Abstract
Many elementary chemical and physical processes such as the breaking of a chemical bond or the vibrational motion of atoms within a molecule take place on a femtosecond (fs = 10−15 s) or picosecond (ps = 10−12 s) time scale. It is now possible to monitor these events as a function of time with temporal resolution well below 100 fs. This capability is based on the pump-probe technique where one optical pulse triggers a reaction and a second delayed optical pulse probes the changes that ensue. To illustrate this capability, the dynamics of ligand motion within a protein are presented. Moving beyond casual observation of a reaction to active control of its outcome requires additional experimental and theoretical effort. To illustrate the concept of control, the effect of optical pulse duration on the vibrational dynamics of a tri-atomic molecule are discussed. The experimental and theoretical resources currently available are poised to make the dream of reaction control a reality for certain molecular systems.
Any chemical process taking place on a large industrial or smaller laboratory scale is the result of the reaction of single molecules in well-defined states. The observed outcome of a chemical reaction is the average over all single intermolecular interactions that might occur. A thorough investigation of the underlying principles of such events requires the ability to monitor them as a function of all external parameters and, in particular, as a function of time. This is an ambitious undertaking because the spatial and temporal scales for molecular motion are small beyond our imagination.
To be more specific, consider the simple internal dynamics of the I2 molecule. The period of the vibrational motion of the nuclei in an electronically excited state is about 300 fs. How can we measure the change of the bond length as a function of time? Conventional spectroscopy can determine the average bond length in a sample of I2 molecules but naturally cannot detect temporal changes taking place on the femtosecond time scale. Moreover, when the duration of the optical radiation used to excite the sample is long compared with its vibrational dynamics, the average value of the bond length does not change with time. In the language of quantum mechanics, which gives the proper framework to describe the interaction between molecules and light, a pulse that is long compared with the vibrational period prepares the molecule in a stationary state. On the other hand, a pulse that is short compared with the time scale of the vibrational motion prepares the ensemble of molecules in a nonstationary state, which also is called a wave packet. For a wave packet, the average bond length does change with time. Consequently, when an ultrashort pulse is sent through the molecular sample, a temporal change in the bond length is initialized. This can be regarded as the starting of a clock and is called the pump process (1). Next, a time-delayed laser pulse, the probe pulse, is applied to the system. Afterward, one measures the signal that results from the interaction of the sample with both pulses. The temporal information is obtained by varying the delay time between the pump and probe pulses. Because the average bond length changes in time, the signal depends on time, thus reflecting the vibrational dynamics. Examples of signals that can be measured include fluorescence, ion yield, or coherently emitted radiation.
Pump-probe schemes similar to that described above have been used to study many elementary processes in biology, chemistry, and physics (2–5). In what follows we will highlight several aspects that were discussed at the meeting.
Probing Molecular Dynamics
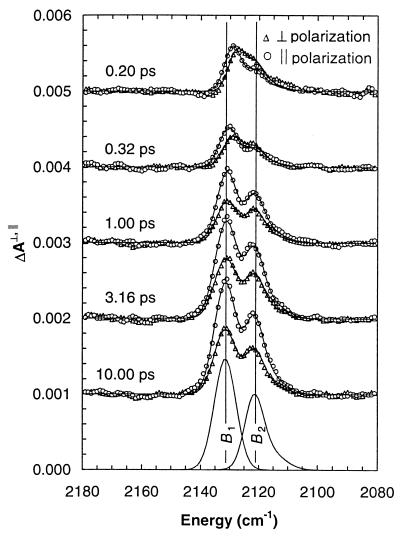
Femtosecond time-resolved IR spectroscopy has been used to probe the dynamics of ligand motion within a protein. The system studied, carbon monoxymyoglobin, is an ideal candidate for detailed investigations; the bound CO can be detached with an ultrashort optical pulse and its subsequent motion can be probed through its IR absorbance spectrum (6–8). By measuring IR spectra with pulses polarized both parallel and perpendicular to the polarization of the photolysis pulse, the time-dependent CO orientation also can be determined. The time-resolved spectra shown in Fig. 1 suggest that CO is quickly transported to a ligand docking site along one of two trajectories. The faster trajectory rotates with a 200-fs time constant and leads to B1, whereas the slower trajectory rotates with a 500-fs time constant and leads to B2. The feature denoted B1 arises when the O end of CO points toward the heme iron, and B2 arises from the opposite orientation. The docking site inhibits the rebinding of CO and thereby facilitates the efficient expulsion of this toxic ligand from the protein.
Figure 1.
Time-resolved polarized IR absorbance spectra of photolyzed carbon monoxymyoglobin. The features labeled B1 and B2 arise from two CO dissociation trajectories, each of which leads to a docking site where CO is constrained to lie in a direction nearly orthogonal to its orientation when bound to the heme.
To go beyond the view of spectroscopy, ultrafast structural techniques are being developed. For example, time-resolved x-ray crystal structures of carbon monoxymyoglobin have been acquired at the European Synchrotron and Radiation Facility in Grenoble, France with 7.5-ns time resolution (9). Further experiments by Wulff and collaborators there are being carried out with about 100-ps time resolution. Ultrafast electron diffraction also is being developed (10, 11). Given the current pace of new technological developments, the prospects for directly monitoring the motion of nuclei throughout the course of a chemical reaction are becoming ever brighter.
Control of Molecular Dynamics
In a subsequent step, theoretical and experimental efforts have been undertaken to develop control concepts for chemical reactions. For small molecules such as Na3, it was shown that ultrashort pulses can prepare a particular state of the system and thus can trigger a characteristic dynamical behavior (12). The effect of selective state preparation is achieved by variation of the exciting pump pulse duration. Whereas a 120-fs pulse prepares a symmetrical vibrational motion with a period of 320 fs, a pulse of 1.5-ps length results in a quite different vibrational dynamics, with temporal changes taking place over several ps.
One ambitious aim is to actively control the outcome of a chemical reaction that is driven by a laser pulse (13, 14). A primary question is whether selective control of molecular dynamics can be realized in larger molecular systems. For example, the energy absorbed from the laser field might rapidly dissipate among the many vibrational and rotational degrees of freedom of the molecule and might, as a consequence, compromise selectivity. It is encouraging to note that most recent theoretical studies on isomerization reactions predict that active control of such reactions in larger molecules is possible (15). Nevertheless, this capability has to be verified experimentally. Especially promising experimental approaches are those that use the concept of feedback, where the response of the molecules to the external fields serves as input in the design of laser pulses (spectral and temporal properties) that optimally direct the reaction along a preferred channel (16). Recent results suggest that feedback-based learning algorithms provide an efficient means for designing optical pulses that optimize a desired outcome (17). Although this technology will not become industrially important (a mole of photons is a very expensive reagent), it certainly will help to develop a deeper understanding of the interactions between light and matter as well as molecular reactivity.
References
- 1.Zewail A H. Sci Am. 1990;262:107–114. [Google Scholar]
- 2.Zewail A H. Femtochemistry. 1 and 2. Singapore: World Scientific; 1994. [Google Scholar]
- 3.Manz J, Wöste L, editors. Femtosecond Chemistry. Weinheim: VCH; 1995. [Google Scholar]
- 4.Chergui M, editor. Femtochemistry. Singapore: World Scientific; 1996. [Google Scholar]
- 5.Sundström V, editor. Femtochemistry and Femtobiology. London: Imperial College Press; 1997. [Google Scholar]
- 6.Lim M, Jackson T A, Anfinrud P A. Science. 1995;269:962–965. doi: 10.1126/science.7638619. [DOI] [PubMed] [Google Scholar]
- 7.Lim M, Jackson T A, Anfinrud P A. J Chem Phys. 1995;102:4355–4366. [Google Scholar]
- 8.Lim M, Jackson T A, Anfinrud P A. Nat Struct Biol. 1997;4:209–214. doi: 10.1038/nsb0397-209. [DOI] [PubMed] [Google Scholar]
- 9.Srajer V, Teng T, Ursby T, Pradervand C, Ren Z, Adachi S, Schildkamp W, Bourgeois D, Wulff M, Moffat K. Science. 1996;274:1726–1729. doi: 10.1126/science.274.5293.1726. [DOI] [PubMed] [Google Scholar]
- 10.Williamson J C, Cao J, Ihee H, Frey H, Zewail A H. Nature (London) 1997;386:159–162. [Google Scholar]
- 11.Dantus M, Kim S B, Williamson J C, Zewail A H. J Phys Chem. 1994;98:2782–2796. [Google Scholar]
- 12.Reischl B, de Vivie-Riedle R, Rutz S, Schreiber E. J Chem Phys. 1996;104:8857–8864. [Google Scholar]
- 13.Gaspard P, Burghardt I, editors. Advances in Chemical Physics. Vol. 707. New York: Wiley; 1997. Chemical Reactions and Their Control on the Femtosecond Time Scale, XXTH Solvay Conference on Chemistry. [Google Scholar]
- 14.Manz J. In: Femtochemistry and Femtobiology. Sundström V, editor. London: Imperial College Press; 1997. pp. 80–318. [Google Scholar]
- 15.Manz J, Sundermann K, de Vivie-Riedle R. Chem Phys. 1998;290:415–422. [Google Scholar]
- 16.Zhu W, Botina J, Rabitz H. J Chem Phys. 1998;108:1953–1963. [Google Scholar]
- 17.Assion A, Baumert T, Bergt M, Brixner T, Kiefer B, Seyfried V, Strehle M, Gerber G. Science. 1998;282:919–922. doi: 10.1126/science.282.5390.919. [DOI] [PubMed] [Google Scholar]