Abstract
Objectives
Our aim was to determine if total parenteral nutrition (TPN)–induced pancreatic atrophy and Hsp70 expression attenuates cerulein-induced pancreatitis in rats.
Methods
Rats were randomized to a 7-day course of saline infusion plus a semipurified diet or TPN, with or without an intravenous cerulein injection or vehicle on day 7, and killed 1 or 6 hours after the injection. Based on a pilot study, 1 hour was the primary time point. Pancreatic atrophy was determined by mass, protein, and DNA contents. Pancreatic heat shock protein 70 (Hsp70) expression was measured by Western analysis. Histological examination of the pancreas assessed for edema, inflammation, vacuolization, and apoptosis. Serum amylase activity was measured using the Phadebas assay. Pancreatic trypsinogen activation was measured using a fluorometric substrate assay.
Results
The saline-infused rats fed orally gained significantly more weight than TPN rats. The TPN decreased the pancreatic mass and protein content and the protein-DNA ratio and increased the pancreatic DNA content compared with the saline. The TPN increased the pancreatic Hsp70 expression by 91% compared with the saline. The TPN reduced the cerulein-induced pancreatic histological edema, the vacuolization, and the inflammation compared with the saline. The increase in the serum amylase level after cerulein injection was significantly attenuated, and trypsinogen activation was reduced in TPN animals compared with the saline group.
Conclusions
Lack of luminal nutrients with a 7-day course of TPN provides moderate protection against cerulein-induced pancreatitis in rats.
Keywords: total parenteral nutrition, pancreatitis, heat shock protein 70, cerulean, rat, TPN
Acute pancreatitis is a common clinical problem, with 250,000 inpatient admissions occurring each year in the United States alone,1 resulting in direct health care costs of 2.2 billion dollars.2 Most episodes of acute pancreatitis are mild-moderate and self-limiting, requiring only a period of fasting and supportive care until the pancreatic inflammation subsides. However, up to 20% of episodes of acute pancreatitis result in pancreatic necrosis,3 leading to increased rates of multisystem organ failure, infection, and mortality.4 The transition from mild-moderate acute pancreatitis to severe acute pancreatitis and pancreatic necrosis is not completely understood, and there are currently no therapies available to prevent this transition. However, there is a growing body of literature showing that the course of severe acute pancreas, once established, can be improved with nutritional interventions such as enteral feeding5 and supplementation with inflammatory and immune modulators.6
One of the initial events in pancreatitis is the premature activation of trypsin, which according to one theory, is due to aberrant colocalization of zymogen granules with lysosomal vacuoles containing cathepsin B. Cathepsin B is a hydrolase that activates trypsinogen, and the activated trypsin enzyme is released into the cytosol where it has the ability to activate itself and other intracellular digestive enzymes in a cascade that results in autodigestion and pancreatic injury.7 This colocalization theory is supported by a study showing that cathepsin B inhibition prevents intracellular zymogen activation without affecting the colocalization of zymogens and lysosomal hydrolases in response to cerulein stimulation.8 Given that pancreatitis is difficult to attenuate once it has occurred, prevention of pancreatitis by blocking the activation of trypsinogen is an obvious therapeutic goal.
Heat shock protein 70 (Hsp70) is an inducible cytosolic protein that acts as a molecular chaperone and is involved in protein folding, degradation, membrane transport, and prevention of aggregation.9 Experimental Hsp70 induction has been accomplished using hyperthermic stress10 or warm-water immersion,11 catecholamine injection12 and ischemia,13 and sodium arsenite injection.14 Several authors have shown that Hsp70 induction with hyperthermia before infusion with the secretagogue cerulein, a cholecystokinin (CCK) analogue, can prevent the onset of pancreatitis in rats10,15 and that inhibition of Hsp70 expression with antisense oligonucleotides abolishes this protective effect of hyperthermic stress.16Hsp70 seems to prevent cerulein-induced acinar injury by preventing intrapancreatic trypsinogen activation,16 a critical initial step in pancreatitis. Furthermore, selective ablation of the Hsp70 gene in mice significantly enhances basal trypsin activity in the gland.17Hsp70 induction also results in reduced levels of downstream mediators of inflammation such as tumor necrosis factor α, intercellular adhesion molecule 1, and nuclear factor κB.18 We have shown previously that a 7-day course of total parenteral nutrition (TPN) and a lack of luminal nutrients induce pancreatic atrophy and Hsp70 expression in rats.19 The purpose of this study was to determine if TPN-induced pancreatic Hsp70 expression attenuates cerulein-induced pancreatic injury in the rat.
MATERIALS AND METHODS
Animals and Experimental Design
The animal facilities and research protocols were approved by the University of Wisconsin Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (Harlan, Madison, Wis) initially weighing 121 to 145 g were housed in individual stainless steel cages with unlimited access to water in a room maintained at 22°C on a 12:12-hour light-dark cycle. The rats were adapted to the facility for 5 to 7 days while being fed a semipurified powdered diet ad libitum.20
The night before surgery, the animals were fasted and were randomly assigned to undergo placement of a jugular venous catheter and postoperative infusion of TPN or saline for 7 days. A nonsurgical group of rats fed the semipurified diet ad libitum were included for reference. On the day of surgery (day 0), the rats were anesthetized by inhalation of isofluorane (Isoflo; Abbot Laboratories, North Chicago, Ill) and underwent placement of an infusion catheter in the superior vena cava as previously described.20,21 The infusion of TPN or saline began immediately after surgery and was increased gradually to provide 100% of the nutritional needs in the TPN group (discussed later), with equal infusion rates for animals receiving TPN or saline. All animals had ad libitum access to water.
In an initial pilot experiment, rats were again randomized to undergo the following treatments on day 7: injection of cerulein (10 μg/kg of body weight; Sigma-Aldrich, St Louis, Mo) for 1 or 6 hours and injection of vehicle (phosphate-buffered saline) for 1 or 6 hours. The animals were then anesthetized, and the pancreas and blood were collected. Based on the results of the pilot study, there were no significant differences in the measured end points between the 1- and 6-hour injections within each group, and the 1-hour injection was chosen for subsequent experiments.
Composition of the TPN Solution and the Oral Diet
The TPN solution was prepared aseptically as a total nutrient admixture using commercial preparations of amino acids, dextrose, and lipid emulsions. Electrolytes, vitamins, trace elements, and choline were added according to reported requirements of the TPN-fed rat.20 The rat TPN solutions have a caloric density of approximately 4.2 kJ/mL and provide 1.5 g N·kg−1·d−1 and 32% of nonprotein energy from lipids. The TPN or the saline solution was infused via a syringe infusion pump (Harvard Apparatus, Inc, Holliston, Mass) at rates of 1.0 mL/h on day 0, 1.2 mL/h on day 1, 1.6 mL/h on day 2, 1.8 mL/h on day 3, 2.0 mL/h on day 4, and 2.2 mL/h on days 5 to 7 to provide 987 kJ/kg per day. Orally fed animals, including those in the saline group, had free access to a nutritionally complete, semipurified powdered diet with a macronutrient content comparable to the TPN solution.20
Pancreas Mass and Cellularity
The pancreas was removed, flushed with ice-cold 0.9% saline, and blotted dry. The entire pancreas was weighed intact, and then a portion was excised to measure tissue edema. Additional portions of each pancreas were fixed in 10% buffered formalin and paraffin embedded, and 5-μm sections were stained with hematoxylin and eosin for histological analysis. The remainder of the pancreas was immediately frozen in liquid nitrogen and stored at −80°C until ready for analysis. The pancreas was homogenized in 2 mL of 20-mmol/L sodium phosphate (NaH2PO4) buffer (pH 7.4). Pancreas protein concentration was determined using a bicinchoninic acid protein assay (Pierce Chemicals, Rockford, Ill), and pancreatic DNA content was measured using a Hoechst reagent assay (Calbiochem, La Jolla, Calif).22 The ratio of protein to DNA in the pancreas expressed as concentrations was determined.
Pancreas Western Immunoblotting
Homogenized pancreatic tissue samples were centrifuged for 30 minutes at 21,000g at 4°C, and the supernatant was collected. The protein concentration of the supernatant was determined using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, Calif). The supernatant was prepared for electrophoresis by adding a sodium dodecyl sulfate sample buffer and boiling for 5 minutes. Pancreatic soluble proteins (400 μg) were separated in polyacrylamide gels and transferred onto nitrocellulose membranes. Membranes were blocked in Tris-buffered saline containing 5% nonfat dry milk and 3% Tween 20. The membranes were cut at the 50-kd marker, and the upper portion was incubated with an anti-Hsp70 mouse monoclonal antibody (1:1000 dilution; Stressgen, Victoria, British Columbia, Canada); and the lower portion, with an anti-CRHSP24 polyclonal antibody23 (1:1000 dilution) for 90 minutes at room temperature. After incubation with horseradish peroxidase-conjugated secondary antibodies, immunoreactivity was detected by enhanced chemiluminescence. Band intensity was quantified by light densitometry using OptiQuant version 03.00 software package (Packard Instruments, Meriden, Conn).
Histological Examination of the Pancreas
Hematoxylin and eosin–stained pancreas sections were evaluated in a blinded manner by a single pathologist using a scale adapted from Rongione et al24 that assigns a grade from 0 to 4 for the degree of edema, inflammation, vacuolization, and apoptosis 1 hour after cerulein or vehicle injection.
Enzyme Activity
Serum was separated from collected blood and stored at −70°C until use. Serum amylase activity was measured using the Phadebas Amylase Test (Magle, Lund, Sweden). Pancreas trypsin activity was measured as previously described.25 Briefly, pancreas homogenates were thawed and diluted in 3 volumes of buffer containing 50-mmol/L Tris base, 150-mmol/L NaCl, 1-mmol/L CaCl2, and 0.1-mg/mL bovine serum albumin. The samples were then homogenized with a pestle and centrifuged at 1500g for 10 minutes at 4°C. The supernatant was collected, and protein was measured by the Bio-Rad protein assay. Trypsin activity in the supernatant was measured by flourometry using a 100-μmol/L Boc-Gln-Ala-Arg-MCA substrate.
Statistical Analysis
The SAS version 8.2 (SAS Institute, Cary, NC) was used for the statistical analysis. The differences between the treatment groups were examined by 1-way analysis of variance (ANOVA). General linear models were used to analyze the main effects of the treatments and their interactions. The 1- and 6-hour time points were combined as 1 group for amylase activity because there were no significant time effects. All other comparisons were limited to the 1-hour time point. All values are presented as mean ± SE; P ≤ 0.05 was considered statistically significant.
RESULTS
Body Weight
The animals in all the groups gained weight over the experiment (Fig. 1). Orally fed animals had greater weight gain than animals maintained with TPN, and TPN had a significant main effect to decrease weight gain (P < 0.0001). However, there were no significant differences in the mean body weight gain based on the treatment with cerulein. Previous studies using this well-characterized TPN model have confirmed that nitrogen retention increases each day in all TPN rats.26
FIGURE 1.
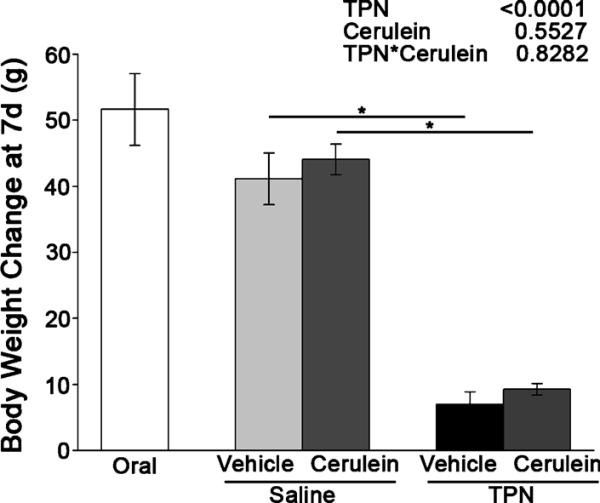
Increase in body weight (in grams) in the orally fed controls, the saline-infused, orally fed surgical controls, and the TPN-fed rats after 7 days of treatment followed by cerulein or vehicle injection. The data are expressed as mean ± SE; n = 6 to 9. *P < 0.05. The main effects of TPN, cerulein injection, and their interaction as indicated by 2-way ANOVA are shown at the top right.
Total Parenteral Nutrition Induces Pancreatic Atrophy and Hsp70 Induction
Lack of luminal nutrients in TPN animals treated with vehicle led to a significant decrease in pancreas wet weight (corrected for body mass) of 14% compared with the saline-infused vehicle animals and the orally fed controls (Fig. 2). The TPN had a significant main effect to decrease pancreas wet weight (P < 0.0001). In contrast, the cerulein treatment had a significant main effect to increase pancreas wet weight (P = 0.003). The cerulein-treated, saline-infused animals had a significant 28% increase in pancreas wet weight compared with their vehicle controls, whereas the TPN-infused animals showed a 21% increase.
FIGURE 2.
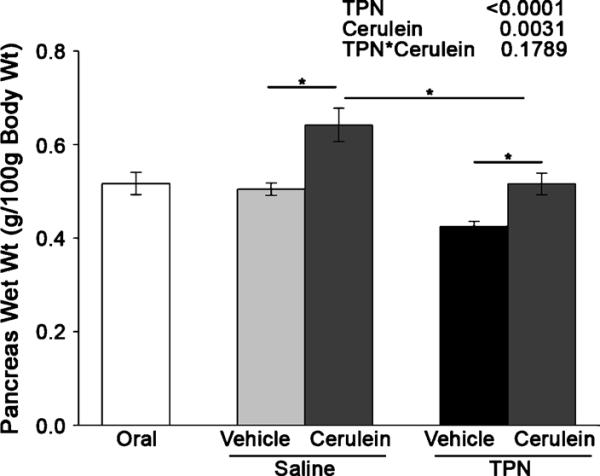
Total parenteral nutrition reduces pancreatic mass. The mass of the pancreas (in grams) was measured in the orally fed controls, the saline-infused, orally fed surgical controls, and the TPN-fed rats after 7 days of treatment followed by cerulein or vehicle injection. The data are expressed as mean ± SE; n = 6 to 9. *P < 0.05. The main effects of TPN, cerulein injection, and their interaction as indicated by 2-way ANOVA are shown at the top right.
Total pancreatic protein was significantly reduced (P < 0.0004) with TPN infusion (Fig. 3A). In addition, pancreas DNA concentration was significantly (P < 0.0001) increased in the animals infused with TPN compared with the saline animals (Fig. 3B). As a consequence, the ratio of protein to DNA in the pancreas decreased significantly (P = 0.001) with TPN infusion (Fig. 3C), indicating that reduction in pancreatic weight due to TPN treatment is primarily due to protein loss and cellular atrophy. Pancreas Hsp70 expression (normalized to CRHSP24) was significantly increased by 91% in the TPN animals treated with vehicle compared with the saline-infused animals and orally fed control animals, which had equal levels of expression (Fig. 4). As a control, there were no differences in the expressions of the calcium-sensitive signaling protein CRHSP24.19,23Hsp70 expression was not assessed in cerulein animals because cerulein is known to increase pancreatic Hsp70 expression regardless of the dietary treatment.27,28
FIGURE 3.
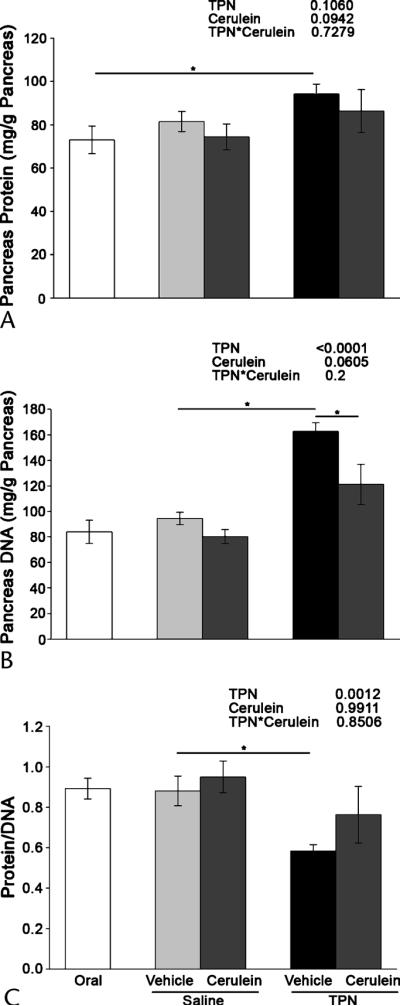
Total parenteral nutrition induces pancreatic atrophy. Pancreatic protein, DNA, and protein-to-DNA ratio were determined in the orally fed controls, the saline-infused, orally fed surgical controls, and the TPN-fed rats after 7 days of treatment followed by cerulein or vehicle injection. A, Pancreatic protein was measured by bicinchoninic acid colorimetric assay. B, Pancreatic DNA concentration was measured using the Hoescht reagent and a fluorometric assay. C, Protein-to-DNA ratio. The data are expressed as mean ± SE; n = 6 to 9. *P < 0.05. The main effects of TPN, cerulein injection, and their interaction as indicated by 2-way ANOVA are shown at the top right.
FIGURE 4.
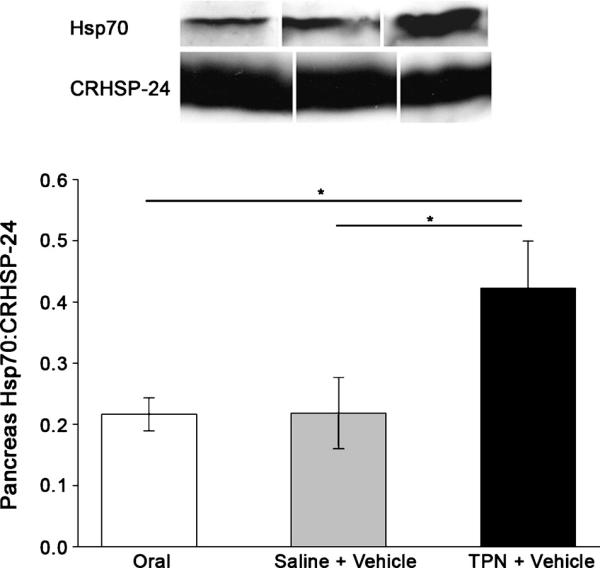
HSP70 is induced in TPN-fed rats. Pancreatic Hsp70 and CRHSP24 were measured in the orally fed controls, the saline-infused, orally fed surgical controls, and the TPN-fed rats after 7 days of treatment and vehicle injection by immunoblot analysis using anti-Hsp70 (1:1000) and anti-CRHSP24 (1:1000) antibodies. The band intensity was measured by densitometry and expressed as Hsp70-to-CRHSP24 ratio. Data are expressed as mean ± SE; n = 8. *P G 0.05.
Total Parenteral Nutrition Attenuates Cerulein-Induced Inflammation
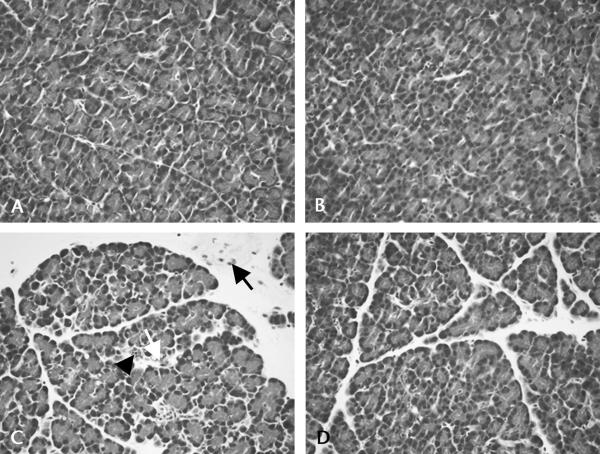
The pancreatic inflammatory response 1 hour after cerulein injection was less severe in the TPN-infused rats than in the saline-infused rats based on the histological analysis (Table 1, Figs. 5A–D). After cerulein-injection, the TPN-infused animals had a 32% reduction in pancreas edema, a 41% decrease in inflammatory cell infiltration, and a significant 23% reduction in pancreas vacuolization compared with the saline-infused animals. The TPN infusion led to a significant 25% reduction in total inflammation after cerulein injection compared with the saline-infused rats. Moreover, TPN showed a significant main effect to reduce pancreatic total inflammation (P < 0.05), and there was a weak interaction between TPN and cerulein (P = 0.09), suggesting that the increase in pancreatic histological inflammation after cerulein injection is smaller when rats are maintained with TPN. In contrast, there was only a small reduction in acinar apoptosis and no significant changes in gross pancreas edema (pancreatic water content, expressed as percent of wet weight) with cerulein infusion in either the TPN or the saline-infused groups. In addition to the inflammatory changes noted on the histological examination, there was a decrease in pancreatic zymogen granule staining in the TPN-infused animals (Figs. 5B, D) compared with the saline-infused, orally fed animals (Figs. 5A, C). This is consistent with the reduction in pancreatic mass and protein noted earlier.
TABLE 1.
Histological Assessment of Pancreatic Edema, Inflammation, Vacuolization, and Apoptosis at 1 Hour
| Saline |
TPN |
||||
|---|---|---|---|---|---|
| Oral | Vehicle | Cerulein | Vehicle | Cerulein | |
| Edema | 0.56 ± 0.24*† | 0.00 ± 0† | 1.73 ± 0.27‡ | 0.18 ± 0.12† | 1.17 ± 0.34*‡ |
| Inflammation | 0.11 ± 0.11* | 0.29 ± 0.18* | 1.13 ± 0.40‡ | 0.13 ± 0.12* | 0.67 ± 0.33*‡ |
| Vacuolization | 0.00 ± 0† | 0.00 ± 0† | 2.38 ± 0.18‡ | 0.00 ± 0† | 1.83 ± 0.40* |
| Apoptosis | 0.67 ± 0.24*‡ | 0.14 ± 0.14* | 1.25 ± 0.37‡ | 0.00 ± 0* | 1.17 ± 0.60‡ |
| Total | 1.33 ± 0.29† | 0.38 ± 0.18† | 6.73 ± 0.65‡ | 0.45 ± 0.25† | 5.08 ± 0.70* |
| Edema: |
| 0: Absent |
| 1: Mild, diffuse expansion of interlobar septa |
| 2: Moderate, diffuse expansion interacinar septa |
| 3: Severe, diffuse expansion intercellular septa |
| Inflammation: |
| 0: Absent |
| 1: 1–5 leukocytes per 10 HPF |
| 2: 6–10 leukocytes per 10 HPF |
| 3: >10 leukocytes per 10 HPF |
| Vacuolization: |
| 0: Absent |
| 1: Mild/focal |
| 2: Moderate/diffuse |
| 3: Severe |
| Apoptosis: |
| 0: Absent |
| 1: 1–4 apoptotic cells per 10 HPF |
| 2: 5–10 apoptotic cells per 10 HPF |
| 3: >10 apoptotic cells per 10 HPF |
Total parenteral nutrition attenuates cerulein-induced pancreatic edema, inflammation, and vacuolization at 1 hour. Values are expressed as mean ± SE; n = 6 to 9 animals per group. The mean values in a row with different symbols are significantly different by 1-way ANOVA.
Adapted from Rongione et al.24
FIGURE 5.
Total parenteral nutrition induces acinar cell atrophy and attenuates cerulein-induced pancreatic inflammation. Compared with the saline-infused, orally fed control animals (A), there was decreased acidic staining of zymogen granules in the apical cytoplasm of acini from the TPN-infused control animals (B) as seen on hematoxylin and eosin–stained sections. There were increased pathologic responses to cerulein infusion in the saline rats (C) compared with the TPN rats (D). Note the prominent inflammatory cells (arrow), the vacuolization (open arrow), and the apoptosis (arrowhead) in C.
Total Parenteral Nutrition Attenuates Pancreatitis-Associated Enzyme Activity
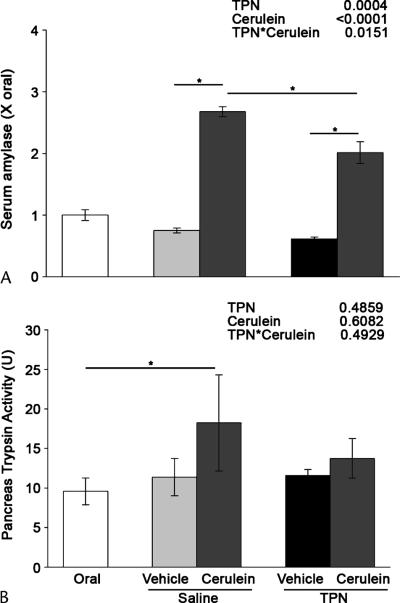
The serum amylase levels 1 to 6 hours after cerulein treatment were significantly higher in the saline animals (2.67 × the oral control) compared with the TPN animals (2.01 × oral control, Fig. 6A). There were significant main effects for decreased serum amylase level with TPN (P = 0.0004) and increased serum amylase level with cerulein treatment (P < 0.0001). In addition, TPN significantly attenuated the rise in serum amylase level after cerulein injection (TPN × cerulein = 0.02), which reflects the larger rise in serum amylase level with cerulein treatment in saline animals (356% > with vehicle) compared with the TPN animals (330% > with vehicle).
FIGURE 6.
Total parenteral nutrition attenuates pancreatitis-associated enzyme activity. Serum amylase and trypsin activities were measured in the orally fed controls, the saline-infused, orally fed surgical controls, and the TPN-fed rats after 7 days of treatment followed by cerulein or vehicle injection. A, Serum amylase level was measured using the Phadebas Amylase Test. B, Trypsin activity was measured using a fluorometric substrate assay. U = activity per time per microgram total protein. The data are expressed as mean ± SE; n = 5 to 13. *P < 0.05. The main effects of TPN, cerulein injection, and their interaction as indicated by 2-way ANOVA are shown at the top right.
Pancreatic trypsin activity (measured as activity per time per microgram total protein) increased 60% after cerulein treatment in the saline-infused animals (18.2 vs 11.4 in the vehicle-treated animals; Fig. 6B), which was significantly increased compared with the orally fed controls (9.6). In contrast, trypsin activity in the TPN-infused animals increased by only 18% with the cerulein treatment (13.7 vs 11.6 in the vehicle-treated controls) and was not statistically different from the orally fed controls.
DISCUSSION
The removal of enteral nutrients during TPN results in a reduction in pancreatic wet weight and protein content,29 as well as a stress response in the gut30 and the pancreas19 characterized by the induction of Hsp70 expression. Hsp27 and Hsp60 expressions, however, do not change in this model.19 This study is in agreement with previous findings in the pancreas and demonstrates that preconditioning rats with TPN and an absence of luminal nutrients provide a moderate protective effect against mild secretagogue-induced pancreatitis. Others have shown that preconditioning with stressful stimuli can induce pancreatic Hsp70 expression and protect against secretagogue-induced pancreatitis in fed rodents. Wagner et al10 demonstrated that induction of Hsp70 expression with thermal stress has a protective effect against cerulein-induced pancreatitis in rats. Using cultured pancreatic lobules, Bhagat et al16 showed that the addition of antisense oligonucleotides to inhibit Hsp70 expression abrogated its protective effects against cerulein-induced trypsinogen activation, directly implicating Hsp70 in this process. Likewise, hyperthermia-induced heat shock protein expression offered protection against the more severe arginine-induced pancreatitis in rats.31 However, hyperthermia is not a clinically relevant method of inducing HSP synthesis or does not elicit a specific stress response. In this respect, TPN offers a more clinically relevant model to study mechanisms of pancreatitis and its prevention because it allows complete dietary control with the option to study specific dietary, nutrient, or pharmacologic agents.
Parenteral nutrition affects the exocrine pancreas through the lack of gastrointestinal stimulation due to the absence of luminal nutrients and the unique profile of nutrients infused systemically. Total parenteral nutrition has been shown to change systemic levels of gastrointestinal hormones, the most important being a reduction in serum CCK.32 In rodents, CCK plays a major role in stimulating the production and secretion of digestive enzymes, and infusion of CCK during TPN prevents the pancreatic hypotrophy seen with a lack of luminal nutrients.33 In addition to a reduction in CCK, the lack of luminal nutrients results in failure to initiate the vagal-mediated enteropancreatic reflex, leading to a reduction in the production and secretion of pancreatic enzymes.34
The macronutrient components of TPN may also have individual effects on the exocrine pancreas structure and function. Whereas intraduodenal fat is a potent stimulator of CCK release, intravenous (IV) fat does not affect fasting circulating plasma CCK levels.35 Intraduodenal protein also stimulates the release of CCK; however, the effect of IV amino acids on CCK levels has not been established. Although infusion of IV amino acids alone presumably maintains pancreatic amino acid concentrations, this is insufficient to promote digestive enzyme synthesis because there is a marked decrease in pancreatic amylase and lipase contents.19,36 Intraduodenal glucose does not stimulate the pancreas, and exclusive IV glucose feeding results in marked pancreatic atrophy.36 However, data on the effect that IV glucose has on exocrine pancreatic function is conflicting, with evidence pointing to both decreases37 and increases38 in secretory and synthetic function. What is apparent is that luminal fats and proteins stimulate pancreatic exocrine function, IV amino acids mitigate pancreatic hypotrophy, and IV glucose may stimulate synthesis of pancreatic enzymes. Thus, a mixed regimen of IV amino acids and lipids with enteral carbohydrate feeding may provide greater suppression of pancreatic stimulation and improve the protective effect of parenteral nutrition on pancreatitis while maintaining intestinal mucosal integrity.
The current study confirms the findings from a previous study that a 7-day course of TPN decreased pancreatic mass and total protein and increased DNA concentration and Hsp70 expression.19 However, there are slight differences between the 2 studies. Compared with the previous study, the current study found that TPN induced a 2-fold increase in pancreatic Hsp70 expression, whereas the previous study showed a 3-fold induction. Likewise, histological examination of acini revealed that the loss of acidic zymogen granule staining in response to TPN was less pronounced than the extensive loss of zymogen granules seen in the previous study. The current study also showed no change in apoptosis, demonstrating pancreatic cellular atrophy rather than a loss of cells. Previously, TPN was shown to increase pancreatic apoptosis compared with saline infusion.19 These differences in outcomes may be due to the smaller animal size in the current study (165 vs 262 G) and the prior study measuring apoptosis by terminal deoxynucleotidyl transferase deoxyuridine 5-triphosphate nick end labeling and staining.
The induction of Hsp70 in the pancreas and the small intestine seen during TPN was previously attributed to a lack of luminal nutrient stimulation and atrophy of the gut because this was not seen in the intestine when coinfusing the growth factor GLP-2 that prevents mucosal atrophy.19 Moreover, the intestinal atrophy seen in hibernating animals is also associated with significant Hsp70 induction.30 Infusion of glutamine or arginine is known to induce Hsp70 expression. Ziegler et al39 reported that parenteral glutamine increases Hsp70 levels in the plasma of patients with critical illnesses. Similarly, arginine infusion of rats to induce necrotizing pancreatitis also caused a marked induction of Hsp70.40 The TPN solution used in these studies contained arginine (865 mg/100 mL) and glutamine (627 mg/100 mL). Thus, we cannot rule out that arginine or glutamine in the TPN solution played a role in Hsp70 induction; however, our preliminary data indicate that placing animals on a protein-free diet for 6 days causes significant pancreatic atrophy with a marked induction of Hsp70 (data not shown). Regardless of the mechanism, the dietary-induced expression of so-called vitagenes including Hsp70, heme-oxygenase, thioredoxin reductase, and sirtuins play an important role in mediating cytoprotective functions.41
Although the TPN-induced Hsp70 expression in our study showed only a moderate protective effect against experimental pancreatitis, others have shown more profound effects using hyperthermia42 and β-agonists.12 There are several possible reasons for this. The increase in Hsp70 with TPN more than the control levels in this study and our previous work19 (91%–200%) is much lower than that seen using hyperthermia (up to 9-fold42). Thus, it is possible that the lower Hsp70 expression with TPN provides moderate protection against acinar injury. Second, the dose of cerulein (10 μg/kg) in our study was lower than the 20- to 50-μg/kg dose used in other studies.12,42 Finally, larger rats (120–145 g) were used in the current study than in others (70–100 g42,43). Indeed, conflicting results have been reported for the effects of Hsp70 in pancreatitis. In 2 studies that demonstrated Hsp70 induction with sodium arsenite, Bhagat et al43 reported a protective effect against cerulein-induced pancreatitis whereas Rakonczay et al14 failed to show protection. The study of Bhagat et al43 used rats that were 70 to 100 g, whereas Rakonczay et al14 used rats that were 250 to 300 g. Moreover, the study of Rakonczay et al14 used a much higher dose of secretagogue (3 subcutaneous 75-μg/kg injections) compared with the study of Bhagat et al43 (a single 20-μg/kg intraperitoneal injection). This suggests that animal size and levels of Hsp70 induction and secretagogue are important factors in interpreting the outcomes of animal studies of pancreatitis.
A systematic review of human studies examining nutrition support in acute pancreatitis demonstrated that enteral nutrition, when compared with parenteral nutrition, is associated with a significant reduction in infectious morbidity and length of stay, a trend toward reduced organ failure, and no effect on mortality.44 However, TPN still has a role in acute pancreatitis because many patients are not able to be fed enterally either because of intolerance or a lack of enteral access. In addition, there are human data that suggest that parenteral nutrition is superior to standard therapy (dextrose-containing IV fluids and starvation) in severe acute pancreatitis, with decreased infections, length of stay, and mortality.45 Furthermore, the inferior outcomes with parenteral nutrition compared with enteral nutrition in pancreatitis are primarily related to increased infectious morbidity and multisystem organ failure, outcomes that have the potential to be modified with the addition of immune-enhancing and anti-inflammatory supplements. Rat studies of acute necrotizing pancreatitis have demonstrated that the infusion of omega-3 fatty acids reduces mortality, infections, and systemic inflammation.46 Indeed, human studies of severe acute pancreatitis show decreased pulmonary and renal complications after omega-3 fatty acid treatment47 and reduced infectious morbidity with glutamine-supplemented parenteral nutrition.6
In conclusion, we have demonstrated that pancreatic atrophy and induction of Hsp70 expression after 7 days of TPN and an absence of luminal nutrients offer moderate protection against mild cerulein-induced pancreatitis, likely via reduced activation of trypsinogen and a reduction in pancreatic digestive enzyme production due to absent enteral stimulation. This model offers the opportunity to test the effects of dietary and nutrient manipulation on Hsp70 regulation and inflammatory responses in the setting of acute pancreatitis.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases grants R01-DK-07088 (to G.E. Groblewski) and R01-DK-42835-16 (to D.M. Ney). This work was also supported by the United States Department of Agriculture Cooperative State Research, Education and Extension Services project WISO4958 (to G.E. Groblewski). M.C. Koopmann and M.D. Baumler were supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases Training grant T32-DK-007665.
REFERENCES
- 1.Brown A, Young B, Morton J, et al. Are health related outcomes in acute pancreatitis improving? An analysis of national trends in the U.S. from 1997 to 2003. JOP. 2008;9(4):408–414. [PubMed] [Google Scholar]
- 2.Fagenholz PJ, Fernandez-del Castillo C, Harris NS, et al. Direct medical costs of acute pancreatitis hospitalizations in the United States. Pancreas. 2007;35(4):302–307. doi: 10.1097/MPA.0b013e3180cac24b. [DOI] [PubMed] [Google Scholar]
- 3.Tran DD, Cuesta MA. Evaluation of severity in patients with acute pancreatitis. Am J Gastroenterol. 1992;87(5):604–608. [PubMed] [Google Scholar]
- 4.Baron TH, Morgan DE. Acute necrotizing pancreatitis. N Engl J Med. 1999;340(18):1412–1417. doi: 10.1056/NEJM199905063401807. [DOI] [PubMed] [Google Scholar]
- 5.Olah A, Pardavi G, Belagyi T, et al. Early nasojejunal feeding in acute pancreatitis is associated with a lower complication rate. Nutrition. 2002;18(3):259–262. doi: 10.1016/s0899-9007(01)00755-9. [DOI] [PubMed] [Google Scholar]
- 6.Fuentes-Orozco C, Cervantes-Guevara G, Mucino-Hernandez I, et al. l-Alanyl-l-glutamine–supplemented parenteral nutrition decreases infectious morbidity rate in patients with severe acute pancreatitis. JPEN J Parenter Enteral Nutr. 2008;32(4):403–411. doi: 10.1177/0148607108319797. [DOI] [PubMed] [Google Scholar]
- 7.Saluja AK, Donovan EA, Yamanaka K, et al. Cerulein-induced in vitro activation of trypsinogen in rat pancreatic acini is mediated by cathepsin B. Gastroenterology. 1997;113(1):304–310. doi: 10.1016/s0016-5085(97)70108-2. [DOI] [PubMed] [Google Scholar]
- 8.Van Acker GJ, Weiss E, Steer ML, et al. Cause-effect relationships between zymogen activation and other early events in secretagogue-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292(6):G1738–G1746. doi: 10.1152/ajpgi.00543.2006. [DOI] [PubMed] [Google Scholar]
- 9.Polier S, Dragovic Z, Hartl FU, et al. Structural basis for the cooperation of Hsp70 and Hsp110 chaperones in protein folding. Cell. 2008;133(6):1068–1079. doi: 10.1016/j.cell.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Wagner AC, Weber H, Jonas L, et al. Hyperthermia induces heat shock protein expression and protection against cerulein-induced pancreatitis in rats. Gastroenterology. 1996;111(5):1333–1342. doi: 10.1053/gast.1996.v111.pm8898648. [DOI] [PubMed] [Google Scholar]
- 11.Rakonczay Z, Jr, Takacs T, Mandi Y, et al. Water immersion pretreatment decreases pro-inflammatory cytokine production in cholecystokinin-octapeptide-induced acute pancreatitis in rats: possible role of HSP72. Int J Hyperthermia. 2001;17(6):520–535. doi: 10.1080/02656730110081785. [DOI] [PubMed] [Google Scholar]
- 12.Frossard JL, Bhagat L, Lee HS, et al. Both thermal and non-thermal stress protect against caerulein induced pancreatitis and prevent trypsinogen activation in the pancreas. Gut. 2002;50(1):78–83. doi: 10.1136/gut.50.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warzecha Z, Dembinski A, Ceranowicz P, et al. Ischemic preconditioning inhibits development of edematous cerulein-induced pancreatitis: involvement of cyclooxygenases and heat shock protein 70. World J Gastroenterol. 2005;11(38):5958–5965. doi: 10.3748/wjg.v11.i38.5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rakonczay Z, Jr, Mandi Y, Kaszaki J, et al. Induction of HSP72 by sodium arsenite fails to protect against cholecystokinin-octapeptide-induced acute pancreatitis in rats. Dig Dis Sci. 2002;47(7):1594–1603. doi: 10.1023/a:1015883522648. [DOI] [PubMed] [Google Scholar]
- 15.Weber H, Wagner AC, Jonas L, et al. Heat shock response is associated with protection against acute interstitial pancreatitis in rats. Dig Dis Sci. 2000;45(11):2252–2264. doi: 10.1023/a:1026459001195. [DOI] [PubMed] [Google Scholar]
- 16.Bhagat L, Singh VP, Hietaranta AJ, et al. Heat shock protein 70 prevents secretagogue-induced cell injury in the pancreas by preventing intracellular trypsinogen activation. J Clin Invest. 2000;106(1):81–89. doi: 10.1172/JCI8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang JH, Ryu JK, Yoon YB, et al. Spontaneous activation of pancreas trypsinogen in heat shock protein 70.1 knock-out mice. Pancreas. 2005;31(4):332–336. doi: 10.1097/01.mpa.0000183377.04295.c3. [DOI] [PubMed] [Google Scholar]
- 18.Frossard JL, Pastor CM, Hadengue A. Effect of hyperthermia on NF-kappaB binding activity in cerulein-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2001;280(6):G1157–G1162. doi: 10.1152/ajpgi.2001.280.6.G1157. [DOI] [PubMed] [Google Scholar]
- 19.Baumler MD, Nelson DW, Ney DM, et al. Loss of exocrine pancreatic stimulation during parenteral feeding suppresses digestive enzyme expression and induces Hsp70 expression. Am J Physiol Gastrointest Liver Physiol. 2007;292(3):G857–G866. doi: 10.1152/ajpgi.00467.2006. [DOI] [PubMed] [Google Scholar]
- 20.Dahly EM, Guo Z, Ney DM. Alterations in enterocyte proliferation and apoptosis accompany TPN-induced mucosal hypoplasia and IGF-I-induced hyperplasia in rats. J Nutr. 2002;132(7):2010–2014. doi: 10.1093/jn/132.7.2010. [DOI] [PubMed] [Google Scholar]
- 21.Lasekan JB, Rivera J, Hirvonen MD, et al. Energy expenditure in rats maintained with intravenous or intragastric infusion of total parenteral nutrition solutions containing medium- or long-chain triglyceride emulsions. J Nutr. 1992;122(7):1483–1492. doi: 10.1093/jn/122.7.1483. [DOI] [PubMed] [Google Scholar]
- 22.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 23.Groblewski GE, Yoshida M, Bragado MJ, et al. Purification and characterization of a novel physiological substrate for calcineurin in mammalian cells. J Biol Chem. 1998;273(35):22738–22744. doi: 10.1074/jbc.273.35.22738. [DOI] [PubMed] [Google Scholar]
- 24.Rongione AJ, Kusske AM, Kwan K, et al. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology. 1997;112(3):960–967. doi: 10.1053/gast.1997.v112.pm9041259. [DOI] [PubMed] [Google Scholar]
- 25.Grady T, Saluja A, Kaiser A, et al. Edema and intrapancreatic trypsinogen activation precede glutathione depletion during caerulein pancreatitis. Am J Physiol. 1996;271(1 Pt 1):G20–G26. doi: 10.1152/ajpgi.1996.271.1.G20. [DOI] [PubMed] [Google Scholar]
- 26.Yang H, Grahn M, Schalch DS, et al. Anabolic effect of IGF-I coinfused with total parenteral nutrition in dexamethasone-treated rats. Am J Physiol Endocrinol Metab. 1994;266(5 Pt 1):E690–E698. doi: 10.1152/ajpendo.1994.266.5.E690. [DOI] [PubMed] [Google Scholar]
- 27.Ethridge RT, Ehlers RA, Hellmich MR, et al. Acute pancreatitis results in induction of heat shock proteins 70 and 27 and heat shock factor-1. Pancreas. 2000;21(3):248–256. doi: 10.1097/00006676-200010000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Weber CK, Gress T, Muller-Pillasch F, et al. Supramaximal secretagogue stimulation enhances heat shock protein expression in the rat pancreas. Pancreas. 1995;10(4):360–367. doi: 10.1097/00006676-199505000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Fan BG, Axelson J, Sternby B, et al. Total parenteral nutrition affects the tropic effect of cholecystokinin on the exocrine pancreas. Scand J Gastroenterol. 1997;32(4):380–386. doi: 10.3109/00365529709007688. [DOI] [PubMed] [Google Scholar]
- 30.Carey HV, Mangino MJ, Southard JH. Changes in gut function during hibernation: implications for bowel transplantation and surgery. Gut. 2001;49(4):459–461. doi: 10.1136/gut.49.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tashiro M, Ernst SA, Edwards J, et al. Hyperthermia induces multiple pancreatic heat shock proteins and protects against subsequent arginine-induced acute pancreatitis in rats. Digestion. 2002;65(2):118–126. doi: 10.1159/000057713. [DOI] [PubMed] [Google Scholar]
- 32.Mashako MN, Bernard C, Cezard JP, et al. Effect of total parenteral nutrition, constant rate enteral nutrition, and discontinuous oral feeding on plasma cholecystokinin immunoreactivity in children. J Pediatr Gastroenterol Nutr. 1987;6(6):948–952. doi: 10.1097/00005176-198711000-00022. [DOI] [PubMed] [Google Scholar]
- 33.Mok KT, Meng HC. Intestinal, pancreatic, and hepatic effects of gastrointestinal hormones in a total parenteral nutrition rat model. JPEN J Parenter Enteral Nutr. 1993;17(4):364–369. doi: 10.1177/0148607193017004364. [DOI] [PubMed] [Google Scholar]
- 34.Niebergall-Roth E, Singer MV. Enteropancreatic reflexes mediating the pancreatic enzyme response to nutrients. Auton Neurosci. 2006;125(1–2):62–69. doi: 10.1016/j.autneu.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 35.de Boer SY, Masclee AA, Jebbink MC, et al. Effect of intravenous fat on cholecystokinin secretion and gallbladder motility in man. JPEN J Parenter Enteral Nutr. 1992;16(1):16–19. doi: 10.1177/014860719201600116. [DOI] [PubMed] [Google Scholar]
- 36.Fan BG, Ake AS. Influence of an intravenous infusion of amino acids and glucose on the pancreatic exocrine in rats. Chin Med J (Engl) 2004;117(11):1659–1664. [PubMed] [Google Scholar]
- 37.Nakajima S, Magee DF. Inhibition of exocrine pancreatic secretion by glucagon and d-glucose given intravenously. Can J Physiol Pharmacol. 1970;48(5):299–305. doi: 10.1139/y70-049. [DOI] [PubMed] [Google Scholar]
- 38.Fan BG, Andren-Sandberg A. I.v. hypertonic glucose stimulates the exocrine pancreas in rat. JPEN J Parenter Enteral Nutr. 2006;30(1):40–44. doi: 10.1177/014860710603000140. [DOI] [PubMed] [Google Scholar]
- 39.Ziegler TR, Ogden LG, Singleton KD, et al. Parenteral glutamine increases serum heat shock protein 70 in critically ill patients. Intensive Care Med. 2005;31(8):1079–1086. doi: 10.1007/s00134-005-2690-5. [DOI] [PubMed] [Google Scholar]
- 40.Tashiro M, Schafer C, Yao H, et al. Arginine induced acute pancreatitis alters the actin cytoskeleton and increases heat shock protein expression in rat pancreatic acinar cells. Gut. 2001;49(2):241–250. doi: 10.1136/gut.49.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calabrese V, Cornelius C, Mancuso C, et al. Cellular stress response: a novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem Res. 2008;33(12):2444–2471. doi: 10.1007/s11064-008-9775-9. [DOI] [PubMed] [Google Scholar]
- 42.Bhagat L, Singh VP, Song AM, et al. Thermal stress-induced HSP70 mediates protection against intrapancreatic trypsinogen activation and acute pancreatitis in rats. Gastroenterology. 2002;122(1):156–165. doi: 10.1053/gast.2002.30314. [DOI] [PubMed] [Google Scholar]
- 43.Bhagat L, Singh VP, Dawra RK, et al. Sodium arsenite induces heat shock protein 70 expression and protects against secretagogue-induced trypsinogen and NF-kappaB activation. J Cell Physiol. 2008;215(1):37–46. doi: 10.1002/jcp.21286. [DOI] [PubMed] [Google Scholar]
- 44.McClave SA, Chang WK, Dhaliwal R, et al. Nutrition support in acute pancreatitis: a systematic review of the literature. JPEN J Parenter Enteral Nutr. 2006;30(2):143–156. doi: 10.1177/0148607106030002143. [DOI] [PubMed] [Google Scholar]
- 45.Xian-li H, Qing-jiu M, Jian-guo L, et al. Effect of total parenteral nutrition (TPN) with and without glutamine dipeptide supplmentation on outcome in severe acute pancreatitis (SAP) Clinical Nutrition Supplements. 2004;1:43–47. [Google Scholar]
- 46.Alhan E, Turkyilmaz S, Ercin C, et al. Effects of omega-3 fatty acids on acute necrotizing pancreatitis in rats. Eur Surg Res. 2006;38(3):314–321. doi: 10.1159/000094019. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Li W, Li N, et al. Omega-3 fatty acids-supplemented parenteral nutrition decreases hyperinflammatory response and attenuates systemic disease sequelae in severe acute pancreatitis: a randomized and controlled study. JPEN J Parenter Enteral Nutr. 2008;32(3):236–241. doi: 10.1177/0148607108316189. [DOI] [PubMed] [Google Scholar]