Abstract
Although the signaling pathways underlying exercise-induced cardiac adaptation have been extensively studied, little is known about the molecular mechanisms that result in the response of the heart to pregnancy. The objective of this study was to define the morphological, functional, and gene expression patterns that define the hearts of pregnant mice, and to identify the signaling pathways that mediate this response. Mice were divided into three groups: nonpregnant diestrus control, midpregnancy, and late pregnancy. Both time points of pregnancy were associated with significant cardiac hypertrophy. The prosurvival signaling cascades of Akt and ERK1/2 were activated in the hearts of pregnant mice, while the stress kinase, p38, was decreased. Given the activation of Akt in pregnancy and its known role in cardiac hypertrophy, the hypertrophic response to pregnancy was tested in mice expressing a cardiac-specific activated (myristoylated) form of Akt (myrAkt) or a cardiac-specific constitutively active (antipathologic hypertrophic) form of its downstream target, glycogen synthase kinase 3β (caGSK3β). The pregnancy-induced hypertrophic responses of hearts from these mice were significantly attenuated. Finally, we tested whether pregnancy-associated sex hormones could induce hypertrophy and alter signaling pathways in isolated neonatal rat ventricular myocytes (NRVMs). In fact, progesterone, but not estradiol treatment increased NRVM cell size via phosphorylation of ERK1/2. Inhibition of MEK1 effectively blocked progesterone-induced cellular hypertrophy. Taken together, our study demonstrates that pregnancy-induced cardiac hypertrophy is mediated by activation of Akt and ERK1/2 pathways.
Keywords: pregnancy, cardiac hypertrophy, protein kinase B, Akt, glycogen synthase kinase 3β, mitogen activated protein kinase
pregnancy is associated with a prolonged cardiac volume overload that results in cardiac hypertrophy with no induction of the fetal gene program (11). In this respect, pregnancy-induced cardiac hypertrophy is characterized as physiological cardiac hypertrophy just as is exercise-induced cardiac hypertrophy (12). However, pregnancy is accompanied by significant changes in the hormonal milieu and the volume overload and increased heart rate are continuous, while the stimulus for the cardiac growth response to exercise is intermittent by comparison. For these reasons, the setting is distinct, and little is known about the signaling and molecular events associated with the cardiac adaptation during pregnancy. Thus the objective of this study was to define the morphological, functional, and gene expression patterns that define the hearts of pregnant mice, and to identify the signaling pathways that mediate this response during mid- and late stage of pregnancy.
The phosphatidylinositol-3-kinase (PI3K)/Akt pathway has been implicated as important for exercise-induced cardiac hypertrophy (34). Studies from wild-type (WT) mice (20) and transgenic mouse models (9) suggest that Akt signaling is important in exercise-induced cardiac hypertrophy as well as in cardiac protection against pathological insults (24). For example, mice with targeted disruption of the Akt1 gene (Akt1−/−) do not undergo exercise-induced cardiac hypertrophy but demonstrate exacerbated pressure overload-induced cardiac hypertrophy compared with WT mice (9). Mice expressing a cardiac-specific activated (myristoylated) form of Akt (myrAkt) have significantly enlarged hearts at baseline and are protected against pathological insults (24). One of the downstream targets of Akt is glycogen synthase kinase 3β (GSK3β). Unlike other kinases, GSK3β is active at baseline and inactivated by phosphorylation primarily by Akt upon stimulation (1). For example, phosphorylation of GSK3β is significantly increased in response to voluntary wheel running (20) and treadmill exercise training (16). Conversely, mice expressing constitutively active GSK3β (caGSK3β) in their hearts have been shown to be resistant to pathological hypertrophy (1). Given their known roles in cardiac hypertrophy, we measured the phosphorylation status of Akt and some of its downstream targets during pregnancy. Next, we assessed the cardiac response to pregnancy of myrAkt and caGSK3β mice to test the hypothesis that both Akt and GSK3β are important mediators of pregnancy induced cardiac hypertrophy.
Next, we investigated mitogen activated protein kinase (MAPK) pathways during mid- and late stage of pregnancy because the levels of circulating estradiol and progesterone gradually increase with advancing gestational age (38) and it has been suggested that estradiol alters cardiac morphology and function via activation of MAPK pathways (19, 33). For example, pressure overload-induced cardiac hypertrophy is accompanied by an increase in p38 phosphorylation, and estradiol pellet implantation in ovariectomized mice, which attenuates pathological hypertrophy, is accompanied by a decrease in p38 phosphorylation (33). In adult rat cardiomyocytes, a high concentration of estradiol (1 nM) has antihypertrophic effects when cells are treated with the α1-adrenoreceptor agonist phenylephrine (PE) via a decrease in p38 phosphorylation (19). However, a low concentration of estradiol (1 pM) induces cardiomyocyte hypertrophy through ERK1/2 activation (19). Although the role of estradiol in the cardiovascular system has been widely studied by implanting estradiol pellets in ovariectomized mice (33) or exogenous estradiol treatment of cardiac myocytes (19), the other major pregnancy hormone, progesterone, has not been studied in combination with estradiol in the context of the heart. Moreover, alterations in MAPK signaling have been reported in response to pregnancy. Phosphorylation of p38 is decreased in hearts of late pregnancy (LP) rats (13), while phosphorylation of ERK1/2 is increased in early pregnancy in mice and rats (21) but remains unchanged in LP in rats (13, 21). However, whether the hormonal changes play a role in modulating the activities of MAPK signaling pathways during pregnancy has not been reported. Thus we tested the hypothesis that the hormonal changes during pregnancy play a role in modulating cardiac cell size and the signaling pathways seen in vivo would be recapitulated in cell culture.
Here, we investigated cardiac morphology, function, gene expression, and signaling molecules at different time points, including midpregnancy (MP) and LP, in WT mice. We found that Akt and MAPK signaling pathways were altered during pregnancy. The pregnancy-induced hypertrophic responses of hearts from myrAkt and caGSK3β mice were significantly attenuated. Hormonal treatment of NRVMs did not change the activities of Akt and p38 in contrast to what is seen in the left ventricular tissues of pregnant mice. However, treatment of NRVMs with progesterone resulted in increased phosphorylation of ERK1/2 and cellular protein content along with increased cell size. An inhibitor of ERK1/2 signaling blocked this cellular hypertrophy. Conversely, estrogen had no effects on either cell size or MAPK activity. Taken together, we conclude that pregnancy-induced cardiac hypertrophy is mediated by activation of Akt and ERK1/2 signaling cascades.
MATERIALS AND METHODS
Animals.
Three-month-old virgin female C57Bl/6 mice (WT) were mated with a proven breeder male C57Bl/6 mouse. The presence of a copulatory plug was counted as day 1 of pregnancy, and birth most often occurs at day 20. The male mouse was removed once a copulatory plug was detected. Pregnant mice were studied at 11 days of gestation (midstage of pregnancy, MP) and 18–19 days of gestation (late stage of pregnancy, LP). We checked estrus cycle by the appearance of the vagina (7) and diestrus cycle virgin female mice served as nonpregnant controls (NP) since estradiol level is an important mediator of cardiac morphology and function (19). The transgenic mouse model expressing an autosomally inherited cardiac specific myristoylated form of Akt (myrAkt) (24) or a cardiac-specific constitutively active form of GSK3β (caGSK3β) containing a serine9 to alanine mutation (1) were on a C57Bl/6 background and characterized previously (1, 24). Each transgenic mouse model was bred and offspring were genotyped by PCR for the presence of the specific transgene. Cardiac hypertrophy in response to pregnancy in transgenic mice was compared with WT mice since the hypertrophic responses of nontransgenic littermates (NTG) were equivalent to those of WT mice. Mice with reabsorbed pups were excluded from the study. Animals were housed in a temperature- and light-controlled room with food and water available ad libitum. All of the animals were handled and euthanized under the guidelines of the University of Colorado Animal Care and Use Committee.
Cardiac tissue collection, quantitative real time PCR (qRT-PCR) analysis, and western blot analysis.
At a given time point, mice were weighed and then killed by cervical dislocation after inhalation of isoflurane. Hearts were rapidly excised and washed in PBS to allow blood to be pumped out of the cardiac chambers and coronary vessels. The hearts were trimmed of connective tissue, vascular tissue, and atria. The ventricles were blotted dry and weighed. After right ventricle removal, the left ventricle was weighed, immediately frozen in liquid nitrogen, and stored at −80°C for RNA or protein extraction.
Total RNA was isolated from frozen left ventricular tissue by using Tri Regent according to the manufacturer's instructions (Molecular Research Center, Cincinnati, OH). Two micrograms of total RNA was used to synthesize cDNA with High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, CA) with random primers according to the manufacturer's instructions. Gene expression was determined by qRT-PCR using SYBR Green dye with gene specific primer sets and an Applied Biosysterms 7500 Real-Time PCR system. The levels of all candidate genes were normalized to 18S rRNA and results were plotted as fold changes relative to NP. Primers for 18S, ANF, βMyHC, BNP, and SERCA were listed previously (23). Additional primers were α-skeletal actin, forward 5′-CGACATCAGGAAGGACCTGTATGCC-3′, reverse 5′-AGCCTCGTCGTACTCCTGCTTGG-3′; αMyHC, forward 5′-ACATTCTTCAGGATTCTCTG-3′, reverse 5′-CTCCTTGTCATCAGGCAC-3′; phospholamban, forward 5′-GTTGTGCCCTTTTTCTACAC-3′, reverse 5′-AGAGAGAGCAGATTTGTGG-3′, Hsf1, forward 5′-AACGTCCCGGCCTTCCTAA-3′, reverse 5′-AGATGAGCGCGTCTGTGTC-3′; C/EBPβ, forward 5′-ACGACTTCCTCTCCGACCTCT-3′, reverse 5′-CGAGGCTCACGTAACCGTAGT-3′
To evaluate protein expression levels, left ventricular tissues were homogenized in standard lysis buffer as described previously (20). LDS sample buffer (Invitrogen) was added to the homogenized sample and was then incubated for 10 min at 70°C. Twenty to thirty micrograms of total protein was separated by SDS-PAGE. Separated proteins were transferred to a nitrocellulouse membrane. The membrane was immunoblotted using the following primary antibodies: Akt, mTOR, p70S6kinase, ERK, p38 MAPK, JNK (all from Cell Signaling), GSK3β (Santa Cruz Biotechnology), and their corresponding phosphorylated forms. Total protein content was analyzed after stripping the phospho-blots, and the ratio of phospho- to total protein was used to represent the degree of activity of corresponding proteins. GAPDH was used as an internal control. We also tried another method: we ran two blots, one for phosphoprotein normalized to GAPDH and the other for total protein and normalized to GAPDH. After that, phosphoprotein to total protein ratios were used. The results were the same. Four to six animals were used per group with two to three technical replicates per animal, and a representative blot was shown.
Serum estradiol and progesterone levels.
Serum estradiol and progesterone were measured with a Beckman Coulter Access II (UCH-CTRC Lab, Denver, CO). For estradiol, the coefficient of variation (CV) of values from 60 pg/ml to 4,800 pg/ml was <5%, and the assay sensitivity was 10.0 pg/ml. For progesterone, the highest calibrator is 40 ng/ml. If the results were >40 ng/ml, we extrapolated the curve beyond 40 by using a data reduction program. We measured estradiol and progesterone levels at 11, 13, 18, and 19 days of gestation to see changes in hormone levels throughout pregnancy.
Histological analysis.
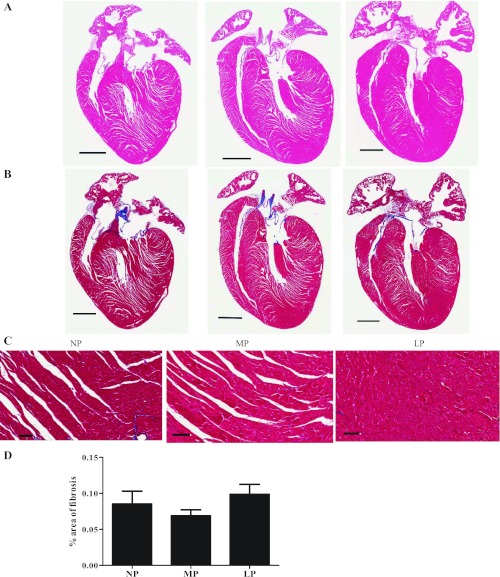
Hearts were rapidly excised, rinsed in cold phosphate-buffered saline, and weighed. The whole heart was fixed in 10% phosphate-buffered formalin for 24 h at room temperature and then placed into 70% ethanol at 4°C until processed. Samples were processed, embedded in paraffin, sectioned, and stained with either hematoxylin-eosin to visualize tissue architecture or Masson's trichrome stain to analyze fibrosis by Premier Histology (Longmont, CO). Trichrome stained slides were digitally scanned with an Aperio ScanScope. Percent of fibrosis was calculated utilizing a modified positive pixel count algorithm.
Assessment of LV functions by echocardiography.
LV function was assessed by M-mode echocardiography. For WT mice, echocardiography was performed using the high-resolution VisualSonics (Toronto, ON) ultrasound bioimager equipped with a 707B 30 MHz transducer. Mice were lightly sedated with isoflurane (3% induction, 1–1.5% maintenance) and placed in a supine position. The isoflurane concentration was adjusted to maintain a heart rate of ∼500 beats/min. Standard parasternal long-axis and midpapillary short-axis views were obtained. For myrAkt and GSK3β mice, we used Sonos 5500 with a 15-MHz phased linear array ultrasound transducer. Mice were induced with 3% isoflurane for 2 min and then maintained on 2% isoflurane via a nosecone for the duration of the experiment. Echocardiography of WT mice and transgenic mice was performed in two different facilities with two different instruments, and read by two different readers during the course of this project; thus we cannot directly compare WT echocardiography data to transgenic mouse data.
Treatment of neonatal rat ventricular myocytes (NRVMs).
NRVMs were prepared as described (32). The concentrations of hormone used in this study reflected the hormone levels that we measured in sera of pregnant mice. Cells were serum starved for 24 h before progesterone (300 nM) and/or estradiol (500 pM) treatment. PE (20 μM) served as a positive control for a prohypertrophic stimulus. Cells were collected 48 h after the addition of hormones. PD98059 (10 μM), a specific inhibitor of MEK1, was added 1 h before treatment with progesterone. Total cellular protein was quantified by the Pierce 660-nm protein assay. DNA concentration was measured using Hoechst 33258 dye as previously described (32) and total protein content was normalized to DNA. NRVMs were immunostained for α-actinin to measure cell size. Over 100 cells from randomly chosen images were analyzed per each experimental condition with Image J (NIH). We used triplicate wells from the same cell isolation for each condition, and data were the means ± SE of six independent experiments.
Data and statistical analysis.
All results are expressed as means ± standard error of mean (SE). Statistical significance was tested with analysis of variance (ANOVA) followed by Fisher's least significant difference (LSD) for multiple group comparisons. P < 0.05 was regarded as significant among groups.
RESULTS
Functional and molecular profiles of pregnancy-induced cardiac hypertrophy.
There were statistically significant increases in left ventricle mass (LV) normalized to tibial length (TL) in both MP and LP of WT mice (Table 1). We also calculated relative wall thickness to ventricular diameter (2× posterior wall/LV diameter at diastole) from echocardiographic parameters (Table 2) to determine whether pregnancy-induced cardiac hypertrophy is eccentric or concentric. The relative wall thicknesses to ventricular diameter (0.33 in NP, 0.34 in MP, and 0.33 in LP, respectively) did not change during pregnancy, indicating that pregnancy-induced cardiac hypertrophy was physiological. Left ventricular diameter and left ventricular volume at diastole were significantly increased in MP, but not LP. Both parameters were significantly increased at systole in LP, which resulted in significantly decreased systolic function, indicated by the percent fractional shortening (%FS). This was also true of the percent ejection fraction (%EF) (Table 2). To determine whether fibrosis accompanied the systolic dysfunction seen in LP, we stained sections with Masson's trichrome to reveal interstitial collagen deposition in hearts from pregnant mice. As indicated in Fig. 1, histological cross sections of NP, MP, and LP hearts did not demonstrate any changes in fibrosis.
Table 1.
Morphometric data from NP, MP, and LP of WT, myrAkt, and caGSK3β mice
| NP | MP | LP | |
|---|---|---|---|
| WT mice | n = 13 | n = 12 | n = 18 |
| BW, g | 21.41 ± 0.30 | 25.59 ± 0.46* | 38.05 ± 0.78*† |
| LV, mg | 72.40 ± 1.36 | 80.00 ± 1.51* | 87.30 ± 1.49*† |
| LV/TL, mg/mm | 4.23 ± 0.07 | 4.58 ± 0.08* | 4.99 ± 0.08*† |
| myrAkt mice | n = 8 | n = 8 | n = 8 |
| BW, g | 23.26 ± 0.55 | 28.71 ± 0.93* | 41.11 ± 2.70*† |
| LV, mg | 128.8 ± 6.31‡ | 143.0 ± 7.54 | 150.0 ± 7.89 |
| LV/TL, mg/mm | 7.38 ± 0.36‡ | 8.15 ± 0.40 | 8.50 ± 0.43 |
| caGSK3β | n = 15 | n = 9 | n = 9 |
| BW, g | 22.55 ± 0.35 | 27.24 ± 0.61* | 38.78 ± 1.15*† |
| LV, mg | 67.21 ± 1.21‡ | 72.62 ± 1.43 | 75.01 ± 2.53* |
| LV/TL, mg/mm | 3.95 ± 0.09‡ | 4.16 ± 0.07 | 4.24 ± 0.16 |
Values are means ± SE; n = number of mice per group. WT, wild type; BW, body weight; LV, left ventricle; TL, tibial length; NP, nonpregnant control; MP, 11 days gestation; LP, 18–19 days gestation. P ≤ 0.05:
significantly different from NP;
, significantly different from MP;
, significantly different from NP of WT mice.
Table 2.
Echocardiographic assessment of WT mice
| LV M-Mode | NP (n = 9) | MP (n = 9) | LP (n = 8) |
|---|---|---|---|
| LV AW, mm | |||
| Diastolic | 0.74 ± 0.02 | 0.79 ± 0.05 | 0.82 ± 0.05 |
| Systolic | 1.10 ± 0.06 | 1.11 ± 0.05 | 1.08 ± 0.05 |
| LV PW, mm | |||
| Diastolic | 0.64 ± 0.04 | 0.68 ± 0.02 | 0.65 ± 0.02 |
| Systolic | 1.03 ± 0.06 | 1.08 ± 0.06 | 0.96 ± 0.02 |
| LV diameter, mm | |||
| Diastolic | 3.86 ± 0.03 | 4.02 ± 0.03* | 4.00 ± 0.06 |
| Systolic | 2.50 ± 0.07 | 2.61 ± 0.08 | 2.87 ± 0.03*† |
| LV volume, mm3 | |||
| Diastolic | 64.24 ± 1.12 | 71.11 ± 1.43* | 70.08 ± 2.44 |
| Systolic | 22.69 ± 1.65 | 25.16 ± 1.90 | 31.41 ± 0.92*† |
| LV %EF | 64.85 ± 2.18 | 64.58 ± 2.63 | 54.99 ± 1.37*† |
| LV %FS | 35.14 ± 1.67 | 35.16 ± 1.20 | 28.21 ± 1.92*† |
Values are expressed as means ± standard error of mean (SE) n = number of mice per group. AW, anterior wall; PW, posterior wall; EF, ejection fraction; FS, fractional shortening.
P ≤ 0.05, significantly different from NP;
P ≤ 0.05, significantly different from MP.
Fig. 1.
A: histological analysis of nonpregnancy (NP), midpregnancy (MP), and late pregnancy (LP) heart sections stained with hematoxylin and eosin (H and E). B: Masson's trichrome staining indicated that fibrosis was not induced during pregnancy. Scale bar, 0.5 mm. C: higher magnification (20×) of image in B. Scale bar, 0.625 mm. D: bar graph is average of % area of fibrosis from 3 hearts per group. Values are means ± SE.
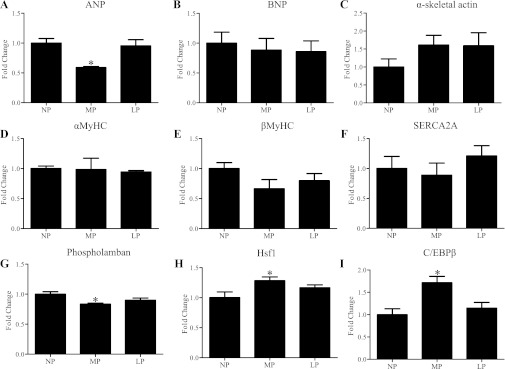
Previously, Eghbali et al. (11) investigated the expression of genes [i.e., α-myosin heavy chain (MyHC), βMyHC, atrial natriuretic peptide (ANP), phospholamban, cardiac Ca2+-ATPase of the sarcoplasmic reticulum (SERCA2A)] whose changes in expression are typically associated with pathological cardiac hypertrophy and found these genes were not altered in LP in mice (Fig. 2). Given that %FS was decreased in LP, we hypothesized that there might be pathological molecular changes in the heart prior to these observed functional changes. Therefore, we profiled expression of a number of genes in both MP and LP, including several genes that have been investigated previously in LP (11) as well as brain natriuretic peptide (BNP), α-skeletal actin, heat shock transcription factor 1 (Hsf1), and C/EBPβ (Fig. 2). Analysis of gene expression revealed that the expression of ANP and phospholamban was significantly decreased in MP (Fig. 2), while mRNA levels of ANP, BNP, α-skeletal actin, αMyHC, βMyHC, SERCA2A, and phospholamban were not changed in LP (Fig. 2). While the physiological stimulus of exercise has been shown to increase the expression of Hsf1 (30) and decrease the expression of C/EBPβ (4), both mRNAs were significantly increased in MP but not in LP (Fig. 2).
Fig. 2.
qRT-PCR of selected genes in hearts of wild-type (WT) mice. Values are means ± SE expressed as fold change relative to nonpregnant control group (NP). qRT-PCR was performed in triplicate with a minimum of 4 independent left ventricular samples at different time points. The levels of all candidate genes were normalized to 18S rRNA. See Glossary for definition of abbreviations in A–I. *P < 0.05, significantly different from NP.
The Akt signaling pathway is activated during pregnancy.
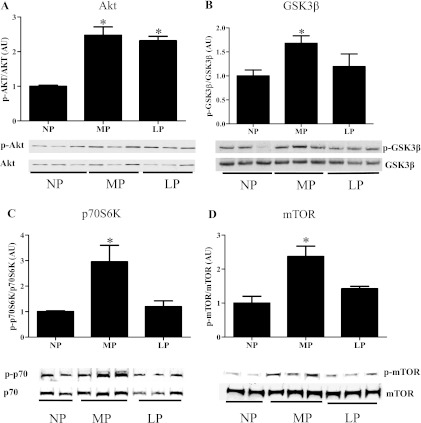
It is generally accepted that the PI3K/Akt pathway is an important mediator of exercise-induced cardiac hypertrophy (34). However, there are conflicting reports regarding the role of Akt signaling in pregnancy-induced cardiac hypertrophy. For example, two studies (14, 21) showed increased phosphorylation of Akt in LP in mice and rats, while another study (13) showed decreased phosphorylation of Akt in LP in rats (13). In addition, the downstream targets of Akt have not been investigated in this context. The activity of Akt, as assessed by the ratio of phospho-Akt to total Akt, was significantly increased in both MP and LP (Fig. 3A). The downstream targets of Akt, including glycogen synthase kinase3β (GSK3β), ribosomal S6 protein kinase (p70S6K), and mammalian target of rapamycin (mTOR), showed increased phosphorylation in MP but not in LP (Fig. 3, B–D). Collectively, our data show that while Akt was activated in both MP and LP, phosphorylation of these downstream targets of Akt was limited to MP only.
Fig. 3.
Pregnancy modulates Akt and its downstream targets in WT mice. A: Akt phosphorylation was significantly increased during MP and LP. B: GSK3β phosphorylation was significantly increased in MP. C: p70S6K phosphorylation was significantly increased in MP. D: mTOR phosphorylation was significantly increased in MP. Five to six animals were used per group with 2 to 3 technical replicates per animal, and a representative blot was shown. *P ≤ 0.05, significantly different from NP.
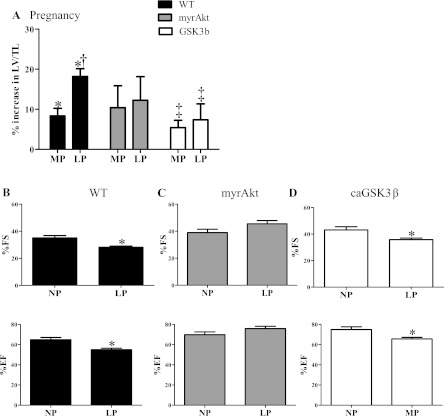
Since the phosphorylation-mediated activation of Akt was significantly increased during pregnancy, we tested the hypothesis that myrAkt mice would not undergo further hypertrophy during pregnancy. As previously reported (24), myrAkt mice had significantly enlarged hearts at baseline (Table 1) compared with WT mice. Consistent with our hypothesis, the hearts of myrAkt mice did not exhibit a WT hypertrophic response to pregnancy (Fig. 4A). Additionally, unlike WT mice, in which LP is associated with the highest degree of cardiac hypertrophy and impaired systolic function (Fig. 4, A and B), cardiac function was maintained in LP in the myrAkt mice (Fig. 4C), consistent with the established cardioprotective effects of Akt (6).
Fig. 4.
Cardiac mass adaptation in response to pregnancy of WT, myrAkt, and caGSK3β mice. A: in WT, the percent increases in left ventricular mass (LV) normalized to tibial length (LV/TL) were 8.3% and 18.2% in MP (n = 12) and LP (n = 18), respectively, compared with NP (n = 13). In myrAkt mice, LV/TL was increased 10.4% and 15.2% in MP (n = 8) and LP (n = 8), respectively, compared with NP (n = 8). In caGSK3β mice, LV/TL was increased 5.4% and 7.4% in MP (n = 9) and LP (n = 9), respectively, compared with NP (n = 17). B: %fractional shortening (%FS) and %ejection fraction (%EF) measured by echocardiography were significantly decreased in LP (n = 9 for NP and n = 8 for LP) in WT mice. C: %FS and %EF were maintained in LP in myrAkt mice (n = 11/group). D: %FS and %EF were significantly decreased in LP in caGSK3β mice (n = 5/group). Values are means ± SE. For abbreviations, see Glossary. *P < 0.05, significantly different from NP; †P < 0.05, significantly different from MP; ‡P < 0.05, significantly different from WT.
GSK3β is a negative regulator of pathological cardiac hypertrophy that is inactivated by Akt-mediated phosphorylation (1). Since phosphorylation (inactivation) of GSK3β was significantly increased in MP in WT animals when there was significant cardiac hypertrophy, we hypothesized that caGSK3β mice would be blocked in their hypertrophic response to pregnancy. Unlike WT or myrAkt mice, caGSK3β mice had difficultly becoming pregnant and had smaller litters compared with WT (7.3 ± 0.5 pups vs.8.6 ± 0.4 pups; caGSK3β vs. WT; P = 0.06). The LV/TL of caGSK3β mice was significantly smaller than that of WT mice at baseline as previously reported (1). caGSK3β mice did not demonstrate a significant hypertrophic response to pregnancy in MP and LP. (Fig. 4A). Our results indicate that inactivation of GSK3β by phosphorylation is required for a normal cardiac hypertrophic response to pregnancy. However, similar to WT mice, cardiac systolic function was significantly decreased in the caGSK3β mice in LP (Fig. 4D). Taken together, the results in the myrAkt and caGSK3β mice indicate that both Akt and GSK3β phosphorylation state are involved in pregnancy-induced cardiac hypertrophy.
The role of MAPK signaling in pregnancy-induced cardiac hypertrophy.
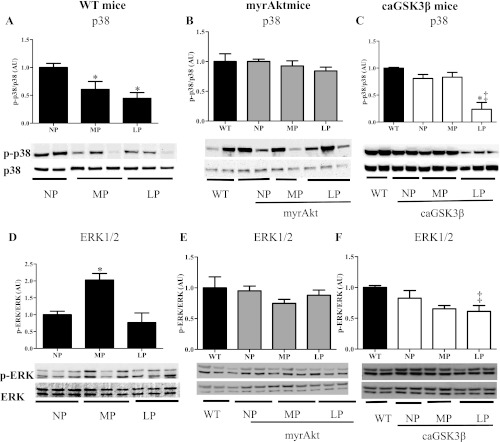
Previous studies have suggested that MAPK signaling is altered in the heart during pregnancy (13, 21), and it has been documented that estradiol changes cardiac morphology and function through MAPK signaling pathways (19). However, the causal role for changes in MAPK signaling in pregnancy-induced cardiac hypertrophy has not been established. Thus we investigated the activity of several members of the MAPK family during both MP and LP. In agreement with published data (13), we found that phosphorylation of p38 was significantly decreased in both MP and LP (Fig. 5A), while phosphorylation of ERK1/2 was significantly increased at MP and returned to NP levels by LP (Fig. 5D). Next, we tested whether pregnancy-induced regulation of p38 and ERK1/2 was preserved in myrAkt and GSK3β mice. Because both myrAkt and GSK3β mice did not exhibit a WT hypertrophic response, we did not expect the activity of p38 and ERK1/2 to be altered in these mice during pregnancy. The phosphorylation of p38 and ERK1/2 was no different from WT mice at baseline in both myrAkt and caGSK3β mice. And, while the phosphorylation of p38 (Fig. 5 B) and ERK1/2 (Fig. 5E) was unchanged in pregnant myrAkt mice, both were significantly decreased in LP in caGSK3β mice (Fig. 5, C and F). Taken together, these results suggest that ERK1/2 activation, but not a reduction in p38 activity, is important in cardiac growth during pregnancy.
Fig. 5.
Pregnancy modulates MAPK signaling in hearts of WT and GSK3β mice, but not in hearts of myrAkt. p38 phosphorylation was significantly decreased during MP and LP in WT mice (A) but not in myrAkt (B). p38 phosphorylation was significantly decreased in LP in caGSK3β mice (C). ERK1/2 phosphorylation was significantly increased in MP but returned to NP levels in WT mice (D), but no alterations were observed in myrAkt mice (E); alterations were observed in LP caGSK3β mice (F). Five to six animals were used per group with 2 to 3 technical replicates per animal, and a representative blot was shown. *P ≤ 0.05, significantly different from NP; ‡P < 0.05, significantly different from WT.
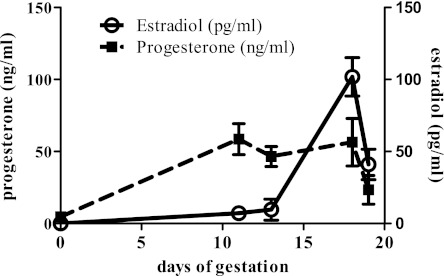
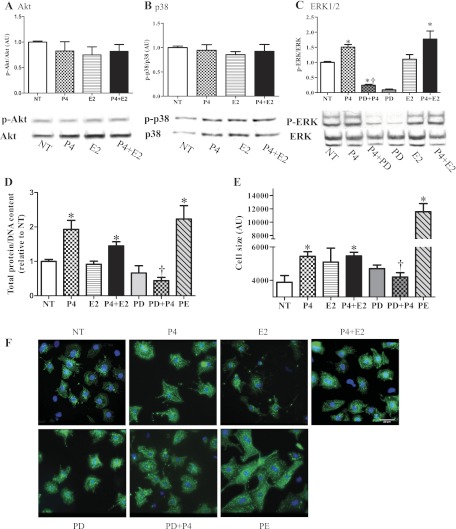
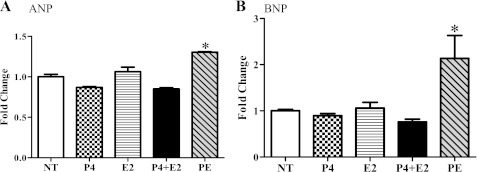
To address the role of hormones in modulating the signaling in pregnancy-mediated cardiac hypertrophy, we first measured serum estradiol and progesterone levels throughout pregnancy since the reported serum estradiol and progesterone levels during pregnancy in mice are quite variable (25, 29, 38). Serum estradiol was undetectable (<10 pg/ml) in the NP control group, significantly increased at MP (7.24 ± 2.51 pg/ml or 26.59 ± 9.21 pM) and maximal at LP (101.8 ± 13.36 pg/ml or 373.55 ± 49.06 pM). Serum progesterone levels gradually increased and peaked at MP (58.57 ± 10.78 ng/ml or 186.25 ± 34.29 nM) and these levels were maintained through LP (Fig. 6). We then used NRVMs to determine the mechanistic roles of estradiol and progesterone in regulating cardiac myocyte size and signaling pathways. Hormonal treatment of NRVMs did not change the activities of Akt (Fig. 7A) and p38 (Fig. 7B) in contrast to what is seen in the left ventricular tissues of pregnant mice. However, progesterone did increase phosphorylation of ERK1/2 (Fig. 7C). Therefore, the ERK1/2 activation seen in MP in vivo is recapitulated in vitro by progesterone treatment. Moreover, progesterone also increased total protein content (Fig. 7D) and cell size (Fig. 7, E and F), indicating cardiomyocyte hypertrophy. Interestingly, despite estradiol's reported antihypertrophic effects at 1 nM concentration (19), it was not able to block progesterone-mediated NRVM hypertrophy (Fig. 7, E and F). Unlike the pathological response of NRVMs to PE, the progesterone-mediated increase in protein content and cell size was not accompanied by fetal gene induction, such as ANP or BNP (Fig. 8), suggesting that progesterone mediated a physiological cellular hypertrophic response. To determine whether progesterone-mediated cellular hypertrophy requires activation of ERK1/2, we pretreated NRVMs with PD98059 to block ERK1/2 phosphorylation, followed by progesterone treatment. PD98059 (10 μM) effectively blocked the phosphorylation of ERK1/2 and the cellular hypertrophic responses (Fig. 7, B–E). As a control, treatment with PD98059 alone did not affect protein content nor cell size (Fig. 7, C–E). Our result agrees with a previous in vitro study (36) demonstrating that without hypertrophic stimuli, 10 μM PD98059 does not change total protein or cell size. Collectively, our results strongly suggest that progesterone induces cardiomyocyte hypertrophy via increased phosphorylation of ERK1/2.
Fig. 6.
Hormonal changes during pregnancy. Serum estradiol (E2) levels were below 10 pg/ml from NP to MP and peaked at LP and declined with approaching parturition. Serum progesterone levels were significantly increased at MP, maintained until approaching parturition.
Fig. 7.
The pregnancy hormone, progesterone (P4), causes neonatal rat ventricular myocyte (NRVM) hypertrophy via ERK signaling pathways. NRVMs treated with 300 nM P4, 500 pM estradiol (E2), or combinations of progesterone and estradiol (P4 + E2) did not show a change in Akt phosphorylation (A) or p38 phosphorylation (B), but P4 increased ERK1/2 phosphorylation (C). PD98059 (PD) blocked progesterone-induced ERK1/2 phosphorylation. We used triplicate wells from the same cell isolation for each condition, and data are the means ± SE of 6 independent experiments. D: total cellular protein normalized to DNA content was significantly increased by P4 and P4 + E2, but PD abolished the progesterone-induced increase in total protein concentration; 20 μM phenylephrine (PE) was used as a positive control. E: cell size was significantly increased by P4, P4 + E2, and PE treatment. P4-mediated cardiomyocyte hypertrophy was blocked by PD treatment. F: NRVMs were stained with α-actinin and cell size was measured. Representative images of 3 independent experiments are shown; scale bar is 20 μm. *P ≤ 0.05, significantly different from nontreated serum free group (NT); †P ≤ 0.05, significantly different from P4-treated group.
Fig. 8.
Pregnancy hormones did not change expression of fetal genes in NRVMs. A and B: mRNA levels measured by qRT-PCR. ANP (A) and BNP (B) were not altered with pregnancy hormones. PE was used as a positive control. *P ≤ 0.05, significantly different from nontreated serum free group (NT).
DISCUSSION
Pregnancy-induced cardiac hypertrophy has been generally viewed as a physiological adaptation (12) similar to exercise-induced cardiac hypertrophy. Several of our findings regarding pregnancy are similar to changes seen in exercise: 1) fibrosis was not induced (Fig. 1); 2) the majority of the fetal genes remained unchanged in LP (Fig. 2); 3) ANP was decreased (10) and Hsf1 was increased (30) in MP (Fig. 2); 4) left ventricular diameter and volume at diastole (3) were increased in MP (Table 2); and 5) the ratio of wall thickness to ventricular radius did not change during pregnancy. These results all resemble the adaption in response to exercise training (3, 17). However, pregnancy-induced cardiac hypertrophy also displays feature(s) distinct from exercise-induced hypertrophy: 1) C/EBPβ was increased in MP (Fig. 2), while downregulation of C/EBPβ following exercise has been suggested as a signature of exercise-induced cardiac hypertrophy (4); and 2) we and others (11, 28, 31) found that systolic function, as indicated by %FS or %EF, was decreased in LP and this is distinct from exercise in terms of preserved or enhanced contractile function. These findings are in somewhat contradiction to the notion that stroke volume and cardiac output are increased during pregnancy in humans (8, 26). Cardiac output is the product of stroke volume (SV) and heart rate (HR). While augmented SV is the major contributor to an increase in cardiac output following exercise training (2), increased cardiac output during pregnancy is mainly due to an increase in HR (26). Stroke volume (SV) has been reported to either increase until MP and then level off until near term (8) or to show a nonstatistically significant increase (26). Thus the mechanisms responsible for an increase in cardiac output between exercise (i.e., increased SV) and pregnancy (i.e., increased HR) are different. We found cardiac output is the same among groups (data not shown). Since we performed echocardiography when mice were anesthetized, therefore HR of all three groups of mice is the same, eliminating the increased HR of the pregnant mice. In addition, although stroke volume and cardiac output are maintained in our study, we found %FS is decreased, indicating that stroke volume and cardiac output can be preserved at lower %FS with higher LV end-diastolic volumes, which we have shown in Table 2. However, we find a significant increase in LV end-systolic volume, which explains how the pumping capacity of the heart is compromised at LP. Since %FS is decreased despite a continuous decrease in vascular resistance in LP in humans (26), a decrease in %FS seen in both humans and rodents indicates that contractile function of the heart is decreased in LP. Although decreased systolic function is associated with late stages of pregnancy, it appears to be short-term and transient because it recovers to control levels after delivery (31). For this reason, we favor the notion that pregnancy-induced cardiac hypertrophy should continue to be considered as a type of physiological cardiac hypertrophy, but one that is distinct from exercise-induced cardiac hypertrophy.
We demonstrated that phosphorylation of most of the signaling molecules in the prosurvival cascade we measured (except for Akt) was maximal at MP, while hypertrophy peaked in LP. These results support the previous findings that signaling molecules are activated rapidly and precede the increase in heart weight in response to exercise (15, 20). Importantly, our data from the myrAkt and caGSK3β mice suggest cross-talk between MAPK and Akt/GSK3β pathways. For example, maximal activation of Akt inhibits ERK1/2 activation (24), and inhibition of GSK3β significantly increases ERK1/2 phosphorylation (35). Indeed, ERK1/2 activity is not changed with pregnancy in myrAkt mice, and is significantly decreased in LP in caGSK3β mice. We find that phosphorylation of p38 is significantly decreased in both WT and caGSK3β mice, but the caGSK3β mice do not exhibit cardiac hypertrophy in response to pregnancy. Thus we speculate that p38 activity may not play a role in cardiac growth in response to pregnancy, but may play an important role in cardiac remodeling processes such as fibrosis and apoptosis since downregulation of p38 attenuates fibrosis and apoptosis in response to pressure overload (37) and we found no induction of fibrosis during pregnancy (Fig. 1).
The increase in phosphorylation of ERK1/2 in MP, presumably as a consequence of progesterone stimulation, being associated with the development of physiological hypertrophy, contradicts previous observations made by others in transgenic mouse models. For example, mice expressing cardiac-specific constitutively active MAPK kinase 1 (MEK1), which is immediately upstream of ERK1/2, but does not activate JNK or p38, have concentric hypertrophy with improved cardiac function (5). On the other hand, hearts of mice deficient in ERK1/2 show a preferential eccentric hypertrophy (18) with significantly decreased cardiac function and these mice have slightly more hypertrophy in response to pressure overload (18). These results suggest that the ERK1/2 pathway may be responsible for limiting the extent of eccentric growth, which may be applicable to the physiological hypertrophy induced with pregnancy. Taken together, it is possible that the activation of these prosurvival pathways and downregulation of stress kinases serve to mitigate damage induced by what would appear to be a harmful prolonged volume overload to preserve a more physiologic phenotype. Supporting this concept, progesterone induced activation of ERK1/2 in NRVMs did not induce any fetal gene program (Fig. 8), which presumably represents a physiological profile rather than pathological as seen with PE treatment.
We found that estradiol at concentrations close to those of LP (500 pM) did not cause changes in cell size or signaling molecules and did not block progesterone's prohypertrophic activity. Studies from adult rat ventricular myocytes demonstrate that >100 pM of estradiol alone does not induce cardiomyocyte hypertrophy or ERK1/2 phosphorylation, while this concentration of estradiol attenuates cardiomyocyte hypertrophy induced by PE (19). These and our results suggest that estradiol may only be antihypertrophic when pathological stimuli are present. Next, NRVMs treated with progesterone plus estrogen, at concentrations similar to LP, have increases in cell size and protein content (Fig. 7, D-F) that agree with our in vivo data showing that LP showed highest cardiac hypertrophy (Fig. 4A). However, progesterone plus estrogen increased phosphorylation of ERK1/2 (Fig. 7C), which is consistent with our data at MP but not LP (phosphorylation of ERK1/2 is not significantly different in LP and NP: Fig. 5D). In addition, progesterone did not change Akt activity (Fig. 7A), which is also upregulated in MP and LP (Fig. 3A). Since upregulation of Akt is well-documented in response to both exercise (34) and volume-overload models (27), we speculate that the increases in Akt activity seen in hearts from pregnant mice may be largely due to hemodynamic overload secondary to increased blood volume during pregnancy and not to the changes in the hormonal milieu (22). In addition, the lack of difference in the phosphorylation of ERK1/2 level seen in hearts from LP mice may be due to the crosstalk between Akt and ERK such that increased phosphorylation of Akt in LP may inhibit further activation of ERK1/2 in hearts from LP mice.
In summary, we find that pregnancy-induced cardiac adaptation is accompanied by modulation of Akt and ERK1/2 pathways. The results from caGSK3β mice indicate that phosphorylation of GSK3β (inactivation) and ERK1/2 (activation) are important because caGSK3β mice demonstrate significantly attenuated pregnancy-induced cardiac hypertrophy and decreased phosphorylation of ERK1/2 in LP. Our findings also suggest that there is cross-talk between ERK1/2 activation and the PI3K/Akt/GSK3β pathway during the development of pregnancy-induced cardiac hypertrophy. Importantly, the results from NRVMs suggest that progesterone is the key hormone regulating cell size via ERK1/2 activation. Taken together, these data provide new insights into the molecular mechanisms that regulate pregnancy-induced cardiac hypertrophy and indicate that while pregnancy-induced cardiac hypertrophy displays many of the hallmarks of physiological hypertrophy, the pregnant heart displays a unique molecular and functional signature.
GRANTS
This work was supported by a National Heart, Lung, and Blood Institute Grant to L. A. Leinwand (HL-50560) and by an American Heart Association Postdoctoral Fellowship (0920040G) to E. Chung.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.C. conception and design of research; E.C. and F.Y. performed experiments; E.C. analyzed data; E.C., F.Y., and L.A.L. interpreted results of experiments; E.C. prepared figures; E.C. drafted manuscript; E.C., F.Y., and L.A.L. edited and revised manuscript; L.A.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We greatly appreciate Margaret Isenhart for the care of the mice. We thank Kelly Ambler and Amy R. Perry for echocardiography. We greatly appreciate Murray Esler, Timothy Hacker, and Robert Weiss for helping us interpret our echocardiography data. We greatly appreciate Ann S. Robinson for the neonatal rat ventricular myocytes preparations. We also thank Mike Antczak, Kristen K. Barthel, Massimo Buvoli, and Brooke C. Harrison for critical reading of the manuscript and technical advice. We are grateful to Anthony Rosenzweig for myrAkt mice and Eric N. Olson for caGSK3β mice.
GLOSSARY
- Akt
Protein kinase B
- ANP
Atrial natriuretic peptide
- BNP
Brain natriuretic peptide
- C/EBPβ
CCAAT/enhancer binding protein (C/EBP), beta
- EF
Ejection fraction
- ERK
Extracellular signal-regulated kinase
- FS
Fractional shortening
- GSK3β
Glycogen synthase kinase 3β
- Hsf1
Heat shock transcription factor 1
- JNK
c-Jun NH2-terminal kinase
- LP
Late pregnancy
- LV
Left ventricle
- MP
Midpregnancy
- mTOR
Mammalian target of rapamycin
- MyHC
Myosin heavy chain
- NRVMs
Neonatal rat ventricular myocytes
- NP
Nonpregnant diestrus cycle
- p70S6K
Ribosomal S6 protein kinase
- PI3K
Phosphatidylinositol 3-kinase
- SERCA2A
Cardiac Ca2+-ATPase of the sarcoplasmic reticulum
- TL
Tibial length
- WT
Wild type
REFERENCES
- 1. Antos CL, McKinsey TA, Frey N, Kutschke W, McAnally J, Shelton JM, Richardson JA, Hill JA, Olson EN. Activated glycogen synthase-3beta suppresses cardiac hypertrophy in vivo. Proc Natl Acad Sci USA 99: 907–912, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barnard RJ, Duncan HW, Baldwin KM, Grimditch G, Buckberg GD. Effects of intensive exercise training on myocardial performance and coronary blood flow. J Appl Physiol 49: 444–449, 1980 [DOI] [PubMed] [Google Scholar]
- 3. Bocalini D, Carvalho E, de Sousa A, Levy R, Tucci P. Exercise training-induced enhancement in myocardial mechanics is lost after 2 weeks of detraining in rats. Eur J Appl Physiol 109: 909–914, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Boström P, Mann N, Wu J, Quintero PA, Plovie ER, Panáková D, Gupta RK, Xiao C, MacRae CA, Rosenzweig A, Spiegelman BM. C/EBP[beta] controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell 143: 1072–1083, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, Kitsis RN, Molkentin JD. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J 19: 6341–6350, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ceci M, Gallo P, Santonastasi M, Grimaldi S, Latronico MVG, Pitisci A, Missol-Kolka E, Scimia MC, Catalucci D, Hilfiker-Kleiner D, Condorelli G. Cardiac-specific overexpression of E40K active Akt prevents pressure overload-induced heart failure in mice by increasing angiogenesis and reducing apoptosis. Cell Death Differ 14: 1060–1062, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Champlin AK, Dorr DL, Gates AH. Determining the stage of the estrous cycle in the mouse by the appearance of the vagina. Biol Reprod 8: 491–494, 1973 [DOI] [PubMed] [Google Scholar]
- 8. Clapp JF, Capeless E. Cardiovascular function before, during, and after the first and subsequent pregnancies. Am J Cardiol 80: 1469–1473, 1997 [DOI] [PubMed] [Google Scholar]
- 9. DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation 113: 2097–2104, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Diffee GM, Seversen EA, Stein TD, Johnson JA. Microarray expression analysis of effects of exercise training: increase in atrial MLC-1 in rat ventricles. Am J Physiol Heart Circ Physiol 284: H830–H837, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Eghbali M, Deva R, Alioua A, Minosyan TY, Ruan H, Wang Y, Toro L, Stefani E. Molecular and functional signature of heart hypertrophy during pregnancy. Circ Res 96: 1208–1216, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Eghbali M, Wang Y, Toro L, Stefani E. Heart hypertrophy during pregnancy: a better functioning heart? Trends Cardiovasc Med 16: 285–291, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Gonzalez AMD, Osorio JC, Manlhiot C, Gruber D, Homma S, Mital S. Hypertrophy signaling during peripartum cardiac remodeling. Am J Physiol Heart Circ Physiol 293: H3008–H3013, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, Luchtefeld M, Poli V, Schneider MD, Balligand JL, Desjardins F, Ansari A, Struman I, Nguyen NQN, Zschemisch NH, Klein G, Heusch G, Schulz R, Hilfiker A, Drexler H. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 128: 589–600, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Iemitsu M, Maeda S, Jesmin S, Otsuki T, Kasuya Y, Miyauchi T. Activation pattern of MAPK signaling in the hearts of trained and untrained rats following a single bout of exercise. J Appl Physiol 101: 151–163, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Iemitsu M, Maeda S, Miyauchi T, Matsuda M, Tanaka H. Gene expression profiling of exercise-induced cardiac hypertrophy in rats. Acta Physiol Scand 185: 259–270, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Jin H, Yang R, Li W, Lu H, Ryan AM, Ogasawara AK, Van Peborgh J, Paoni NF. Effects of exercise training on cardiac function, gene expression, and apoptosis in rats. Am J Physiol Heart Circ Physiol 279: H2994–H3002, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Kehat I, Davis J, Tiburcy M, Accornero F, Saba-El-Leil MK, Maillet M, York AJ, Lorenz JN, Zimmermann WH, Meloche S, Molkentin JD. Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth/novelty and significance. Circ Res 108: 176–183, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kilic A, Javadov S, Karmazyn M. Estrogen exerts concentration-dependent pro-and anti-hypertrophic effects on adult cultured ventricular myocytes. Role of NHE-1 in estrogen-induced hypertrophy. J Mol Cell Cardiol 46: 360–369, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Konhilas JP, Maass AH, Luckey SW, Stauffer BL, Olson EN, Leinwand LA. Sex modifies exercise and cardiac adaptation in mice. Am J Physiol Heart Circ Physiol 287: H2768–H2776, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lemmens K, Doggen K, De Keulenaer GW. Activation of the neuregulin/ErbB system during physiological ventricular remodeling in pregnancy. Am J Physiol Heart Circ Physiol 300: H931–H942, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Longo LD. Maternal blood volume and cardiac output during pregnancy: a hypothesis of endocrinologic control. Am J Physiol Regul Integr Comp Physiol 245: R720–R729, 1983 [DOI] [PubMed] [Google Scholar]
- 23. Luckey SW, Walker LA, Smyth T, Mansoori J, Messmer-Kratzsch A, Rosenzweig A, Olson EN, Leinwand LA. The role of Akt/GSK-3[beta] signaling in familial hypertrophic cardiomyopathy. J Mol Cell Cardiol 46: 739–747, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R, Rosenzweig A. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem 277: 22896–22901, 2002 [DOI] [PubMed] [Google Scholar]
- 25. McCormack JT, Greenwald GS. Progesterone and oestradiol-17b concentrations in the peripheral plasma during pregnancy in the mouse. J Endocrinol 62: 101–107, 1974 [DOI] [PubMed] [Google Scholar]
- 26. Mesa A, Jessurun C, Hernandez A, Adam K, Brown D, Vaughn WK, Wilansky S. Left ventricular diastolic function in normal human pregnancy. Circulation 99: 511–517, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Miyamoto T, Takeishi Y, Takahashi H, Shishido T, Arimoto T, Tomoike H, Kubota I. Activation of distinct signal transduction pathways in hypertrophied hearts by pressure and volume overload. Basic Res Cardiol 99: 328–337, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Mone SM, Sanders SP, Colan SD. Control mechanisms for physiological hypertrophy of pregnancy. Circulation 94: 667–672, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Piekorz RP, Gingras S, Hoffmeyer A, Ihle JN, Weinstein Y. Regulation of progesterone levels during pregnancy and parturition by signal transducer and activator of transcription 5 and 20α-hydroxysteroid dehydrogenase. Mol Endocrinol 19: 431–440, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Sakamoto M, Minamino T, Toko H, Kayama Y, Zou Y, Sano M, Takaki E, Aoyagi T, Tojo K, Tajima N, Nakai A, Aburatani H, Komuro I. Upregulation of heat shock transcription factor 1 plays a critical role in adaptive cardiac hypertrophy. Circ Res 99: 1411–1418, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Schannwell CM, Zimmermann T, Schneppenheim M, Plehn G, Marx R, Strauer BE. Left ventricular hypertrophy and diastolic dysfunction in healthy pregnant women. Cardiology 97: 73–78, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Strait JB, Martin JL, Bayer A, Mestril R, Eble DM, Samarel AM. Role of protein kinase C-ε in hypertrophy of cultured neonatal rat ventricular myocytes. Am J Physiol Heart Circ Physiol 280: H756–H766, 2001 [DOI] [PubMed] [Google Scholar]
- 33. van Eickels M, Grohe C, Cleutjens JPM, Janssen BJ, Wellens HJJ, Doevendans PA. 17beta-estradiol attenuates the development of pressure-overload hypertrophy. Circulation 104: 1419–1423, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Wakatsuki T, Schlessinger J, Elson EL. The biochemical response of the heart to hypertension and exercise. Trends Biochem Sci 29: 609–617, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Wang Q, Zhou Y, Wang X, Evers BM. Glycogen synthase kinase-3 is a negative regulator of extracellular signal-regulated kinase. Oncogene 25: 43–50, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xiao L, Pimental DR, Amin JK, Singh K, Sawyer DB, Colucci WS. MEK1/2-ERK1/2 mediates α1-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes. J Mol Cell Cardiol 33: 779–787, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Yamaguchi O, Higuchi Y, Hirotani S, Kashiwase K, Nakayama H, Hikoso S, Takeda T, Watanabe T, Asahi M, Taniike M, Matsumura Y, Tsujimoto I, Hongo K, Kusakari Y, Kurihara S, Nishida K, Ichijo H, Hori M, Otsu K. Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc Natl Acad Sci USA 100: 15883–15888, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang L, Fishman MC, Huang PL. Estrogen mediates the protective effects of pregnancy and chorionic gonadotropin in a mouse model of vascular injury. Arterioscler Thromb Vasc Biol 19: 2059–2065, 1999 [DOI] [PubMed] [Google Scholar]