Abstract
Host defenses against viral infections depend on a complex interplay of innate (nonspecific) and adaptive (specific) components. In the early stages of infection, innate mechanisms represent the main line of host defense, acting to limit the spread of virus in host tissues prior to the induction of the adaptive immune response. Serum and lung fluids contain a range of lectins capable of recognizing and destroying influenza A viruses (IAV). Herein, we review the mechanisms by which soluble endogenous lectins mediate anti-IAV activity, including their role in modulating IAV-induced inflammation and disease and their potential as prophylactic and/or therapeutic treatments during severe IAV-induced disease.
1. General Features of Influenza Virus
Influenza virus causes a highly contagious, acute infection of the upper and lower respiratory tract that is responsible for millions of deaths annually, predominantly in the elderly and in those with underlying pulmonary and cardiac disease [1]. Influenza viruses belong to the family Orthomyxoviridae, which consists of type A, B, and C influenza viruses, Thogotovirus, and Isavirus. While all 3 types of influenza virus infect humans, only influenza A viruses (IAVs) have the capacity to induce both epidemics and pandemics. Type B influenza viruses are associated with yearly epidemics whereas type C viruses rarely cause severe disease [2].
IAV is an enveloped virus with a genome comprising 8 segments of single-stranded, negative sense RNA encoding at least 10 proteins, including hemagglutinin (HA) and neuraminidase (NA), which are expressed on the virion surface. HA and, to a lesser extent, NA represent major targets for recognition by neutralizing antibodies and IAVs are divided into subtypes based on serological and genetic differences in their HA and NA proteins and the genes that encode them. At present, at least 16 HA and 9 NA subtypes of IAV have been identified, all of which are found amongst aquatic birds [2, 3]. In humans, only certain HA (H1, H2, and H3) and NA (N1 and N2) subtypes have been involved in epidemics and pandemics. Other subtypes of avian or equine origin (e.g., H5N1, H7N7, and H9N2) have infected humans but have not spread efficiently in the human population. These findings do, however, highlight the potential for mutation and/or genetic reassortment to facilitate entry and transmission of novel IAV in the human population.
Antigenic variation is the mechanism by which new subtypes or strains of IAV emerge in humans (reviewed in [4]). Antigenic shift is the most profound type of antigenic variation and results in the emergence of a virus that possesses novel HA and/or NA, to which the human population is immunologically naïve. Antigenic drift describes the gradual accumulation of point mutations in the genome of viruses that have been circulating in humans for an extended time. Mutations in viral RNA can lead to changes in the amino acid sequence of HA or NA and variants with changes in key antigenic epitopes will be selected as they can escape neutralization by preexisting antibodies. Antigenic variation via shift (which can result in pandemics) and drift (which can result in epidemics) ensures continued presence of IAV in the human population despite immunity arising from previous vaccination and/or infection.
1.1. Glycosylation of IAV Glycoproteins
The HA is a trimeric glycoprotein that mediates attachment of IAV to sialic acid (SA) on host cells as well as fusion of the viral and endosomal membranes during cell entry (reviewed in [5]). NA is a tetramer that enzymatically cleaves SA from glycoproteins, thereby facilitating release of progeny virions from the surface of infected cells and preventing self-aggregation of virus [6]. The “balance” between the receptor-binding and receptor-destroying activities of HA and NA, respectively, is a critical factor determining the efficiency of host cell infection by different IAV strains [7, 8].
Glycosylation of HA and NA occurs in the endoplasmic reticulum (ER) and in the Golgi apparatus in a process similar to that of host cell glycoproteins. HA/NA can express a mixture of high mannose (branched structures terminating in mannose), complex (structures terminating in different combinations of galactose, N-acetyl-galactosamine (GalNAc), fucose, or SA), or hybrid-type oligosaccharides [9, 10]. Biosynthesis of N-glycans begins in the rough endoplasmic reticulum (ER) with the transfer of a 14-sugar precursor (usually comprising 3 glucose, 9 mannose, and 2 N-acetyl-glucosamine (GlcNAc) molecules) from a polyisoprenol derivative to the nascent polypeptide chain. Transport of the glycoprotein is accompanied by trimming of terminal glucose and mannose residues in the ER and Golgi to generate high-mannose type glycans. Further processing, including the attachment of additional GlcNAc, fucose, galactose, and SA in the Golgi apparatus, results in the generation of complex and hybrid-type glycans. The oligosaccharides expressed on both HA and NA are exclusively N-linked glycans attached to the Asn residue of Asn-X-Ser/Thr, where X represents any amino acid except proline [11, 12]. Complex oligosaccharides on IAV HA/NA are not sialylated and bear terminal galactose/GlcNAc/fucose due to removal of terminal SA by the viral NA [13].
Oligosaccharide diversity of HA/NA depends on the number and location of potential N-glycosylation sites in the viral genome as well as the size and composition of the attached glycans. Certain regions of HA have conserved glycosylation sites or must remain free of oligosaccharides for the formation, transport, and/or maintenance of functional HA. The combinations of high-mannose, complex, and hybrid type glycans present on IAV glycoproteins are also determined by the particular biosynthetic and trimming enzymes provided by the host cell in which the virus was grown [14]. Acquisition of glycosylation sites on HA through antigenic drift has been proposed to mask or modify antigenic sites, thereby preventing recognition by neutralizing antibodies elicited to previous virus strains [15–17]. Consequently, pandemic IAV strains derived from zoonotic reservoirs generally have fewer potential N-linked glycosylation sites on the head of HA compared to seasonal IAV strains that have circulated in the human population for many years [17–19]. In addition to increased numbers, positional conversion of glycosylation sites (i.e., acquisition of a new glycosylation site accompanied by loss of an existing site) represents an additional mechanism proposed to favour evasion of antibody-mediated immunity and therefore persistence of IAV in humans [18]. The presence of potential N-linked glycosylation sites in the amino acid sequence of HA does not always correspond to the presence of attached glycans and some sites may not be glycosylated efficiently. To date, the detailed structure of the glycans carried at particular glycosylation sites has not been well established for most strains of IAV.
2. General Features of Animal Lectins
Lectins are nonenzymatic proteins that bind selectively to specific carbohydrate structures. Over the last 20 years, numerous lectins have been isolated and characterized from mammals, birds, and other animals. Unlike plant lectins, which are often grouped into families based on taxonomy, animal lectins often exhibit structural similarities even when derived from diverse phyla. Therefore, animal lectins are generally classified on the basis of an evolutionarily conserved carbohydrate recognition domain (CRD) [20] and on this basis they can be grouped into families, including C-type, S-type (or galectins), P-type (or Man-6-P lectins), Ig-type (or Siglecs), R-type, and F-type lectins (reviewed in [21]). Other lectins do not contain these CRDs but have evolved equivalent carbohydrate-binding characteristics, including pentraxins and glycosaminoglycan-binding proteins. C-type and S-type lectins, as well as pentraxins, have been reported to display lectin-mediated anti-IAV activity in vitro and/or in vivo and will be discussed further in this review.
2.1. C-Type Lectins
C-type lectins are a family of animal proteins that display Ca2+-dependent (C-type) carbohydrate-binding activity and mediate a range of biological processes including endocytosis, cell-to-cell adhesion, turnover of serum glycoproteins, and pathogen recognition. The C-type lectin CRD contains conserved residue motifs that are characteristic of the domain and are responsible for Ca2+-dependent binding activity [20, 22]. In general terms, C-type lectins bind either derivatives of mannose (Man-type ligands) or galactose (Gal-type ligands) [22, 23] and may be further subdivided into endocytic lectins and collectins on the basis of structural similarities. Endocytic C-type lectins include type I (e.g., macrophage mannose receptor (MMR)) and type II (e.g., macrophage galactose-type lectin (MGL) and DC-specific ICAM-3 grabbing non-integrin (DC-SIGN)) transmembrane proteins and these have been implicated as attachment and entry receptors for IAV infection [24, 25]. The collectins, so called because of their collagenous domains, are soluble C-type lectins that have been implicated as a major component of innate host defense against IAV infection (Figure 1). The mechanisms by which collectins mediate anti-IAV activity, their role in innate host defense, and their potential as therapeutic agents to ameliorate IAV-induced disease will be discussed further in this paper.
Figure 1.
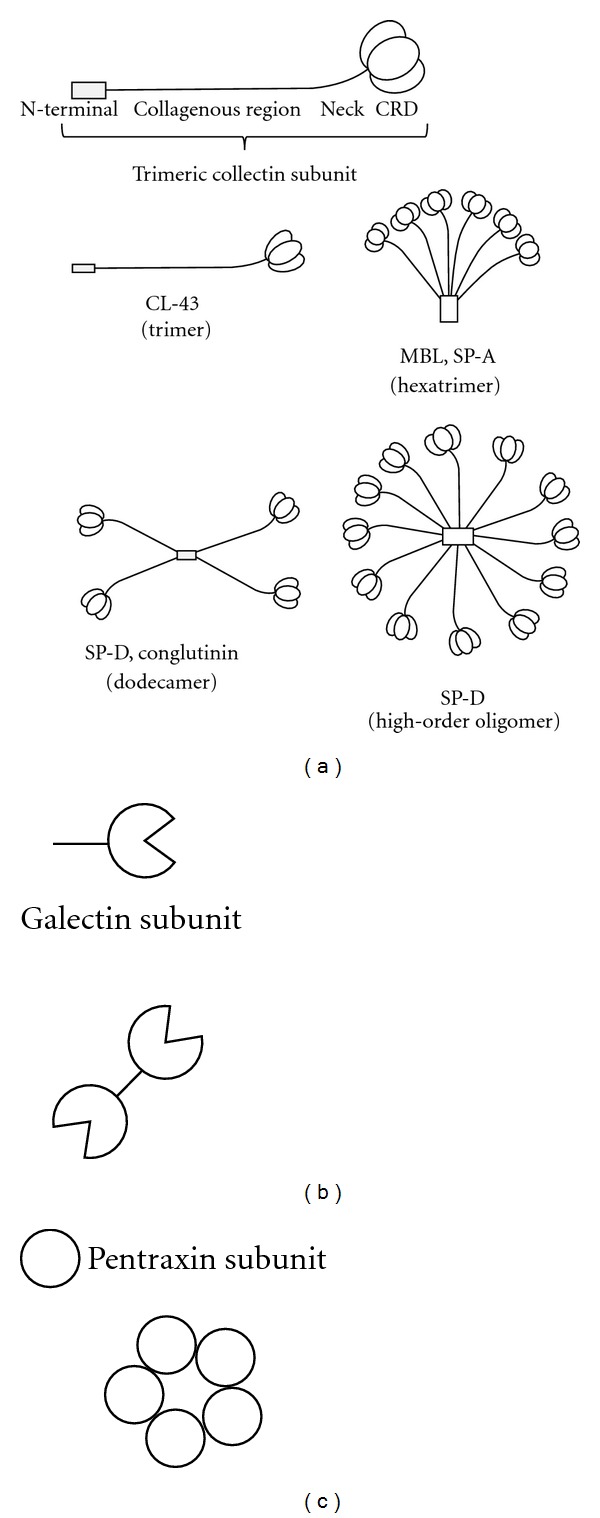
Subunit organization and assembly of mammalian lectins with antiviral activity against IAV. (a) Soluble C-type lectins of the collectin superfamily. Collectin subunits are comprised of 3 identical or similar polypeptide chains associated together to form triple helices. Each polypeptide chain bears a C-terminal CRD attached to a short “neck” region, a longer collagenous domain, and a noncollagenous N-terminal domain. CL-43 exists as a trimer whereas other collectins associate together to form multimers with characteristic “cruciform-like” (e.g., SP-D or conglutinin) or “bouquet-like” structures (e.g., MBL, SP-A) or higher-order oligomers (e.g., SP-D). (b) S-type lectins or galectins are a family of β-galactosidase-binding lectins containing homologous CRDs. Galectin-1 consists of a single CRD and a short N-terminal sequence and can form noncovalent dimers. (c) Pentraxin subunits are characterized by an 8 amino acid pentraxin signature (H-x-C-x-S/T-W-x-S/T, where x is any amino acid). Subunits assemble into multimers, usually noncovalently associated pentameric or decameric structures.
2.2. S-Type Lectins
S-type lectins, also known as galectins, do not require divalent cations for ligand binding, display specificity for β-galactosyl-containing glycoconjugates, and mediate a diverse range of biological functions (Figure 1; reviewed in [26]). They are widely distributed among animal tissues and may be located both inside (in the cytoplasm and/or nucleus) and outside cells (on the cell surface and/or within extracellular spaces). To date, 15 galectins have been identified, all of which contain a conserved CRD (reviewed in [27]). Galectin-1, -3, -8, and -9 are found in human airways, the primary site of IAV infection. Recently, galectin-1 was implicated as an effector in innate host defense during IAV infection [28].
2.3. Pentraxins
Like collectins, the pentraxins are a family of lectin-like proteins highly conserved during evolution and characterized by a multimeric (usually pentameric) structure. The classic short pentraxins, C-reactive protein (CRP) and serum amyloid P-component (SAP), are produced by the liver in response to inflammatory signals and are the main acute phase reactants in human and mouse, respectively [29]. Moreover, they share a structural organization that comprises 5 or 10 identical units arranged in a pentameric radial symmetry (Figure 1). PTX3, the prototype of the long pentraxins, shares similarities with short pentraxins but contains an unrelated N-terminal domain, is produced by different cell types, and binds to a different spectrum of ligands [30]. Pentraxins display lectin-like properties, including binding to carbohydrates and related structures in a Ca2+-dependent manner. SAP was originally isolated based on its ability to bind to the 4,6-cyclin pyruvate acetal group of β-D-galactose [31] and has also been implicated in lectin-mediated interactions with IAV [32, 33].
3. Collectins in Innate Host Defense against Influenza Virus Infection
Collectins are present in serum and mucosal secretions and recognize particular patterns of carbohydrates expressed on the surface of bacteria, fungi, protozoa, and viruses. To date, nine distinct collectins have been identified: surfactant protein (SP)-A, SP-D, mannose-binding lectin (MBL), collectin placenta 1 (CL-P1), collectin liver 1 (CL-L1), and collectin kidney 1 (CL-K1 or collectin-11) as well as the bovine-specific collectins CL-43, CL-46, and conglutinin. The basic structural unit of collectins is a trimer of 3 associated polypeptides. Each polypeptide consists of a short N-terminal domain, a triple helical collagen domain of variable length, a neck region, and a CRD (Figure 1). The neck region induces polypeptide trimerization, which is further stabilized by the collagen domain. Trimers can associate to form higher-order multimeric complexes through disulfide-stabilized interactions at the N-terminus, thereby increasing binding avidity for microbes and other ligands [34]. MBL and SP-A are commonly composed of six associated trimers (octadecamers or 18-mers), which resemble a bouquet of flowers. In contrast, SP-D, conglutinin, and CL-46 are assembled into dodecamers (12-mers) of four or more trimeric subunits and form a cruciform-like structure. With the exception of CL-P1, all collectins described to date show ligand-binding preference for Man-type sugars over Gal-type sugars [23] (see Table 1). Several reviews provide detailed description of the structural and functional features of the collectins [23, 34–36]. In the current paper, we will focus on the relevance of collectins to immunity during IAV infection.
Table 1.
Comparison of monosaccharide specificity of animal lectins with anti-IAV activity.
| Lectina | Family | Monosaccharide preferencesb | Binding to IAV |
|---|---|---|---|
| hSP-D | C-type | L-Fuc > Mann > Gluc > Gal > GlcNAc [142] | Yes |
| hMBL | C-type | GlcNAc > L-Fuc, Mann > ManNAc > Gluc > GalNAc [74] | Yes |
| hCL-L1 | C-type | Mann = L-Fuc = Gal > GlcNAc > GalNAc [94] | — |
| hCL-K1 | C-type | L-Fuc > Mann > ManNAc > GlcNAc > Gal [96] | Yes |
| bConglutinin | C-type | GlcNAc > Mann, L-Fuc > Gluc > ManNAc > GalNAc [74] | Yes |
| bCL-43 | C-type | Mann > ManNAc > L-Fuc > GlcNAc > Gluc > GalNAc [112] | Yes |
| bCL-46 | C-type | GlcNAc > ManNAc > Mann, Gluc, L-Fuc > Gal [143] | Yes |
| hGalectin-1 | S-type | Gal > GalNAc > GlcNAc, Mann [144] | Yes |
| hSAP | Pentraxin | Mann > GlcNAc > L-Fuc, Gal, GalNAc [32] | Yes |
aData reported for human (h) or bovine (b) lectins as indicated.
bAbbreviation of monosaccharides: L-Fuc: L-fucose; Mann: D-mannose; Gal: D-galactose; ManNAc: N-acetyl-D-mannosamine; GlcNAc: N-acetyl-D-glucosamine; GalNAc: N-acetyl-D-galactosamine.
—: not reported to date.
Nonspecific inhibitors of IAV, first described in the 1940s, have been classified as α, β, or γ inhibitors based on their chemical composition and properties (reviewed in [37]). The first evidence of the anti-IAV activities of collectins was provided by Anders et al. who demonstrated that β inhibitors in bovine and mouse serum were Ca2+-dependent mannose-binding lectins that bound to glycans on the globular head of IAV HA, thereby blocking the ability of the HA to bind to sialylated cell-surface receptors [38]. Subsequent studies identified conglutinin and MBL as the major β inhibitors in bovine and mouse serum, respectively [39]. In contrast to the lectin-mediated actions of β inhibitors, γ inhibitors are sialylated glycoproteins that act independently of Ca2+ by competing with sialylated cell-surface receptors for binding to HA [40, 41]. Today, collectins are known to mediate a range of anti-IAV activities including (but not limited to) inhibition of IAV HA and NA, neutralization of virus infectivity, virus aggregation, increased IAV uptake by neutrophils, and opsonization of virus to enhance neutrophil respiratory burst responses to IAV (see Table 2). As discussed in this review, there is growing evidence defining the role of particular collectins in anti-IAV immunity and researchers are now exploring their potential as therapeutic agents for the treatment of IAV-associated disease.
Table 2.
Summary of antiviral activities mediated by animal lectins against IAV.
| Lectina | HI | NA inhibition | Neutralization | Aggregation | Neutrophil | Selected references | |
|---|---|---|---|---|---|---|---|
| (i) ↑ uptake | (ii) ↑ H2O2 | ||||||
| hSP-D | Yes | Yes | Yes | Yes | Yes | Yes | [42, 60, 61, 87] |
| hMBL | Yes | Yes | Yes | Yes | Yes | Yes | [60, 85–88] |
| hCL-K1 | — | — | Yes | — | — | — | [96] |
| bConglutinin | Yes | Yesb | Yes | Yes | Yes | Yes | [39, 60, 105–107] |
| bCL-43 | Yes | Yes | Yes | No | Yes | No | [60, 103, 115, 145] |
| bCL-46c | Yes | — | Yes | Yes | Yes | — | [115, 116] |
| hGalectin-1 | Yes | — | Yes | — | — | — | [28] |
| hSAP | Yes | — | Yes | — | — | — | [32, 33] |
aData reported for human (h) or bovine (b) lectins as indicated.
bReported using recombinant chimeric protein containing the N-terminus and collagen domain of rat SP-D expressed with the neck and CRD of conglutinin.
cReported using CL-46 NCRDs.
—: not reported to date.
3.1. SP-D
SP-D and SP-A are constitutively present in lung fluids of the respiratory tract, the major site of IAV infection. SP-D acts as a classical β inhibitor and its CRDs bind to mannose-rich glycans expressed on IAV HA and NA [42–44]. In contrast, SP-A inhibits IAV in a Ca2+-independent manner by binding of viral HA to a sialylated glycan expressed within the SP-A CRD, thereby blocking the ability of HA to interact with cellular receptors [45]. Findings that (i) removal of the SP-A glycan completely abrogated anti-IAV activity and (ii) SP-A was effective against the poorly glycosylated A/PR/8/34 (PR8, H1N1) strain of IAV [46] are consistent with SP-A acting as a γ inhibitor rather than a β-type lectin inhibitor. Thus, SP-A and SP-D bind to similar regions on the HA molecule but do so via distinct mechanisms. For the purposes of this paper we will focus on lectin-mediated recognition of IAV by SP-D.
Human influenza is primarily an infection of the upper respiratory tract but can spread to the lower airways resulting in viral pneumonia and/or predispose to secondary bacterial infection. SP-D, present in nasal washings and bronchoalveolar fluids (BALF) from naïve mice, is upregulated following IAV infection and appears to be the major IAV inhibitor in the airways of mice [44, 47–50]. Moreover, SP-D has been shown to contribute to anti-IAV activity in BAL from healthy human volunteers [42, 51] and to enhance aggregation and neutrophil uptake of pathogens commonly associated with secondary bacterial pneumonia such as Streptococcus pneumoniae and Staphlococcus aureus [52].
SP-D preparations from different species show varying proportions of dodecamers (four homotrimeric subunits), multimers of dodecamers, and/or single trimeric subunits [53, 54]. The degree of oligomerization is an important factor modulating anti-IAV activity and dodecamers and multimers exhibit greater antiviral activities than trimers [55–57]. In general terms, SP-D binds to Man-type oligosaccharides (see Table 1) although the CRD from human, mouse, and rat SP-D showed distinct preferences for particular saccharide ligands [54]. SP-D binds to glycans on the IAV envelope glycoproteins to mediate hemagglutination inhibition (HI), inhibition of NA activity, virus aggregation, and neutralization of virus infectivity [42, 44, 58–60] (Table 2). Binding of SP-D also promotes uptake of IAV by neutrophils and protects neutrophils against IAV-induced neutrophil dysfunction [42, 61]. However, neutrophil proteases can cleave SP-D and chronic neutrophilic inflammation in the airways may result in degradation of SP-D and account for the increased risk of IAV infection associated with heavy smoking or cystic fibrosis [62, 63]. There are several polymorphisms in the coding sequence of human SP-D, including one at the codon corresponding to amino acid residue 11 (Met11Thr). Moreover, the Thr/Thr11 variant assembles as trimers rather than higher-order multimeric species [64] and these display reduced anti-IAV activity in vitro [56, 64].
SP-D binds directly to oligosaccharides expressed on either IAV HA or NA [44, 59], yet the susceptibility of different IAV strains to SP-D correlates with the degree of glycosylation of HA. Seasonal “drift” strains of H3N2 IAV with increased glycosylation on the globular head of HA were more susceptible to neutralization by SP-D and showed reduced virulence in mice [44]. Furthermore, Hartshorn et al. used hemagglutination inhibition (HI) assays to implicate specific sites on the HA of H3N2 and H1N1 subtype IAV as targets for recognition by recombinant human (Rh) SP-D [58]. Recently, site-directed mutagenesis and reverse genetics were utilized to define the contribution of specific glycans on H3N2 [50, 65] and H1N1 [66] IAV in determining sensitivity to SP-D and to virulence in mice. Collectively, these studies indicated that both the number and the position of potential glycosylation sites on the globular head of the HA modulate the avidity of SP-D binding and therefore the potency of antiviral activity against different IAV.
SP-D-deficient mice (SP-D−/−) display aberrant pulmonary surfactant homeostasis, including progressive pulmonary inflammation, emphysema, macrophage activation, and surfactant phospholipid accumulation [67–69] and correction of alveolar homeostasis required the collagenous domain of SP-D [70, 71]. Following infection with highly glycosylated IAV, SP-D−/− mice show increased virus replication, airway inflammation, and disease [47, 48, 50] (summarized in Table 3) and these effects could be corrected by overexpression of wild type or mutant SP-D proteins in the airways [70]. Mannan, a complex polymer of mannose residues, efficiently blocks the anti-IAV activities of SP-D in vitro and intranasal treatment of wild-type mice with mannan prior to and during infection with a highly glycosylated H3N2 IAV strain resulted in enhanced viral replication, pulmonary inflammation, and death [49]. Studies in a mouse model have also suggested that compromise of SP-D-mediated defenses by elevated glucose levels might be an important factor contributing to the increased disease severity of IAV-induced disease in diabetic mice [72].
Table 3.
Virulence of different IAVs in mice genetically deficient in particular animal lectins.
| Mice deficient in | IAV straina | Susceptibility to IAV infection | Virus growth in lungs | Comments | References |
|---|---|---|---|---|---|
| SP-D | Phil/82 | ↑ | ↑ | ↑ lung inflammation | [47, 48] |
| Phil/82Δ167 | ND | ND | Lacking Asn165 = less sensitive to SP-D | [47] | |
| Mem71 | ND | ND | Strain less sensitive to SP-D than Phil/82 | [48] | |
|
| |||||
| MBL-A/C | Phil/82 | ↑ | ↑ | ↑ inflammation and lung injury | [84] |
| Cal/09 | ↓ | ND | ↓ inflammation and lung injury | [93] | |
| H9N2/G1 | ↓ | ↓ | ↓ inflammation and lung injury | [93] | |
|
| |||||
| Galectin-1 | WSN/33 | ↑ | — | HI and neutralization against IAV in vitro | [28] |
|
| |||||
| SAP | PR8, WS/33, Shg/24 | ND | ND | HI and neutralization against IAV in vitro | [133] |
aIAV strains used: A/Philippines/82 (Phil/82, H3N2); A/Mem71H-BelN (Mem/71, H3N1); A/WSN/33 (WSN/33, H1N1); A/PR/8/34 (PR8, H1N1), A/WS/33 (WS/33, H1N1), A/California/04/09 (Cal/09, H1N1 pdm), A/Shanghai/24/90 (Shg/24, H3N2), A/Quail/Hong Kong/61/97 (H9N2/G1).
↑, ↓, or ND: increased, decreased, or not different to wild-type mice, respectively.
—: not reported to date.
3.2. MBL
MBL binds to oligosaccharides with characteristic chemical and spatial features that are expressed on the surface of a wide range of pathogens, including bacteria, fungi, and viruses (reviewed in [73]). In general terms, MBL recognizes Man-type sugars and Haurum et al. reported that MBL binding to mannan was inhibited by monosaccharides such as GlcNAc, mannose, L-fucose, and N-acetyl-mannosamine but not by galactose or GalNAc [74] (Table 1). Following binding to pathogens, MBL can activate the lectin complement pathway via MBL-associated serine proteases (MASPs), resulting in complement-mediated lysis and/or opsonophagocytosis [75, 76]. Binding of MBL-MASP complexes to appropriate ligands also activates coagulation via thrombin-like activity [77, 78]. MBL is unique among the collectins in its ability to activate complement and coagulation.
Although humans express a single MBL gene, two homologous forms of MBL, termed MBL-A and MBL-C, have been identified in rodents [79, 80]. These two forms of rodent MBL are 50% homologous, display distinct but overlapping ligand-binding specificity, and bind to MASPs to activate complement [81]. Multiple structural and promoter polymorphisms in the human MBL2 gene can result in low levels and/or dysfunctional MBL. Moreover, MBL deficiency has been associated with predisposition to numerous infectious diseases, including respiratory infections (reviewed in [82]).
SP-A and SP-D are constitutively expressed in the lungs whereas MBL is predominantly a serum protein. MBL is rarely detected in BALF from healthy humans or mice, although significant levels have been detected during airway inflammation [83], including that associated with IAV infection of mice [44]. Recently, affinity purification and western blot confirmed the presence of MBL in BALF from healthy mice, albeit at levels that were below detection by ELISA [84].
MBL has been reported to mediate a range of anti-IAV activities in vitro, including inhibition of virus-induced hemagglutination [85, 86], blocking of NA enzymatic activity [60, 87], opsonization for enhanced neutrophil reactivity to IAV [85, 86], and neutralization of virus infectivity [84, 86–88] (Table 2). These activities are complement-independent and shared by other mammalian collectins such as SP-D and conglutinin. In addition, MBL-mediated activation of complement can induce irreversible inactivation of IAV [89] and promote lysis of IAV-infected cells [90]. Chang et al. recently reported that recombinant human MBL (RhMBL) and murine MBL-A/-C could neutralize IAV infectivity but that preferential activation of the lectin complement pathway and thrombin-like activity by MBL-A and MBL-C, respectively, was associated with enhanced MBL-mediated neutralization [84].
Lectin blot analysis indicates that MBL can bind to both HA and NA glycoproteins [44, 88, 91], yet as for SP-D, the degree of glycosylation on the head of IAV HA is the critical factor determining sensitivity to MBL. Seasonal H1N1 IAVs expressing 3-4 potential glycosylation sites on the head of HA were more sensitive to neutralization by RhMBL compared to poorly glycosylated strains such as PR8 (H1N1) or A/California/04/09 (Cal/09, A(H1N1) 2009 pdm) [87]. Similarly, H3N2 IAVs carrying 4-5 potential glycosylation sites on the head of HA were highly sensitive to MBL in mouse serum [44]. Current evidence suggests that inhibition of NA activity also results from steric hindrance of the active site of NA by SP-D/MBL bound to mannose-rich glycans on the viral HA [44, 60, 87].
The role of MBL in vivo during IAV infections remains controversial. Chang et al. reported MBL-deficient mice to be more susceptible to A/Philippines/82 (Phil/82, H3N2), a highly glycosylated IAV strain that is readily neutralized by MBL [84] (Table 3). MBL showed some binding to A(H1N1) 2009 pdm, yet these viruses were resistant to MBL-mediated neutralization [87, 92] and MBL-null mice infected with A(H1N1) 2009 pdm (or H9N2/G1) IAV showed reduced disease severity and airway inflammation [93] (Table 3), arguing that MBL may be a risk factor during certain IAV infections. Thus, high avidity interaction between MBL and IAV may result in protective antiviral responses whereas low avidity binding may be insufficient to mediate anti-IAV function, resulting in detrimental MBL-induced pro-inflammatory responses and severe disease.
3.3. Other Collectins
In addition to “classical collectins” such as SP-D and MBL, much less is known regarding the general structure and function of “novel collectins” such as CL-L1, CL-P1, and CL-K1 (collectin 11) and there are limited data regarding their role in anti-IAV immunity. CL-L1, predominantly produced by the liver, is a cytosolic protein with weak lectin function [94] suggesting a different biological function to that of the classical collectins. CL-P1, a membrane-associated collectin, is expressed on vascular endothelial cells and recognizes oxidized low-density lipoprotein as well as bacteria and yeasts [95]. To date, no interactions between IAV and CL-L1 or CL-P1 have been reported. Collectin 11, a MASP-associated plasma lectin, was recently shown to inhibit IAV-induced hemagglutination and to neutralize virus infectivity [96]. Moreover, collectin 11 is expressed in airway bronchioles [97] and as a circulating plasma protein [96, 98] and may therefore represent an innate barrier to IAV infection and/or transude from the plasma to inflamed airways in a manner analogous to that reported for MBL [44, 83].
3.4. Bovine, Porcine, and Avian Collectins
3.4.1. Bovine Collectins
Conglutinin was the first described lectin of animal origin and was originally discovered by its ability to agglutinate (conglutinate) erythrocytes coated with antibody and complement [99]. It is distinct from other collectins as it binds iC3b, a component of the complement pathway [100, 101]. While most collectins have been identified in multiple species, conglutinin, CL-43, and CL-46 appear to be restricted to the species Bovidae and characterization of their respective genes indicates that they are descendants of an ancestral SP-D gene [102]. Conglutinin and CL-46 form dodecameric structures similar to those of SP-D whereas CL-43 occurs naturally as trimers [102–104]. The evolutionary pressures that have resulted in the presence of additional collectins in Bovidae are not known. Hartshorn et al. proposed that the combined anti-IAV activities of conglutinin, CL-43, and CL-46 might explain why cattle do not represent a natural reservoir for IAV infection, despite living in close proximity to other natural hosts of IAV, such as birds, pigs, horses, and humans [103].
Conglutinin acts as a β inhibitor of IAV, binding to oligosaccharides on the tip of the HA spike in a Ca2+-dependent manner [39, 105] to inhibit virus-induced hemagglutination, aggregation, and neutralization of virus infectivity [39, 105–109] (Table 2). Opsonization with conglutinin also enhanced neutrophil respiratory burst responses after exposure to IAV [107]. Lectin blot studies have demonstrated that MBL and SP-D bind to both HA and NA, yet conglutinin binds only to HA [88, 106, 110, 111], and this has been proposed as one factor contributing to its relatively poor ability to inhibit viral spreading to adjacent cells [108]. Of the different IAV strains and subtypes tested, most H1N1 and H3N2 subtypes were susceptible to conglutinin with the exception of the poorly glycosylated PR8 (H1N1) strain [39, 105, 107]. Mutants of H3N2 or H1N1 IAV lacking a single glycosylation site from the tip of the HA spike (Asn165-H3 numbering or Asn104-H1 numbering, respectively) were also markedly resistant to conglutinin [38, 39].
CL-43, like conglutinin, is a potent inhibitor of IAV; however this lectin exhibits distinct monosaccharide binding properties [112] (see Table 1) and forms trimers but not other high-order multimers [113]. The potency of various SP-D preparations in mediating HI and virus neutralization has been strongly associated with its degree of multimerization [85, 114], yet trimers of CL-43 mediated HI and neutralizing activity against IAV to levels equivalent to those of fully multimerized SP-D and were markedly more potent than trimeric SP-D preparations [103]. Collectin-induced aggregation of IAV has also been strongly associated with the ability of SP-D, MBL, or conglutinin preparations to enhance neutrophil respiratory burst responses and to increase neutrophil uptake of IAV [61, 85]. CL-43 did not induce viral aggregation and did not enhance IAV-induced generation of H2O2; however neutrophil uptake of IAV was increased [103] (Table 2), most likely via mechanisms distinct to those of other collectins. Recently, trimers composed of the neck and CRD (NCRD) of CL-46 were also shown to neutralize IAV infectivity, inhibit virus-induced hemagglutination, induce aggregation of IAV, and to increase neutrophil uptake of IAV [115, 116].
3.4.2. Porcine SP-D
Porcine (p)SP-D is unique amongst the mammalian SP-Ds described to date in that it contains a sialic acid-rich N-glycan in its CRD [117, 118]. Human and porcine SP-A also express sialylated N-glycans in their respective CRDs and mediate Ca2+-independent anti-IAV activity that was abrogated by the removal of this glycan [45, 118]. In contrast, pSP-D displayed dual mechanisms of anti-IAV activity by mediating (i) Ca2+-dependent binding of the pSP-D CRD to oligosaccharides of IAV HA, and (ii) binding of IAV HA to sialic acid expressed by pSP-D. Initial binding of pSP-D to mannose-rich glycans on HA appears to be critical and secondary interactions between IAV HA and sialylated glycans on pSP-D do not take place in the absence of lectin-mediated binding [117, 118]. The sialylated glycan expressed on pSP-D was shown to enhance virus aggregation and neutralization as well as neutrophil responses to IAV [118]. Accordingly, pSP-D isolated from porcine BAL and recombinant pSP-D displayed more potent anti-IAV activity compared to human or rat SP-D and were active against a broader spectrum of IAV strains [118, 119]. Together these data suggest that pulmonary innate defenses may be particularly efficient against IAV in pigs and represent an important barrier limiting disease severity. IAV strains show limited antigenic variation in swine compared to humans [120], and this may reflect the shorter lifespan of farmed animals. Moreover, restriction of IAV infection by porcine innate defenses might also limit the induction of antibody-mediated adaptive immune responses in pigs that are required to drive antigenic drift.
3.4.3. Chicken Collectins
Avian species, including domestic poultry, represent an important natural reservoir for IAV infection. Ducks, chickens, and turkeys may all be farmed in close proximity to humans and other IAV reservoirs including pigs and horses, thereby increasing the potential for interspecies transmission. It is well established that mammalian collectins mediate anti-IAV activity. In chickens, 5 collectins have been described to date, namely, MBL [121], SP-A (cSP-A), and chicken collectins 1, 2, and 3 (cCL-1, -2 and -3) which resemble the mammalian proteins CL-L1, collectin 11, and CL-P1, respectively [122]. In addition, chicken lung lectin (cLL) was identified as a C-type lectin but not as a collectin due to its lack of a collagenous domain [122]. Phylogenetic analyses suggest that the homologue of mammalian SP-D has been deleted in chickens and that a bird-specific duplication of the SP-A gene may have given rise to cLL [123]. cLL inhibited the hemagglutination activity of Phil/82 (H3N2; highly glycosylated HA) and PR8 (H1N1; poorly glycosylated HA) to similar levels but did not neutralize either virus strain [124]. At present the mechanisms underlying cLL-IAV interactions are not fully understood and further studies are required to determine the role of avian collectins in anti-IAV immunity.
4. Other Mammalian Lectins with Anti-Influenza Activity
Galectins are S-type animal lectins characterized by a conserved CRD that recognizes galactose-containing oligosaccharides. Recently, lectin-mediated binding of galectin-1 to IAV was shown to inhibit IAV-induced hemagglutination and to reduce virus production in MDCK cells [28]. Galectin-1 bound to a range of IAV subtypes (H1N1, H2N2, H3N2, H5N2, and H6N1) with micromolar Kd values suggesting broad antiviral activity and binding was inhibited by lactose, but not by mannose (Table 1). Importantly, galectin-1 was upregulated in the lungs of IAV-infected mice and mice lacking endogenous galectin-1 showed reduced survival compared to wild-type mice [28]. Together, these data are the first to demonstrate the importance of galectins in anti-IAV immunity.
The short pentraxin SAP has been reported to act as a β inhibitor against IAV, binding in a Ca2+-dependent manner to glycans on the viral HA to mediate HI and neutralize virus infectivity [32, 33] (Table 2). In vitro, human SAP inhibited a range of type A (H1N1, H2N2, and H3N2) and type B viruses and antiviral activity was blocked by the inclusion of mannose, consistent with recognition of Man-type glycans on the IAV. PTX3 also mediates anti-IAV activity; however this appears to be mediated by binding of the IAV HA to SA residues expressed on PTX3 [125] in a manner analogous to that described for SP-A. As lectin-mediated recognition of IAV by PTX3 does not appear to contribute to its anti-IAV activity, it will not be discussed further in this paper.
5. Pandemic IAV and Lectin-Mediated Defenses
When pandemic IAVs first emerge, they generally carry little glycosylation on the head of HA but acquire additional glycosylation sites as they continue to circulate in the human population. In 1968, H3N2 IAV associated with “Hong Kong flu” carried 2 potential glycosylation sites on the HA head whereas recent H3N2 viruses carry 6 to 7 sites [50, 58]. Pandemic H1N1 strains associated with the 1918 “Spanish flu” had only 1 potential glycosylation site (Asn104) on the globular head of HA but by the 1950s had acquired a total of 4 to 5 sites [17, 18, 126]. Asn104 was also the only site expressed on the head of HA of A(H1N1) 2009 pdm viruses associated with the 2009 “Swine flu” pandemic [17, 87, 126] and few variants with altered HA glycosylation have been detected to date [18]. In the future it is likely that A(H1N1) pdm viruses will develop resistance to the current vaccine, perhaps through the evolution of mutants with altered HA glycosylation patterns that favour resistance to preexisting neutralizing antibodies.
The degree of glycosylation on the globular head of HA is a critical factor in determining sensitivity of different IAV strains to the antiviral activities of collectins. Consequently, pandemic IAVs tend to be resistant to collectins when compared to seasonal IAV strains. For example, human SP-D and MBL inhibited IAV-induced hemagglutinating activity, blocked the enzymatic activity of NA, and neutralized the infectivity of seasonal H1N1, but not pandemic A(H1N1) 2009 pdm IAV [87]. Chimeric viruses expressing the HA of 1918 (H1), 1957 (H2), 1968 (H3), and 2009 (H1) pandemic IAV in the context of a seasonal H1N1 IAV were poorly recognized by RhSP-D in vitro and were associated with enhanced lower respiratory tract pathology in IAV-infected mice [127], consistent with the notion that sensitivity to SP-D might limit the ability of IAV to invade the lower airways and induce severe disease. In the mouse model, weak interactions between MBL and A(H1N1) 2009 pdm viruses have also been implicated in the induction of MBL-induced proinflammatory responses, suggesting that MBL might be a risk factor associated with severe disease during pandemic IAV infections [93]. However, no association was observed between phenotypic MBL deficiency and predisposition to A(H1N1) 2009 pdm in exposed health care workers [128].
Highly pathogenic avian influenza (HPAI) H5N1 IAV infections have been associated with very severe disease and fatality in humans and are still considered to be a potentially serious pandemic threat [129]. Microarray analysis and quantitative RT-PCR showed SP-D expression to be reduced in lungs from two fatal cases of H5N1 IAV infection when compared to healthy patients or patients with acute respiratory distress syndrome [130], although other studies noted no major changes in SP-D expression in the airways of H5N1-infected ferrets [131] or macaques [132]. In addition, 2 human H5N1 strains that were less glycosylated than recent seasonal H1N1 and H3N2 strains were also resistant to SP-D in vitro [58], consistent with the notion that SP-D-mediated defenses may be ineffective against H5N1 strains. Future studies will determine the susceptibility of a range of different avian IAV to SP-D as well as the potential of animal lectins as prophylactic and/or therapeutic treatments during severe H5N1-induced disease. As noted previously, lectins are expressed in the respiratory and digestive systems of chickens. As these are the major sites of IAV replication and dissemination, understanding their role in avian host defense is particularly important as they may act to control disease and limit viral transmission.
6. Therapeutic Potential of Recombinant Animal Lectins during IAV Infection
Understanding mechanisms by which mammalian lectins mediate antiviral activity against IAV is an important first step towards development of novel lectin-based therapeutic and prophylactic agents. Treatment of lectin-deficient mice with purified recombinant lectins can reverse the enhanced susceptibility associated with IAV infection. For example, intraperitoneal injection of RhMBL or intratracheal administration of RhSP-D reduced titres of Phil/82 in the lungs of MBL null mice [84] or SP-D null mice [48], respectively, to levels equivalent to those of wild-type animals. Yang et al. demonstrated that intranasal treatment of IAV-infected C57BL/6 mice with recombinant galectin-1 enhanced survival, reduced viral load, and attenuated lung inflammation and injury [28]. In addition, intranasal treatment of BALB/c mice with human SAP 1 hr prior to infection with A/Japan/57 (Jap/57, H2N2) reduced disease severity [33] although Herbert et al. reported that intraperitoneal injection of C57BL/6 mice with human SAP had no effect on disease outcome [133]. The particular route of lectin administration will be one factor modulating the effectiveness of exogenous animal lectins in ameliorating IAV-associated disease. Moreover, IAV strains show major differences in susceptibility to animal lectins [44, 50, 58], highlighting the importance of assessing therapeutic and/or prophylactic potential against a range of seasonal and pandemic IAV strains.
6.1. Recombinant Chimeric Lectins (RCLs)
RCLs represent a tailored approach exploiting particular anti-IAV properties of different pattern recognition molecules. For example, chimeric lectins comprising the collagenous domain of SP-D in conjunction with the neck and CRD of MBL displayed more potent anti-IAV activities compared to either SP-D or MBL alone [114, 134]. Similarly, expression of the conglutinin neck and CRD domains in conjunction with the collagenous domain of SP-D resulted in increased anti-IAV activity [43]. An RCL bearing the CRD of MBL and the collagenous domains of L-ficolin (an additional innate pattern recognition receptor) showed enhanced activation of the lectin complement pathway and anti-IAV activity as well as reduced thrombin-like and FXa-like coagulation enzyme activities compared to RhMBL alone [135], representing further refinement towards potent therapeutics against IAV which might display reduced side effects in a clinical setting.
6.2. Recombinant Truncated Lectins
Recombinant truncated NCRD trimers of SP-D represent an appealing therapeutic option as they can be produced in large quantities and lack the collagen domain associated with potential pro-inflammatory effects of SP-D [136]. However, NCRDs of human or rodent SP-D bound effectively to various SP-D ligands but displayed only weak inhibitory activity against IAV in vitro [54–56, 137]. Subsequent studies demonstrated that cross-linking of NCRDs and/or mutagenesis of specific CRD residues represent complimentary strategies that enhanced anti-IAV activity of SP-D NCRDs [138, 139]. The lectin domains of serum collectin NCRDs (e.g., MBL, conglutinin, CL-43, or CL-46) show intrinsically greater anti-IAV activity compared to the NCRDs of SP-D [43, 103, 134]. For example, CL-43 NCRDs were more potent than SP-D NCRDs and CL-43 showed a distinctive three amino acid insertion (RAK) in the flanking region of the carbohydrate binding site that was absent in SP-D. Insertion of 3 amino acids (RAK or AAA) into the SP-D NCRD enhanced anti-IAV activity relative to the wild-type SP-D NCRD [115, 137].
Alignment of serum collectins with SP-D has also revealed nonconservative substitutions at amino acid positions 325 and 343 which are highly exposed on opposing ridges of the carbohydrate-binding groove [140]. Recent studies have substituted these residues (alone or in combination) into the SP-D NCRD in an attempt to enhance ligand binding and anti-IAV activity [54, 116, 138, 140]. Crouch et al. recently demonstrated that a mutant of the human SP-D NCRD that contained 2 amino acid substitutions (D325A + R343V) displayed enhanced anti-IAV activity in vitro compared to mutants which expressed either substitution alone [140]. Moreover, intratracheal coadministration of D325A + R343V NCRD reduced morbidity and increased viral clearance in mice infected with a reassortant of A/WSN/33 bearing a glycosylated HA, demonstrating the potential of exogenous recombinant NCRD preparations to enhance antiviral activity and reduce disease severity in vivo.
6.3. Recombinant Lectins with Modified N-Glycans
pSP-D is unique in that it is the only SP-D species known to carry a sialylated sugar in its CRD domain [117]. Simultaneous binding of the CRD to glycans on HA/NA of IAV as well as HA-mediated recognition of terminal SA on the N-glycan expressed by the pSP-D CRD enhances antiviral activity and broadens the range of virus strains inhibited by pSP-D [118]. Recently, van Eijk et al. produced functionally active recombinant pSP-D (RpSP-D) and attempted to enhance the anti-IAV properties of recombinant hSP-D (RhSP-D) by introducing N-linked glycans into its CRD [141]. RpSP-D displayed more potent anti-IAV activity than RhSP-D but introduction of an SA-rich glycan at the same location as in the CRD of pSP-D did not enhance the anti-IAV activity of RhSP-D. Moreover, introduction of glycans at other sites within the CRD of RhSP-D interfered with protein secretion and/or lectin function. Therefore, further studies are required to determine the most effective manner in which glycan modification within the CRD can be utilized to augment lectin-mediated recognition and anti-IAV activities of RhSP-D.
7. Conclusions
It is clear that endogenous lectins are important contributors to pulmonary defense against IAV via direct recognition of free virus (and virus-infected cells) as well as modulating airway inflammation and disease. While classical collectins such as MBL and SP-D have been studied in detail, much less is known regarding antiviral activities of other collectins such as CL-L1, CL-K1 (collectin 11) and avian lectins, or the role that they might play in vivo. Recent findings that chickens lack SP-D but contain an additional lectin (cLL) [123] and that pig SP-D contains unique structural features, including an extra glycosylation site in its CRD [118], highlight the importance of understanding species differences and particular biochemical features that can modulate the anti-IAV activity of endogenous lectins. This in turn will inform the development of highly effective novel lectin constructs that may be used effectively to prevent and/or treat influenza infections in humans. Finally, understanding the role of endogenous lectins in natural reservoirs (including humans, pigs and avian species) and animal models (e.g., ferrets and mice) of IAV infection will aid in our understanding of factors that may limit interspecies transmission of IAV and/or modulate disease severity.
Authors' Contribution
W. C. Ng, M. D. Tate, A. G. Brooks, and P. C. Reading all contributed to the planning, writing and preparation of this paper.
Acknowledgments
This work was supported by Project Grant 1032079 from the National Health & Medical Research Council (NHMRC) of Australia. W. C. Ng is a recipient of an NHMRC Biomedical (Dora Lush) Postgraduate Research Scholarship. The Melbourne WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health and Ageing.
References
- 1.Barker WH, Mullooly JP. Pneumonia and influenza deaths during epidemics. Implications for prevention. Archives of Internal Medicine. 1982;142(1):85–89. [PubMed] [Google Scholar]
- 2.Wright PF, Neumann G, Kawaoka Y. Fields Virology. 5th edition. Vol. 2. Philadelphia, Pa, USA: Lippincott Williams & Wilkins; 2007. Orthomyxoviruses. [Google Scholar]
- 3.Fouchier RAM, Munster V, Wallensten A, et al. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. Journal of Virology. 2005;79(5):2814–2822. doi: 10.1128/JVI.79.5.2814-2822.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy BR, Webster RG. Fields Virology. Philadelphia, Pa, USA: Lippincott-Raven; 1996. Orthomyxoviruses. [Google Scholar]
- 5.Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annual Review of Biochemistry. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 6.Gong J, Xu W, Zhang J. Structure and functions of influenza virus neuraminidase. Current Medicinal Chemistry. 2007;14(1):113–122. doi: 10.2174/092986707779313444. [DOI] [PubMed] [Google Scholar]
- 7.Mitnaul LJ, Matrosovich MN, Castrucci MR, et al. Balanced hemagglutinin and neuraminidase activities are critical for efficient replication of influenza A virus. Journal of Virology. 2000;74(13):6015–6020. doi: 10.1128/jvi.74.13.6015-6020.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner R, Matrosovich M, Klenk HD. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Reviews in Medical Virology. 2002;12(3):159–166. doi: 10.1002/rmv.352. [DOI] [PubMed] [Google Scholar]
- 9.Basak S, Pritchard DG, Brown AS, Compans RW. Glycosylation sites of influenza viral glycoproteins: characterization of tryptic glycopeptides from the A/USSR(H1N1) hemagglutinin glycoprotein. Journal of Virology. 1981;37(2):549–558. doi: 10.1128/jvi.37.2.549-558.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward CW, Dopheide TA. Amino acid sequence and oligosaccharide distribution of the haemagglutinin from an early Hong Kong influenza virus variant A/Aichi/2/68 (X-31) Biochemical Journal. 1981;193(3):953–962. doi: 10.1042/bj1930953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imperiali B, Hendrickson TL. Asparagine-linked glycosylation: specificity and function of oligosaccharyl transferase. Bioorganic and Medicinal Chemistry. 1995;3(12):1565–1578. doi: 10.1016/0968-0896(95)00142-5. [DOI] [PubMed] [Google Scholar]
- 12.Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annual Review of Biochemistry. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 13.Basak S, Tomana M, Compans RW. Sialic acid is incorporated into influenza hemagglutinin glycoproteins in the absence of viral neuraminidase. Virus Research. 1985;2(1):61–68. doi: 10.1016/0168-1702(85)90060-7. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura K, Compans RW. Host cell- and virus strain-dependent differences in oligosaccharides of hemagglutinin glycoproteins of influenza A viruses. Virology. 1979;95(1):8–23. doi: 10.1016/0042-6822(79)90397-0. [DOI] [PubMed] [Google Scholar]
- 15.Abe Y, Takashita E, Sugawara K, Matsuzaki Y, Muraki Y, Hongo S. Effect of the addition of oligosaccharides on the biological activities and antigenicity of influenza A/H3N2 virus hemagglutinin. Journal of Virology. 2004;78(18):9605–9611. doi: 10.1128/JVI.78.18.9605-9611.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skehel JJ, Stevens DJ, Daniels RS. A carbohydrate side chain on hemagglutinins of Hong Kong influenza viruses inhibits recognition by a monoclonal antibody. Proceedings of the National Academy of Sciences of the United States of America. 1984;81(6):1779–1783. doi: 10.1073/pnas.81.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei CJ, Boyington JC, Dai K, et al. Cross-neutralization of 1918 and 2009 influenza viruses: role of glycans in viral evolution and vaccine design. Science Translational Medicine. 2010;2(24):p. 24ra21. doi: 10.1126/scitranslmed.3000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun S, Wang Q, Zhao F, Chen W, Li Z. Glycosylation site alteration in the evolution of influenza a (H1N1) viruses. PLoS One. 2011;6(7) doi: 10.1371/journal.pone.0022844. Article ID e22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang M, Gaschen B, Blay W, et al. Tracking global patterns of N-linked glycosylation site variation in highly variable viral glycoproteins: HIV, SIV, and HCV envelopes and influenza hemagglutinin. Glycobiology. 2004;14(12):1229–1246. doi: 10.1093/glycob/cwh106. [DOI] [PubMed] [Google Scholar]
- 20.Drickamer K. Two distinct classes of carbohydrate-recognition domains in animal lectins. Journal of Biological Chemistry. 1988;263(20):9557–9560. [PubMed] [Google Scholar]
- 21.Drickamer K, Taylor ME. Biology of animal lectins. Annual Review of Cell Biology. 1993;9:237–264. doi: 10.1146/annurev.cb.09.110193.001321. [DOI] [PubMed] [Google Scholar]
- 22.Drickamer K. C-type lectin-like domains. Current Opinion in Structural Biology. 1999;9(5):585–590. doi: 10.1016/s0959-440x(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 23.Veldhuizen EJA, Van Eijk M, Haagsman HP. The carbohydrate recognition domain of collectins. FEBS Journal. 2011;278(20):3930–3941. doi: 10.1111/j.1742-4658.2011.08206.x. [DOI] [PubMed] [Google Scholar]
- 24.Londrigan SL, Turville SG, Tate MD, Deng Y-M, Brooks AG, Reading PC. N-linked glycosylation facilitates sialic acid-independent attachment and entry of influenza A viruses into cells expressing DC-SIGN or L-SIGN. Journal of Virology. 2011;85(6):2990–3000. doi: 10.1128/JVI.01705-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Upham JP, Pickett D, Irimura T, Anders EM, Reading PC. Macrophage receptors for influenza a virus: role of the macrophage galactose-type lectin and mannose receptor in viral entry. Journal of Virology. 2010;84(8):3730–3737. doi: 10.1128/JVI.02148-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabinovich GA, Liu FT, Hirashima M, Anderson A. An emerging role for galectins in tuning the immune response: lessons from experimental models of inflammatory disease, autoimmunity and cancer. Scandinavian Journal of Immunology. 2007;66(2-3):143–158. doi: 10.1111/j.1365-3083.2007.01986.x. [DOI] [PubMed] [Google Scholar]
- 27.Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert Reviews in Molecular Medicine. 2008;10(17, article e17) doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
- 28.Yang M-L, Chen Y-H, Wang S-W, et al. Galectin-1 binds to influenza virus and ameliorates influenza virus pathogenesis. Journal of Virology. 2011;85(19):10010–10020. doi: 10.1128/JVI.00301-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantovani A, Garlanda C, Doni A, Bottazzi B. Pentraxins in innate immunity: from C-reactive protein to the long pentraxin PTX3. Journal of Clinical Immunology. 2008;28(1):1–13. doi: 10.1007/s10875-007-9126-7. [DOI] [PubMed] [Google Scholar]
- 30.Inforzato A, Bottazzi B, Garlanda C, Valentino S, Mantovani A. Pentraxins in humoral innate immunity. Advances in Experimental Medicine and Biology. 2012;946:1–20. doi: 10.1007/978-1-4614-0106-3_1. [DOI] [PubMed] [Google Scholar]
- 31.Hind CRK, Collins PM, Renn D. Binding specificity of serum amyloid P component for the pyruvate acetal of galactose. Journal of Experimental Medicine. 1984;159(4):1058–1069. doi: 10.1084/jem.159.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersen O, Ravn KV, Sørensen IJ, Jonson G, Holm Nielsen E, Svehag S-E. Serum amyloid P component binds to influenza A virus haemagglutinin and inhibits the virus infection in vitro. Scandinavian Journal of Immunology. 1997;46(4):331–337. doi: 10.1046/j.1365-3083.1997.d01-147.x. [DOI] [PubMed] [Google Scholar]
- 33.Horváth A, Andersen I, Junker K, et al. Serum amyloid P component inhibits influenza A virus infections: in vitro and in vivo studies. Antiviral Research. 2001;52(1):43–53. doi: 10.1016/s0166-3542(01)00158-9. [DOI] [PubMed] [Google Scholar]
- 34.Seaton BA, Crouch EC, McCormack FX, Head JF, Hartshorn KL, Mendelsohn R. Structural determinants of pattern recognition by lung collectins. Innate Immunity. 2010;16(3):143–150. doi: 10.1177/1753425910368716. [DOI] [PubMed] [Google Scholar]
- 35.Gupta G, Surolia A. Collectins: sentinels of innate immunity. BioEssays. 2007;29(5):452–464. doi: 10.1002/bies.20573. [DOI] [PubMed] [Google Scholar]
- 36.Van De Wetering JK, Van Golde LMG, Batenburg JJ. Collectins: players of the innate immune system. European Journal of Biochemistry. 2004;271(7):1229–1249. doi: 10.1111/j.1432-1033.2004.04040.x. [DOI] [PubMed] [Google Scholar]
- 37.Gottschalk A, Belyavin G, Biddle F. Glycoproteins Their Composition, Structure and Function. New York, NY, USA: Elsevier; 1972. Glycoproteins as influenza virus haemagglutinin inhibitors and as cellular virus receptors. [Google Scholar]
- 38.Anders EM, Hartley CA, Jackson DC. Bovine and mouse serum β inhibitors of influenza A viruses are mannose-binding lectins. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(12):4485–4489. doi: 10.1073/pnas.87.12.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartley CA, Jackson DC, Anders EM. Two distinct serum mannose-binding lectins function as β inhibitors of influenza virus: identification of bovine serum β inhibitor as conglutinin. Journal of Virology. 1992;66(7):4358–4363. doi: 10.1128/jvi.66.7.4358-4363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanaoka K, Pritchett TJ, Takasaki S, et al. 4-O-Acetyl-N-acetylneuraminic acid in the N-linked carbohydrate structures of equine and guinea pig α2-macroglobulins, potent inhibitors of influenza virus infection. Journal of Biological Chemistry. 1989;264(17):9842–9849. [PubMed] [Google Scholar]
- 41.Pritchett TJ, Paulson JC. Basis for the potent inhibition of influenza virus infection by equine and guinea pig α2-macroglobulin. Journal of Biological Chemistry. 1989;264(17):9850–9858. [PubMed] [Google Scholar]
- 42.Hartshorn KL, Crouch EC, White MR, et al. Evidence for a protective role of pulmonary surfactant protein D (SP-D) against influenza A viruses. Journal of Clinical Investigation. 1994;94(1):311–319. doi: 10.1172/JCI117323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hartshorn KL, Sastry KN, Chang D, White MR, Crouch EC. Enhanced anti-influenza activity of a surfactant protein D and serum conglutinin fusion protein. American Journal of Physiology. 2000;278(1):L90–L98. doi: 10.1152/ajplung.2000.278.1.L90. [DOI] [PubMed] [Google Scholar]
- 44.Reading PC, Morey LS, Crouch EC, Anders EM. Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. Journal of Virology. 1997;71(11):8204–8212. doi: 10.1128/jvi.71.11.8204-8212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benne CA, Kraaijeveld CA, Van Strijp JAG, et al. Interactions of surfactant protein A with influenza A viruses: binding and neutralization. Journal of Infectious Diseases. 1995;171(2):335–341. doi: 10.1093/infdis/171.2.335. [DOI] [PubMed] [Google Scholar]
- 46.Mikerov AN, White M, Hartshorn K, Wang G, Floros J. Inhibition of hemagglutination activity of influenza a viruses by SP-A1 and SP-A2 variants expressed in CHO cells. Medical Microbiology and Immunology. 2008;197(1):9–12. doi: 10.1007/s00430-007-0051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hawgood S, Brown C, Edmondson J, et al. Pulmonary collectins modulate strain-specific influenza A virus infection and host responses. Journal of Virology. 2004;78(16):8565–8572. doi: 10.1128/JVI.78.16.8565-8572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LeVine AM, Whitsett JA, Hartshorn KL, Crouch EC, Korfhagen TR. Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. Journal of Immunology. 2001;167(10):5868–5873. doi: 10.4049/jimmunol.167.10.5868. [DOI] [PubMed] [Google Scholar]
- 49.Tate MD, Brooks AG, Reading PC. Inhibition of lectin-mediated innate host defences in vivo modulates disease severity during influenza virus infection. Immunology and Cell Biology. 2011;89(3):482–491. doi: 10.1038/icb.2010.113. [DOI] [PubMed] [Google Scholar]
- 50.Vigerust DJ, Ulett KB, Boyd KL, Madsen J, Hawgood S, McCullers JA. N-linked glycosylation attenuates H3N2 influenza viruses. Journal of Virology. 2007;81(16):8593–8600. doi: 10.1128/JVI.00769-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hartshorn KL, White MR, Mogues T, Ligtenberg T, Crouch E, Holmskov U. Lung and salivary scavenger receptor glycoprotein-340 contribute to the host defense against influenza A viruses. American Journal of Physiology. 2003;285(5):L1066–L1076. doi: 10.1152/ajplung.00057.2003. [DOI] [PubMed] [Google Scholar]
- 52.Hartshorn KL, Crouch E, White MR, et al. Pulmonary surfactant proteins A and D enhance neutrophil uptake of bacteria. American Journal of Physiology. 1998;274(6):L958–L969. doi: 10.1152/ajplung.1998.274.6.L958. [DOI] [PubMed] [Google Scholar]
- 53.Crouch E, Persson A, Chang D, Heuser J. Molecular structure of pulmonary surfactant protein D (SP-D) Journal of Biological Chemistry. 1994;269(25):17311–17319. [PubMed] [Google Scholar]
- 54.Crouch EC, Smith K, McDonald B, et al. Species differences in the carbohydrate binding preferences of surfactant protein D. American Journal of Respiratory Cell and Molecular Biology. 2006;35(1):84–94. doi: 10.1165/rcmb.2005-0462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tecle T, White MR, Sorensen G, et al. Critical role for cross-linking of trimeric lectin domains of surfactant protein D in antiviral activity against influenza A virus. Biochemical Journal. 2008;412(2):323–329. doi: 10.1042/BJ20071663. [DOI] [PubMed] [Google Scholar]
- 56.Hartshorn KL, White MR, Tecle T, et al. Reduced influenza viral neutralizing activity of natural human trimers of surfactant protein D. Respiratory Research. 2007;8, article 9 doi: 10.1186/1465-9921-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.White M, Kingma P, Tecle T, et al. Multimerization of surfactant protein D, but not its collagen domain, is required for antiviral and opsonic activities related to influenza virus. Journal of Immunology. 2008;181(11):7936–7943. doi: 10.4049/jimmunol.181.11.7936. [DOI] [PubMed] [Google Scholar]
- 58.Hartshorn KL, Webby R, White MR, et al. Role of viral hemagglutinin glycosylation in anti-influenza activities of recombinant surfactant protein D. Respiratory research. 2008;9, article 65 doi: 10.1186/1465-9921-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hartshorn KL, White MR, Voelker DR, Coburn J, Zaner K, Crouch EC. Mechanism of binding of surfactant protein D to influenza A viruses: importance of binding to haemagglutinin to antiviral activity. Biochemical Journal. 2000;351(2):449–458. [PMC free article] [PubMed] [Google Scholar]
- 60.Tecle T, White MR, Crouch EC, Hartshorn KL. Inhibition of influenza viral neuraminidase activity by collectins. Archives of Virology. 2007;152(9):1731–1742. doi: 10.1007/s00705-007-0983-4. [DOI] [PubMed] [Google Scholar]
- 61.Hartshorn KL, Reid KBM, White MR, et al. Neutrophil deactivation by influenza A viruses: mechanisms of protection after viral opsonization with collectins and hemagglutination-inhibiting antibodies. Blood. 1996;87(8):3450–3461. [PubMed] [Google Scholar]
- 62.Hirche TO, Crouch EC, Espinola M, et al. Neutrophil serine proteinases inactivate surfactant protein D by cleaving within a conserved subregion of the carbohydrate recognition domain. Journal of Biological Chemistry. 2004;279(26):27688–27698. doi: 10.1074/jbc.M402936200. [DOI] [PubMed] [Google Scholar]
- 63.White MR, Tecle T, Crouch EC, Hartshorn KL. Impact of neutrophils on antiviral activity of human bronchoalveolar lavage fluid. American Journal of Physiology. 2007;293(5):L1293–L1299. doi: 10.1152/ajplung.00266.2007. [DOI] [PubMed] [Google Scholar]
- 64.Leth-Larsen R, Garred P, Jensenius H, et al. A common polymorphism in the SFTPD gene influences assembly, function, and concentration of surfactant protein D. Journal of Immunology. 2005;174(3):1532–1538. doi: 10.4049/jimmunol.174.3.1532. [DOI] [PubMed] [Google Scholar]
- 65.Tate MD, Job ER, Brooks AG, Reading PC. Glycosylation of the hemagglutinin modulates the sensitivity of H3N2 influenza viruses to innate proteins in airway secretions and virulence in mice. Virology. 2011;413(1):84–92. doi: 10.1016/j.virol.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 66.Tate MD, Brooks AG, Reading PC. Specific sites of N-linked glycosylation on the hemagglutinin of H1N1 subtype influenza A virus determine sensitivity to inhibitors of the innate immune system and virulence in mice. Journal of Immunology. 2011;187(4):1884–1894. doi: 10.4049/jimmunol.1100295. [DOI] [PubMed] [Google Scholar]
- 67.Ikegami M, Na CL, Korfhagen TR, Whitsett JA. Surfactant protein D influences surfactant ultrastructure and uptake by alveolar type II cells. American Journal of Physiology. 2005;288(3):L552–L561. doi: 10.1152/ajplung.00142.2004. [DOI] [PubMed] [Google Scholar]
- 68.Ikegami M, Whitsett JA, Jobe A, Ross G, Fisher J, Korfhagen T. Surfactant metabolism in SP-D gene-targeted mice. American Journal of Physiology. 2000;279(3):L468–L476. doi: 10.1152/ajplung.2000.279.3.L468. [DOI] [PubMed] [Google Scholar]
- 69.Korfhagen TR, Sheftelyevich V, Burhans MS, et al. Surfactant protein-D regulates surfactant phospholipid homeostasis in vivo. Journal of Biological Chemistry. 1998;273(43):28438–28443. doi: 10.1074/jbc.273.43.28438. [DOI] [PubMed] [Google Scholar]
- 70.Kingma PS, Zhang L, Ikegami M, Hartshorn K, McCormack FX, Whitsett JA. Correction of pulmonary abnormalities in Sftpd-/- mice requires the collagenous domain of surfactant protein D. Journal of Biological Chemistry. 2006;281(34):24496–24505. doi: 10.1074/jbc.M600651200. [DOI] [PubMed] [Google Scholar]
- 71.Zhang L, Ikegami M, Korfhagen TR, et al. Neither SP-A nor NH2-terminal domains of SP-A can substitute for SP-D in regulation of alveolar homeostasis. American Journal of Physiology. 2006;291(2):L181–L190. doi: 10.1152/ajplung.00015.2006. [DOI] [PubMed] [Google Scholar]
- 72.Reading PC, Allison J, Crouch EC, Margot Anders E. Increased susceptibility of diabetic mice to influenza virus infection: compromise of collectin-mediated host defense of the lung by glucose? Journal of Virology. 1998;72(8):6884–6887. doi: 10.1128/jvi.72.8.6884-6887.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takahashi K, Ip WKE, Michelow IC, Ezekowitz RAB. The mannose-binding lectin: a prototypic pattern recognition molecule. Current Opinion in Immunology. 2006;18(1):16–23. doi: 10.1016/j.coi.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haurum JS, Thiel S, Haagsman HP, Laursen SB, Larsen B, Jensenius JC. Studies on the carbohydrate-binding characteristics of human pulmonary surfactant-associated protein A and comparison with two other collectins: Mannan-binding protein and conglutinin. Biochemical Journal. 1993;293(3):873–878. doi: 10.1042/bj2930873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fujita T, Matsushita M, Endo Y. The lectin-complement pathway—its role in innate immunity and evolution. Immunological Reviews. 2004;198:185–202. doi: 10.1111/j.0105-2896.2004.0123.x. [DOI] [PubMed] [Google Scholar]
- 76.Takahashi M, Mori S, Shigeta S, Fujita T. Role of MBL-associated serine protease (MASP) on activation of the lectin complement pathway. Advances in Experimental Medicine and Biology. 2007;598:93–104. doi: 10.1007/978-0-387-71767-8_8. [DOI] [PubMed] [Google Scholar]
- 77.Krarup A, Wallis R, Presanis JS, Gál P, Sim RB. Simultaneous activation of complement and coagulation by MBL-associated serine protease 2. PLoS One. 2007;2(7, article no. e623) doi: 10.1371/journal.pone.0000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Presanis JS, Hajela K, Ambrus G, Gál P, Sim RB. Differential substrate and inhibitor profiles for human MASP-1 and MASP-2. Molecular Immunology. 2004;40(13):921–929. doi: 10.1016/j.molimm.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 79.Sastry R, Wang JS, Brown DC, Ezekowitz RA, Tauber AI, Sastry KN. Characterization of murine mannose-binding protein genes Mbl1 and Mbl2 reveals features common to other collectin genes. Mammalian Genome. 1995;6(2):103–110. doi: 10.1007/BF00303252. [DOI] [PubMed] [Google Scholar]
- 80.Takahashi K. Lessons learned from murine models of mannose-binding lectin deficiency. Biochemical Society Transactions. 2008;36(6):1487–1490. doi: 10.1042/BST0361487. [DOI] [PubMed] [Google Scholar]
- 81.Hansen S, Thiel S, Willis A, Holmskov U, Jensenius JC. Purification and characterization of two mannan-binding lectins from mouse serum. Journal of Immunology. 2000;164(5):2610–2618. doi: 10.4049/jimmunol.164.5.2610. [DOI] [PubMed] [Google Scholar]
- 82.Eisen DP. Mannose-binding lectin deficiency and respiratory tract infection. Journal of Innate Immunity. 2010;2(2):114–122. doi: 10.1159/000228159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fidler KJ, Hilliard TN, Bush A, et al. Mannose-binding lectin is present in the infected airway: a possible pulmonary defence mechanism. Thorax. 2009;64(2):150–155. doi: 10.1136/thx.2008.100073. [DOI] [PubMed] [Google Scholar]
- 84.Chang WC, White MR, Moyo P, et al. Lack of the pattern recognition molecule mannose-binding lectin increases susceptibility to influenza A virus infection. BMC Immunology. 2010;11, article 64 doi: 10.1186/1471-2172-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hartshorn KL, White MR, Shepherd V, Reid K, Jensenius JG, Crouch EC. Mechanisms of anti-influenza activity of surfactant proteins A and D: comparison with serum collectins. American Journal of Physiology. 1997;273(6):L1156–L1166. doi: 10.1152/ajplung.1997.273.6.L1156. [DOI] [PubMed] [Google Scholar]
- 86.Hartshorn KL, Sastry K, White MR, et al. Human mannose-binding protein functions as an opsonin for influenza A viruses. Journal of Clinical Investigation. 1993;91(4):1414–1420. doi: 10.1172/JCI116345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Job ER, Deng YM, Tate MD, et al. Pandemic H1N1 influenza a viruses are resistant to the antiviral activities of innate immune proteins of the collectin and pentraxin superfamilies. Journal of Immunology. 2010;185(7):4284–4291. doi: 10.4049/jimmunol.1001613. [DOI] [PubMed] [Google Scholar]
- 88.Kase T, Suzuki Y, Kawai T, et al. Human mannan-binding lectin inhibits the infection of influenza a virus without complement. Immunology. 1999;97(3):385–392. doi: 10.1046/j.1365-2567.1999.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Anders EM, Hartley CA, Reading PC, Ezekowitz RAB. Complement-dependent neutralization of influenza virus by a serum mannose-binding lectin. Journal of General Virology. 1994;75(3):615–622. doi: 10.1099/0022-1317-75-3-615. [DOI] [PubMed] [Google Scholar]
- 90.Reading PC, Hartley CA, Ezekowitz RAB, Anders EM. A serum mannose-binding lectin mediates complement dependent lysis of influenza virus-infected cells. Biochemical and Biophysical Research Communications. 1995;217(3):1128–1136. doi: 10.1006/bbrc.1995.2886. [DOI] [PubMed] [Google Scholar]
- 91.Malhotra R, Haurum JS, Thiel S, Sim RB. Binding of human collectins (SP-A and MBP) to influenza virus. Biochemical Journal. 1994;304(2):455–461. doi: 10.1042/bj3040455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tokunaga H, Ushirogawa H, Ohuchi M. The pandemic (H1N1) 2009 influenza virus is resistant to mannose-binding lectin. Virology Journal. 2011;8, article 50 doi: 10.1186/1743-422X-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ling MT, Tu W, Han Y, et al. Mannose-binding lectin contributes to deleterious inflammatory response in pandemic H1N1 and avian H9N2 infection. Journal of Infectious Diseases. 2012;205(1):44–53. doi: 10.1093/infdis/jir691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ohtani K, Suzuki Y, Eda S, et al. Molecular cloning of a novel human collectin from liver (CL-L1) Journal of Biological Chemistry. 1999;274(19):13681–13689. doi: 10.1074/jbc.274.19.13681. [DOI] [PubMed] [Google Scholar]
- 95.Ohtani K, Suzuki Y, Eda S, et al. The membrane-type Collectin CL-P1 is a scavenger receptor on vascular endothelial cells. Journal of Biological Chemistry. 2001;276(47):44222–44228. doi: 10.1074/jbc.M103942200. [DOI] [PubMed] [Google Scholar]
- 96.Hansen S, Selman L, Palaniyar N, et al. Collectin 11 (CL-11, CL-K1) is a MASP-1/3-associated plasma collectin with microbial-binding activity. Journal of Immunology. 2010;185(10):6096–6104. doi: 10.4049/jimmunol.1002185. [DOI] [PubMed] [Google Scholar]
- 97.Motomura W, Yoshizaki T, Ohtani K, et al. Immunolocalization of a novel collectin CL-K1 in murine tissues. Journal of Histochemistry and Cytochemistry. 2008;56(3):243–252. doi: 10.1369/jhc.7A7312.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yoshizaki T, Ohtani K, Motomura W, et al. Comparison of human blood concentrations of collectin kidney 1 and mannan-binding lectin. Journal of Biochemistry. 2012;151(1):57–64. doi: 10.1093/jb/mvr114. [DOI] [PubMed] [Google Scholar]
- 99.Bordet J, Gay FP. Sur les relations des sensibilisatrices avec l'alexine. Annales de l'Institut Pasteur. 1906;20:467–498. [Google Scholar]
- 100.Lachmann PJ. Conglutinin and Immunoconglutinins. Advances in Immunology. 1967;6:479–527. doi: 10.1016/s0065-2776(08)60527-1. [DOI] [PubMed] [Google Scholar]
- 101.Lachmann PJ, Müller-Eberhard HJ. The demonstration in human serum of “conglutinogen-activating factor” and its effect on the third component of complement. Journal of Immunology. 1968;100(4):691–698. [PubMed] [Google Scholar]
- 102.Hansen S, Holmskov U. Lung surfactant protein D (SP-D) and the molecular diverted descendants: conglutinin, CL-43 and CL-46. Immunobiology. 2002;205(4-5):498–517. doi: 10.1078/0171-2985-00150. [DOI] [PubMed] [Google Scholar]
- 103.Hartshorn KL, Holmskov U, Hansen S, et al. Distinctive anti-influenza properties of recombinant collectin 43. Biochemical Journal. 2002;366(1):87–96. doi: 10.1042/BJ20011868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rothmann AB, Mortensen HD, Holmskov U, Hojrup P. Structural characterization of bovine collectin-43. European Journal of Biochemistry. 1997;243(3):630–635. doi: 10.1111/j.1432-1033.1997.t01-1-00630.x. [DOI] [PubMed] [Google Scholar]
- 105.Wakamiya N, Okuno Y, Sasao F, et al. Isolation and characterization of conglutinin as an influenza A virus inhibitor. Biochemical and Biophysical Research Communications. 1992;187(3):1270–1278. doi: 10.1016/0006-291x(92)90440-v. [DOI] [PubMed] [Google Scholar]
- 106.Eda S, Suzuki Y, Kase T, et al. Recombinant bovine conglutinin, lacking the N-terminal and collagenous domains, has less conglutination activity but is able to inhibit haemagglutination by influenza A virus. Biochemical Journal. 1996;316(1):43–48. doi: 10.1042/bj3160043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hartshorn KL, Sastry K, Brown D, et al. Conglutinin acts as an opsonin for influenza A viruses. Journal of Immunology. 1993;151(11):6265–6273. [PubMed] [Google Scholar]
- 108.Kawai T, Kase T, Suzuki Y, et al. Anti-influenza A virus activities of mannan-binding lectins and bovine conglutinin. Journal of Veterinary Medical Science. 2007;69(2):221–224. doi: 10.1292/jvms.69.221. [DOI] [PubMed] [Google Scholar]
- 109.Suzuki Y, Eda S, Kawai T, et al. Characterization of recombinant bovine conglutinin expressed in a mammalian cell. Biochemical and Biophysical Research Communications. 1997;238(3):856–860. doi: 10.1006/bbrc.1997.7402. [DOI] [PubMed] [Google Scholar]
- 110.Eda S, Suzuki Y, Kawai T, et al. Structure of a truncated human surfactant protein D is less effective in agglutinating bacteria than the native structure and fails to inhibit haemagglutination by influenza A virus. Biochemical Journal. 1997;323(2):393–399. doi: 10.1042/bj3230393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Eda S, Suzuki Y, Kawai T, et al. Characterization of Truncated Human Mannan-Binding Protein (MBP) Expressed in Escherichia coli. Bioscience, Biotechnology and Biochemistry. 1998;62(7):1326–1331. doi: 10.1271/bbb.62.1326. [DOI] [PubMed] [Google Scholar]
- 112.Holmskov U, Teisner B, Willis AC, Reid KBM, Jensenius JC. Purification and characterization of a bovine serum lectin (CL-43) with structural homology to conglutinin and SP-D and carbohydrate specificity similar to mannan-binding protein. Journal of Biological Chemistry. 1993;268(14):10120–10125. [PubMed] [Google Scholar]
- 113.Holmskov U, Laursen SB, Malhotra R, et al. Comparative study of the structural and functional properties of a bovine plasma C-type lectin, collectin-43, with other collectins. Biochemical Journal. 1995;305(3):889–896. doi: 10.1042/bj3050889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.White MR, Crouch E, Chang D, Hartshorn KL. Increased antiviral and opsonic activity of a highly multimerized collectin chimera. Biochemical and Biophysical Research Communications. 2001;286(1):206–213. doi: 10.1006/bbrc.2001.5373. [DOI] [PubMed] [Google Scholar]
- 115.Hartshorn KL, White MR, Smith K, et al. Increasing antiviral activity of surfactant protein D trimers by introducing residues from bovine serum collectins: dissociation of mannan-binding and antiviral activity. Scandinavian Journal of Immunology. 2010;72(1):22–30. doi: 10.1111/j.1365-3083.2010.02409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hartshorn KL, White MR, Tecle T, Sorensen G, Holmskov U, Crouch EC. Viral aggregating and opsonizing activity in collectin trimers. American Journal of Physiology. 2010;298(1):L79–L88. doi: 10.1152/ajplung.00223.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Van Eijk M, White MR, Batenburg JJ, et al. Interactions of influenza A virus with sialic acids present on porcine surfactant protein D. American Journal of Respiratory Cell and Molecular Biology. 2004;30(6):871–879. doi: 10.1165/rcmb.2003-0355OC. [DOI] [PubMed] [Google Scholar]
- 118.Van Eijk M, White MR, Crouch EC, et al. Porcine pulmonary collectins show distinct interactions with influenza A viruses: role of the N-linked oligosaccharides in the carbohydrate recognition domain. Journal of Immunology. 2003;171(3):1431–1440. doi: 10.4049/jimmunol.171.3.1431. [DOI] [PubMed] [Google Scholar]
- 119.Hillaire MLB, van Eijk M, van Trierum SE, et al. Assessment of the antiviral properties of recombinant porcine SP-D against various influenza A viruses in vitro. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0025005. Article ID e25005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brown IH. The epidemiology and evolution of influenza viruses in pigs. Veterinary Microbiology. 2000;74(1-2):29–46. doi: 10.1016/s0378-1135(00)00164-4. [DOI] [PubMed] [Google Scholar]
- 121.Laursen SB, Dalgaard TS, Thiel S, et al. Cloning and sequencing of a cDNA encoding chicken mannan-binding lectin (MBL) and comparison with mammalian analogues. Immunology. 1998;93(3):421–430. doi: 10.1046/j.1365-2567.1998.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hogenkamp A, Van Eijk M, Van Dijk A, Van Asten AJAM, Veldhuizen EJA, Haagsman HP. Characterization and expression sites of newly identified chicken collectins. Molecular Immunology. 2006;43(10):1604–1616. doi: 10.1016/j.molimm.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 123.Hughes AL. Evolution of the lung surfactant proteins in birds and mammals. Immunogenetics. 2007;59(7):565–572. doi: 10.1007/s00251-007-0218-6. [DOI] [PubMed] [Google Scholar]
- 124.Hogenkamp A, Isohadouten N, Reemers SSN, et al. Chicken lung lectin is a functional C-type lectin and inhibits haemagglutination by influenza A virus. Veterinary Microbiology. 2008;130(1-2):37–46. doi: 10.1016/j.vetmic.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 125.Reading PC, Bozza S, Gilbertson B, et al. Antiviral activity of the long chain pentraxin PTX3 against influenza viruses. Journal of Immunology. 2008;180(5):3391–3398. doi: 10.4049/jimmunol.180.5.3391. [DOI] [PubMed] [Google Scholar]
- 126.Igarashi M, Ito K, Yoshida R, Tomabechi D, Kida H, Takada A. Predicting the antigenic structure of the pandemic (H1N1) 2009 influenza virus hemagglutinin. PLoS One. 2010;5(1) doi: 10.1371/journal.pone.0008553. Article ID e8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Qi L, Kash JC, Dugan VG, et al. The ability of pandemic influenza virus hemagglutinins to induce lower respiratory pathology is associated with decreased surfactant protein D binding. Virology. 2011;412(2):426–434. doi: 10.1016/j.virol.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Eisen DP, Marshall C, Dean MM, et al. No association between mannose-binding lectin deficiency and H1N1 2009 infection observed during the first season of this novel pandemic influenza virus. Human Immunology. 2011;72(11):1091–1094. doi: 10.1016/j.humimm.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.WHO. Avian Influenza. http://www.who.int/mediacentre/factsheets/avian_influenza/en/
- 130.Kongchanagul A, Suptawiwat O, Boonarkart C, et al. Decreased expression of surfactant protein D mRNA in human lungs in fatal cases of H5N1 avian influenza. Journal of Medical Virology. 2011;83(8):1410–1417. doi: 10.1002/jmv.22105. [DOI] [PubMed] [Google Scholar]
- 131.Cameron CM, Cameron MJ, Bermejo-Martin JF, et al. Gene expression analysis of host innate immune responses during lethal H5N1 infection in ferrets. Journal of Virology. 2008;82(22):11308–11317. doi: 10.1128/JVI.00691-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cillóniz C, Shinya K, Peng X, et al. Lethal influenza virus infection in macaques is associated with early dysregulation of inflammatory related genes. PLoS pathogens. 2009;5(10) doi: 10.1371/journal.ppat.1000604. Article ID e1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Herbert J, Hutchinson WL, Carr J, et al. Influenza virus infection is not affected by serum amyloid P component. Molecular Medicine. 2002;8(1):9–15. [PMC free article] [PubMed] [Google Scholar]
- 134.White MR, Crouch E, Chang D, et al. Enhanced antiviral and opsonic activity of a human mannose-binding lectin and surfactant protein D chimera. Journal of Immunology. 2000;165(4):2108–2115. doi: 10.4049/jimmunol.165.4.2108. [DOI] [PubMed] [Google Scholar]
- 135.Chang WC, Hartshorn KL, White MR, et al. Recombinant chimeric lectins consisting of mannose-binding lectin and L-ficolin are potent inhibitors of influenza A virus compared with mannose-binding lectin. Biochemical Pharmacology. 2011;81(3):388–395. doi: 10.1016/j.bcp.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gardai SJ, Xiao YQ, Dickinson M, et al. By binding SIRPα or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115(1):13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]
- 137.Crouch E, Tu Y, Briner D, et al. Ligand specificity of human surfactant protein D: expression of a mutant trimeric collectin that shows enhanced interactions with influenza A virus. Journal of Biological Chemistry. 2005;280(17):17046–17056. doi: 10.1074/jbc.M413932200. [DOI] [PubMed] [Google Scholar]
- 138.Crouch E, Hartshorn K, Horlacher T, et al. Recognition of mannosylated ligands and influenza a virus by human surfactant protein D: contributions of an extended site and residue 343. Biochemistry. 2009;48(15):3335–3345. doi: 10.1021/bi8022703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.White MR, Boland P, Tecle T, et al. Enhancement of antiviral activity of collectin trimers through cross-linking and mutagenesis of the carbohydrate recognition domain. Journal of Innate Immunity. 2010;2(3):267–279. doi: 10.1159/000272313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Crouch E, Nikolaidis N, McCormack FX, et al. Mutagenesis of surfactant protein D informed by evolution and x-ray crystallography enhances defenses against influenza A virus in vivo. Journal of Biological Chemistry. 2011;286(47):40681–40692. doi: 10.1074/jbc.M111.300673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Van Eijk M, Bruinsma L, Hartshorn KL, et al. Introduction of N-linked glycans in the lectin domain of surfactant protein D: impact on interactions with influenza A viruses. Journal of Biological Chemistry. 2011;286(23):20137–20151. doi: 10.1074/jbc.M111.224469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lu J, Willis AC, Reid KBM. Purification, characterization and cDNA cloning of human lung surfactant protein D. Biochemical Journal. 1992;284(3):795–802. doi: 10.1042/bj2840795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hansen S, Holm D, Moeller V, et al. CL-46, a novel collectin highly expressed in bovine thymus and liver. Journal of Immunology. 2002;169(10):5726–5734. doi: 10.4049/jimmunol.169.10.5726. [DOI] [PubMed] [Google Scholar]
- 144.Lee RT, Ichikawa Y, Allen HJ, Lee YC. Binding characteristics of galactoside-binding lectin (galaptin) from human spleen. Journal of Biological Chemistry. 1990;265(14):7864–7871. [PubMed] [Google Scholar]
- 145.Reading PC, Holmskov U, Anders EM. Antiviral activity of bovine collectins against rotaviruses. Journal of General Virology. 1998;79(9):2255–2263. doi: 10.1099/0022-1317-79-9-2255. [DOI] [PubMed] [Google Scholar]


