Abstract
The properties of the voltage-dependent H+ channel have been studied in lung epithelial cells for many years, and recently HVCN1 mRNA expression has been linked directly to H+ channel function in lung epithelium. The H+ channel is activated by strong membrane depolarization, intracellular acidity, or extracellular alkalinity. Early on it was noted that these are surprising physiological channel characteristics when considering that lung epithelial cells have rather stable membrane potentials and a well pH-buffered intracellular milieu. This raised the question under which conditions the H+ channel is active in lung epithelium and what is its physiological function there. Current understanding of the HVCN1 H+ channel in lung epithelial acid secretion, its activation by an alkaline mucosal extracellular pH, and its role in the regulation of the mucosal pH of the lung has resulted in a model of mucosal pH regulation based on the parallel function of the HVCN1 H+ channel and the CFTR HCO3- channel, which suggests that HVCN1 is a critical factor that maintains a neutral surface pH in the lung.
INTRODUCTION
Lung epithelial cells were the first intact mammalian cell system in which voltage-dependent H+ channels were identified. Using primary cultures of rat alveolar type II cells, DeCoursey (1) originally described a plasma membrane H+ conductance in whole cell patch clamp recordings. The H+ conductance was shown to be active during membrane depolarization and extracellular alkalinity supported current activation. The activation by extracellular alkalinity was later found to be governed by the inside-to-outside H+ gradient such that the H+ conductance could be expected to activate physiologically by intracellular acidification, extracellular alkalinization, or membrane depolarization (2). In type II cells it was empirically determined that the threshold potential (Vthreshold) at which the H+ conductance activates is linearly related to the transmembrane inside-to-outside H+ gradient (ΔpH) and the membrane potential by Vthreshold = -40 mV ΔpH + 20 mV (ref. (2)). For epithelial cells that do not depolarize significantly this relation represents a substantial hurdle for the activation of the H+ conductance. For example, in the absence of a transmembrane pH gradient, activation of the H+ conductance would require a depolarization of the membrane potential above 20 mV, which would be unusual for any epithelial cell. Also, when considering a normal apical membrane potential of airway cells of Va=-24 mV, a pH-gradient of 1.1 pH units would be required for H+ channel activation.
Similar voltage and pH-dependent characteristics have been found for the H+ conductance that is present in phagocytes (3-6). These cells, however, greatly depolarize their plasma membrane potential during the respiratory burst and measurably acidify their cytosol, and the H+ conductance functions to recover the intracellular pH from an acid load and to repolarize the membrane potential (7, 8). In contrast, lung epithelial cells have a rather stable membrane potential, and the intracellular pH changes little (2). The discrepancy between the functional properties of the H+ conductance and the conditions present in lung epithelial cells has been noted as soon as the H+ conductance was identified in type II cells (9), and the physiological function of the H+ conductance in these cells was initially rather unclear (9).
The purpose of this review is to shed some light into the physiological function of the voltage-dependent H+ conductance in lung epithelia by particularly considering the context of an epithelial setting. I will first review epithelial characteristics of the lung, cellular pH regulation, the pH of the lung lining fluid, and transepithelial and membrane potentials in polarized epithelial cells as they would affect the activity of the H+ conductance. Then, the involvement of the H+ conductance in acid secretion and the regulation of the pH of the airway surface liquid will be considered. In contrast to the role of the H+ conductance in phagocytes, I make the argument here that its function in the lung epithelium is the reacidification of an alkaline lung lining fluid.
ANATOMICAL CONSIDERATIONS
The lung is made of 23 generations of branching airway tubes where in most cases each airway divides to form two smaller airways. Starting at the trachea (generation 0) and terminating at the alveolar sac (generation 23) the function and cellular composition of the epithelium that lines the lung changes gradually. The terms “airway epithelium” or “tracheobronchial epithelium” usually refer to the ciliated epithelium as it is found in the nasal cavity, trachea, and bronchi until approximately generation 10 of the airways (Fig. 1A). This part of the lung is lined with a moderately-tight epithelium with a transepithelial electrical resistance of 300-400 Ω·cm2 (10). Epithelial tightness is a functional feature that determines the ability of the epithelium to maintain different apical and basolateral membrane potentials (Va and Vb, respectively); epithelial tightness is also critical to maintain concentration gradients, including pH gradients, between the airway surface liquid (ASL, the thin layer of fluid that lines the epithelium) and the serosa. Structurally, epithelial tightness results from the organization of the tight junctional cell contacts which serve to form an epithelial barrier and define the two distinct membranes, the apical (facing the lumen) and the basolateral (facing the serosa; Fig. 1). The transepithelial resistance is governed by the parallel transcellular and paracellular conductive pathways. The two principal cell types that form the airway epithelial barrier are ciliated cells and goblet cells. Ciliated cells comprise 50% to 70% of the epithelium; they are ~50 μm tall and continuously move the ASL with their cilia. Goblet cells release mucus that forms a normally quite thin layer that floats on top the periciliary fluid. ASL usually refers to the periciliary fluid plus the overlying mucus blanket. In normal airways, the ASL is ~10-20 μm high resulting in a volume of ~1-2 μl per square centimeter epithelial area (11). The small volume of the ASL is a determinant of the concentrations of factors secreted by the epithelium into the ASL including pH buffers and protons. The ASL contains a number of antibacterial factors and bicarbonate and its normal pH is regulated around neutral, although large variations and extremes between pH~4.5 and 8.5 have been reported for a number of airway conditions ((12) and see below). The major function of the airway epithelium is to form an innate defense barrier, filter air, and kill microbes.
Fig. 1. The two major epithelia of the lung.
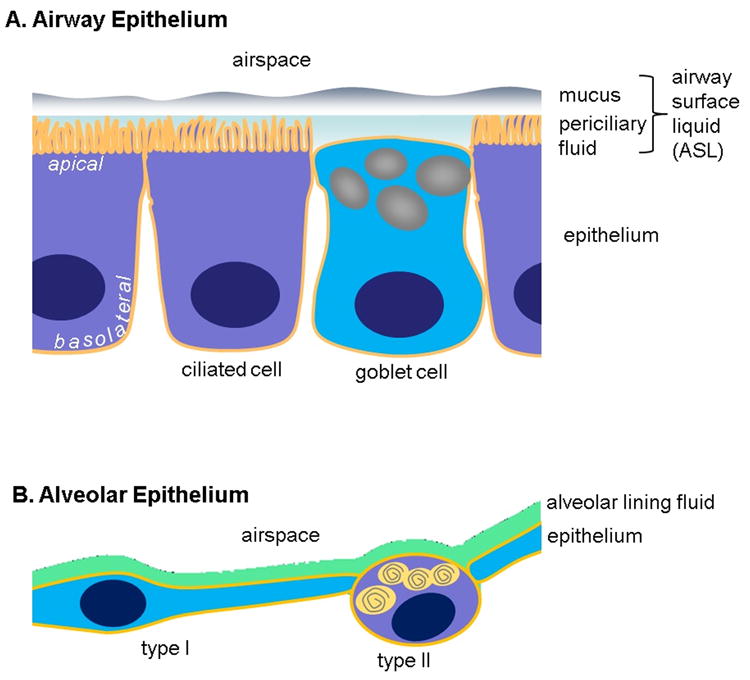
A. The proximal airways (nasal cavity, trachea, bronchi, conducting bronchioles) are lined with a ciliated, columnar epithelium. The major two cell types that from the epithelial barrier are ciliated and mucus-producing goblet cells. The luminal surface is lined by the airway surface liquid (ASL) that is composed of the periciliary fluid (in which the cilia beat) and overlayed by a mucus blanket. The major function of the airway epithelium is innate defense. B. The alveoli in the distal lung are lined by the very thin alveolar epithelium composed of large and squamous type I cells and of small, surfactant-producing type II cells. The alveolar epithelium is lined by an extremely thin alveolar lining fluid. The major functions of the alveolar epithelium are gas exchange and innate defense.
Starting at generation 17, the airways become respiratory bronchioles with an increasing number of alveoli budding off, until generation 23, where the airways terminate in an alveolar sac. Each alveolus (diameter roughly 0.25 mm) is lined by the extremely thin alveolar epithelium. It is composed of very large but thin type I cells (13) and small, cuboidal type II cells, which are characterized by lamellar bodies as the storage organelle of surfactant. Alveoli are the sites of gas exchange. Alveoli are lined with an extremely thin fluid film with a volume of ~10-20 nl/cm2 and a corresponding height of 0.1-0.2 μm, which is maintained by the surface properties of the surfactant generated by type II cells. This is by far the thinnest fluid film on any epithelium and as a result epithelial secretions result in correspondingly high luminal concentration changes. In contrast to the epithelium of the upper airways, the alveolar epithelium is extremely tight with transepithelial resistances of >2000 Ω·cm2 (14-16). A high transepithelial resistance helps to depolarize the apical membrane potential (see below) and to maintain large concentration gradients between the alveolar lining fluid and the serosa.
THE H+ CONDUCTANCE IN LUNG EPITHELIA
A voltage-dependent H+ conductance has been found in type II cells and every other type of lung epithelial cell line that has been investigated. The epithelial cell types investigated are of tracheobronchial origin or alveolar type II cells, and a few commonly used cell lines (Table 1) which expressed H+ conductances with biophysical characteristics similar to the initial reports in rat type II cells, which are 1) strong voltage-dependence, slow time constants of current activation, block by Zn2+, and dependence on the pH gradient (17-19). Interestingly, investigations performed in confluent, polarized airway epithelia allowed to localize the H+ conductance to the apical (that is, luminal) membrane and to establish its involvement in airway epithelial acid secretion (Table 1). This has been confirmed further in freshly excised human nasal tissue samples (20) supporting the physiological role of the H+ conductance in epithelial acid secretion. Thus, it became clear that a H+ conductance is present and active in the epithelia of the lung to support acid secretion into the luminal compartment.
Table 1.
Proton conductance and acid secretion of lung epithelial cells
| Lung Cell Type | Species | Characteristics | Whole cell conductance, (pS/pF) | Epithelial acid Secretion nmole·h-1·cm-2 | reference |
|---|---|---|---|---|---|
| Ciliated | Human | Primary tracheal | 20 | 410 | (17, 18) |
| 460 | |||||
| Mixed | Human | Primary sinonasal | -- | 1070 2) | (20) |
| Calu-3 | Human | Serous-like gland cell line | 12 | 22 | (18) |
| JME | Human | Nasal epithelial cell line, CF genotype | 9 | 125 | (17, 19) |
| Type II | Human | Primary fetal alveolar | 10 3) | 80 | (14) |
| Type II | Rat | Primary alveolar | 150 1) | -- | (2) |
| A549 | Human | Type II-like cell line | 11 1) | -- | (76) |
Whole cell conductance is given at 0 mV for recordings at room temperature; epithelial acid secretion is the Zn2+-sensitive fraction of total at a mucosal pH of 7.3.
original value adjusted to one pH unit gradient;
Original value adjusted to mucosal pH of 7.3 using the pH-dependence in (19);
H. Fischer, unpublished;
, not determined.
When considering the biophysical characteristics of the H+ conductance, cellular pH, and membrane potentials, the observation that a H+ conductance contributes to acid secretion raises the question of 1) how the H+ conductance is physiologically activated in lung epithelium, and 2) what is its role in epithelial function. For comparison, the best described function of the H+ conductance is in innate defense in phagocytes (21) where it is activated in parallel with the NOX2 NADPH oxidase to kill phagocytosed microbes during the respiratory burst (Fig. 2A). NADPH oxidases are plasma membrane proteins that transfer electrons from intracellular NADPH across the membrane to form extracellular superoxide and kill microbes. The phagosomal membrane is strongly depolarized by the electron transport by NOX2. During this process, large amounts of intracellular acid are generated by the oxidation of NADPH. Activated neutrophils acidify their cytosol at a rate of 0.1 pH/min and depolarize their membrane potential from a resting value of -60 mV to +50 mV (7, 21). As a result, the plasma membrane H+ conductance activates (by both membrane depolarization and intracellular acidification) leading to a massive release of acid from the cell and a repolarization of the plasma membrane (5, 22). In phagocytes, the role of the H+ conductance is closely linked to the activity of NOX2 where it serves as an acid relief valve to recover from intracellular acid loading by NOX2 activity and to repolarize the membrane potential (21).
Fig. 2. Comparison of HVCN1 in phagocytes compared to Lung epithelial cells.
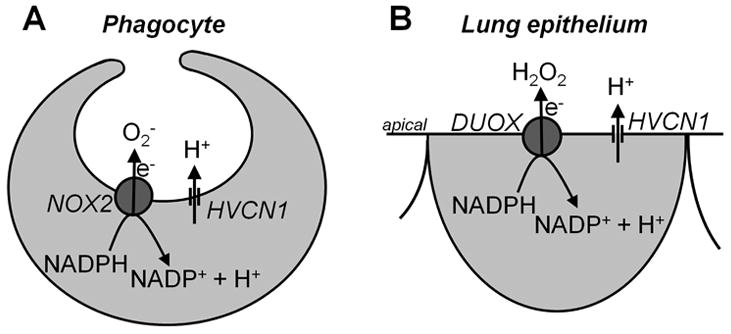
A. In phagocytes the HVCN1 H+ channel operates to support NOX2 activity by maintaining intracellular pH and membrane potential. NOX2 transfers electrons (e-) from intracellular NADPH to extracellular O2 to form O2-. This process generates intracellular H+. B. Initial proposal of H+ channel function in lung epithelial cells was modeled after phagocytes as a similar arrangement of HVCN1 with the DUOX NADPH oxidase. There, DUOX transfers e- to form extracellular H2O2 and similarly generates intracellular H+; for proper stoichiometry see (77). NOX2, NADPH oxidase 2; HVCN1, voltage-dependent H+ channel 1; DUOX, dual NADPH oxidase.
Interestingly, epithelial cells of the lung express their own NADPH oxidase based on the DUOX isoforms (14, 18) and, similar to phagocytes, lung epithelial cells kill bacteria and are part of the innate defense system. When both the DUOX NADPH oxidase and the H+ conductance were identified in lung epithelial cells (18) it appeared that the H+ conductance in lung epithelium serves a similar function as in phagocytes and is required to release intracellular H+ during DUOX activity (23). Accordingly, an initial model by us for the function of the H+ channel in lung epithelium was closely modeled after phagocytes, as shown in Fig. 2B. However, later it became clear that DUOX has only very small effects on pHi (14, 18), which is well buffered by the pH-regulating membrane transporters present in lung epithelial cells. Nevertheless, local acidic pHi compartments near the apical membrane have been discussed (23, 24) and might affect this conclusion. Acidic pHi compartments at the apical membrane could be caused by apical DUOX or the tightly packed apical mitochondria in airways (25); however, a local acidic pHi at the apical membrane has not been observed experimentally.
DUOX also shows relatively low rates of H2O2 production in comparison to NOX2 (26) and, by extension, small transmembrane electron currents, which in the presence of the dominating Na+ and Cl- conductances in lung epithelia are expected to show little effects on the membrane potential. These findings suggested that, in contrast to NOX2, DUOX does not necessarily require a H+ channel for its function because lung epithelial cells express mechanisms to stably regulate the intracellular pH and the membrane potentials. This suggests that the role of the H+ channel in the lung is quite different from the one in phagocytes. To support this argument, below I describe in detail the mechanisms that determine the membrane potentials and the intracellular pH in lung epithelial cells.
MEMBRANE POTENTIALS
A significant determinant of the activity of the H+ conductance is the membrane potential. The lung epithelium is a salt transporting tissue and the membrane potential of the apical membrane (Va) is governed by its major conductances, which are the apical Na+ and Cl- conductances (Fig. 3). The basolateral membrane potential (Vb) is largely governed by its K+ conductance. The primary ion transport function of lung epithelia is Na+ absorption across apical Na+ channels driven by the basolaterally located Na+-K+-ATPase. Recycling of K+ across the basolateral membrane results in a hyperpolarization of the cell, and Cl- distributes passively across the apical membrane according to its electrochemical driving forces. Although there are a number of other ion channels expressed in lung epithelium, including the apical H+ channel, their contributions to the membrane potentials can be safely neglected owing to the high Na+ and Cl- conductances. For comparison, the apical Na+ and Cl- conductance in rabbit airways was found to be 1.5 and 2 mS/cm2, respectively, and the basolateral conductance about 3 mS/cm2 (27). These can be physiologically upregulated, and in Calu-3 cells, an astounding forskolin-stimulated apical Cl- conductance of 200 mS/cm2 has been reported (28). For comparison, the apical H+ conductance in human airway epithelium was found to be 9 μS/cm2 (17) indicating that its effect on Va can be safely ignored.
Fig. 3. Major ion transport and equivalent circuit model for lung epithelium.
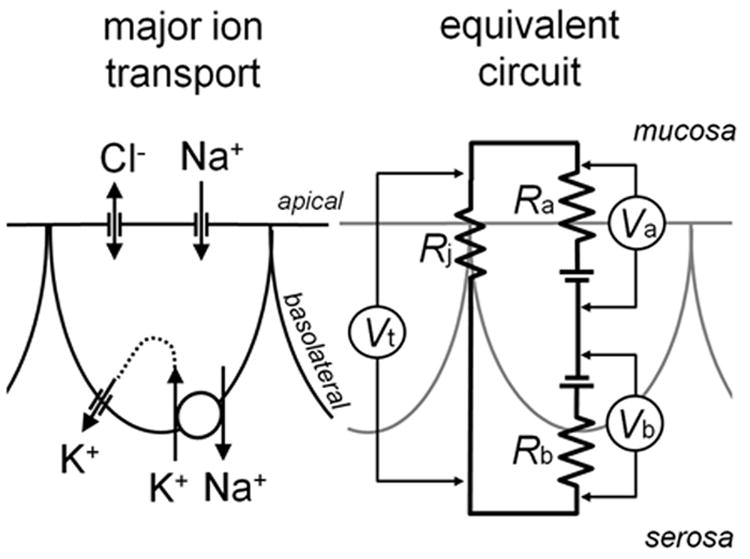
Left, major ion conductances are shown and lumped together. Lung epithelia absorb Na+ across apical Na+ channels driven by a basolateral Na+-K+-ATPase; K+ is recycled across basolateral K+ channels. Cl- distributes passively over the apical membrane. Right, arrangement and activity of transport mechanisms results in an equivalent circuit model where Ra and Rb represent the Thévenin equivalents of the apical and basolateral membrane resistances and Va and Vb the corresponding membrane potentials. Note that Va is reported inside-vs-mucosa, Vb inside-vs-serosa, and Vt mucosa-vs-serosa, which results in the relation Vt = Vb - Va for practical purposes. Rj represent the Thévenin equivalent of the paracellular, junctional resistance over which Vt drops.
Membrane potentials in primary tracheobronchial human epithelia have been found to be comparably stable during normal ion transport activity. Under control conditions, during basal Na+ absorption, the apical membrane potential is approximately Va = -24 mV and the basolateral membrane potential Vb = -34 mV (29-32). Regulation of conductances in either membrane result in voltage changes in both membranes owing to their arrangement in a 3-resistor electrical circuit formed by the apical, basolateral, and junctional resistors (Ra, Rb, and Rj; see Fig. 3). The differential expression of the major ion conductances in the two membranes and the selective ion gradients towards the mucosal and the serosal compartment result in distinct electromotive forces across the two membrane. However, the difference in the membrane potentials of the apical and basolateral membrane is solely caused by the junctional resistance, Rj, where the voltage difference between Va and Vb drops (Fig. 3). Thus, the tightness of the epithelial monolayer (that is, the value of Rj relative to Ra+Rb) determines the depolarization of Va with respect to Vb. In leaky epithelia, where Rj is small compared to Ra+Rb, there is little difference between Va and Vb, and as a result the transepithelial potential, Vt, is small. The difference Vb-Va is readily measured experimentally as Vt. In primary tracheal cultures Vt is approximately -10 mV. Interestingly, in measurements of the nasal potential difference in human subjects, an average Vt of -19 mV is reported as the normal value (33) suggesting that in vivo Va is significantly more depolarized than Vb compared to in vitro epithelial cultures.
Va and Vb hyperpolarize in presence of the Ca2+-agonist histamine by ΔVa = -5 mV and ΔVb = -6 mV (29), which is likely governed by the activation of basolateral Ca2+-activated K+ channels. Va and Vb are slightly depolarized when stimulated by the cAMP agonist isoproterenol by ΔVa = 1.5 mV and ΔVb = 2 mV (34), which is caused by the activation of the apical Cl- conductance. For comparison, the serous cell-like Calu-3 airway cell line expresses very high amounts of apical CFTR Cl- channels. When Calu-3 epithelia were stimulated with the cAMP-agonist forskolin to activate the CFTR Cl- conductance, Va depolarized from -52 mV to -21 mV, and, correspondingly, Vb depolarized from -60 mV to -44 mV (28). These membrane depolarizations are the largest reported for any lung epithelium. Nevertheless, in comparison to the depolarizations seen in phagocytes and the requirements for the activation of the H+ conductance, the depolarizations in airway cells appear small.
In alveolar cells, in comparison, membrane potentials have been little studied owing to their complicated anatomy in vivo and the lack of good cell culture models for alveolar epithelia. In vivo, the alveolar epithelium is composed of type I and type II cells (Fig. 1B); however, type I cells are difficult to harvest and culture, and type II cells dedifferentiate quickly into a type I-like phenotype in culture. As a result, there is little information about membrane potentials in these cells or in an intact epithelium. In two early reports on freshly isolated single type II cells, a resting membrane potential of -27 mV (rat lung, using a membrane potential dye (35)) or -63 mV (rabbit lung, using the Rb+ distribution potential (36)) has been found, and Matalon recently estimated -40 mV(37). None of these studies allowed estimates of apical or basolateral potentials because single cells were used. However, the transepithelial potential of confluent human primary type II cell monolayers is -9 mV (14), or in rat type II monolayers, -10 mV (38). There is no transepithelial information about type I cells but given a similar expression of channel types in type I and type II cells (13), these values are likely found in intact alveoli as well. Thus, although Va and Vb have not been measured in alveolar cells, Va is expected to be ~10 mV depolarized compared to Vb. It should be noted that some studies found relatively small transepithelial potentials (39), including a study of microimpaled intact alveoli in the rabbit (Vt = 3.5 mV; (40)).
Stimulation of type II monolayers with a cAMP agonist activates both apical CFTR Cl- channels and ENaC Na+ channels and results in a transepithelial hyperpolarization by -6 mV (41) suggesting a depolarization of Va by 6 mV. For comparison, selective membrane potentials have been measured in H441 cells, which are Clara-like, small-airway cells but share some transport characteristics with type II cells. H441 cells had a baseline Va of -43 mV and during stimulation with forskolin Va depolarized to -18 mV by way of activation of the apical ENaC Na+ conductance (42).
These studies of membrane potentials in both airway and alveolar epithelia suggest that the apical membrane is approximately 10 mV more depolarized than the basolateral membrane. The absolute membrane potentials change only little during epithelial transport regulation when compared to the requirements of the voltage-dependent regulation of the H+ conductance. The reported depolarizations in primary cells are in the order of <10 mV and only cell lines with high CFTR or ENaC expression depolarize Va somewhat more (i.e., Calu-3 and H441 cells). However, the relatively small changes of Va suggest that the apical H+ conductance is only little regulated by the membrane voltage in lung epithelial cells.
pH GRADIENTS
Because the airway pH, and in particular the pH of the luminal surface varies widely (12), and the threshold potential for H+ channel activation is sensitively regulated by the pH gradient, the pH gradient across the apical membrane is a candidate as a regulator of H+ channel activity in lung epithelium.
The pH of the airways has been measured over many years using pH electrodes placed on the luminal epithelial surface and, more recently, using pH sensitive fluorescent dyes (12). The earliest reliable measurement of normal ASL pH by Kyle et al. (43) reported a pH of 6.85 in ferret trachea using a pH-sensitive microelectrode. This value under resting conditions has been confirmed using the fluorescent, pH-sensitive dye BCECF (as dextran to maintain extracellular localization) in bovine tracheal cultures (pH 6.81; (44)) and in basal human airway gland secretions (pH 6.90; (45)). When determining the ASL pH indirectly by measuring the pH of an unbuffered solution that is perfused over the airway mucosa, a pH of 6.93 and 6.85 was found in pig and human airway epithelium, respectively (17, 46). A similar value (pH 6.92) was found in vivo in rabbit alveoli using pH sensitive microelectrodes (47). These studies, using different systems and techniques, confirmed the initial measurements by Kyle et al. and suggest that the normal pH of the ASL is pH 6.85.
Very large variations of ASL pH have been reported in measurements in human subjects for a number of disease conditions indicating that the apical membrane can be exposed to large pH changes. Measurements in vivo in human subjects are currently mainly done by using the exhaled breath condensate, tracheal aspirates, or bronchoalveolar lavage fluid as reporters of ASL pH. Comparison of pH values between different studies and techniques is somewhat limited by the different sample processing techniques, and, in particular, the maintenance of the CO2 content in the samples (48), however, the inclusion of normal control values in each study allows for a relative comparison. In a study of exhaled breath condensate, normal average pH was 7.65, which dropped in asthmatics to an average of pH 5.23, and extreme values of pH 4.5 to pH 8.5 were reported (49). In another study, the pH of the tracheal aspirate from neonates was found to be alkaline, ranging between 5.8 and 8.8 (50) and with average pH of 7.8. Bronchoalveolar lavage fluid sampled from neonates is on average acidic with a pH of 5.5 but ranges up to pH 7.5 during respiratory distress (51). In cystic fibrosis, exhaled breath condensate was found to be ~1 pH unit more acidic than normal (52, 53), and in pulmonary tuberculosis the exhaled breath was 0.5 pH units more acidic than normal (range pH 4 to 8.2 (54)). The origin of the pH changes in the lung are not well investigated but likely relate to acid and base secretion by the epithelium (12). These observations indicate that the lung epithelium is exposed to sizeable luminal pH changes of several pH units.
Intracellular pH (pHi) has been studied in both airway and alveolar epithelia. The most reliable measurement of pHi in airways is a study by Paradiso et al. (55) who used confluent, polarized human cultures in presence of HCO3-/CO2 buffered solutions. Use of these conditions appears critical when considering the effect on membrane potentials, transporter activities, and buffering systems on pHi. Paradiso et al. (55) reported an average pHi of 6.95. For comparison, other studies in single cells or HCO3- free solutions reported a more alkaline pHi of 7.1 – 7.4 (14, 55-58). Under physiological conditions, pHi is stably buffered by basolateral mechanisms (Fig. 4A) including the Na+-H+ exchanger (57, 59), the Na+-HCO3- co-transporter (60), and the Cl--HCO3- anion exchanger (55, 61). The activity of these transporters is tightly regulated by pHi so that any intracellular acid or base excess is removed across the basolateral membrane. Any observed changes of pHi are small and are only observed experimentally when these major pH regulating membrane transporters are inhibited.
Fig. 4. Airway epithelial H+ and HCO3- transport determines ASL pH.
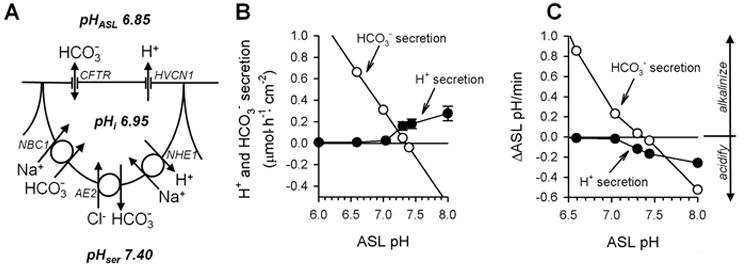
A. Distribution of H+ and HCO3- transporters in lung epithelium. The HVCN1 H+ conductance and the CFTR HCO3- conductance are expressed apically. HVCN1 conducts H+ only outwardly while CFTR conducts HCO3- both ways. The Na+-H+ exchanger NHE1, the Cl--HCO3- anion exchanger AE2, and the Na+-HCO3- cotransporter NBC1 are present in the basolateral membrane. Resting pH values for the airway surface liquid (pHASL), intracellularly (pHi), and serosally (pHser) are given. For stoichiometry of transporters see (28, 78). B. ASL pH determines airway H+ and HCO3- secretion. HCO3- secretion (open symbols) is modeled for pHi 6.95; Va -24 mV; apical, linear, voltage-independent HCO3- conductance 0.4 mS/cm2; average pCO2 3 kPa. H+ secretion data (filled symbols) are mean±SE compiled from previous measurements (17, 19). C. Predicted change of ASL pH by H+ and HCO3- secretion. Calculated from rates in B using buffer capacities for the ASL from ref. (65) (range 6.5 to 15 mM/pH) and an ASL volume of 1.5 μl/cm2. CFTR, cystic fibrosis transmembrane conductance regulator.
A stably buffered pHi in combination with a variable extracellular pH is likely to affect the activity of the apical H+ conductance. This also suggests that the regulation of the H+ conductance by the extracellular pH is involved in the acidification of the ASL. This contrasts from its role in phagocytes, where its role is acid release and alkalinization of the cytosol.
PROTON CHANNELS GOVERN PROTON SECRETION
H+ channels are present in cell types all along the respiratory tract epithelium (Table 1). When investigating intact, polarized epithelia in Ussing chambers using the pH stat technique, these cell types also secrete substantial amounts of acid into the mucosal (luminal) medium. However, the relation between H+ channel activity in whole cell patch clamp measurements and epithelial acid secretion was initially difficult to make.
The identification of the role of H+ channels in epithelial acid secretion was complicated by the fact that airway epithelia express at least three functional mechanisms for H+ secretion in their apical membrane including the vacuolar-type H+-ATPase, the non-gastric (colonic) H+-K+-ATPase, and H+ channels (for details see (12)). Initially it was unclear why three parallel mechanisms for acid secretion are present in the apical membrane; however, when investigating airway epithelia in presence of an acidic luminal pH of around 6, low rates of acid secretion are observed (~5 nmole·h-1·cm-2, (19), compare to rates in Table 1). These are likely mediated by ATP-dependent processes based on the observation that H+ secretion occurs against an H+ gradient. In contrast, when the mucosal medium is alkalinized, acid secretion increases in parallel with the pH gradient, and a substantial fraction of the acid secretion is Zn2+ sensitive (Table 1, and (19)). This suggests that the ATP-dependent mechanisms operate at a low rate and provide basal H+ secretion even against an H+ gradient, while H+ channels allow for large rates of acid secretion when the ASL pH is alkaline.
Additional evidence for a role of H+ channels in epithelial acid secretion came from the similar sensitivity to Zn2+ and the similar dependency on pH gradients of whole cell H+ currents and epithelial acid secretion. Even more convincing evidence is based on functional characteristics of a misfunctional mutation of HVCN1 (19). In a study of epithelial acid secretion and whole cell H+ currents in primary airway cultures we found a tracheal isolate that expressed the M91T HVCN1 mutant. Acid secretion measured in these cultures was characterized by a reduced sensitivity to an alkaline pH such that approximately a 0.5 pH-unit larger mucosal pH was required to activate acid secretion compared to normal. A similar behavior was found when the M91T HVCN1 mutant was recombinantly expressed and investigated with whole cell patch clamping, where again larger bath-to-pipette pH gradients were required for H+ current activation compared to wild type HVCN1. Thus, the functionally distinct characteristics of the M91T HVCN1 mutant were found in acid secretion by epithelia and in whole cell patch clamp measurements and directly related H+ channel activity to acid secretion.
PROTON CHANNELS REGULATE ASL pH
Since H+ channels are evidently present and active in the lung epithelium, we wondered under which conditions the electrochemical H+ gradient would be outward and large enough to activate the H+ conductance. Because both the intracellular pH and the membrane potential are relatively stable, we focused in a recent investigation on the role of the ASL pH in the activation of apical H+ channels and H+ secretion (19). The pH-dependence of Zn2+-sensitive, H+ channel-mediated acid secretion is shown in Fig. 4B, filled circles. At a mucosal pH of 7.3 and above, acid secretion is active while at a mucosal pH of 7 and below no acid is secreted. Thus, when considering the resting ASL pH of 6.85 (Fig. 4A), the H+ channel is likely closed and opens only when the ASL pH turns alkaline. In general, the pH gradient-driven activation of H+ currents is consistent with the observed pH dependence of H+ currents in phagocytes; however, the H+ gradient in phagocytes is generated by intracellular acidity, while in lung epithelium it appears to be governed by extracellular alkalinity.
This has two major consequence for the function of H+ channels in lung epithelium. First, in contrast to phagocytes, where H+ channels are involved in the regulation of pHi, H+ channels in lung epithelial cells appear little involved in the regulation of pHi. Second, the observed activation of H+ channels by extracellular alkalinity will re-acidify an alkaline ASL pH, which indicates the H+ channel in the regulation of the extracellular pH.
Alkalinization of the ASL is likely caused by epithelial HCO3- secretion. Figure 4B displays, in comparison, the predicted effect of ASL pH on both H+ and HCO3- secretion by the epithelium. Because both H+ and HCO3- move passively across the apical membrane, both H+ and HCO3- secretion are driven by Va and the apical transmembrane pH gradient. Alkalinization of the airways is thought to be mainly related to the activity of the cAMP-activated CFTR Cl- channel1) in the apical membrane of lung epithelia. CFTR shows a voltage-independent and linear conductance for HCO3- that is ~20% compared to Cl- (62, 63) and CFTR mediates HCO3- secretion by airway epithelia (60, 64). When modeling airway epithelial HCO3- secretion for an apical HCO3- conductance of 0.4 mS/cm2 with Va = -24 mV and pHi = 6.95 then gradient-driven HCO3- secretion across the apical membrane linearly increases with decreasing pH for all ASL pH values <7.35 (Fig. 4B). Accordingly, at high ASL pH values CFTR may mediate HCO3- absorption.
Because the volume of the airway surface liquid is very small, these rates of H+ and HCO3- secretion are expected to affect the ASL pH. The estimated effects of H+ and HCO3- secretion on changes of the ASL pH are shown in Fig. 4C. Rates of ASL pH changes were calculated from ΔASL pH = J / (β · v), where J is the H+ or HCO3- flux, β is the pH-dependent buffer capacity of the ASL (from Holma (65) for respiratory secretions), and v is the ASL volume (1.5 μl/cm2). The resulting rates are quite fast (Fig. 4C). For example, an alkaline ASL pH of 8 is predicted to re-acidify within a few minutes back to pH 7 based on H+ channel activity (with an initial rate of -0.25 pH/min); in a cAMP-stimulated epithelium with active CFTR this is predicted to re-acidify even faster. These calculations suggest a role for the H+ channel in the re-acidification of the ASL to values near pH 7, where the H+ channel is closed. Because the H+ channel is expressed in parallel to CFTR, the activities of both channels are predicted to mutually complement each other during the regulation of the ASL pH. We speculate that the activities of CFTR and HVCN1 in airways provide a feedback control system for the regulation of pH ASL as depicted in Fig. 5. The normal set-point under resting conditions is pH~7 (activation of H+ conductance; Fig. 4B) for this system, although this set-point is dependent on Va. A disturbance of ASL pH results in a H+ or HCO3- secretion as a response and adjustment of ASL pH. At an acidic ASL pH, the H+ channel is closed but HCO3- is secreted along its electrochemical gradient through CFTR and alkalinizes the ASL. At an alkaline pH ASL, H+ channels are open and secrete acid to acidify the ASL. HCO3- absorption might support the effect of acid secretion on ASL pH, however, the H+ channel appears better positioned to perform this function because it is selectively activated by the alkaline pH, while CFTR is not pH-activated, but instead is regulated through the cAMP/PKA system (e.g., requires activation by β agonists).
Fig. 5. Proposed role for HVCN1 in ASL pH regulation.
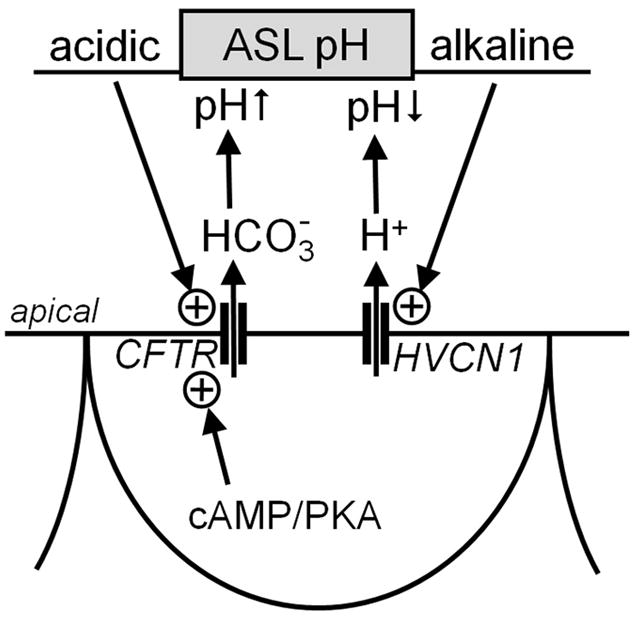
The CFTR HCO3- channel and the HVCN1 H+ channel provide a system for the regulation of the pH of the ASL. The ASL pH is the control variable to that governs H+ or HCO3- secretion into the ASL. An alkaline ASL pH activates HVCN1 and provides a driving force for H+ secretion. An acidic ASL pH provides a driving force for HCO3- secretion, however, requires additional activation of CFTR by the cAMP/PKA system. Arrow with circled + indicates activation. PKA, protein kinase A.
This model can be used to explain the observed acidic or alkaline ASL pH values in disease conditions. In inflammatory airway diseases an alkaline ASL pH is generally observed (12). Inflamed airways are characterized by increased Ca2+ signaling (66) and break-down of the junctional resistance (67, 68), both of which result in a hyperpolarization of the apical membrane. As a result, HCO3- secretion is favored and the H+ channel is inactivated, which shifts the set-point of the regulatory feedback to an alkaline value. On the other hand, an acidic ASL pH is observed in cystic fibrosis (12). Cystic fibrosis airways are characterized by a depolarized apical membrane (10) and a misfunctional CFTR, which results in a shift of the set-point to an acidic ASL and an inhibition of CFTR as a control element in this model.
Further information might be generated from HVCN1 knockout mouse models that have been introduced recently (69-72) in comparison or in combination with CFTR knockout mice. In mice, ASL pH is regulated by CFTR as shown by an acidification of the ASL when CFTR is blocked (73) or in CFTR knockout mice (74). As of the writing of this review, HVCN1 H+ channel activity has not been studied in mouse lung, however, investigations of mouse lung epithelium derived from HVCN1 knockout mice may shed additional light into the function of HVCN1 is lung epithelial biology.
Acknowledgments
I thank Terry Machen for helpful comments on the manuscript. Work in the author’s laboratory is funded by the National Institutes of Health (HL86323) and the CF Foundation (FISCHER10G0). I thank the Beverly M. Folger Foundation for support.
Footnotes
In this review, the apical HCO3- conductance is mainly modeled for the cAMP-activated CFTR channel because its role in HCO3- secretion is established. The Ca-activated Cl- channel is similarly conductive for HCO3- (75) and present in the apical membrane, however, its role in HCO3- secretion is currently unclear.
References
- 1.DeCoursey T. Hydrogen ion currents in rat alveolar epithelial cells. Biophys J. 1991;60:1243–1253. doi: 10.1016/S0006-3495(91)82158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cherny V, Markin V, DeCoursey T. The voltage-activated hydrogen ion conductance in rat alveolar epithelial cells is determined by the pH gradient. J Gen Physiol. 1995;105:861–896. doi: 10.1085/jgp.105.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapus A, Romanek R, Qu AY, Rotstein OD, Grinstein S. A pH-sensitive and voltage-dependent proton conductance in the plasma membrane of macrophages. J Gen Physiol. 1993;102:729–760. doi: 10.1085/jgp.102.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeCoursey T, Cherny V. Potential, pH, and arachidonate gate hydrogen ion currents in human neutrophils. Biophys J. 1993;65:1590–1598. doi: 10.1016/S0006-3495(93)81198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demaurex N, Grinstein S, Jaconi M, Schlegel W, Lew DP, Krause KH. Proton currents in human granulocytes: regulation by membrane potential and intracellular pH. J Physiol Lond. 1993;466:329–344. [PMC free article] [PubMed] [Google Scholar]
- 6.DeCoursey TE, Cherny VV, DeCoursey AG, Xu W, Thomas LL. Interactions between NADPH oxidase-related proton and electron currents in human eosinophils. J Physiol (Lond) 2001;535:767–781. doi: 10.1111/j.1469-7793.2001.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grinstein S, Furuya W. Cytoplasmic pH regulation in phorbol ester-activated human neutrophils. Am J Physiol Cell Physiol. 1986;251:C55–65. doi: 10.1152/ajpcell.1986.251.1.C55. [DOI] [PubMed] [Google Scholar]
- 8.DeCoursey TE. Interactions between NADPH oxidase and voltage-gated proton channels: why electron transport depends on proton transport. FEBS Letters. 2003;555:57–61. doi: 10.1016/s0014-5793(03)01103-7. [DOI] [PubMed] [Google Scholar]
- 9.Lukacs GL, Kapus A, Nanda A, Romanek R, Grinstein S. Proton conductance of the plasma membrane: properties, regulation, and functional role. Am J Physiol Cell Physiol. 1993;265:C3–14. doi: 10.1152/ajpcell.1993.265.1.C3. [DOI] [PubMed] [Google Scholar]
- 10.Willumsen NJ, Boucher RC. Shunt resistance and ion permeabilities in normal and cystic fibrosis airway epithelia. Am J Physiol Cell Physiol. 1989;256:C1054–1063. doi: 10.1152/ajpcell.1989.256.5.C1054. [DOI] [PubMed] [Google Scholar]
- 11.Widdicombe JH. Volume of airway surface liquid in health and disease. Am J Respir Crit Care Med. 2002;165:1566. doi: 10.1164/ajrccm.165.11.165111. [DOI] [PubMed] [Google Scholar]
- 12.Fischer H, Widdicombe JH. Mechanisms of acid and base secretion by the airway epithelium. J Membr Biol. 2006;211:139–150. doi: 10.1007/s00232-006-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobbs LG, Johnson MD, Vanderbilt J, Allen L, Gonzalez R. The great big alveolar TI cell: evolving concepts and paradigms. Cell Physiol Biochem. 2010;25:55–62. doi: 10.1159/000272063. [DOI] [PubMed] [Google Scholar]
- 14.Fischer H, Gonzales LK, Kolla V, Schwarzer C, Miot F, et al. Developmental Regulation of Duox1 Expression and Function in Human Fetal Lung Epithelial Cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1506–L1514. doi: 10.1152/ajplung.00029.2007. [DOI] [PubMed] [Google Scholar]
- 15.Lubman RL, Danto SI, Crandall ED. Evidence for active H+ secretion by rat alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 1989;257:L438–445. doi: 10.1152/ajplung.1989.257.6.L438. [DOI] [PubMed] [Google Scholar]
- 16.Jiang X, Ingbar DH, O’Grady SM. Adrenergic stimulation of Na+ transport across alveolar epithelial cells involves activation of apical Cl- channels. Am J Physiol Cell Physiol. 1998;275:C1610–1620. doi: 10.1152/ajpcell.1998.275.6.C1610. [DOI] [PubMed] [Google Scholar]
- 17.Fischer H, Widdicombe JH, Illek B. Acid secretion and proton conductance in human airway epithelium. Am J Physiol Cell Physiol. 2002;282:C736–743. doi: 10.1152/ajpcell.00369.2001. [DOI] [PubMed] [Google Scholar]
- 18.Schwarzer C, Machen TE, Illek B, Fischer H. NADPH oxidase-dependent acid production in airway epithelial cells. J Biol Chem. 2004;279:36454–36461. doi: 10.1074/jbc.M404983200. [DOI] [PubMed] [Google Scholar]
- 19.Iovannisci D, Illek B, Fischer H. Function of the HVCN1 proton channel in airway epithelia and a naturally occurring mutation, M91T. J Gen Physiol. 2010;136:35–46. doi: 10.1085/jgp.200910379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho D-Y, Hajighasemi M, Hwang PH, Illek B, Fischer H. Proton secretion in freshly excised sinonasal mucosa from asthma and sinusitis patients. Am J Rhinol Allergy. 2009;23:10–13. doi: 10.2500/ajra.2009.23.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy R, DeCoursey TE. Charge compensation during the phagocyte respiratory burst. Biochim Biophys Acta. 2006;1757:996–1011. doi: 10.1016/j.bbabio.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 22.DeCoursey TE, Morgan D, Cherney VV. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature. 2003;422:531–534. doi: 10.1038/nature01523. [DOI] [PubMed] [Google Scholar]
- 23.Fischer H. Mechanism and function of DUOX in epithelia of the lung. Antiox Redox Signal. 2009;11:2453–2465. doi: 10.1089/ars.2009.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeCoursey TE. Hypothesis: do voltage-gated H+ channels in alveolar epithelial cells contribute to CO2 elimination by the lung? Am J Physiol Cell Physiol. 2000;278:C1–C10. doi: 10.1152/ajpcell.2000.278.1.C1. [DOI] [PubMed] [Google Scholar]
- 25.Ribeiro CMP, Paradiso AM, Livraghi A, Boucher RC. The Mitochondrial Barriers Segregate Agonist-induced Calcium-dependent Functions in Human Airway Epithelia. J Gen Physiol. 2003;122:377–387. doi: 10.1085/jgp.200308893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ameziane-El-Hassani R, Morand S, Boucher J-L, Frapart Y-M, Apostolou D, et al. Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J Biol Chem. 2005;280:30046–30054. doi: 10.1074/jbc.M500516200. [DOI] [PubMed] [Google Scholar]
- 27.Poulsen A, Klausen T, Pedersen P, Willumsen N, Frederiksen O. Nucleotide regulation of paracellular Cl- permeability in natural rabbit airway epithelium. Pflugers Arch. 2005 doi: 10.1007/s00424-005-0023-8. [DOI] [PubMed] [Google Scholar]
- 28.Tamada T, Hug MJ, Frizzell RA, Bridges RJ. Microelectrode and impedance analysis of anion secretion in Calu-3 cells. J Pancreas (Online) 2001;2:219–228. [PubMed] [Google Scholar]
- 29.Clarke LL, Paradiso AM, Boucher RC. Histamine-induced Cl- secretion in human nasal epithelium: responses of apical and basolateral membranes. Am J Physiol Cell Physiol Cell Physiol. 1992;263:C1190–1199. doi: 10.1152/ajpcell.1992.263.6.C1190. [DOI] [PubMed] [Google Scholar]
- 30.Cotton CU, Stutts MJ, Knowles MR, Gatzy JT, Boucher RC. Abnormal apical cell membrane in cystic fibrosis respiratory epithelium. An in vitro electrophysiologic analysis. J Clin Invest. 1987;79:80–85. doi: 10.1172/JCI112812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willumsen NJ, Boucher RC. Intracellular pH and its relationship to regulation of ion transport in normal and cystic fibrosis human nasal epithelia. J Physiol Lond. 1992;455:247–269. doi: 10.1113/jphysiol.1992.sp019300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willumsen NJ, Davis CW, Boucher RC. Cellular Cl- transport in cultured cystic fibrosis airway epithelium. Am J Physiol Cell Physiol. 1989;256:C1045–C1053. doi: 10.1152/ajpcell.1989.256.5.C1045. [DOI] [PubMed] [Google Scholar]
- 33.Boyle MP, Diener-West M, Milgram L, Knowles M, Foy C, et al. A Multicenter Study of the Effect of Solution Temperature on Nasal Potential Difference Measurements. Chest. 2003;124:482–489. doi: 10.1378/chest.124.2.482. [DOI] [PubMed] [Google Scholar]
- 34.Willumsen NJ, Boucher RC. Activation of an apical Cl- conductance by Ca2+ ionophores in cystic fibrosis airway epithelia. Am J Physiol Cell Physiol. 1989;256:C226–233. doi: 10.1152/ajpcell.1989.256.2.C226. [DOI] [PubMed] [Google Scholar]
- 35.Castranova V, Jones GS, Miles PR. Transmembrane potential of isolated rat alveolar type II cells. 1983;54:1511–1517. doi: 10.1152/jappl.1983.54.6.1511. [DOI] [PubMed] [Google Scholar]
- 36.Gallo R, Finkelstein J, Notter R. Characterization of the plasma and mitochondrial membrane potentials of alveolar type II cells by the use of ionic probes. Biochim Biophys Acta. 1984;771:217–227. doi: 10.1016/0005-2736(84)90536-4. [DOI] [PubMed] [Google Scholar]
- 37.Matalon S, Lazrak A, Jain L, Eaton DC. Biophysical properties of sodium channels in lung alveolar epithelial cells. J Appl Physiol. 2002;93:1852–1859. doi: 10.1152/japplphysiol.01241.2001. [DOI] [PubMed] [Google Scholar]
- 38.Cheek JM, Kim KJ, Crandall ED. Tight monolayers of rat alveolar epithelial cells: bioelectric properties and active sodium transport. Am J Physiol Cell Physiol. 1989;256:C688–693. doi: 10.1152/ajpcell.1989.256.3.C688. [DOI] [PubMed] [Google Scholar]
- 39.Matalon S. Mechanisms and regulation of ion transport in adult mammalian alveolar type II pneumocytes. Am J Physiol Cell Physiol. 1991;261:C727–C738. doi: 10.1152/ajpcell.1991.261.5.C727. [DOI] [PubMed] [Google Scholar]
- 40.Nielson DW. Electrolyte composition of pulmonary alveolar subphase in anesthetized rabbits. J Appl Physiol. 1986;60:972–979. doi: 10.1152/jappl.1986.60.3.972. [DOI] [PubMed] [Google Scholar]
- 41.Bove PF, Grubb BR, Okada SF, Ribeiro CMP, Rogers TD, et al. Human Alveolar Type II Cells Secrete and Absorb Liquid in Response to Local Nucleotide Signaling. J Biol Chem. 2010;285:34939–34949. doi: 10.1074/jbc.M110.162933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazrak A, Matalon S. cAMP-induced changes of apical membrane potentials of confluent H441 monolayers. Am J Physiol Lung Cell Mol Physiol. 2003;285:L443–L450. doi: 10.1152/ajplung.00412.2002. [DOI] [PubMed] [Google Scholar]
- 43.Kyle H, Ward JP, Widdicombe JG. Control of pH of airway surface liquid of the ferret trachea in vitro. J Appl Physiol. 1990;68:135–140. doi: 10.1152/jappl.1990.68.1.135. [DOI] [PubMed] [Google Scholar]
- 44.Jayaraman S, Song Y, Verkman AS. Airway surface liquid pH in well-differentiated airway epithelial cell cultures and mouse trachea. Am J Physiol Cell Physiol. 2001;281:C1504–1511. doi: 10.1152/ajpcell.2001.281.5.C1504. [DOI] [PubMed] [Google Scholar]
- 45.Thiagarajah JR, Song Y, Haggie PM, Verkman AS. A small molecule CFTR inhibitor produces cystic fibrosis-like submucosal gland fluid secretions in normal airways. FASEB J. 2004 doi: 10.1096/fj.03-1248fje. 03-1248fje. [DOI] [PubMed] [Google Scholar]
- 46.Inglis SK, Wilson SM, Olver RE. Secretion of acid and base equivalents by intact distal airways. Am J Physiol Lung Cell Mol Physiol. 2003;284:L855–862. doi: 10.1152/ajplung.00348.2002. [DOI] [PubMed] [Google Scholar]
- 47.Nielson DW, Goerke J, Clements JA. Alveolar subphase pH in the lungs of anesthetized rabbits. PNAS. 1981;78:7119–7123. doi: 10.1073/pnas.78.11.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jackson AS, Sandrini A, Campbell C, Chow S, Thomas PS, Yates DH. Comparison of Biomarkers in Exhaled Breath Condensate and Bronchoalveolar Lavage. 2007;175:222–227. doi: 10.1164/rccm.200601-107OC. [DOI] [PubMed] [Google Scholar]
- 49.Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, et al. Endogenous airway acidification. Implications for asthma pathophysiology. Am J Resp Crit Care Med. 2000;161:694–699. doi: 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]
- 50.Paget-Brown AO, Hunt JF, Gaston B. Tracheal aspirate pH is alkaline in pre-term human infants. Eur Respir J. 2007;30:840–842. doi: 10.1183/09031936.00015507. [DOI] [PubMed] [Google Scholar]
- 51.Zecca E, De Luca D, Baroni S, Vento G, Tiberi E, Romagnoli C. Bile Acid-Induced Lung Injury in Newborn Infants: A Bronchoalveolar Lavage Fluid Study. 2008;121:e146–149. doi: 10.1542/peds.2007-1220. [DOI] [PubMed] [Google Scholar]
- 52.Ojoo JC, Mulrennan SA, Kastelik JA, Morice AH, Redington AE. Exhaled breath condensate pH and exhaled nitric oxide in allergic asthma and in cystic fibrosis. Thorax. 2005;60:22–26. doi: 10.1136/thx.2003.017327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tate S, MacGregor G, Davis M, Innes JA, Greening AP. Airways in cystic fibrosis are acidified: detection by exhaled breath condensate. Thorax. 2002;57:926–929. doi: 10.1136/thorax.57.11.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ngamtrakulpanit L, Yu Y, Adjei A, Amoah G, Gaston B, Hunt J. Identification of intrinsic airway acidification in pulmonary tuberculosis. Global J Health Sci. 2010;2:106–110. doi: 10.5539/gjhs.v2n1p106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paradiso AM, Coakley RD, Boucher RC. Polarized distribution of HCO3- transport in human normal and cystic fibrosis nasal epithelia. J Physiol (Lond) 2003;548:203–218. doi: 10.1113/jphysiol.2002.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paradiso AM. ATP-activated basolateral Na+/H+ exchange in human normal and cystic fibrosis airway epithelium. Am J Physiol Lung Cell Mol Physiol. 1997;273:L148–158. doi: 10.1152/ajplung.1997.273.1.L148. [DOI] [PubMed] [Google Scholar]
- 57.Paradiso AM. Identification of Na+-H+ exchange in human normal and cystic fibrotic ciliated airway epithelium. Am J Physiol Lung Cell Mol Physiol. 1992;262:L757–764. doi: 10.1152/ajplung.1992.262.6.L757. [DOI] [PubMed] [Google Scholar]
- 58.Murphy R, Cherny VV, Morgan D, DeCoursey TE. Voltage-gated proton channels help regulate pHi in rat alveolar epithelium. Am J Physiol Lung Cell Mol Physiol. 2005;288:L398–408. doi: 10.1152/ajplung.00299.2004. [DOI] [PubMed] [Google Scholar]
- 59.Lubman RL, Crandall ED. Polarized distribution of Na+-H+ antiport activity in rat alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 1994;266:L138–147. doi: 10.1152/ajplung.1994.266.2.L138. [DOI] [PubMed] [Google Scholar]
- 60.Devor DC, Bridges RJ, Pilewski JM. Pharmacological modulation of ion transport across wild-type and ΔF508 CFTR-expressing human bronchial epithelia. Am J Physiol Cell Physiol. 2000;279:C461–479. doi: 10.1152/ajpcell.2000.279.2.C461. [DOI] [PubMed] [Google Scholar]
- 61.Lubman R, Danto S, Chao D, Fricks C, Crandall E. Cl--HCO3- exchanger isoform AE2 is restricted to the basolateral surface of alveolar epithelial cell monolayers. Am J Respir Cell Mol Biol. 1995;12:211–219. doi: 10.1165/ajrcmb.12.2.7865219. [DOI] [PubMed] [Google Scholar]
- 62.Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA. 1994;91:5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Illek B, Yankaskas JR, Machen TE. cAMP and genistein stimulate HCO3- conductance through CFTR in human airway epithelia. Am J Physiol Lung Cell Mol Physiol. 1997;272:L752–761. doi: 10.1152/ajplung.1997.272.4.L752. [DOI] [PubMed] [Google Scholar]
- 64.Devor DC, Singh AK, Lambert LC, DeLuca A, Frizzell RA, Bridges RJ. Bicarbonate and chloride secretion in Calu-3 human airway epithelial cells. J Gen Physiol. 1999;113:743–760. doi: 10.1085/jgp.113.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holma B. Influence of buffer capacity and pH-dependent rheological properties of respiratory mucus on health effects due to acidic pollution. Sci Total Environ. 1985;41:101–123. doi: 10.1016/0048-9697(85)90181-0. [DOI] [PubMed] [Google Scholar]
- 66.Ribeiro CMP, Paradiso AM, Carew MA, Shears SB, Boucher RC. Cystic Fibrosis Airway Epithelial Ca2+i Signaling: the mechanism for the larger agonist-mediated Ca2+i signals in human cystic fibrosis airway epithelia. J Biol Chem. 2005;280:10202–10209. doi: 10.1074/jbc.M410617200. [DOI] [PubMed] [Google Scholar]
- 67.Vermeer PD, Denker J, Estin M, Moninger TO, Keshavjee S, et al. MMP9 modulates tight junction integrity and cell viability in human airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2009;296:L751–762. doi: 10.1152/ajplung.90578.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heijink IH, van Oosterhout A, Kapus A. Epidermal growth factor receptor signalling contributes to house dust mite-induced epithelial barrier dysfunction. Eur Respir J. 2010;36:1016–1026. doi: 10.1183/09031936.00125809. [DOI] [PubMed] [Google Scholar]
- 69.Capasso M, Bhamrah M, Henley T, Boyd R, Langlais C, et al. HVCN1 modulates BCR signal strength via regulation of BCR-dependent generation of reactive oxygen species. Nat Immunol. 2010;11:265–272. doi: 10.1038/ni.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.El Chemaly A, Okochi Y, Sasaki M, Arnaudeau S, Okamura Y, Demaurex N. VSOP/Hv1 proton channels sustain calcium entry, neutrophil migration, and superoxide production by limiting cell depolarization and acidification. J Exp Med. 2010;207:129–139. doi: 10.1084/jem.20091837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okochi Y, Sasaki M, Iwasaki H, Okamura Y. Voltage-gated proton channel is expressed on phagosomes. Biochem Biophys Res Commun. 2009;382:274–279. doi: 10.1016/j.bbrc.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 72.Ramsey IS, Ruchti E, Kaczmarek JS, Clapham DE. Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst. Proc Natl Acad Sci USA. 2009;106:7642–7647. doi: 10.1073/pnas.0902761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Song Y, Thiagarajah J, Verkman AS. Sodium and Chloride Concentrations, pH, and Depth of Airway Surface Liquid in Distal Airways. J Gen Physiol. 2003;122:511–519. doi: 10.1085/jgp.200308866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jayaraman S, Song Y, Vetrivel L, Shankar L, Verkman AS. Noninvasive in vivo fluorescence measurement of airway-surface liquid depth, salt concentration, and pH. J Clin Invest. 2001;107:317–324. doi: 10.1172/JCI11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qu Z, Hartzell HC. Anion permeation in Ca2+-activated Cl- channels. J Gen Physiol. 2000;116:825–844. doi: 10.1085/jgp.116.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DeCoursey TE, Cherny VV. Voltage-activated hydrogen ion currents. J Membr Biol. 1994;141:203–223. doi: 10.1007/BF00235130. [DOI] [PubMed] [Google Scholar]
- 77.DeCoursey TE. Voltage-Gated Proton Channels Find Their Dream Job Managing the Respiratory Burst in Phagocytes. Physiology. 2010;25:27–40. doi: 10.1152/physiol.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krouse ME, Talbott JF, Lee MM, Joo NS, Wine JJ. Acid and base secretion in the Calu-3 model of human serous cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1274–1283. doi: 10.1152/ajplung.00036.2004. [DOI] [PubMed] [Google Scholar]


