Abstract
Objective. To study the impact of the neutral endopeptidase (NEP)/neuropeptides (NPs) axis and nuclear factor kappa B (NFκB) as predictors of prostate-specific antigen (PSA) recurrence after radical prostatectomy (RP). Patients and Methods. 70 patients with early-stage PC were treated with RP and their tumor samples were evaluated for expression of NEP, endothelin-1 (ET-1) and NFκB (p65). Time to PSA recurrence was correlated with the examined parameters and combined with preoperative PSA level, Gleason score, pathological TNM (pT) stage, and surgical margin (SM) assessment. Results and Limitations. Membranous expression of NEP (P < 0.001), cytoplasmic ET-1 (P = 0.002), and cytoplasmic NFκB (P < 0.001) were correlated with time to PSA relapse. NEP was associated with ET-1 (P < 0.001) and NFκB (P < 0.001). ET-1 was also correlated with NFκB (P < 0.001). NEP expression (P = 0.017), pT stage (P = 0.013), and SMs (P = 0.036) were independent predictors of time to PSA recurrence. Conclusions. There seems to be a clinical model of NEP/NPs and NFκB pathways interconnection, with their constituents following inverse patterns of expression in accordance with their biological roles and molecular interrelations.
1. Introduction
Neuropeptides (NPs) constitute a family of potent vasoconstrictor peptides with mitogenic properties relevant to carcinogenesis. Endothelin-1 (ET-1) is a major representative, consistently implicated in prostate cancer (PC) progression through induction of proliferation of PC cells in vitro, while in vivo, increased ET-1 levels were detected in plasma and tissue specimens from patients with castrate-resistant PC [1–3].
The enzyme responsible for cleavage and inactivation of ET-1 and other bioactive NPs is neutral endopeptidase (NEP or CD10). NEP is a cell surface peptidase normally expressed by various tissues, including prostate [4, 5] but its loss of expression has been correlated with tumor progression to castration resistance by allowing NPs growth-promoting effects [6].
Nuclear factor kappa B (NFκB) is a transcription factor known for its prosurvival and antiapoptotic roles in various types of neoplasia. The most studied form of NFκB is the heterodimer formed by the p50 and RelA (p65) proteins. Emerging preclinical evidence implicates NFκB in growth, survival, angiogenesis, and metastatic progression of PC cells [7, 8]. Constitutive activation of NFκB has been detected in castrate-resistant PC xenografts and in PC tissues [9].
Since the continuously increasing list of prognostic biomarkers candidate for clinical use in PC and given the central role played by the NEP/NPs and NFκB pathways in PC progression, gross data from immunohistochemical studies of radical prostatectomy (RP) specimens has accumulated. However, the existence of conflicting results as well as the lack of an integrated investigation of both pathways in the same study still hampers proper interpretation of data towards refining the already existing prognostic models of the disease.
2. Patients and Methods
2.1. Patient Selection
The study enrolled patients over 18 years old with histologically newly diagnosed, early-stage PC, admitted to the Department of Urology, University Hospital of Larissa. All patients of the study underwent an open retropubic radical prostatectomy. Patients were hormone and treatment (chemotherapy and radiotherapy) naïve at the time of surgery. No history of previous reproductive or endocrine diseases was reported. The study was approved by the Internal Review Board of our institution and written informed consent was provided by all patients before study entry.
Patient demographics (age) as well as clinico-pathological parameters, including preoperative PSA level, pT stage and Gleason score of the primary tumor, lymph node status, SMs, PSA recurrence, and survival data were recorded.
Hematoxylin and eosin-stained tissue sections from 70 RP specimens were examined by a single, blinded histopathologist, based on the availability of both adequate followup and representative pathology specimens. The radical prostatectomy specimens were processed using a whole-mount technique. Evaluation of histopathological characteristics was made according to recommendations of the 2004 World-Health-Organization-(WHO-) sponsored International Consultation on Prediction of Patients Outcome in Prostate Cancer meeting [10]. Cases were divided into 2 Gleason groups: low (≤3 + 4; n = 50) and high (≥4 + 3; n = 20), as there were no lower Gleason score (2, 3, 4) samples based on the established 3-group histopathological criteria of current literature (low, medium, and high). Cases were also grouped according to pT stage into either organ-confined disease (pT ≤ 2; n = 42) or advanced tumors extending beyond the prostatic capsule (pT ≥ 3; n = 28). pT3 group consisted of 11 patients with pT3a disease and 16 patients of pT3b stage; however due to the small number of patients, no additional subgroup analysis was performed. With regard to preoperative PSA levels, patients were categorized in 2 subgroups: <10 ng/mL and ≥10 ng/mL. The majority of patients had a preoperative PSA level <10 ng/mL (n = 60), while they also displayed negative SM (n = 49) and lymph node status (n = 53). The latter was not included in most of our statistical analyses due to missing information regarding a significant number of patients (n = 11 or 15.7%). Patients' clinical and pathological characteristics are depicted in Table 1.
Table 1.
Patients' clinicopathological characteristics (n = 70) and associations with time to PSA relapse.
| Characteristic | Subgroup | n (%) | P |
|---|---|---|---|
| Age (years) | ≤65 | 31 (44.3) | 0.277 |
| range 47–75 | >65 | 39 (55.7) | |
| pre-op PSA (ng/mL) | <10 | 60 (85.7) | 0.143 |
| range 2.8–23.9 | ≥10 | 10 (14.3) | |
| pT stage | ≤2 | 42 (60.0) | <0.001 |
| ≥3 | 28 (40.0) | ||
| Gleason score | ≤7 (3 + 4) | 50 (46.8) | <0.001 |
| ≥7 (4 + 3) | 20 (44.2) | ||
| SMs | (−) | 49 (70.0) | 0.004 |
| (+) | 21 (30.0) | ||
| LN status | N0 | 53 (75.7) | 0.072 |
| N1 | 6 (8.6) | ||
| Nx | 11 (15.7) |
Pre-op PSA: preoperative PSA; pT stage: pathologic TNM stage; SMs: surgical margins; LN: lymph node; n: number of patients; P: statistical value.
2.2. Immunohistochemical (IHC) Procedures
The RP specimens were fixed in 10% buffered formalin solution and embedded in paraffin blocks. Serial sections (4 μm) from selected 1 or 2 paraffin blocks of each case were obtained. Tissue blocks were chosen based on the presence of both, the primary and the secondary architectural Gleason pattern of prostate adenocarcinoma, as determined on hematoxylin and eosin sections. Sections were deparaffinised in xylene and rehydrated through decreasing alcohols. Antigen unmasking for NFκB was achieved by treating sections in a 6 mM citrate buffer (pH 6) for a total of 20 min in a microwave oven at 850 Watt. Antigen unmasking for NEP and ET-1 was achieved by boiling sections in Trilogy reagent (Cell Marque, Rocklin, CA) for a total of 1 hour in a commercially available steamer. After quenching endogenous peroxidase with 3% hydrogen peroxide solution for 10 min, slides were incubated at room temperature for 30 minutes with the following primary mouse monoclonal antibodies: against p65 subunit of NFκB (clone F-6, 1 : 500 dilution, Santa Cruz Biotechnology, Inc., CA) and anti-NEP (clone 56C6, 1 : 30 dilution, DAKO, Denmark). Adjacent sections were incubated overnight at 4°C with mouse monoclonal antibody against ET-1 (clone TRET-485, 1 : 100 dilution, SIGMA, UK). Staining was developed with substrate chromogen solution (EnVision, DAKO, Glostrup, Denmark) and diaminobenzidine for 10 minutes. Slides were counterstained with Harris hematoxylin for 1 minute, dehydrated, and mounted with DPX solution.
2.3. Assessment of IHC Staining
Assessment of IHC staining was made according to evaluations in previous studies. NFκB immunostaining was cytoplasmic. NEP immunostaining was membranous and apical cytoplasmic. ET-1 immunostaining was cytoplasmic. Intensity of immunostaining for all 3 examined parameters was evaluated, using a score from 0 to 3 (0: no staining 1: weak staining, 2: moderate staining, and 3: strong staining) compared with the background [9, 11, 12]. Weak and moderate staining (0–2) versus strong staining (3) were considered for statistical analysis of NFκB and ET-1, classified as low and high expression, respectively [9, 12]. High NEP expression was defined as a score of 2 or greater (2-3) and low expression as a score of less than 2 (0-1) [11].
The weak ET-1 staining category was assessed to be less than that of endothelial cells, the moderate ET-1 staining category was determined to be equal to that of endothelial cells, and the intense ET-1 staining category exhibited more than that of endothelial cells. The normal adjacent prostate gland was used as an internal control marker for the evaluation of NEP and NFκB expression. IHC reaction was glandular for all tested parameters (NEP, ET-1, and NFκB).
2.4. Study Endpoints
Our objective was to investigate possible interrelations between IHC expression of NEP, ET-1, and NFκB as well as their potential correlations with preoperative PSA level, Gleason score, pT stage, and SMs in patients with hormone naïve PC undergoing RP. We further examined the putative prognostic role of these parameters in association with time to PSA failure. The response variable, time to PSA recurrence, was defined as the time from RP to the time of the first detectable (nonzero) PSA measurement >0.2 ng/mL.
2.5. Statistical Analysis
The Fisher's and χ 2 tests were used to explore associations between NEP, ET-1, NFκB expression and Gleason score, tumor stage, and SM status. Pearson's correlation coefficient was reported to determine the correlation between NEP, ET-1, NFκB expression and preoperative PSA levels. The Kaplan-Meier method was used to determine the effect of each categorical variable on PSA relapse-free survival, and the log-rank test was used to compare PSA relapse-free survival differences within each variable. For PSA recurrence-free survival analysis at the univariate and multivariate level, the Cox proportional hazards model was used to estimate hazard ratios (HR) with 95% confidence intervals (CI). Statistical significance was determined by using two-tailed P values and was reported at P < 0.05 level. Statistical analysis was performed using SPSS (SPSS for Windows, version 15.0, SPSS, Chicago, IL).
3. Results
Thirty-eight (54%) patients developed PSA recurrence during followup and 32 (46%) did not have a PSA relapse. Two patients (2.8%) expired. The estimated median follow-up time, as calculated by the reverse Kaplan-Meier method was 30 months (range 12–86) while the median time to PSA recurrence was 56 months (range 1–74). Among relapsed patients, 22 received combined androgen blockade (CAB), 1 received local radiotherapy, and 9 patients were treated with a combination of CAB and radiotherapy (5 in sequential order and 4 concurrently).
3.1. NEP Is Associated with Grade, Stage, and Time to PSA Relapse
According to level of NEP expression, patients were divided into a group of low (n = 36, 51.4%) and another of high NEP IHC expression (n = 34, 48.6%). In univariate analysis, we observed a significant association of NEP with Gleason score, as the majority of high NEP-expressing tumors (30/34 or 88.2%) correlated with low Gleason score (P = 0.003). Similar was the case for the association between NEP and pT stage, with 25/34 or approximately 73.5% of tumors with high NEP expression correlating with low pT stage (P = 0.030) (Table 2).
Table 2.
Correlations between levels of NEP, ET-1, NFκB expression, and clinicopathological characteristics.
| Characteristic | NEP | P | ET-1 | P | NFκB | P | |||
|---|---|---|---|---|---|---|---|---|---|
| low | high | low | high | low | high | ||||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||||
| Pre-op PSA | |||||||||
| <10 | 28 (96.6) | 34 (82.9) | 0.076 | 32 (94.1) | 30 (83.3) | 0.277 | 35 (83.3) | 27 (96.4) | 0.191 |
| ≥10 | 1 (3.4) | 7 (17.1) | 2 (5.9) | 6 (16.7) | 7 (16.7) | 1 (3.6) | |||
| Gleason score | |||||||||
| ≤7 (3 + 4) | 20 (55.6) | 30 (88.2) | 0.003 | 19 (55.9) | 31 (86.1) | 0.008 | 37 (74) | 6 (30) | 0.001 |
| ≥7 (4 + 3) | 16 (44.4) | 4 (11.8) | 15 (44.1) | 5 (13.9) | 13 (26) | 14 (70) | |||
| pT stage | |||||||||
| ≤2 | 17 (47.2) | 25 (73.5) | 0.030 | 14 (41.2) | 28 (77.8) | 0.003 | 32 (76.2) | 11 (39.3) | 0.003 |
| ≥3 | 19 (52.8) | 9 (26.5) | 20 (58.8) | 8 (22.2) | 10 (23.8) | 17 (60.7) | |||
| SMs | |||||||||
| (−) | 22 (61.1) | 27 (79.4) | 0.121 | 20 (58.8) | 29 (80.6) | 0.068 | 33 (76.7) | 16 (59.3) | 0.180 |
| (+) | 14 (38.9) | 7 (20.6) | 14 (41.2) | 7 (19.4) | 10 (23.3) | 11 (40.7) | |||
Pre-op PSA: preoperative PSA; pT stage: pathologic TNM stage; SMs: surgical margins; n: number of patients; P: statistical value.
The expression of NEP was found to be associated with time to PSA recurrence (r = 0.485), (P < 0.001) (Table 2, Figure 1). There were no statistically significant correlations between NEP and preoperative PSA levels (P = 0.076) or NEP and SMs (P = 0.121) (Table 2).
Figure 1.
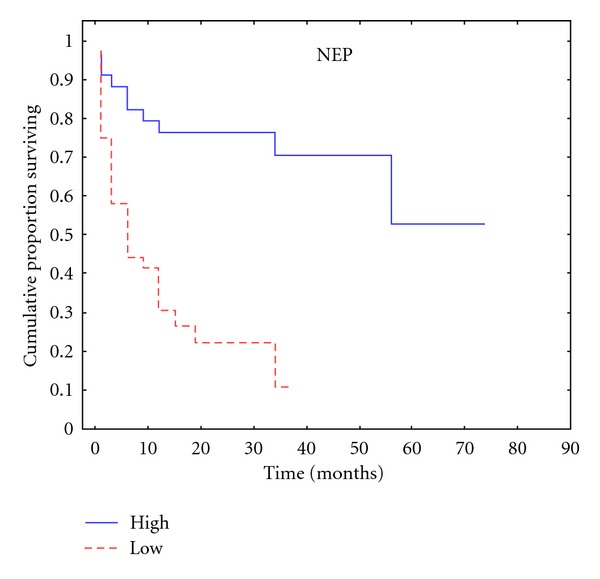
Kaplan-Meier plot according to NEP expression for time to PSA relapse.
3.2. ET-1 Is Associated with Grade, Stage, and Time to PSA Relapse
ET-1 expression was equally divided in two groups of either low (n = 34, 48.6%) or high (n = 36, 51.4%) immunoreactivity. Elevated ET-1 expression was correlated with more advanced disease, evidenced by both pT stage (P = 0.003) and Gleason score (P = 0.008) in univariate analysis (Table 2). ET-1 was also found to be an indicator of biochemical progression as its expression correlated with a smaller time interval from RP until PSA relapse (r = −0.375), (P < 0.001) (Table 2, Figure 2). There were no statistically significant correlations between ET-1 and preoperative PSA levels (P = 0.277) or ET-1 and SMs (P = 0.068) (Table 2).
Figure 2.
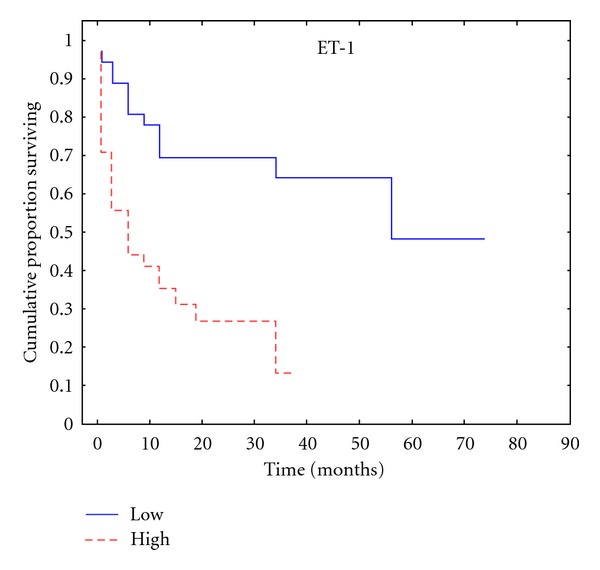
Kaplan-Meier plot according to ET-1 expression for time to PSA relapse.
3.3. NFκB Expression Is Correlated with Grade, Stage, and Time to PSA Relapse
NFκB staining was found to be low in 60% of patients (n = 42) whereas high in 40% of the cohort (n = 28). There was an association of NFκB expression with both stage (P = 0.003) and grade (P = 0.002) in univariate analysis (Table 2). High NFκB expression was also found to significantly correlate with a shortened time to PSA recurrence (r = −0.432), (P < 0.001) (Table 2, Figure 3). There were no statistically significant correlations between NFκB and preoperative PSA levels (P = 0.191) or NFκB and SMs (P = 0.180) (Table 2).
Figure 3.
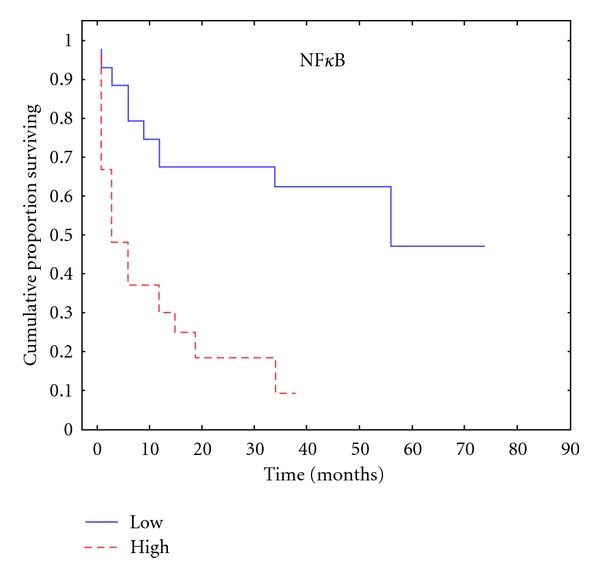
Kaplan-Meier plot according to NFκB expression for time to PSA relapse.
3.4. NEP Expression Is Associated with ET-1 and NFκB Expression
In univariate analysis, a significant correlation was found between IHC expression of NEP and ET-1 (P < 0.001). Same was the case for the NEP-NFκB relationship (P < 0.001) (Table 3). ET-1 and NFκB expressions were also interrelated (P < 0.001). IHC expression patterns of NEP, ET-1, and NFκB are depicted in Figures 4, 5 and 6.
Table 3.
Correlations between NEP, ET-1, and NFκB expression.
| Variables | NEP | HR [95% CI] | P | |
|---|---|---|---|---|
| Low | High | |||
| n (%) | n (%) | |||
| ET-1 | ||||
| Low | 3 | 31 | 0.016 [0.003–0.071] | <0.001 |
| High | 31 | 5 | ||
| NFκB | ||||
| Low | 10 | 33 | 0.012 [0.001–0.097] | <0.001 |
| High | 26 | 1 | ||
n: number of patients; HR: hazard ratio; CI: confidence interval; P: statistical value.
Figure 4.
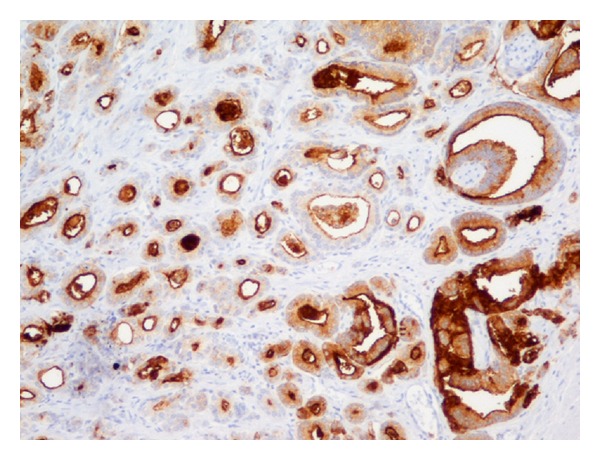
Prostate adenocarcinoma Gleason pattern 3. Strong membranous and apical cytoplasmic IHC staining for NEP (×200).
Figure 5.
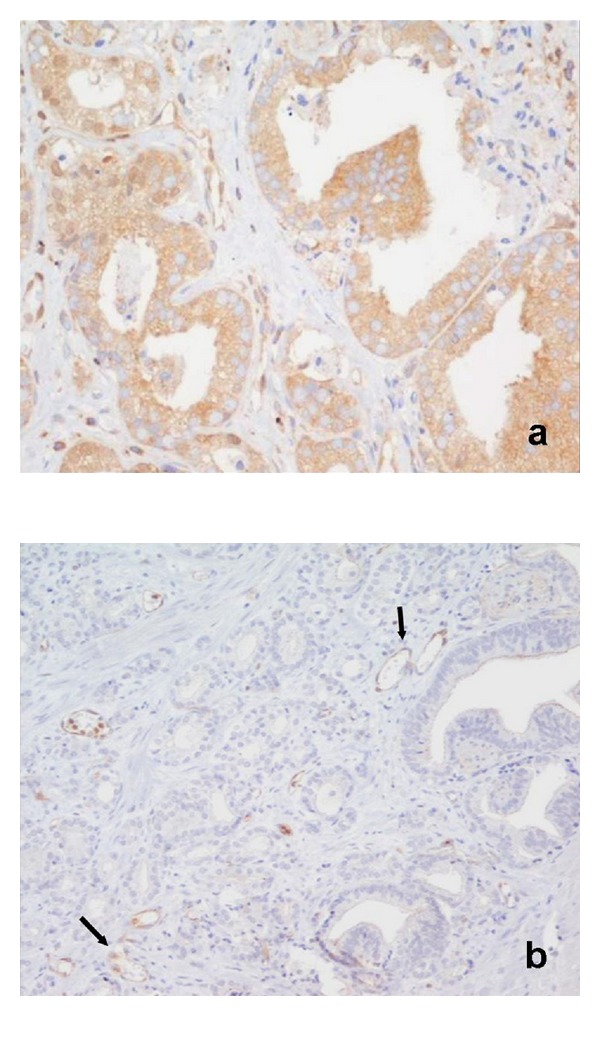
Prostate adenocarcinoma Gleason pattern 3. The same area as in Figure 4. (a) Weak-to-moderate cytoplasmic IHC staining for NFκB (×400); (b) Negative immunoreactivity for ET-1. Positive marker the capillary endothelium (arrows) (×200).
Figure 6.
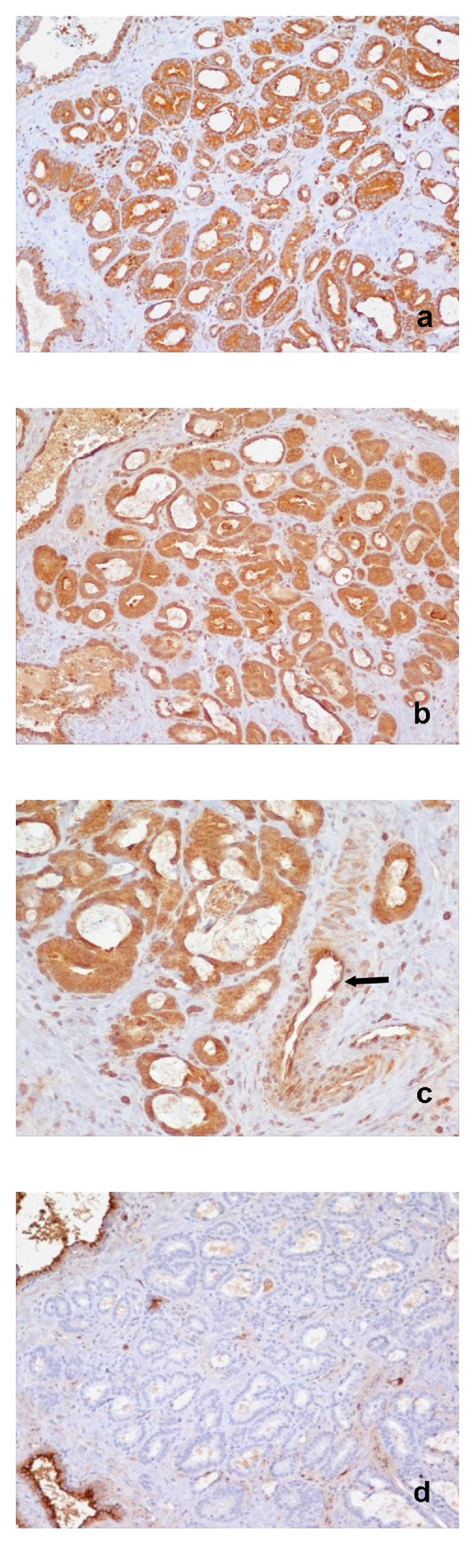
Prostate adenocarcinoma Gleason pattern 3. (a) Strong cytoplasmic IHC staining for NFκB (×200); (b) strong cytoplasmic IHC staining for ET-1 (×200); (c) strong cytoplasmic IHC staining for ET-1. Positive marker the capillary endothelium (arrow) (×400); (d) negative immunoreactivity for NEP. Positive marker the normal prostate glands (×200).
3.5. Other Univariate Correlations
pT stage (P < 0.001), Gleason score (P < 0.001), and SM status (P = 0.004) were related with PSA relapse-free survival (Table 1). There was no statistically significant correlation between age (P = 0.277), preoperative PSA (P = 0.143) or lymph node status (P = 0.072), and time to PSA recurrence, respectively (Table 1). Preoperative PSA level was associated with pT stage (P = 0.016) but not with Gleason score (P = 0.123). Stage and grade were interrelated (P < 0.001).
3.6. NEP, pT Stage and SMs Are Independent Predictors of Time to PSA Relapse
In multivariate analysis, there was a significant association between NEP expression and time to PSA recurrence after controlling for preoperative PSA, tumor stage, Gleason score, SMs, NFκB, and ET-1 (P = 0.017; 95% [CI] = 0.228 [0.068–0.766]). pT stage (P = 0.013; 95% [CI] = 3.025 [1.257–7.277] and SM status (P = 0.036; 95% [CI] = 2.061 [1.049–4.051]) also retained their significance as predictors of time to PSA recurrence (Table 4).
Table 4.
Multivariate Cox regression analysis.
| Variable | Groups | HR [95% CI] | P |
|---|---|---|---|
| NEP | low vs high | 4.386 [1.305–14.706] | 0.017 |
| ET-1 | low vs high | 0.636 [0.167–2.422] | 0.507 |
| NFκB | low vs high | 0.851 [0.273–2.652] | 0.781 |
| pre-op PSA | <10 vs ≥10 | 0.334 [0.101–1.097] | 0.071 |
| Gleason score | ≤7 (3 + 4) vs ≥7 (4 + 3) | 0.637 [0.276–1.473] | 0.291 |
| pT stage | ≤2 vs ≥3 | 0.330 [0.137–0.795] | 0.013 |
| SMs | (−) vs (+) | 0.485 [0.247–0.953] | 0.036 |
pT stage: pathologic TNM stage; SMs: surgical margins; pre-op PSA: preoperative PSA; HR: hazard ratio; CI: confidence interval; P: statistical value; vs = versus.
4. Discussion
In this study we have simultaneously examined the expression of major components of two systems that have separately been correlated with PC progression, the NEP/NPs, and the NFκB pathway.
We have confirmed the previously reported association between expression of components of the endothelin axis and stage, grade [11, 13, 14]. We have also observed an association of ET-1 expression with time to PSA recurrence, in accordance with results from Rosenblatt et al. [12] who demonstrated that both the intensity and the combination product of intensity and extent of ET-1 immunoreactivity (IRp) but not the staining extent alone predicted biochemical relapse in a large-scale study of 287 PC specimens from RP. Recurrence-free survival in patients with strong ET-1 staining was shorter than in those with weaker expression [12].
Moreover, we have observed that the aggressiveness of the examined tumors as evidenced by increasing grade and advanced stage coincided with loss of membranous NEP, justifying the latter's biological role in attenuation of oncogenic signaling induced by NEP substrates. Accordingly, a significant association was observed between NEP and time to PSA recurrence.
The prognostic relevance of NEP expression in early PC is a field of controversies between studies regarding differences in localization and level of expression patterns as well as differences in correlation or lack thereof with clinicopathologic parameters, including PSA relapse-free survival. Loss or decreased expression of NEP was observed in 219 prostatectomy specimens of patients with PC compared with normal prostate and prostatic intraepithelial neoplasia (PIN), although no correlation of NEP expression with clinical parameters (Gleason score and pathologic stage) or outcome (biochemical recurrence) was observed in these series [15]. In another series of 223 patients, loss of membranous NEP expression was significantly associated with a shorter time to PSA relapse [16]. In contrast, high NEP expression was associated with higher PSA recurrence in a cohort of 87 patients [17], in which 34 patients were lymph node positive and were not included in the recurrence analysis. The most extensive series investigating NEP as a predictor of PC reports on 2385 patients treated by RP in a single institution during a 14-year time period [18]. In these series, the authors observed a PSA recurrence-free survival decline from membranous over membrane-cytoplasmic to exclusively cytoplasmic NEP expression [18]. However, the best prognosis was observed with NEP negativity, which is a finding against the results of several preclinical models [6, 19], including ours [20] and is also at odds with the results of Osman et al. [16], with the rest of the results of this same series predicting a worse prognosis for a nonfunctional localization of NEP as well as the results of our present study. One could hypothesize that the mechanism promoting methylation of the NEP promoter and NEP expression loss inhibits also expression of cancer-promoting genes with the net effect being an improvement in prognosis. Alternatively, an artifact of loss of extracellular NEP during tissue processing in at least part of the samples remains a possibility. Importantly, staining intensity was not considered for analysis in their study.
Our findings regarding the clinical usefulness of NFκB corroborate previous data indicative of association between NFκB expression and grade [21, 22] and significant improvement of the prediction of outcome with the combination of Gleason score and nuclear NFκB staining [23]. NFκB has also been reported as an independent prognostic indicator of recurrence in groups with positive SMs [24], or lymph node invasion [25]. A few reports have emphasized on subcellular NFκB compartmentalization, with reproducible results regarding the correlation of either cytoplasmic [26], or nuclear immunoreactivity [27] with PSA failure. In general, despite differences in localization between studies, high NFκB is correlated with inferior outcome.
An issue that has been so far underestimated, despite its already established preclinical significance, concerns the potential interrelations between expression of NEP, ET-1, and NFκB in early PC. In this study we coexamined all three parameters together at the clinical level and demonstrated that NEP expression is related to ET-1 and NFκB expression, with the majority of high NEP-expressing cells demonstrating a low ET-1 and NFκB expression level and reversely.
In multivariate analysis including preoperative PSA, tumor stage, grade, SMs, ET-1, and NFκB expression, only pT stage, SMs, and NEP retained their importance in predicting PSA recurrence after RP. In fact, the risk of PSA relapse was approximately 4.386-fold greater in patients with low NEP expression compared to men with high expression of membranous NEP. In addition, this risk was greater than the risk derived from advanced pT stage (3.025 fold) and positive SM status (2.061 fold). These data confirm that there is a strong association between NEP expression and PSA relapse in hormone naïve PC patients and a strong rational for integration of NEP in the armamentarium of existing prognostic tools for early prediction of PC recurrence. Intriguingly, the emergence of significant NEP, ET-1, and NFκB interrelations (Table 3) illustrates the clinical relevance of two pathways that might be in close communication. Our results implicate the existence of two clinicopathological patterns of hormone naïve PC with regard to the time of PSA recurrence after RP. The first pattern is featuring high NEP expression and low ET-1 and NFκB expression and predicts a prolonged PSA relapse-free survival compared to the second one, which involves attenuation of NEP expression accompanied by elevated ET-1 and NFκB levels and is characterized by a shortened PSA relapse-free survival.
Indeed, at the molecular level, there seems to be a functional link between the NEP/NPs and NFκB pathways. NFκB has been suggested as a mediator of the antiapoptotic actions of NPs, given that prosurvival signaling initiated by NPs involves activation of the kinases PI3K and Akt, which then activates NFκB by phosphorylating its inhibitor, IκB [28, 29]. In a recent in vitro work, we have revealed an inverse baseline expression pattern of the NEP/NPs and NFκB/proteasome pathways in androgen-dependent and androgen-independent PC cells [20]. Further we have observed that NPs were able to induce NFκB nuclear translocation and DNA binding, an event that was much more pronounced in androgen-independent cell lines that display low levels of NEP. These events were reversed by NP inhibitors (Patrikidou et al. unpublished data). The current study is the first linking, at a clinical level, NEP expression with ET-1 and NFκB expression. These results complement our in vitro data on PC cell lines.
Nevertheless, several limitations of the present study should be considered. It should be acknowledged that the small number of patients included in the present study does not permit to draw unequivocal conclusions. Further, lowest Gleason scores (2, 3, and 4) are not represented in the study population and this might also blunt the validity of our results, although it might be hypothesized that differences between expression of NEP, ET-1, and NFκB might be even more pronounced based on the underlying biology of the disease in its earliest phase. The evaluation of SMs, although clearly defined may be either misinterpreted due to the presence of crush, thermal, and electrocautery artifacts [30] or clinically less relevant in locally advanced disease [31]. Retrospective review of our prospectively collected data and the relatively short median followup of patients may have also biased our results. Moreover, the prognostic values of NEP, ET-1, and NFκB might have been better established if they had been compared to predicted outcomes of validated nomograms. Finally, our study was not intended to be all inclusive of current prognostic markers such as seminal vesicle involvement, tumor marker ploidy status, and proliferation indexes.
In conclusion, our study was designed in an effort to offer an integrated approach of the role of the NEP/endothelin and NFκB pathways in the clinical course of PC patients. The relationship between recurrence (given the heterogeneity of the population), stage and grade of the tumors, and outcome was an exploratory (hypothesis generating) analysis and the primary goal of this study was to demonstrate a relationship between NEP/ET-1 and NFκB signaling. Although further prospective evaluation to confirm this interaction is required, the strong preclinical model of PC evolution based on aberration of both NEP/NPs and NFκB pathways seems to play a role in the clinical setting. Most importantly, the significant interrelations that we have suggested to exist between these two systems, if confirmed in a large prospective cohort, might encourage not only the incorporation of their pathological assessment into the current model of predictive factors but also the concept of their concurrent pharmacological inhibition to enable a greater therapeutic benefit in tumors with aggressive biology.
References
- 1.Nelson JB, Hedican SP, George DJ, et al. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nature Medicine. 1995;1(9):944–949. doi: 10.1038/nm0995-944. [DOI] [PubMed] [Google Scholar]
- 2.Nelson JB, Chan-Tack K, Hedican SP, et al. Endothelin-1 production and decreased endothelin b receptor expression in advanced prostate cancer. Cancer Research. 1996;56(4):663–668. [PubMed] [Google Scholar]
- 3.Grant ES, Brown T, Roach A, Williams BC, Habib FK. In vitro expression of endothelin-1 (ET-1) and the ETA and ETB et receptors by the prostatic epithelium and stroma. Journal of Clinical Endocrinology and Metabolism. 1997;82(2):508–513. doi: 10.1210/jcem.82.2.3724. [DOI] [PubMed] [Google Scholar]
- 4.Liu AY, LaTray L, van Den Engh G. Changes in cell surface molecules associated with in vitro culture of prostatic stromal cells. Prostate. 2000;44:303–312. doi: 10.1002/1097-0045(20000901)44:4<303::aid-pros7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 5.Usmani BA, Harden B, Maitland NJ, Turner AJ. Differential expression of neutral endopeptidase-24.11 (neprilysin) and endothelin-converting enzyme in human prostate cancer cell lines. Clinical Science. 2002;103(48):314S–317S. doi: 10.1042/CS103S314S. [DOI] [PubMed] [Google Scholar]
- 6.Papandreou CN, Usmani B, Geng Y, et al. Neutral endopeptidase 24.11 loss in metastatic human prostate cancer contributes to androgen-independent progression. Nature Medicine. 1998;4(1):50–57. doi: 10.1038/nm0198-050. [DOI] [PubMed] [Google Scholar]
- 7.Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-κB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20(31):4188–4197. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- 8.Chen CD, Sawyers CL. NF-κB activates prostate-specific antigen expression and is upregulated in androgen-independent prostate cancer. Molecular and Cellular Biology. 2002;22(8):2862–2870. doi: 10.1128/MCB.22.8.2862-2870.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweeney C, Li L, Shanmugam R, et al. Nuclear factor-κB is constitutively activated in prostate cancer in vitro and is overexpressed in prostatic intraepithelial neoplasia and adenocarcinoma of the prostate. Clinical Cancer Research. 2004;10(16):5501–5507. doi: 10.1158/1078-0432.CCR-0571-03. [DOI] [PubMed] [Google Scholar]
- 10.Epstein JI, Amin M, Boccon-Gibod L, et al. Prognostic factors and reporting of prostate carcinoma in radical prostatectomy and pelvic lymphadenectomy specimens. Scandinavian Journal of Urology and Nephrology Supplement. 2005;216:34–63. doi: 10.1080/03008880510030932. [DOI] [PubMed] [Google Scholar]
- 11.Godara G, Pecher S, Jukic DM, et al. Distinct patterns of endothelin axis expression in primary prostate cancer. Urology. 2007;70(1):209–215. doi: 10.1016/j.urology.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Rosenblatt R, Valdman A, Cheng L, et al. Endothelin-1 expression in prostate cancer and high grade prostatic intraepithelial neoplasia. Analytical & Quantitative Cytology & Histology. 2009;31:137–142. [PubMed] [Google Scholar]
- 13.Gohji K, Kitazawa S, Tamada H, Katsuoka Y, Nakajima M. Expression of endothelin receptor a associated with prostate cancer progression. Journal of Urology. 2001;165(3):1033–1036. [PubMed] [Google Scholar]
- 14.Montironi R, Mazzucchelli R, Barbisan F, et al. Immunohistochemical expression of endothelin-1 and endothelin-A and endothelin-B receptors in high-grade prostatic intraepithelial neoplasia and prostate cancer. European Urology. 2007;52(6):1682–1690. doi: 10.1016/j.eururo.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 15.Freedland SJ, Seligson DB, Liu AY, et al. Loss of cd10 (neutral endopeptidase) is a frequent and early event in human prostate cancer. Prostate. 2003;55(1):71–80. doi: 10.1002/pros.10202. [DOI] [PubMed] [Google Scholar]
- 16.Osman I, Yee H, Taneja SS, et al. Neutral endopeptidase protein expression and prognosis in localized prostate cancer. Clinical Cancer Research. 2004;10(12):4096–4100. doi: 10.1158/1078-0432.CCR-04-0120. [DOI] [PubMed] [Google Scholar]
- 17.Dall’Era MA, True LD, Siegel AF, Porter MP, Sherertz TM, Liu AY. Differential expression of cd10 in prostate cancer and its clinical implication. Bmc Urology. 2007;7, article no. 3 doi: 10.1186/1471-2490-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fieischmann A, Schlomm T, Huland H, et al. Distinct subcellular expression patterns of neutral endopeptidase (cd10) in prostate cancer predict diverging clinical courses in surgically treated patients. Clinical Cancer Research. 2008;14(23):7838–7842. doi: 10.1158/1078-0432.CCR-08-1432. [DOI] [PubMed] [Google Scholar]
- 19.Sumitomo M, Shen R, Nanus DM. Involvement of neutral endopeptidase in neoplastic progression. Biochimica Et Biophysica Acta. 2005;1751(1):52–59. doi: 10.1016/j.bbapap.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Patrikidou A, Vlachostergios PJ, Voutsadakis IA, et al. Inverse baseline expression pattern of the nep/neuropeptides and nfκb/proteasome pathways in androgen-dependent and androgen-independent prostate cancer cells. Cancer Cell International. 2011;11, article no. 13 doi: 10.1186/1475-2867-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonnell TJ, Chari NS, Cho-Vega JH, et al. Biomarker expression patterns that correlate with high grade features in treatment naive, organ-confined prostate cancer. BMC Medical Genomics. 2008;1, article 1 doi: 10.1186/1755-8794-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuñez C, Cansino JR, Bethencourt F, et al. Tnf/il-1/nik/nf-κb transduction pathway: a comparative study in normal and pathological human prostate (benign hyperplasia and carcinoma) Histopathology. 2008;53(2):166–176. doi: 10.1111/j.1365-2559.2008.03092.x. [DOI] [PubMed] [Google Scholar]
- 23.Lessard L, Mes-Masson AM, Lamarre L, Wall L, Lattouf JB, Saad F. Nf-κb nuclear localization and its prognostic significance in prostate cancer. Bju International. 2003;91(4):417–420. doi: 10.1046/j.1464-410x.2003.04104.x. [DOI] [PubMed] [Google Scholar]
- 24.Fradet V, Lessard L, Bégin LR, Karakiewicz P, Mes Masson AM, Saad F. Nuclear factor-κB nuclear localization is predictive of biochemical recurrence in patients with positive margin prostate cancer. Clinical Cancer Research. 2004;10(24):8460–8464. doi: 10.1158/1078-0432.CCR-04-0764. [DOI] [PubMed] [Google Scholar]
- 25.Lessard L, Karakiewicz PI, Bellon-Gagnon P, et al. Nuclear localization of nuclear factor-κB p65 in primary prostate tumors is highly predictive of pelvic lymph node metastases. Clinical Cancer Research. 2006;12(19):5741–5745. doi: 10.1158/1078-0432.CCR-06-0330. [DOI] [PubMed] [Google Scholar]
- 26.Ross JS, Kallakury BVS, Sheehan CE, et al. Expression of nuclear factor-κb and iκbα proteins in prostatic adenocarcinomas: correlation of nuclear factor-κB immunoreactivity with disease recurrence. Clinical Cancer Research. 2004;10(7):2466–2472. doi: 10.1158/1078-0432.ccr-0543-3. [DOI] [PubMed] [Google Scholar]
- 27.Domingo-Domenech J, Mellado B, Ferrer B, et al. Activation of nuclear factor-κB in human prostate carcinogenesis and association to biochemical relapse. British Journal of Cancer. 2005;93(11):1285–1294. doi: 10.1038/sj.bjc.6602851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osman I, Dai J, Mikhail M, et al. Loss of neutral endopeptidase and activation of protein kinase B (Akt) is associated with prostate cancer progression. Cancer. 2006;107(11):2628–2636. doi: 10.1002/cncr.22312. [DOI] [PubMed] [Google Scholar]
- 29.Levine L, Lucci JA, Pazdrak B, et al. Bombesin stimulates nuclear factor κb activation and expression of proangiogenic factors in prostate cancer cells. Cancer Research. 2003;63(13):3495–3502. [PubMed] [Google Scholar]
- 30.Evans AJ, Henry PC, Van Der Kwast TH, et al. Interobserver variability between expert urologic pathologists for extraprostatic extension and surgical margin status in radical prostatectomy specimens. American Journal of Surgical Pathology. 2008;32(10):1503–1512. doi: 10.1097/PAS.0b013e31817fb3a0. [DOI] [PubMed] [Google Scholar]
- 31.Ploussard G, Agamy MA, Alenda O, et al. Impact of positive surgical margins on prostate-specific antigen failure after radical prostatectomy in adjuvant treatment-naïve patients. Bju International. 2011;107(11):1748–1754. doi: 10.1111/j.1464-410X.2010.09728.x. [DOI] [PubMed] [Google Scholar]


