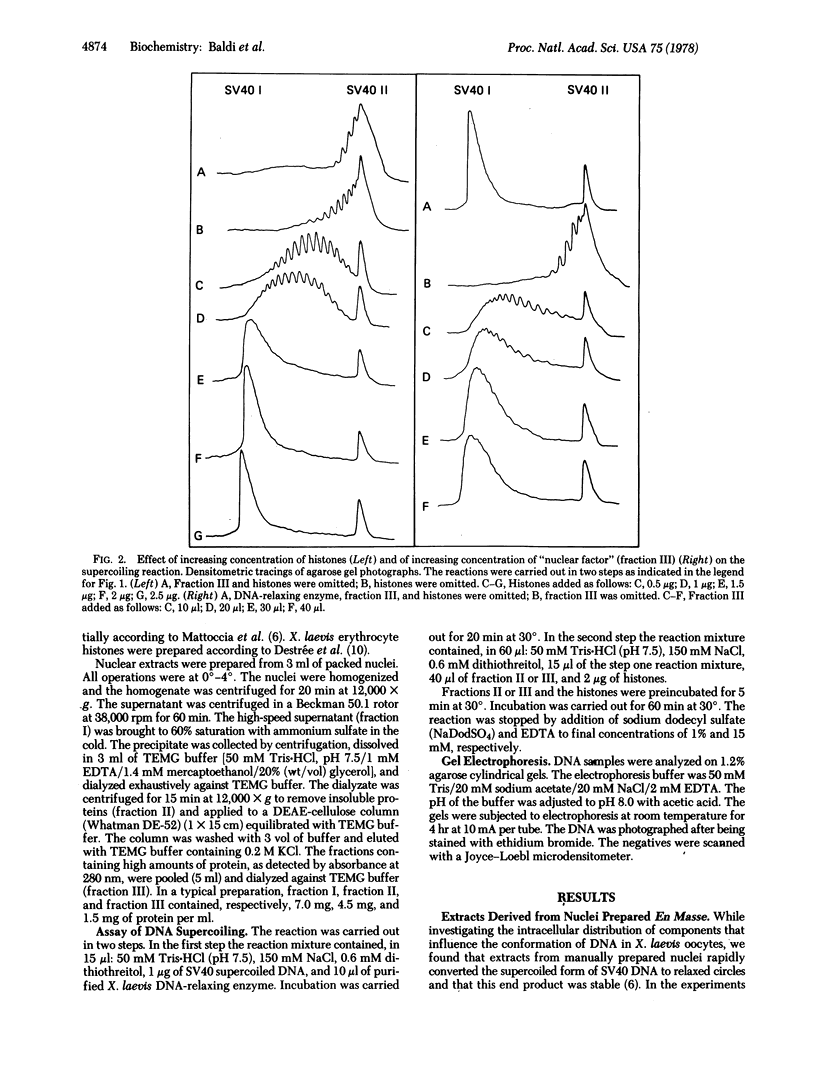
Abstract
A purified system is described for the introduction of negative supercoils into simian virus 40 DNA. The system consists of histones H2A, H2B, H3, and H4, DNA-relaxing enzyme, and a purified factor from Xenopus laevis stage 6 oocyte nuclei. The nuclei are prepared en masse by the technique of F. Scalenghe, M. Buscaglia, C. Steinheil, and M. Crippa [(1978) Chromosoma 60, 299-308]. The supercoiled simian virus 40 DNA prepared by this method is indistinguishable from simian virus 40 supercoiled DNA prepared from infected monkey cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson E. D., Woodland H. R. Histone synthesis in early amphibian development: histone and DNA syntheses are not co-ordinated. J Mol Biol. 1974 Sep 15;88(2):263–285. doi: 10.1016/0022-2836(74)90481-1. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Destrée O. H., d' Adelhart Toorop H. A., Charles R. Analysis of histones from different tissues and embryos of Xenopus laevis (Daudin). II. Qualitative and quantitative aspects of nuclear histones during early stages of development. Cell Differ. 1973 Oct;2(4):229–242. doi: 10.1016/0045-6039(73)90011-0. [DOI] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germond J. E., Hirt B., Oudet P., Gross-Bellark M., Chambon P. Folding of the DNA double helix in chromatin-like structures from simian virus 40. Proc Natl Acad Sci U S A. 1975 May;72(5):1843–1847. doi: 10.1073/pnas.72.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Keller W. Determination of the number of superhelical turns in simian virus 40 DNA by gel electrophoresis. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4876–4880. doi: 10.1073/pnas.72.12.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D., Morris N. R. Assembly of SV40 chromatin in a cell-free system from Xenopus eggs. Cell. 1977 Feb;10(2):237–243. doi: 10.1016/0092-8674(77)90217-3. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Micrococcus luteus DNA gyrase: active components and a model for its supercoiling of DNA. Proc Natl Acad Sci U S A. 1978 May;75(5):2098–2102. doi: 10.1073/pnas.75.5.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoccia E., Attardi D. G., Tocchini-Valentini G. P. DNA-relaxing activity and endonuclease activity in Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4551–4554. doi: 10.1073/pnas.73.12.4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalenghe F., Buscaglia M., Steinheil C., Crippa M. Large scale isolation of nuclei and nucleoli from vitellogenic oocytes of Xenopus laevis. Chromosoma. 1978 May 16;66(4):299–308. doi: 10.1007/BF00328531. [DOI] [PubMed] [Google Scholar]
- Schorderet-Slatkine S. Action of progesterone and related steroids on oocyte maturation in Xenopus laevis. An in vitro study. Cell Differ. 1972 Aug;1(3):179–189. doi: 10.1016/0045-6039(72)90027-9. [DOI] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Macasaet F. Simian virus 40 deoxyribonucleic acid synthesis: analysis by gel electrophoresis. J Virol. 1972 Oct;10(4):599–604. doi: 10.1128/jvi.10.4.599-604.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodland H. R., Adamson E. D. The synthesis and storage of histones during the oogenesis of Xenopus laevis. Dev Biol. 1977 May;57(1):118–135. doi: 10.1016/0012-1606(77)90359-1. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Gurdon J. B., Price J. Nuclear localisation of an oocyte component required for the stability of injected DNA. Nature. 1977 Jul 14;268(5616):150–152. doi: 10.1038/268150a0. [DOI] [PubMed] [Google Scholar]