Abstract
Numerous evidences from prevention studies in humans, support the existence of an association between green tea polyphenols consumption and a reduced cancer risk. Prostate cancer is one of the most frequently diagnosed male neoplasia in the Western countries, which is in agreement with this gland being particularly vulnerable to oxidative stress processes, often associated with tumorigenesis. Tea polyphenols have been extensively studied in cell culture and animal models where they inhibited tumor onset and progression. Prostate cancer appears a suitable target for primary prevention care, since it grows slowly, before symptoms arise, thus offering a relatively long time period for therapeutic interventions. It is, in fact, usually diagnosed in men 50-year-old or older, when even a modest delay in progression of the disease could significantly improve the patients quality of life. Although epidemiological studies have not yet yielded conclusive results on the chemopreventive and anticancer effect of tea polyphenols, there is an increasing trend to employ these substances as conservative management for patients diagnosed with less advanced prostate cancer. Here, we intend to review the most recent observations relating tea polyphenols to human prostate cancer risk, in an attempt to outline better their potential employment for preventing prostate cancer.
1. Introduction
In the past decade, prostate cancer (PCa) has been one of the most frequently diagnosed male neoplasias in the Western countries, and despite recent important progress, it continues to represent a major cause of cancer-related mortality. The reasons of this high incidence are unknown. Racial and ethnic differences in PCa incidence and mortality are well known, with African-American men being at the greatest risk for diagnosis, followed by Caucasian and Hispanic men. Asian-Americans seem to be at the lowest risk for PCa [1]. Generally, PCa appears to be sporadically inherited (less than 10%). These observations highlight the hypothesis that interactions between multiple genetic and ambient factors are significative determinant in PCa development.
Diet is believed one of the most probable and determinant environmental risk factors. The hypothesis results were strengthened by ecological studies showing that the PCa incidence rapidly increases in Asian immigrants that have assimilated Western diet and way of living, and in Asian men that, although living in their original countries, are contaminated by Western lifestyle, tending to substitute soy, tea, fish, fruits, and vegetables consumption with red meat and fatty food [2]. The molecular mechanisms, through which racial, genetic, environmental conditions affect PCa development, are still a matter of discussion.
Numerous experimental evidences suggest that both dietary and lifestyle factors act by promoting chronic inflammation and/or oxidative stress leading to DNA damage, epigenetic modifications, or other alterations associated with cancer initiation. Altogether, the experimental data so far produced suggest that antioxidant and anti-inflammatory agents may play a promising role for PCa prevention [3]. In fact, (1) the proliferative inflammatory atrophy (PIA) has been proposed as a precursor to prostatic intraepithelial neoplasia (PIN) that merges with high-grade PIN (HGPIN) in about 34% of cancerous lesions [4]. Chronic inflammation may damage epithelial cells and lead to proliferative lesions, likely PIN lesions, and prostatic carcinomas precursors [5]; (2) several evidences have suggested that oxidative stress, following from the imbalance of reactive oxygen species (ROS) production and cellular antioxidant defences, is one of the most critical aging-associated factor on prostate carcinogenesis. Cumulative ROS effect possibly results in lipids, proteins, and DNA damage [6]. Prostate gland is known to be particularly vulnerable to oxidative stress, probably because of inflammation and hormonal deregulation processes and epigenetic modifications, frequently occurring in the organ.
It is worth to underline that PCa is a suitable target for primary chemopreventive interventions, since it is a unique malignancy that generally grows very slowly, before symptoms arise. As a consequence, it offers a relatively long time period for therapeutic interventions and, because of its long latency, it is typically diagnosed in 50-year-old men or older, when even a modest delay in the disease progression could significantly improve the patient quality of life.
Considering that most of the known chemotherapeutic treatments against PCa carry side effects risk, there is an increasing trend to employ conservative management for patients diagnosed with less advanced PCa, that may not require treatment. These types of tumors, in fact, are relatively indolent, almost never relapse after local therapy, and probably require a simple watchful waiting.
In order to obtain new additional opportunities improving nontoxic chemopreventive strategies, dietary substances consumption, especially tea polyphenols, can represent an important clinical challenge.
Tea, the most popular worldwide consumed beverage after water, obtained from the dried leaves of the plant Camellia sinensis, has been studied extensively for its effects on cancer prevention. The major polyphenols in green tea, generally known as catechins, (-)-epigallocatechin-3-gallate (EGCG), (-)-epigallocatechin (EGC), (-)-epicatechin-3-gallate (ECG), and (-)-epicatechin (EC), display meta-5,7-dihydroxyl groups on the A ring [7] and di- or trihydroxyl groups on the B ring, that represent the principal site of antioxidant reactions [8]. EGCG and ECG, harboring D ring (gallate), present maximal antioxidant activity (Figure 1). The above characteristics allow tea polyphenols to react with ROS (superoxide radical, singlet oxygen, hydroxyl/peroxyl radical, peroxynitrite) [9] and prevent, as strong metal ion chelators, ROS output following several compounds auto-oxidation. Due to the ability of acting as good donors for hydrogen bonding, an accurate prediction about tea polyphenols solubility and permeability remains, at the moment, an elusive target [10].
Figure 1.
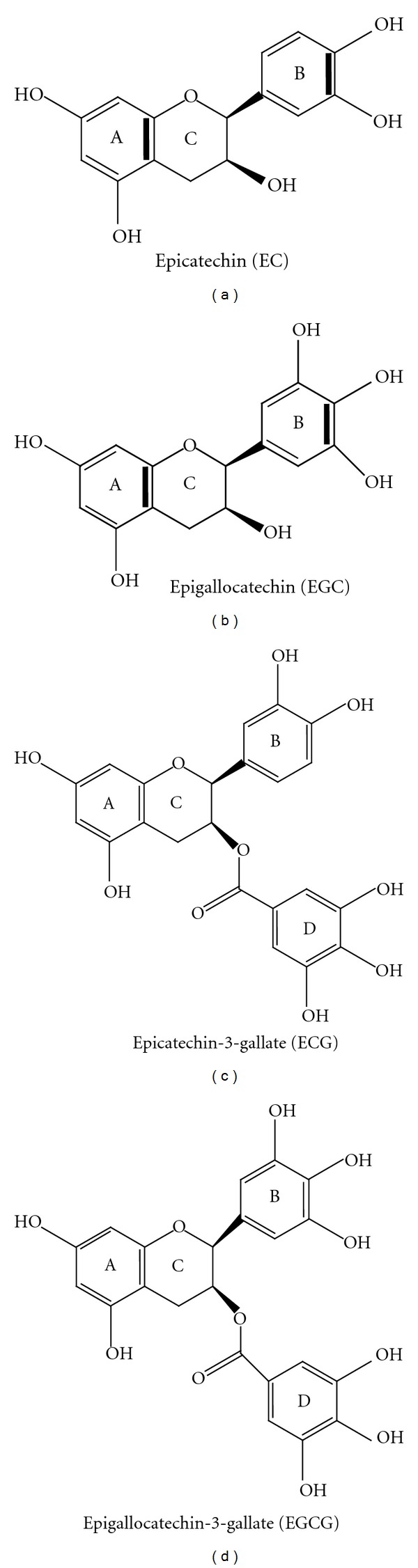
Chemical structure of the green tea catechins obtained from the dried leaves of the plant Camellia sinensis.
Tea polyphenols have been shown to inhibit tumorigenesis and tumor progression, at different organ sites, in different animal models for human cancer. Many evidences highlighted that these compounds affect enzyme activities and signal transduction pathways, resulting in cell proliferation suppression, apoptosis enhancement, as well as angiogenesis and cell invasion inhibition, finally inhibiting the development of the disease.
To date, an association between green tea polyphenols consumption and reduced cancer risk is also supported by human cancer prevention studies, although epidemiological evidences have not yet yielded conclusive results on their chemopreventive and anticancer effect against PCa, possibly owing to different confounding factors [11].
2. Mechanisms of GTCs Action in PCa Cell Lines
Several mechanisms involved in GTCs inhibition of cancer formation/progression are recently reviewed by numerous Authors [12–15]. Certainly, GTCs, through their antioxidant activity, are able to quench ROS and chelate transition metals, produced during all the carcinogenesis stage. However, it has been reported that also GTCs can be a source of ROS generation, inducing oxidative stress and consequently activating apoptotic pathways [16].
GTCs, and especially EGCG, are capable of modulating a plethora of cell signalling pathways crucial for cancer cells transformation and survival, including, but not limited to, the mitogen-activated protein kinase (MAP-kinase), the nuclear factor-kappa B (NFκB), and the insulin-like growth factor (IGF)/IGF-1 receptor pathways.
With regard to the prostate-specific processes GTCs are able to affect androgen receptor (AR) downregulation and prostate-specific antigen (PSA) expression [14, 17].
Here below, we report the most probable GTCs mechanisms of action in some PCa cell lines.
2.1. Inhibition of Cell Proliferation and Cell Cycle Arrest
GTCs exhibit ant-proliferative effects versus both androgen-sensitive and androgen-insensitive human PCa cells. The effect is mediated by cell cycle deregulation and cell death induction [18].
We showed that GTCs action is cancer specific, since GTCs is capable of inducing growth arrest both in SV-40 immortalized prostate epithelial cells (PNT1a) and in tumorigenic androgen-independent PCa cells (PC3), while normal human prostate epithelial cells were not significantly affected, even when EGCG was administered at higher doses [19]. The IC50 of EGCG ranges from about 40 to about 200 μM, depending on the cell line type (LNCap < PNT1a < DU145 < PC3), as well as the length of the experiment, ranging from 24 to 72 hours [18, 20]. Our results were confirmed by other authors in normal fibroblasts [20, 21]. Both in LnCap and DU145 cells, EGCG treatment resulted in the cell cycle arrest at G0/G1 phase (dose dependent) and the cyclin kinase inhibitor WAF1/p21 and apoptosis induction, irrespective of the AR presence or p53 cell status. Cells harbouring an active p53 protein respond to lower EGCG doses than cells with a mutated p53 [18].
It was shown that EGCG treatment of LNCaP and DU145 cells causes an induction of G1-phase cyclin kinase inhibitors, that, in turn, inhibit the cyclin-cycline-dependent kinase (CDK) complexes operative in the G0/G1 phase, thereby causing a cell cycle arrest, a possible irreversible process ultimately leading to apoptotic cell death [22].
2.2. Apoptosis
Hastak et al. [23] reported that EGCG induces apoptosis in LNCaP cells mainly through modulation of two related pathways: (i) p53 stabilization by specific phosphorylation and downregulation of murine double minute 2 protein (MDM2), mediated by the tumor suppressor p14ARF; (ii) negative regulation of NFκB activity, which results in reduction of the proapoptotic Bcl2 family protein expression. EGCG-induced p53 stabilization causes up-regulation of its downstream targets WAF1/p21 and Bax; consequently, EGCG produces a change in the Bax/Bcl2 ratio, shifting the balance between pro-/antiapoptotic proteins in favor of apoptosis. This first event triggers the caspases cascade activation, followed by poly-ADP-Ribose polymerase (PARP) cleavage and chromatin fragmentation. Inactivation of p53, by using Small Interfering RNA, renders LnCap cells more resistant to EGCG-mediated apoptosis. On the other hand, stable transfection of PC3 cells (that are endowed with a mutated and inactivated p53 protein) with a cDNA encoding wild-type p53, allows to by-pass their resistance to EGCG-mediated apoptosis [24]. Ablation of p21 or Bax confers a growth advantage to the cells through inhibition of the mitochondrial pathway of caspase-dependent apoptosis.
Tumour necrosis factor-related apoptosis-inducing ligand/Apo2L ligand (TRAIL/Apo2L) is believed a promising candidate for cancer therapy, even if emergence of drug resistance limiting its potential use occurs.
Siddiqui et al. [25] reported that EGCG treatment sensitizes TRAIL-resistant LNCaP cells to TRAIL-mediated apoptosis through modulation of apoptotic pathways. TRAIL/Apo2L, when combined with EGCG, exhibited enhanced cells apoptotic activity, characterized by three major molecular events: (i) increase of PARP cleavage; (ii) pro-/antiapoptotic Bcl2 family proteins modulation, favoring apoptosis; (iii) synergistic inhibition of apoptosis inhibitors and concomitant increase in caspase activation.
2.3. Anti- or Pro-Oxidant Effects and Activation of Phase II Detossifying Enzymes
GTCs were shown to suppress cell growth and induce apoptosis in DU145, through increasing ROS formation and mitochondrial depolarization. Although the molecular mechanisms are still not clear, GTCs-induced apoptosis is not related to the members of Bcl2 family, as EGCG did not alter Bcl2, BclX(L) and BAD expression, in this cell line [26].
Nuclear factor-E2-related factor 2 (Nrf2) is a transcription factor that plays a pivotal role in the antioxidant response and oxidative stress, through activation of phase II detoxifying or antioxidant enzymes [27, 28]. EGCG, as well as other electrophile natural compounds, are able to activate a core antioxidant responsive element (EpRe), present in the promoter region of many genes involved in the cellular response to oxidative stress [28, 29]. A widely accepted model for induction of EpRe-mediated antioxidant gene expression, plausible also for EGCG, involves phosphorylation of Nrf2, leading to enhanced Nrf2 accumulation and subsequent EpRe binding [28].
Activator Protein-1 (AP-1) is a redox-sensitive transcription factor that transduces modifications of the cellular redox status, modulating the expression of genes, including pro-survival genes, in responses to oxidative and electrophilic stresses.
Nair et al. [30] demonstrated that EGCG, when administered to PC3 cells together with sulforaphane (SFN) is able to reduce AP-1 induction. The authors also confirmed, by “in silico” analyses, the presence of conserved transcription factor binding site in the Nrf2 and AP-1 promoter region, suggesting that gene expression changes induced by SFN and EGCG, could be mediated via concerted modulation of the Nrf2 and AP-1 pathways [30].
2.4. Modulation of the NFκB Signalling and Inhibition of Inflammation Pathways
NFκB is a redox sensitive transcription factor, often overexpressed in tumor and cancer cell lines, that has been suggested to regulate a variety of cellular functions, including inflammation, immune response, growth, and cell death. NFκB resides in the cytoplasm, bound to its inhibitor IκB; once the inhibitor is released, NFκB phosphorylation occurs, followed by its translocation to the nucleus.
EGCG has been shown to decrease the DNA binding activity of NFκB, and reduce the expression of the p65 subunit of NFκB in LNCaP cells, stimulated by tumour necrosis factor alfa. NFκB over-expression is an important target in PCa due to the regulation of various downstream targets that include the cylcooxygenase-2 proteins (COX-2) [23].
In the LNCaP (androgen-dependent) and PC3 (androgen independent) prostate cancer cells, EGCG was shown to inhibit mitogen stimulated COX-2 expression through a mechanism probably involving the regulation of transcription factors, like NFκB, and not the direct binding to the enzyme [31]. Moreover, LnCaP and PC3 cells treatment with a combination of EGCG and COX-2 inhibitors resulted in: (i) enhanced cell growth inhibition; (ii) caspase-dependent induction of apoptosis and PARP cleavage; (iii) inhibition of peroxisome proliferator-activated receptor gamma; (iv) NFκB inhibition, when compared with the effects of the two singularly employed agents, suggesting that they play a synergistic role [32].
Matrix metalloproteinases (MMPs), generally involved in extracellular matrix degradation, result overexpressed in PCa and play an important role in tumor progression and invasion. NFκB is the key transcription factor involved in the regulation of MMPs genes.
In DU145 cells Vayalil and Katiyar [33] demonstrated that EGCG inhibits MMP-2 and MMP-9 (inactive and active form expression) through a dose-dependent phosphorylation inhibition of the extracellular signal-regulated kinase (ERK1/2) and p38 pathways. Inhibition of the activation of transcription factors c-jun and NFκB also occurs.
Siddiqui et al. [25] observed that EGCG, administered in combination with TRAIL, can inhibit LNCaP cells invasion and migration potential. The authors found the effect is mediated through inhibition of the expression of the following factors: vascular endothelial growth factor (VEGF), urokinase plasminogen activator (uPA), and angiopoietin 1 and 2. A significant inhibition in both MMP-2 and -9 protein expression and activity occurs, in the presence of up-regulation of the tissue inhibitor of metalloproteinases 1.
2.5. Insulin-Like Growth Factor (IGF) Axis, Mitogen-Activated Protein (MAP) Kinases Pathway, Phosphoinositide-3 Kinase (PI3K/AKT) Pathway
It has been suggested that the IGF axis plays a relevant role in PCa onset and development. Binding of IGF1 to its cognate receptor activates the intracellular tyrosine kynase domain, that produces phosphorylation of many protein substrates, including members of the MAP kinase cascade and PI3K/AKT. MAP kinases and the PI3K/AKT pathway are both involved in the complex modulation of signalling pathways, which regulates cellular processes like proliferation, survival/death, and motility, usually altered in carcinogenesis.
It has been reported that EGCG inhibits IGF-1 receptor activity with an IC50 of 14 μM [34]. Treatment of DU145 and LNCap cells with subapoptotic EGCG doses reduces IGF-induced growth [35].
Siddiqui et al. [36] found that EGCG is able to: (i) decrease PI3K and phospho-Akt levels and (ii) increase ERK1/2 level in both DU145 and LNCaP cells. Treatment of PC3 cells with EGCG results in activation of the ERK1/2 pathway, that is, not dependent by mitogen-extracellular signal-regulated kinase (MEK), the immediate upstream kinase responsible for ERK1/2 activation, suggesting an MEK-independent signalling mechanism. Pretreatment of PC3 cells with a PI3K inhibitor partially reduced both EGCG-induced ERK1/2 phosphorylation and cell proliferation inhibition. These results suggest that ERK1/2 activation via a MEK-independent and PI3-K-dependent signalling pathway is partially responsible for the antiproliferative EGCG effects in PC3 cells [20].
2.6. Androgen Receptor (AR) and Prostate-Specific Antigen (PSA)
Experimental evidences suggest that androgens are involved in PCa development and progression, being AR the essential mediator for androgen action. Detailed mechanisms of AR activation and modified function in PCa are reviewed in [37–39]. Briefly, the AR is a nuclear receptor activated through binding of its cognate ligands, such as testosterone and 5alfa-dihydrotestosterone, and consequent dissociation by the heat shock proteins (normally bounded to it in the resting state). The activation process involves several coactivators recruitments. Activated AR up regulates the transcription of genes containing androgen response elements in their promoters as PSA gene that, specifically expressed in prostate, has been widely utilized for PCa screening, in the last 20 years [40]. A very recent report by means of a fluorescence resonance energy transfer (FRET) based assay provides evidence that EGCG is a direct antagonist of androgen action.
EGCG is capable to physically interact with the ligand-binding domain of AR by replacing a high-affinity-labeled ligand (IC50: 0.4 μM) [41].
In different LNCaP sublines, EGCG suppresses cell proliferation, PSA expression, and AR transcriptional activity, at concentration comprised in the 10–20 μM range [42, 43]. The effect on PSA expression might be related to reduction of AR activity, but it should also be considered, that EGCG, in vitro, can down regulate PSA by direct action on transcription and translation mechanisms [44, 45].
3. PCa Chemoprevention by Green Tea Polyphenols in Transgenic Mouse Model
Progress toward understanding the PCa biology has been slow due to the few animal research models of tumour onset and progression, available to study the spectrum of this uniquely human disease. Genetically engineered mice are being increasingly employed for delineating the molecular mechanisms of PCa development and the potential of new compounds as chemotherapeutic/chemopreventive drugs against it. Animal models present a rapid tumor growth comparing to the long latency of human PCa, which, on the other hand, makes the disease an ideal target for chemoprevention strategies. Mouse dorsolateral prostate lobe is functionally equivalent to human prostate peripheral zone, from where the majority of human cancer originate [46]. Preclinical studies with GTCs or with pure EGCG, administered at a human achievable doses, have been conducted in both transgenic animals and xenograft tumor models, in which murine and human cell lines, derived from primary tumor or metastasis, have been implanted subcutaneously [47, 48] or injected intraprostatically [49].
To the aim of studying human CaP, autochthonous murine models appear more suitable than orthotopic cell lines transplantation. In fact, transgenic mice exhibit sets of interactions between the different cellular, tissue and hormonal compartments appropriate to human prostate. Among the several lines of transgenic mice generated models, the transgenic adenocarcinoma of mouse prostate (TRAMP) has been well characterized and employed for a number of pre-clinical trials. Mice expressing the transgene display progressive forms of prostatic disease that histologically resemble human PCa, from mild intraepithelial neoplasia (PIN) to poor differentiate adenocarcinoma phase (PD) and finally to metastatic spread [50]. In the TRAMP model, the SV40 early genes (T and t antigens, Tag) are under the control of the minimal rat Probasin promoter −426/+28—fragment [50], which renders the transgene expression androgen dependent, restricting it to the epithelial cells of the dorsolateral and ventral prostate lobes, thus abrogating p53 and Rb function [51] and inactivating protein phosphatase 2A (PP2A), specifically in this tissue [52].
In many experimental studies conducted by Gupta et al. [53] and by other authors [54–57], TRAMP mice aged from 8 to 32 weeks, received 0.1% oral infusion of a 95% GTCs enriched mixture. The animals, when compared to water-fed TRAMP mice, presented a significant delay in primary tumor incidence and almost complete metastases inhibition; prostate and genitourinary tract weight, a well-known tumor growth index, was decreased (64% and 72%, resp.), correlating with the reduced expression of Proliferating Cell Nuclear Antigen (PCNA).
The insulin-like growth factor pathway IGF/IGFBP-3 has been suggested to regulate PCa growth and development through its gradually increased activation during cancer progression.
After GTCs administration TRAMP mice showed a significant decrease in the IGF/IGFBP-3 ratio [54], accompanied by an inhibition of the downstream signaling cascade that involves both PI3K/AKT and MAPK pathways. Also, a parallel inhibition of vascular endothelial growth factor (VEGF), matrix metalloproteinases MMP-2, and MMP-9 expression were demonstrated [55]. Furthermore, the authors showed that metastasis-promoting Mts1 (S100A4) level, that, as a rule increases in cancer development, resulted in markedly decreased, E-cadherin level, that is progressively lost during cancer progression, where restored [56]. Also, the NFκB pathway activity, generally activated as a function of tumor grade, was reduced after 32 weeks of EGCG treatment, at a time when a shift in balance between Bax and Bcl2, favoring apoptosis, also occurred [57].
Under similar experimental conditions, we observed that, while 100% of TRAMP mice underwent PCa at 24 weeks of age, exhibiting tumor cell transendothelial passage in the absorbing lymphatic vessels, only 20% of the animals receiving 0.3% of GTCs (oral infusion), developed the neoplasm [19, 58–60]. In TRAMP mice presenting tumor growth arrest, a sequence of events were demonstrated, such as downregulation of H3 histone (a process usually affecting chromatin structure and gene expression), upregulation of growth arrest-Specific gene 1 (GAS1) and suppression of Mini-Chromosome Maintenance protein 7 (MCM7), a marker of DNA synthesis, essential for its replication, that is aberrantly expressed in various cancer types.
Interestingly, the level of the secretory protein clusterin (CLU) and mRNA, dramatically downregulated with the disease onset and development, resulted to accumulate progressively in the prostate after GTCs administration and remain undetectable in the 20% of animals that presented PCa, in spite of receiving GTCs solution. In correlation with these observations, when tumor progression was inhibited, organelles committed to protein synthetic and secretory activities, as endoplasmic reticulum and Golgi apparatus, appeared significantly reduced in the prostate, suggesting possibly protein posttranslational processes alterations [60]. We suggest that CLU might participate in the chemopreventive action exerted by GTCs in TRAMP mice [19].
Harper et al. [61], after treating a cohort of TRAMP mice with almost pure 0.06% EGCG in drinking water, demonstrated that the compound can act only by slowing PCa progression. EGCG inhibited early (12-week-old mice), but not late (28-week-old mice) PCa stage. The treatment resulted in many various effects: AR, IGF1, and its receptor decreased level, apoptosis-reduced cell proliferation, and phospho-extracellular signal-regulated kinases 1/2, cyclooxygenase-2, and inducible nitric oxide synthase reduced activities. To a better interpretation of the data, it is worthwhile noting that green tea polyphenols bioavailability and transformation are key factors that can limit the compound activities in vivo; EGCG, as a single agent, may present a low bioavailability and/or slow its rapid metabolism, when stabilized by the naturally occurring mixture of green polyphenols. In addition, attention has to be paid when comparing experimental works on TRAMP mice colonies obtained through different background strains. In fact, the genetic background may have a profound effect on some aspects of tumor initiation/progression in this animal model.
The latest experimental work from Adhami et al. [62] confirmed the efficacy of 0.1% GTCs in drinking water to TRAMP mice, starting at defined stages of PCa onset and progression: 6 weeks (normal prostate), 12 weeks (PIN), 18 weeks (well-differentiated adenocarcinoma, WD), 28 weeks (poorly-differentiated adenocarcinoma, PD), finally cancer development. Tumor-free survival was indeed extended to 38 weeks in the 6 weeks group mice, but only to 24 weeks in the 18 weeks group, compared with 19 weeks in water-fed controls. Additionally, IGF and its downstream targets were significantly inhibited only when intervention was initiated at early stages.
The study design was conceived as a response to the outcomes of human clinical trials, based on alternative and complimentary green tea therapy, showing minimal effect on hormone-refractory PCa stage (CRPC) [63, 64], but lower incidence on high grade PIN stage [65, 66].
Extensive laboratory studies in multiple animal models consistently show the GTCs inhibitory activities against prostate carcinogenesis [14] and suggest the importance of identifying the most vulnerable PCa stages towards GTCs chemoprevention in humans.
4. Possible PCa Chemoprevention by Green Tea Polyphenols in Humans
4.1. PCa: Statistics and Importance for Chemoprevention Strategies
PCa is the second most common cancer in American men with a 1 in 6 lifetime risk of developing it. The National Cancer Institute (NCI) estimated that approximately 217,730 new cases PCa would have been diagnosed in 2010 and there would have been approximately 32,050 PCa deaths [67]. In Europe, PCa has become the most common non-skin cancer neoplasm among men, with an estimated 382,000 cases occurred in 2008 [68]. Incidence has increased rapidly over the past two decades, and rates are dramatically influenced by PSA testing, as well as by latent cancer detection in prostate surgery.
According to a recent report from the prostate, lung, colorectal, and ovarian cancer screening trial, six annual PCa screening programs (10 years followup results) led to increased number of diagnosed cancers, in the screening cohorts, but no cancer-specific survival advantage was seen for the group. Therefore, the real possibility to “overdiagnose” and “over-treat” cancers which are not life threatening, must be carefully taken in account [69]. A reasonable explanation for the low efficacy of invasive radical intervention, which are the gold standard in clinical practice after a diagnosis of confined PCa, relies on the fact that the disease is biologically heterogeneous and its natural history is almost unpredictable. Some cancers growth slowly, showing indolent course, while others are very aggressive, quickly progressing to advanced metastatic stage, that is almost an incurable disease [70]. Therefore, it is important and urgent to search for prevention strategy, effective when the disease is still at an early and potentially curable stage. PCa is an ideal candidate for chemoprevention, because it has a high prevalence, a long latency, and it is potentially lethal. Preventive strategies could carry a high economic benefit on the healthcare system reducing the costs associated with PCa diagnosis and therapy, moreover, it might have a deep positive impact on the patients quality of life, reducing the morbidity associated with radical surgery (incontinence and impotence) [71].
4.2. Epidemiological Evidence of Green Tea Efficacy in PCa Chemoprevention
PCa etiology is multifactorial, but the marked disparity in its incidence between “Eastern” and “Western” cultures suggests that dietary and lifestyle factors play an important role in the disease development and progression. This is strongly supported by migratory studies showing that Asian men, who relocate to the United States of America and adopt a western lifestyle, have a significantly higher PCa risk, when compared to their native Asian counterparts [72, 73].
During the last two decades, the relationship between tea consumption and cancer has been a subject of research interest for many investigators. Unfortunately at present, many epidemiological studies present conflicting results about the green tea role in cancer prevention. Recently, an exploratory meta-analysis of observational studies supported the hypothesis that green, but not black tea, may have a protective effect against PCa. A total of six epidemiological studies, including two case-control studies as well as four cohort studies, evaluated green tea role in reducing developing PCa risk [74–79]. A borderline statistical significant decrease in the disease development is presented with increasing green tea intake and, moreover, only case-control studies (odds ratio, OR = 0.43; 95% CI: 0.25–0.73), but not prospective cohort studies (OR = 1.00; 95% CI: 0.66–1.53) reached a statistically significant result [80].
Negative and conflicting results in epidemiological data may be due to study design pitfalls and to many hardly controllable variables, like tea infusion composition and way of preparation (temperature), GTCs bioavailability, diet and the lifestyle of the people included in the study, and last but not least, genetic differences in the ability to metabolize GTCs.
4.3. Pharmacokinetic Studies, Phase I Clinical Study, GTCs Metabolism and Tissue Distribution
Polyphenon E is a GTCs-enriched and defined product, virtually caffeine free (<0,5%) produced by Mitsui Norin Co. Ltd, a new drug investigated by the Food and Drug Administration. It contains 80% to 98% total catechins by weight, with EGCG main component, accounting for 50% to 75% of the material.
Chow et al. [81, 82] performed several pharmacokinetic phase I studies (in healthy volunteers) utilizing Polyphenon E (capsules) to determine the systemic tea polyphenols availability after a single or multiple dose and various dosing condition (200, 400, 600, 800 mg of EGCG). In the single-dose study, Polyphenon E was compared to pure EGCG for differences in pharmacokinetic parameters [81]; after its administration, at the four dose levels above mentioned, the average EGCG peak plasma concentration (Cmax) was 72.7 ± 66.4, 125.3 ± 50.4, 165.7 ± 126.9, and 377.6 ± 149.8 ng/mL. Similar results were obtained after pure EGCG administration, at the same concentration, suggesting that GTCs and Polyphenon E do not have any effect on the EGCG pharmacokinetics. It should be noted that tea catechins bioavailability is quite low in humans, resulting in plasma concentrations 5 to 50 times less than concentrations shown to exert biological activities in vitro [14].
In the multiple dose study, the same authors evaluated effects and safety following chronic Polyphenon E administration [82], concluding GTCs do not accumulate in the body and are safe for human health.
A third pharmacokinetic study from the same authors [83], evaluated the role of fasted or fed state on Polyphenon E bioavailability (400, 800, or 1200 mg of EGCG), concluding that Cmax of free catechins (EGCG, EGC, EC) can be increased of about 3-folds, when administered in a fasted state. The first study demonstrating that GTCs can be detected in the prostate tissue after green tea consumption has shown that after a short period (5 days) of green tea continuous intake (1.42 L of brewed tea divided in 5 daily doses), the prostate GTCs concentration were 0.1, 0.043, 0.040, 0.0021 (nmol/g tissue) for EGC, EC, EGCG, and ECG, respectively [84].
4.4. Proof of Principles and Phase II Studies of Green Tea Efficacy for PCa Chemoprevention and Treatment
There have been 5 intervention studies evaluating the GTCs effect on PCa treatment or prevention [63–65, 85, 86]. Three were single-arm open-label phase II trials, performed in patients diagnosed with a castration resistant PCa (CRPC) [63, 64, 86] and one was a pilot proof of concept study, performed to evaluate GTCs ability to reduce cancer incidence in a well-defined cohort of patients, bearing premalignant lesions (HGPIN) [65].
The most recent study, a randomized, double-blind, placebo-controlled phase II trial evaluated the effect of short-term Polyphenon E administration in men with PCa scheduled to undergo radical prostatectomy [85].
The primary endpoint of the trial reported in [64] was to determine the capacity of nonstandardized green tea powder to produce a PSA base level decline in 42 men with clinical CRPC evidence. Only 1 patient, among 42, manifested a transitory 50% decreased PSA level from baseline, during the 6 months followup. The negative result of this study needs to be critically considered; it should be taken into account that the enrolled population comprised men with an advanced cancer stage, that acquired resistance to standard hormone deprivation therapy, and that a nonstandardized GTCs preparation was employed.
Choan et al. [63] published the results of a study aimed to evaluate the effect of a standardized green tea extract (250 mg GTCs/day) on PSA level or measurable marks of the disease progression, after a minimum of 2 months therapy. Only 15, out of the enrolled 19 patients, completed at least 2 months therapy and all of them exhibited a progressive disease in the first 4 months. In addition, a very small population with an advanced cancer stage, unresponsive to previously administered hormonal therapy, was considered.
On the basis of encouraging results obtained by us and others in the PCa chemoprevention with animal models [19, 53, 62], our research group performed a proof of concept trial in a well-selected patients cohort at high risk to develop PCa [65]. We enrolled 60 patients with HGPIN diagnosis, that were randomly assigned to receive, according to a double-blind procedure, a standardized GTCs formulation (600 mg/day), or identical placebo capsules for 1 year. GTCs composition, virtually caffeine free, was total catechins 75.7%; EGC, 5.5%; EC, 12.2%; EGCG, 51.9%; ECG. All the patients, during the study, were subjected to regular prostate biopsy (6 and 12 months after the study beginning) to asses differences in PCa values between the two arms. Total serum PSA concentration was measured at 3, 6, 9, and 12 months, to check whether GTCs administration would reduce the value. Because concomitant benign prostate hypertrophy (BPH) was very common among the patients enrolled, we also evaluated the International prostate symptom score (IPSS) and quality of life (QoL) after 3 months of GTCs administration in a subcohort of patients with low urinary tract symptoms (LUTSs), as a further secondary end point. We found that 9 out of 30 patients receiving placebo developed PCa, while only 1 out of 30 patients receiving GTCs was found PCa positive after prostate biopsy. We did not find any significant difference in PSA values between the 2 arms, but we found a significant IPSS and QoL scores improvement.
Our results point the attention on the fact that GTCs should be considered interesting and promising natural compounds for PCa chemoprevention, while they are almost ineffective in case of full-developed metastatic neoplasia (CRPC). We also found that PCa incidence remained low in patients belonging to the GTCs-arm, even two years after therapy suspension [66]. This result may suggest that chemoprevention activity and clinical benefits achieved with GTCs are stable over time. We hope that our data will be confermed by a large phase II study, now ongoing, sponsored by NCI. This trial is aimed to enroll about 300 HGPIN patients to be given 400 mg/day of Polyphenon E for PCa prevention. (Study of Polyphenon E in men with high-grade prostatic intraepithelial neoplasia, Protocol IDs: MCC-15008, R01 CA12060-01A1, NCT00596011).
McLarty et al. [86] published the results of a study aimed to evaluate the effect of short term (median treatment period 34.5 days) GTCs administration in a population of PCa diagnosed patients, scheduled for radical prostatectomy. The supplementation, performed with an high, well-tolerated, Polyphenon E dose (1.3 g GTCs/day) produced a significant decrease of biomarkers relevant for PCa development, like HGF (hepatocyte growth factor), VEGF (vascular endothelial growth factor), IGF-1 (insulin growth factor-1) and IGFBP-3 (insulin growth factor binding protein-3). These results support a potential GTCs role in PCa chemoprevention and treatment in early confined stage.
Disappointingly, the positive Polyphenon E influence resulted not statistically significative according to the results of a randomized, double-blind, placebo-controlled trial, having a study design strictly close to that of McLarty [85]. The reason(s) for the discrepancy between the two studies remains to be elucidated, but it should be considered that the lack of a control group in the single arm study made it easier to gain statistical significance. On the other hand, it may indicate that future studies would need a larger population to show a statistically significant difference in systemic biomarkers and Polyphenon E, or generally standardized GTCs formulation, should be preferentially tested in longer-term intervention studies and in a precancerous model, where its effects have a best chance to be demonstrated.
5. Molecular Mechanisms for Green Tea Polyphenols Anticancer Activity in PCa: An Emerging Role for Epigenetics
5.1. Modulation of Epigenetic Mechanisms
In the last five years, GTCs have been shown to be able to modulate all the principal epigenetic mechanisms: DNA methylation, regulation of chromatin structure (through histone posttranslational modifications), and alterations of noncoding miRNAs. Epigenetics is defined as changes in gene expression that do not involve changes in the DNA sequence. Importantly, these changes are both reversible and heritable through division of somatic cells, making epigenetic regulation of gene expression a dynamic process that plays a crucial role in a vast number of biological processes including development, cell differentiation, stem cell maintenance, and tissue homeostasis [87]. Deregulation of epigenetic mechanisms is found in numerous diseases and in virtually every kind of cancer. This observation, together with the dynamicity, and therefore the potential reversibility, of epigenetic mechanisms sparked the interest in developing epigenetic drugs capable to modulate DNA methylation and chromatin structure in cancer cells. In the last few decades, several epigenetic drugs have been developed and are now either tested in clinical trials or already established in the clinical care, even if the drugs, besides modulating epigenetic mechanisms, appear to exert a certain degree of cytotoxicity.
The observation that GTCs, together with other dietary polyphenols, can regulate specific epigenetic features of premalignant and malignant cells opens new possibilities for epigenetic therapy.
5.2. DNA Methylation
DNA methylation is an important epigenetic determinant in gene expression. As a matter of fact, it participates in the maintenance of DNA integrity and stability, in chromosomal modification, and development of mutations. Generally, DNA hypermethylation is associated with genes inactivation and global genomic hypomethylation is associated to chromosomal instability induction. The DNA methylation occuring on the carbon-5 position of cytosine residues within a CpG dinucleotide sequence, represents the most studied epigenetic marker. In normal tissues, the process shows a bivalent function: CpG sites clustered in regulatory regions, within promoters and enhancers (CpG islands), are usually unmethylated, allowing for genes to be expressed; while sparse CpG sites (throughout the genome) are usually methylated, contributing to genome stability [88]. DNA methylation is crucially dysregulated in the vast majority of cancers, where CpG islands become hypermethylated, causing silencing of many genes, involved in cell cycle regulation, tumor suppression, DNA repair enzymes, receptors activity, and apoptosis, while the sparse CpG sites are usually subject to hypomethylation, favoring genomic instability, a common feature of cancer etiology [89].
Epigenetic analysis offers a potential noninvasive blood marker, complementary to PSA, for a preliminary PCa diagnosis. Since inhibition of DNA methyltransferases (DNMTs) may prevent hypermethylation and silencing of tumor suppressor key genes, it is reasonable to suppose that enzyme inhibition, along with histone deacetylase activation, may contribute to cancer treatment and or carcinogenesis prevention.
In human prostate cancer PC3 cells, as well as in other cell lines, Fang et al. [90] demonstrated that EGCG decreases total DNMTs activity, leading to reactivation of several genes silenced by methylation, such as retinoic acid receptor beta (RARb), which expression results increased by lowering the methylation levels of its promoter. This is the first demonstration of such an activity of a commonly consumed dietary constituent.
Recently, it has been reported that exposure of human prostate cancer LnCaP cells to GTCs causes a time-and concentration-dependent reactivation of the expression of the gluthathione-S-transferase p1 gene (GSTP1) [91]. The gene, mostly studied for methylation in PCa, results hypermethylated in almost 90% of the tumors [92]; in fact, it is recognized as a molecular PCa hallmark. Cells treatment with GTCs results in GSTP1 promoter demethylation, associated with a significant reduction of DNMT1 activity. Interestingly, the treatment does not cause global sparse CpG sites hypomethylation, contributing to maintain genome stability. The authors present GTCs as excellent candidates for epigenetic chemoprevention against PCa, even more favorable than the most commonly DNMT inhibitors employed, such as 5-aza-2′deoxycitidine.
There is growing evidence that the epigenetic mechanisms that impact DNA methylation and histone status, also contribute to genomic instability. Instead, GTCs administration lacks of toxicity, do not reactivate the prometastatic gene S100P, as a reverse response of 5-aza-2′deoxycitidine administration, and is able to alter chromatin modeling by histone acetylation (the second global epigenetic mechanism of gene regulation, see below) [91].
Almost at the same time, Morey Kinney et al. [93] showed that GTCs administration to TRAMP mice (spontaneously developing prostate adenocarcinoma) had very little effect on DNA methylation. They have found that 5-methyl-deoxycitosine level, together with methylation levels of B1 repetitive elements and the Mage-a8 gene, that are correlated to the phenomenon, remained unchanged in wild-type and TRAMP mice prostate. Also, they performed a genome-wide DNA methylation profiling of the HpaII tiny fragment enrichment by ligation-mediated PCR (HELP) [94] and found no significant hypomethylation. Their study, however, arises some doubts on the interpretation of the data: most of the analysis is directed towards sparse CpG site, rather than CpG islands. Indeed, the lack of global hypomethylation in GTCs fed mice agree with the above results obtained in LnCaP cells [91]. Second, but most important, in their study, GTCs treatment did not cause chemopreventive effects on PCa development, in contrast with previous reports by other authors [19, 53]. The different composition of the GTCs preparation used by Morey Kinney et al. [93] might account for this discrepancy, since it contained only about half of the ECGC concentration (35%) relative to the other polyphenols, when compared to the preparations used in other studies (51.9% in [19] and 62% in [53]).
Among the still growing number of experiments aimed to clarify the GTCs power as epigenetic drugs against PCa, there is plenty of evidence that the GTCs can interfere with DNA methylation pathways in vitro, leading, also, to the reactivation of the tumor suppressor p16, known to be involved in the cell cycle regulation [95].
Three different molecular mechanisms, at least, have been proposed to explain GTCs effects on the DNA methylation process (Figure 2). In human models, GTCs are demonstrated to be readily methylated, by catechol-O-methyltransferase (COMT) which utilizes S-Adenosyl-L-methionine (SAM) as a methyl donor, producing equimolar concentration of S-adenosyl-L-homocysteine (SAH). It should be noted that SAM is an indispensable methyl donor for DNMTs and SAH is a potent inhibitor of DNMTs activity. Since GTCs administration causes both decreased SAM and increased SAH cellular concentrations, the hypothesis is proposed that GTCs may act by perturbing the regulatory physiological homeostasis of the two molecules [96].
Figure 2.
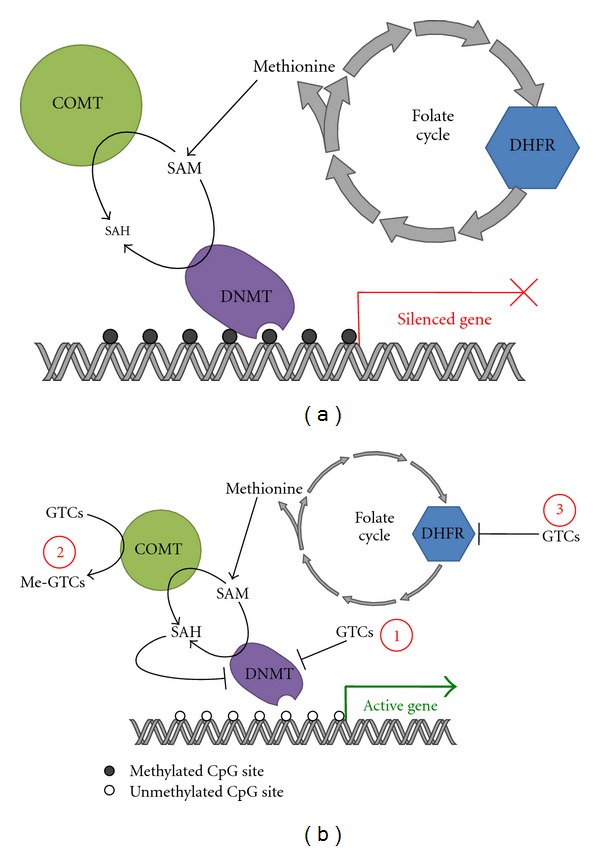
Mechanisms of inhibition of DNMTs by GTCs. (a) In a cancer environment, specific genes are silenced by hypermethylation of their promoters. DNA hypermethylation is catalysed by DNMTs, which use as a substrate SAM and release SAH as a by-product. (b) Within this context GTCs are able to inhibit DNMTs through three distinct mechanisms. (1) Direct inhibition. (2) GTCs are methylated by COMT, resulting in a depletion of SAM and accumulation of the DNMT inhibitor SAH. (3) Direct inhibition of DHFR, resulting in disruption of the folate cycle that influences negatively the levels of SAM. Inhibition of DNMTs ultimately results in DNA hypomethylation and re-expression of previously repressed genes.
This mechanistic explanation is supported by Lee et al. [97]. The authors, utilizing multiple modelling tools, have shown that the inhibitory effect exerted by several polyphenols on human DNMT1 is increased after in vitro COMT addition. In these experiments, EGCG resulted the much more potent DNMT1 inhibitor, able to act also independently of the COMT presence. In addition to the indirect action on DNA methylation demonstrated by [96], EGCG is able to inhibit DNMT1 by directly binding to the enzyme [90], with the gallic acid moiety of the molecule playing a crucial role in the interaction (Figure 2).
In human cell lines, EGCG is shown to act as an antifolate compound and disturb the folic acid metabolism by inhibiting dihydrofolate reductase. Since folic acid physiologically modulates DNA methylation, Navarro-Peran et al. [95] hypothesize that many molecular effects imparted by EGCG administration might be explained by this kind of interference with DNA methylation (Figure 2).
5.3. Chromatin Structure
Changes in the chromatin condensation, a crucial mechanism to control gene expression in eukaryotic cells [98], is regulated by at least eight posttranslational modifications of histones, such as acetylation, methylation, phosphorylation, poly-ADP-ribosylation, ubiquitination, glycosylation, and so forth, predominantly occurring on lysine residues [99]. Combinations of the different modifications constitute the histone code that defines the actual or potential transcriptional state [100] through proteins-DNA interaction, which in turns regulates gene expression.
Among the most studied processes, histone acetylation/deacetylation is finely regulated by balancing the activity of acetyl-transferases (HATs) and histone deacetylases (HDACs) enzyme families; inhibition of the latter rapidly results in histone hyperacetylation. Acetyl groups added to lysine residues by HATs, mainly on H3 and H4 histones, neutralizes aminoacid positive charge and consequently loosens DNA binding to histone complexes, ultimately resulting in chromatin relaxation and gene activation [99].
Being HDACs overexpression implicated in protecting cancer cells from genotoxic insults, many HDAC inhibitors synthesized in order to favor tumor suppressor genes re-expression and silence DNA repair pathways, are gaining a momentum as a novel cancer therapy. Raiendran et al. [101], however, indicate how dietary phytochemicals, by affecting the epigenome, and also can trigger DNA damage and repair mechanisms.
With regard to PCa, Pandey et al. [91] have observed a time-dependent inhibition of the total HDACs activity, after GTCs administration to LnCaP cells, correlating with mRNA and HDAC1-2-3 classes decrease.
Recently, GTCs administration was shown to inhibit HDAC1 activity and protein expression in PCa cell lines as LnCaP (harboring wild-type p53) and p53-null PC3 (lacking p53). When administered together a proteasome inhibitor, GTCs effect resulted prevented, indicating that HDAC1 is proteasoma degradated. HDAC1 inhibition was followed by up regulation of the cell cycle inhibitors WAF1/p21, ultimately inducing G0/G1 phase cell cycle arrest and apoptosis in both cell lines, irrespective of their p53 status [102].
A sustained DNA damage response, coupled with insufficient repair mechanisms, may be a pivotal mechanism for apoptosis induction in cancer cells exposed to dietary phytochemicals. The above findings provide new insight into the GTCs mechanisms, suggesting a novel approach to prevention and/or therapy.
5.4. microRNAs
microRNAs (miRNAs) comprise a novel class of endogenous, small, noncoding RNAs that control gene expression by acting on target mRNAs for degradation and/or translational repression. Transcription of miRNA genes and protein-coding genes shares common regulatory mechanisms: miRNA genes can be embedded in the introns of protein coding genes or can derive from their own transcript units in intergenic regions of the genome. When miRNA genes are located within introns of protein-coding genes, primary miRNA biogenesis is controlled by the same transcriptional mechanisms as the parent gene. In contrast, an independent miRNA gene will have its own transcriptional controls. Interestingly, multiple miRNAs can be produced within a single transcript, each of which can act independently [103, 104]. The long primary nuclear miR transcript (“pri-miRNA”) undergoes maturation by the RNase-III Drosha/Dgcr8 enzyme complex, generating a precursor miRNA (“pre-miRNA”) that is exported from the nucleus by exportin-5 [105]. A second cleavage takes place in the cytoplasm, involving the action of a complex containing another RNAase III enzyme named Dicer [106], to generate a single-strand mature miRNA. Mature miRNA, is usually incorporated in RNA-Induced Silencing Complex, a complex of proteins that is responsible for silencing the target mRNA [107]. Translational inhibition or degradation of targeted mRNA transcripts is due to imperfect or perfect base pairing between positions 2 to 8 from the 5′miRNA (also known as the seed sequence), with the 3′Un-Translated Region of their target mRNAs [104].
miRNAs are emerging as important regulators of gene expression and we expect them to be key players in intracellular signalling, thus enabling to close gaps of knowledge in molecular pathways. Pathogenic roles of miRNAs were initially described in cancer and miRNAs have now become a hot topic in medical research [108].
5.5. microRNA in PCa
To our knowledge, by employing miRNA microarray analysis, 7 miRNAs (miR-145, miR-141, miR-125, miR-1, miR-133, miR-106b, and miR-16) have been found downregulated in PCa [109–111]. In addition, the chromosomal region containing the miR-15/16 cluster and miR-101 is often lost during PCa progression. Table 1 summarizes recent publications on miRNAs in PCa.
Table 1.
miRNAs involved in PCa.
Bonci et al. [112] demonstrated that reduced levels of miR-15/16 are associated with PCa growth and due to an increase in the protein levels of their target genes Bcl2, cyclin D1, and WNT3A. Conversely, overexpression of miR-15/16 suppresses tumor growth and induces its regression. Expression of miR-101 inversely correlates with upregulation of its target, enhancer of zezte homolog 2 (Ezh2), which is highly expressed in CRPC [113].
miR-331-3p and miR-449a are downregulated in PCa, contributing to cancer growth by overexpressing their targets ErbB2 [114] and HDAC1, respectively [115]. Poliseno et al. [116] demonstrated that miR-22 and the miR-106b~25 cluster are overexpressed in PCa, and potentiate cellular transformation both in vitro and in vivo. Intronic miR-106b~25 cluster cooperates with its host gene MCM7 in cellular transformation both in vitro and in vivo.
Other tumor suppressor miRNAs in PCa are miR-34 expecially in p53-deficient PCa cells [117] and miR-330, which suppresses E2F-1 and E2F-1-mediated AKT phosphorylation [118]. In PCa cells, miR-21 is induced by stimulation of androgen receptors and mediates hormone-dependent and -independent cell growth [119]; in contrast, miR-221 increases in androgen-independent tumors [120]. MiR-146a, which is reduced in androgen-independent PCa cells, inhibits proliferation and cell invasion by targeting of RhO-activated protein Kinase 1 [121].
miRNAs that have been detected in human serum and plasma specimens, and circulating miRNA profiles, have now been associated with cancer, as an emerging class of diagnostic and prognostic biomarkers. Specifically in PCa, the expression of miR-141 has been found to be elevated in the plasma of PCa patients [122], where levels of miR-141 in PCa were able to predict tumor progression, when compared with other validated prostate biomarkers [123]. Finally, miR-141, miR-298, and miR-375 were also found to be upregulated at differential levels in the serum of men with CPRC [124].
5.6. microRNAs in PCa and Polyphenols
Recently, studies support a growing interest in the chemopreventive role of dietary agents such as polyphenols, as modulators of miRNA profiles in cancer progression and prevention [125]; (Table 2).
Table 2.
miRNAs modulated by polyphenols in PCa.
Although the chemopreventive effects of EGCG, as well as other polyphenols, have been largely demonstrated in PCa by us [19, 59, 126] and other groups (reviewed in [127]), only few reports discussed the regulatory effect of miRNA expression by dietary components in PCa. At the moment, the first and only study that clearly correlated miRNA-regulation with catechins treatment in PCa is performed by Siddiqui et al. [41]. The authors studying the role of androgen receptor (AR) in both early and advanced stage of PCa etiology, showed that the androgen-regulated miRNA-21 and the tumor suppressor miRNA-330, commonly regarded to play a role in PCa, are, respectively, down- and upregulated in a xenograft mice model for PCa, following EGCG treatment. These findings strengthen EGCG as an androgen receptor signalling antagonist, which can block AR gene expression and cell growth in the human PCa cells. EGCG is suggested as a chemotherapeutic agent against CPRC.
Basing on experiments performed in pancreatic cells with the phytochemical substance curcumin by Bao et al. [128], it appears worthwhile investigating the EGCG effect on miR-200 and miR-21 expression in PCa cells. Interestingly, the increase of the two above miRNAs is correlated to the induction of the critical tumor suppressor gene PTEN, frequently defective in PCa.
A study examined the effect of the phytochemical compound genistein on minichromosome maintenance (MCM) genes, commonly dysregulated in cancer, showing that MCM2 expression genes is higher in PCa samples, whereas miR-1296 was significantly downregulated [129]. Genistein induced miR-1296 expression and subsequently downregulated the MCM2 expression, along with cell cycle arrest in S-phase. This study is worth of our attention, since we demonstrated a dramatic MCM7 genes suppression by GTCs in the prostate of TRAMP mice [59]; analyses of therapeutic effect of catechins on miR-1296 and on miR-106b~25 cluster, request further investigations.
Finally, utilizing miRNA microarrays, Dhar et al. [130] found that miR-17-92 and miR-106ab clusters, with well-recognized oncogenic properties, were significantly down-regulated after treatment with the phytochemical compound resveratrol.
To corroborate the hypothesis that EGCG may act as a negative tumorigenesis process, downregulating oncogenes miRNAs and/or upregulating tumor suppressor genes miRNAs, more definitive informations are needed.
6. Conclusion and Future Perspective
Despite the routinary employment of intermediate-risk prognosticators such as PSA, Gleason score, and T-category, PCa remains a complicate malignancy, exhibiting high heterogeneity features both when present in latent, clinically indolent, and in progressively more aggressive stages, leading to the “hormone refractory state” (CRPC). This is the most devastating PCa form, representing the terminal stage of transition from the androgen dependence stage, against which, at the moment, no curative therapy exists.
Although radical prostatectomy is curative in the majority of patients with clinically localized PCa, up to 40% of them fail to respond to local therapy and develop PSA recurrence, as a sign of metastatic growth; ultimately, many of these patients die from their disease. While latent PCa appears similarly frequent in men with different ethnic background, residing in culturally diverse geographic locations, experimental evidences show that the quality of diet and lifestyle factors may carry the mechanisms triggering the transition to the “hormone-refractory state” and, at the same time, may be the target for the action of anticancer prevention.
These considerations support the increasing use of complementary and/or alternative therapies such as developing a diet-based combinatorial approach. Chemopreventive action of a naturally occurring, nontoxic agent, such as green tea polyphenols, could be useful in the PCa management. Just a postponement of the “hormone-refractory state” or the maintenance of the androgen dependence would produce chronic instead of terminal PCa. On the other hand, there is growing consensus that a large subset of patients do not require aggressive treatment. Recent preclinical evidences showing that GTCs can inhibit cell cycle, induce apoptosis, and modulate several signalling pathways, strengthened by experiments with animal models, have demonstrated the possible utilization of these compounds in selected human PCa stages for preventing its development (Table 3).
Table 3.
Principal progressive phases in human PCa development.
| Definition | Acronyms | STAGE of PCa development | STAGE where CTCs could be possibly used |
|---|---|---|---|
| Proliferative Inflammatory Atrophy | PIA | Precursor of cancer initiation | YES |
| Prostatic Intraepithelial Neoplasia | MILD PIN | PreMalignancy | YES |
| HIGH PIN | Malignancy | YES | |
| Adenocarcinoma | WD | Well-differentiated Cancer | NO |
| PD | POOR differentiated Cancer | NO | |
| Fully Developed Metastatic Neoplasia | CRPC | Hormone refractory castration resistant Terminal cancer | NO |
To date, although additional investigations are needed, a real cancer preventive activity by tea polyphenols has not been consistently observed in the few studies with humans versus the several studies with animal models so far achieved. Table 4 suggests two main differences between humans and animals studies: (1) relatively low quantities of GTCs intake by patients, as compared to TRAMP mice (it is conceivable that GTCs availability in the prostate is different in mouse than in humans; on the other hand pharmacokinetics studies demonstrate EGCG limited systemic concentrations); (2) various confounding factors (as genetic differences, diet, lifestyle and, etc.) present during the patients treatment, unlike the controlled conditions to which animals are subjected to optimize cancer prevention effect.
Table 4.
Studies on the effect of GTCs administration to TRAMP mice and humans with PCa.
| Experimental groups | GTCs dose g/100 mL [oral administration] | Formulation (%) | PCa phases age-dependent (weeks) | PCa inhibition (%) | References |
|---|---|---|---|---|---|
| TRAMP mice | 0.06 | GTCsa | Start 5; end 12 | 83 | [61] |
| EGCG (93) | |||||
| 0.1 | GTCsb: | Start 8; end 32 | 42 | [53] | |
| EGCG (62) | |||||
| ECG (24) | |||||
| EGC (5) | |||||
| EC (6) | |||||
| 0.1 | GTCsb: | [62] | |||
| EGCG (62) | Start 6; end 38 | ~50 | |||
| ECG (24) | |||||
| EGC (5) | Start 18; end 24 | ~20 | |||
| EC (6) | |||||
| 0.3 | GTCsc: | Start 8; end 24 | 80 | [19] | |
| EGC (5.5), | |||||
| EC (12.2) | |||||
| EGCG (51.9) | |||||
| ECG (6.1) | |||||
|
| |||||
| GTCs dose mg/day [oral administration] | GTCs providers | PCa phases | Percentage (%) of PCa inhibition | References | |
|
| |||||
| HUMANS | 500 | Sabinsa Corporation | CRPC | No effect | [63] |
| 6000 | Unilever | CRPC | No effect | [64] | |
| 1300 | Polyphenon E Matsui Norin, | CRPC | Mild effect | [86] | |
| 600 | Polyphenon E Matsui Norin | HGPIN | 33 | [65] | |
| 800 | Polyphenon E Matsui Norin | CRPC | No effect | [85] | |
GTCsa: Roche, GTCsb: natural resources and products, GTCsc: isolated by the investigators; CRPC (castration resistant prostate cancer), HGPIN (high grade prostatic intraepithelial neoplasia).
In the future, efforts will be needed to monitor GTCs administration to humans starting from the standardization of optimal GTCs compositions and doses, and continuing by enhancing GTCs bioavailability through nanocapsules or liposome deliver (nanochemoprevention). Simultaneous check of plasmatic and bioptic EGCG levels will be indispensable; it remains to be determined whether EGCG undergo autooxidation in the prostate gland, as it occurs in cell culture.
Certainly novel biomarkers focused not only on PCa epithelial cells but also on cell interaction with the extracellular microenvironment, that will improve the ability to detect PCa, predict lethality, and monitor response to therapies (many markers validated in experimental studies are not yet introduced as routine diagnostics).
Substantial information on tea polyphenols chemopreventive activity will emerge from long-term and rigorous clinical trials on well-designed cohorts of patients. Although it may appear simplistic that clinical PCa heterogeneity is attributable to underlying molecular heterogeneity, clinical trials still have to consider the need of cohorts with definite cancer risk, classified by their gene expression signature, that represents a clinically relevant genetic biomarker, independent of the current diagnostic variables. PCa develops via a limited number of alternatively preferred genetic pathways, providing genetic subtype (molecular classification) that may constitute a framework for investigating PCa and possibly explain the clinical heterogeneity of the disease with regard to both tumor progression and therapeutic response.
Conclusively, to avoid difficulties in the interpretation of the data, the future tea polyphenols protocols should take into account the following: (1) standardization in the preparation of the substances employed, choice of their doses and concentrations, systematically tested in plasma as well in biopsies; (2) more accurate stratification of a large number of the patients utilized for the analysis and selection of an appropriate treatment duration.
Acknowledgment
The authors would like to thank the Istituto Nazionale Biostrutture e Biosistemi (INBB), Roma, Italy, Polyphenon Pharma, New York, USA, Yorkshire Cancer Research (Project Grant Y256), and The Freemasons' Grand Charity for financial support of Davide Pellacani.
Abbreviations
- AR:
Androgen receptor
- PCa:
Prostate cancer
- EC:
Epicatechin
- ECG:
(-)-Epicatechin-3-gallate
- EC:
(-)-Epicatechin
- EGC:
(-)-Epigallocatechin
- EGCG:
(-)-Epigallocatechin-3-gallate
- GTCs:
Green tea catechins
- miRNA:
MicroRNAs
- ROS:
Reactive oxygen species
- PSA:
Prostatic specific antigen
- TRAMP:
Transgenic adenocarcinoma of mouse prostate.
References
- 1.Moyad MA, Carroll PR. Lifestyle recommendations to prevent prostate cancer—part I: time to redirect our attention? Urologic Clinics of North America. 2004;31(2):289–300. doi: 10.1016/j.ucl.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Namiki M, Akaza H, Lee SE, et al. Prostate cancer working group report. Japanese Journal of Clinical Oncology. 2010;40(1):i70–i75. doi: 10.1093/jjco/hyq130. [DOI] [PubMed] [Google Scholar]
- 3.DeWeese TL, Hruszkewycz AM, Marnett LJ. Oxidative stress in chemoprevention trials. Urology. 2001;57(4):137–140. doi: 10.1016/s0090-4295(00)00959-6. [DOI] [PubMed] [Google Scholar]
- 4.Konishi N, Shimada K, Ishida E, Nakamura M. Molecular pathology of prostate cancer. Pathology International. 2005;55(9):531–539. doi: 10.1111/j.1440-1827.2005.01865.x. [DOI] [PubMed] [Google Scholar]
- 5.Nelson WG, De Marzo AM, DeWeese TL, et al. The role of inflammation in the pathogenesis of prostate cancer. Journal of Urology. 2004;172(5):S6–S12. doi: 10.1097/01.ju.0000142058.99614.ff. [DOI] [PubMed] [Google Scholar]
- 6.Minelli A, Bellezza I, Conte C, Culig Z. Oxidative stress-related aging: a role for prostate cancer? Biochimica et Biophysica Acta. 2009;1795(2):83–91. doi: 10.1016/j.bbcan.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Balentine DA, Wiseman SA, Bouwens LCM. The chemistry of tea flavonoids. Critical Reviews in Food Science and Nutrition. 1997;37(8):693–704. doi: 10.1080/10408399709527797. [DOI] [PubMed] [Google Scholar]
- 8.Valcic S, Burr JA, Timmermann BN, Liebler DC. Antioxidant chemistry of green tea catechins. New oxidation products of (-)-epigallocatechin gallate and (-)-epigallocatechin from their reactions with peroxyl radicals. Chemical Research in Toxicology. 2000;13(9):801–810. doi: 10.1021/tx000080k. [DOI] [PubMed] [Google Scholar]
- 9.Sang S, Tian S, Meng X, et al. Theadibenzotropolone A, a new type pigment from enzymatic oxidation of (-)-epicatechin and (-)-epigallocatechin gallate and characterized from black tea using LC/MS/MS. Tetrahedron Letters. 2002;43(40):7129–7133. [Google Scholar]
- 10.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced Drug Delivery Reviews. 2001;46(1–3):3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 11.Mak JC. the potential role of green tea catechins in various disease therapies: progress and promise. Clinical and Experimental Pharmacology and Physiology. 2012;39(3):265–273. doi: 10.1111/j.1440-1681.2012.05673.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Zhang HY. Cancer preventive mechanisms of the green tea polyphenol (-)-epigallocatechin-3-gallate. Molecules. 2007;12(5):946–957. doi: 10.3390/12050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adhami VM, Ahmad N, Mukhtar H. Molecular targets for green tea in prostate cancer prevention. Journal of Nutrition. 2003;133(7):2417S–2424S. doi: 10.1093/jn/133.7.2417S. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JJ, Bailey HH, Mukhtar H. Green tea polyphenols for prostate cancer chemoprevention: a translational perspective. Phytomedicine. 2010;17(1):3–13. doi: 10.1016/j.phymed.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan N, Adhami VM, Mukhtar H. Review: green tea polyphenols in chemoprevention of prostate cancer: preclinical and clinical studies. Nutrition and Cancer. 2009;61(6):836–841. doi: 10.1080/01635580903285056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddiqui IA, Saleem M, Adhami VM, Asim M, Mukhtar H. Tea beverage in chemoprevention and chemotherapy of prostate cancer. Acta Pharmacologica Sinica. 2007;28(9):1392–1408. doi: 10.1111/j.1745-7254.2007.00693.x. [DOI] [PubMed] [Google Scholar]
- 17.Yang CS, Wang H, Li GX, Yang Z, Guan F, Jin H. Cancer prevention by tea: evidence from laboratory studies. Pharmacological Research. 2011;64(2):113–122. doi: 10.1016/j.phrs.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Gupta S, Ahmad N, Nieminen AL, Mukhtar H. Growth inhibition, cell-cycle dysregulation, and induction of apoptosis by green tea constituent (-)-epigallocatechin-3-gallate in androgen-sensitive and androgen-insensitive human prostate carcinoma cells. Toxicology and Applied Pharmacology. 2000;164(1):82–90. doi: 10.1006/taap.1999.8885. [DOI] [PubMed] [Google Scholar]
- 19.Caporali A, Davalli P, Astancolle S, et al. The chemopreventive action of catechins in the TRAMP mouse model of prostate carcinogenesis is accompanied by clusterin over-expression. Carcinogenesis. 2004;25(11):2217–2224. doi: 10.1093/carcin/bgh235. [DOI] [PubMed] [Google Scholar]
- 20.Albrecht DS, Clubbs EA, Ferruzzi M, Bomser JA. Epigallocatechin-3-gallate (EGCG) inhibits PC-3 prostate cancer cell proliferation via MEK-independent ERK1/2 activation. Chemico-Biological Interactions. 2008;171(1):89–95. doi: 10.1016/j.cbi.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. Journal of the National Cancer Institute. 1997;89(24):1881–1886. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- 22.Gupta S, Hussain T, Mukhtar H. Molecular pathway for (-)-epigallocatechin-3-gallate-induced cell cycle arrest and apoptosis of human prostate carcinoma cells. Archives of Biochemistry and Biophysics. 2003;410(1):177–185. doi: 10.1016/s0003-9861(02)00668-9. [DOI] [PubMed] [Google Scholar]
- 23.Hastak K, Gupta S, Ahmad N, Agarwal MK, Agarwal ML, Mukhtar H. Role of p53 and NF-κB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene. 2003;22(31):4851–4859. doi: 10.1038/sj.onc.1206708. [DOI] [PubMed] [Google Scholar]
- 24.Hastak K, Agarwal MK, Mukhtar H, Agarwal ML. Ablation of either p21 or Bax prevents p53-dependent apoptosis induced by green tea polyphenol epigallocatechin-3-gallate. FASEB Journal. 2005;19(7):789–791. doi: 10.1096/fj.04-2226fje. [DOI] [PubMed] [Google Scholar]
- 25.Siddiqui IA, Malik A, Adhami VM, et al. Green tea polyphenol EGCG sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated apoptosis and synergistically inhibits biomarkers associated with angiogenesis and metastasis. Oncogene. 2008;27(14):2055–2063. doi: 10.1038/sj.onc.1210840. [DOI] [PubMed] [Google Scholar]
- 26.Chung LY, Cheung TC, Kong SK, et al. Induction of apoptosis by green tea catechins in human prostate cancer DU145 cells. Life Sciences. 2001;68(10):1207–1214. doi: 10.1016/s0024-3205(00)01020-1. [DOI] [PubMed] [Google Scholar]
- 27.Nair S, Li W, Kong ANT. Natural dietary anti-cancer chemopreventive compounds: redox-mediated differential signaling mechanisms in cytoprotection of normal cells versus cytotoxicity in tumor cells. Acta Pharmacologica Sinica. 2007;28(4):459–472. doi: 10.1111/j.1745-7254.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- 28.Na HK, Surh YJ. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food and Chemical Toxicology. 2008;46(4):1271–1278. doi: 10.1016/j.fct.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Chen C, Yu R, Owuor ED, Tony Kong AN. Activation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Archives of Pharmacal Research. 2000;23(6):605–612. doi: 10.1007/BF02975249. [DOI] [PubMed] [Google Scholar]
- 30.Nair S, Barve A, Khor TO, et al. Regulation of Nrf2-and AP-1-mediated gene expression by epigallocatechin-3-gallate and sulforaphane in prostate of Nrf2-knockout or C57BL/6J mice and PC-3 AP-1 human prostate cancer cells. Acta Pharmacologica Sinica. 2010;31(9):1223–1240. doi: 10.1038/aps.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hussain T, Gupta S, Adhami VM, Mukhtar H. Green tea constituent epigallocatechin-3-gallate selectively inhibits COX-2 without affecting COX-1 expression in human prostate carcinoma cells. International Journal of Cancer. 2005;113(4):660–669. doi: 10.1002/ijc.20629. [DOI] [PubMed] [Google Scholar]
- 32.Adhami VM, Malik A, Zaman N, et al. Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo. Clinical Cancer Research. 2007;13(5):1611–1619. doi: 10.1158/1078-0432.CCR-06-2269. [DOI] [PubMed] [Google Scholar]
- 33.Vayalil PK, Katiyar SK. Treatment of epigallocatechin-3-gallate inhibits matrix metalloproteinases-2 and -9 via inhibition of activation of mitogen-activated protein kinases, c-jun and NF-κB in human prostate carcinoma DU-145 cells. Prostate. 2004;59(1):33–42. doi: 10.1002/pros.10352. [DOI] [PubMed] [Google Scholar]
- 34.Li M, He Z, Ermakova S, et al. Direct inhibition of insulin-like growth factor-I receptor kinase activity by (-)-epigallocatechin-3-gallate regulates cell transformation. Cancer Epidemiology Biomarkers and Prevention. 2007;16(3):598–605. doi: 10.1158/1055-9965.EPI-06-0892. [DOI] [PubMed] [Google Scholar]
- 35.Thomas F, Patel S, Holly JMP, Persad R, Bahl A, Perks CM. Dihydrotestosterone SEnsitises LNCaP cells to death induced by epigallocatechin-3-Gallate (EGCG) or an IGF-I receptor inhibitor. Prostate. 2009;69(2):219–224. doi: 10.1002/pros.20873. [DOI] [PubMed] [Google Scholar]
- 36.Siddiqui IA, Adhami VM, Afaq F, Ahmad N, Mukhtar H. Modulation of phosphatidylinositol-3-kinase/protein kinase B- and mitogen-activated protein kinase-pathways by tea polyphenols in human prostate cancer cells. Journal of Cellular Biochemistry. 2004;91(2):232–242. doi: 10.1002/jcb.10737. [DOI] [PubMed] [Google Scholar]
- 37.Saraon P, Jarvi K, Diamandis EP. Molecular alterations during progression of prostate cancer to androgen independence. Clinical Chemistry. 2011;57:1366–1375. doi: 10.1373/clinchem.2011.165977. [DOI] [PubMed] [Google Scholar]
- 38.Ryan CJ, Tindall DJ. Androgen receptor rediscovered: the new biology and targeting the androgen receptor therapeutically. Journal of Clinical Oncology. 2011;29:3651–3658. doi: 10.1200/JCO.2011.35.2005. [DOI] [PubMed] [Google Scholar]
- 39.Basu S, Tindall DJ. Androgen action in prostate cancer. Hormones and Cancer. 2010;1(5):223–228. doi: 10.1007/s12672-010-0044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedrich MJ. Debate continues on use of PSA testing for early detection of prostate cancer. Journal of the American Medical Association. 2011;305(22):2273–2276. doi: 10.1001/jama.2011.777. [DOI] [PubMed] [Google Scholar]
- 41.Siddiqui IA, Asim M, Hafeez BB, Adhami VM, Tarapore RS, Mukhtar H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB Journal. 2011;25(4):1198–1207. doi: 10.1096/fj.10-167924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chuu CP, Chen RY, Kokontis JM, Hiipakka RA, Liao S. Suppression of androgen receptor signaling and prostate specific antigen expression by (-)-epigallocatechin-3-gallate in different progression stages of LNCaP prostate cancer cells. Cancer Letters. 2009;275(1):86–92. doi: 10.1016/j.canlet.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren F, Zhang S, Mitchell SH, Butler R, Young CYF. Tea polyphenols down-regulate the expression of the androgen receptor in LNCaP prostate cancer cells. Oncogene. 2000;19(15):1924–1932. doi: 10.1038/sj.onc.1203511. [DOI] [PubMed] [Google Scholar]
- 44.Pezzato E, Sartor L, Dell’Aica I, et al. Prostate carcinoma and green tea: PSA-triggered basement membrane degradation and MMP-2 activation are inhibited by (-)epigallocatechin-3-gallate. International Journal of Cancer. 2004;112(5):787–792. doi: 10.1002/ijc.20460. [DOI] [PubMed] [Google Scholar]
- 45.Siddiqui IA, Zaman N, Aziz MH, et al. Inhibition of CWR22Rν1 tumor growth and PSA secretion in athymic nude mice by green and black teas. Carcinogenesis. 2006;27(4):833–839. doi: 10.1093/carcin/bgi323. [DOI] [PubMed] [Google Scholar]
- 46.Huss WJ, Maddison LA, Greenberg NM. Autochthonous mouse models for prostate cancer: past, present and future. Seminars in Cancer Biology. 2001;11(3):245–259. doi: 10.1006/scbi.2001.0373. [DOI] [PubMed] [Google Scholar]
- 47.Sartor L, Pezzato E, Donà M, et al. Prostate carcinoma and green tea: (-)epigallocatechin-3-gallate inhibits inflammation-triggered MMP-2 activation and invasion in murine tramp model. International Journal of Cancer. 2004;112(5):823–829. doi: 10.1002/ijc.20496. [DOI] [PubMed] [Google Scholar]
- 48.Liao S, Umekita Y, Guo J, Kokontis JM, Hiipakka RA. Growth inhibition and regression of human prostate and breast tumors in athymic mice by tea epigallocatechin gallate. Cancer Letters. 1995;96(2):239–243. doi: 10.1016/0304-3835(95)03948-v. [DOI] [PubMed] [Google Scholar]
- 49.Zhou JR, Yu L, Zhong Y, Blackburn GL. Soy phytochemicals and tea bioactive components synergistically inhibit androgen-sensitive human prostate tumors in mice. Journal of Nutrition. 2003;133(2):516–521. doi: 10.1093/jn/133.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greenberg NM, DeMayo F, Finegold MJ, et al. Prostate cancer in a transgenic mouse. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(8):3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dilworth SM. Cell alterations induced by the large T-antigens of SV40 and polyoma virus. Seminars in Cancer Biology. 1990;1(6):407–414. [PubMed] [Google Scholar]
- 52.Sontag E, Fedorov S, Kamibayashi C, Robbins D, Cobb M, Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell. 1993;75(5):887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- 53.Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(18):10350–10355. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adhami VM, Siddiqui IA, Ahmad N, Gupta S, Mukhtar H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Research. 2004;64(23):8715–8722. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- 55.Kaplan PJ, Mohan S, Cohen P, Foster BA, Greenberg NM. The insulin-like growth factor axis and prostate cancer: lessons from the transgenic adenocarcinoma of mouse prostate (TRAMP) model. Cancer Research. 1999;59(9):2203–2209. [PubMed] [Google Scholar]
- 56.Saleem M, Adhami VM, Ahmad N, Gupta S, Mukhtar H. Prognostic significance of metastasis-associated protein S100A4 (Mts1) in prostate cancer progression and chemoprevention regimens in an autochthonous mouse model. Clinical Cancer Research. 2005;11(1):147–153. [PubMed] [Google Scholar]
- 57.Siddiqui IA, Shukla Y, Adhami VM, et al. Suppression of NFκB and its regulated gene products by oral administration of green tea polyphenols in an autochthonous mouse prostate cancer model. Pharmaceutical Research. 2008;25(9):2135–2142. doi: 10.1007/s11095-008-9553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Azzali G. Tumor cell transendothelial passage in the absorbing lymphatic vessel of transgenic adenocarcinoma mouse prostate. American Journal of Pathology. 2007;170(1):334–346. doi: 10.2353/ajpath.2007.060447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCarthy S, Caporali A, Enkemann S, et al. Green tea catechins suppress the DNA synthesis marker MCM7 in the TRAMP model of prostate cancer. Molecular Oncology. 2007;1(2):196–204. doi: 10.1016/j.molonc.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davalli P, Rizzi F, Caldara GF, et al. Chronic administration of green tea extract to TRAMP mice induces the collapse of Golgi apparatus in prostate secretory cells and results in alterations of protein post- translational processing. Journal of Clinical Oncology. 2011;39:1521–1527. doi: 10.3892/ijo.2011.1136. [DOI] [PubMed] [Google Scholar]
- 61.Harper CE, Patel BB, Wang J, Eltoum IA, Lamartiniere CA. Epigallocatechin-3-gallate suppresses early stage, but not late stage prostate cancer inTRAMP mice: mechanisms of action. Prostate. 2007;67(14):1576–1589. doi: 10.1002/pros.20643. [DOI] [PubMed] [Google Scholar]
- 62.Adhami VM, Siddiqui IA, Sarfaraz S, et al. Effective prostate cancer chemopreventive intervention with green tea polyphenols in the TRAMP model depends on Thestage of the disease. Clinical Cancer Research. 2009;15(6):1947–1953. doi: 10.1158/1078-0432.CCR-08-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choan E, Segal R, Jonker D, et al. A prospective clinical trial of green tea for hormone refractory prostate cancer: an evaluation of the complementary/alternative therapy approach. Urologic Oncology. 2005;23(2):108–113. doi: 10.1016/j.urolonc.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 64.Jatoi A, Ellison N, Burch PA, et al. A phase II trial of green tea in the treatment of patients with androgen independent metastatic prostate carcinoma. Cancer. 2003;97(6):1442–1446. doi: 10.1002/cncr.11200. [DOI] [PubMed] [Google Scholar]
- 65.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Research. 2006;66(2):1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 66.Brausi M, Rizzi F, Bettuzzi S. Chemoprevention of human prostate cancer by green tea catechins: two years later. A follow-up update. European Urology. 2008;54(2):472–473. doi: 10.1016/j.eururo.2008.03.100. [DOI] [PubMed] [Google Scholar]
- 67.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer Journal for Clinicians. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 68.Bray F, Lortet-Tieulent J, Ferlay J, Forman D, Auvinen A. Prostate cancer incidence and mortality trends in 37 European countries: an overview. European Journal of Cancer. 2010;46(17):3040–3052. doi: 10.1016/j.ejca.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 69.Studer UE, Collette L. Prostate cancer: to screen or not to screen? Nature Reviews Urology. 2009;6(6):299–301. doi: 10.1038/nrurol.2009.92. [DOI] [PubMed] [Google Scholar]
- 70.Crawford ED. Understanding the epidemiology, natural history, and key pathways involved in prostate cancer. Urology. 2009;73(5):S4–S10. doi: 10.1016/j.urology.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 71.Diamandis EP. Early prostate cancer antigen-2: a controversial prostate cancer biomarker? Clinical Chemistry. 2010;56(4):542–544. doi: 10.1373/clinchem.2009.140061. [DOI] [PubMed] [Google Scholar]
- 72.Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, Mack TM. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. British Journal of Cancer. 1991;63(6):963–966. doi: 10.1038/bjc.1991.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haenszel W, Kurihara M. Studies of Japanese migrants. I. Mortality from cancer and other diseases among Japanese in the United States. Journal of the National Cancer Institute. 1968;40(1):43–68. [PubMed] [Google Scholar]
- 74.Zheng J, Yang B, Huang T, Yu Y, Yang J, Li D. Green tea and black tea consumption and prostate cancer risk: an exploratory meta-analysis of observational studies. Nutrition and Cancer. 2011;63(5):663–672. doi: 10.1080/01635581.2011.570895. [DOI] [PubMed] [Google Scholar]
- 75.Severson RK, Nomura AMY, Grove JS, Stemmermann GN. A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Research. 1989;49(7):1857–1860. [PubMed] [Google Scholar]
- 76.Allen NE, Sauvaget C, Roddam AW, et al. A prospective study of diet and prostate cancer in Japanese men. Cancer Causes and Control. 2004;15(9):911–920. doi: 10.1007/s10552-004-1683-y. [DOI] [PubMed] [Google Scholar]
- 77.Jian L, Xie LP, Lee AH, Binns CW. Protective effect of green tea against prostate cancer: a case-control study in southeast China. International Journal of Cancer. 2004;108(1):130–135. doi: 10.1002/ijc.11550. [DOI] [PubMed] [Google Scholar]
- 78.Sonoda T, Nagata Y, Mori M, et al. A case-control study of diet and prostate cancer in Japan: possible protective effect of traditional Japanese diet. Cancer Science. 2004;95(3):238–242. doi: 10.1111/j.1349-7006.2004.tb02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kikuchi N, Ohmori K, Shimazu T, et al. No association between green tea and prostate cancer risk in Japanese men: the Ohsaki Cohort study. British Journal of Cancer. 2006;95(3):371–373. doi: 10.1038/sj.bjc.6603230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kurahashi N, Sasazuki S, Iwasaki M, Inoue M. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. American Journal of Epidemiology. 2008;167(1):71–77. doi: 10.1093/aje/kwm249. [DOI] [PubMed] [Google Scholar]
- 81.Chow HHS, Cai Y, Alberts DS, et al. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and Polyphenon E. Cancer Epidemiology Biomarkers and Prevention. 2001;10(1):53–58. [PubMed] [Google Scholar]
- 82.Chow HHS, Cai Y, Hakim IA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clinical Cancer Research. 2003;9(9):3312–3319. [PubMed] [Google Scholar]
- 83.Chow HHS, Hakim IA, Vining DR, et al. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of polyphenon E in healthy individuals. Clinical Cancer Research. 2005;11(12):4627–4633. doi: 10.1158/1078-0432.CCR-04-2549. [DOI] [PubMed] [Google Scholar]
- 84.Henning SM, Aronson W, Niu Y, et al. Tea polyphenols and theaflavins are present in prostate tissue of humans and mice after green and black tea consumption. Journal of Nutrition. 2006;136(7):1839–1843. doi: 10.1093/jn/136.7.1839. [DOI] [PubMed] [Google Scholar]
- 85.Nguyen MM, Ahmann FR, Nagle RB, et al. Randomized, double-blind, placebo controlled trial of polyphenon E in prostate cancer patients before prostatectomy: evaluation of potential chemopreventive activities. Cancer Prevention Research. 2012;5:290–298. doi: 10.1158/1940-6207.CAPR-11-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McLarty J, Bigelow RLH, Smith M, Elmajian D, Ankem M, Cardelli JA. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prevention Research. 2009;2(7):673–682. doi: 10.1158/1940-6207.CAPR-08-0167. [DOI] [PubMed] [Google Scholar]
- 87.Portela A, Esteller M. Epigenetic modifications and human disease. Nature Biotechnology. 2010;28(10):1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- 88.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genetics. 2003;33:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 89.Feinberg AP, Tycko B. The history of cancer epigenetics. Nature Reviews Cancer. 2004;4(2):143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 90.Fang MZ, Wang Y, Ai N, et al. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Research. 2003;63(22):7563–7570. [PubMed] [Google Scholar]
- 91.Pandey M, Shukla S, Gupta S. Promoter demethylation and chromatin remodeling by green tea polyphenols leads to re-expression of GSTP1 in human prostate cancer cells. International Journal of Cancer. 2010;126(11):2520–2533. doi: 10.1002/ijc.24988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meiers I, Shanks JH, Bostwick DG. Glutathione S-transferase pi (GSTP1) hypermethylation in prostate cancer: review 2007. Pathology. 2007;39(3):299–304. doi: 10.1080/00313020701329906. [DOI] [PubMed] [Google Scholar]
- 93.Morey Kinney SR, Zhang W, Pascual M, et al. Lack of evidence for green tea polyphenols as DNA methylation inhibitors in murine prostate. Cancer Prevention Research. 2009;2(12):1065–1075. doi: 10.1158/1940-6207.CAPR-09-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Suzuki M, Greally JM. DNA methylation profiling using HpaII tiny fragment enrichment by ligation-mediated PCR (HELP) Methods. 2010;52(3):218–222. doi: 10.1016/j.ymeth.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Navarro-Perán E, Cabezas-Herrera J, Campo LSD, Rodríguez-López JN. Effects of folate cycle disruption by the green tea polyphenol epigallocatechin-3-gallate. International Journal of Biochemistry and Cell Biology. 2007;39(12):2215–2225. doi: 10.1016/j.biocel.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 96.Zhu BT, Patel UK, Cai MX, Conney AH. O-methylation of tea polyphenols catalyzed by human placental cytosolic catechol-o-methyltransferase. Drug Metabolism and Disposition. 2000;28(9):1024–1030. [PubMed] [Google Scholar]
- 97.Lee WJ, Shim JY, Zhu BT. Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Molecular Pharmacology. 2005;68(4):1018–1030. doi: 10.1124/mol.104.008367. [DOI] [PubMed] [Google Scholar]
- 98.Kornberg RD, Lorch Y. Chromatin-modifying and -remodeling complexes. Current Opinion in Genetics and Development. 1999;9(2):148–151. doi: 10.1016/S0959-437X(99)80022-7. [DOI] [PubMed] [Google Scholar]
- 99.Kouzarides T. Chromatin Modifications and Their Function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 100.Turner BM. Cellular memory and the histone code. Cell. 2002;111(3):285–291. doi: 10.1016/s0092-8674(02)01080-2. [DOI] [PubMed] [Google Scholar]
- 101.Rajendran P, Ho E, Williams DE, Dashwood RH. Dietary phytochemicals, HDAC inhibition, and DNA damage/repair defects in cancer cells. Clinical Epigenetics. 2011;3(1, article 4) doi: 10.1186/1868-7083-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thakur VS, Gupta K, Gupta S. Green tea polyphenols causes cell cycle arrest and apoptosis in prostate cancer cells by suppressing class I histone deacetylases. Carcinogenesis. 2012;33:377–384. doi: 10.1093/carcin/bgr277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zeng Y, Cullen BR. Recognition and cleavage of primary microRNA transcripts. Methods in Molecular Biology. 2006;342:49–56. doi: 10.1385/1-59745-123-1:49. [DOI] [PubMed] [Google Scholar]
- 104.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 105.Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes and Development. 2003;17(24):3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nature Reviews Genetics. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 107.Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell. 2005;123(4):631–640. doi: 10.1016/j.cell.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 108.Couzin J. MicroRNAs make big impression in disease after disease. Science. 2008;319(5871):1782–1784. doi: 10.1126/science.319.5871.1782. [DOI] [PubMed] [Google Scholar]
- 109.Ambs S, Prueitt RL, Yi M, et al. Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Research. 2008;68(15):6162–6170. doi: 10.1158/0008-5472.CAN-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ozen M, Creighton CJ, Ozdemir M, Ittmann M. Widespread deregulation of microRNA expression in human prostate cancer. Oncogene. 2008;27(12):1788–1793. doi: 10.1038/sj.onc.1210809. [DOI] [PubMed] [Google Scholar]
- 111.Porkka KP, Pfeiffer MJ, Waltering KK, Vessella RL, Tammela TLJ, Visakorpi T. MicroRNA expression profiling in prostate cancer. Cancer Research. 2007;67(13):6130–6135. doi: 10.1158/0008-5472.CAN-07-0533. [DOI] [PubMed] [Google Scholar]
- 112.Bonci D, Coppola V, Musumeci M, et al. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nature Medicine. 2008;14(11):1271–1277. doi: 10.1038/nm.1880. [DOI] [PubMed] [Google Scholar]
- 113.Varambally S, Cao Q, Mani RS, et al. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science. 2008;322(5908):1695–1699. doi: 10.1126/science.1165395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Epis MR, Giles KM, Barker A, Kendrick TS, Leedman PJ. miR-331-3p regulates ERBB-2 expression and androgen receptor signaling in prostate cancer. Journal of Biological Chemistry. 2009;284(37):24696–24704. doi: 10.1074/jbc.M109.030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Noonan EJ, Place RF, Pookot D, et al. MiR-449a targets HDAC-1 and induces growth arrest in prostate cancer. Oncogene. 2009;28(14):1714–1724. doi: 10.1038/onc.2009.19. [DOI] [PubMed] [Google Scholar]
- 116.Poliseno L, Salmena L, Riccardi L, et al. Identification of the miR-106b∼25 microRNA cluster as a proto-oncogenic PTEN-targeting intron that cooperates with its host gene MCM7 in transformation. Science Signaling. 2010;3(117):p. ra29. doi: 10.1126/scisignal.2000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rokhlin OW, Scheinker VS, Taghiyev AF, Bumcrot D, Glover RA, Cohen MB. MicroRNA-34 mediates AR-dependent p53-induced apoptosis in prostate cancer. Cancer Biology and Therapy. 2008;7(8):1288–1296. doi: 10.4161/cbt.7.8.6284. [DOI] [PubMed] [Google Scholar]
- 118.Lee KH, Chen YL, Yeh SD, et al. MicroRNA-330 acts as tumor suppressor and induces apoptosis of prostate cancer cells through E2F1-mediated suppression of Akt phosphorylation. Oncogene. 2009;28(38):3360–3370. doi: 10.1038/onc.2009.192. [DOI] [PubMed] [Google Scholar]
- 119.Ribas J, Ni X, Haffner M, et al. miR-21: an androgen receptor-regulated microRNA that promotes hormone-dependent and hormone-independent prostate cancer growth. Cancer Research. 2009;69(18):7165–7169. doi: 10.1158/0008-5472.CAN-09-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun T, Wang Q, Balk S, Brown M, Lee GSM, Kantoff P. The role of microrna-221 and microrna-222 in Androgen- independent prostate cancer cell lines. Cancer Research. 2009;69(8):3356–3363. doi: 10.1158/0008-5472.CAN-08-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lin SL, Chiang A, Chang D, Ying SY. Loss of mir-146a function in hormone-refractory prostate cancer. RNA. 2008;14(3):417–424. doi: 10.1261/rna.874808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gonzales JC, Fink LM, Goodman OB, Jr., Symanowski JT, Vogelzang NJ, Ward DC. Comparison of circulating MicroRNA 141 to circulating tumor cells, lactate dehydrogenase, and prostate-specific antigen for determining treatment response in patients with metastatic prostate cancer. Clinical Genitourinary Cancer. 2011;9:39–45. doi: 10.1016/j.clgc.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 124.Selth LA, Townley S, Gillis JL, et al. Discovery of circulating microRNAs associated with human prostate cancer using a mouse model of disease. doi: 10.1002/ijc.26405. International Journal of Cancer. In press. [DOI] [PubMed] [Google Scholar]
- 125.Schmid HP, Fischer C, Engeler DS, Bendhack ML, Schmitz-Dräger BJ. Nutritional aspects of primary prostate cancer prevention. Recent Results in Cancer Research. 2011;188:101–107. doi: 10.1007/978-3-642-10858-7_8. [DOI] [PubMed] [Google Scholar]
- 126.Scaltriti M, Belloni L, Caporali A, et al. Molecular classification of green tea catechin-sensitive and green tea catechin-resistant prostate cancer in the TRAMP mice model by quantitative real-time PCR gene profiling. Carcinogenesis. 2006;27(5):1047–1053. doi: 10.1093/carcin/bgi287. [DOI] [PubMed] [Google Scholar]
- 127.Yang CS, Wang X. Green tea and cancer prevention. Nutrition and Cancer. 2010;62:931–937. doi: 10.1080/01635581.2010.509536. [DOI] [PubMed] [Google Scholar]
- 128.Bao B, Ali S, Banerjee S, et al. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Research. 2012;72:335–345. doi: 10.1158/0008-5472.CAN-11-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Majid S, Dar AA, Saini S, et al. Regulation of minichromosome maintenance gene family by MicroRNA-1296 and genistein in prostate cancer. Cancer Research. 2010;70(7):2809–2818. doi: 10.1158/0008-5472.CAN-09-4176. [DOI] [PubMed] [Google Scholar]
- 130.Dhar S, Hicks C, Levenson AS. Resveratrol and prostate cancer: promising role for microRNAs. Molecular Nutrition and Food Research. 2011;55(8):1219–1229. doi: 10.1002/mnfr.201100141. [DOI] [PubMed] [Google Scholar]


