Abstract
Endothelial nitric oxide (NO) synthase (NOS) has been shown to contribute to enhanced vascular function after exercise training. Recent studies have revealed that relatively low concentrations of reactive oxygen species can contribute to endothelium-dependent vasodilation under physiological conditions. We tested the hypothesis that exercise training enhances endothelial function via endothelium-derived vasodilators, NO and superoxide/H2O2, in the underlying setting of chronic coronary artery occlusion. An ameroid constrictor was placed around the proximal left circumflex coronary artery to induce gradual occlusion in Yucatan miniature swine. At 8 wk postoperatively, pigs were randomly assigned to sedentary (pen-confined) or exercise-training (treadmill-run: 5 days/wk for 14 wk) regimens. Exercise training significantly enhanced concentration-dependent, bradykinin-mediated dilation in cannulated collateral-dependent arterioles (∼130 μm diameter) compared with sedentary pigs. NOS inhibition reversed training-enhanced dilation at low bradykinin concentrations in collateral-dependent arterioles, although increased dilation persisted at higher bradykinin concentrations. Total and phosphorylated (Ser1179) endothelial NOS protein levels were significantly increased in arterioles from collateral-dependent compared with the nonoccluded region, independent of exercise. The H2O2 scavenger polyethylene glycol-catalase abolished the training-enhanced bradykinin-mediated dilation in collateral-dependent arterioles; similar results were observed with the SOD inhibitor diethyldithiocarbamate. Fluorescence measures of bradykinin-stimulated H2O2 levels were significantly increased by exercise training, independent of occlusion. The NADPH inhibitor apocynin significantly attenuated bradykinin-mediated dilation in arterioles of exercise-trained, but not sedentary, pigs and was associated with significantly increased protein levels of the NADPH subunit p67phox. These data provide evidence that, in addition to NO, the superoxide/H2O2 signaling pathway significantly contributes to exercise training-enhanced endothelium-mediated dilation in collateral-dependent coronary arterioles.
Keywords: chronic coronary occlusion, ischemic heart disease, hydrogen peroxide
an imbalance between vasodilator and vasoconstrictor influences of endothelium is a critical consequence of the pathogenic process of endothelial dysfunction, which is characterized by decreased vasodilation, a proinflammatory state, and smooth muscle proliferation (2, 4). An important feature of this altered vasoreactivity is impaired nitric oxide (NO) bioavailability and/or elevated oxidant stress (22, 30). Previous studies have shown that exercise training enhances endothelium-dependent relaxation and endothelial NO synthase (eNOS) mRNA expression and protein levels in coronary arteries and arterioles of control animals (29, 45). Exercise training has also been reported to enhance the contribution of NO and K+ channel activity to basal tone, as well as increase protein content of eNOS and phosphorylated eNOS (Ser1179) in small coronary arteries from pigs subjected to chronic coronary artery occlusion/stenosis (19). Exercise training-induced improvements in vascular function have been shown to contribute to enhanced myocardial perfusion and contractile function in vascular disease states (16, 17). However, despite remarkable evidence for therapeutic benefits of physical activity, the primary mechanisms by which regular exercise improves vascular function in the setting of coronary artery disease have not been fully elucidated.
While reactive oxygen species (ROS) have been implicated in the development of clinical pathophysiology of the cardiovascular system, additional studies have suggested that, at relatively low concentrations, ROS, such as superoxide and H2O2, can function as physiological signaling molecules (8, 9, 11, 27). Furthermore, recent studies have revealed that inhibition of NADPH oxidase reduced bradykinin-induced superoxide and H2O2 production (26) and attenuated endothelium-dependent dilation in human coronary arterioles (26) and rat skeletal muscle arterioles (46). Interestingly, a small number of recent reports indicate that the ROS H2O2 may function as a potential mediator of exercise training-induced adaptations in vascular reactivity (46). Thus we sought to assess the contribution of the NO and superoxide/H2O2 signaling pathways to exercise training-enhanced, endothelium-dependent dilation in collateral-dependent coronary arterioles and to begin to explore the underlying mechanisms that may contribute to adaptations in these signaling pathways.
MATERIALS AND METHODS
Experimental animals and surgical procedures.
All procedures were in accordance with “Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training” and were approved by the Institutional Animal Care and Use Committee at Texas A & M University in accordance with the Association for the Accreditation of Laboratory Animal Care procedures. In addition, all protocols and methods conformed to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals [Department of Health and Human Services Publication No. (NIH) 85-23, Office of Science and Health Reports, Bethesda, MD]. Adult female Yucatan miniature swine (Sinclair Research Center, Auxvasse, MO) were surgically instrumented with an ameroid constrictor around the proximal left circumflex coronary (LCX) artery, as described in detail previously (21, 33). Animals were preanesthetized with glycopyrrolate (0.004 mg/kg im), midazolam (0.5 mg/kg im), and ketamine (20 mg/kg im), and surgical anesthesia was induced with 3% isoflurane. Animals were then intubated, and anesthesia was maintained with 2–3% isoflurane-balance O2 during aseptic surgery. During the surgical procedure, animals received the following drugs as necessary: pancuronium (0.1 mg/kg) or vecuronium bromide (0.1 mg/kg) and lidocaine (1 mg/kg iv). Immediately following surgery, pigs received ketoprofen (Ketofen, 3.0 mg/kg iv). Prior to surgery and during surgical recovery, animals received buprenorphine hydrochloride (0.1 mg/kg iv) or butorphanol tartrate (0.5 mg/kg) every 3–6 h as needed for pain relief. The antibiotic Naxcel (cetiofur sodium, 4 mg/kg im) was administered 24 h before surgery, immediately prior to surgery, and for 2 days following surgery. For the most efficient use of our pigs, we utilize multiple tissue preparations (e.g., vascular, cardiac, skeletal muscle, blood, and cerebral) from each animal, so that numerous experiments can be conducted concurrently. Thus, while a large number of pigs were used for the studies described here, we make every effort to maximize the use of these animals.
Exercise training.
At 8 wk postoperatively, animals were randomly assigned to a sedentary (n = 48) or an exercise-training (n = 44) group. Sedentary pigs were confined to their pens, while exercise-trained animals underwent a progressive treadmill exercise-training program, 5 days/wk for 14 wk, as described previously (12, 15, 20). At termination, skeletal muscle citrate synthase activity and heart-to-body weight ratio were measured to evaluate effectiveness of the exercise-training regimen, as described previously (15, 21).
Preparation of coronary arterioles.
After the 14-wk exercise-training protocol or sedentary confinement, pigs were anesthetized using xylazine (Rompun, 2.25 mg/kg im), ketamine (35 mg/kg im), and pentothal sodium (30 mg/kg iv), and heparin was administered (1,000 U/kg iv). Animals were intubated and ventilated with room air, and a left lateral thoracotomy was performed in the fourth intercostal space. Hearts were removed, placed in Krebs bicarbonate buffer (0–4°C), and weighed. Visual inspection at the ameroid occluder during dissection of the LCX artery indicated 100% occlusion in all animals used in this study. With the aid of a dissection microscope, size-matched arterioles (∼130 μm) were isolated from subepicardial regions of the nonoccluded left anterior descending (LAD) artery and the collateral-dependent LCX artery in areas free from infarct.
Microvessel cannulation and experimental protocols.
Isolated arterioles were transferred to a Lucite vessel chamber containing physiological saline solution, cannulated, and pressurized for assessment of vascular reactivity, as described in detail previously (18). Arterioles underwent a 1-h equilibration period, during which the vessels established a stable level of basal tone. Arterioles were further preconstricted with endothelin-1 until a preconstriction level of ∼30–70% of maximal diameter was attained. For experiments in which pharmacological inhibitors were utilized, arterioles were preconstricted to the same level (∼30–70%) using the inhibitor plus endothelin-1, as previously described (18). Pharmacological inhibitors included the NOS inhibitor Nω-nitro-l-arginine methyl ester (l-NAME, 300 μM), the H2O2 scavenger polyethylene glycol (PEG)-catalase (1,000 U/ml), an inhibitor of SOD [diethyldithiocarbamate (DETC), 1 mM], and an inhibitor of NADPH oxidase (apocynin, 100 μM). Additional vehicle control data were collected in the presence of the PEG compound at the same concentration (2.5 mg/ml) used in the PEG-catalase studies. Concentration-response curves were determined in response to cumulative concentrations of bradykinin or nitroprusside. All drugs were added directly to the tissue bath. Because our preliminary experiments suggested that arterioles subjected to repeated exposures to bradykinin exhibit tachyphylaxis, each arteriole underwent only a single concentration-response curve to bradykinin in the absence or presence of selective inhibitors.
Immunoblots.
Additional coronary arterioles (∼100–150 μm diameter, 10–15 mm total length) were dissected from the nonoccluded and collateral-dependent myocardial regions, quick-frozen in liquid N2, and stored at −80°C for immunoblot analysis. Arterioles were homogenized in 40 μl of 2× lysis buffer [20 mM Tris·HCl, 50 mM NaCl, 0.1% Triton X-100, 1% each protease and phosphatase inhibitor cocktails (catalog nos. 539131 and 524625, respectively, Calbiochem), and 3 mM EGTA] by freeze-thaw cycles and vortexed approximately six to eight times. Protein concentration was determined by bicinchoninic acid protein assay kit (Pierce). Arteriole lysate (12 μg of total protein) was subjected to 12.5% SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed overnight with primary antibody, as described in detail previously (12). Primary antibody dilutions were as follows: eNOS (1:250), phosphorylated eNOS (Ser1179, 1:750), the NADPH oxidase subunits Nox1 (1.1 μg/ml), Nox2 (1:500), Nox4 (1:500), p47phox (1:1,250), and p67phox (1:250), and β-actin (1:5,000) at 4°C overnight. After they were washed, the membranes were incubated with the appropriate horseradish peroxidase-conjugated species-specific anti-IgG (diluted 1:50,000–1:100,000 depending on primary antibody) for 2 h at 25°C. Peroxidase activity was detected using SuperSignal West Dura substrate. Scanning densitometry was used to quantify signal density from luminograms. Normalization for potential loading differences was accomplished using the ratio of densitometry signals for proteins of interest to β-actin.
Fluorescent detection of H2O2.
In additional studies, bradykinin-mediated changes in H2O2 were detected in real time with the cell-permeable fluorescent indicator 5-(and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (DCF). Pressurized coronary arterioles (∼100–150 μm) were loaded intraluminally by delivery of DCF (5 μM) into the arteriole through a cannulating micropipette. Flow was stopped, and the dye was incubated in the arteriole for 10 min. Flow was resumed for an additional 10 min to eliminate the fluorescent indicator from the arterioles and perfusion pipettes. The arteriole was allowed to equilibrate for an additional 10 min prior to start of experimentation. Albumin-free solutions were utilized to avoid potential interference with DCF fluorescence detection (47). After equilibration, a region of interest was selected, and changes in fluorescence intensity were observed (NIS Elements, Nikon) using an epifluorescence microscopy system. Arterioles were excited with a 175-W xenon arc lamp with a 475-nm interference filter (Lambda DG-4, Sutter Instruments). Fluorescence emission was captured at 515 nm every 30 s and reflected to an interline transfer, progressive-scan, cooled charge-coupled device video camera (CoolSNAP HQ, Photometrics) with a dichroic mirror. The microscope was equipped with an ×10 oil immersion objective, numerical aperture of 0.3. Basal fluorescence was obtained for 3 min; then arterioles were exposed to bradykinin (10−12 or 10−10 M), and fluorescent images were acquired every 30 s for 5 min. An equal number of arterioles from each of the four vessel treatment groups received 10−12 or 10−10 M bradykinin. Arterioles were exposed to exogenous H2O2, PEG-catalase, and nitroprusside in various combinations to verify specificity of DCF. Although DCF is oxidized by other peroxides in addition to H2O2, other investigators have demonstrated complete inhibition of agonist-stimulated fluorescence by addition of catalase (3, 24, 51).
Solutions and pharmacological agents.
l-NAME, apocynin, PEG-catalase, PEG, and DETC were purchased from Sigma. Endothelin-1 was purchased from Peninsula Laboratories. DCF was obtained from Invitrogen. Primary antibodies against the following proteins were utilized for these studies: eNOS (catalog no. 610297) and phosphorylated eNOS (Ser1179, catalog no. 612392) purchased from BD Biosciences; Nox1 (catalog no. abs5831) from Abcam; Nox4 (catalog no. NB110-58851) and β-actin (catalog no. NB400-501) from Novus; and Nox2 (catalog no. SC-20782), p47phox (catalog no. SC-14015), and p67phox (catalog no. SC-7662) from Santa Cruz Biotechnology. Physiological saline solution contained 145 mM NaCl, 4.7 mM KCl, 2.0 mM CaCl2, 1.17 mM MgSO4, 3.0 mM MOPS, 1.2 mM NaH2PO4, 5.0 mM glucose, 2.0 mM pyruvate, 0.02 mM EDTA, and 1% bovine serum albumin, pH 7.4.
Data presentation and analysis.
Citrate synthase, body weight, and heart-to-body weight ratio were compared between sedentary and exercise-trained pigs using Student's t-tests. Vasodilation responses to bradykinin are presented as the percent increase in internal diameter relative to the maximal possible dilation as follows: % = [(Dss − Dpre)/(Dmax − Dpre)]/100, where Dss is the steady-state internal diameter in response to each bradykinin concentration, Dpre is the baseline diameter following preconstriction, and Dmax is the maximal diameter of the arteriole determined by exposure to nitroprusside at the end of each experiment. Bradykinin- and nitroprusside-mediated dilation were evaluated by repeated-measures two-way ANOVA. Immunoblot and DCF fluorescence were analyzed using two-way ANOVA. Mean differences were ascertained using Bonferroni's multiple comparison tests when a main effect was significant by ANOVA. For all analyses, P ≤ 0.05 was considered significant. Values are means ± SE; n reflects the number of animals studied. When more than one arteriole from the nonoccluded or collateral-dependent myocardial region was used from one animal in identical protocols, the responses from these arterioles were averaged before data analyses were conducted.
RESULTS
Effectiveness of the exercise-training program.
Efficacy of the 14-wk progressive treadmill exercise-training regimen was demonstrated by significant increases in skeletal muscle oxidative enzyme capacity and an increased heart-to-body weight ratio in exercise-trained compared with sedentary animals. Citrate synthase activity increased significantly in muscle from exercise-trained (n = 44) compared with sedentary (n = 48) pigs as follows: deltoid muscle (44.3 ± 1.6 vs. 34.4 ± 1.1 μmol·min−1·g−1) and the medial (44.2 ± 1.6 vs. 35.1 ± 1.3 μmol·min−1·g−1), lateral (39.2 ± 1.0 vs. 29.9 ± 0.8 μmol·min−1·g−1), and long (31.4 ± 1.2 vs. 26.0 ± 0.8 μmol·min−1·g−1) heads of the triceps brachii muscle. Although body weight did not differ between sedentary and exercise-trained animals at the time of death (35.1 ± 0.7 and 33.7 ± 0.7 kg, respectively), heart-to-body weight ratio was significantly greater from exercise-trained (n = 44) than sedentary (n = 48) pigs (5.3 ± 0.1 vs. 4.6 ± 0.1 g/kg).
Characteristics of arterioles.
Maximal (passive) intraluminal diameters (Dmax) of cannulated coronary arterioles, determined using nitroprusside (100 μM), were not significantly different between the four treatment groups (Table 1). The level of preconstriction was also similar across these groups (Table 1), and the concentration of endothelin-1 required to attain this level of preconstriction was not significantly different between arteriole groups (Table 1).
Table 1.
Dimensional characteristics of cannulated coronary arterioles
| n | Maximal Diameter, μm | Preconstriction, % | ET-1, nM | |
|---|---|---|---|---|
| Nonoccluded | ||||
| Sedentary | 46 | 129 ± 4 | 60 ± 3 | 0.27 ± 0.02 |
| Exercise-trained | 40 | 134 ± 5 | 62 ± 3 | 0.26 ± 0.02 |
| Collateral-dependent | ||||
| Sedentary | 44 | 135 ± 6 | 58 ± 3 | 0.30 ± 0.03 |
| Exercise-trained | 44 | 133 ± 4 | 63 ± 2 | 0.27 ± 0.03 |
Values are means ± SE; n, number of arterioles. %Preconstriction, percentage of maximal diameter to which arterioles were preconstricted; ET-1, concentration of endothelin-1 required to obtain %preconstriction. No significant differences exist.
Effects of exercise training and occlusion on bradykinin-mediated dilation.
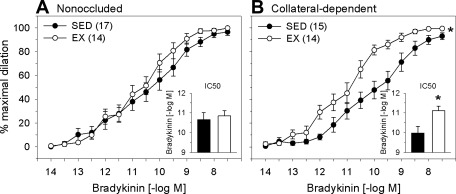
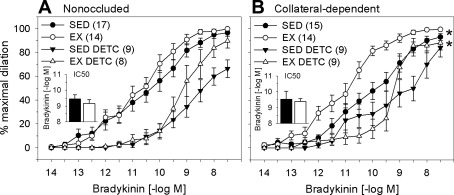
We examined the effect of exercise training and chronic coronary occlusion on bradykinin-mediated dilation in coronary arterioles isolated from nonoccluded and collateral-dependent myocardial regions. Our data revealed that exercise training did not alter bradykinin-mediated dilation in arterioles from the nonoccluded region (Fig. 1A). In contrast, bradykinin-mediated dilation was significantly enhanced in collateral-dependent arterioles from exercise-trained compared with sedentary animals (Fig. 1B). Bradykinin-mediated dilation was not statistically different in collateral-dependent compared with nonoccluded coronary arterioles of sedentary and exercise-trained pigs. Evaluation of IC50 revealed that sensitivity to bradykinin was not altered by exercise training in the nonoccluded region (Fig. 1A, inset). In contrast, exercise training significantly increased the sensitivity of arterioles from the collateral-dependent region to bradykinin (Fig. 1B, inset).
Fig. 1.
Effect of chronic occlusion and exercise training on bradykinin-mediated vasodilation in porcine coronary arterioles. A: bradykinin-mediated dilation was not significantly altered by exercise training in arterioles of the nonoccluded region. B: exercise training significantly enhanced bradykinin-mediated dilation in the collateral-dependent region. IC50 values (insets) revealed that sensitivity to bradykinin was not altered by exercise training in the nonoccluded region but was significantly increased in collateral-dependent arterioles of exercise-trained (EX) compared with sedentary (SED) pigs. Values are means ± SE of number of animals in parentheses. *P ≤ 0.05.
Role of NO in bradykinin-mediated vasodilation.
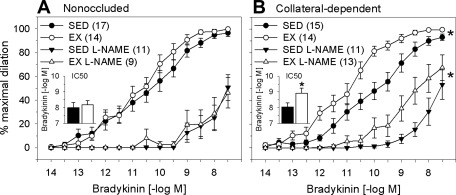
We evaluated the contribution of NO to exercise-training-enhanced, bradykinin-mediated vasodilation using the NOS inhibitor l-NAME. NOS inhibition significantly attenuated bradykinin-mediated dilation in nonoccluded and collateral-dependent arterioles of sedentary and exercise-trained animals. NOS inhibition similarly reduced bradykinin-mediated dilation in arterioles from the nonoccluded region of sedentary and exercise-trained animals (Fig. 2A). In collateral-dependent arterioles, l-NAME corrected the exercise-training-enhanced, endothelium-mediated dilation at lower bradykinin concentrations. However, l-NAME did not reverse the training-enhanced vasodilation at higher bradykinin concentrations (Fig. 2B), and significant vasodilation persisted that was insensitive to NO synthase (NOS) inhibition in collateral-dependent coronary arterioles from trained animals. In the presence of l-NAME, IC50 values were similar in arterioles from the nonoccluded region of sedentary and exercise-trained pigs (Fig. 2A, inset). In contrast, enhanced sensitivity to bradykinin in arterioles from the collateral-dependent region of exercise-trained pigs persisted in the presence of l-NAME (Fig. 2B, inset).
Fig. 2.
Effect of the nitric oxide synthase ·(NOS) inhibitor nitro-l-arginine methyl ester (l-NAME, 300 μM) on bradykinin-mediated dilation of arterioles. NOS inhibition significantly attenuated dilation in nonoccluded (A) and collateral-dependent (B) arterioles of sedentary and exercise-trained pigs. In collateral-dependent arterioles, NOS inhibition corrected the exercise-training-enhanced, endothelium-mediated dilation at lower bradykinin concentrations; training-enhanced vasodilation persisted at higher bradykinin concentrations. IC50 values revealed that, in the presence of l-NAME, sensitivity to bradykinin was similar in arterioles from the nonoccluded region of sedentary and exercise-trained pigs (A, inset). Enhanced sensitivity to bradykinin in arterioles from the collateral-dependent region of exercise-trained pigs persisted in the presence of l-NAME (B, inset). Values are means ± SE of number of animals in parentheses. *P ≤ 0.05.
Smooth muscle responsiveness to nitroprusside.
To examine the possibility that arteriolar smooth muscle responsiveness to NO was altered by occlusion or exercise training, we determined concentration-response curves of coronary arterioles to the endothelium-independent NO donor nitroprusside (10−10–10−4 M). Concentration-dependent dilation responses to nitroprusside were not significantly different in coronary arterioles isolated from the collateral-dependent and nonoccluded myocardial regions of sedentary or exercise-trained animals (data not shown).
Total and phosphorylated eNOS protein levels.
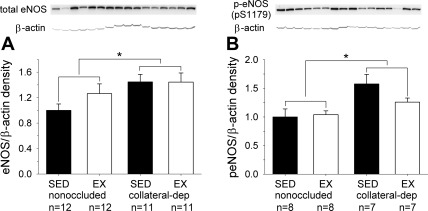
On the basis of our finding that NO contributed to exercise-training-enhanced dilation at lower bradykinin concentrations, we examined the effect of occlusion and exercise training on protein levels of total and phosphorylated (Ser1179) eNOS. Figure 3 provides representative immunoblots for total and phosphorylated eNOS, as well as β-actin as loading control, using arterioles from the nonoccluded and collateral-dependent regions of sedentary and exercise-trained pigs. Total (Fig. 3A) and phosphorylated (Fig. 3B) eNOS protein levels were significantly increased in coronary arterioles from the collateral-dependent region compared with the nonoccluded region. Exercise training did not significantly alter total or phosphorylated eNOS protein levels.
Fig. 3.
Effect of chronic occlusion and exercise training on total endothelial NOS (eNOS) and phosphorylated eNOS (p-eNOS, Ser1179) protein levels. Total (A) and phosphorylated (B) eNOS protein levels were significantly increased in coronary arterioles from the collateral-dependent region compared with the nonoccluded region, independent of exercise-training status. Protein was quantified by densitometry analysis, normalized to β-actin, and expressed relative to the density of nonoccluded arterioles of sedentary pigs. Values are means ± SE; n, number of animals. *P ≤ 0.05.
Role of H2O2 in bradykinin-mediated vasodilation.
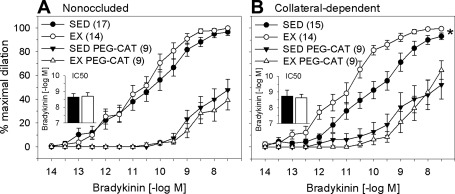
Because NOS inhibition did not fully correct the exercise-training-enhanced bradykinin-mediated dilation, we determined the contribution of the candidate endothelium-derived hyperpolarizing factor H2O2 to bradykinin-induced dilation using the cell-permeable H2O2 scavenger PEG-catalase. PEG-catalase significantly attenuated bradykinin-mediated dilation in all arteriole treatment groups (Fig. 4). Scavenging of H2O2 similarly reduced bradykinin-mediated dilation in arterioles from the nonoccluded region of sedentary and exercise-trained pigs (Fig. 4A). Importantly, PEG-catalase abolished the difference in bradykinin-mediated dilation between arterioles of the collateral-dependent myocardial region in exercise-trained and sedentary animals (Fig. 4B). In the presence of PEG-catalase, IC50 values were similar in arterioles from the nonoccluded region of sedentary and exercise-trained pigs (Fig. 4A, inset). Importantly, treatment with PEG-catalase reversed the enhanced sensitivity to bradykinin observed under control conditions in arterioles from the collateral-dependent region of exercise-trained pigs (Fig. 4B, inset). Vehicle-control experiments revealed that bradykinin-mediated dilation was not significantly altered by inclusion of an equivalent concentration of the PEG compound in the tissue bath (n = 5).
Fig. 4.
Effect of the H2O2 scavenger polyethylene glycol-catalase (PEG-CAT) on bradykinin-mediated dilation of arterioles. PEG-CAT significantly attenuated dilation in nonoccluded (A) and collateral-dependent (B) arterioles of sedentary and exercise-trained pigs. Exercise-training-enhanced dilation in collateral-dependent arterioles was reverse in the presence of PEG-CAT. In the presence of PEG-CAT, IC50 values were similar in arterioles from the nonoccluded region of sedentary and exercise-trained pigs (A, inset). Treatment with PEG-CAT reversed the enhanced sensitivity to bradykinin that was observed under control conditions in arterioles from the collateral-dependent region of exercise-trained pigs (B, inset). Values are means ± SE of number of animals in parentheses. *P ≤ 0.05.
Effects of SOD inhibition on bradykinin-mediated vasodilation.
We also examined the effect of the SOD inhibitor DETC on bradykinin-mediated dilation in nonoccluded and collateral-dependent arterioles from sedentary and exercise-trained pigs. DETC specifically inhibits the copper-containing SOD isoforms Cu,Zn-SOD and extracellular SOD (6), reducing the conversion of superoxide to H2O2. Treatment with DETC significantly diminished bradykinin-mediated dilation in all arteriole treatment groups (Fig. 5). SOD inhibition similarly attenuated bradykinin-mediated dilation in arterioles from the nonoccluded region of sedentary and exercise-trained pigs (Fig. 5A). Notably, DETC treatment had a more pronounced effect in collateral-dependent arterioles of exercise-trained than sedentary pigs (Fig. 5B), similar to that observed in the presence of PEG-catalase in Fig. 5B. Despite the more marked attenuation of bradykinin-mediated dilation with DETC after exercise training, a significant interaction effect between collateral-dependent arterioles of sedentary and exercise-trained pigs persisted, although post hoc analysis revealed that responses differed only at one concentration (3 nM) of bradykinin (Fig. 5B). In the presence of DETC, IC50 values were similar in arterioles from the nonoccluded region of sedentary and exercise-trained pigs (Fig. 5A, inset). Similar to that observed with PEG-catalase, treatment with DETC reversed the enhanced sensitivity to bradykinin that was observed under control conditions in arterioles from the collateral-dependent region of exercise-trained pigs (Fig. 5B, inset).
Fig. 5.
Effect of the SOD inhibitor diethyldithiocarbamate (DETC) on bradykinin-mediated vasodilation in coronary arterioles. DETC significantly attenuated dilation in nonoccluded (A) and collateral-dependent (B) arterioles of sedentary and exercise-trained pigs. Exercise-training-enhanced dilation in collateral-dependent arterioles was nearly reversed in the presence of DETC. In the presence of DETC, IC50 values were similar in arterioles from the nonoccluded region of sedentary and exercise-trained pigs (A, inset). Treatment with DETC reversed the enhanced sensitivity to bradykinin that was observed under control conditions in arterioles from the collateral-dependent region of exercise-trained pigs (B, inset). Values are means ± SE of number of animals in parentheses. *P ≤ 0.05.
Bradykinin-induced H2O2 production.
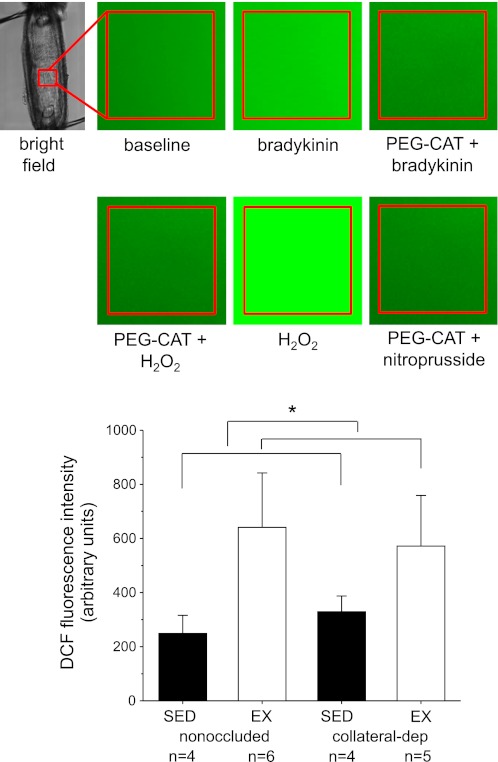
We utilized the fluorescent indicator DCF as a measure of bradykinin-stimulated H2O2 in cannulated arterioles (Fig. 6). Our preliminary studies with DCF demonstrated that treatment with PEG-catalase (1,000 U/ml) abolished bradykinin-stimulated DCF fluorescence and exogenous application of H2O2 (3 mM) markedly stimulated DCF fluorescence in arterioles, demonstrating specificity of the fluorescent dye for H2O2 (Fig. 6A), as confirmed previously by other investigators (3, 24, 51). Arterioles designated sedentary nonoccluded in Fig. 6 were obtained from the LAD-supplied region of occluded (n = 2), sham-operated (n = 1), or control, nonoccluded (n = 1) pigs. Mean responses from arterioles of the nonoccluded region (LAD) of occluded pigs were not different from the mean responses of the same region (LAD) of nonoccluded pigs. Arterioles from other treatment groups were collected from designated regions of occluded pigs. The peak cytosolic DCF signal in response to bradykinin stimulation was calculated by subtraction of basal fluorescence from the peak fluorescence response to bradykinin. Bradykinin stimulation significantly increased DCF fluorescence in coronary arterioles of exercise-trained animals to a greater extent than that observed in sedentary pigs, with no effect of occlusion (Fig. 6B).
Fig. 6.
Fluorescence measures of H2O2 in cannulated porcine coronary arterioles by 5-(and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (DCF). A: representative fluorescence images of arterioles at baseline, in response to bradykinin in the absence and presence of PEG-CAT (1,000 U/ml), in response to exogenously applied H2O2 (3 mM) in the absence and presence of PEG-CAT, and in response to the NO donor nitroprusside (100 μM) in the presence of PEG-CAT. B: DCF fluorescence was significantly increased in nonoccluded and collateral-dependent coronary arterioles of exercise-trained compared with sedentary pigs. Values are means ± SE; n, number of animals. *P ≤ 0.05.
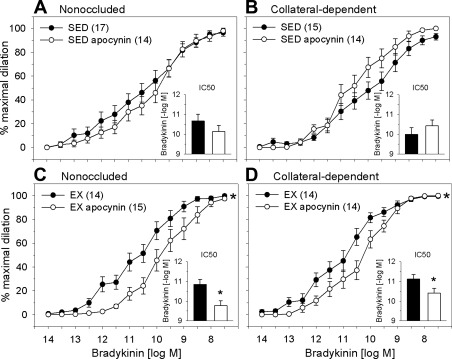
Effect of apocynin on bradykinin-mediated vasodilation.
In the vasculature, NADPH oxidase is considered a primary source of superoxide anion, the precursor to H2O2 production (13, 14, 39). We examined the effect of the NADPH oxidase inhibitor apocynin on bradykinin-mediated dilation. Pretreatment with apocynin did not significantly alter bradykinin-induced dilation in arterioles from the nonoccluded (Fig. 7A) or collateral-dependent (Fig. 7B) region of sedentary pigs. In contrast to these findings, apocynin significantly attenuated bradykinin-mediated dilation in arterioles from nonoccluded (Fig. 7C) and collateral-dependent (Fig. 7D) myocardial regions of exercise-trained animals. Treatment with apocynin did not significantly alter the sensitivity to bradykinin in arterioles from the nonoccluded or collateral-dependent regions of sedentary pigs (Fig. 7, A and B, insets). On the other hand, the concentration necessary to elicit 50% dilation in response to bradykinin was significantly increased in arterioles from the nonoccluded and collateral-dependent regions of exercise-trained pigs (Fig. 7, C and D, insets).
Fig. 7.
Effect of the NADPH oxidase inhibitor apocynin on bradykinin-mediated dilation of arterioles. Apocynin did not significantly alter bradykinin-mediated dilation in nonoccluded (A) or collateral-dependent (B) arterioles of sedentary pigs. Apocynin significantly attenuated dilation in nonoccluded (C) and collateral-dependent (D) arterioles of exercise-trained pigs. In the presence of apocynin, IC50 values revealed that sensitivity to bradykinin was not altered in arterioles from the nonoccluded and collateral-dependent regions of sedentary pigs (A and B, insets). Apocynin significantly reduced sensitivity to bradykinin in arterioles from the nonoccluded and collateral-dependent regions of exercise-trained pigs (C and D, insets). Values are means ± SE of number of animals in parentheses. *P ≤ 0.05.
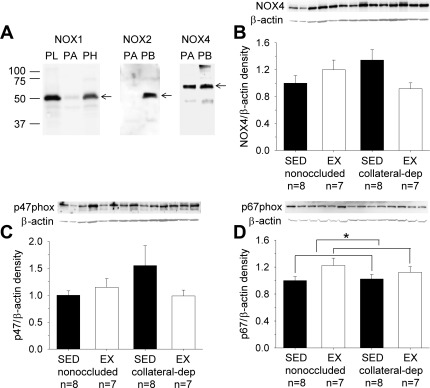
NADPH oxidase subunit protein levels.
We examined protein levels of the NADPH oxidase subunits p47phox, p67phox, and Nox4. In preliminary studies (Fig. 8A), we characterized the protein levels of Nox1, Nox2, and Nox4, specific homologs that are reported to be most abundant in vascular cells (26, 28). Our findings revealed that while we were able to detect Nox1 and Nox2 in pig tissues (e.g., brain, lung, and/or myocardium), the protein levels of these two homologs in pig arterioles were of very low abundance. On the other hand, Nox4 protein was abundant in pig arterioles and, thus, was further studied across arteriole treatment groups. Figure 8, B–D, shows representative immunoblots of Nox4, p47phox, and p67phox, as well as β-actin as loading control, using arterioles from the nonoccluded and collateral-dependent regions of sedentary and exercise-trained pigs. Immunoblot analyses for Nox4 (Fig. 8B) and p47phox (Fig. 8C) revealed no significant effect of occlusion or exercise training on arteriolar protein levels. In contrast, exercise training produced a small, but statistically significant, increase in p67phox protein levels (Fig. 8D), independent of occlusion.
Fig. 8.
Effect of chronic occlusion and exercise training on protein levels of NADPH oxidase subunits. Preliminary evaluation of the Nox family of isoforms revealed that Nox1 and Nox2 were poorly expressed in porcine coronary arterioles (PA), whereas Nox4 was abundant (A). Subsequent evaluation across groups revealed that neither Nox4 (B) nor p47phox (C) was significantly altered by occlusion or exercise training. In contrast, p67phox protein (D) was significantly increased by exercise training, independent of occlusion. Protein was quantified as described in Fig. 3 legend. PL, pig lung; PH, pig heart; PB, pig brain. Values are means ± SE; n, number of animals. *P ≤ 0.05.
DISCUSSION
In this study, we document the novel finding that exercise training increases the contribution of the superoxide/H2O2 signaling pathway to endothelium-dependent vasodilation in collateral-dependent coronary arterioles. Specifically, our data indicate that H2O2 significantly contributes to bradykinin-mediated dilation in arterioles from nonoccluded and collateral-dependent regions of sedentary and exercise-trained pigs. Importantly, we provide two data sets that suggest that the elimination of H2O2, via PEG-catalase or DETC, nearly abolished the exercise-training-enhanced dilation in collateral-dependent arterioles. Furthermore, H2O2 levels were significantly increased in arterioles from exercise-trained pigs, independent of occlusion. Our additional unique data reveal that the NADPH oxidase inhibitor apocynin significantly attenuated bradykinin-mediated dilation in arterioles from nonoccluded and collateral-dependent myocardial regions of exercise-trained, but not sedentary, pigs. Consistent with these findings, the NADPH oxidase subunit p67phox was increased by exercise training, independent of occlusion.
Previous studies have reported that H2O2 signaling contributes to endothelium-dependent bradykinin-mediated dilation in coronary arterioles of heart disease patients (26). However, our studies are the first to demonstrate that exercise training produces adaptations in H2O2 signaling that correct impaired endothelium-dependent dilation in coronary arterioles from ischemic hearts. In support of our data, studies in rat skeletal muscle arterioles revealed that exercise training increased NO and superoxide anion production in response to increased intraluminal flow and that enhanced superoxide, or its dismutation to H2O2, contributed to the exercise-training-induced improvements in flow-mediated dilation (46). Previous data reported by our group have also revealed that exercise training corrects impaired adenosine-mediated dilation in collateral-dependent arterioles, which also appeared to be mediated by NO and H2O2 (48). Our complementary evaluation of bradykinin-stimulated arteriolar H2O2 production revealed that exercise training increased H2O2 levels in arterioles from the nonoccluded and collateral-dependent region. Taken together with our vascular reactivity studies, our data indicate that enhanced H2O2 levels contribute significantly to adaptations in the coronary microcirculation following exercise training. Furthermore, adaptations in H2O2 signaling with exercise training appear to compensate for loss of endothelium-dependent vasodilation in the collateral-dependent region.
It is curious that while bradykinin-stimulated H2O2 levels were elevated in nonoccluded and collateral-dependent arterioles of exercise-trained pigs, dilation to bradykinin was augmented only in collateral-dependent arterioles after exercise training. Furthermore, no difference in the effect of PEG-catalase was noted in nonoccluded arterioles of sedentary and exercise-trained pigs. Although these findings are difficult to reconcile, we postulate that potential cross talk between various signaling pathways of vasodilation may alter the contribution of individual pathways, limiting overall dilation. Other investigators have cited reports of the interaction and interdependence of multiple pathways of endothelium-derived vasodilators (53). Furthermore, we reported previously that multiple mediators appear to be enhanced by exercise training in the underlying setting of ischemic heart disease and proposed that this may reflect redundant pathways that cooperate to preserve arterial relaxation/dilation (1). Alternatively, combined chronic occlusion and exercise training may stimulate increased expression and/or activity of signaling pathway components downstream of H2O2 that are not altered by exercise training alone, enhancing vasodilation in collateral-dependent arterioles of exercise-trained compared with sedentary pigs.
It is also interesting that, in the presence of DETC, dilation at high bradykinin concentrations was more pronounced in nonoccluded and collateral-dependent arterioles of exercise-trained than sedentary pigs. This effect did not attain statistical significance in arterioles from the nonoccluded region and attained statistical significance at only one concentration of bradykinin (3 nM) in arterioles from the collateral-dependent region. Because DETC specifically inhibits the copper-containing SOD isoforms Cu,Zn-SOD and extracellular SOD (6), activity of Mn-SOD may have contributed to the persistent bradykinin-mediated dilation at high concentrations in arterioles from exercise-trained pigs. In the presence of DETC, another vasodilatory mediator or pathway (H2O2-independent) also may have partially compensated to promote dilation at high bradykinin concentrations; however, one would speculate that the same mediator would have contributed in the presence of PEG-catalase also.
Our findings also support a role for an increased contribution of NOS to exercise-training-enhanced dilation at low bradykinin concentrations, despite the finding that enhanced dilation persisted in the presence of NOS inhibition at higher bradykinin concentrations. Our additional data demonstrate that eNOS and phosphorylated eNOS protein levels were significantly enhanced in coronary arterioles from the collateral-dependent region compared with the nonoccluded region, independent of exercise status. Thus the exercise-training-enhanced dilation at low bradykinin concentrations in the collateral-dependent region likely was not attributable to changes in eNOS or phosphorylated eNOS protein levels. These data suggest that another regulatory mechanism of eNOS enzyme activity likely played a role in the enhanced eNOS contribution at low bradykinin concentrations. Our eNOS protein data in coronary arterioles are consistent with our previous report that eNOS and phosphorylated eNOS protein levels were enhanced in epicardial artery segments by chronic occlusion, but not by exercise training (52). Furthermore, these findings are in contrast to our previous report that protein content of eNOS and phosphorylated eNOS (Ser1179) was elevated in small arteries from exercise-trained animals, with the greatest effect in collateral-dependent vasculature (19, 29). Interestingly, it has been reported previously that exercise training produces variable and heterogenous adaptations in total eNOS protein content depending on vessel size (29).
In the present study, apocynin significantly attenuated bradykinin-mediated vasodilation in arterioles from nonoccluded and collateral-dependent regions in exercise-trained animals. These findings suggest that NADPH oxidase provides a significant source of superoxide that either directly, or indirectly through its dismutation to H2O2, contributes to bradykinin-mediated dilation in coronary arterioles from exercise-trained pigs. These data are in contrast to previously reported rat skeletal muscle arteriole data, in which apocynin reduced flow-induced vasodilation in soleus muscle arterioles of young and old sedentary rats but not in those of exercise-trained animals (46). However, these investigators did demonstrate that exercise training augmented the relative increase in superoxide production by intraluminal flow, suggesting an exercise-training-induced increase in superoxide levels by a source other than NADPH oxidase (46).
ROS are produced by a variety of intracellular mechanisms, including mitochondrial sources, xanthine oxidase, NADPH oxidase, cytochrome P-450, cyclooxygenases, lipoxygenases, and uncoupled eNOS (2). NADPH oxidase is generally regarded as a predominant source of superoxide in the cardiovascular system (37, 40). In our study, exercise training significantly elevated the arteriolar protein level of the NADPH oxidase subunit p67phox, independent of occlusion. Previous studies have documented that exercise training did not alter p67phox protein levels in coronary arterioles from control animals (43), while p67phox was reduced and p47phox protein levels were not altered by exercise training in aortic endothelial cells isolated from the same animal model (42). However, exercise training has been reported to increase protein content of p47phox and p67phox, but not gp91phox, in rat neutrophils (32). Differences in animal models and cell and tissue types may account for the discrepancy in findings across the literature regarding the effects of exercise training on NADPH oxidase subunit expression levels. Furthermore, NADPH oxidase is composed of several subunits, so coordination of these subunits may contribute to altered enzyme activity, independent of protein content (42). In our study, exercise training resulted in increased protein levels of p67phox and an increase in the contribution of NADPH oxidase to bradykinin-mediated dilation, both independent of occlusion. Taken together, these data suggest that enhanced p67phox protein levels after exercise training may have contributed to increased NADPH oxidase activity.
In present study, we also found that Nox4 protein levels appeared to be more abundant than Nox1 and Nox2 in porcine coronary arterioles. Consistent with these data, previous studies also have reported that Nox4 is expressed at markedly higher levels than Nox2 in vascular endothelial cells (50), suggesting that Nox4 may play a more pronounced role in vascular endothelial cells. Nox4 is thought to act as an oxygen sensor and contribute to increased ROS generation and cell proliferation (23, 31). In our pig model, exercise training bouts likely cause periods of ischemia/hypoxia in the collateral-dependent region (41); thus we postulate that Nox4-induced superoxide generation may be altered in the collateral-dependent region, despite no change in protein levels. Additionally, it has been suggested that Nox4 may generate more H2O2 than superoxide and, thus, contribute directly to H2O2-mediated vasodilation (26, 34).
Study limitations.
Although DCF is often used experimentally to detect intracellular H2O2, literature suggests that it also reacts with other ROS and that confirmation of H2O2 detection by DCF is generally accomplished by treatment with PEG-catalase (7). On the basis of our findings that PEG-catalase eliminated the bradykinin-stimulated DCF signal in our cannulated microvessels, we postulated that the DCF signal was representative of H2O2. On the other hand, use of PEG-catalase may influence the equilibrium flux across the handling processes of other ROS through the creation of a H2O2 sink. For example, scavenging of H2O2 may increase the forward flux of superoxide through SOD, altering the concentration of other ROS that may have been detected by DCF. Similarly, use of PEG-catalase in our studies of bradykinin-mediated dilation may have also altered other ROS upstream of H2O2. Although use of the SOD inhibitor DETC provided additional evidence suggesting that the exercise-training-enhanced bradykinin-mediated dilation was attributable to H2O2, the specificity of DETC is equivocal (25).
Furthermore, inclusion of experiments with combined l-NAME and PEG-catalase treatment may have provided additional insight into a potential source of ROS in these studies, as well as the interaction of these two mediators. Drouin et al. (8) demonstrated that N-nitro-l-arginine (NOS inhibitor) + catalase had no additive inhibitory effects on acetylcholine-mediated dilation compared with catalase alone in mouse cerebral arterioles. They ultimately concluded that uncoupled eNOS was the source for superoxide/H2O2 generation in their model (8). On the basis of our data, we anticipate that pretreatment of arterioles with l-NAME + PEG-catalase would have an additive effect in arterioles from exercise-trained pigs. These findings would suggest that the PEG-catalase-sensitive component is, at least in part, independent of NOS. We base our prediction on our finding that bradykinin-mediated dilation had a significant component that was apocynin-sensitive (Fig. 7), suggesting that an NADPH oxidase-derived ROS contributed to bradykinin-induced dilation in arterioles from exercise-trained pigs. Because arterioles from sedentary pigs did not demonstrate sensitivity to apocynin, there is potential that pretreatment with l-NAME + PEG-catalase would not have an additive effect. Future studies will more thoroughly explore potential sources of ROS, as well as downstream signaling pathway components and their potential interaction, that may contribute to exercise-training-enhanced, endothelium-dependent vasodilation.
Clinical implications and conclusions.
Despite extensive evidence for therapeutic benefits of physical activity, the cellular and molecular mechanisms by which regular exercise improves vascular function in the setting of coronary artery disease are only beginning to be explored. Understanding adaptive responses of the coronary microcirculation to disease and interventional therapies is important in coronary artery disease, because these small arteries and arterioles determine coronary vascular resistance and, therefore, coronary blood flow (33). Historically, the production of ROS has been implicated in the development of cardiovascular pathophysiology (5, 38). Interestingly, accumulating evidence indicates that relatively low concentrations of ROS can function as important signaling molecules under physiological conditions and may be involved in normal regulation of vascular structure and function (10, 35, 36, 44, 49). Our current studies provide new evidence that exercise training appears to enhance the contribution of ROS to improvements in endothelium-mediated dilation in collateral-dependent coronary arterioles distal to chronic coronary artery occlusion/stenosis. These exciting findings suggest the novel concept that the superoxide/H2O2 signaling pathway, as well as the NO signaling pathway, may play critical roles in the adaptive responses of the coronary microcirculation in the diseased heart in response to exercise training.
GRANTS
These studies were supported by National Institutes of Health Grants R01-HL-064931 (C. L. Heaps and J. L. Parker) and R01-GM-046441 (Principal Investigator, Paul A. Lindahl).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.X. and C.L.H. are responsible for conception and design of the research; W.X. performed the experiments; W.X. and C.L.H. analyzed the data; W.X., J.L.P., and C.L.H. interpreted the results of the experiments; W.X. and C.L.H. prepared the figures; W.X. drafted the manuscript; W.X., J.L.P., and C.L.H. edited and revised the manuscript; W.X., J.L.P., and C.L.H. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the technical and surgical expertise of Mildred Mattox and excellent technical contributions of Jeff Bray and Erin Ashmore. The authors thank Drs. Guichun Han, Katrin Hinrichs, and Maya Scott for discussions pertaining to these studies and Drs. Jessica Garber Morales and Paul Lindahl for contributions of relevant preliminary electron paramagnetic resonance studies.
REFERENCES
- 1. Boyle RR, Heaps CL. Exercise training enhances multiple endothelium-derived vasodilatory pathways in coronary arteries from ischemic hearts (Abstract). FASEB J 25: 1056.4, 2011 [Google Scholar]
- 2. Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Carter WO, Narayanan PK, Robinson JP. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J Leukoc Biol 55: 253–258, 1994 [DOI] [PubMed] [Google Scholar]
- 4. Celermajer DS. Endothelial dysfunction: does it matter? Is it reversible? J Am Coll Cardiol 30: 325–333, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Chrissobolis S, Faraci FM. The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends Mol Med 14: 495–502, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Didion SP, Kinzenbaw DA, Schrader LI, Faraci FM. Heterozygous CuZn superoxide dismutase deficiency produces a vascular phenotype with aging. Hypertension 48: 1072–1079, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension 49: 717–727, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drouin A, Thorin-Trescases N, Hamel E, Falck JR, Thorin E. Endothelial nitric oxide synthase activation leads to dilatory H2O2 production in mouse-cerebral arteries. Cardiovasc Res 73: 73–81, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Drouin A, Thorin E. Flow-induced dilation is mediated by Akt-dependent activation of endothelial nitric oxide synthase-derived hydrogen peroxide in mouse cerebral arteries. Stroke 40: 1827–1833, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faraci FM. Hydrogen peroxide: watery fuel for change in vascular biology. Arterioscler Thromb Vasc Biol 26: 1931–1933, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Faraci FM. Reactive oxygen species: influence on cerebral vascular tone. J Appl Physiol 100: 739–743, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Fogarty JA, Delp MD, Muller-Delp JM, Laine GA, Parker JL, Heaps CL. Neuropilin-1 is essential for enhanced VEGF(165)-mediated vasodilatation in collateral-dependent coronary arterioles of exercise-trained pigs. J Vasc Res 46: 152–161, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fukai T, Siegfried MR, Ushio-Fukai M, Griendling KK, Harrison DG. Modulation of extracellular superoxide dismutase expression by angiotensin II and hypertension. Circ Res 85: 23–28, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494–501, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Griffin KL, Laughlin MH, Parker JL. Exercise training improves endothelium-mediated vasorelaxation after chronic coronary occlusion. J Appl Physiol 87: 1948–1956, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 107: 3152–3158, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, Schoene N, Schuler G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med 342: 454–460, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Heaps CL, Jeffery EC, Laine GA, Price EM, Bowles DK. Effects of exercise training and hypercholesterolemia on adenosine activation of voltage-dependent K+ channels in coronary arterioles. J Appl Physiol 105: 1761–1771, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heaps CL, Mattox ML, Kelly KA, Meininger CJ, Parker JL. Exercise training increases basal tone in arterioles distal to chronic coronary occlusion. Am J Physiol Heart Circ Physiol 290: H1128–H1135, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heaps CL, Parker JL, Sturek M, Bowles DK. Altered calcium sensitivity contributes to enhanced contractility of collateral-dependent coronary arteries. J Appl Physiol 97: 310–316, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heaps CL, Sturek M, Price EM, Laughlin MH, Parker JL. Exercise training restores adenosine-induced relaxation in coronary arteries distal to chronic occlusion. Am J Physiol Heart Circ Physiol 278: H1984–H1992, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104: 2673–2678, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T, Hoidal J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-β1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol 296: L489–L499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kang LS, Reyes RA, Muller-Delp JM. Aging impairs flow-induced dilation in coronary arterioles: role of NO and H2O2. Am J Physiol Heart Circ Physiol 297: H1087–H1095, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kober T, Konig I, Weber M, Kojda G. Diethyldithiocarbamate inhibits the catalytic activity of xanthine oxidase. FEBS Lett 551: 99–103, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Larsen BT, Bubolz AH, Mendoza SA, Pritchard KA, Jr, Gutterman DD. Bradykinin-induced dilation of human coronary arterioles requires NADPH oxidase-derived reactive oxygen species. Arterioscler Thromb Vasc Biol 29: 739–745, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Larsen BT, Gutterman DD, Sato A, Toyama K, Campbell WB, Zeldin DC, Manthati VL, Falck JR, Miura H. Hydrogen peroxide inhibits cytochrome p450 epoxygenases: interaction between two endothelium-derived hyperpolarizing factors. Circ Res 102: 59–67, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285: R277–R297, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Laughlin MH, Pollock JS, Amann JF, Hollis ML, Woodman CR, Price EM. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol 90: 501–510, 2001 [DOI] [PubMed] [Google Scholar]
- 30. LeBlanc AJ, Shipley RD, Kang LS, Muller-Delp JM. Age impairs Flk-1 signaling and NO-mediated vasodilation in coronary arterioles. Am J Physiol Heart Circ Physiol 295: H2280–H2288, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee YM, Kim BJ, Chun YS, So I, Choi H, Kim MS, Park JW. NOX4 as an oxygen sensor to regulate TASK-1 activity. Cell Signal 18: 499–507, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Levada-Pires A, Lambertucci R, Mohamad M, Hirabara S, Curi R, Pithon-Curi T. Exercise training raises expression of the cytosolic components of NADPH oxidase in rat neutrophils. Eur J Appl Physiol 100: 153–160, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Liu YP, Gutterman DD. Vascular control in humans: focus on the coronary microcirculation. Basic Res Cardiol 104: 211–227, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared with other NADPH oxidases. Cell Signal 18: 69–82, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest 106: 1521–1530, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res 92: E31–E40, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Mohazzab KM, Kaminski PM, Wolin MS. NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium. Am J Physiol Heart Circ Physiol 266: H2568–H2572, 1994 [DOI] [PubMed] [Google Scholar]
- 38. Mueller CF, Laude K, McNally JS, Harrison DG. ATVB in focus: redox mechanisms in blood vessels. Arterioscler Thromb Vasc Biol 25: 274–278, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Pagano PJ, Ito Y, Tornheim K, Gallop PM, Tauber AI, Cohen RA. An NADPH oxidase superoxide-generating system in the rabbit aorta. Am J Physiol Heart Circ Physiol 268: H2274–H2280, 1995 [DOI] [PubMed] [Google Scholar]
- 40. Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97: 1916–1923, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roth DM, Maruoka Y, Rogers J, White FC, Longhurst JC, Bloor CM. Development of coronary collateral circulation in left circumflex ameroid-occluded swine myocardium. Am J Physiol Heart Circ Physiol 253: H1279–H1288, 1987 [DOI] [PubMed] [Google Scholar]
- 42. Rush JW, Turk JR, Laughlin MH. Exercise training regulates SOD-1 and oxidative stress in porcine aortic endothelium. Am J Physiol Heart Circ Physiol 284: H1378–H1387, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Rush JWE, Laughlin MH, Woodman CR, Price EM. SOD-1 expression in pig coronary arterioles is increased by exercise training. Am J Physiol Heart Circ Physiol 279: H2068–H2076, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Sato A, Sakuma I, Gutterman DD. Mechanism of dilation to reactive oxygen species in human coronary arterioles. Am J Physiol Heart Circ Physiol 285: H2345–H2354, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ Res 74: 349–353, 1994 [DOI] [PubMed] [Google Scholar]
- 46. Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol 587: 3885–3897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Subramaniam R, Fan XJ, Scivittaro V, Yang JQ, Ha CE, Petersen CE, Surewicz WK, Bhagavan NV, Weiss MF, Monnier VM. Cellular oxidant stress and advanced glycation endproducts of albumin: caveats of the dichlorofluorescein assay. Arch Biochem Biophys 400: 15–25, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Thengchaisri N, Shipley R, Ren Y, Parker J, Kuo L. Exercise training restores coronary arteriolar dilation to NOS activation distal to coronary artery occlusion: role of hydrogen peroxide. Arterioscler Thromb Vasc Biol 27: 791–798, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Ungvari Z, Wolin MS, Csiszar A. Mechanosensitive production of reactive oxygen species in endothelial and smooth muscle cells: role in microvascular remodeling? Antioxid Redox Signal 8: 1121–1129, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal 7: 308–317, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor WR, Griendling KK. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension 32: 488–495, 1998 [DOI] [PubMed] [Google Scholar]
- 52. Zhou M, Widmer RJ, Xie W, Jimmy Widmer A, Miller MW, Schroeder F, Parker JL, Heaps CL. Effects of exercise training on cellular mechanisms of endothelial nitric oxide synthase regulation in coronary arteries after chronic occlusion. Am J Physiol Heart Circ Physiol 298: H1857–H1869, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhou MS, Raij L. Cross-talk between nitric oxide and endothelium-derived hyperpolarizing factor: synergistic interaction? J Hypertens 21: 1505–1512, 2003 [DOI] [PubMed] [Google Scholar]