Abstract
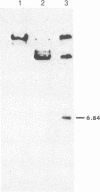
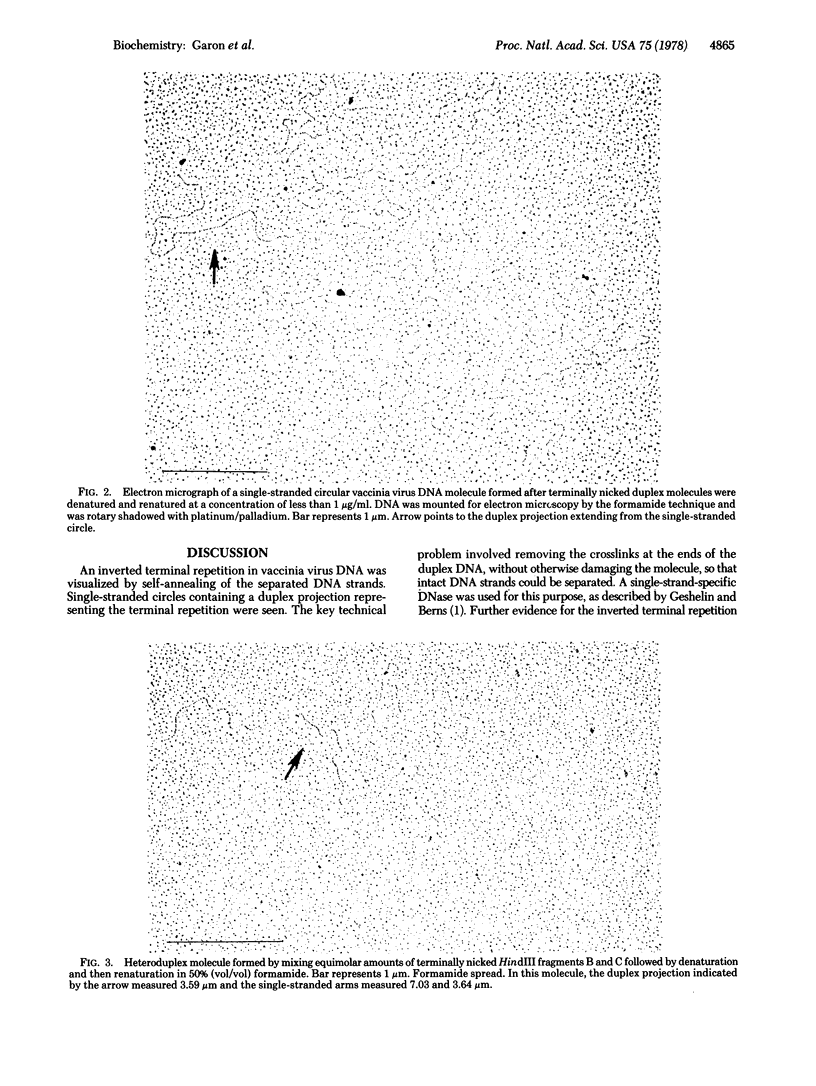
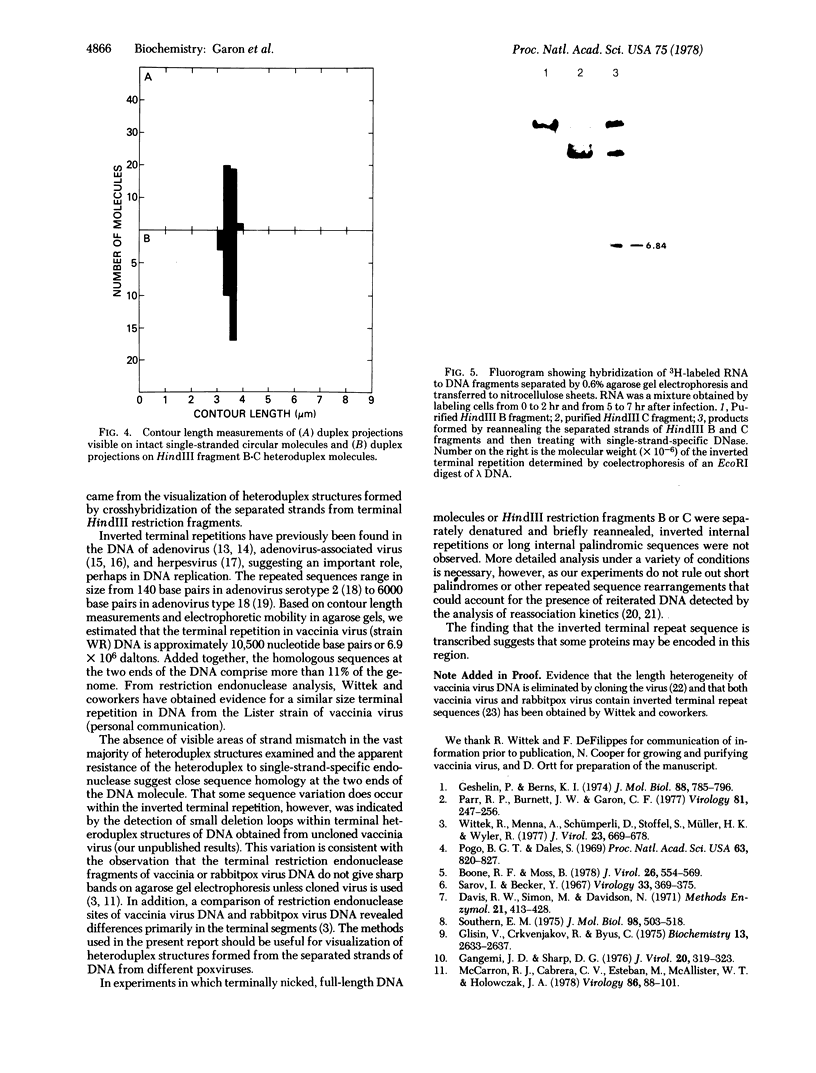
An inverted terminal repetition was observed in DNA molecules extracted from vaccinia virus. The repeated sequence was visualized by (i) nicking the hairpin loops present of the ends of vaccinia virus DNA, (ii) separating the strands of DNA by alkali denaturation, (iii) allowing the single strands to self-anneal, and (iv) examining the DNA with an electron microscope. Single-stranded circular molecules, each of which contained a duplex projection (3.54 +/- 0.12 micron) representing the terminal repetition, readily formed. Similar size projections were also seen in heteroduplex structures formed by crosshybridization of the separated strands of the two terminal HindIII restriction fragments. Based on contour length measurements and the electrophoretic mobility of the isolated inverted terminal repetition, a molecular weight of approximately 6.9 X 10(6), equivalent to about 10,500 nucleotide base pairs, was estimated. Evidence was obtained from DNA-RNA hybridization studies that the terminal repetition is transcribed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berns K. I., Kelly T. J., Jr Letter: Visualization of the inverted terminal repetition in adeno-associated virus DNA. J Mol Biol. 1974 Jan 15;82(2):267–271. doi: 10.1016/0022-2836(74)90344-1. [DOI] [PubMed] [Google Scholar]
- Boone R. F., Moss B. Sequence complexity and relative abundance of vaccinia virus mRNA's synthesized in vivo and in vitro. J Virol. 1978 Jun;26(3):554–569. doi: 10.1128/jvi.26.3.554-569.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangemi J. D., Sharp D. G. Use of a restriction endonuclease in analyzing the genomes from two different strains of vaccinia virus. J Virol. 1976 Oct;20(1):319–323. doi: 10.1128/jvi.20.1.319-323.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon C. F., Berry K. W., Rose J. A. A unique form of terminal redundancy in adenovirus DNA molecules. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2391–2395. doi: 10.1073/pnas.69.9.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geshelin P., Berns K. I. Characterization and localization of the naturally occurring cross-links in vaccinia virus DNA. J Mol Biol. 1974 Oct 5;88(4):785–796. doi: 10.1016/0022-2836(74)90399-4. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Grady L. J., Paoletti E. Molecular complexity of vaccinia DNA and the presence of reiterated sequences in the genome. Virology. 1977 Jun 15;79(2):337–341. doi: 10.1016/0042-6822(77)90361-0. [DOI] [PubMed] [Google Scholar]
- Koczot F. J., Carter B. J., Garon C. F., Rose J. A. Self-complementarity of terminal sequences within plus or minus strands of adenovirus-associated virus DNA. Proc Natl Acad Sci U S A. 1973 Jan;70(1):215–219. doi: 10.1073/pnas.70.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron R. J., Cabrera C. V., Esteban M., McAllister W. T., Holowczak J. A. Structure of vaccinia DNA: analysis of the viral genome by restriction endonucleases. Virology. 1978 May 1;86(1):88–101. doi: 10.1016/0042-6822(78)90010-7. [DOI] [PubMed] [Google Scholar]
- Parr R. P., Burnett J. W., Garon C. F. Ultrastructural characterization of the Molluscum contagiosum virus genome. Virology. 1977 Sep;81(2):247–256. doi: 10.1016/0042-6822(77)90141-6. [DOI] [PubMed] [Google Scholar]
- Pedrali-Noy G., Weissbach A. Evidence of a repetitive sequence in vaccinia virus DNA. J Virol. 1977 Oct;24(1):406–407. doi: 10.1128/jvi.24.1.406-407.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Two deoxyribonuclease activities within purified vaccinia virus. Proc Natl Acad Sci U S A. 1969 Jul;63(3):820–827. doi: 10.1073/pnas.63.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. J., Arrand J. R., Keller W. The length of the terminal repetition in adenovirus-2 DNA. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3829–3833. doi: 10.1073/pnas.71.10.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarov I., Becker Y. Studies on vaccinia virus DNA. Virology. 1967 Nov;33(3):369–375. doi: 10.1016/0042-6822(67)90112-2. [DOI] [PubMed] [Google Scholar]
- Sedat J. W., Kelly R. B., Sinsheimer R. L. Fractionation of nucleic acid on benzoylated-naphthoylated DEAE cellulose. J Mol Biol. 1967 Jun 28;26(3):537–540. doi: 10.1016/0022-2836(67)90321-x. [DOI] [PubMed] [Google Scholar]
- Sheldrick P., Berthelot N. Inverted repetitions in the chromosome of herpes simplex virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):667–678. doi: 10.1101/sqb.1974.039.01.080. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wittek R., Menna A., Schümperli D., Stoffel S., Müller H. K., Wyler R. HindIII and Sst I restriction sites mapped on rabbit poxvirus and vaccinia virus DNA. J Virol. 1977 Sep;23(3):669–678. doi: 10.1128/jvi.23.3.669-678.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Müller H. K., Wyler R. Length heterogeneity in the DNA of vaccinia virus is eliminated on cloning the virus. FEBS Lett. 1978 Jun 1;90(1):41–46. doi: 10.1016/0014-5793(78)80293-2. [DOI] [PubMed] [Google Scholar]
- Wolfson J., Dressler D. Adenovirus-2 DNA contains an inverted terminal repetition. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3054–3057. doi: 10.1073/pnas.69.10.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]