Abstract
Despite the escalating prevalence in the aging population, few therapeutic options exist to treat patients with peripheral arterial disease. Application of intermittent pneumatic leg compressions (IPC) is regarded as a promising noninvasive approach to treat this condition, but the clinical efficacy, as well the mechanistic basis of action of this therapy, remain poorly defined. We tested the hypothesis that 2 wk of daily application of IPC enhances exercise tolerance by improving blood flow and promoting angiogenesis in skeletal muscle in a model of peripheral arterial insufficiency. Male Sprague-Dawley rats were subjected to bilateral ligation of the femoral artery and randomly allocated to treatment or sham groups. Animals were anesthetized daily and exposed to 1-h sessions of bilateral IPC or sham treatment for 14–16 consecutive days. A third group of nonligated rats was also studied. Marked increases in treadmill exercise tolerance (∼33%, P < 0.05) and improved muscle performance in situ (∼10%, P < 0.05) were observed in IPC-treated animals. Compared with sham-treated controls, blood flow measured with isotope-labeled microspheres during in situ contractions tended to be higher in IPC-treated animals in muscles composed of predominantly fast-twitch white fibers, such as the plantaris (∼93%, P = 0.02). Capillary contacts per fiber and citrate synthase activity were not significantly altered by IPC treatment. Collectively, these data indicate that IPC improves exercise tolerance in a model of peripheral arterial insufficiency in part by enhancing blood flow to collateral-dependent tissues.
Keywords: peripheral arterial disease, intermittent pneumatic compressions, exercise tolerance
peripheral arterial disease (PAD) affects nearly 8 million individuals in the United States (35) and is increasingly prevalent among the elderly (30). One of the most striking features of the disease is the marked deterioration in functional capacity and poor quality of life experienced by these individuals (27, 28). Compared with healthy counterparts, patients with PAD have lower levels of ambulatory activity and greatly diminished exercise tolerance (15, 40). Surprisingly, despite the escalating prevalence, few of the noninvasive and surgical treatment options for PAD have been shown to promote meaningful long-term improvements in functional performance in these patients. For example, despite promoting modest short-term benefits (26, 29), surgical revascularization is costly, can only be performed in a selected group of patients, and often requires recurrent hospitalizations for repeated procedures (25). Likewise, supervised exercise training, thought to be the best single option for symptomatic individuals, is not accessible for the vast majority of the patients (34). Therefore, there is a clear need to develop more accessible and cost-effective therapies for patients with PAD.
Application of intermittent pneumatic compressions (IPC) recently emerged as a promising treatment option for patients with PAD (9, 12, 19, 33). This treatment consists of daily sessions of compressions applied to the foot and calf or calf only at pressures ranging from 65 to 120 mmHg (9, 12, 19, 33). Since IPC is a home-based therapy, it essentially overcomes many of the limitations associated with the other therapeutic options. Furthermore, this strategy is easily applicable, does not require direct supervision, and is painless, which increases the chances of patient adherence to the treatment (10). Most importantly, available randomized clinical trials with stable claudicants also indicate that long-term IPC treatment might improve functional capacity, limb hemodynamics, and quality of life in these patients (9, 12, 19, 33).
Despite the growing excitement with this novel approach, very few studies have been devoted to determine the physiological adaptations to long-term IPC therapy (45). Furthermore, none of the available trials employed a placebo-controlled design, making it difficult to discern the real benefits of this therapy. We have recently developed a model in rats to study the mechanistic basis associated with IPC application (36, 37). Using a preclinical model of peripheral arterial insufficiency, we first documented that a single session of compressions upregulates the expression of inflammatory chemokines in the compressed skeletal muscle [monocyte chemoattractant protein-1 (MCP-1) and chemokine (C-X-C motif) ligand 1 (CXCL-1)] and isolated segments of a major collateral artery (MCP-1) (36). Given the key role of these factors in vascular remodeling and inflammation, these initial studies prompted us to evaluate the long-term consequences of repeated exposures to IPC treatment.
In the present study, we evaluated the impact of 14–16 days of daily IPC on 1) skeletal muscle performance, 2) exercise tolerance, 3) blood flow, 4) oxidative capacity, and 5) capillary contacts per fiber in a preclinical model of peripheral arterial insufficiency. Based on our previous findings (36, 37) and other reports (45), we hypothesized that IPC would improve blood flow capacity and increase capillary contacts per fiber in the leg of treated animals. We further predicted that, combined, these adaptations would enhance exercise tolerance of treated animals compared with sham-treated controls.
METHODS
Animals.
Seventy male Sprague-Dawley rats (body weight 300–350 g) were used in the present investigation. Animals were housed in pairs in temperature (24°C) and light-controlled (12:12-h light-dark cycle) rooms. Rat chow and water were available ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Missouri.
Femoral artery ligation.
Bilateral ligation of the femoral artery was performed as described previously (32). Animals were anesthetized with a mixture of ketamine (100 mg/kg) and acepromazine (0.5 mg/kg), and their femoral arteries were exposed and isolated immediately distal to the inguinal ligament. A ligature (3–0 silk) was placed tightly around each femoral artery ∼3 mm distal to the inguinal ligament. In the nonligated control animals, the same surgical procedure was performed except for the ligation of the artery. The animals were allowed 10 days of recovery before the experiments. In this model, calf blood flow in sedentary animals reaches near peak values within 7–10 days after ligation of the femoral artery (32).
Experimental design.
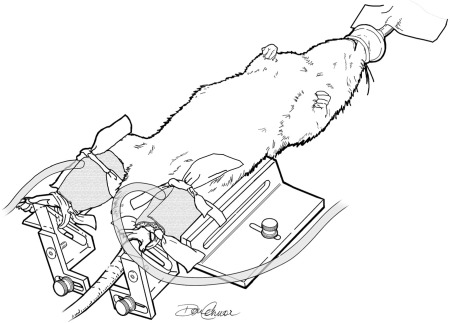
Ten days following bilateral ligation of the femoral artery, rats were randomly allocated to either a ligated IPC-treated group or to a ligated sham-treated group. Rats were anesthetized daily with isoflurane (VetOne) and placed on a heated custom-made stage (Fig. 1). Two small tapered cuffs were firmly wrapped around the calf and secured in place with elastic rubber bands (Thera-Band; The Hygenic, Akron, OH). The inflatable bladders in the cuffs were designed to compress the entire circumference of the leg. The feet were also secured on a pedal to prevent cuff displacement during the therapy application. The cuffs were then connected to the pneumatic pump, and the IPC treatment was applied for 1 h. The temperature of the animals was checked periodically and maintained between 37 and 38°C. This protocol was repeated daily in groups of three animals at a time for 14–16 consecutive days. Animals in the ligated sham-treated group underwent the exact same procedures, except for the application of compressions. A third group of nonligated control animals was also studied. These animals underwent a sham surgery and remained in their cages for the duration of the study. Functional measurements and tissue sampling were performed ∼24 h following the completion of the last treatment/sham session. The experimental design is outlined in Fig. 2. In the first subset of animals (n = 35), we studied muscle performance in situ. At the end of the in situ protocol, blood flow to the left calf muscles was determined in all ligated animals (ligated sham treated and ligated IPC treated) with isotope labeled microspheres. In addition, muscle samples from the right leg were harvested for determination of citrate synthase activity in eight nonligated controls, ligated sham treated, and eight ligated IPC treated. Since IPC/sham treatment was applied to three animals at a time, but we could only kill two animals per day, the treatment duration varied between 14 and 16 days in this protocol. A second subset of animals (n = 17) (center of Fig. 2) was used for determination of muscle capillary contacts per fiber using histochemical analysis. Finally, as shown in Fig. 2, treadmill exercise tolerance was determined in a third subset of animals (n = 18).
Fig. 1.
Schematic illustration of the setup used for intermittent pneumatic compressions (IPC) application. Animals were kept under isoflurane anesthesia throughout the intervention period. Custom-made tapered cuffs (12 × 4, 5 × 3.5 cm) were snugly wrapped around the calves and secured in place using elastic bands.
Fig. 2.
Experimental design. Experiments ·were completed in 70 Sprague-Dawley rats divided in three subsets (n, no. of animals). In each of the three sets of experiments, rats were allocated to three groups: nonligated control, ligated sham treated, and ligated IPC treated. At the end of the in situ muscle performance experiments, blood flow was determined with isotope-labeled microspheres (left leg), and muscle samples were harvested for measurement of citrate synthase activity (right leg). *Two animals in the ligated sham-treated group and two animals in the ligated IPC-treated group were excluded from the blood flow analysis due to technical difficulties and/or inadequate mixing of the microspheres in the circulation. Blood flows were not determined in the nonligated controls. +Citrate synthase activity was only determined in randomly selected animals, as it was not necessary to measure this in all animals.
IPC device.
A custom version of the ArtAssist pump (ACI Medical, San Marcos, CA) was used for IPC application in the leg. This device applies 12 compressions/min (2-s inflation/3-s deflation) at a pressure of 120 mmHg. These parameters were chosen based on our laboratory's previous reports that a high frequency of compressions seems to evoke greater and more reproducible changes in the expression of inflammatory genes in the compressed muscle and in isolated segments of collateral arteries (36). Cuff inflation time with this device is ∼0.25 s, while deflation time is ∼0.33 s.
Muscle performance in situ.
Muscle performance was evaluated in the gastrocnemius-plantaris-soleus (GPS) group (24). Like in other mammals, the GPS muscles in rats are composed of different fiber types of contrasting physiological and biochemical properties (1). For example, the soleus muscle is composed of predominantly slow-twitch, red fibers (∼84%) that are resistant to fatigue, while the gastrocnemius muscle is largely composed of low-oxidative, fast-twitch white fibers that exhibit poor fatigue resistance (1). In this protocol, rats were initially anesthetized with inactin (100 mg/kg ip) and maintained on a 1–2% isoflurane-O2 mixture during the surgical procedures. Three catheters (polyethylene-50 tubing) were implanted as follows: one catheter was placed in the aortic arch via the left carotid artery for microsphere infusion and mean arterial pressure monitoring, a second catheter was placed in the tail artery for blood withdraw, and a third catheter was placed in the left jugular vein for supplemental anesthesia administration (inactin, 0.25 ml/h). The left GPS muscle group was then prepared for in situ stimulation, as described previously (24). Briefly, after the skin was removed from the entire hindlimb, the left trunk of the sciatic nerve was isolated, ligated, and cut. The calcaneal tendon was also carefully isolated, cut, and tied to a metal rod. To secure the limb, a piece of umbilical tape (J-25UB Jorgensen Laboratories, Loveland, CO) was inserted under the patellar tendon and firmly tied to a metal post. The animal was then transferred to a temperature-regulated (37°C) Plexiglas chamber, and the tendon was connected to a lever system (Series 300B Lever System, Cambridge Technology, Cambridge, MA) for measurement of tension development. The distal portion of the sciatic nerve was placed over a platinum electrode connected to a stimulator (model S48, Grass Instruments, Quincy, MA). Last, a thermometer probe was placed on the anterior-medial portion of the leg, and plastic film was gently wrapped around the leg to minimize water loss. The preparation was maintained at 36–37°C with a heat lamp throughout the entire experiment. After 10–15 min of equilibration, the GPS muscle complex was stimulated with supramaximal square waves (6 V, 0.1-ms duration), and the length that produced the maximal tension development was determined. An incremental protocol of twitch contractions at 1, 2, 3, 4, and 5 Hz with 10 min in each frequency was employed. At the end of the 5-Hz period, the frequency of stimulation was returned to 3 Hz for 5 min for determination of muscle blood flow.
Muscle blood flow determination.
Blood flow to individual muscles of the leg, which is dependent on the collateral circuit, was determined in the occluded animals at the end of the in situ protocol, when the muscles were contracting at a rate of 3 Hz. We chose this contraction frequency because it has been previously shown that blood flows to some muscle sections during isometric twitch contractions tend to decline at frequencies higher than 3 Hz due to mechanical impedance to flow (24). Stable isotope-labeled microspheres (Iridium, Lanthanum, Lutetium; BioPAL) of 15-μm diameter were used for blood flow studies (37). A suspension of microspheres was vortexed for 30 s and infused (∼0.5 ml) into the carotid catheter, followed by a saline flush over ∼20 s. About 10 s before the infusion of microspheres, withdrawal of the reference blood sample began at a rate of 500 μl/min via the caudal artery catheter. Adequate mixing of the microspheres in the circulation was verified by comparing flows from the left and right kidney (ratio of flows in left kidney to right kidney = 0.94 ± 0.07) (48). On completion of the protocol, rats were euthanized, and muscle samples from the left leg were carefully dissected, weighed, and placed immediately into sample vials for blood flow determination. The samples were dried overnight (at 37°C) and shipped to BioPhysics Assay Laboratory, for analysis (BioPAL, Worcester, MA) (37). Muscle samples were also dissected from the right leg for determination of citrate synthase activity (see below). Blood flows (ml·min−1·100 g−1) were calculated as:
| (1) |
where RBS is reference blood sample, and CPM is counts per minute (48).
Citrate synthase activity.
Muscle sections (∼100 mg) from the plantaris and red and white portions of the gastrocnemius were carefully dissected and snap frozen in liquid nitrogen and stored in −80°C. Citrate synthase activity was determined spectrophotometrically according to the method of Srere (41).
Histological analysis.
In the muscle capillarity experiments, rats were anesthetized with a mixture of ketamine (100 mg/kg) and acepromazine (0.5 mg/kg), and the tibialis anterior (TA) and plantaris muscles were harvested. Sections from the midportion of the muscles were mounted on a cork with tissue freezing medium and frozen in liquid nitrogen-cooled isopentane for later cutting of 10-μm-thick sections using a cryostat. For determination of muscle capillary contacts per fiber, frozen sections were fixed in acetone and stained for alkaline phosphatase (AP) (5). Sites of AP activity appear pink-red to red. For the analytic procedures, a square grid counting frame was placed over the image of a muscle section. Within each muscle section, all analyses were always performed on several different areas that were each in the same position relative to the long axis of the section and the section boundaries. Sections were examined using an Olympus BX60 photomicroscope (Olympus, Melville, NY) and photographed with Spot Insight digital camera (Diagnostic Instruments, Sterling Heights, MI). Capillary contacts per fiber were measured by counting three fields taken at ×20 magnification or a minimum of 100 fibers. A single operator who was blinded to treatment group's identities performed the selection of target areas, photography, and image analysis. The intraobserver coefficient of variation for capillary counting was 0.013%.
Exercise tolerance.
A graded treadmill exercise test to fatigue was employed for the assessment of exercise tolerance before and after the intervention, as described previously (52). Animals were familiarized with running on a motor-driven treadmill for 5 min/day (15–20 m/min) for 5 consecutive days before femoral artery ligation or sham surgery. Animals were allowed to recover from the surgery for 10 days, as described above. The exercise test consisted of three stages performed at a fixed incline (10% grade): animals began to run at 15 m/min for 10 min, followed by a second period of 10 min at 20 m/min and a final stage in which speed was increased to 25 m/min and kept constant. Animals were considered fatigued and removed from the treadmill when they could no longer maintain the treadmill speed, despite repeated encouragement achieved by electrical shocks. An experienced investigator, blinded to the animals' identity, was responsible for determining the point of fatigue.
Statistical analysis.
All statistical comparisons were performed using a commercially available software (Sigmaplot 11, Systat Software, San Jose, CA). Tension development, running capacity, and blood flow responses were compared using ANOVA for repeated measures and Tukey's procedure for post hoc analysis. Animal's characteristics, capillary density, and citrate synthase activity were compared between the ligated IPC-treated and ligated sham-treated groups using Student's t-tests. Significance was accepted at the P < 0.05 level. Values are presented as means ± SE.
RESULTS
Muscle performance in situ.
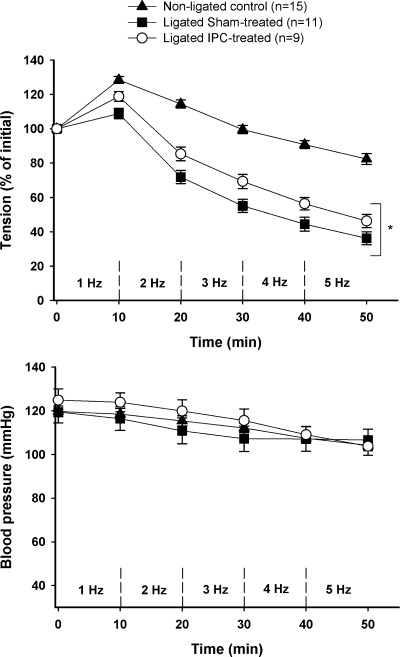
Tissue mass and initial muscle force development of the GPS muscle group are shown in Table 1. There were no differences between ligated sham-treated and ligated IPC-treated animals for initial tension development or tissue masses. Nonligated controls tended to be heavier (P = 0.08) and had greater calf muscle mass than the ligated counterparts (P < 0.05). The profile of muscle tension development and blood pressure responses to in situ stimulation are shown in Fig. 3. The ability to sustain tension development was markedly improved in the ligated IPC-treated group compared with ligated sham-treated animals (Fig. 3). When adjusting for possible differences in blood pressure, the estimated difference in tension development between ligated IPC treated and ligated sham treated was ∼10% (confidence interval 2.26, 16.21) (P = 0.012). As expected, tension development in nonligated controls was substantially higher than in both groups of ligated animals throughout the stimulation period (P < 0.01).
Table 1.
Body weight, tissue weight, and initial force development
| Nonligated Controls | Ligated Sham Treated | Ligated IPC Treated | |
|---|---|---|---|
| n | 15 | 11 | 9 |
| Body weight, g | 363 ± 4 | 345 ± 5 | 358 ± 8 |
| Muscles weight, mg | |||
| Soleus | 156 ± 2* | 136 ± 3 | 142 ± 4 |
| Plantaris | 456 ± 11* | 394 ± 4 | 401 ± 10 |
| Gastrocnemius | 2,182 ± 47* | 1,982 ± 36 | 1,972 ± 58 |
| Tibialis anterior | 729 ± 19‡ | 665 ± 18 | 647 ± 22 |
| Initial muscle tension, g | 360 ± 7† | 409 ± 14 | 386 ± 20 |
Values are means ± SE; n, no. of animals. IPC, intermittent pneumatic compressions. P < 0.05:
nonligated controls vs. ligated sham treated and ligated IPC treated;
nonligated controls vs. ligated sham-treated;
nonligated controls vs. ligated IPC treated.
Fig. 3.
Developed tension of the gastrocnemius-plantaris-soleus muscle group (top) and mean blood pressure (bottom) during in situ stimulation. Values are means ± SE; n, no. of animals. *P < 0.05 vs. ligated sham treated.
Exercise tolerance.
To further study the impact of IPC treatment on functional capacity, we performed a graded exercise test on a treadmill before and after the IPC/sham intervention. Body weight was similar between occluded groups before (sham treated: 336 ± 6 g and IPC treated: 344 ± 8 g, P = 0.4) and after (sham treated: 330 ± 6 g and IPC treated: 333 ± 5 g, P = 0.7) the intervention. Predictably, the net external work performed by both ligated groups during the incremental exercise test was similar. In agreement to what was observed in the in situ protocol, ligated IPC-treated animals had a marked improvement in exercise performance (Fig. 4). Compared with the pretreatment tests, animals in the ligated IPC-treated group showed significant increases in running time (P = 0.03), while in ligated sham treated there was a minor, nonsignificant change (P = 0.23). This ∼33% increase in exercise tolerance in ligated IPC-treated animals is especially meaningful, as the extension of running time was observed during running at the highest treadmill speed.
Fig. 4.
Exercise tolerance determined during a graded treadmill exercise test to fatigue. Values are means ± SE. *P < 0.05 vs. preintervention.
Muscle blood flow.
Blood flows to individual muscles of the distal hindlimbs are shown in Table 2. Two animals in the ligated sham-treated group and two animals in the ligated IPC-treated group were excluded due to technical difficulties and/or inadequate mixing of the microspheres in the circulation. For comparison, we have included blood flows obtained during treadmill exercise in this model by Prior and coworkers (32). Comparison with these reference blood flow values reveals that we were able to obtain near maximal blood flows in tissues composed of predominantly fast-twitch white fibers, such as the plantaris muscle and the white and mixed portions of the gastrocnemius using an in situ muscle stimulation protocol (Table 2). Exposure to IPC treatment led to a trend for improved blood flow across all muscle sections composed of fast-twitch white fibers (Table 2), but this increase was statistically significant only in the plantaris muscle. Plantaris muscle blood flow was nearly 94% higher in the ligated IPC-treated animals compared with ligated sham-treated controls (P = 0.02).
Table 2.
Blood flow to individual muscles in the leg during muscle contractions in situ
| Ligated Sham Treated | Ligated IPC Treated | Treadmill Blood Flows (from Ref. 32) | |
|---|---|---|---|
| n | 9 | 7 | |
| Soleus | 48 ± 12 | 45 ± 3 | 100 ± 6.3 |
| Plantaris | 31 ± 8 | 60 ± 9* | 58 ± 5.1 |
| Red gastrocnemius | 68 ± 15 | 84 ± 13 | 113 ± 6.1 |
| White gastrocnemius | 28 ± 8 | 36 ± 4 | 23 ± 2.2 |
| Mixed gastrocnemius | 43 ± 9 | 53 ± 4 | 42 ± 1.4 |
| Tibialis anterior | 34 ± 9 | 50 ± 11 | 73 ± 3.2 |
Values are means ± SE in ml · min−1 · 100 g−1; n, no. of animals.
P < 0.05 vs. ligated sham treated.
Oxidative capacity.
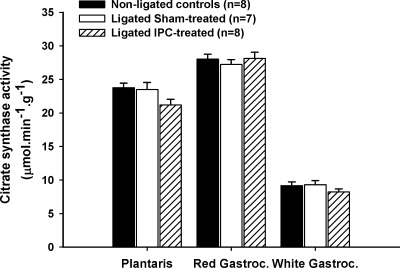
As shown in Fig. 5, the activity of citrate synthase, a well-accepted marker of oxidative capacity in rat skeletal muscle, was comparable between groups (Fig. 5).
Fig. 5.
Citrate synthase activity in the red and white muscle sections of the gastrocnemius and in the plantaris muscles. Values are means ± SE; n, no. of animals.
Capillary contacts per fiber.
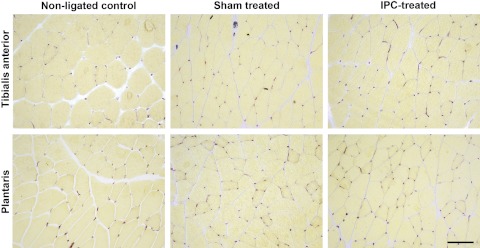
Representative micrographs of histochemical staining for AP in cross sections of the TA and plantaris muscles are shown in Fig. 6. The number of capillary contacts per fiber tended to be higher in the TA of ligated IPC-treated animals compared with the ligated sham-treated group, but the difference did not reach statistical significance (P = 0.16) (Fig. 7). No group differences were found for this variable in the plantaris muscle (P = 0.33).
Fig. 6.
Representative micrographs of histochemical staining for alkaline phosphatase in cross sections of the tibialis anterior and plantaris muscles. Scale bar = 50 μm.
Fig. 7.
Average capillary contacts per fiber in the tibialis anterior and plantaris muscles. Values are means ± SE; n, no. of animals.
DISCUSSION
This is the first sham-controlled study to investigate the effects of IPC therapy on muscle performance and blood flow in a preclinical model of peripheral artery insufficiency. We report that as little as 2 wk of daily IPC intervention enhances exercise tolerance and increase blood flow to the plantaris muscle in rats with bilateral ligation of the femoral artery. Furthermore, IPC does not seem to meaningfully impact muscle capillarization or oxidative capacity, at least when applied for this time duration. Collectively, these findings shed new light on the therapeutic potential of IPC for patients with PAD, as well as on the mechanistic basis behind the observed benefits.
Methodological considerations.
A major prerequisite for the translation of findings from preclinical studies is that the model employed mimics the pathophysiological characteristics of the disease observed in the clinical scenario. The animal model employed in this experiment has been well characterized and extensively used to investigate potential therapeutic approaches to treat patients with PAD (17, 46). One of the major advantage of this model is that, in accordance with what is observed clinically in patients with intermittent claudication, blood flow at rest is minimally altered, but it is impaired during increases in metabolic demand, such as during exercise (46). On the other hand, the animals utilized in the present study are relatively young, and, as opposed to the typical slow progression of luminal obstruction in atherosclerotic disease, these animals are subjected to an acute ischemic insult. Regarding the effect of age, it is worth noting that, in this model, age is no barrier for vascular adaptations evoked by interventions, such as exercise training or pharmacological therapy (47, 51). Hence, we believe that this model is useful to explore the physiological adaptations to novel therapeutic strategies such as IPC.
It has been suggested that the placebo effect in studies involving medical devices can be particularly enhanced (20). Thus, to make meaningful insights into the clinical value of mechanical instruments, such as pneumatic pumps, it is imperative that a sham-treated group is included in the study design. Recent trials with mechanical therapies, such as enhanced external counterpulsation, have begun to utilize a sham-controlled design in which patients assigned to the control group are exposed to a sham pump instead of no intervention (3). Unfortunately, none of the available randomized trials with IPC therapy in stable patients with intermittent claudication have administrated a sham treatment, which raises questions about the true efficacy of these devices (31). The use of preclinical models is advantageous in that regard. In the present study, ligated sham-treated animals underwent the exact same interventions of the ligated IPC-treated group, including cuff placement in the limbs, but did not receive the intermittent compressions. Accordingly, our results of improved exercise tolerance and muscle performance constitute the first unequivocal piece of evidence that IPC treatment improves functional exercise performance in peripheral arterial insufficiency.
Collateral-dependent blood flow.
A major goal of the present investigation was to dissect out the possible factors that underlie the observed changes in exercise performance with IPC treatment. Aiming to determine the possible contribution of collateral-dependent blood flow, we used isotope-labeled microspheres to estimate blood flow to the calf muscle during contractions (Table 2). A major strength of this approach, regarded as the gold standard for determination of collateral blood flow, is that exercise can efficiently minimize downstream resistance within the lower limb musculature, thereby allowing for a substantial increase in blood flow (2). As illustrated in Table 2, it is apparent that our muscle stimulation protocol was successful in producing meaningful changes in blood flow. Indeed, the blood flow values we report here for some of the calf muscles, such as the plantaris, and white and mixed portions of the gastrocnemius, are within the range previously documented in treadmill studies in this animal model (4, 23, 49) (Table 2). On the other hand, it should be highlighted that blood flows to muscles composed of predominantly red, slow-twitch fibers, such as the soleus, are not appreciably altered in this model, especially compared with reference values obtained in vivo (Table 2).
In the in situ preparation, about two-thirds of the contracting muscle mass is composed of fast-twitch white fibers, which are the most fatigable (13). Under the contraction conditions employed in this study, it is predictable that the progressive decline in tension development reflects largely the behavior of these fast-fatigable fibers (18, 43). Based on the oxygen cost of twitch contractions in this model (18), it is expected that oxygen delivery would be insufficient to meet the metabolic needs of the fast-twitch white fibers before that of the red and the slow-twitch muscle fibers (14), which comprise a minor portion of the contracting muscle mass. Thus the energy demand associated with this specific contraction protocol reflects adaptations evoked in the predominantly white sections of the calf muscle (43, 50). With these concepts in mind, it is fair to assume that the marked improvements in muscle performance in animals exposed to IPC therapy stem, at least partially, from an increased blood flow capacity of the predominantly white muscles. This is mostly evident in the plantaris muscle of ligated IPC-treated animals, in which blood flow was nearly 93% higher (P = 0.02) than in ligated sham controls. When combining these predominantly fast-twitch regions, blood flow in ligated IPC-treated animals was, on average, 45% higher than in the ligated sham-treated control group.
The exact mechanism underlying the observed changes in flow is unclear, but examination of prior studies offers some reasonable insights. Experiments in preclinical models (45) and anecdotal studies in PAD patients (44) have documented that long-term IPC therapy seems to enhance the density of the collateral arteries in the treated limbs. One prevailing view sustains that intermittent increases in blood flow and wall shear stress during IPC application could activate the endothelium and initiate the cascade of events associated with collateral growth and remodeling (8, 10, 11). Interestingly, our laboratory has recently shown that one single session of IPC upregulates the mRNA expression of MCP-1, a major player in the early phases of arteriogenesis (14), in isolated segments of collateral arteries in this rat model (36). Taken together, these findings encourage additional, sham-controlled studies to determine the impact of IPC therapy on collateral density in claudicants.
An alternative mechanism that could drive the observed changes in blood flow to the calf muscles is a potential improvement in vasomotor reactivity of the existing collateral arteries. Leg IPC application has been shown to promote local and systemic nitric oxide-dependent vasodilation (6, 22) and also evoke the release of vasoactive neuropeptides, such as substance P and calcitonin gene-related peptide (8). Furthermore, strategies such as exercise training have been shown to enhance endothelium-dependent vasodilation in isolated collaterals in this rat model (7). It is conceivable that the aforementioned episodic changes in blood flow and shear stress could evoke a similar adaptation following IPC therapy. Lending support to this notion, most of the available clinical trials have established that long-term IPC improve postexercise, but not resting ankle-brachial index (9, 19, 33). This has been taken as evidence that functional, but not structural, remodeling of the collateral arteries is the underlying cause of improved exercise performance in patients with intermittent claudication (9, 19, 33). In other words, the unaltered pressures downstream from the occlusion site(s) at rest after IPC treatment would indicate that collateralization is not sufficient to overcome the arterial insufficiency and that improved ankle-brachial indexes following exercise reflect improved functional vasodilation of the existing vessels. Clearly, additional work is necessary to determine the long-term impact of this therapy on vasomotor function.
Muscle capillarization and oxidative capacity.
Besides collateral-dependent blood flow, at least two main additional factors could play a role in the observed changes in muscle performance: increases in capillary contacts per fiber, and oxidative capacity. This is the first study to characterize the effect of IPC treatment on these two variables in a model of peripheral arterial insufficiency. The rationale for assessing changes in capillary contacts per fiber is based on the notion that muscle capillarization is a determinant of oxygen conductance across the muscle and, consequently, one critical factor involved in muscle performance (16). Importantly, our laboratory has previously reported that IPC application increases the expression of factors involved in vascular remodeling in the compressed skeletal muscle (36, 37, 42). Indeed, one single session of compression in this rat model induces a robust upregulation of the chemokines MCP-1 and CXCL-1 in the TA muscle (36). Given the well-known pivotal role of these factors in angiogenesis (21) and overall functional recovery from the ischemic insult in skeletal muscle (39), these observations prompted the hypothesis that repeated exposure to IPC therapy would result in enhanced capillarity in skeletal muscle. Of note, prior evidence in a rat model of soft tissue injury indicated that 2 wk of IPC therapy promote angiogenesis in the healing tendon (8). Contrary to our predictions, we only observed minor increases in capillary contacts per fiber in two of the compressed muscles. In fact, capillary contacts per fiber in the IPC-treated animals were only 7% higher in the TA and 5% higher in the plantaris compared with that in sham-treated controls. Although it is impossible to exclude the hypothesis that these small changes contributed to the improvement in functional capacity, we interpret these findings as an indication that other mechanisms played a major role. These results, combined with the lack of changes in oxidative capacity following the intervention (Fig. 5), suggest that collateral blood flow and/or other undetermined factors probably had a more decisive contribution to the observed improvement in muscle performance in treated animals. It is worth mentioning though that we opted for a rather brief treatment duration that might not have been sufficient to induce detectable changes in capillarity. Whether a more prolonged IPC treatment can indeed change muscle capillary contacts per fiber remains to be determined.
Limitations.
The IPC device employed in this experiment has a frequency of compressions (12 compressions/min) that is four times higher than the one used in most commercially available devices (9, 12, 19). This frequency was chosen based on our laboratory's previous reports that high-frequency IPC promotes greater and more consistent increases in chemokine expression in skeletal muscle compared with a lower frequency of stimulation (37, 38). It should be highlighted though that no clinical studies to date have examined the impact of compression frequency on clinically relevant outcomes in patients with PAD. Thus, given possible species differences in the physiological responses to IPC application, direct extrapolation of the findings from the present study to the clinical scenario requires caution. Further study is needed to establish the optimal IPC stimulation parameters, including frequency and pressure of compressions, for patients with PAD.
Conclusions.
We have shown for the first time that 2 wk of daily applications of intermittent pneumatic leg compressions produces significant improvements in exercise tolerance in a preclinical model of peripheral arterial insufficiency. This functional benefit appears to stem, at least in part, from improved blood flow to collateral-dependent tissues. These novel findings encourage additional efforts to determine the mechanistic basis behind the functional improvements associated with this novel therapeutic strategy.
GRANTS
This research was supported by National Institutes of Health Grants RR-18276 and HL-36088, and a predoctoral pilot grant award from the Clinical and Translational Science Institute at the University of Missouri to B. Roseguini. B. Roseguini is a Fulbright/Brazilian Ministry of Education (Capes) fellow.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.T.R., S.C.N., H.T.Y., R.L.T., and M.H.L. conception and design of research; B.T.R. and H.T.Y. performed experiments; B.T.R. and A.A.A.-E. analyzed data; B.T.R., A.A.A.-E., H.T.Y., R.L.T., and M.H.L. interpreted results of experiments; B.T.R. and A.A.A.-E. prepared figures; B.T.R. drafted manuscript; B.T.R., A.A.A.-E., S.C.N., H.T.Y., R.L.T., and M.H.L. edited and revised manuscript; B.T.R., A.A.A.-E., S.C.N., H.T.Y., R.L.T., and M.H.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Eric Gibson, Ann Melloh, Jennifer Casati, and Alexa Bermudez for invaluable technical assistance and Don Connor for the illustration.
REFERENCES
- 1. Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. Am J Anat 171: 259–272, 1984 [DOI] [PubMed] [Google Scholar]
- 2. Bondke A, Hillmeister P, Buschmann IR. Exact assessment of perfusion and collateral vessel proliferation in small animal models. Circ Res 100: e82–e83, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Braith RW, Conti CR, Nichols WW, Choi CY, Khuddus MA, Beck DT, Casey DP. Enhanced external counterpulsation improves peripheral artery flow-mediated dilation in patients with chronic angina: a randomized sham-controlled study. Circulation 122: 1612–1620, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carr AN, Howard BW, Yang HT, Eby-Wilkens E, Loos P, Varbanov A, Qu A, DeMuth JP, Davis MG, Proia A, Terjung RL, Peters KG. Efficacy of systemic administration of SDF-1 in a model of vascular insufficiency: support for an endothelium-dependent mechanism. Cardiovasc Res 69: 925–935, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Carson FL. Histotechnology: A Self-Instructional Text. Chicago, IL: American Society for Clinical Pathology, 1997 [Google Scholar]
- 6. Chen LE, Liu K, Qi WN, Joneschild E, Tan X, Seaber AV, Stamler JS, Urbaniak JR. Role of nitric oxide in vasodilation in upstream muscle during intermittent pneumatic compression. J Appl Physiol 92: 559–566, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Colleran PN, Li Z, Yang HT, Laughlin MH, Terjung RL. Vasoresponsiveness of collateral vessels in the rat hindlimb: influence of training. J Physiol 588: 1293–1307, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dahl J, Li J, Bring DK, Renstrom P, Ackermann PW. Intermittent pneumatic compression enhances neurovascular ingrowth and tissue proliferation during connective tissue healing: a study in the rat. J Orthop Res 25: 1185–1192, 2007 [DOI] [PubMed] [Google Scholar]
- 9. de Haro J, Acin F, Florez A, Bleda S, Fernandez JL. A prospective randomized controlled study with intermittent mechanical compression of the calf in patients with claudication. J Vasc Surg 51: 857–862, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Delis KT. The case for intermittent pneumatic compression of the lower extremity as a novel treatment in arterial claudication. Perspect Vasc Surg Endovasc Ther 17: 29–42, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Delis KT, Knaggs AL. Duration and amplitude decay of acute arterial leg inflow enhancement with intermittent pneumatic leg compression: an insight into the implicated physiologic mechanisms. J Vasc Surg 42: 717–725, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Delis KT, Nicolaides AN. Effect of intermittent pneumatic compression of foot and calf on walking distance, hemodynamics, and quality of life in patients with arterial claudication: a prospective randomized controlled study with 1-year follow-up. Ann Surg 241: 431–441, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol 80: 261–270, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Demicheva E, Hecker M, Korff T. Stretch-induced activation of the transcription factor activator protein-1 controls monocyte chemoattractant protein-1 expression during arteriogenesis. Circ Res 103: 477–484, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Gardner AW, Montgomery PS, Scott KJ, Afaq A, Blevins SM. Patterns of ambulatory activity in subjects with and without intermittent claudication. J Vasc Surg 46: 1208–1214, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hepple RT, Hogan MC, Stary C, Bebout DE, Mathieu-Costello O, Wagner PD. Structural basis of muscle O2 diffusing capacity: evidence from muscle function in situ. J Appl Physiol 88: 560–566, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Herzog S, Sager H, Khmelevski E, Deylig A, Ito WD. Collateral arteries grow from preexisting anastomoses in the rat hindlimb. Am J Physiol Heart Circ Physiol 283: H2012–H2020, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Hood DA, Gorski J, Terjung RL. Oxygen cost of twitch and tetanic isometric contractions of rat skeletal muscle. Am J Physiol Endocrinol Metab 250: E449–E456, 1986 [DOI] [PubMed] [Google Scholar]
- 19. Kakkos SK, Geroulakos G, Nicolaides AN. Improvement of the walking ability in intermittent claudication due to superficial femoral artery occlusion with supervised exercise and pneumatic foot and calf compression: a randomised controlled trial. Eur J Vasc Endovasc Surg 30: 164–175, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Kaptchuk TJ, Goldman P, Stone DA, Stason WB. Do medical devices have enhanced placebo effects? J Clin Epidemiol 53: 786–792, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of neovascularization. Arterioscler Thromb Vasc Biol 28: 1928–1936, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu K, Chen LE, Seaber AV, Johnson GW, Urbaniak JR. Intermittent pneumatic compression of legs increases microcirculation in distant skeletal muscle. J Orthop Res 17: 88–95, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Lloyd PG, Prior BM, Li H, Yang HT, Terjung RL. VEGF receptor antagonism blocks arteriogenesis, but only partially inhibits angiogenesis, in skeletal muscle of exercise-trained rats. Am J Physiol Heart Circ Physiol 288: H759–H768, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Mackie BG, Terjung RL. Blood flow to different skeletal muscle fiber types during contraction. Am J Physiol Heart Circ Physiol 245: H265–H275, 1983 [DOI] [PubMed] [Google Scholar]
- 25. Mahoney EM, Wang K, Keo HH, Duval S, Smolderen KG, Cohen DJ, Steg G, Bhatt DL, Hirsch AT. Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States. Circ Cardiovasc Qual Outcomes 3: 642–651, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Mazari FA, Khan JA, Carradice D, Samuel N, Abdul Rahman MN, Gulati S, Lee HL, Mehta TA, McCollum PT, Chetter IC. Randomized clinical trial of percutaneous transluminal angioplasty, supervised exercise and combined treatment for intermittent claudication due to femoropopliteal arterial disease. Br J Surg 99: 39–48, 2012 [DOI] [PubMed] [Google Scholar]
- 27. McDermott MM, Greenland P, Ferrucci L, Criqui MH, Liu K, Sharma L, Chan C, Celic L, Priyanath A, Guralnik JM. Lower extremity performance is associated with daily life physical activity in individuals with and without peripheral arterial disease. J Am Geriatr Soc 50: 247–255, 2002 [DOI] [PubMed] [Google Scholar]
- 28. McDermott MM, Liu K, Ferrucci L, Tian L, Guralnik JM, Liao Y, Criqui MH. Greater sedentary hours and slower walking speed outside the home predict faster declines in functioning and adverse calf muscle changes in peripheral arterial disease. J Am Coll Cardiol 57: 2356–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murphy TP, Cutlip DE, Regensteiner JG, Mohler ER, Cohen DJ, Reynolds MR, Massaro JM, Lewis BA, Cerezo J, Oldenburg NC, Thum CC, Goldberg S, Jaff MR, Steffes MW, Comerota AJ, Ehrman J, Treat-Jacobson D, Walsh ME, Collins T, Badenhop DT, Bronas U, Hirsch AT. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation 125: 130–139, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ostchega Y, Paulose-Ram R, Dillon CF, Gu Q, Hughes JP. Prevalence of peripheral arterial disease and risk factors in persons aged 60 and older: data from the National Health and Nutrition Examination Survey 1999–2004. J Am Geriatr Soc 55: 583–589, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Porter JM. Pneumatic limb compression: a free lunch? J Vasc Surg 31: 821–822, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol 287: H2434–H2447, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Ramaswami G, D'Ayala M, Hollier LH, Deutsch R, McElhinney AJ. Rapid foot and calf compression increases walking distance in patients with intermittent claudication: results of a randomized study. J Vasc Surg 41: 794–801, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Regensteiner JG. Exercise rehabilitation for the patient with intermittent claudication: a highly effective yet underutilized treatment. Curr Drug Targets Cardiovasc Haematol Disord 4: 233–239, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation 123: e18–e209, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roseguini BT, Arce-Esquivel AA, Newcomer SC, Laughlin MH. Impact of a single session of intermittent pneumatic leg compressions on skeletal muscle and isolated artery gene expression in rats. Am J Physiol Regul Integr Comp Physiol 301: R1658–R1668, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roseguini BT, Mehmet Soylu S, Whyte JJ, Yang HT, Newcomer S, Laughlin MH. Intermittent pneumatic leg compressions acutely upregulate VEGF and MCP-1 expression in skeletal muscle. Am J Physiol Heart Circ Physiol 298: H1991–H2000, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roseguini BT, Sheldon R, Stroup A, Bell JW, Maurer D, Crist BD, Laughlin MH, Newcomer SC. Impact of chronic intermittent external compressions on forearm blood flow capacity in humans. Eur J Appl Physiol 111: 509–519, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shireman PK. The chemokine system in arteriogenesis and hind limb ischemia. J Vasc Surg 45, Suppl A: A48–A56, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sieminski DJ, Gardner AW. The relationship between free-living daily physical activity and the severity of peripheral arterial occlusive disease. Vasc Med 2: 286–291, 1997 [DOI] [PubMed] [Google Scholar]
- 41. Srere PA. Citrate synthase. Methods Enzymol 13: 3–5, 1969 [Google Scholar]
- 42. Tan X, Qi WN, Gu X, Urbaniak JR, Chen LE. Intermittent pneumatic compression regulates expression of nitric oxide synthases in skeletal muscles. J Biomech 39: 2430–2437, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Terjung RL, Engbretson BM. Blood flow to different rat skeletal muscle fiber type sections during isometric contractions in situ. Med Sci Sports Exerc 20: S124–S130, 1988 [DOI] [PubMed] [Google Scholar]
- 44. van Bemmelen P, Char D, Giron F, Ricotta JJ. Angiographic improvement after rapid intermittent compression treatment [ArtAssist] for small vessel obstruction. Ann Vasc Surg 17: 224–228, 2003 [DOI] [PubMed] [Google Scholar]
- 45. van Bemmelen PS, Choudry RG, Salvatore MD, Goldenberg M, Goldman BI, Blebea J. Long-term intermittent compression increases arteriographic collaterals in a rabbit model of femoral artery occlusion. Eur J Vasc Endovasc Surg 34: 340–346, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Waters RE, Terjung RL, Peters KG, Annex BH. Preclinical models of human peripheral arterial occlusive disease: implications for investigation of therapeutic agents. J Appl Physiol 97: 773–780, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Yang HT, Feng Y. bFGF increases collateral blood flow in aged rats with femoral artery ligation. Am J Physiol Heart Circ Physiol 278: H85–H93, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Yang HT, Feng Y, Allen LA, Protter A, Terjung RL. Efficacy and specificity of bFGF increased collateral flow in experimental peripheral arterial insufficiency. Am J Physiol Heart Circ Physiol 278: H1966–H1973, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Yang HT, Ogilvie RW, Terjung RL. Heparin increases exercise-induced collateral blood flow in rats with femoral artery ligation. Circ Res 76: 448–456, 1995 [DOI] [PubMed] [Google Scholar]
- 50. Yang HT, Ogilvie RW, Terjung RL. Low-intensity training produces muscle adaptations in rats with femoral artery stenosis. J Appl Physiol 71: 1822–1829, 1991 [DOI] [PubMed] [Google Scholar]
- 51. Yang HT, Ogilvie RW, Terjung RL. Training increases collateral-dependent muscle blood flow in aged rats. Am J Physiol Heart Circ Physiol 268: H1174–H1180, 1995 [DOI] [PubMed] [Google Scholar]
- 52. Yang HT, Terjung RL. Angiotensin-converting enzyme inhibition increases exercise tolerance and muscle blood flow in rats with peripheral arterial insufficiency. J Clin Pharmacol 34: 345–355, 1994 [DOI] [PubMed] [Google Scholar]