weber K, Grone E, Helmchen U, Osborn M. Coexpression of keratin and vimentin in damaged and regenerating tubular epithelia of the kidney. Am J Pathol 129: 1– 8, 1987 [PMC free article] [PubMed] [Google Scholar]
- 16. Hill GS. Hypertensive nephrosclerosis. Curr Opin Nephrol Hypertens 17: 266– 270, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Kanellis J, Ma FY, Kandane-Rathnayake R, Dowling JP, Polkinghorne KR, Bennett BL, Friedman GC, Nikolic-Paterson DJ. JNK signalling in human and experimental renal ischaemia/reperfusion injury. Nephrol Dial Transplant 25: 2898– 2908, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Kapoun AM, Gaspar NJ, Wang Y, Damm D, Liu YW, O'Young G, Quon D, Lam A, Munson K, Tran TT, Ma JY, Murphy A, Dugar S, Chakravarty S, Protter AA, Wen FQ, Liu X, Rennard SI, Higgins LS. Transforming growth factor-beta receptor type 1 (TGFbetaRI) kinase activity but not p38 activation is required for TGFbetaRI-induced myofibroblast differentiation and profibrotic gene expression. Mol Pharmacol 70: 518– 531, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. Philos Trans R Soc Lond B Biol Sci 351: 127– 134, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I, Gunn A, Nakagawa Y, Shimano H, Todorov I, Rossi JJ, Natarajan R. TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol 11: 881– 889, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kishimoto H, Hamada K, Saunders M, Backman S, Sasaki T, Nakano T, Mak TW, Suzuki A. Physiological functions of Pten in mouse tissues. Cell Struct Funct 28: 11– 21, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Koesters R, Kaissling B, Lehir M, Picard N, Theilig F, Gebhardt R, Glick AB, Hahnel B, Hosser H, Grone HJ, Kriz W. Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am J Pathol 177: 632– 643, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases–insights from animal models. Kidney Int 67: 404– 419, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Lan R, Geng H, Hwang Y, Mishra P, Skloss WL, Sprague EA, Saikumar P, Venkatachalam MA. A novel wounding device suitable for quantitative biochemical analysis of wound healing and regeneration of cultured epithelium. Wound Repair Regen 18: 159– 167, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liberati NT, Datto MB, Frederick JP, Shen X, Wong C, Rougier-Chapman EM, Wang XF. Smads bind directly to the Jun family of AP-1 transcription factors. Proc Natl Acad Sci USA 96: 4844– 4849, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma FY, Flanc RS, Tesch GH, Han Y, Atkins RC, Bennett BL, Friedman GC, Fan JH, Nikolic-Paterson DJ. A pathogenic role for c-Jun amino-terminal kinase signaling in renal fibrosis and tubular cell apoptosis. J Am Soc Nephrol 18: 472– 484, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Ma FY, Sachchithananthan M, Flanc RS, Nikolic-Paterson DJ. Mitogen activated protein kinases in renal fibrosis. Front Biosci 1: 171– 187, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Ma FY, Tesch GH, Ozols E, Xie M, Schneider MD, Nikolic-Paterson DJ. TGF-β1-activated kinase-1 regulates inflammation and fibrosis in the obstructed kidney. Am J Physiol Renal Physiol 300: F1410– F1421, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20: 1– 17, 1992 [DOI] [PubMed] [Google Scholar]
- 30. Nath KA, Croatt AJ, Haggard JJ, Grande JP. Renal response to repetitive exposure to heme proteins: chronic injury induced by an acute insult. Kidney Int 57: 2423– 2433, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Nath KA, Croatt AJ, Warner GM, Grande JP. Genetic deficiency of Smad3 protects against murine ischemic acute kidney injury. Am J Physiol Renal Physiol 301: F436– F442, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okada H, Kikuta T, Kobayashi T, Inoue T, Kanno Y, Takigawa M, Sugaya T, Kopp JB, Suzuki H. Connective tissue growth factor expressed in tubular epithelium plays a pivotal role in renal fibrogenesis. J Am Soc Nephrol 16: 133– 143, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Park KM, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury and JNK, p38, and MAPK kinase activation by remote ischemic pretreatment. J Biol Chem 276: 11870– 11876, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell 133: 403– 414, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, Yin Y. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell 128: 157– 170, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Sinha D, Wang Z, Price VR, Schwartz JH, Lieberthal W. Chemical anoxia of tubular cells induces activation of c-Src and its translocation to the zonula adherens. Am J Physiol Renal Physiol 284: F488– F497, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Spurgeon KR, Donohoe DL, Basile DP. Transforming growth factor-β in acute renal failure: receptor expression, effects on proliferation, cellularity, and vascularization after recovery from injury. Am J Physiol Renal Physiol 288: F568– F577, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Suzuki A, Yamaguchi MT, Ohteki T, Sasaki T, Kaisho T, Kimura Y, Yoshida R, Wakeham A, Higuchi T, Fukumoto M, Tsubata T, Ohashi PS, Koyasu S, Penninger JM, Nakano T, Mak TW. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity 14: 523– 534, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Suzuki T, Kimura M, Asano M, Fujigaki Y, Hishida A. Role of atrophic tubules in development of interstitial fibrosis in microembolism-induced renal failure in rat. Am J Pathol 158: 75– 85, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Taylor LM, Khachigian LM. Induction of platelet-derived growth factor B-chain expression by transforming growth factor-beta involves transactivation by Smads. J Biol Chem 275: 16709– 16716, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298: F1078– F1094, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Verrecchia F, Vindevoghel L, Lechleider RJ, Uitto J, Roberts AB, Mauviel A. Smad3/AP-1 interactions control transcriptional responses to TGF-beta in a promoter-specific manner. Oncogene 20: 3332– 3340, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Ward JM, Stevens JL, Konishi N, Kurata Y, Uno H, Diwan BA, Ohmori T. Vimentin metaplasia in renal cortical tubules of preneoplastic, neoplastic, aging, and regenerative lesions of rats and humans. Am J Pathol 141: 955– 964, 1992 [PMC free article] [PubMed] [Google Scholar]
- 44. Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest 93: 2175– 2188, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16: 535– 543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yue J, Mulder KM. Requirement of Ras/MAPK pathway activation by transforming growth factor beta for transforming growth factor beta 1 production in a Smad-dependent pathway. J Biol Chem 275: 30765– 30773, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Yuen PS, Dunn SR, Miyaji T, Yasuda H, Sharma K, Star RA. A simplified method for HPLC determination of creatinine in mouse serum. Am J Physiol Renal Physiol 286: F1116– F1119, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Zager RA, Johnson AC, Naito M, Bomsztyk K. Maleate nephrotoxicity: mechanisms of injury and correlates with ischemic/hypoxic tubular cell death. Am J Physiol Renal Physiol 294: F187– F197, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J 23: 1155– 1165, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang Y, Feng XH, Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced transcription. Nature 394: 909– 913, 1998 [DOI] [PubMed] [Google Scholar]