Abstract
Diseases that result in muscle weakness, e.g., heart failure, are characterized by elevated sphingomyelinase (SMase) activity. In intact muscle, SMase increases oxidants that contribute to diminished muscle force. However, the source of oxidants, specific processes of muscle contraction that are dysfunctional, and biochemical changes underlying the weakness elicited by SMase remain unknown. We tested three hypotheses: 1) SMase-induced depression of muscle force is mediated by mitochondrial reactive oxygen species (ROS), 2) SMase depresses force and calcium sensitivity of the contractile apparatus, and 3) SMase promotes oxidation and phosphorylation of myofibrillar proteins. Our experiments included intact muscle bundles, permeabilized single fibers, and isolated myofibrillar proteins. The mitochondrial-targeted antioxidant d-Arg-2′,6′-dimethyl-Tyr-Lys-Phe-NH2, decreased cytosolic oxidants and protected intact muscle bundles from weakness stimulated by SMase. SMase depressed maximal calcium-activated force by 20% in permeabilized single fibers (in kN/m2: control 117 ± 6; SMase 93 ± 8; P < 0.05). Calcium sensitivity of permeabilized single fibers decreased from 5.98 ± 0.03 (control) to 5.91 ± 0.02 (SMase; P < 0.05). Myofibrillar protein nitrotyrosines, carbonyls, and phosphorylation were unaltered by SMase. Our study shows that the fall in specific force of intact muscle elicited by SMase is mediated by mitochondrial ROS and can be attributed largely to dysfunction of the contractile apparatus.
Keywords: skeletal muscle, mitochondria, single fiber, sphingolipids, oxidative stress
sphingomyelinase (smase) is part of a group of enzymes that participate in the turnover of sphingolipids (16, 26, 33). Sphingolipids play critical roles in cellular homeostasis (25, 26). The sphingolipid ceramide, a product of sphingomyelin hydrolysis by SMase, impairs muscle glucose uptake (51), stimulates apoptosis (7, 41), disrupts mitochondrial oxidative phosphorylation (21), and increases cellular production of reactive oxygen species (ROS) and reactive nitrogen species (56). Heart failure and sepsis are diseases associated with a debilitating loss of skeletal muscle force and heightened activity of serum SMase (37, 57). Exogenous SMase decreases maximal tetanic force production in intact mouse skeletal muscle (20), which suggests that elevated SMase activity leads to muscle weakness in disease states (18). However, the mechanisms of muscle weakness elicited by SMase remain unclear.
Hypothesis I: SMase-induced depression of muscle force is mediated by mitochondrial ROS.
SMase and its reaction product, ceramide, increase ROS in whole muscle and cultured muscle cells (20). The nonspecific antioxidant N-acetylcysteine exerted partial protection against SMase-induced depression of force, suggesting ROS as mediators of SMase-induced muscle weakness. The exact source of ROS production elicited by SMase is unknown. In nonmuscle cells, SMase and ceramide stimulate ROS production by mitochondria (38, 56). In recent years, Szeto and Schiller (53) developed a mitochondria-targeted polypeptide with antioxidant action [d-Arg-2′,6′-dimethyl-Tyr-Lys-Phe-NH2 (SS-31)], which decreases skeletal muscle mitochondrial ROS emission (2) and protects from disuse-induced muscle weakness (44). To test hypothesis I, we determined the effects of the mitochondrial antioxidant SS-31 on cytosolic oxidants and force of skeletal muscle exposed to exogenous SMase.
Hypothesis II: SMase depresses force and calcium sensitivity of the contractile apparatus.
Heightened activity of SMase could disrupt any of the three main processes involved in muscle force production: 1) membrane depolarization, 2) sarcoplasmic reticulum calcium ion (Ca2+) release, and 3) Ca2+-activated force of the contractile apparatus. ROS, which are increased by SMase in skeletal muscle, are known to depress sarcolemmal excitability (54) and force of the contractile apparatus (3, 8, 32, 35). Of the three main processes mentioned above, Ca2+-activated force of the contractile apparatus seems to be the most sensitive to physiological levels of ROS (3, 45, 48). Therefore, we hypothesized that SMase depresses force and calcium sensitivity of the contractile apparatus.
Hypothesis III: SMase promotes oxidation and phosphorylation of myofibrillar proteins.
Oxidation of select myofibrillar proteins decreases force and Ca2+ sensitivity of the contractile apparatus (13, 29, 35, 43, 46). Similarly, the phosphorylation state of myofibrillar proteins modulates maximal force and Ca2+ sensitivity of the contractile apparatus (15, 40, 52). Ceramide activates protein kinases and phosphatases (25, 39). Activation of protein kinases and phosphatases that react with myofibrillar proteins can also be a secondary effect of ROS stimulated by SMase (5, 58). Hence, we hypothesized that SMase promotes oxidation and phosphorylation of myofibrillar proteins.
We used intact diaphragm bundles, chemically permeabilized single fibers, and myofibrillar proteins isolated from intact skeletal muscle bundles to test our hypotheses.
METHODS
Animals.
We studied C57BL/6J mice (6–8 wk old; Harlan, Indianapolis, IN). Animals were maintained in a 12:12-h dark:light cycle and received water and food ad libitum. Each animal was deeply anesthetized by inhalation of isoflurane (Aerrane, Baxter Healthcare, Deerfield, IL) and killed by removal of vital organs after cervical dislocation. All procedures conformed to the regulations for use and care of laboratory animals in the United States and were approved by the Institutional Animal Care and Use Committee of the University of Kentucky.
Experimental solutions.
In experiments using bundles of intact diaphragm fibers, we used bicarbonate-buffered physiological solution (in mM: 137 NaCl, 5 KCl, 1 MgSO4, 1 NaH2PO4, 24 NaHCO3, and 2 CaCl2), equilibrated with 95% O2–5% CO2 (pH ∼7.4). SS-31 (100 μM) was dissolved in the muscle buffer solution. For permeabilized single-fiber experiments, we used relaxing (in mM: 100 KCl, 20 imidazole, 4 ATP, 2 EGTA, and 7 MgCl2; pH adjusted to 7.0 using KOH), permeabilizing (1% Triton X-100 in relaxing solution), and pCa solutions, where pCa = −log10[Ca2+]. pCa solutions (pH 7.0 at 22°C) contained free Ca2+, ranging from 1 nM (pCa 9.0) to 32 μM (pCa 4.5), added as CaCl2, and (in mM) 20 imidazole, 14.5 creatine phosphate, 7 EGTA, 4 MgATP, and 1 free Mg2+. The pH of pCa solutions was adjusted to 7.0 at 22°C using KOH, and ionic strength was adjusted to 180 mM by adding KCl as needed. All chemicals were from Sigma-Aldrich (St. Louis, MO), except for CaCl2 (Orion ISE Calibration Standard, Thermo Scientific, Hudson, NH), used in pCa solution, ATP (Roche, Indianapolis, IN), and Triton X-100 (Thermo Scientific).
In vitro treatment.
We dissected two diaphragm fiber bundles/animal and mounted both separately for in vitro treatment, as described recently (20). Briefly, we tied the rib to a glass rod and attached the central tendon to a force transducer (BG Series, 100 g, Kulite, Leonia, NJ) using silk suture (4-0). The fiber bundle was placed in a water-jacketed organ bath containing Krebs buffer, continuously gassed with 95% O2–5% CO2. Prior to the experimental treatment, we positioned the fiber bundle at the length that elicited the highest twitch force (Lo). Fiber bundles were treated with SMase (0.5 U/ml) or diluent control (0.15% glycerol) in Krebs solution for 60 min at 37°C. For experiments using SS-31, muscles were pretreated with buffer plus vehicle (control) or buffer containing SS-31 for 15 min before adding recombinant SMase to the bath (total exposure: SMase 45 min; SS-31 or vehicle 60 min). SMase was purchased from Sigma-Aldrich (Staphylococcus aureus; Cat. No. S8633). SS-31 was purchase from the W. M. Keck Foundation (Large Scale Peptide Synthesis Laboratory, Yale University, New Haven, CT). After treatment, we processed fiber bundles using protocols described below to test each hypothesis.
Cytosolic oxidant activity.
This technique was described in depth in our recent study (20). Briefly, we used the fluorochrome probe 2′,7′-dichlorofluorescin diacetate (DCFH-DA; Molecular Probes, Eugene, OR) at 20 μM to measure overall cytosolic oxidant activity (36, 47). The DCFH-DA crosses the cell membrane and is cleaved into DCFH by intracellular esterases. DCFH is oxidized into 2′,7′-dichlorofluorescein (DCF), which emits the fluorescence signal in proportion to the oxidants in the cytosol. The measurement area is ∼0.27 mm2, and fluorescence from the entire field of view is quantified and recorded for statistical analysis (4). In our previous study, we observed that SMase increased cytosolic oxidants (20). In the present study, our focus was on SS-31 effects on cytosolic oxidants in diaphragm preparations exposed to SMase. Hemidiaphragms used in positive-control studies (SMase vs. vehicle) were incubated in DCFH for 30 min before SMase exposure (20). In experiments focused on the effects of SS-31 on DCF fluorescence, hemidiaphragms were incubated in DCFH (20 μM), with or without SS-31 (10 μM), for 15 min prior to the addition of SMase (0.5 U/ml). After 30 min of incubation with SMase, we measured the oxidized derivative (DCF; 480 nm excitation, 520 nm emissions) and corrected for background emissions using an epifluorescence microscope (Eclipse TE2000, Nikon Instruments, Melville, NY), a charge-coupled device camera (CoolSNAP ES, Photometrics, Tucson, AZ), and a computer-controlled shutter in the excitation light pathway.
Contractile function of intact fiber bundles.
Fiber bundles were stimulated to contract using electrical field stimulation (supramaximal voltage, 0.25–0.30 ms pulse, and 250–300 ms train duration) via platinum electrodes at frequencies of 1–300 Hz. We measured force using a digital oscilloscope and computed cross-sectional area using muscle weight and Lo (14) to calculate specific force (in kN/m2). To test the role of mitochondrial ROS using SS-31, we measured skeletal muscle force at 37°C. All solutions used for measurement of muscle force contained D-tubocurarine (25 μM).
Permeabilized single-fiber mechanics.
We prepared permeabilized single fibers using a technique similar to that described by Campbell and Moss (9), as adapted for diaphragm muscle by our group (27). Immediately after SMase exposure (see In vitro treatment above), diaphragm fiber bundles were immersed in ice-cold relaxing solution; bundles used for isolation of single fibers were not subjected to electrically stimulated contractions. Fiber bundles were subdivided, tied to glass capillary tubes, and placed in skinning solution for 3 h at 4°C to achieve chemical permeabilization. We stored permeabilized fiber bundles in 50% v/v glycerol/relaxing solution at −20°C for up to 3 days before experiments. On the day of the experiment, we isolated single fibers from permeabilized bundles in ice-cold relax solution and mounted segments of individual fibers between a force transducer (403B, Aurora Scientific, Ontario, Canada) and a motor arm (312B, Aurora Scientific). After mounting the fiber, the temperature of the apparatus was set to 22°C and maintained throughout the experiment. Upon reaching the desired temperature, fibers were positioned at a sarcomere length of ∼2.60 μm in relax solution. We excluded from analyses single fibers that had no visible striation pattern at pCa 4.5 or developed force at pCa 6.4 > 10% maximum force. The latter responses were observed in two fibers, were not evident in previous studies of mouse diaphragm (27), and may relate to expression of type I myosin heavy chain (22).
Fiber length and diameter were measured using video microscopy. The fiber was transferred to pCa 9.0. Calcium-activated force was elicited by immersing the fiber in pCa solutions (22°C), ranging from 6.4 to 4.5. In each solution, force was allowed to plateau. We then performed a quick-release step by rapidly shortening the fiber by 20% of the segment length for 20 ms before returning to the initial length. The force response to this procedure was fitted using a single exponential equation starting at the residual force (9) to determine the rate of tension redevelopment (ktr). Experiments were performed and analyzed using SLControl software (10). The force–pCa relationship of individual fibers was analyzed using a four-parameter Hill equation (Prism 5.0b, GraphPad Software, La Jolla, CA): F = Fpas + Fo(10−pCa)nH/[(10−pCa)nH + (10−pCa50)nH], where Fpas is passive force, Fo is maximal active force, nH is the Hill coefficient, and pCa50 is the pCa that elicits half-maximal activation. Fo was also calculated by subtracting baseline force (pCa 9.0) from maximal Ca2+-activated force (pCa 4.5). We determined fiber “rundown” as the decrease in force at pCa 4.5 from the beginning to the end of the experiment. We studied three to five fibers/diaphragm fiber bundle (two fiber bundles/animal, control, and SMase; total of four mice). Data from individual fibers were fitted using the Hill equation, and parameters from fibers of the same bundle were averaged to yield a mean value for each animal.
Myofibrillar protein separation.
After SMase treatment, diaphragm muscles were quickly frozen in liquid nitrogen and stored at −80°C until further analysis. Myofibrillar proteins were separated by rinsing the diaphragm in ice-cold relax buffer (in mM: 75 KCl, 20 MOPS, pH 7, 2 MgCl2, 2 EGTA, 1 NaN3, 4 phosphocreatine, 1 ATP, 1 DTT, and 1 benzamidine) and homogenizing in relax plus 1% Triton, 10 mM EDTA, and protease, kinase, and phosphatase inhibitors (in mM: 30 sodium fluoride, 200 PMSF, 1 leupeptin, 1 pepstatin A, 400 EDTA, and 200 sodium orthovanadate). The homogenate was pelleted (P1) at 16,100 g (4°C) for 5 min and washed three times by resuspension in standard rigor buffer (in mM: 75 KCl, 20 MOPS, pH 7, 2 MgCl2, 2 EGTA, and 1 NaN3) plus Triton and inhibitors, followed by 5 min on ice and centrifugation at 16,100 g (4°C) for 5 min. P1 was then rinsed in standard rigor buffer and pelleted (P2) at 1,500 g (4°C) for 1 min. P2 was resuspended in K-60 (in mM: 60 KCl, 20 MOPS, and 2 MgCl2, pH 7.0) plus BSA (1 mg/ml) and inhibitors and pelleted (P3) at 1,500 g (4°C) for 1 min. P3 was resuspended in 100 μl K-60, BSA and inhibitors, plus 100 μl of 2 mM DTT in K-60 solution mixed 1:1 with glycerol and stored at −80°C.
Western blot analysis and protein phosphorylation.
To pellet myofibrils for analysis, P3 (see above) was thawed and centrifuged at 1,500 g for 2 min at 4°C. P3 was resuspended in 200 μl urea sample buffer (in M: 8 urea, 0.05 Tris, pH 6.8, 0.075 DTT, and 0.05% bromophenol blue) and heated at 98°C for 5 min. Myofibrillar proteins were fractionated on 15% Tris-HCl polyacrylamide gels (Criterion precast gels, Bio-Rad, Hercules, CA) at 200 V for 50 min (PowerPac HC, Bio-Rad). Gels were either transferred to membrane for Western blot analysis or fixed for testing protein phosphorylation. Total protein was measured by staining gels with SimplyBlue (Invitrogen, Life Technologies, Grand Island, NY).
Proteins were transferred to reduced-fluorescence polyvinylidene difluoride membrane (Immobilon-FL, Millipore, Billerica, MA) at 150 mA for ∼18 h at 4°C. Membranes were blocked in Odyssey blocking buffer (LI-COR, Lincoln, NE) for 6–8 h at room temperature. Primary antibodies were diluted 1:1,000 in blocking buffer, mixed 1:1 with PBS plus 0.2% Tween, and incubated overnight at room temperature, followed by four, 5-min washes. Carbonyl groups on myofibrillar protein side chains were detected by derivatization to 2,4-dinitrophenylhydrazone (DNPH) prior to loading protein aliquots on the gel (OxyBlot protein oxidation kit, Millipore). Isolated myofibrils (100 μg) were resuspended in 25 μl of 20 mM HEPES (pH 7.4) plus 100 μl of 10 mM DNPH in 2 M HCl [adapted from Barreiro et al. (6)] and incubated for 15 min at room temperature. We used aliquots of myofibrillar protein extracts, which did not undergo DNPH derivatization, to test for nonspecific binding of the primary antibody. Membranes were probed with anti-DNPH (OxyBlot kit). Membranes were then incubated with fluorescence-conjugated secondary antibodies in Odyssey blocking buffer, mixed 1:1 with PBS–0.2% Tween plus 0.01% SDS for 30 min, followed by four, 5-min washes. Fluorescent secondary antibodies were used for detection (goat anti-mouse Alexa-680, Molecular Probes/Invitrogen, Life Technologies; goat anti-rabbit IRD800, Rockland Antibodies & Assays, Gilbertsville, PA). After drying, membrane fluorescence was imaged, and results were quantified using the Odyssey infrared imaging system (LI-COR).
To detect phosphorylated myofibrillar proteins, gels were fixed in 10% glacial acetic acid/50% methanol (3×, 20 min), washed in water (6×, 5 min), stained in Pro-Q Diamond phosphoprotein gel stain (Molecular Probes/Invitrogen, Life Technologies) for 90 min, and de-stained (3×, 25 min in 15% 1.2-propanediol and 50 mM sodium acetate, pH 4). After transfer to fresh de-stain solution, gels were incubated overnight and washed in water (3×, 5 min). Gels were then scanned using a Typhoon imager (GE Healthcare, Piscataway, NJ), and images were analyzed using Odyssey software (LI-COR).
Statistics.
All comparisons were performed using commercially available software (Prism 5.0b, GraphPad Software; SigmaPlot v. 11, Systat Software, San Jose, CA). We used repeated measures ANOVA and Bonferroni's test for multiple comparisons to analyze the force-frequency relationship of intact fiber bundles. All other variables and parameters were analyzed using a paired t-test. Data from parameters describing the force–pCa relationship and ktr of individual permeabilized single fibers were averaged within each hemidiaphragm to obtain a representative value for each animal under the two conditions (control and SMase; n = 4/group). These were used for statistical comparisons among muscles. We preferred this conservative approach for analysis of single-fiber data to avoid pseudoreplication and minimize the risks of type I error. However, the outcome was essentially the same as that obtained by testing data from all fibers individually. Data are presented as mean ± SE. Statistical significance was accepted when P < 0.05.
RESULTS
SS-31 decreases cytosolic oxidant activity in SMase-treated diaphragm.
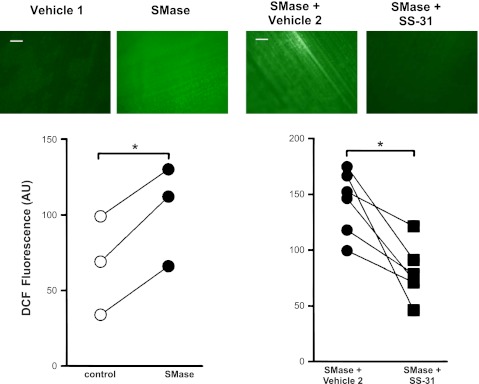
DCF fluorescence was unchanged by SS-31 under control conditions (SS-31 = 98 ± 8% control; P = 0.296 vs. control). However, DCF fluorescence of SMase-treated muscles decreased ∼45% by SS-31 in muscles treated with SMase (Fig. 1), suggesting that mitochondrial ROS contribute to the rise in cytosolic oxidants stimulated by SMase.
Fig. 1.
d-Arg-2′,6′-dimethyl-Tyr-Lys-Phe-NH2 (SS-31) decreases cytosolic oxidant activity in sphingomyelinase (SMase)-treated muscles. Images show 2′,7′-dichlorofluorescin (DCF) fluorescence in diaphragm bundles. Original scale bars (white) = 100 μm. All images were obtained at the same magnification. Data are arbitrary units of DCF fluorescence from the entire field of view in paired hemidiaphragms treated with SMase (0.5 U/ml), SS-31/SMase (100 μM), and respective vehicles. *P < 0.05 by t-test.
SS-31 protects against depression of force caused by SMase.
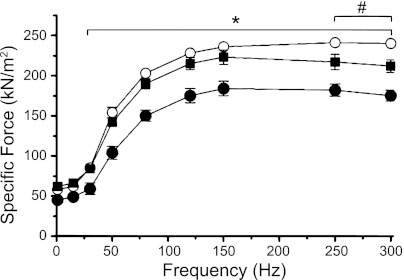
SMase depressed tetanic diaphragm force (Fig. 2; 30–150 Hz) results, consistent with our previous study (20). In muscles treated with SS-31/SMase, forces—elicited by stimulus frequencies, ranging from 1 to 150 Hz—were essentially the same as in vehicle controls. At 250 and 300 Hz, SS-31 protection of force was partial (Fig. 2). The relationship between relative force (% maximal tetanic) and stimulation frequency was unchanged by SMase and SS-31 (data not shown). These findings are consistent with our hypothesis that mitochondrial ROS cause depression of force after exposure to SMase. Subsequent results address potential targets and biochemical effects of ROS that might depress skeletal muscle force.
Fig. 2.
SS-31 protects against SMase-induced depression of specific force (kN/m2). Force measured at 37°C, normalized for cross-sectional area; n = 5/group. Groups are control (○), SS-31/SMase (■), and SMase (•). *P < 0.05 for control or SS-31/SMase vs. SMase; #P < 0.05 for control vs. SS-31/SMase. Statistical analysis: repeated measures ANOVA and Bonferroni's post hoc test.
Mechanics of permeabilized single fibers.
Permeabilized single fibers are unstable at 37°C, and experiments were performed at 22°C. In muscle, studies at room temperature depress cellular ROS production (4) and can blunt force depression via ROS-mediated mechanisms [ref. (17) and unpublished observations]. Therefore, in preliminary experiments, we tested for temperature dependence of SMase effects by measuring force of intact bundles at room temperature. The depression of force elicited by SMase was similar when force was measured at room temperature (data not shown) and 37°C (Fig. 2).
We performed experiments using a total of 31 permeabilized single fibers. Fibers accepted for formal analyses had segment lengths of 995 ± 91 μm (control) and 1,046 ± 34 μm (SMase). Cross-sectional areas were 870 ± 155 μm2 (control) and 1,050 ± 150 μm2 (SMase). Overall fiber rundown during the protocol was 10 ± 1.6% and 7.5 ± 0.4% in control and SMase, respectively. None of these variables differed between groups.
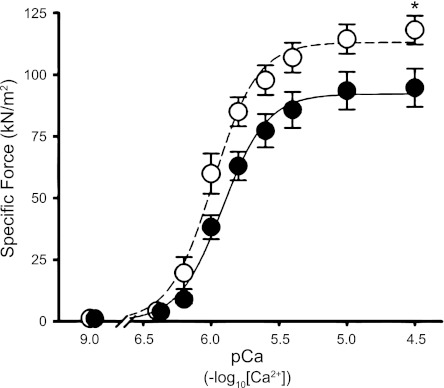
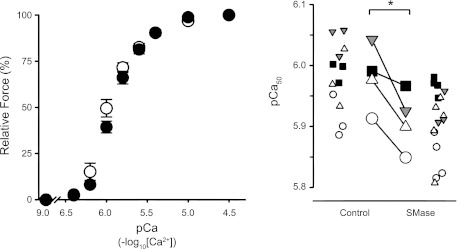
SMase treatment decreased specific force by 20 ± 5% at pCa 4.5 (Fig. 3). There was no difference in Fpas (pCa 9.0) between control and SMase groups (Fig. 3). Thus maximal Ca2+-activated force (force at pCa 4.5 − force at pCa 9.0) decreased from 117 ± 6 kN/m2 in control fibers to 93 ± 8 kN/m2 in SMase-treated fibers (P < 0.05; n = 4 muscles; mean of three to five fibers/muscle). SMase also decreased calcium sensitivity of the contractile apparatus, as shown by lower pCa50 values (Fig. 4). nH describes the slope of the sigmoidal force–pCa relationship and was unaltered by SMase treatment (control 2.82 ± 0.32; SMase 2.71 ± 0.21; n = 4 animals/group).
Fig. 3.
SMase depresses maximal specific force of myofibrillar proteins. Specific force in permeabilized single fibers. Solid and dashed lines depict line of best fit using the Hill equation. Data are from 12 (control) and 16 (SMase) permeabilized single fibers, which were averaged into 3–5 fibers/animal (n = 4). *P < 0.05.
Fig. 4.
SMase decreases calcium sensitivity of myofibrillar proteins. Left: data from Fig. 3, expressed as percentage of force at pCa 4.5 (where pCa = −log10[Ca2+]), to illustrate decrease in calcium ion (Ca2+) sensitivity. Right: pCa that elicited 50% of maximal calcium-activated force (pCa50). pCa50 was determined from fitting the data using the Hill equation for each individual fiber. Unique symbols depict data from individual fibers (small symbols) and averaged values for each animal (large symbols). For further details, see Fig. 3. *P < 0.05.
SMase exposure had marginal effects on fiber mechanics during the quick-release step. Residual force at pCa 4.5 tended to be less after SMase exposure (in kN/m2: control 62 ± 9; SMase 44 ± 4; P < 0.07). The ktr following the short release at pCa 4.5 tended to be slower with SMase treatment (in s−1: control 54 ± 2; SMase 45 ± 3; P < 0.11).
Post-translational modifications of myofibrillar proteins.
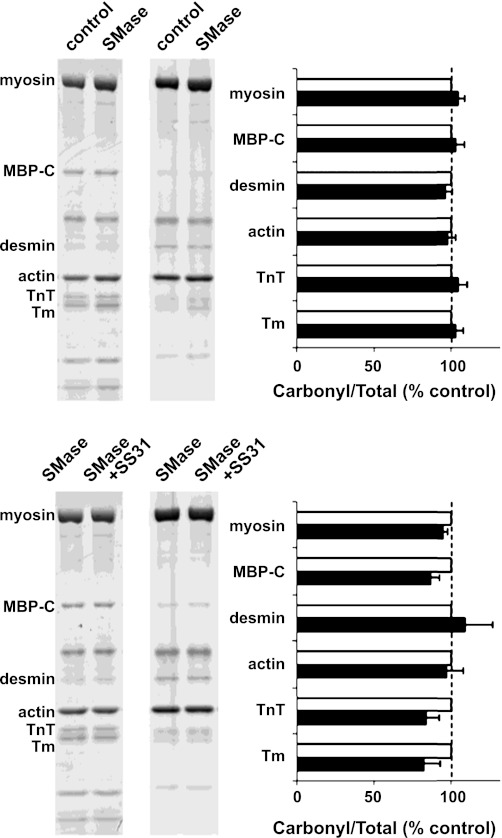
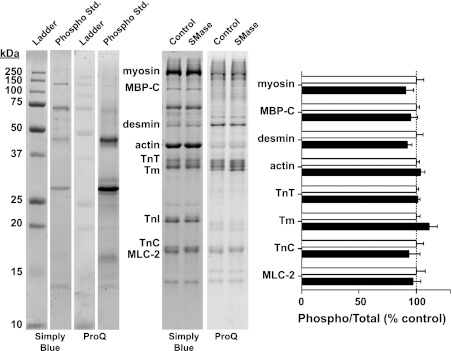
SMase exposure did not increase oxidation of myofibrillar proteins measured by DNPH-derivatized carbonyls (Fig. 5) or nitrotyrosines (data not shown). Enzyme-mediated modifications, phosphorylation (Fig. 6), and ubiquitin conjugation (data not shown) were also unaffected, nor did we observe changes in gel migration patterns of myofibrillar proteins stained with SimplyBlue.
Fig. 5.
Myofibrillar protein ·carbonyls are unchanged by SMase. Western blot images are from representative samples showing total protein gel (left pair of lanes) and probing of protein carbonyls by OxyBlot (right pair of lanes). Horizontal bar graph shows integrated intensity of protein carbonyls normalized for total protein signal/band (top: n = 3; bottom: n = 5). MBP-C, myosin-binding protein C; TnT, troponin T; Tm, tropomyosin.
Fig. 6.
Phosphorylation status of myofibrillar proteins. Left: representative samples of Western blot after probing with SimplyBlue for total protein and Pro-Q for phosphoprotein stains. SimplyBlue and Pro-Q staining of bands on ladder and phospho standard (std.) are consistent with Pro-Q probing phosphorylated proteins. TnI, troponin I; MLC, myosin light chain; TnC, troponin C. The fiber type distribution in mouse diaphragm is ∼10% type I, 30% type IIa, and 60% type IId/x (24). Thus both slow and fast isoforms of myofibrillar proteins are present in the gel, with a predominance of fast isoforms. Right: ratio of phosphorylated to total protein signal, obtained from bands on gels stained with Pro-Q and SimplyBlue, respectively. Data are expressed as percentage of mean from controls (n = 7).
DISCUSSION
In the present study, we sought to test three hypotheses: 1) SMase-induced depression of muscle force is mediated by mitochondrial ROS, 2) SMase depresses force and calcium sensitivity of the contractile apparatus, and 3) SMase promotes oxidation and phosphorylation of myofibrillar proteins. Our results support the first two hypotheses, but we found no evidence of carbonylation or phosphorylation of myofibrillar proteins as mechanisms for SMase-induced muscle weakness.
SS-31 lowers cytosolic oxidants and protects against depression of force caused by SMase.
SMase increases ceramide content in muscle cells (20). Ceramide enhances ROS production by mitochondria and NADPH oxidase (38, 56). Exogenous SMase increased cytosolic oxidants that lead to depression of muscle force (20). We found that SS-31 lowered cytosolic oxidants in SMase-treated muscles but not in controls. SS-31 preferentially localizes to the inner mitochondrial membrane and diminishes mitochondrial ROS in several cell types (2, 53, 59). The antioxidant effect of SS-31 arises from the presence of tyrosine (direct ROS scavenger) and facilitation of electron transport in the inner mitochondrial membrane (53, 55). SS-31 lowers mitochondrial ROS emission in skeletal muscle (2, 44) and has no effect on NADPH oxidase activity (44). SS-31 also prevented the increase in cytosolic oxidants and depression of force elicited by SMase—findings that support mitochondrial ROS as mediators of muscle weakness elicited by SMase. We cannot exclude the possibility that very low levels of cytosolic SS-31 might affect oxidant activity via a nonmitochondrial mechanism. However, SS-31 levels in the cytosol are two to three orders of magnitude lower than in mitochondria, which concentrate SS-31. This makes a cytosolic mechanism of action less likely. In combination, data from this study and our previous report (20) suggest that exogenous SMase increases ceramide in skeletal muscle, leading mitochondria to release ROS into the cytosol, which impairs myofibrillar protein function.
In our previous study, the antioxidant N-acetyl cysteine (NAC) exerted partial protection against depression of force elicited by SMase (20). The distribution of NAC to mitochondria is limited (53), and its antioxidant effects are dose dependent, which may explain the partial efficacy of NAC in protecting muscle force from SMase effects. SS-31 protection against SMase-induced weakness was also partial at 250 and 300 Hz (Fig. 2). After SMase exposure, tetanic force displays a “descending limb” at stimulus frequencies higher than 150 Hz (Fig. 2). The decreasing force at high-stimulus frequencies, plateau region in controls, suggests that SMase impairs membrane excitability and voltage-dependent Ca2+ release. A similar response is expected from control diaphragm muscles if stimulus frequencies are increased progressively beyond 300 Hz, as electrical pulses will reach the sarcolemma during the membrane refractory period. Because SS-31 did not normalize the descending limb of the force-frequency relationship, we consider that this effect of SMase is independent of mitochondrial ROS—likely a direct consequence of sphingomyelin hydrolysis on the biophysical properties of the membrane.
SMase promotes weakness through impairment of muscle contractile apparatus.
SMase effects on muscle, mentioned above, were evident at the lower temperatures necessary for studies in permeabilized single fibers. Weakness of an intact muscle can stem from impairments of membrane excitability, Ca2+ release, and/or myofibrillar protein function. Our experiments using permeabilized single fibers show that the primary determinant of weakness elicited by SMase is dysfunction of myofibrillar proteins (Fig. 3). The decrease in maximal calcium-activated force suggests a lower number of force-generating cross bridges and/or less force/cross bridge.
We also found a decrease in calcium sensitivity of the contractile apparatus. The change in pCa50 indicates a 25% increase in Ca2+ concentration necessary to elicit half-maximal force after SMase exposure. The mechanisms that control Ca2+ sensitivity in skeletal muscle are complex and not well understood. One possibility is that bound cross bridges activate the thin filament through a cooperative mechanism that facilitates further cross-bridge binding. This cooperative mechanism would lead to a steep tension-pCa curve and a relatively high pCa50 value (low Ca concentration). If this is the case, a reduction in the number of bound cross bridges in SMase-treated fibers could explain both the reduced force and lower pCa50. Other potential mechanisms involving the troponin (Tn)-tropomyosin complex are possible as well.
Post-translation modifications of myofibrillar proteins.
The decrease in maximal calcium-activated force and calcium sensitivity can be caused by covalent modifications, for example, oxidation (8, 29, 35), or phosphorylation of myofibrillar proteins (23, 34). Based on the SMase-induced increase in cytosolic oxidants and blunting of the depression of force by antioxidant treatment (20), we tested for oxidation of myofibrillar proteins. Several myofibrillar proteins are susceptible to oxidative and nitrosative modifications that impair function (12, 13, 29, 30, 42, 46). Notably, carbonylation of actin and tropomyosin are associated with myocardial contractile dysfunction in heart failure (11). In our experiments, the abundance of carbonyl groups or nitrotyrosines was unchanged in myofibrillar proteins of diaphragm bundles exposed to SMase for 1 h in vitro. A longer exposure to SMase may be necessary to elicit increases in myofibrillar protein carbonyls. However, our data indicate that myofibrillar protein carbonylation does not mediate the muscle weakness that we observed after SMase exposure.
Oxidation of thiol groups in myofibrillar proteins is an alternative cause for depression of maximal force and calcium sensitivity in single fibers (19, 32, 35). Disulfide exchange reactions [e.g., S-glutathiolation (35)] and formation of intermolecular disulfide bridges are potential modifications documented in myofibrillar proteins (11). However, thiol oxidation could not be detected using the reducing conditions required for optimal isolation of myofibrillar proteins (12, 13). Our preliminary attempts to isolate myofibrillar proteins from diaphragm bundles under nonreducing conditions resulted in low myofibrillar protein yields that precluded resolution of thiol oxidation using physiological concentrations of H2O2 (unpublished observations). More sophisticated methods will be necessary to define the myofbrillar redox proteomics that underlie the decrease in calcium sensitivity and maximal force triggered by SMase.
Ceramide activates protein kinases and phosphatases (23, 35). In cardiac myocytes, phosphorylation of TnI lowers Ca2+ sensitivity of the contractile apparatus (22), and dephosphorylation of both tropomyosin (48) and myosin-binding protein C (31) depresses maximal Ca2+-activated force. These effects are similar to those elicited by SMase in diaphragm bundles, and it is therefore possible that some of the functional changes elicited in the current experiments may have been produced by phosphorylation or dephosphorylation of skeletal tropomyosin (28), TnI (49), and/or myosin-binding protein C (1). However, our measurements with Pro-Q Diamond did not reveal statistically significant differences in the phosphorylation state of any of these sarcomeric proteins.
Limitations and methodological aspects.
Exogenous SMase acts on sphingomyelin localized on the extracellular leaflet of the plasma membrane (33, 50). This mechanism of action and downstream products will closely reflect the effects of secretory SMase in vivo (33, 50). However, the effects of SMase on skeletal muscle of patients will be a result of prolonged exposure to SMase. Our findings represent short-term effects of SMase on skeletal muscle (60 min). Whereas we anticipate that signaling pathways stimulated by SMase will be similar for acute vs. prolonged exposure to SMase, post-translational modifications undetected within 60 min might be seen after a prolonged period of heightened SMase activity. Thus our experiments may underestimate the impact of SMase on skeletal muscle contractile properties.
Conclusions.
Mitochondrial ROS increase cytosolic oxidants in muscle exposed to SMase, leading to a decrease in specific force. Muscle weakness elicited by SMase occurs largely due to decreases in calcium sensitivity and calcium-activated force of the contractile apparatus. Changes in the carbonylation or phosphorylation state of myofibrillar proteins are not involved in the contractile impairment elicited by SMase. We speculate that SMase results in muscle weakness due to oxidation of thiol groups in myofibrillar proteins.
GRANTS
Support for this study was provided by a National Institute of Arthritis and Musculoskeletal and Skin Diseases grant to M. B. Reid (R01 AR055974). L. F. Ferreira received funding from the American Heart Association (09POST2020082) and National Heart, Lung, and Blood Institute (1K99HL098453-01). K. S. Campbell was supported by a National Heart, Lung, and Blood Institute grant (HL 090749).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.F.F., S.S., K.S.C., and M.B.R. conception and design of research; L.F.F., J.S.M., S.S., and J.D.S. performed experiments; L.F.F., J.S.M., S.S., J.D.S., K.S.C., and M.B.R. analyzed data; L.F.F., J.S.M., S.S., J.D.S., K.S.C., and M.B.R. interpreted results of experiments; L.F.F., J.S.M., and J.D.S. prepared figures; L.F.F. drafted manuscript; L.F.F., J.S.M., K.S.C., and M.B.R. edited and revised manuscript; L.F.F., J.S.M., S.S., J.D.S., K.S.C., and M.B.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Laura A. A. Gilliam for technical support in our experiments using intact muscle bundles and isolation of myofibrillar proteins.
REFERENCES
- 1. Ackermann MA, Kontrogianni-Konstantopoulos A. Myosin binding protein-C slow is a novel substrate for protein kinase A (PKA) and C (PKC) in skeletal muscle. J Proteome Res 10: 4547–4555, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119: 573–581, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol 509: 565–575, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arbogast S, Reid MB. Oxidant activity in skeletal muscle fibers is influenced by temperature, CO2 level, and muscle-derived nitric oxide. Am J Physiol Regul Integr Comp Physiol 287: R698–R705, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Avner BS, Hinken AC, Yuan C, Solaro RJ. H2O2 alters rat cardiac sarcomere function and protein phosphorylation through redox signaling. Am J Physiol Heart Circ Physiol 299: H723–H730, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barreiro E, Gea J, Di FM, Kriazhev L, James S, Hussain SN. Protein carbonyl formation in the diaphragm. Am J Respir Cell Mol Biol 32: 9–17, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Birbes H, El Bawab S, Obeid LM, Hannun YA. Mitochondria and ceramide: intertwined roles in regulation of apoptosis. Adv Enzyme Regul 42: 113–129, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Callahan LA, She ZW, Nosek TM. Superoxide, hydroxyl radical, and hydrogen peroxide effects on single-diaphragm fiber contractile apparatus. J Appl Physiol 90: 45–54, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Campbell KS, Moss RL. History-dependent mechanical properties of permeabilized rat soleus muscle fibers. Biophys J 82: 929–943, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campbell KS, Moss RL. SLControl: PC-based data acquisition and analysis for muscle mechanics. Am J Physiol Heart Circ Physiol 285: H2857–H2864, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Canton M, Menazza S, Sheeran FL, Polverino de Laureto P, Di Lisa F, Pepe S. Oxidation of myofibrillar proteins in human heart failure. J Am Coll Cardiol 57: 300–309, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Canton M, Neverova I, Menabo R, Van EJ, Di LF. Evidence of myofibrillar protein oxidation induced by postischemic reperfusion in isolated rat hearts. Am J Physiol Heart Circ Physiol 286: H870–H877, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Canton M, Skyschally A, Menabo R, Boengler K, Gres P, Schulz R, Haude M, Erbel R, Di Lisa F, Heusch G. Oxidative modification of tropomyosin and myocardial dysfunction following coronary microembolization. Eur Heart J 27: 875–881, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Close RI. Dynamic properties of mammalian skeletal muscles. Physiol Rev 52: 129–197, 1972 [DOI] [PubMed] [Google Scholar]
- 15. Colson BA, Bekyarova T, Locher MR, Fitzsimons DP, Irving TC, Moss RL. Protein kinase A-mediated phosphorylation of cMyBP-C increases proximity of myosin heads to actin in resting myocardium. Circ Res 103: 244–251, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cowart LA. A novel role for sphingolipid metabolism in oxidant-mediated skeletal muscle fatigue. Focus on “Sphingomyelinase stimulates oxidant signaling to weaken skeletal muscle and promote fatigue”. Am J Physiol Cell Physiol 299: C549–C551, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Diaz PT, Brownstein E, Clanton TL. Effects of N-acetylcysteine on in vitro diaphragm function are temperature dependent. J Appl Physiol 77: 2434–2439, 1994 [DOI] [PubMed] [Google Scholar]
- 18. Doehner W, Bunck AC, Rauchhaus M, von Haehling S, Brunkhorst FM, Cicoira M, Tschope C, Ponikowski P, Claus RA, Anker SD. Secretory sphingomyelinase is upregulated in chronic heart failure: a second messenger system of immune activation relates to body composition, muscular functional capacity, and peripheral blood flow. Eur Heart J 28: 821–828, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Dutka TL, Mollica JP, Lamb GD. Differential effects of peroxynitrite on contractile protein properties in fast- and slow-twitch skeletal muscle fibers of rat. J Appl Physiol 110: 705–716, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Ferreira LF, Moylan JS, Gilliam LA, Smith JD, Nikolova-Karakashian M, Reid MB. Sphingomyelinase stimulates oxidant signaling to weaken skeletal muscle and promote fatigue. Am J Physiol Cell Physiol 299: C552–C560, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species Role of mitochondrial glutathione. J Biol Chem 272: 11369–11377, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Geiger PC, Cody MJ, Sieck GC. Force-calcium relationship depends on myosin heavy chain and troponin isoforms in rat diaphragm muscle fibers. J Appl Physiol 87: 1894–1900, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev 80: 853–924, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Gregorevic P, Plant DR, Leeding KS, Bach LA, Lynch GS. Improved contractile function of the mdx dystrophic mouse diaphragm muscle after insulin-like growth factor-I administration. Am J Pathol 161: 2263–2272, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gulbins E, Li PL. Physiological and pathophysiological aspects of ceramide. Am J Physiol Regul Integr Comp Physiol 290: R11–R26, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9: 139–150, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Hardin BJ, Campbell KS, Smith JD, Arbogast S, Smith J, Moylan JS, Reid MB. TNF-α acts via TNFR1 and muscle-derived oxidants to depress myofibrillar force in murine skeletal muscle. J Appl Physiol 104: 694–699, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Heeley DH. Investigation of the effects of phosphorylation of rabbit striated muscle alpha alpha-tropomyosin and rabbit skeletal muscle troponin-T. Eur J Biochem 221: 129–137, 1994 [DOI] [PubMed] [Google Scholar]
- 29. Hertelendi Z, Toth A, Borbely A, Galajda Z, van der Velden J, Stienen GJ, Edes I, Papp Z. Oxidation of myofilament protein sulfhydryl groups reduces the contractile force and its Ca2+ sensitivity in human cardiomyocytes. Antioxid Redox Signal 10: 1175–1184, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Heusch P, Canton M, Aker S, van de Sand A, Konietzka I, Rassaf T, Menazza S, Brodde OE, Di Lisa F, Heusch G, Schulz R. The contribution of reactive oxygen species and p38 mitogen-activated protein kinase to myofilament oxidation and progression of heart failure in rabbits. Br J Pharmacol 160: 1408–1416, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kunst G, Kress KR, Gruen M, Uttenweiler D, Gautel M, Fink RH. Myosin binding protein C, a phosphorylation-dependent force regulator in muscle that controls the attachment of myosin heads by its interaction with myosin S2. Circ Res 86: 51–58, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Lamb GD, Posterino GS. Effects of oxidation and reduction on contractile function in skeletal muscle fibres of the rat. J Physiol 546: 149–163, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marchesini N, Hannun YA. Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochem Cell Biol 82: 27–44, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Moss RL, Fitzsimons DP. Regulation of contraction in mammalian striated muscles—the plot thick-ens. J Gen Physiol 136: 21–27, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Murphy RM, Dutka TL, Lamb GD. Hydroxyl radical and glutathione interactions alter calcium sensitivity and maximum force of the contractile apparatus in rat skeletal muscle fibres. J Physiol 586: 2203–2216, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murrant CL, Reid MB. Detection of reactive oxygen and reactive nitrogen species in skeletal muscle. Microsc Res Tech 55: 236–248, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Nethery D, DiMarco A, Stofan D, Supinski G. Sepsis increases contraction-related generation of reactive oxygen species in the diaphragm. J Appl Physiol 87: 1279–1286, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Nikolova-Karakashian MN, Reid MB. Sphingolipid metabolism, oxidant signaling, and contractile function of skeletal muscle. Antioxid Redox Signal 15: 2501–2517, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nikolova-Karakashian MN, Rozenova KA. Ceramide in stress response. Adv Exp Med Biol 688: 86–108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Noland TA, Jr., Kuo JF. Protein kinase C phosphorylation of cardiac troponin I and troponin T inhibits Ca(2+)-stimulated MgATPase activity in reconstituted actomyosin and isolated myofibrils, and decreases actin-myosin interactions. J Mol Cell Cardiol 25: 53–65, 1993 [DOI] [PubMed] [Google Scholar]
- 41. Novgorodov SA, Chudakova DA, Wheeler BW, Bielawski J, Kindy MS, Obeid LM, Gudz TI. Developmentally regulated ceramide synthase 6 increases mitochondrial Ca2+-loading capacity and promotes apoptosis. J Biol Chem 286: 4644–4658, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pinto JR, de Sousa VP, Sorenson MM. Redox state of troponin C cysteine in the D/E helix alters the C-domain affinity for the thin filament of vertebrate striated muscle. Biochim Biophys Acta 1810: 391–397, 2011 [DOI] [PubMed] [Google Scholar]
- 43. Powell SR, Gurzenda EM, Wahezi SE. Actin is oxidized during myocardial ischemia. Free Radic Biol Med 30: 1171–1176, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Powers SK, Hudson MB, Nelson WB, Talbert EE, Min K, Szeto HH, Kavazis AN, Smuder AJ. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit Care Med 39: 1749–1759, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88: 1243–1276, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Prochniewicz E, Lowe DA, Spakowicz DJ, Higgins L, O'Conor K, Thompson LV, Ferrington DA, Thomas DD. Functional, structural, and chemical changes in myosin associated with hydrogen peroxide treatment of skeletal muscle fibers. Am J Physiol Cell Physiol 294: C613–C626, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reid MB, Haack KE, Franchek KM, Valberg PA, Kobzik L, West MS. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol 73: 1797–1804, 1992 [DOI] [PubMed] [Google Scholar]
- 48. Reid MB, Lannergren J, Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha: involvement of muscle myofilaments. Am J Respir Crit Care Med 166: 479–484, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Sancho Solis R, Ge Y, Walker JW. A preferred AMPK phosphorylation site adjacent to the inhibitory loop of cardiac and skeletal troponin I. Protein Sci 20: 894–907, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schissel SL, Schuchman EH, Williams KJ, Tabas I. Zn2+-stimulated sphingomyelinase is secreted by many cell types and is a product of the acid sphingomyelinase gene. J Biol Chem 271: 18431–18436, 1996 [DOI] [PubMed] [Google Scholar]
- 51. Straczkowski M, Kowalska I, Baranowski M, Nikolajuk A, Otziomek E, Zabielski P, Adamska A, Blachnio A, Gorski J, Gorska M. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia 50: 2366–2373, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Sweeney HL, Bowman BF, Stull JT. Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am J Physiol Cell Physiol 264: C1085–C1095, 1993 [DOI] [PubMed] [Google Scholar]
- 53. Szeto HH, Schiller PW. Novel therapies targeting inner mitochondrial membrane-from discovery to clinical development. Pharm Res 28: 2669–2679, 2011 [DOI] [PubMed] [Google Scholar]
- 54. van der Poel C, Edwards JN, Macdonald WA, Stephenson DG. Effect of temperature-induced reactive oxygen species production on excitation-contraction coupling in mammalian skeletal muscle. Clin Exp Pharmacol Physiol 35: 1482–1487, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Winterbourn CC, Parsons-Mair HN, Gebicki S, Gebicki JM, Davies MJ. Requirements for superoxide-dependent tyrosine hydroperoxide formation in peptides. Biochem J 381: 241–248, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Won JS, Singh I. Sphingolipid signaling and redox regulation. Free Radic Biol Med 40: 1875–1888, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Wong ML, Xie B, Beatini N, Phu P, Marathe S, Johns A, Gold PW, Hirsch E, Williams KJ, Licinio J, Tabas I. Acute systemic inflammation up-regulates secretory sphingomyelinase in vivo: a possible link between inflammatory cytokines and atherogenesis. Proc Natl Acad Sci USA 97: 8681–8686, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wright VP, Reiser PJ, Clanton TL. Redox modulation of global phosphatase activity and protein phosphorylation in intact skeletal muscle. J Physiol 587: 5767–5781, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem 279: 34682–34690, 2004 [DOI] [PubMed] [Google Scholar]