Abstract
Most nanomaterials enter the natural environment as nano-enabled products, which are typically composites with primary nanoparticles bound on substrates or embedded in liquid or solid matrices. The environmental risks associated with these products are expected to differ from those associated with the as-produced particles. This article presents a case study on the end-of-life emission of a commercial prototype polymer/quantum-dot (QD) composite used in solid-state lighting for homes. We report the extent of cadmium release upon exposure to a series of environmental and biological simulant fluids, and track the loss of QD-characteristic fluorescence as a marker for chemical damage to the CdSe/ZnS nanoparticles. Measured cadmium releases after 30-day exposure range from 0.007-1.2 mg/g of polymer, and the higher values arise for low-pH simulants containing nitric or gastric acid. Centrifugal ultrafiltration and ICP was used to distinguish soluble cadmium from particulate forms. The leachate is found to contain soluble metals with no evidence of free QDs or QD-containing polymeric debris. The absence of free nanoparticles suggests that this product does not raise nanotechnology-specific environmental issues associated with degradation and leaching, but is more usefully regarded as a conventional chemical product that is a potential source of small amounts of soluble cadmium.
INTRODUCTION
A major international effort is underway to identify and manage the potential risks of nanotechnology to human health and the environment.1-4 Most research in this area focuses on primary nanomaterials, whose behavior is directly relevant to occupational risks in nanomanufacturing facilities.5 Consumer and environmental risks, in contrast, are most often associated with nano-enabled products, which are typically composites in which primary nanomaterials are bound on substrates or embedded in liquid or solid matrices.6 A particularly common product formulation is a polymeric matrix with embedded nanomaterials that enhance the optical, mechanical, or electronic properties or impart wholly new function to the base polymer.
It is widely understood that the consumer and environmental risks associated with nano-enabled consumer products may differ from those of the primary particles on which most current research is based. Embedded nanoparticles (NPs) typically have lower exposure potential than free particles,7 but exposure can occur in principle if there is damage to or degradation of the polymer matrix during use, misuse, or disposal.8,9 The exposure can be to liberated particles, chemical components of degraded particles, or particle-containing polymer wear debris.
In one example, Wohlleben et al.9 reported that no free nanomaterials were liberated by sanding or weathering of polymeric and cementitious nanocomposites. While the wear debris contained nanofillers, some of which appeared on the debris particle surfaces, there was no apparent toxicological effect of the nanofiller component of the composite debris. It is a challenge to study nanocomposite risks in any general way, because each commercial product is a unique formulation, and both the degradation processes and the resulting debris depend on the specific manner in which the product is stressed during use or disposal. Nevertheless, more information and data are needed on the important topic of nanocomposite risks, and in light of the challenges cited above, a practical way forward is in the consideration of commercial product case studies.
One emerging nano-enabled consumer product is the Quantum Light™ Optic of QD Vision Inc. (Lexington, MA). The optic is an acrylate polymer with embedded quantum dots (QDs) used as a faceplate on light-emitting diode (LED) lamps to shift the output spectrum to more closely resemble incandescent lamps, which have higher consumer acceptance. LED lamps are more efficient than incandescent lamps and their widespread acceptance would lead to significant energy savings and environmental protection. For illustration, today’s typical luminous efficiencies of LED and incandescent lamps are 113 Lm/W and 17 Lm/W respectively,10 and complete substitution in the world market would lead to approximately 1,000 TWh/yr energy savings and 200 million tones reduction in CO2 emissions.11 The use of quantum dots for spectral correction saves an additional 25 - 40% of energy relative to LEDs with broadband downconversion material such as rare-earth phosphors. QD-enabled LED lighting products with 250 Lm/W are within reach in the coming decade. Quantum dots absorb blue light and re-emit the energy through fluorescence downconversion at longer wavelengths in a very narrow band (~30 nm full-width at half of maximum (FWHM)) of color, while conventional phosphors have a typical FWHM of 90 - 120 nm, resulting in significant deep red and infrared emission. Realizing the energy and environmental benefits of QD-enhanced LED technologies will require careful consideration of their potential risk and net benefits as nanoproducts.
Quantum dots in the form of suspended primary particles have been the subject of numerous environment and health-oriented studies, reviewed by Hardman12 and Pelley et al..13 QDs have been reported to be benign or toxic depending on the specific material, biological system, dose, and length of test.12,13 Adverse responses have been attributed to associated cadmium ions, NP surfaces, or a combination of the two.14,15 Many studies have considered the effects of QD formulation, in the sense that toxicity is reported to be sensitive to the presence and chemical nature of stabilizing shells, ligands, and other conjugated moieties.12,13 Essentially all of the studies reviewed12,13 focus on primary QDs rather than composites.
A subset of the literature deals with the issue of QD stability. Mahendra et al.16 report bacterial toxicity of quantum dots due to cadmium or selenite ion, but only after a pre-weathering step under acidic or basic conditions to destabilize the dots and cause ion release. Navarro et al.17 provide evidence for degradation of quantum dots in soil and study both particle and ion mobility in soil columns. King-Heiden et al.14 report little QD degradation during zebrafish exposure, and a toxicity mechanism that involves both particles and associated ions. Hardman12 notes that adverse effects are more typically seen in longer term studies. This may reflect the time required for the relevant toxicity pathways, or the increased toxicity of QDs over time due to slow degradation and release of toxic cadmium and selenium species. A number of studies report toxicity mitigation by inclusion of a ZnS shell13, which may reflect stabilization of the CdSe core to ion release or passivation of surface reactions. These degradation studies also focus on primary quantum dots rather than composite materials. Relevant to polymer composites is a significant literature on (pure) polymer degradation, and there have been several published life cycle analyses on polymer/nanoclay composites.18,19 To our knowledge, there have been no studies of the environmental or health implications of quantum dot composite materials.
The quantum dot product chosen for this case study is not a structural material that is likely to be drilled, sawed or otherwise modified by consumers, so the main issue in environmental risk is end-of-life behavior. Common environmental fates include landfilling, incineration, improper disposal and product recycling. In this study we focus on landfilling, where the optics will be exposed to fluid phases and may leach heavy metals, liberated QDs or particle-containing polymeric debris. Cadmium, selenium, and zinc are known environmental toxicants. If present in soluble form in the leachate, they represent chemical pollutants whose risks may be assessed by conventional methods without the need for new information on unique properties or behaviors that emerge at the nanoscale. If NPs are liberated, however, they may have environmental mobilities, bioaccumulation propensities, and toxicities that are different from the soluble forms of the constituent elements. The presence of NPs in leachate does not necessarily imply an elevated risk, but does imply uncertainty that warrants special consideration and possibly new regulation. A key question for risk assessment and regulation of nanocomposites, therefore, is whether or not disposal leads to NP liberation to the natural environment.20
The present article examines the end-of-life behavior of the Quantum Light™ Optic as a case study in nanocomposite environmental risks. The study focuses on the extent and forms of Cd release using a prototype optic in commercial size and form and a range of fluid simulants. A particular interest is to determine whether this material liberates NPs following shredding and long-term fluid exposure, which would imply a nanotechnology-specific environmental issue, or whether the product liberates soluble cadmium and is more usefully regarded as a conventional chemical product that is a potential source of leachate fluids containing small amounts of soluble cadmium.
MATERIALS AND METHODS
Materials
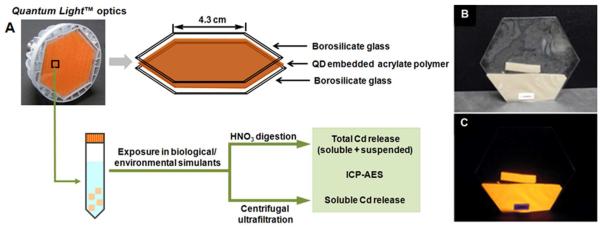
Prototype Quantum Light™ optics were provided by QD Vision Inc. (Lexington, MA). The optic consists of two borosilicate glass plates with a thin, hard, polygonal sheet of acrylate polymer matrix embedded with QDs (Fig. 1A). The QDs employed in the optic were approximately 50/50-wt% CdSe/ZnS core/shell structures with a core diameter of ~5 nm.21 The polymer-QD nanocomposite had a beige color under visible light (Fig. 1B) and a bright orange color under UV irradiation (Fig. 1C). The bare CdSe/ZnS QDs suspended in toluene and a QD-free optic assembly were also provided as reference samples. Eight environmental or biological simulant fluids were used (Table 1). All solutions were prepared in deionized (DI) water (18.3 M · cm). Pepsin (from porcine gastric mucosa) was purchased from Sigma-Aldrich, and natural organic matter (NOM, Suwannee River Humic Acid Standard II and Fulvic Acid Standard I) were purchased from International Humic Substances Society.
Figure 1.
The prototype optic and procedure to determine soluble and particulate cadmium release. (A) Exploded sketch of the optic, and illustration of the leaching procedure. Photograph of optic with part of the polymer removed under (B) visible light and (C) 365 nm UV irradiation.
Table 1.
Environmental and Biological Simulant Fluids
| Simulant fluids | Components | pH |
|---|---|---|
| Nitric acid (positive control) | 1 M HNO3 | 0.16 |
| Dilute peroxide (mild oxidant) | 1 mM H2O2 | 5.81 |
| Phosphate buffered saline (PBS) | 8.01 g/L NaCl, 0.20 g/L KCl, 1.44 g/L Na2HPO4, 0.24 g/L KH2PO4 |
7.38 |
| Gastric acid22 | 2 g/L NaCl, 7 mL/L 37% HCl, 3.2 g/L pepsin | 1.12 |
| Toxicity characteristic leaching procedure extraction fluid #1 (TCLP)*23 |
5.7 mL/L glacial acetic acid and 2.572g/L NaOH | 4.93 |
| Moderately hard reconstituted water (MHRW)24 |
0.096 g/L NaHCO3, 0.06 g/L CaSO4•2H2O, 0.06 g/L MgSO4, 0.004 g/L KCl |
8.02 |
| Humic acid (SHA) | 20 mg/L humic acid in MHRW | 7.71 |
| Fulvic acid (SFA) | 20 mg/L fulvic acid in MHRW | 7.47 |
TCLP standard extraction procedure was not used
Sample Characterization
Bright field and fluorescence images of fresh- and simulant fluid treated-samples were acquired on an Olympus IX71 Inverted Microscope equipped with a UPlanApo 10× objective. The fluorescence images were analyzed using CellSens® Dimension Imaging software to obtain the mean fluorescence intensity of the acquired area (detailed procedure is provided in supporting information, SI). Sample surface morphologies and roughness were examined by atomic force microscope (AFM). AFM samples were prepared by exposing QD-embedded polymer samples to simulant fluids for 2 years. The samples were then rinsed with DI water, dried, and observed with a D3100 AFM (Veeco, Inc.) using contact mode in air. Gravimetric water absorption was determined by immersing pre-rinsed and oven dried QD-embedded polymer in DI water for up to 9 days, after which the weight of polymer was re-measured after gently blotting the surface water. Selected 30-day leachates were subjected to UV-vis and fluorescence analysis with bare CdSe/ZnS QDs as a control for the possible presence of free NPs. QDs were mixed with MHRW and SHA fluids at concentration of 0.5 ~ 1000 mg/L. After rotating at 60 rpm in dark for 10 days, the UV-vis spectra of QD aqueous suspensions were recorded on a V-630 Spectrophotometer (Jasco, MD), and the fluorescence intensity was measured on a SpectraMax M2 multiplate reader (Molecular Devices, CA) with excitation at 490 nm and emission at 606 nm.
Cadmium Release Experiment
The release of cadmium (soluble and/or suspended) from the prototypes (Fig. 1) was tested in eight environmental or biological simulant fluids (Table 1). The original glass-covered optic assembly and QD-embedded acrylate polymer were cut into small pieces (~ 7 mm×7 mm), exposed to simulant fluid at 27.4 mg/mL and 1 mg/mL respectively, and rotated at 60 rpm in dark or under ambient light for up to 30 days. The fluid phases were exchanged completely with fresh simulants after 1, 4, 7, 14 and 30-day exposure, and the cadmium release was measured by inductively coupled plasma-atomic emission spectrometer (ICP-AES) on a Jobin Yvon Emission JY2000 after HNO3 digestion for total (soluble and suspended) cadmium release or after centrifugal ultrafiltration (Amicon Ultra-4 3K MW cutoff, 30 min at 4000 g) to remove particles larger than 1-2 nm for soluble cadmium. Multiple metal analysis (Cd, Se and Zn) was performed on selected lechates using ICP-AES.
Cadmium Partitioning
The ability of liberated cadmium ions to bind to the base polymer was characterized by incubating QD-free polymer in CdCl2 solutions (1 mg/L Cd2+ in DI water or 1 mM H2O2) at a concentration of 1 mg polymer/mL, followed with rotation at 60 rpm in dark and analysis of aqueous cadmium concentration for up to 4 days. Soluble cadmium binding to NOM was determined by measuring Cd2+ depletion in Cd2+ - NOM mixture solutions after removal of NOM with centrifugal ultrafiltration. A kinetic experiment was carried out by adding CdCl2 to MHRW and NOM (20 mg/L SHA or SFA) at a Cd2+ concentration of 1 mg/L followed with rotation at 60 rpm for 0.5 ~ 6 hours, after which free Cd2+ phase was collected with ultrafiltration and analyzed using ICP-AES. Based on the rapid adsorption kinetics, a one-hour equilibration time was chosen to measure equilibrium Cd2+/NOM adsorption isotherms at 25 °C for starting Cd2+ concentrations at 0.02 ~ 20 mg/L.
RESULTS
Characterization of Prototype Optic
The QD-embedded polymer surface appears to be covered with a thin layer of adhesive, which is easily rinsed off with water as shown by AFM (Fig. 2A and B) and optical microscopy (SI, Fig. S1A and B). The samples show bright red/orange fluorescence (Fig. 1C, Fig. S1C) and the presence of surface pores with average diameter of 80 μm. The gravimetric water absorption by the pre-rinsed sample is 0.112 g/g polymer. The AFM images (Fig. 2) exhibit no significant roughening after incubation in peroxide or nitric acid, suggesting the stability of the polymer to the simulant fluids. One sample was immersed in HNO3 over the entire course of this study (two years) and remained intact with the appearance only of shallow surface pits demonstrating the high resistance of the polymer to chemical damage.
Figure 2.
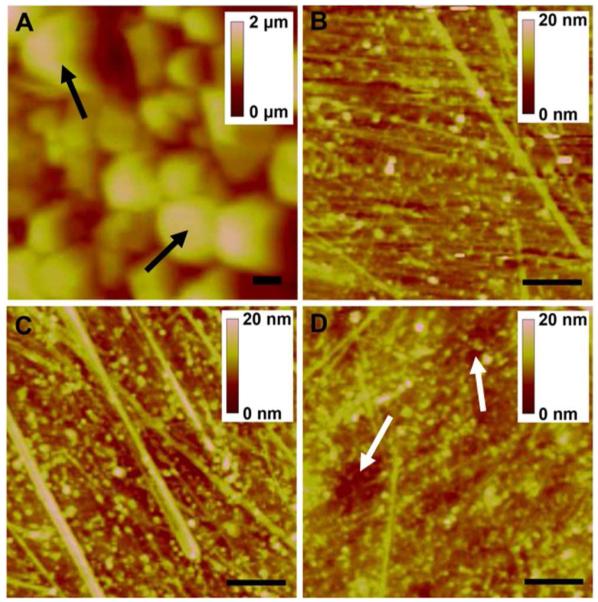
AFM images of QD-embedded polymer that are (A) as-received; (B) rinsed with DI water; (C) incubated in 1 mM H2O2 for 2 years; and (D) incubated in 1M HNO3 for 2 years. Black arrows in (A) show granule like surface adhesive coating, and white arrows in (D) show shallow pits. Scale bars are 1 μm.
Cadmium Release
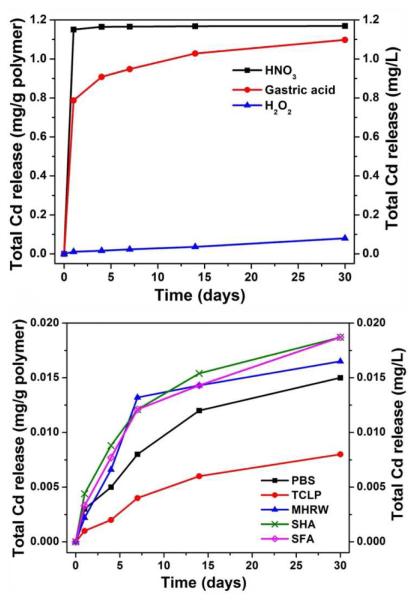
We conducted release experiments in ambient light and in the dark for the full set of simulant fluids, and found no significant effect of light on Cd release (SI, Fig. S2). The remainder of the article reports only data taken in the dark. Figure 3 gives the time-resolved total (soluble and suspended) cadmium release from QD-embedded polymer. Nitric and gastric acids may destroy the ZnS shell,25,26 which facilitates the acidic etching of CdSe core26,27and the mobilization of Cd. Hydrogen peroxide (1 mM) released less than 7 % of the amount released in HNO3 over 30-day exposure, which is lower than the 16% reported by Derfus et al.28 for mercaptoacetic acid capped CdSe QDs after 24 hr H2O2 exposure. To understand whether Cd could be solubilized from the dots but remain complexed within the polymer, we characterized the ability of Cd2+ to adsorb on QD-free polymer samples (SI, Fig. S3). The 24-hr exposure of QD-free polymer to 1 mg/L Cd2+ gave 0.002 mg Cdad/g polymer in DI water and 0.004 mg Cdad/g polymer in 1 mM H2O2. Based on these controls, the complexation of solubilized Cd2+ to the polymer has a negligible effect on the data in Fig. 3. We then performed a more complete elemental analysis of the 30-day filtrates (Fig. 4). The CdSe/ZnS QDs used in the prototype optic are protected by a thick (50 wt%) ZnS shell that improves chemical stability. Figure 4 shows that less than 8% Zn2+ is liberated after 30-day H2O2 exposure, suggesting ZnS shell is only partially dissolved, possibly due to the embedded structure of QDs in polymer matrix and the formation of slightly soluble Zn(OH) (Ksp =3×10−17) as an oxidation product29.
Figure 3.
Time-resolved total cadmium release from QD-embedded polymer. Experiments were conducted with 1 mg polymer/mL solution at 25 °C in the dark.
Figure 4.
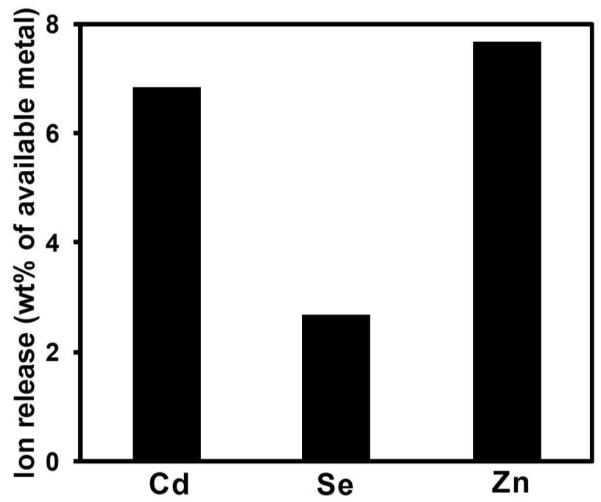
Multiple element release from QD-embedded polymer after 30-day exposure in 1 mM H2O2, suggesting ZnS shell was partially dissolved.
The Fig. 3 and 4 experiments were conducted on polymer sections removed from the glass cover plates in the prototype optic to simulate breakage during handling and disposal. Experiments on pieces of the full prototype optic show that the glass plates provide negligible shielding (SI, Fig. S4A), which is likely due to the ease with which the polymer is separated from its protective housing during rotation in the incubation step (SI, Fig. S4B).
Forms of Released Cadmium – Ion versus Nanoparticle
Of particular interest in this study are the forms of released cadmium – does the prototype liberate NPs or only soluble cadmium? Cadmium release was tracked over 30-day fluid exposure after HNO3 digestion for total cadmium (particles and ions) or after centrifugal ultrafiltration for soluble cadmium. Figure 5 gives the accumulated 30-day total Cd release versus soluble Cd release, indicating that soluble ions are the predominant cadmium species. The only significant difference between total and soluble cadmium release was observed in the environmental simulants containing NOM (SHA, SFA). We considered the possibility that the insoluble Cd in those two filtrates was: (i) free QD NPs, (ii) polymer debris containing QDs, (iii) Cd2+ bound to NOM macromolecules that are captured on the 3k MW ultrafilter and reported as condensed-phase cadmium. Possibilities (i) and (ii) could arise if the polymer degrades to produce hydrophobic debris that adheres to the bulk optic, and the debris transports to the filtrate only in the presence of NOM that acts as a surfactant. In a related observation, Navarro et al.30 reported that NOM facilitated phase transfer of organic ligand capped CdSe QDs from organic solvent to the water phase. Here we used UV-vis and fluorescence spectrometry to look for suspended QDs in the filtrates but could not detect any spectral features characteristic of QDs. Control experiments with bare QDs and SHA revealed detection limits of 50-100 mg/L, which are too high for this technique alone to be sufficient in ruling out (i) and (ii).
Figure 5.
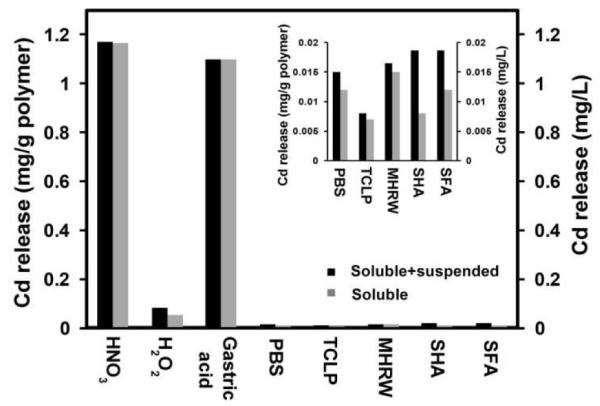
Forms of Cd release from QD-embedded polymer, showing soluble Cd is the predominant form in most filtrates. Exposure for 30 days under 60 rpm rotation in the dark.
To assess possibility (iii), we characterized the binding of Cd2+ to both SHA and SFA under conditions existing in the fluid phases of this study. We first studied the kinetics of binding (SI, Fig. S5A), from which a suitable exposure time was chosen for measurement of equilibrium adsorption isotherms (SI, Fig. S5B). The maximum adsorption capacity of Cd2+ on NOM is 0.156 mg/mg SHA and 0.131 mg/mg SFA using Langmuir-Freundlich (LF) model31,32 fitting (SI for details). These isotherms can be used to show that Cd2+/SHA and Cd2+/SFA complexes fully account for the difference between soluble and total Cd release without the need to invoke particle release (see discussion).
Fluorescence as a probe for QD damage
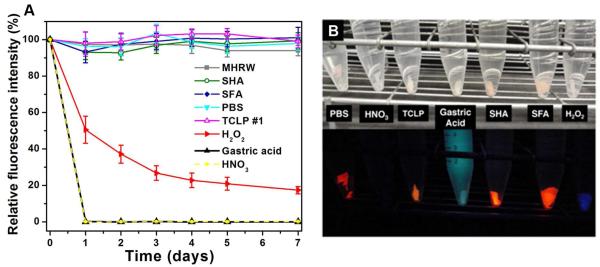
Fluorescence microscopy was also used to monitor QD degradation. Figure 6A shows the QD polymer film loses fluorescence signal rapidly in HNO3 and gastric acid in parallel with fast cadmium release. Fluorescence bleaching is more pronounced at the edge and sometimes in the vicinity of pores (SI, Fig. S6), and is easily observed under UV irradiation (Fig. 6B). In most other simulants (PBS, TCLP, MHRW, SHA, and SFA) the fluorescence intensity remains constant as a result of polymer matrix shielding action, consistent with the low Cd release. Cadmium release is also low in 1 mM H2O2; however, there is a continuous drop in fluorescence intensity. It has been reported that CdSe QDs are susceptible to oxidation by hydroperoxides,33 O2/photons,34 cyclohexenyl peroxide,35 and the formation of CdSeOx at the CdSe/ZnS interface has been suggested to cause blue shift and decay of the primary fluorescence peak.34 We also considered the possibility that Cd and Se are first mobilized and then precipitated in the H2O2 experiment, but the relevant oxide phases are too soluble to support this hypothesis. The most likely mechanism is oxidative damage to the QDs and loss of fluorescence with minimal Cd/Se leaching.
Figure 6.
Fluorescence as a probe for QD damage. (A) Time-resolved relative fluorescence intensity of QD-embedded polymer during fluid exposure. (B) Photographs of QD-embedded polymer after 30-days exposure under visible light (top) and 365 nm UV irradiation (bottom). Note that the light blue color in gastric acid is characteristic of pepsin,36 and the dark blue color of the polymer in H2O2 is possibly the result of light scattering.
DISCUSSION
Most nanomaterials are sold as formulated composites, and more information is needed to understand and manage their environmental risks. Here as a case study we examined the end-of-life stability of a commercial prototype quantum-dot/acrylate polymer composite that is a component in an emerging consumer product for advanced LED lighting. We report cadmium release (1.10-1.20 mg/g polymer) into two low-pH simulant fluids (1 M HNO3 and gastric acid) and much smaller releases (< 0.10 mg/g polymer) from other simulants (1 mM H2O2, PBS, TCLP, MHRW, SHA and SFA). The cadmium release is accompanied by loss of the QD-characteristic fluorescence, indicating chemical damage to the CdSe/ZnS NPs. The release is time dependent over 30 days but is not ambient light dependent. In addition, there is no apparent structural damage to the acrylate polymer matrix during exposure. Peroxide treatment destroys dot fluorescence and optical absorption but does not release appreciable cadmium, which is consistent with QD damage and formation of insoluble oxide phases, and suggested the loss of fluorescence of QD-composites alone is not sufficient to indicate the release of QDs or their components.
A central question for assessing nano-specific risk is whether the prototypes release free quantum dots or suspended polymeric debris containing embedded QDs. Centrifugal ultrafiltration through regenerated cellulose filters (3k MW cutoff) removes even 1-2 nm particles37 and here demonstrates that the Cd release is in soluble form not particulate form. The data for humic and fulvic acid simulants initially appeared to be exceptions, as they showed a significant difference between unfiltered (total) Cd and filtered (soluble only) Cd. We thought initially that QDs or polymer debris were being liberated from the product but remain reattached to the polymer film by hydrophobic forces and thus did not report as suspended particulate unless NOM was present. Humic and fulvic acids are known to aid in the dispersion and phase transfer of hydrophobic nanomaterials.30,38 An alternative hypothesis, however, is that the NOM binds soluble Cd and are removed themselves by ultrafiltration causing a part of the soluble Cd to report as “suspended matter”. The clear color of the filtrate indicates that NOM is captured by the ultrafilter, but the amount of complexed Cd they contain is unknown. The measured adsorption isotherms for Cd2+/SHA and Cd2+/SFA (Fig. S5B) were used to estimate the bound Cd2+ in equilibrium with the free Cd2+ in the leachate. Figure 7 (also see SI, Fig. S7) shows that Cd complexation on SHA/SFA can fully account for difference between filtered and unfiltered leachates. We thus find no evidence for the release of QDs or QD-containing polymeric debris in any of the simulant fluids, which is consistent with our separate observations that the filtrates lack any QD-characteristic fluorescence and that there is no apparent degradation of the polymer matrix.
Figure 7.
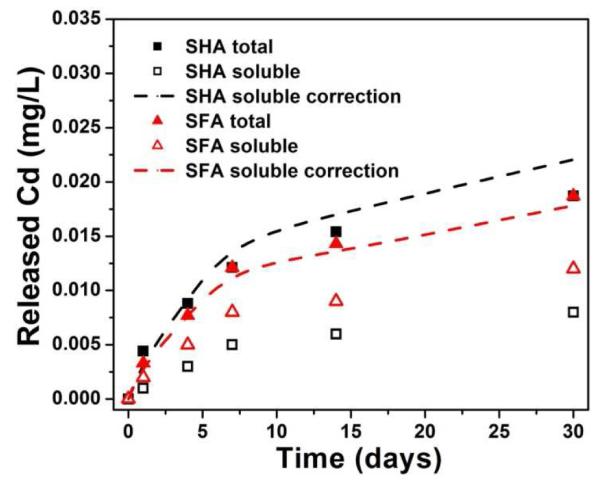
Total and soluble Cd release from QD-embedded polymer in the presence of SHA and SFA (points) and correction of the soluble release for Cd2+ bound to NOM (dashed lines). Experiments were conducted in the dark for 30 days. The correction was calculated from the Langmuir-Freundlich isotherms in Fig. 6, and strongly suggests the difference between total and soluble Cd is due to adsorption on NOM.
The data provide some insight into the release mechanism. The complete loss of color and fluorescence, along with the extent of Cd release in low-pH media, suggests bulk solvent penetration rather than surface leaching. For the ~100 μm thick polymer films, diffusion times can be estimated as L2/Deff, where Deff= Dθ/τ using the 11% water infiltration to estimate porosity, θ, a tortuosity factor, τ of 4, and a typical liquid-phase D value of 10−5 cm2/s. The characteristic diffusion time for decomposition products estimated in this way is of order ~5 min (see SI for details), consistent with the release data in low-pH media. We suggest that the release rate for the neutral pH simulants is limited by dissolution reaction kinetics rather than water infiltration or product diffusion.
Overall, our data suggest a mechanism of solution infiltration, CdSe/ZnS partial dissolution, and out-diffusion of the dissolution products from the polymer matrix, which remains intact. This material thus behaves like conventional Cd-containing material rather than a nano-product, though of course the nanoscale nature of the CdSe/ZnS certainly plays a role in the rates and extents of Cd release. It is difficult to say if this “in situ NP degradation” mechanism is a general trend in NP/polymer composites, and this difficulty is an inherent limitation in the case study approach. One could nevertheless imagine that the leaching of soluble vs. nano-particulate material is governed by the relative rates of polymer and NP degradation. Stable hydrophilic polymers can imbibe water and allow attack and slow dissolution of NP components to produce soluble NP degradation products in the filtrate, as observed here. In contrast, a polymer that degrades readily could lose structural integrity and liberate NPs or NP-containing polymer debris that would warrant additional consideration. While there is much interest in the environmental benefits of biodegradable materials, in the case of NP composites it may be more desirable to have non-biodegradable matrices that either sequester the NP filler indefinitely, or allow it to degrade in situ as observed here. There are also needs to build standard release testing procedure for better assessing the potential environmental impact of NP composites.
Supplementary Material
ACKNOWLEDGEMENT
Financial support was provided by EPA STAR grant R833862, and the Superfund Research Program of the National Institute of Environmental Health Sciences, P42ES013660. We gratefully acknowledge Joseph Orchardo at Brown University for the technical support in ICP-AES.
Footnotes
Supporting Information. Detailed procedure for analyzing fluorescence intensity of QD-embedded polymer, Langmuir-Freundlich (LF) fitting of Cd2+ adsorption on NOM, estimation of characteristic diffusion time, optical images of fresh QD-embedded polymer, 30-day total Cd release under dark and light exposure, time-dependent Cd2+ adsorption on QD-free polymer, comparison of Cd release from base polymer and full prototype optic, adsorption kinetics and isotherms of Cd2+ on NOM, fluorescence image of aged sample, and Langmuir-Freundlich correction of soluble Cd release. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1).Colvin VL. The potential environmental impact of engineered nanomaterials. Nat. Biotechnol. 2003;21(10):1166–1170. doi: 10.1038/nbt875. [DOI] [PubMed] [Google Scholar]
- (2).Helland A, Scheringer M, Siegrist M, Kastenholz HG, Wiek A, Scholz RW. Risk assessment of engineered nanomaterials: a survey of industrial approaches. Environ. Sci. Technol. 2008;42(2):640–646. doi: 10.1021/es062807i. [DOI] [PubMed] [Google Scholar]
- (3).Meyer DE, Ann Curran M, Gonzalez MA. An examination of existing data for the industrial manufacture and use of nanocomponents and their role in the life cycle impact of nanoproducts. Environ. Sci. Technol. 2009;43(5):1256–1263. doi: 10.1021/es8023258. [DOI] [PubMed] [Google Scholar]
- (4).Wiesner MR, Lowry GV, Alvarez P, Dionysiou D, Biswas P. Assessing the risks of manufactured nanomaterials. Environ. Sci. Technol. 2006;40(14):4336–4345. doi: 10.1021/es062726m. [DOI] [PubMed] [Google Scholar]
- (5).Liao C, Chiang Y, Chio C. Model-based assessment for human inhalation exposure risk to airborne nano/fine titanium dioxide particles. Sci. Total. Environ. 2008;407(1):165–177. doi: 10.1016/j.scitotenv.2008.09.028. [DOI] [PubMed] [Google Scholar]
- (6).Khanna V, Bakshi BR, Lee LJ. Assessing life cycle environmental implications of polymer nanocomposites. Proceedings of the 2008 IEEE International Symposium on Electronics and the Environment; San Francisco, CA. May 19-21, 2008. [Google Scholar]
- (7).Lee J, Mahendra S, Alvarez PJJ. Nanomaterials in the construction industry: a review of their applications and environmental health and safety considerations. ACS Nano. 2010;4(7):3580–3590. doi: 10.1021/nn100866w. [DOI] [PubMed] [Google Scholar]
- (8).Nguyen T, Pellegrin B, Bernard C, Gu X, Gorham JM, Stutzman P, Stanley D, Shapiro A, Byrd E, Hettenhouser R, Chin J. Fate of nanoparticles during life cycle of polymer nanocomposites. J. Phys.: Conf. Ser. 2011;304(1):012060. [Google Scholar]
- (9).Wohlleben W, Brill S, Meier MW, Mertler M, Cox G, Hirth S, von Vacano B, Strauss V, Treumann S, Wiench K, Ma-Hock L, Landsiedel R. On the lifecycle of nanocomposites: comparing released fragments and their in-vivo hazards from three release mechanisms and four nanocomposites. Small. 2011;7(16):2384–2395. doi: 10.1002/smll.201002054. [DOI] [PubMed] [Google Scholar]
- (10).Khan N, Abas N. Comparative study of energy saving light sources. Renew. Sustain. Energy Rev. 2011;15(1):296–309. [Google Scholar]
- (11).Pimputkar S, Speck JS, Denbaars SP, Nakamura S. Prospects for LED lighting. Nat. Photonics. 2009;3:180–182. [Google Scholar]
- (12).Hardman R. A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ. Health Perspect. 2006;114(2):165–172. doi: 10.1289/ehp.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Pelley JL, Daar AS, Saner MA. State of academic knowledge on toxicity and biological fate of quantum dots. Toxicol. Sci. 2009;112(2):276–296. doi: 10.1093/toxsci/kfp188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).King-Heiden TC, Wiecinski PN, Mangham AN, Metz KM, Nesbit D, Pedersen JA, Hamers RJ, Heideman W, Peterson RE. Quantum dot nanotoxicity assessment using the zebrafish embryo. Environ. Sci. Technol. 2009;43(5):1605–1611. doi: 10.1021/es801925c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Cho SJ, Maysinger D, Jain M, R#x25FB;der B, Hackbarth S, Winnik FM. Long-term exposure to CdTe quantum dots causes functional impairments in live cells. Langmuir. 2007;23(4):1974–1980. doi: 10.1021/la060093j. [DOI] [PubMed] [Google Scholar]
- (16).Mahendra S, Zhu H, Colvin VL, Alvarez PJ. Quantum dot weathering results in microbial toxicity. Environ. Sci. Technol. 2008;42(24):9424–9430. doi: 10.1021/es8023385. [DOI] [PubMed] [Google Scholar]
- (17).Navarro DA, Banerjee S, Watson DF, Aga DS. Differences in soil mobility and degradability between water-dispersible CdSe and CdSe/ZnS quantum dots. Environ. Sci. Technol. 2011;45(15):6343–6349. doi: 10.1021/es201010f. [DOI] [PubMed] [Google Scholar]
- (18).Lloyd SM, Lave LB. Life cycle economic and environmental implications of using nanocomposites in automobiles. Environ. Sci. Technol. 2003;37(15):3458–3466. doi: 10.1021/es026023q. [DOI] [PubMed] [Google Scholar]
- (19).Roes AL, Marsili E, Nieuwlaar E, Patel MK. Environmental and cost assessment of a polypropylene nanocomposite. J. Polym. Environ. 2007;15(3):212–226. [Google Scholar]
- (20).Nowack B, Krug HF, Height M. 120 years of nanosilver history: implications for policy makers. Environ. Sci. Technol. 2011;45(4):1177–1183. doi: 10.1021/es103316q. [DOI] [PubMed] [Google Scholar]
- (21).Coe-Sullivan S, Linton JR, Sadasivan S, Squires EM. Solid state lighting devices including quantum confined semiconductor nanoparticles, an optical component for a solid state lighting device, and methods. 20110103064 US Patent Application. 2011 May 5;
- (22).Hamel SC, Ellickson KM, Lioy PJ. The estimation of the bioaccessibility of heavy metals in soils using artificial biofluids by two novel methods: mass-balance and soil recapture. Sci. Total Environ. 1999;243/244:273–283. doi: 10.1016/s0048-9697(99)00402-7. [DOI] [PubMed] [Google Scholar]
- (23).Test Methods for Evaluating Solid Waste, Physical/Chemical Method. U.S. Environmental Protection Agency; Method 1311 - TCLP, Toxicity Characteristic Leaching Procedure. SW-846. http://www.epa.gov/epawaste/hazard/testmethods/sw846/pdfs/1311.pdf. [Google Scholar]
- (24).Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. 5th ed. U.S. Environmental Protection Agency Office of Water; Washington, DC: 2002. http://water.epa.gov/scitech/methods/cwa/wet/upload/2007_07_10_methods_wet_disk2_atx.pdf. [Google Scholar]
- (25).Liu H, Ni Y, Han M, Liu Q, Xu Z, Hong J, Ma X. A facile template-free route for synthesis of hollow hexagonal ZnS nano- and submicro-spheres. Nanotech. 2005;16(12):2908–2912. [Google Scholar]
- (26).Wang L, Nagesha DK, Selvarasah S, Dokmeci MR, Carrier RL. Toxicity of CdSe nanoparticles in Caco-2 cell cultures. J. Nanobiotechnology. 2008;6:11. doi: 10.1186/1477-3155-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Guo W, Li JJ, Wang YA, Peng X. Luminescent CdSe/CdS core/shell nanocrystals in Dendron boxes: superior chemical, photochemical and thermal stability. J. Am. Chem. Soc. 2003;125(13):3901–3909. doi: 10.1021/ja028469c. [DOI] [PubMed] [Google Scholar]
- (28).Derfus AM, Chan WCW, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Letters. 2004;4(1):11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Jennings SR, Dollhopf DJ, Inskeep WP. Acid production from sulfide minerals using hydrogen peroxide weathering. Appl. GeoChem. 2000;15(2):235–243. [Google Scholar]
- (30).Navarro DAG, Watson DF, Aga DS, Banerjee S. Natual organic matter-mediated phase transfer of quantum dots in the aquatic environment. Environ. Sci. Technol. 2009;43(3):677–682. doi: 10.1021/es8017623. [DOI] [PubMed] [Google Scholar]
- (31).Janoš P, Sypecká J, Mlčkovská P, Kuráň P, Pilařová V. Removal of metal ions from aqueous solutions by sorption onto untreated low-rank coal (oxihumolite) Separ. Purif. Technol. 2007;53(3):322–329. [Google Scholar]
- (32).Umpleby RJ, Baxter SC, Chen Y, Shah RN, Shimizu KD. Characterization of molecularly imprinted polymers with the Langmuir-Freundlich isotherm. Anal. Chem. 2001;73(19):4584–4591. doi: 10.1021/ac0105686. [DOI] [PubMed] [Google Scholar]
- (33).Hay KX, Waisundara VY, Zong Y, Han M, Huang D. CdSe nanocrystals as hydroperoxide scavengers: a new approach to highly sensitive quantification of lipid hydroperoxides. Small. 2007;3(2):290–293. doi: 10.1002/smll.200600390. [DOI] [PubMed] [Google Scholar]
- (34).van Sark WGJHM, Frederix PLTM, Bol AA, Gerritsen HC, Meijerink A. Blueing, bleaching, and blinking of single CdSe/ZnS quantum dots. Chem. Phys. Chem. 2002;3(10):871–879. [Google Scholar]
- (35).Kim YT, Kim J, Lee J, Chae HK. Post-synthesis emission control of CdSe-ZnS quantum dots by surface oxidation through in situ generation of oxidant under mild reaction conditions. Chem. Lett. 2009;38(8):862–863. [Google Scholar]
- (36).Lakowicz JR, Weber G. Quenching of protein fluorescence by oxygen. Detection of structural fluctuations in proteins on the nanosecond time scale. BioChem. 1973;12(21):4171–4179. doi: 10.1021/bi00745a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Liu J, Hurt RH. Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ. Sci. Technol. 2010;44(6):2169–2175. doi: 10.1021/es9035557. [DOI] [PubMed] [Google Scholar]
- (38).Li Q, Xie B, Hwang Y, Xu Y. Kinetics of C60 fullerene dispersion in water enhanced by natural organic matter and sunlight. Environ. Sci. Technol. 2009;43(10):3574–3579. doi: 10.1021/es803603x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.