Abstract
Splanchnic hemodynamics and tilt table tolerance were assessed after an infusion of placebo or octreotide acetate, a somatostatin analog whose vascular effects are largely confined to the splanchnic circulation. We hypothesized that reductions in splanchnic blood flow (SpBF) and splanchnic vascular conductance (SpVC) would be related to improvements in tilt table tolerance. In randomized, double-blind, crossover trials, hemodynamic variables were collected in 14 women and 16 men during baseline, 70° head-up tilt (HUT), and recovery. A repeated-measures analysis of variance was used to compare changes from baseline with respect to sex and condition. HUT elicited an increase in heart rate and decreases in mean arterial pressure, cardiac index, stroke index, and systemic vascular conductance. Additionally, SpVC and non-SpVC were lower during HUT. Octreotide reduced SpBF and SpVC and increased systemic vascular conductance and non-SpVC. Changes in SpBF and SpVC between supine and HUT were smaller in women (P < 0.05). Tilt table tolerance was increased after administration of octreotide [median tilt time: 15.7 vs. 37.0 min (P < 0.05) and 21.8 vs. 45.0 min (P < 0.05) for women and men, respectively]. A significant relationship existed between change (Δ) in SpBF (placebo-octreotide) and Δtilt time in women (Δtilt time = 2.5–0.0083 ΔSpBF, P < 0.01), but not men (Δtilt time = 3.41–0.0008 ΔSpBF, P = 0.59). In conclusion, administration of octreotide acetate improved tilt table tolerance, which was associated with a decrease in SpVC. In women, but not men, the magnitude of reduction in SpBF was positively associated with improvements in tilt tolerance.
Keywords: orthostatic intolerance, splanchnic blood flow, octreotide
orthostatic intolerance (OI), the inability to maintain blood pressure (BP) in the upright posture, can affect a wide range of individuals. For instance, OI can manifest itself in individuals who have undergone deconditioning due to bed rest (24, 45), in patients with autonomic dysfunction (16), or in astronauts upon return from spaceflight (25, 34). Additionally, some (9, 29, 46, 47), but not all (13, 42), studies suggest that women are less orthostatically tolerant than men. The mechanism(s) is likely multifactorial; however, because such varied populations can experience OI, it is plausible that these groups share common hemodynamic characteristics that make them more susceptible to presyncope.
We previously reported that women demonstrated a blunted reduction in splanchnic vascular conductance (SpVC) during head-up tilt (HUT) and that the women also tended to be less orthostatically tolerant than the men (18). Our finding was consistent with Arbeille et al. (2), who examined the portal vein constrictor response to HUT after 90 days of bed rest. They identified “tolerant” and “nontolerant” individuals, noting that the portal vein cross-sectional area decreased from supine to HUT in the tolerant individuals; however, this was not observed in the nontolerant group (2). Taken together, these findings (2, 18) suggest that the inability to adequately vaso- and venoconstrict the splanchnic circulation influences an individual's tolerance to an orthostatic stress. Additionally, these studies imply that therapies targeting splanchnic vasoconstriction and vasoconstrictor reserve should improve orthostatic tolerance.
While several approaches may be used to elicit splanchnic vasoconstriction, administration of a somatostatin (sst) analog offers a quasi-selective intervention because of the preferential distribution of sst2 and sst5 receptors in the gut. For example, Hoeldtke et al. (17) administered octreotide acetate, an sst analog with a biological half-life considerably longer than native sst, to patients with postprandial hypotension. They noted improvements in BP maintenance that were greater than those observed with midodrine, an α1-adrenergic agonist (17). In a separate study, Hoeldtke et al. (16) reported improvements with standing times in patients with OI after administration of octreotide. While Hoeldtke et al. (16, 17) did not measure splanchnic blood flow (SpBF), their findings support the idea that selective constriction of the splanchnic circulation could lead to improvements in BP maintenance. However, to the best of our knowledge, no group has fully characterized splanchnic and systemic hemodynamics after the administration of the octreotide with respect to orthostatic tolerance.
Thus the purpose of this randomized, double-blinded crossover study was as follows: 1) to characterize splanchnic and systemic hemodynamics in response to octreotide acetate; and 2) to determine the effect of selective splanchnic vasoconstriction on tilt table tolerance. We hypothesized that octreotide acetate would reduce SpBF and SpVC, and that these reductions would lead to improvements in tilt table tolerance. Additionally, we hypothesized that the improvement in tilt tolerance would be greater in the women.
MATERIALS AND METHODS
Subjects
Thirty subjects (14 women, 16 men) gave written, informed consent to participate in the study, which was approved by the Institutional Review Board at the Pennsylvania State University. Descriptive characteristics of the subjects are outlined in Table 1. Exclusionary criteria included the following: smoking, body mass index <20 kg/m2 or ≥30 kg/m2, hormonal contraceptive use, autonomic dysfunction or clinically diagnosed OI, peripheral vascular disease, and allergies to local anesthetics, shellfish, iodides, penicillin, or sulphonamides.
Table 1.
Subject characteristics
| Women | Men | |
|---|---|---|
| Age, yr | 23 ± 6 | 23 ± 5 |
| Height, cm | 164.6 ± 6.6* | 180.0 ± 6.3 |
| Weight, kg | 62.6 ± 12.5* | 82.2 ± 7.4 |
| BMI, kg/m2 | 23 ± 3 | 25 ± 2 |
| AD, m2 | 1.68 ± 0.18* | 2.01 ± 0.11 |
| V̇o2max, ml · kg−1 · min−1 | 39.3 ± 6.3* | 47.1 ± 6.6 |
| Hematocrit, % | 38 ± 3* | 44 ± 2 |
| Plasma volume, ml/kg | 29.4 ± 10.1 | 32.3 ± 4.0 |
| Blood volume, ml/kg | 50.1 ± 11.2* | 57.8 ± 7.4 |
Values are means ± SD. BMI, body mass index; AD, body surface area; V̇o2max, maximal oxygen uptake.
Significantly different from men, P < 0.05.
Subjects were admitted to the General Clinical Research Center at the Pennsylvania State University for study visits and were instructed to fast for at least 8 h; they also refrained from alcohol intake for 24 h and caffeine intake for 48 h before each study visit. Female participants were tested during the early follicular phase of the menstrual cycle (days 2–7). Minson et al. (27) previously reported a direct relationship between muscle sympathetic nerve activity and plasma estrogen concentration. Thus the early follicular phase was chosen because estrogen would be the lowest; this should have maximized our ability to detect any sex differences because sympathetic activity differences between the sexes should have been the greatest. Female subjects submitted urine samples for pregnancy testing during screening, as well as on the day of the study.
Experimental Design
Subjects completed a total of five visits. On the first visit, subjects had a 12-lead resting electrocardiogram and BP measurements, and blood drawn for a complete blood count, basic blood chemistry profile, and coronary risk profile. Subjects returned within 1 wk for a physical exam, evaluation of medical history, and a maximal graded treadmill exercise test. The splanchnic extraction ratio of indocyanine green (ICG) and the plasma volume were determined during the third visit. During the fourth and fifth visits, subjects underwent a tilt tolerance test with and without octreotide; these last two visits were randomly assigned. Blood was also collected in the female subjects on the fourth and fifth visits for hormone analyses.
Measurements
Extraction ratio of ICG and plasma volume determination.
We determined the hepatic extraction ratio of ICG (Akorn, Lake Forest, IL), based on a two-compartment model (14). The subject had one antecubital intravenous (18–20 gauge) catheter placed into each arm. The left arm was used for bolus injection and infusion, whereas the right arm was used for blood sample withdrawal. Subjects were supine for a minimum of 30 min before withdrawal of an aliquot of blood to serve as a spectrophotometer blank, followed by the bolus injection of 0.5 mg/kg ICG. After the intravenous bolus injection, a 3-ml blood sample was withdrawn every 5 min, followed by a 3-ml saline flush. Samples were centrifuged at 3,000 rpm for 20 min, and the plasma concentration of ICG was measured by spectrophotometry (805 nm for absorbance and 910 nm for turbidity). A separate extraction ratio was calculated for each subject from the exponential decay of the plasma disappearance curve of ICG (SigmaPlot 9.0, San Rafael, CA) fit utilizing the Marquardt-Levenberg algorithm. In addition to the extraction ratio, plasma volume was estimated for each subject from the extrapolated time zero plasma concentration of dye.
Hormone analyses.
Blood was withdrawn during the fourth and fifth visits for analysis of estrogen, progesterone, luteinizing hormone, and follicle-stimulating hormone. Plasma was separated and frozen at −80°C and sent to the Pennsylvania State University Hershey Medical Center Core Laboratory (Hershey, PA) for analysis of hormone concentration via radioimmunoassay.
Tilt tolerance test.
The placebo and octreotide conditions were randomly assigned during the visits when tilt tolerance was assessed. On these visit days, the subjects received a brief physical exam by a General Clinical Research Center clinician, followed by catheter placement (one antecubital in each arm, the right arm for ICG and placebo/octreotide, and the left arm for blood sample withdrawal). Subjects were then moved to the laboratory and instrumented for electrocardiogram (3-lead ECG; Hewlett-Packard 78534A) and BP (via arterial applanation tonometry and brachial oscillometric determinations, Colin 7000; Colin Medical Instruments). Subjects were instructed to lay supine on a modified tilt table (model OT-9003, Omni Technologies) with arms outstretched and supported at the level of the heart. The room temperature was kept thermoneutral at 24 ± 1°C for all testing.
Fifty minutes after instrumentation, subjects received an infusion of either placebo (50 ml saline) or octreotide acetate (100 μg or 125 μg octreotide acetate mixed with 50 ml of saline for the women and men, respectively) over 15 min. The doses were chosen based on the average weight of each sex. Ninety minutes after instrumentation and baseline measurements, the subject was tilted 70° head-up. Subjects were instructed to stand quietly with feet shoulder width apart. Subjects remained in this position for 45 min, until presyncope, or at the subject's request to stop. Presyncope was defined as loss of hemodynamic stability [decrease in BP >20/10 mmHg and/or rapid decline in heart rate (HR) >25 beats/min], diaphoresis, nausea, light headedness, and/or hyperventilation. At the onset of presyncope, the subject was placed in the Tredelenburg position (−10°) until BP and HR stabilized. Once BP and HR were stabilized, the subject was moved to supine. Twenty minutes of recovery data were collected in the supine position.
HR and BP.
HR and beat-by-beat BP were sampled continuously at 1,000 and 100 Hz, respectively, and stored in beat-to-beat format using a customized data collection program. BP was also obtained via the oscillometric method for determinations of systemic vascular conductance (SVC) and SpVC. Radial artery tonometric estimates of intra-arterial BP have been validated previously during HUT (40).
Cardiac output and stroke volume.
Before data recording began, the subject was instructed on how to perform the cardiac output (Q̇c) rebreathing maneuver and was allowed to perform at least two practice maneuvers. We used the acetylene (C2H2) rebreathing technique as described by Triebwasser et al. (44), which has been previously validated against direct Fick and thermodilution (19). A mass spectrometer (Perkin-Elmer MGA 1000) was used to analyze the gas concentrations during the 20-s rebreathing period. Q̇c was measured every 10-min during baseline and recovery, except during the infusion of placebo or octreotide. A Q̇c measurement was obtained upon HUT and then every 10 min during tilt. To correct for size differences, Q̇c was normalized to cardiac index (Q̇i) (Q̇i = Q̇c/BSA), where body surface area (BSA) = 0.202·kg0.425·m0.725 (11). Stroke volume (SV) was calculated as Q̇c/HR and normalized to SV index (SVi = SV/BSA).
SpBF.
SpBF was determined from continuous infusion of ICG (14). Blank samples for spectrophotometry were drawn before the ICG bolus and infusion. Time zero began on the priming of the bolus injection, followed immediately by continuous infusion of ICG (0.5 mg/min) until the experiment was completed. Every 5 min throughout baseline, tilt, and recovery, a 3-ml blood sample was drawn, followed by a 3-ml saline flush. Plasma ICG concentration was determined in the same manner as the extraction ratio procedure. To determine SpBF, we first determined splanchnic plasma flow (SpPF) by the following equation: SpPF = {I − [(Ca2 − Ca1)/dt]·PV}/(ER·Ca), where I is infusion rate (mg/min); Ca2 and Ca1 are ICG concentrations at times 2 and 1, respectively; dt is time between samples; PV is plasma volume; ER is extraction ratio of ICG; and Ca is ICG concentration. SpBF was then calculated as SpPF/(1 − hematocrit).
Vascular conductance.
Systemic vascular conductance (SVC) [SVC = Q̇c/mean arterial pressure (MAP)] was calculated using BP obtained via the oscillometric method. SpVC was calculated as SpVC = SpBF/MAP. Non-SpVC was calculated from the difference between SVC and SpVC (non-SpVC = SVC − SpVC). Since the numerator and the denominator scale to body size, the quotients (i.e., conductance) were not normalized to body size.
Data Summary and Statistical Analyses
Data were summarized as 5-min averages. The infusion of octreotide or placebo started 50 min after the subject was placed in the supine position and was complete 25 min before tilting began. Baseline was designated as minute 80 to minute 85, or 10 min before tilt. For those completing the entire 45-min tilt, minutes 40–45 were used to represent the tilt stage. For those individuals who became presyncopal, we used a 3-min average of data during the last 5 min of the tilt with the 2 min immediately preceding tilt termination excluded from analyses. This approach avoided the statistical problems associated with subject dropouts during the tilt by considering all subjects at a similar orthostatic stress (i.e., hemodynamic stability just before presyncope). Recovery included data collected 10 min after the subject was returned to the supine position.
Kaplan-Meier survival analysis and Mood's median test were used to assess differences in tilt tolerance between the sexes and conditions. Sex differences were analyzed using repeated-measures ANOVA (sex × stage × treatment). Data were expressed as changes from baseline (after the infusion) to HUT (Δbaseline). A repeated-measures ANOVA was also used to assess the time course of treatment (pre and post) and HUT using a baseline before infusion of octreotide or placebo. Tukey post hoc analysis was used when significance was found. The LIFEREG procedure in SAS was used to assess the relationship between the change in SpBF and the change in time between the two conditions. LIFEREG allows for regression analysis to be performed on right censored data, since we ended tilt tests at 45 min, if individuals did not become presyncopal. Analyses were performed using SAS 9.1 (Cary, NC). Data are presented as means ± SD. In all cases, differences with P < 0.05 were considered significant.
RESULTS
Table 1 summarizes the subject characteristics. The groups were different in height, weight, BSA, maximum O2 uptake, hematocrit, and blood volume (ml/kg), but age, body mass index, and plasma volume (ml/kg) were not different between the groups. Table 2 outlines the baseline hormonal data for the women for both experimental visits. There was no difference in estrogen, progesterone, luteinizing hormone, or follicle-stimulating hormone between the two visits.
Table 2.
Baseline hormonal profile
| Placebo | Octreotide | |
|---|---|---|
| Estrogen, pg/ml | 26 ± 10 | 25 ± 8 |
| Progesterone, ng/ml | 0.5 ± 0.4 | 0.4 ± 0.3 |
| Luteinizing hormone, mIU/ml | 5 ± 3 | 4 ± 2 |
| Follicle-stimulating hormone, mIU/ml | 8 ± 3 | 8 ± 2 |
Values are means ± SD; n = 11, estrogen; n = 6, progesterone; n = 7, luteinizing hormone; n = 11, follicle- stimulating hormone.
Tilt Tolerance
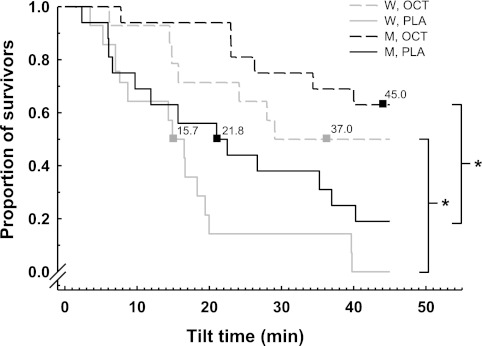
Figure 1 illustrates the median tilt times and proportion of individuals surviving 70° HUT after administration of placebo or octreotide. Both women and men demonstrated improvements in tilt time after administration of octreotide, where 7 out of 14 women (vs. 0 during placebo) and 10 out of 16 men (vs. 3 during placebo) completed 45 min of tilt. Median tilt times were improved from 15.7 to 37.0 min (P < 0.05) for the women and 21.8 to 45.0 min (P < 0.05) for the men. There was no difference between the sexes in the median tilt time after the administration of octreotide.
Fig. 1.
Median tilt times and proportion of individuals surviving 70° head-up tilt (HUT) after administration of placebo (PLA) or octreotide (OCT). Both women (W) and men (M) demonstrated improvements in tilt time after administration of OCT, where 7 of 14 women and 10 of 16 men completed 45 min of tilt. Median tilt times were improved from 15.7 to 37.0 min (P < 0.05) for the women and 21.8 to 45.0 min (P < 0.05) for the men. There was no difference between the sexes in the median tilt time after the administration of OCT. *Difference between conditions, P < 0.05.
Systemic Hemodynamics
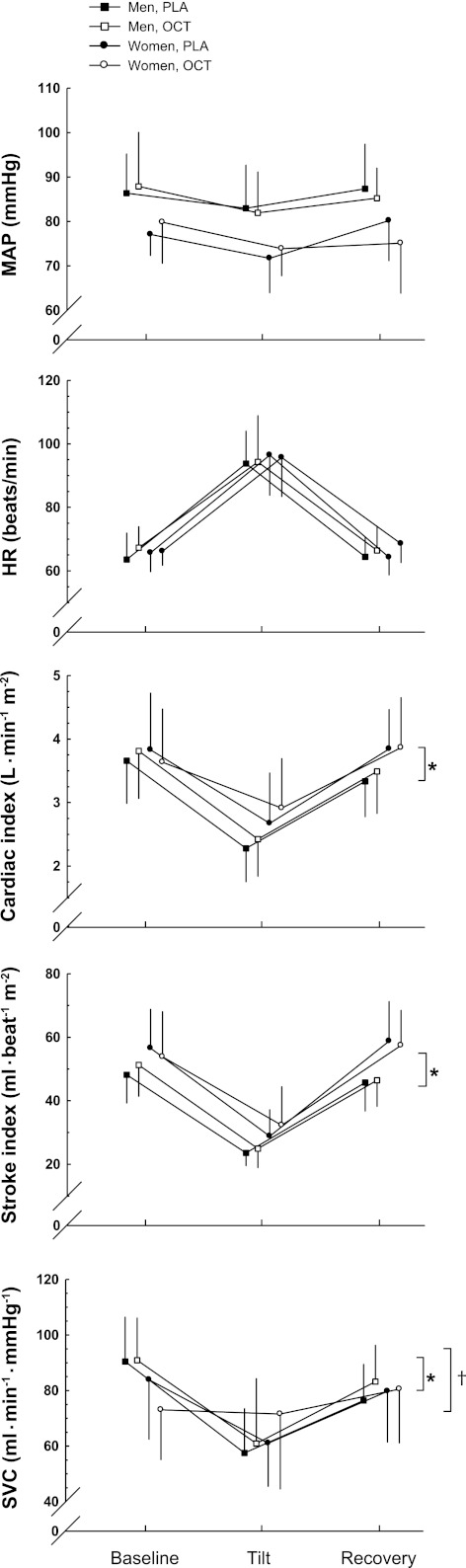
HUT induced significant changes in all hemodynamic variables (P < 0.01) (Table 3). Table 3 also shows that women had lower MAP (main effect, P < 0.01), higher Q̇i (main effect, P < 0.05), higher SVi (main effect, P < 0.05), and lower SVC (main effect, P < 0.05) compared with the men. Changes (Δ, tilt-baseline, Fig. 2) in MAP and HR, elicited by tilt, were not different between the sexes. However, ΔQ̇i and ΔSVi were smaller in the women (both P < 0.05). Octreotide induced an increase in SVC in the women, which is likely the contributing factor to an overall sex effect (P < 0.001) and treatment effect of octreotide (P < 0.05).
Table 3.
Hemodynamic variables
| Women |
Men |
||||||
|---|---|---|---|---|---|---|---|
| Baseline | Tilt d | Recovery | Baseline | Tilt | Recovery | Significance | |
| Placebo | |||||||
| MAP, mmHg | 77 ± 5 | 72 ± 8 | 80 ± 9 | 86 ± 9 | 83 ± 10 | 87 ± 10 | a |
| HR, beats/min | 66 ± 6 | 96 ± 13 | 64 ± 6 | 64 ± 8 | 94 ± 10 | 64 ± 6 | |
| Q̇i, l · min−1 · m−2 | 3.83 ± 0.90 | 2.67 ± 0.79 | 3.84 ± 0.62 | 3.66 ± 0.67 | 2.28 ± 0.53 | 3.33 ± 0.56 | a |
| SVi, ml · beat−1 · m−2 | 56.54 ± 12.39 | 28.81 ± 8.35 | 58.78 ± 12.46 | 48.19 ± 8.92 | 23.55 ± 4.11 | 45.74 ± 9.09 | a |
| SVC, ml · min−1 · mmHg−1 | 83.87 ± 21.50 | 61.05 ± 15.53 | 79.73 ± 18.29 | 90.47 ± 15.95 | 57.51 ± 16.10 | 76.43 ± 13.11 | a,c |
| SpBF, ml/min | 1,174 ± 243 | 884 ± 300 | 1,087 ± 217 | 1,671 ± 391 | 1,124 ± 273 | 1,637 ± 447 | b |
| SpVC, ml · min−1 · mmHg−1 | 14.83 ± 3.60 | 13.14 ± 4.28 | 12.99 ± 3.70 | 19.59 ± 4.94 | 15.59 ± 5.05 | 18.67 ± 5.05 | b |
| Non-SpVC, ml · min−1 · mmHg−1 | 69.04 ± 21.57 | 47.91 ± 15.34 | 66.30 ± 16.77 | 70.87 ± 14.19 | 41.92 ± 14.62 | 57.76 ± 13.60 | a,c |
| Octreotide | |||||||
| MAP, mmHg | 80 ± 9 | 74 ± 6 | 75 ± 11 | 88 ± 12 | 82 ± 9 | 85 ± 7 | a |
| HR, beats/min | 66 ± 4 | 96 ± 12 | 69 ± 6 | 67 ± 7 | 94 ± 15 | 66 ± 8 | |
| Q̇i, l · min−1 · m−2 | 3.63 ± 0.84 | 2.91 ± 0.78 | 3.87 ± 0.79 | 3.81 ± 0.75 | 2.42 ± 0.59 | 3.49 ± 0.67 | a |
| SVi, ml · beat−1 · m−2 | 53.82 ± 14.24 | 32.29 ± 12.10 | 57.40 ± 11.09 | 51.23 ± 9.94 | 24.94 ± 6.09 | 46.43 ± 8.29 | a |
| SVC, ml · min−1 · mmHg−1 | 73.06 ± 17.93 | 71.54 ± 26.97 | 80.50 ± 19.33 | 90.91 ± 15.31 | 60.97 ± 23.44 | 83.19 ± 13.23 | a |
| SpBF, ml/min | 1,004 ± 201 | 697 ± 180 | 920 ± 164 | 1,496 ± 426 | 1,023 ± 291 | 1,644 ± 418 | b |
| SpVC, ml · min−1 · mmHg−1 | 11.82 ± 2.85 | 10.40 ± 2.40 | 9.78 ± 4.03 | 16.76 ± 4.53 | 12.29 ± 3.68 | 19.27 ± 7.31 | b,e |
| Non-SpVC, ml · min−1 · mmHg−1 | 61.24 ± 16.61 | 61.14 ± 25.44 | 70.72 ± 18.30 | 74.15 ± 16.32 | 48.68 ± 21.84 | 63.91 ± 17.38 | a |
Values are means ± SD. MAP, mean arterial pressure; HR, heart rate; Q̇i, cardiac index; SVi, stroke volume index; SVC, systemic vascular conductance; SpBF, splanchnic blood flow; SpVC, splanchnic vascular conductance. a Difference between sexes, main effect; b change in baseline sex × stage interaction; c change in baseline difference between treatments; d difference induced by head-up tilt, and e difference pre- and postinfusion: P < 0.05.
Fig. 2.
Change (Δ) in hemodynamic variables from baseline to HUT. ΔMean arterial pressure (MAP) and Δheart rate (HR) were comparable between the sexes; however, women demonstrated a smaller Δcardiac index and Δstroke volume index compared with the men. OCT induced an increase in systemic vascular conductance (SVC) in the women, which is likely the contributing factor to an overall sex effect and treatment effect of OCT. Values are means ± SD. *Difference between sexes, P < 0.05. †Difference between treatments, P < 0.05.
Splanchnic Hemodynamics
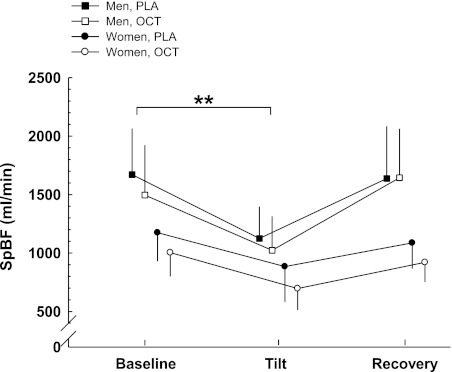
In most individuals, we saw an immediate reduction (20 ± 19%, P < 0.01) in SpBF after the infusion of octreotide was initiated, where the nadir was achieved within 10 min. However, baseline SpBF (30 min after the infusion was initiated) was not statistically different between the placebo and octreotide conditions (Table 3). HUT elicited a decrease in SpBF (Fig. 3; P < 0.01). Women demonstrated a smaller ΔSpBF compared with the men (P < 0.01).
Fig. 3.
ΔSplanchnic blood flow (SpBF) from baseline to HUT. The women demonstrated a smaller ΔSpBF compared with the men. Values are means ± SD. **Difference between sexes in the change between supine and HUT, P < 0.01.
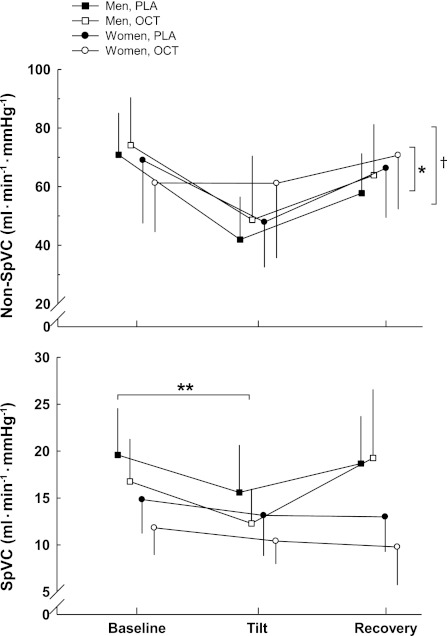
SpVC and non-SpVC (Fig. 4) were lower during tilt compared with supine (P < 0.01). Women had higher non-SpVC compared with the men (P < 0.01). The women showed little change in ΔSpVC compared with the men (P < 0.05). As expected, SpVC (Table 3) was reduced after administration of octreotide (P < 0.05). With respect to non-SpVC, octreotide elicited a treatment effect in the women that likely contributes to the overall treatment effect of octreotide, where non-SpVC was increased after the administration of the drug (P < 0.05).
Fig. 4.
ΔSplanchnic vascular conductance (SpVC) and non-SpVC from baseline to HUT. ΔSpVC was attenuated in the women where they showed little change from baseline compared with the men. OCT elicited a treatment effect in non-SpVC in the women that likely contributes to the overall treatment effect of OCT. Values are means ± SD. *Difference between sexes, P < 0.01. **Difference between sexes in the change between supine and HUT, P < 0.05. †Difference between treatments, P < 0.05.
Relationship between SpBF and tilt table tolerance.
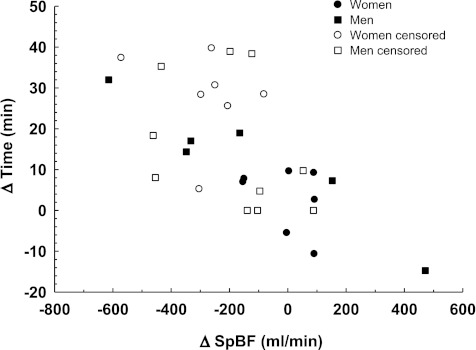
To explore the impact of the reduction in SpBF induced by octreotide on tilt tolerance, we examined the differences in average SpBF during tilt for both conditions (ΔSpBF) and assessed its relationship with changes in tilt tolerance (Δtilt time) (Fig. 5). Regression analysis indicated a significant relationship existed between ΔSpBF induced by octreotide acetate and Δtilt time in women (Δtilt time = 2.5 − 0.0083 ΔSpBF, P < 0.01), but not men (Δtilt time = 3.41 − 0.0008 ΔSpBF, P = 0.59).
Fig. 5.
The LIFEREG procedure revealed that a significant relationship existed between the ΔSpBF and Δtilt time in women (Δtilt time = 2.5 − 0.0083 ΔSpBF, P = 0.0051), but not men (Δtilt time = 3.41 − 0.0008 ΔSpBF, P = 0.59). The open symbols correspond to those points that were right censored with respect to time. Thus Δtilt time may be greater in these individuals than what is actually illustrated.
DISCUSSION
What we present that differs from past investigations is an assessment of splanchnic and systemic hemodynamics in humans after octreotide administration and its corresponding effect on tilt table tolerance. The principal findings from this investigation are as follows: 1) administration of the 100- to 125-μg octreotide acetate significantly reduced SpBF and SpVC and improved tilt table tolerance; this improvement was comparable between the sexes, contrary to our hypothesis; 2) on tilting, women demonstrated a blunted response in splanchnic hemodynamics compared with men; and 3) the systemic effects of octreotide acetate differed in the women. Thus the following section addresses the vascular effects of octreotide acetate, the functional implications of decreasing SpBF and SpVC, and the potential reasons for the different responses observed in women after octreotide administration.
The Vascular Effects of sst
The pharmacology and physiology of octreotide have been reviewed extensively (21, 35, 36). Briefly, five sst receptor subtypes (sst1–5) are expressed in the brain, stomach, liver, kidneys, intestines, and pancreas (33). Receptor subtypes sst2 and sst5 are preferentially located in the liver, stomach, and pancreas (33). Octreotide acetate is a synthetic octapeptide and is a sst analog that demonstrates high affinity for sst2 and sst5 and intermediate affinity for sst3 (36). The relatively long half-life of octreotide, ∼100 min vs. 1–3 min for native sst (41), makes it attractive for experimental and therapeutic manipulation of the splanchnic vascular bed.
The exact mechanism of octreotide, in terms of reducing SpBF, has not been clearly defined, but there are three potential candidates: modulation of gut vasodilatory hormones (23), an indirect vasoconstrictive effect via potentiation of vasoconstrictors such as endothelin-1 (48), and a direct vasoconstrictive effect (30). During pilot testing for the present study, we initially administered octreotide as a bolus injection and noted an immediate and dramatic pressor response (similar to phenylephrine); others (5, 32) have also reported near immediate reductions in SpBF. Thus the rapidity in which octreotide induces a reduction in SpBF suggests, at least, an initial direct vascular effect. It is likely that vasoconstriction occurs through an inositol 1,4,5-trisphosphate-mediated increase in Ca2+ release (30). μ-Opioid receptors have been identified in the circular muscle layer in the gastrointestinal tract (4). Murthy and Makhlouf (30) reported that administration of [d-Ala2, N-MePhe4, Gly-ol5]enkephalin, a μ-opioid receptor agonist, elicited smooth muscle contraction. Therefore, the initial rapid vasoconstriction may not be a direct effect of sst binding to its receptor, per se, but an indirect effect that acts through opioid receptors. Although there is compelling evidence to support this assertion, we cannot exclude the other potential mechanisms outlined above, which may occur after the initial decline in SpBF.
The Functional Implication of Reducing SpBF and SpVC
We previously reported that women were less able to vasoconstrict the splanchnic circulation because they showed no significant reduction in SpVC between supine and HUT (18). Because the splanchnic circulation is highly compliant and receives a large proportion of Q̇c during resting conditions, a gravity-induced shift in blood volume promotes pooling in compliant regions such as the gut (38). Individuals lacking the ability to adequately constrict this circulation reduce the effective circulating blood volume, decreasing venous return, cardiac preload, and, therefore, Q̇c; these reductions challenge the maintenance of BP (38). We previously outlined the candidate reasons for the blunted response observed in the splanchnic circulation of women (18).
When women were exposed to octreotide acetate in the present investigation, there was a significant improvement in tilt table tolerance. However, we also noted a minimal vasoconstrictive effect of HUT during octreotide and placebo conditions with both SpVC and SpBF in women. This finding is generally consistent with studies of other circulations in women (7, 12, 22).
Although octreotide did not induce larger changes in SpBF or SpVC during HUT, it produced a lower flow and conductance compared with placebo conditions. The resultant diversion of blood from compliant to noncompliant circulations may increase the effective circulating blood volume (37, 50). Wong and Sheriff (49) recently reported modest but significant increases in right atrial pressure after administration of octreotide acetate in the conscious dog. Thus we speculate that right atrial filling pressure was supported by a reduction in blood flow to the gut.
It is not clear why the relationship between the change in SpBF and the change in tilt table tolerance was only significant in women. However, there are two plausible explanations for this finding. First, a greater percentage of the men's data were right censored because three men completed 45 min of tilt, not only during the octreotide trial, but during the placebo trial as well. Thus we do not have an assessment of the true improvement due to the octreotide intervention. This concern holds true for any subject's data that was censored during tilt. Alternatively, this finding might represent true physiological differences between women and men in terms of the vasoconstrictor response. For example, Kneale et al. (22) reported that women may have greater β2-adrenergic sensitivity compared with men. This suggests that differences in adrenergic receptor sensitivity and/or density might exist, which means it is also possible that women may have enhanced sensitivity to sst. Thus selective constriction with a sst analog (nonadrenergic pathway) could translate to larger improvements for this group.
Extending our findings to other populations that might experience splanchnic pooling, such as individuals having undergone bed rest (1, 2), we might speculate that octreotide administration would successfully improve orthostatic tolerance and BP maintenance. Additionally, bed rest mimics exposure to microgravity (10); therefore, we reason that astronauts could also benefit from administration of octreotide, namely on landing day when they are reintroduced to Earth's gravitational field.
Potential Explanations for the Divergent Response in SVC and Non-SpVC in Women After Octreotide Administration
Unexpectedly, we found a treatment effect of octreotide in non-SpVC. Even more unexpectedly, we found differences between the sexes with this response. An increase in SVC and non-SpVC suggests that another circulation is vasodilating in response to octreotide. Because it receives a large proportion of Q̇c at rest, the renal circulation is a logical consideration for the vasodilatory response. The effect of octreotide on renal blood flow has been reported as increase (20) or no change (31). One consistent finding, however, indicates that renin is significantly reduced after octreotide is administered (20, 39, 43). This may be of functional importance, since, during states of reduced renal perfusion pressure, such as during HUT, angiotensin II induces constriction in both the splanchnic and renal beds (6). Therefore, removal or blunting of renin activity could translate into increases in SVC and non-SpVC. Extending this hypothesis, it is tempting to speculate that the differential effect of octreotide in women can be explained by the interaction of estrogen and renin, since estrogen has been shown to modulate plasma renin activity (15). For example, Hirshoren et al. (15) examined several cardiovascular regulatory hormones throughout the menstrual cycle, reporting that the low-estrogen phase (consistent with our study population) was associated with the lowest renin concentration (15).
Octreotide and Its Effect on the Autonomic and Enteric Nervous Systems
Neurons in the rostral ventrolateral medulla (RVLM) are opioid receptor rich (26) and are located in a key brain structure involved with cardiovascular regulation. Previous studies (8, 28) have indicated that microinjections of sst or its agonists into the RVLM of rats induces a substantial decrease in MAP (8, 28), as well as a marked decrease in splanchnic sympathetic nerve activity (28). These studies demonstrate the region-specific actions of sst. That is, an intravenous infusion of a sst analog did not induce the hypotensive response seen with a microinjection into the RVLM in other studies. This is likely due to the route of administration, since an intravenous infusion would be associated with drug degradation (via blood and tissues, such as the liver), leading to a smaller concentration that would eventually pass the blood-brain barrier (3). However, we cannot exclude the possibility that octreotide also altered cardiovascular regulation through other mechanisms, such as through a direct effect on the autonomic and enteric nervous systems.
In conclusion, populations unable to adequately vasoconstrict the splanchnic region might be susceptible to OI. We pharmacologically induced splanchnic vasoconstriction with a sst analog, which decreased SpBF and SpVC. These reductions led to improvements in tilt table tolerance in both women and men. Similar to previous reports that women demonstrate an attenuated constrictor response, the women in our study showed a blunted response in splanchnic hemodynamics to HUT. However, only in the women was the improvement in tilt time significantly related to the reduction in SpBF induced by octreotide.
GRANTS
This study was supported by National Aeronautics and Space Administration (NASA) NNJ04HF45G (to J. A. Pawelczyk), National Institutes of Health MO1RR10732 (to General Clinical Research Center), and the NASA Harriet G. Jenkins Pre-doctoral fellowship (to S. S. Jarvis).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.S.J., J.P.F., M.J.C., and J.A.P. conception and design of research; S.S.J., J.P.F., M.J.C., and J.A.P. performed experiments; S.S.J. analyzed data; S.S.J., J.P.F., M.J.C., and J.A.P. interpreted results of experiments; S.S.J. prepared figures; S.S.J. drafted manuscript; S.S.J., J.P.F., M.J.C., and J.A.P. edited and revised manuscript; S.S.J., J.P.F., M.J.C., and J.A.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Sandy Smithmyer, Christopher Sirolli, Matt Hinkley, the General Clinical Research Center, and Critical Care Systems for assistance with this study.
REFERENCES
- 1. Arbeille P, Kerbeci P, Mattar L, Shoemaker JK, Hughson R. Insufficient flow reduction during LBNP in both splanchnic and lower limb areas is associated with orthostatic intolerance after bedrest. Am J Physiol Heart Circ Physiol 295: H1846–H1854, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Arbeille PP, Besnard SS, Kerbeci PP, Mohty DM. Portal vein cross-sectional area and flow and orthostatic tolerance: a 90-day bed rest study. J Appl Physiol 99: 1853–1857, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Banks WA, Schally AV, Barrera CM, Fasold MB, Durham DA, Csernus VJ, Groot K, Kastin AJ. Permeability of the murine blood-brain barrier to some octapeptide analogs of somatostatin. Proc Natl Acad Sci U S A 87: 6762–6766, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bitar KN, Makhlouf GM. Selective presence of opiate receptors on intestinal circular muscle cells. Life Sci 37: 1545–1550, 1985 [DOI] [PubMed] [Google Scholar]
- 5. Bosch J, Kravetz D, Rodes J. Effects of somatostatin on hepatic and systemic hemodynamics in patients with cirrhosis of the liver: comparison with vasopressin. Gastroenterology 80: 518–525, 1981 [PubMed] [Google Scholar]
- 6. Boulpaep EL. Regulation of arterial pressure and cardiac output. In: Medical Physiology, edited by Boron WF, Boulpaep EL. Philadelphia, PA: Saunders, 2003, p. 534–557 [Google Scholar]
- 7. Bowyer L, Brown MA, Jones M. Vascular reactivity in men and women of reproductive age. Am J Obstet Gynecol 185: 88–96, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Burke PG, Li Q, Costin ML, McMullan S, Pilowsky PM, Goodchild AK. Somatostatin 2A receptor-expressing presympathetic neurons in the rostral ventrolateral medulla maintain blood pressure. Hypertension 52: 1127–1133, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Convertino VA. Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol Regul Integr Comp Physiol 275: R1909–R1920, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Dorfman TA, Levine BD, Tillery T, Peshock RM, Hastings JL, Schneider SM, Macias BR, Biolo G, Hargens AR. Cardiac atrophy in women following bed rest. J Appl Physiol 103: 8–16, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 17: 863–871, 1916 [PubMed] [Google Scholar]
- 12. Ergul A, Shoemaker K, Puett D, Tackett RL. Gender differences in the expression of endothelin receptors in human saphenous veins in vitro. J Pharmacol Exp Ther 285: 511–517, 1998 [PubMed] [Google Scholar]
- 13. Geelen G, Laitinen T, Hartikainen J, Lansimies E, Bergstrom K, Niskanen L. Gender influence on vasoactive hormones at rest and during a 70 degrees head-up tilt in healthy humans. J Appl Physiol 92: 1401–1408, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Grainger SL, Keeling PW, Brown IM, Marigold JH, Thompson RP. Clearance and non-invasive determination of the hepatic extraction of indocyanine green in baboons and man. Clin Sci (Lond) 64: 207–212, 1983 [DOI] [PubMed] [Google Scholar]
- 15. Hirshoren N, Tzoran I, Makrienko I, Edoute Y, Plawner MM, Itskovitz-Eldor J, Jacob G. Menstrual cycle effects on the neurohumoral and autonomic nervous systems regulating the cardiovascular system. J Clin Endocrinol Metab 87: 1569–1575, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Hoeldtke RD, Bryner KD, Hoeldtke ME, Hobbs G. Treatment of postural tachycardia syndrome: a comparison of octreotide and midodrine. Clin Auton Res 16: 390–395, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Hoeldtke RD, Horvath GG, Bryner KD, Hobbs GR. Treatment of orthostatic hypotension with midodrine and octreotide. J Clin Endocrinol Metab 83: 339–343, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Jarvis SS, Florian JP, Curren MJ, Pawelczyk JA. Sex differences in vasoconstrictor reserve during 70 deg head-up tilt. Exp Physiol 95: 184–193, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Jarvis SS, Levine BD, Prisk GK, Shykoff BE, Elliott AR, Rosow E, Blomqvist CG, Pawelczyk JA. Simultaneous determination of the accuracy and precision of closed-circuit cardiac output rebreathing techniques. J Appl Physiol 103: 867–874, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Kalambokis G, Economou M, Fotopoulos A, Al Bokharhii J, Pappas C, Katsaraki A, Tsianos EV. The effects of chronic treatment with octreotide versus octreotide plus midodrine on systemic hemodynamics and renal hemodynamics and function in nonazotemic cirrhotic patients with ascites. Am J Gastroenterol 100: 879–885, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Katz MD, Erstad BL. Octreotide, a new somatostatin analogue. Clin Pharm 8: 255–273, 1989 [PubMed] [Google Scholar]
- 22. Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol 36: 1233–1238, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Law SF, Woulfe D, Reisine T. Somatostatin receptor activation of cellular effector systems. Cell Signal 7: 1–8, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Levine BD, Zuckerman JH, Pawelczyk JA. Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation 96: 517–525, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Meck JV, Waters WW, Ziegler MG, deBlock HF, Mills PJ, Robertson D, Huang PL. Mechanisms of postspaceflight orthostatic hypotension: low alpha1-adrenergic receptor responses before flight and central autonomic dysregulation postflight. Am J Physiol Heart Circ Physiol 286: H1486–H1495, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Milner TA, Pickel VM, Reis DJ. Ultrastructural basis for interactions between central opioids and catecholamines. I. Rostral ventrolateral medulla. J Neurosci 9: 2114–2130, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Miyawaki T, Goodchild AK, Pilowsky PM. Activation of mu-opioid receptors in rat ventrolateral medulla selectively blocks baroreceptor reflexes while activation of delta opioid receptors blocks somato-sympathetic reflexes. Neuroscience 109: 133–144, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Montgomery LD, Kirk PJ, Payne PA, Gerber RL, Newton SD, Williams BA. Cardiovascular responses of men and women to lower body negative pressure. Aviat Space Environ Med 48: 138–145, 1977 [PubMed] [Google Scholar]
- 30. Murthy KS, Makhlouf GM. Opioid mu, delta, and kappa receptor-induced activation of phospholipase C-beta 3 and inhibition of adenylyl cyclase is mediated by Gi2 and G(o) in smooth muscle. Mol Pharmacol 50: 870–877, 1996 [PubMed] [Google Scholar]
- 31. Ottesen LH, Aagaard NK, Kiszka-Kanowitz M, Rehling M, Henriksen JH, Pedersen EB, Flyvbjerg A, Bendtsen F. Effects of a long-acting formulation of octreotide on renal function and renal sodium handling in cirrhotic patients with portal hypertension: a randomized, double-blind, controlled trial. Hepatology 34: 471–477, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Panes J, Pique JM, Bordas JM, Casadevall M, Teres J, Bosch J, Rodes J. Effect of bolus injection and continuous infusion of somatostatin on gastric perfusion in cirrhotic patients with portal-hypertensive gastropathy. Hepatology 20: 336–341, 1994 [PubMed] [Google Scholar]
- 33. Patel YC, Srikant CB. Somatostatin receptors. Trends Endocrinol Metab 8: 398–405, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Platts SH, Ziegler MG, Waters WW, Mitchell BM, Meck JV. Midodrine prescribed to improve recurrent post-spaceflight orthostatic hypotension. Aviat Space Environ Med 75: 554–556, 2004 [PubMed] [Google Scholar]
- 35. Reichlin S. Somatostatin. N Engl J Med 309: 1495–1501, 1983 [DOI] [PubMed] [Google Scholar]
- 36. Reynaert H, Geerts A. Pharmacological rationale for the use of somatostatin and analogues in portal hypertension. Aliment Pharmacol Ther 18: 375–386, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Rowell LB. Human Circulation: Regulation During Physical Stress. New York: Oxford Press, 1986 [Google Scholar]
- 38. Rowell LB. Human Cardiovascular Control. New York: Oxford University Press, 1993 [Google Scholar]
- 39. Sabat M, Guarner C, Soriano G, Bulbena O, Novella MT, Ortiz J, Ricart E, Villanueva C, Rosello J, Rodriguez J, Balanzo J. Effect of subcutaneous administration of octreotide on endogenous vasoactive systems and renal function in cirrhotic patients with ascites. Dig Dis Sci 43: 2184–2189, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Sato T, Nishinaga M, Kawamoto A, Ozawa T, Takatsuji H. Accuracy of a continuous blood pressure monitor based on arterial tonometry. Hypertension 21: 866–874, 1993 [DOI] [PubMed] [Google Scholar]
- 41. Sheppard M, Shapiro B, Pimstone B, Kronheim S, Berelowitz M, Gregory M. Metabolic clearance and plasma half-disappearance time of exogenous somatostatin in man. J Clin Endocrinol Metab 48: 50–53, 1979 [DOI] [PubMed] [Google Scholar]
- 42. Shvartz E, Meyerstein N. Tilt tolerance of young men and young women. Aerospace Med 41: 253–255, 1970 [PubMed] [Google Scholar]
- 43. Sieber C, Gnadinger M, Del Pozo E, Shaw S, Weidmann P. Effect of a new somatostatin analogue SMS 201–995 (Sandostatin) on the renin-aldosterone axis. Clin Endocrinol (Oxf) 28: 25–32, 1988 [DOI] [PubMed] [Google Scholar]
- 44. Triebwasser JH, Johnson RL, Burpo RP, Campbell JC, Reardon WC, Blomqvist CG. Noninvasive determination of cardiac output by a modified acetylene rebreathing procedure utilizing mass spectrometer measurements. Aviat Space Environ Med 48: 203–209, 1977 [PubMed] [Google Scholar]
- 45. Watenpaugh DE, O'Leary DD, Schneider SM, Lee SM, Macias BR, Tanaka K, Hughson RL, Hargens AR. Lower body negative pressure exercise plus brief postexercise lower body negative pressure improve post-bed rest orthostatic tolerance. J Appl Physiol 103: 1964–1972, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Waters WW, Ziegler MG, Meck JV. Postspaceflight orthostatic hypotension occurs mostly in women and is predicted by low vascular resistance. J Appl Physiol 92: 586–594, 2002 [DOI] [PubMed] [Google Scholar]
- 47. White DD, Montgomery LD. Pelvic blood pooling of men and women during lower body negative pressure. Aviat Space Environ Med 67: 555–559, 1996 [PubMed] [Google Scholar]
- 48. Wiest R, Tsai MH, Groszmann RJ. Octreotide potentiates PKC-dependent vasoconstrictors in portal-hypertensive and control rats. Gastroenterology 120: 975–983, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Wong BJ, Sheriff DD. Impact of a somatostatin analog on vascular capacity in conscious dogs (Abstract). FASEB J 21: A949, 2007 [Google Scholar]
- 50. Zidon TM, Sheriff DD. Diversion of blood flow from noncompliant to compliant vasculature in awake dogs: mechanical impact on right atrial pressure. Am J Physiol Heart Circ Physiol 290: H217–H223, 2006 [DOI] [PubMed] [Google Scholar]