Abstract
The everyday experience of stepping onto a stationary escalator causes a stumble, despite our full awareness that the escalator is broken. In the laboratory, this “broken escalator” phenomenon is reproduced when subjects step onto an obviously stationary platform (AFTER trials) that was previously experienced as moving (MOVING trials) and attests to a process of motor adaptation. Given the critical role of M1 in upper limb motor adaptation and the potential for transcranial direct current stimulation (tDCS) to increase cortical excitability, we hypothesized that anodal tDCS over leg M1 and premotor cortices would increase the size and duration of the locomotor aftereffect. Thirty healthy volunteers received either sham or real tDCS (anodal bihemispheric tDCS; 2 mA for 15 min at rest) to induce excitatory effects over the primary motor and premotor cortex before walking onto the moving platform. The real tDCS group, compared with sham, displayed larger trunk sway and increased gait velocity in the first AFTER trial and a persistence of the trunk sway aftereffect into the second AFTER trial. We also used transcranial magnetic stimulation to probe changes in cortical leg excitability using different electrode montages and eyeblink conditioning, before and after tDCS, as well as simulating the current flow of tDCS on the human brain using a computational model of these different tDCS montages. Our data show that anodal tDCS induces excitability changes in lower limb motor cortex with resultant enhancement of locomotor adaptation aftereffects. These findings might encourage the use of tDCS over leg motor and premotor regions to improve locomotor control in patients with neurological gait disorders.
Keywords: locomotor aftereffect, transcranial direct current stimulation, motor adaptation, eyeblink conditioning, transcranial magnetic stimulation
the “broken escalator” phenomenon describes the unusual sensation and transient imbalance experienced when walking onto a stationary escalator, as frequently occurs on the underground (subway or metro) stations. Gait adaptation to varying terrain is essential to maintain balance in everyday life and requires the selection of an appropriate motor strategy. In the experimental setting, stepping onto a stationary platform that was previously moving elicits a locomotor aftereffect (Reynolds and Bronstein 2003) that attests to a process of motor adaptation. Motor adaptation refers to a motor learning process that occurs over a short time course of minutes to hours and allows effective motor control in the face of an external perturbation (Bastian 2008). It involves modulation of previously learned motor skills and differs from the acquisition of a new motor skill, a form of procedural motor learning, which often takes longer to achieve (Shadmehr and Krakauer 2008).
The expression of a learned motor skill may be variously voluntary or involuntary (Fitts and Peterson 1964; Newell 1991). Indeed, voluntary and involuntary motor control mechanisms are relevant to the broken escalator effect since one conundrum is the inability to suppress the motor aftereffect despite full awareness that the escalator is in fact broken, which implies the selection of a previously learned motor response (Reynolds and Bronstein 2003). The broken escalator aftereffect is, however, dissipated with the second trial as a result of deadaptation, a type of error-based learning. In addition, the selection of a motor response is influenced by prior learning and the frequency of exposure to the motor context (Bronstein et al. 2009; Gherri et al. 2009) that is also presumably under cortical control. Perhaps the most salient rationale for invoking a cortical mechanism in the broken escalator phenomenon is that the release of the aftereffect is context-dependent, i.e., it will only occur if the subject walks on the sled that was used during the adaptation (MOVING) phase (Reynolds and Bronstein 2004).
There is substantial evidence highlighting the cortical influences over locomotor control. For example, patients with gray matter frontal lobe dysfunction display difficulties initiating and sustaining locomotion (Nutt et al. 1993), implying a role in locomotor initiation processes. Evidence from cats suggests that the corticospinal tract plays a crucial role in the modification and resetting of the locomotor rhythm (Armstrong 1988; Drew 1991). In humans, it is well-recognized that the primary motor cortex is involved in the automatic execution of lower limb learned motor plans (Christensen et al. 2000), whereas loops of the prefrontal cortex regulate and plan complex actions relevant to gait (Freund and Hummelsheim 1985). The importance of cortical control on human gait is highlighted by the clinical higher-level gait disorders (Nutt et al. 1993) and is supported by functional neuroimaging studies (Bakker et al. 2007; Wang et al. 2008). The neural correlates of motor adaptation are therefore thought to involve M1 and the premotor cortex (Krebs et al. 1998; Shadmehr and Holcomb 1997).
Transcranial magnetic stimulation (TMS) (Baraduc et al. 2004; Hadipour-Niktarash et al. 2007; Muellbacher et al. 2002; Richardson et al. 2006; Robertson et al. 2005) and transcranial direct current stimulation (tDCS) (Hunter et al. 2009) studies have highlighted the involvement of the primary motor cortex (M1) in the different stages of motor skill learning. For example, repetitive TMS (rTMS) to M1 disrupts consolidation, a strengthening of memory that occurs between practice sessions, if it is applied within 6 h (Muellbacher et al. 2002) or 2 h (Robertson et al. 2005) of initial skill acquisition. If applied before learning, subsequent consolidation can be blocked (Richardson et al. 2006). Hadipour-Niktarash et al. (2007) used single-pulse TMS to show that M1 is involved in retention of arm movement adaptation when TMS is applied immediately following the arm movement. When low-frequency (inhibitory) rTMS (<1 Hz) was applied over M1 after arm reaching with no force fields, but before adaptation, the acquisition of learned novel force field-induced dynamics during adaptation was not affected. However, movement errors were greater 1 day later when subjects repeated the reaching tasks with no force perturbations (Richardson et al. 2006). Low-frequency rTMS applied to M1 shortly after force-field motor adaptation, however, did not disrupt subsequent consolidation of the newly formed internal model (Baraduc et al. 2004). Furthermore, when TMS was delivered immediately after each reaching trial during adaptation to visuomotor rotation, subjects showed fewer deadaptation trials to return to baseline values for target errors (Hadipour-Niktarash et al. 2007). These results suggest a possible role of M1 in motor deadaptation of upper limb motor learning. The broken escalator paradigm therefore offers an opportunity to explore the role of M1 and premotor cortex in lower limb locomotor adaptation.
We posed two associated questions: first, can anodal tDCS over M1 and premotor cortex alter locomotor adaptation? Based on the upper limb adaptation data (Hunter et al. 2009; Richardson et al. 2006), we predicted that increasing the excitability of the primary motor and premotor cortical leg areas using anodal tDCS would increase the amplitude of the forward sway and gait velocity in the first AFTER trial (aftereffect). We further hypothesized that anodal tDCS over M1 would prolong the aftereffect given the role of M1 in memory retention (Galea et al. 2011). To test our hypothesis, we applied anodal tDCS over Cz to induce prolonged excitability changes over M1 and premotor cortex bilaterally before the broken escalator paradigm. Second, are the behavioral changes associated with tDCS related to neurophysiological changes in M1 cortical excitability of the legs, and can these neurophysiological changes be predicted using a computational model? We used TMS to probe changes in cortical leg excitability before and after tDCS to M1 using different electrode montages and simulated the current flow of tDCS on the human brain via a geometrically simple (Ferdjallah et al. 1996) finite element model in these different tDCS montages. We used this model to confirm that one of these montages would induce greater current density in M1 bilaterally and would therefore preferentially increase lower limb excitability of both legs.
MATERIALS AND METHODS
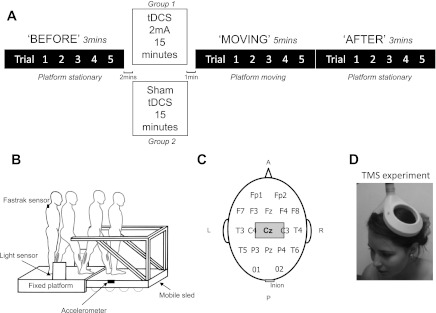
Local ethical approval and participant informed written consent were obtained. Thirty healthy participants, 15 females, mean age 25.5 yr (range 17–32 yr), and 15 males, mean age 30.1 yr (range 22–44 yr), took part in the study. All participants were right-handed and right-footed. The participants were allocated to 2 separate groups for testing. The “real tDCS” group (7 males and 8 females) received tDCS at 2 mA over 15 min, and “sham tDCS” group (8 males and 7 females) received sham stimulation (see Procedure, below) for 15 min. Both groups performed 5 “BEFORE” trials, 5 MOVING trials, and 5 AFTER trials (Fig. 1A). All participants were naïve to both the task and tDCS stimulation.
Fig. 1.
A: experimental sequence. Subjects received either real or sham stimulation while seated in an office chair. Transcranial direct current stimulation (tDCS; or sham) lasted for 15 min exactly, but trial times shown are approximate. B: the motorized sled is enveloped by the fixed platform but remains freely mobile. Its movement is initiated by the subject's leg via a light sensor. Ankle flexor [medial gastrocnemius (MG)] and extensor [tibialis anterior (TA)] from where electromyogram (EMG) was recorded are shaded. C: international 10-20 EEG electrode placement. The anodal (stimulating) electrode was placed over Cz and covered a region 10–20% anterior to Cz as measured from the midpoint of the electrode. The cathode was placed over the inion. D: transcranial magnetic stimulation (TMS) over Cz using double-cone coil. EMG activity was recorded simultaneously from the right and left TA muscles. A, anterior; P, posterior; L, left; R, right.
Materials
A mobile sled (Reynolds and Bronstein 2003) was powered by two linear induction motors and moved with a maximum velocity of 1.4 m/s. The sled was enveloped by a fixed platform under which it could freely pass (Fig. 1B). Movement of the sled was controlled by a computer, triggered by gait initiation via a leg-activated infrared light switch. On passing through the sensor, after a 600-ms delay, the sled would travel a distance of ∼3.7 m in 4.2 s; maximum velocity was reached at 1.3 s after motion initiation. Acceleration of the sled up to peak velocity was therefore 1.08 m/s2. Sled velocity was recorded using a tachometer.
Subject anteroposterior trunk position was measured using a FASTRAK electromagnetic tracking device (Polhemus, Colchester, VT). The sensor was placed over the C7 vertebra, and the transmitter was attached to the sled. A second FASTRAK sensor was attached to the wall and allowed measurement of sled displacement during the moving trials. Step-timing information was given by foot contact strips overlying the shoes at the level of the metatarsophalangeal joints. A linear accelerometer attached to the sled provided independent accurate information of foot-sled contact timing (Fig. 1B). Electromyogram (EMG) activity from the medial gastrocnemius (MG) and tibialis anterior (TA) muscles of each leg was recorded with bipolar electrodes placed 5 cm apart on the belly of the muscle. The EMG signal was band-pass filtered (10–600 Hz), recorded on a personal computer, and sampled as the other signals at 500 Hz.
Procedure
Moving platform task.
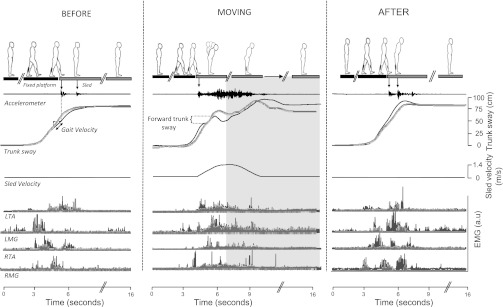
The experimental sequence (Fig. 1A) was as follows: BEFORE (5 trials), MOVING (5 trials), and AFTER (5 trials). Subjects were asked to take their 1st step after an auditory cue (3 “beeps”), at their own pace, with the right leg forward 1st. In the BEFORE trials, the sled remained stationary. Subjects would thus land from the fixed platform onto the sled with the left leg, where they were asked to stop and adopt a quiet stance, with both legs approximately in line until recording was completed (Fig. 2). Each trial lasted 16 s. Depending on which group they were allocated to, on completion of the BEFORE trials, subjects then received either sham stimulation or tDCS stimulation while sitting in an office chair for 15 min. Participants read a magazine and conversed with the experimenters during this period. Before continuing onto the MOVING trials, each participant was shown once how the platform would move. Subjects then performed 5 MOVING trials. The number of learning trials used was chosen on the basis that this locomotor aftereffect can be generated with few adaptation trials (Bunday et al. 2006). Subjects were instructed to avoid the use of the available handrails when stepping on the moving sled unless this was absolutely necessary. When the MOVING trials were complete, all subjects received the warning, “I will now switch the motor off so the platform will not move - it will be stationary as it was in the first 5 trials,” and the motor was ostensibly turned off (subjects were able to hear and see this). All subjects then completed another set of 5 stationary (AFTER) trials.
Fig. 2.
Representative data from 1 subject receiving real tDCS stimulation and 1 subject receiving sham stimulation during the experimental conditions (BEFORE trial 1, MOVING trial 1, and AFTER trial 1). Trunk sway, gait velocity, and EMG activity are shown in light gray for the subject receiving sham stimulation. Trunk sway data in AFTER trial 1 show the characteristic trunk overshoot in addition to increased EMG activity and are more marked in the real stimulation (black lines). Note that the sled velocity profile during the BEFORE and AFTER trials is 0 m/s as the sled remained stationary. The accelerometer was not calibrated as it was just used to detect accurately foot-contact timing (down-pointing arrow) and was subject to interference from the motor in the MOVING trials. a.u, Arbitrary units.
tDCS.
We determined the tDCS electrode montage using evidence from MRI studies (Lagerlund et al. 1993) and three-dimensional probabilistic anatomic correlation techniques (Okamoto et al. 2004) showing that the scalp topography of Cz [international 10-20 EEG system (Klem et al. 1999)] corresponds to lower limb primary motor cortex. Thus a DC stimulating rectangular saline-soaked sponge electrode (10 × 4 cm; surface area 40 cm2) was placed centrally across the scalp to cover a region 10–20% anterior to Cz as measured from the midline of the stimulating electrode (Fig. 1C). The reference electrode (4 × 4 cm) was positioned at the inion. A 2-mA current was delivered by a battery-driven Magstim Eldith DC stimulator (neuroConn, Ilmenau, Germany) between the end of the BEFORE trials and the beginning of the MOVING trials (i.e., just before the adaptation period; Fig. 1A). The current was initially increased by a ramp input over 10 s until reaching 2 mA (current density 0.05 mA/cm2). Stimulation duration of 15 min, as chosen, can result in an excitability change lasting up to 90 min (Nitsche and Paulus 2001). The sham stimulation is identical to the real stimulation condition except that the current drops off to zero after 30 s. Participants reported a “tingle” sensation beneath the electrodes at the beginning of the stimulation for both sham and real conditions, which typically faded away after 10–20 s in both groups. In these experiments, as documented in the literature, naïve participants are unable to distinguish between real and sham tDCS when employed in this fashion (Gandiga et al. 2006) and have similar perception thresholds for sham and real stimulation (Ambrus et al. 2011).
In the second series of experiments, described below, we investigated the changes that occur in lower limb excitability following anodal tDCS over M1 using two different electrode montages (TMS). Given that one of these montages involved placing the reference electrode over the inion, we probed whether cathodal tDCS over the inion induced changes in cerebellar cortex excitability (Eyeblink conditioning). Finally, we modeled the current flow induced by tDCS through a schematic human head using a computational model (A topographic tDCS model) in an attempt to predict the neurophysiological changes observed in our TMS experiments and support the choice of electrode placement in the escalator experiments.
TMS.
To confirm that tDCS was indeed modulating lower limb M1 excitability, we evaluated the effect of tDCS on TMS-induced motor-evoked potentials (MEPs) in TA bilaterally in 16 additional healthy subjects, 2 of whom took part in the main broken escalator locomotor experiment. The gastrocnemius muscle (the activity of which was recorded during the moving platform task) was not tested as it is less readily accessible with TMS, owing to its smaller cortical representation. These TMS experiments were performed in a separate session, at least 2 wk apart from the gait experiments. Magnetic stimuli were delivered to the motor cortex using a Magstim 200 stimulator connected to an angled double-cone coil (Magstim, Whitland, United Kingdom) positioned over the TA hotspot (Fig. 1D), with the current induced in the brain flowing in a posterior-to-anterior direction. Surface EMG was recorded using disposable Ag-AgCl electrodes (Viasys; CareFusion) in a belly-tendon montage. Maximum voluntary contraction (MVC) was determined for all subjects before sampling using forced dorsiflexion against a strap over the dorsum of the foot that was connected to the visual biofeedback (of EMG). Subjects were then asked to contract tonically TA muscles bilaterally to ∼20% of MVC to standardize the muscular activity within and between subjects. Active muscle recording (compared with rest) enables the use of lower TMS output intensities, which ensures higher compliance with the task when using a double-cone coil. The two muscles (right and left TA) were recorded simultaneously. TMS pulses were delivered over the hotspot for bilateral TA, starting at 20% of maximum stimulator output (MSO) and increasing by 5% steps to 60% MSO. The hotspot was taken as the scalp location where the peak-to-peak MEP amplitudes were greater in the target muscle than amplitudes of adjacent scalp locations for a given TMS stimulus intensity. For all subjects, this site was approximately 2–3 cm anterior to the vertex. EMG signals were amplified and band-pass filtered between 20 Hz and 2 kHz, digitized at a sampling rate of 5 kHz, and relayed onto a computer using Signal 3.06 software [Cambridge Electronic Design (CED), Cambridge, United Kingdom]. Electrode placement for tDCS was identical to that used during the main broken escalator experiments. Eight subjects (3 females and 5 males; age range 22–31 yr) received real stimulation, and 8 subjects (4 females and 4 males; age range 22–34 yr) received sham stimulation.
MEPs were defined as peak-to-peak amplitudes of >0.1 mV. MEP peak-to-peak amplitudes were measured from unrectified single traces. Silent-period duration was measured from the end of the MEP to the restoration of background EMG activity and was then averaged for the last 10 frames (highest TMS stimulator output intensity used) for pre- and post-tDCS stimulation blocks. Recruitment curves were constructed based on MEP amplitude as a function of TMS intensity. To detect any tDCS-induced changes, we measured MEP amplitude and the length of the silent periods. Curves were fitted using the Boltzmann equation for a 3-parameter sigmoid fit where a = the upper asymptote, x = TMS intensity, x0 = midpoint value of the curve, and b = slope:
Paired t-test comparisons between pre- and poststimulation were performed for midpoint values of the curves (x0), maximum MEP amplitude, and silent-period duration between tDCS and sham conditions for both right and left TA muscles.
Eyeblink conditioning.
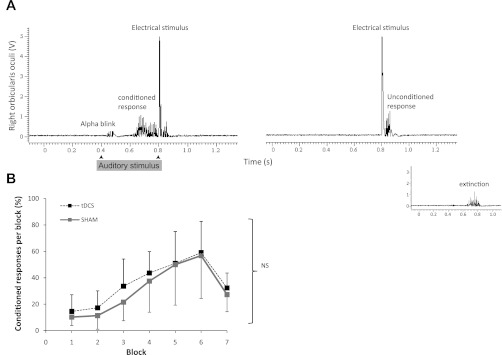
We performed a series of experiments to assess what effect, if any, cathodal stimulation over the inion could have had on cerebellar structures, given the recognized role of the cerebellum in motor learning and adaptation (Galea et al. 2011). We predicted that our electrode montage would spare cerebellar structures, as this is a suboptimal placement to stimulate the cerebellar cortex (Ohtsuka and Enoki 1998). Eyeblink classic conditioning (EBC) is a well-characterized experimental paradigm that is conserved across species and is dependent on the cerebellum (Gerwig et al. 2007). There are, to date, no studies investigating the use of tDCS to modulate EBC. EBC consists of pairing a weak conditioning stimulus (CS) with a strong unconditioning stimulus (US) repeatedly to produce conditioned responses (CRs) consisting of an eyeblink starting before the US. Patients with cerebellar disease are unable to produce CRs (Gerwig et al. 2003, 2005; Jirenhed et al. 2007). EBC was performed as described in Teo et al. (2009). We used a loud (∼80-dB) 200-Hz auditory tone as the CS, lasting 400 ms, played via binaural headphones. The CS produced an acoustic startle (α-blink) within 100 ms after the CS (Fig. 3A). The US was an electric pulse (200-μs pulse width at 5× sensory threshold, ∼1.0 mA) given to the supraorbital nerve 400 ms after the CS to elicit an eyeblink. Repeated pairs of CS and US at 400-ms intervals (i.e., delay eyeblink conditioning) yield CRs occurring within 200 ms before the US, which are independent of basal ganglia and cerebral function (Gormezano and Tait 1976; Sommer et al. 1999).
Fig. 3.
A: representative normalized EMG recording from right orbicularis oculi in response to a 400-ms duration auditory stimulus with onset at 400 ms and electric stimulus at 800 ms. Eyeblink conditioned responses are seen before the electric stimulus. Extinction phase (inset) consisted of conditioning (auditory) stimuli alone. B: averaged data for healthy subjects following 15 min of anodal tDCS or sham stimulation showing the presence of conditioned responses in both groups. NS, not significant.
Conditioning consisted of 6 learning blocks of 11 trials: trials 1-9 were always CS-US pairs, trial 10 was US only, and trial 11 was CS only. A 7th block consisted of 11 CS trials to measure extinction (Fig. 3A, inset). The intertrial interval was randomized with a range of 10–20 s to reduce habituation. A period of 400 ms before the CS was recorded to detect spontaneous blinks. The CS was applied to the left orbicularis oculi in all subjects. EMG was recorded from both orbicularis oculi muscles with the active electrode over the inferior belly and the silent electrode over the lateral canthus. EMG bursts were regarded as CRs when present 200 ms post-CS and before the US. Data analysis consisted of a 2 × 2 factorial repeated-measures ANOVA with within-subject factor block (blocks 1–6) and the between-subject factor group (real tDCS and sham). The tDCS montage was identical to that used in the locomotor experiment described above (tDCS). Eight subjects (4 females) underwent 15 min of real tDCS, and 8 subjects underwent sham stimulation (4 females).
A topographic tDCS model.
We estimated the effect of tDCS on the human brain and compared the current density induced in M1 by two different montages using a finite element model. Such a model represents an idealized geometry of the head and calculates the electric field induced by current flow within this geometry. The geometry was defined using a previously reported four-layer sphere model (Ferdjallah et al. 1996). This model is based on four nested spheres for the scalp (7.65-cm radius), skull (7.18-cm radius), cerebrospinal fluid (CSF; 6.40-cm radius), and brain (6.15-cm radius).
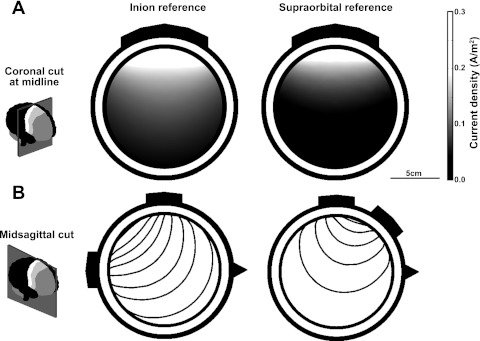
Geometrically simplified models have been used to model tDCS and TMS and for source localization models in EEG and magnetoencephalography (Datta et al. 2008; Vatta et al. 2005). However, it is important to note that this model does not account for either the detailed geometry of the human head or the anisotropic electric conductivity of white matter. Therefore, the model simulations are not intended to provide an absolute value of the exact current density distribution induced by tDCS in the brain. Rather, the model allows us to compare quantitatively the relative current density induced by two different electrode montages. Specifically, we compared the current density induced in the leg area of the primary motor cortex by anodal stimulation (electrode placed at Cz) with the cathode placed on the inion, relative to anodal Cz stimulation with a supraorbital cathode (Fig. 4), as used in the TMS experiments (see TMS).
Fig. 4.
The simulation of a 4-layer finite element model allows visualization of the flow of current beneath the tDCS electrodes. A: the coronal cut through the midline of the model shows the current density at the level of M1 in 2 electrode montages. The anode is placed bilaterally across M1 in both cases, and the reference is either on the inion (left) or on the supraorbital region (right). The distribution shows that the peak of the current density is similar in the 2 cases immediately beneath the anode (0.29 A/m2 with the inion reference vs. 0.28 A/m2 with the supraorbital reference). As shown previously, the current density drops rapidly with distance (Datta et al. 2008), but this decay is more gradual with the inion reference, and the current density stays above threshold (0.1 A/m2) deep into the motor region, hence supporting our choice of electrode positioning. B: the sagittal cut highlights the direction of current flow via the field lines. When the reference is at the inion, the field lines penetrate deeper into the brain, whereas with a supraorbital reference there is more current passing superficially through the brain.
The modeling package COMSOL Multiphysics 3.3 (COMSOL, Stockholm, Sweden) was used to create a three-dimensional geometric representation of the whole head, with tDCS electrodes modeled as two cuboids on the surface of the scalp with dimensions 10 × 4 and 4 × 4 cm. The defined geometry was meshed into tetrahedral elements using the default Delaunay triangulation method in COMSOL. The electric potential distribution induced by stimulation was calculated by solving the Laplace equation:
where V is the potential (measured in volts), σ is the constant conductivity (measured in Siemens per meter), ▽ is the gradient of the potential, and ▽. is the divergence of the resulting vector field. The mean conductivity values of the brain tissue were defined based on previous biological studies with 0.45 S/m for the scalp, 0.06 S/m for the skull, 1.7 S/m for the CSF, and 0.45 S/m for the brain (Gabriel et al. 1996; Rush and Driscoll 1969). The surface of the electrode in contact with the scalp was set to the desired stimulating current in milliamperes using Neumann boundary conditions.
Data Analysis
Pre- and postfoot-sled contact epochs were derived from the sled accelerometer (Fig. 2) and corroborated with the foot contact strip data. Trunk position along the anteroposterior axis was provided by the FASTRAK (Fig. 2). For trials where the sled was kept stationary (BEFORE and AFTER), trunk sway was measured as the maximum forward deviation or “overshoot” of the trunk, relative to the mean final resting stance position in the last 3 s of the trial (Fig. 2). Because of the complex oscillations of the trunk during the MOVING trials, trunk sway was measured as the maximum backward-forward (peak-to-peak) displacement of the trunk before sled deceleration (Bunday and Bronstein 2008). The FASTRAK position sensor was also used to calculate walking velocity, defined as the mean linear trunk velocity in a 0.5-s time window before foot-sled contact (Fig. 2). The EMG signals were rectified and integrated over a 500-ms time window after foot-sled contact. For graphic display, EMG values were normalized with respect to mean BEFORE (trials 3-5) values. Within-subject comparisons were made using one-way repeated-measures ANOVA. Bonferroni multiple comparisons were performed for post hoc analysis of each trial. To assess the presence of the aftereffect, a mean BEFORE value for trials 3-5 for each subject was taken for comparison against AFTER trial 1; a significant (P < 0.05) larger value in the AFTER trials indicated the presence of an aftereffect. To assess the duration or persistence of the aftereffect, AFTER trial 2 was also compared with the mean value of the BEFORE trials (3-5) using a one-way repeated-measure ANOVA. When interactions were found, each AFTER trial was investigated individually. Between-group comparisons (tDCS vs. sham) were carried out using two-way repeated-measures ANOVA. Statistical analysis was done using SPSS 16.0.
RESULTS
We describe the results from the main broken escalator experiments first and the results of the neurophysiological and computational experiments later.
Linear trunk displacement and gait velocity were similar across all subjects in the BEFORE trials, and trunk velocity increased appropriately in the MOVING trials in all subjects. Trunk sway in the MOVING condition was highly variable between individuals, ranging between 11.4 and 40.4 cm in trial 1. Trunk sway reduced considerably in the subsequent MOVING trials as subjects familiarized themselves with sled motion and reached a plateau gait velocity by the second MOVING trial. In the AFTER trials, we found an increase in the magnitude of trunk sway and gait velocity in subjects receiving real tDCS stimulation and a persistence of the aftereffect into the second AFTER trial (Fig. 5). In the sham stimulation group, the aftereffect was only present in the AFTER trial 1.
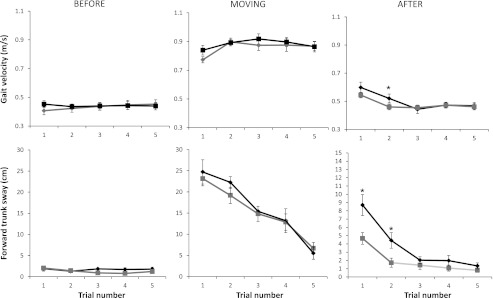
Fig. 5.
Mean (±SE) group data for real (black) and sham (gray) tDCS during conditions BEFORE, MOVING, and AFTER for gait velocity (top) and trunk sway (bottom). The horizontal axis shows the trial number (1-5). Note the presence of the aftereffect in AFTER trial 1 in both groups and to a lesser extent in AFTER trial 2 in the real tDCS stimulation group. The aftereffect is seen as an increase in trunk displacement and gait velocity compared with baseline trials. Note the change in y-axis scaling for trunk forward sway in the AFTER trials for graphic representation. *P < 0.05.
Descriptive results for the real vs. sham stimulation groups have been divided into BEFORE, MOVING, and AFTER conditions. EMG data are reported separately below. The corrected Bonferroni significance level was P = 0.016.
BEFORE
Gait velocity was not significantly different within individuals for real tDCS (P = 0.58) or sham (P = 0.93). There was also no difference in trunk displacement within individuals (P = 0.57 for real stimulation and P = 0.20 for sham). Gait velocity was not significantly different between groups (P = 0.1) in the BEFORE trials (mean velocity 0.42 m/s).
MOVING
To negotiate the accelerating sled, gait velocity before foot-sled contact in the moving trials is expected to increase, and there is a forward trunk displacement to shift the center of mass anteriorly (Reynolds and Bronstein 2003). As in previous studies (Bunday et al. 2006; Reynolds and Bronstein 2003), these changes were observed for all subjects (Fig. 5 for grouped data; individual data not shown). However, no difference was seen in gait velocity (P = 0.55 for real and P = 0.72 for sham) or trunk displacement (P = 0.83 real and P = 0.93 sham) during MOVING trial 1 within individuals. Gait velocity during MOVING trial 1 was not significantly different between stimulation groups (P = 0.67). There was no significant difference in the rate of reduction in trunk sway between the two groups. Subjects receiving real tDCS had a faster gait velocity in MOVING trial than those in the sham group, although this did not reach significance (0.77 m/s for sham, 0.84 m/s for real; P = 0.14). Gait velocity in the first MOVING trial did not correlate with the magnitude of the aftereffect (r = 0.36, P = 0.19 for real tDCS; and r = 0.41, P = 0.23 for sham).
AFTER Trial 1
AFTER trial 1 was compared with the mean of the BEFORE trials (3-5) to determine the presence of an aftereffect. There was a significant increase in gait velocity in both the real (P < 0.001) and sham (P < 0.001) groups. There was also evidence of a trunk sway aftereffect with a significant increase in forward trunk displacement in AFTER trial 1 compared with mean BEFORE values (P < 0.001 for real stimulation and P < 0.001 for sham).
We found a statistically significant difference between tDCS and sham in the size of the locomotor aftereffect and its duration (Fig. 5) as measured by the trunk displacement. The mean forward trunk displacement in AFTER trial 1 was 8.55 cm (SE = 1.25) for real tDCS and 4.66 cm (SE = 0.7) for sham stimulation (P = 0.04). This represents an 83% increase in forward displacement of the trunk in the tDCS group over the sham group. A similar trend was seen in gait velocity [0.6 m/s (tDCS), 0.54 m/s (sham)], although this did not reach significance (P = 0.15). There was no correlation between gait velocity during the moving trials and the magnitude of the aftereffect (as measured by both gait velocity and trunk overshoot). The effect of DC stimulation on trunk displacement was apparent even when subjects that did not have an aftereffect were excluded from the analysis (unpaired t-test, n = 13 for sham, n = 14 for tDCS; P < 0.05).
AFTER Trial 2
In the real tDCS group, trunk displacement was also greater in AFTER trial 2 compared with BEFORE trials (P = 0.0013). Unlike in the real stimulation group, no aftereffect was observed beyond AFTER trial 1 in the sham group, with AFTER trial 2 being no different from the mean of the BEFORE trials (P = 0.13). For both trunk displacement and gait velocity, a locomotor aftereffect was present in AFTER trial 2 in the tDCS group (P = 0.001 and P = 0.001, respectively; Fig. 5) but not in the sham group (P = 0.25).
AFTER Trials 3, 4, and 5
Trunk displacement in AFTER trials 3, 4, and 5 was not different within individuals (P = 0.2 for tDCS and P = 0.12 for sham stimulation). Gait velocity in AFTER trials 3-5 was also not different within individuals (P = 0.24 tDCS and P = 0.36 for sham). There was no significant difference in gait velocity between AFTER trials 3, 4, or 5 and the mean of the BEFORE trials 3-5 in the real stimulation group (P = 0.28) or in the sham stimulation group (P = 0.22). There was no significant difference in gait velocity between AFTER trials 2, 3, 4, or 5 and the mean of the BEFORE trials in the real tDCS group (P = 0.19) or sham group (P = 0.19). Gait velocity in AFTER trials 3-5 was not different between groups (P = 0.75).
EMG Data
A comparison of rectified and integrated EMG activity (BEFORE vs. AFTER) for the left MG muscle in the real and sham groups is shown in Fig. 6. As in previous studies, we have focused on the left MG as the left leg is the first leg to contact the sled and therefore has to absorb the brunt of the initial impact for reducing the forward momentum. As described in previous studies using this paradigm (Reynolds and Bronstein 2003, 2004), EMG activity increases in the MG muscles (particularly the left) just after foot-sled contact. Thus the EMG data also showed the presence of an aftereffect (Fig. 6).
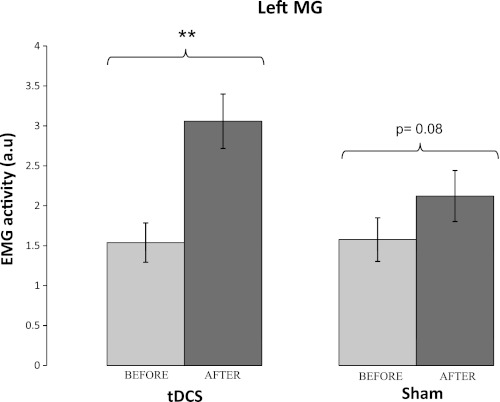
Fig. 6.
EMG activity in the left MG muscles (mean of the BEFORE trials 3-5 vs. AFTER trial 1) for real and sham tDCS groups. EMG activity was greater in the 1st AFTER trial compared with BEFORE trials, consistent with the presence of an aftereffect. Vertical bars represent standard errors. **P < 0.001.
In the tDCS group, significant increases were observed in the left and right MG muscle activity in AFTER trial 1 compared with BEFORE values (P < 0.001 for left MG and P = 0.03 for right MG). EMG activity from the right MG in AFTER trial 1 was also greater than BEFORE values in the sham group (P = 0.018). A similar trend was seen for the left MG in the sham group but did not reach significance (P = 0.075). No significant difference was seen in TA muscles.
Additional Experiments
TMS.
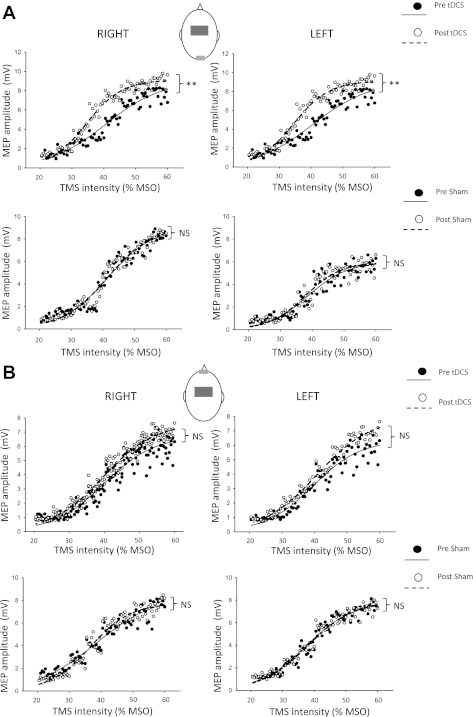
A repeated-measures ANOVA revealed no difference in background EMG activity (at 20% of MVC) across subjects, right and left TA, nor between groups (mean EMG activity 1.29 mV for real tDCS, 1.53 mV for sham; P = 0.26). Averaged data are shown in Fig. 7A (inion cathode) and Fig. 7B (supraorbital cathode). We found a significant increase in MEP amplitudes in right (P < 0.001) and left TA muscles (P < 0.001) following 15 min of 2-mA anodal tDCS using an inion reference electrode (as used in the broken escalator experiment) but not for sham stimulation (P = 0.93 for the left TA and P = 0.43 for the right TA). Concordantly, silent-period durations increased with real stimulation (real tDCS: P = 0.019 for right TA and P = 0.012 for left TA) but not with sham (P = 0.76 right TA and P = 0.95 left TA). Using a supraorbital reference (cathode) electrode did not significantly change MEP amplitude with real tDCS (P = 0.19 for right TA and P = 0.45 for left TA) nor with sham stimulation (P = 0.74 for right TA and P = 0.29 for left TA). There was no overall effect of tDCS using this second montage on silent-period durations in the last 10 frames (P > 0.1). These data provide further evidence that tDCS placement over Cz is able to modulate cortical excitability in leg areas bilaterally. In addition, it supports the simulation results of our computational model, which predicted the neurophysiological effects of tDCS.
Fig. 7.
Averaged group data showing TMS-induced motor-evoked potential (MEP) amplitudes in right and left TA before and after tDCS or sham. A: reference electrode placed at the inion. Top: MEP amplitudes pre- (black spots) and post- (white spots) real tDCS. Bottom: MEP amplitudes pre- and postsham stimulation. MSO, maximum stimulator output. B: supraorbital reference (cathode) electrode. Top: MEP amplitudes pre- (black spots) and post- (white spots) real tDCS. Bottom: MEP amplitudes pre- (black spots) and post- (white spots) sham stimulation. Sigmoid curves were fitted using MATLAB (The MathWorks, Natick, MA). r2 Values ranged from 0.93 to 0.99. Muscle excitability was increased following 15 min of tDCS but not following sham but only when a reference electrode was placed at the inion.
Eyeblink conditioning.
Cathodal effects over the cerebellum would be expected to impair conditioned eyeblink responses. All subjects (n = 8) in the real tDCS and 7 subjects (n = 8) in the sham group acquired the CR. Comparison of CRs per block revealed no differences between real and sham groups [ANOVAtDCS/block, F(1,14) = 0. 54, P = 0.74] with normal CRs in both groups (Fig. 3B). There was a significant effect of block [F(1,14) = 8.78, P = 0.002] with increasing CRs across blocks but no effect of group [F(1,14) = 0.32, P = 0.58]. Latencies of CRs and α-blinks were not statistically significant between groups. Extinction phase responses were analyzed only for subjects who showed CRs. Mean CR in extinction phase was 3.6 (SD = 1.2) in the tDCS group and 3.0 (SD = 1.3) in the sham group (paired t-test; P = 0.26). Representative traces showing unconditioned and conditioned responses are illustrated in Fig. 3A. Thus placing the cathode electrode over the inion does not appear to have significant cerebellar effects.
Topographic tDCS model.
The model simulation (Fig. 4) shows two main differences between anodal M1 stimulation with a reference at the inion compared with a supraorbital reference. First, with a 2-mA stimulating current, the current density induced in the region of brain directly below the anode peaks at 0.29 A/m2 (Fig. 4A). In comparison, when the reference electrode was moved to the supraorbital location, the induced current density in the M1 region was modestly smaller, peaking at 0.28 A/m2, and in particular the current density decay is greater as the depth into the brain increases. Second, the field lines, which show the current path through the brain, show less shunting through the scalp and CSF, penetrating deeper into the brain (Fig. 4B). Specifically, the model predicted that the same 2-mA stimulating anodal current would cause up to 47% less current density beneath the anode using a supraorbital reference electrode.
DISCUSSION
We present the novel finding that anodal tDCS over the primary motor cortex and premotor leg areas increases the magnitude of the broken escalator adaptation aftereffect by 80% and prolongs its duration (normally observed only in the 1st AFTER trial; Reynolds and Bronstein 2003) into the 2nd trial. We provide TMS data demonstrating that anodal tDCS over Cz increases cortical excitability in leg muscles bilaterally and also show that our computational model predicts tDCS-induced leg motor cortex excitability changes.
At first sight, the occurrence of the broken escalator effect itself appears incongruous. Why do healthy subjects display a motor response (a forward “stumble” on a stationary platform) apparently inimical for their stability despite full knowledge of the escalator being broken? Since motor aftereffects are a measure of motor learning in general (Lam et al. 2006; Morton and Bastian 2006), the observed stumble on the stationary platform implies the inappropriate expression of the newly generated motor program acquired when stepping onto the moving platform (Reynolds and Bronstein 2004). Viewed in this light, the increased amplitude of the trunk sway aftereffect reflects an enhancement of the motor adaptation aftereffect.
Our results are consistent with previous data using TMS to explore the role of M1 in upper limb force-field adaptation. Repetitive training of a single ballistic hand movement transiently modifies corticospinal excitability, leading to a favored pattern of motor output (Classen et al. 1998), although the neural substrate for this change is unknown. Studies of visuomotor adaptation or learning in animals have implicated the supplementary motor area (SMA; Wise et al. 1998), premotor cortex (Mitz et al. 1991; Shen and Alexander 1997; Wise et al. 1998), parietal cortex (Eskandar and Assad 1999), and cerebellum (Liu et al. 2003), and this has been confirmed using functional imaging in humans (Krebs et al. 1998). We have shown that stimulation of M1 and premotor cortex before skill acquisition increases locomotor adaptation. One may consider three nonmutually exclusive explanations to account for this.
When the brain encounters environmental or sensory changes in the body, it must choose the most appropriate response for the current situation, using a Bayesian motor decision-making process (Wolpert 2007). The motor response in the escalator task may be determined by weighing prior experience on the moving sled and the current estimate of the platform motion. Indeed, the proportion of subjects who show an aftereffect on this task can be increased from 80 to 100% when the experiment uses a faster platform velocity (Green et al. 2010). Furthermore, autonomic arousal and anxiety about the task all influence the expression of the locomotor aftereffect (Green et al. 2010) via a process of “risk assessment.” This assessment is therefore subject to interactions from the physical properties of the sled and the individual's state of arousal. Premotor areas show prominent activation during motor planning (Alexander and Crutcher 1990; Kurata and Wise 1988; Tanji and Shima 1994; Wise et al. 1997), and there is evidence that the activity of neurons in premotor cortex (Xiao et al. 2006) and SMA (Padoa-Schioppa et al. 2002) during motor planning reflects the dynamics of the upcoming movement. tDCS in its offline mode may thus prevent the selection of a motor program that is contextually appropriate in favor of a cautious approach (“the sled may move after all”), thus generating a larger aftereffect. Recent data have confirmed that premotor regions are involved in involuntary task switching (Boy et al. 2010; Sumner et al. 2007), a function that could potentially be invoked in our subjects' decision to select a gait response appropriate for a moving vs. a stationary platform.
Alternatively, neurostimulation paradigms may impact on connected structures distant to the site of stimulation, including subcortical and brain-stem locomotor circuits such as the basal ganglia, brain-stem locomotor and posture regions (e.g., the midbrain locomotor regions), and spinal circuits. Indeed, it has been shown that noninvasive brain stimulation can alter spinal network excitability. Thus one may consider that tDCS is altering excitability of spinal systems involved in sensorimotor integration (sensory and proprioceptive output from muscles, skin, and joints) to modulate motor output. Spinal cord reflexes can be facilitated using rTMS over the motor cortex, presumably occurring as a result of inhibition of corticospinal projections (Valero-Cabre et al. 2001). Anodal tDCS has also been shown to have effects on spinal network excitability (Roche et al. 2011). Thus, in our experiment, anodal tDCS may alter the excitability of spinal reflex responses to enhance adaptive motor responses. In fact, an interesting characteristic of this locomotor aftereffect is that the aftereffect overrides cognitive control, e.g., it cannot be suppressed at will, and yet it will not appear if the subject walks on a surface other than the training sled (Bronstein et al. 2009; Reynolds and Bronstein 2004). This suggests the involvement of low-order brain-stem locomotor pathways (Lawrence and Kuypers 1968; Matsuyama et al. 2004) that are under involuntary, although cortical in that they are context-dependent, gait control. Contrary to this argument, the spinal effects of anodal tDCS appear to operate online and rapidly wear off when stimulation is discontinued (Roche et al. 2011). In addition, and in contrast to our findings, there is some disagreement in the literature as to whether motor cortex high-frequency rTMS and anodal tDCS increase (Cowan et al. 1986; Roche et al. 2009) or decrease (Roche et al. 2011) spinal cord excitability. Overall, our results suggest that anodal tDCS stimulation may modulate “high-order” cortical networks given the gait parameters that were affected: motor learning, the characteristics of the locomotor aftereffect, and the dissociations between its functional components, namely gait velocity vs. trunk sway. Although tDCS is likely to induce excitability changes over widespread cortical networks, including areas outside M1, we provide direct TMS evidence that tDCS increases lower limb excitability bilaterally, suggesting that M1 is involved in the locomotor aftereffect.
Lastly, the assumption that applying noninvasive brain stimulation with parameters that increase motor cortical excitability (e.g., anodal tDCS) improves motor performance has become generally accepted (Reis and Fritsch 2011). However, it has become clear that there are timing-specific effects of tDCS relative to adaptation or training. Thus anodal tDCS applied to M1 during training improves performance (Antal et al. 2004; Boggio et al. 2006; Galea and Celnik 2009; Nitsche et al. 2003), whereas the application of tDCS before learning or adaptation has produced mixed results with respect to motor performance. It has been proposed that such variability may relate to homeostatic effects when tDCS is applied before training (Stagg et al. 2011). Given that tDCS-associated increases in excitability are likely to be widespread, concurrent synaptic activation induced by motor training could lead to improvements in performance via synaptic specificity (ensuring that the synaptic changes specific to the motor task are enhanced, with pruning of nonspecific synaptic connectivity). A further explanation for our findings, therefore, is that anodal tDCS increased neuronal excitability in a widespread cortical network and that the repetitive locomotor task of walking onto the moving platform reinforced appropriate synaptic changes that led to an enhanced expression of the adaptation aftereffect. This is the first demonstration of enhanced locomotor (lower limb) adaptation aftereffect using anodal tDCS when applied just before adaptation.
One may reasonably assume that the forward trunk displacement component of the aftereffect is directly related to the increased gait velocity, such that a sudden termination of gait leads to the forward overshoot of the trunk. However, gait velocity only contributes to a small degree to forward trunk sway, suggesting that these two aspects of locomotion are largely independent (Bronstein et al. 2009), and the current results further support such a view: the most prominent effect of tDCS was on trunk displacement (largely due to hip flexors) rather than gait velocity. Whether this dissociation is due to tDCS modulation of relevant frontal areas such as the SMA (Fukuyama et al. 1997; Iseki et al. 2008) or to the fact that cortical leg areas are deeper than hip/axial structures (although still affected by tDCS; see Fig. 7) is not clear. Furthermore, we can exclude a direct effect of tDCS on gait velocity per se, as we observed no significant differences in gait approach velocity in MOVING trial 1 between real and sham groups.
It is perhaps surprising that the large increase in the motor aftereffect was not accompanied by recordable changes during the learning phase (MOVING trials). However, this has been a consistent finding in studies exploring the role of M1 in motor learning and retrieval (Galea et al. 2011; Orban de Xivry et al. 2011). For example, anodal tDCS to the contralateral M1 did not result in greater adaptation compared with sham during force-field adaptation, but when the force field was turned off, anodal tDCS induced larger errors in reaching performance, suggesting a role for M1 in retention (Hunter et al. 2009). Indeed, M1 appears to have a distinct functional role in retention, rather than acquisition, processes during adaptive motor learning (Galea et al. 2011), such that anodal tDCS over M1 did not improve the ability to learn from errors but resulted in increased retention (larger aftereffects). This result is consistent with our findings.
Study Limitations
Some methodological aspects of the study deserve mention. It could be argued that a large active electrode (anode) on Cz and a smaller cathode on the inion could cause effects over a number of other central nervous system regions relevant to sensorimotor control and adaptation. In particular, the cerebellum is in anatomic proximity to the inion and is known to be involved in motor adaptation (Jayaram et al. 2011). Most studies of tDCS cerebellar stimulation, however, place the stimulating electrode 3 cm lateral to the inion (Galea et al. 2009; Jayaram et al. 2011) or 2 cm below the inion and 1 cm posterior to the mastoid process (Ferrucci et al. 2008), locations that we avoided. Furthermore, “open-loop” locomotor adaptation experiments in patients with cerebellar damage have shown reduced, and sometimes absent, motor aftereffects (Morton and Bastian 2006). Lastly, anodal cerebellar tDCS has been shown to produce faster adaptation to a visuomotor transformation (Galea et al. 2011). Thus one would not expect cathodal (inhibitory) stimulation over the cerebellum to cause the enhancement of the locomotor aftereffect that we observed. Indeed, we show that cathodal stimulation over the inion does not alter eyeblink conditioning (a phenomenon that relies on the cerebellum; Gerwig et al. 2007), although this cannot be taken as definitive evidence that the cerebellum was not modulated by tDCS in this locomotor task. Nevertheless, our computational model predicted that current flow from Cz to the inion was more likely to have an effect on cortical leg areas than with a supraorbitally placed cathodal electrode, a fact that we were able to corroborate neurophysiologically with TMS. Additionally, this electrode montage enabled us to target midline locomotor cortical structures that span both hemispheres (including bilateral M1). It should be noted that an extracephalic reference electrode, while avoiding cathodal effects over the cortex, may shunt electric current through the skin or even displace the current such that it would not reach the desired cortical regions (Mahmoudi et al. 2011). We cannot comment on the specific brain structures directly affected by the stimulation, which may not be strictly limited to leg regions of the primary motor and premotor cortex. Admittedly, the pathway of least resistance for current flow using tDCS is not known, a potential criticism of this and most tDCS studies. Current flow during tDCS depends on the electrode montage, electrode size, and current intensity used; computational models of brain current flow during tDCS provide an estimation of the current flow and hence the anatomic regions affected by the stimulation. Such models range in complexity from concentric sphere models (Datta et al. 2008; Im et al. 2008; Miranda et al. 2006, 2009) to individualized MRI-based high-resolution models (Datta et al. 2011; Parazzini et al. 2011; Wagner et al. 2007). Our modeling data show that it is possible to predict neurophysiological outcomes with tDCS based on simple models and suggest that such models could help optimize the potential of noninvasive techniques. Finally, the nature of the broken escalator adaptation process that spans several seconds precludes the use of single-pulse or short-burst TMS approaches. Equally, the widespread cortical circuits involved in gait adaptation phenomena render more spatially selective tDCS and rTMS techniques unsuitable. Indeed, rTMS is likely to affect premotor areas in one hemisphere, not both.
Our results may have clinical implications. The finding of an effect of cortical modulation on involuntary locomotor control is of potential interest for patients with gait disorders as tDCS offers the advantage of ease of access. Its relative lack of focus, an apparent disadvantage for physiological experiments, may be welcomed when attempting to modulate complex and distributed cortical networks such as those involved in gait. Indeed, there is evidence supporting the use of tDCS in the treatment of conditions such as stroke (Kim et al. 2006) and Parkinson's disease (Benninger et al. 2010), although this is the first study to assess specifically the effect of bihemispheric anodal tDCS on gait. Results from the current finding suggest that direct noninvasive stimulation of primary motor cortex and premotor areas may be suitable sites to target locomotor adaptive learning. Accordingly, our findings support the use of tDCS for experimental treatment of neurological gait disorders.
GRANTS
These experiments were conducted with a grant from the Medical Research Council.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.K., N.Y., and A.M.B. conception and design of research; D.K., S.Q., M.P., and N.Y. performed experiments; D.K., S.Q., M.P., and N.Y. analyzed data; D.K., N.Y., and A.M.B. interpreted results of experiments; D.K. and N.Y. prepared figures; D.K. drafted manuscript; D.K., S.Q., M.P., and A.M.B. edited and revised manuscript; D.K. and A.M.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank David Buckwell, Paul Strutton, and Michael Gresty for their technical support.
REFERENCES
- Alexander GE, Crutcher MD. Preparation for movement: neural representations of intended direction in three motor areas of the monkey. J Neurophysiol 64: 133–150, 1990 [DOI] [PubMed] [Google Scholar]
- Ambrus GG, Antal A, Paulus W. Comparing cutaneous perception induced by electrical stimulation using rectangular and round shaped electrodes. Clin Neurophysiol 122: 803–807, 2011 [DOI] [PubMed] [Google Scholar]
- Antal A, Nitsche MA, Kincses TZ, Kruse W, Hoffmann KP, Paulus W. Facilitation of visuo-motor learning by transcranial direct current stimulation of the motor and extrastriate visual areas in humans. Eur J Neurosci 19: 2888–2892, 2004 [DOI] [PubMed] [Google Scholar]
- Armstrong DM. The supraspinal control of mammalian locomotion. J Physiol 405: 1–37, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker M, Verstappen CC, Bloem BR, Toni I. Recent advances in functional neuroimaging of gait. J Neural Transm 114: 1323–1331, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraduc P, Lang N, Rothwell JC, Wolpert DM. Consolidation of dynamic motor learning is not disrupted by rTMS of primary motor cortex. Curr Biol 14: 252–256, 2004 [DOI] [PubMed] [Google Scholar]
- Bastian AJ. Understanding sensorimotor adaptation and learning for rehabilitation. Curr Opin Neurol 21: 628–633, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger DH, Lomarev M, Lopez G, Wassermann EM, Li X, Considine E, Hallett M. Transcranial direct current stimulation for the treatment of Parkinson's disease. J Neurol Neurosurg Psychiatry 81: 1105–1111, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Castro LO, Savagim EA, Braite R, Cruz VC, Rocha RR, Rigonatti SP, Silva MT, Fregni F. Enhancement of non-dominant hand motor function by anodal transcranial direct current stimulation. Neurosci Lett 404: 232–236, 2006 [DOI] [PubMed] [Google Scholar]
- Boy F, Husain M, Singh KD, Sumner P. Supplementary motor area activations in unconscious inhibition of voluntary action. Exp Brain Res 206: 441–448, 2010 [DOI] [PubMed] [Google Scholar]
- Bronstein AM, Bunday KL, Reynolds R. What the “broken escalator” phenomenon teaches us about balance. Ann NY Acad Sci 1164: 82–88, 2009 [DOI] [PubMed] [Google Scholar]
- Bunday KL, Bronstein AM. Visuo-vestibular influences on the moving platform locomotor aftereffect. J Neurophysiol 99: 1354–1365, 2008 [DOI] [PubMed] [Google Scholar]
- Bunday KL, Reynolds RF, Kaski D, Rao M, Salman S, Bronstein AM. The effect of trial number on the emergence of the ‘broken escalator’ locomotor aftereffect. Exp Brain Res 174: 270–278, 2006 [DOI] [PubMed] [Google Scholar]
- Christensen LO, Johannsen P, Sinkjaer T, Petersen N, Pyndt HS, Nielsen JB. Cerebral activation during bicycle movements in man. Exp Brain Res 135: 66–72, 2000 [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol 79: 1117–1123, 1998 [DOI] [PubMed] [Google Scholar]
- Cowan JM, Day BL, Marsden C, Rothwell JC. The effect of percutaneous motor cortex stimulation on H reflexes in muscles of the arm and leg in intact man. J Physiol 377: 333–347, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Baker JM, Bikson M, Fridriksson J. Individualized model predicts brain current flow during transcranial direct-current stimulation treatment in responsive stroke patient. Brain Stimul 4: 169–174, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Elwassif M, Battaglia F, Bikson M. Transcranial current stimulation focality using disc and ring electrode configurations: FEM analysis. J Neural Eng 5: 163–174, 2008 [DOI] [PubMed] [Google Scholar]
- Drew T. Visuomotor coordination in locomotion. Curr Opin Neurobiol 1: 652–657, 1991 [DOI] [PubMed] [Google Scholar]
- Eskandar EN, Assad JA. Dissociation of visual, motor and predictive signals in parietal cortex during visual guidance. Nat Neurosci 2: 88–93, 1999 [DOI] [PubMed] [Google Scholar]
- Ferdjallah M, Bostick FX, Jr, Barr RE. Potential and current density distributions of cranial electrotherapy stimulation (CES) in a four-concentric-spheres model. IEEE Trans Biomed Eng 43: 939–943, 1996 [DOI] [PubMed] [Google Scholar]
- Ferrucci R, Mameli F, Guidi I, Mrakic-Sposta S, Vergari M, Marceglia S, Cogiamanian F, Barbieri S, Scarpini E, Priori A. Transcranial direct current stimulation improves recognition memory in Alzheimer disease. Neurology 71: 493–498, 2008 [DOI] [PubMed] [Google Scholar]
- Fitts PM, Peterson JR. Information capacity of discrete motor responses. J Exp Psychol 67: 103–112, 1964 [DOI] [PubMed] [Google Scholar]
- Freund HJ, Hummelsheim H. Lesions of premotor cortex in man. Brain 108: 697–733, 1985 [DOI] [PubMed] [Google Scholar]
- Fukuyama H, Ouchi Y, Matsuzaki S, Nagahama Y, Yamauchi H, Ogawa M, Kimura J, Shibasaki H. Brain functional activity during gait in normal subjects: a SPECT study. Neurosci Lett 228: 183–186, 1997 [DOI] [PubMed] [Google Scholar]
- Gabriel C, Gabriel S, Corthout E. The dielectric properties of biological tissues: I. Literature survey. Phys Med Biol 41: 2231–2249, 1996 [DOI] [PubMed] [Google Scholar]
- Galea JM, Celnik P. Brain polarization enhances the formation and retention of motor memories. J Neurophysiol 102: 294–301, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci 29: 9115–9122, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Vazquez A, Pasricha N, de Xivry JJ, Celnik P. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb Cortex 21: 1761–1770, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol 117: 845–850, 2006 [DOI] [PubMed] [Google Scholar]
- Gerwig M, Dimitrova A, Kolb FP, Maschke M, Brol B, Kunnel A, Boring D, Thilmann AF, Forsting M, Diener HC, Timmann D. Comparison of eyeblink conditioning in patients with superior and posterior inferior cerebellar lesions. Brain 126: 71–94, 2003 [DOI] [PubMed] [Google Scholar]
- Gerwig M, Hajjar K, Dimitrova A, Maschke M, Kolb FP, Frings M, Thilmann AF, Forsting M, Diener HC, Timmann D. Timing of conditioned eyeblink responses is impaired in cerebellar patients. J Neurosci 25: 3919–3931, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwig M, Kolb FP, Timmann D. The involvement of the human cerebellum in eyeblink conditioning. Cerebellum 6: 38–57, 2007 [DOI] [PubMed] [Google Scholar]
- Gherri E, Van Velzen J, Eimer M. The instructed context of a motor task modulates covert response preparation and shifts of spatial attention. Psychophysiology 46: 655–667, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormezano I, Tait RW. The Pavlovian analysis of instrumental conditioning. Pavlov J Biol Sci 11: 37–55, 1976 [DOI] [PubMed] [Google Scholar]
- Green DA, Bunday KL, Bowen J, Carter T, Bronstein AM. What does autonomic arousal tell us about locomotor learning? Neuroscience 170: 42–53, 2010 [DOI] [PubMed] [Google Scholar]
- Hadipour-Niktarash A, Lee CK, Desmond JE, Shadmehr R. Impairment of retention but not acquisition of a visuomotor skill through time-dependent disruption of primary motor cortex. J Neurosci 27: 13413–13419, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T, Sacco P, Nitsche MA, Turner DL. Modulation of internal model formation during force field-induced motor learning by anodal transcranial direct current stimulation of primary motor cortex. J Physiol 587: 2949–2961, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im CH, Jung HH, Choi JD, Lee SY, Jung KY. Determination of optimal electrode positions for transcranial direct current stimulation (tDCS). Phys Med Biol 53: N219–N225, 2008 [DOI] [PubMed] [Google Scholar]
- Iseki K, Hanakawa T, Shinozaki J, Nankaku M, Fukuyama H. Neural mechanisms involved in mental imagery and observation of gait. Neuroimage 41: 1021–1031, 2008 [DOI] [PubMed] [Google Scholar]
- Jayaram G, Galea JM, Bastian AJ, Celnik P. Human locomotor adaptive learning is proportional to depression of cerebellar excitability. Cereb Cortex 21: 1901–1909, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirenhed DA, Bengtsson F, Hesslow G. Acquisition, extinction, and reacquisition of a cerebellar cortical memory trace. J Neurosci 27: 2493–2502, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, You SH, Ko MH, Park JW, Lee KH, Jang SH, Yoo WK, Hallett M. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke 37: 1471–1476, 2006 [DOI] [PubMed] [Google Scholar]
- Klem GH, Luders HO, Jasper HH, Elger C. The ten-twenty electrode system of the International Federation. Electroencephalogr Clin Neurophysiol Suppl 52: 3–6, 1999 [PubMed] [Google Scholar]
- Krebs HI, Brashers-Krug T, Rauch SL, Savage CR, Hogan N, Rubin RH, Fischman AJ, Alpert NM. Robot-aided functional imaging: application to a motor learning study. Hum Brain Mapp 6: 59–72, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata K, Wise SP. Premotor and supplementary motor cortex in rhesus monkeys: neuronal activity during externally- and internally-instructed motor tasks. Exp Brain Res 72: 237–248, 1988 [DOI] [PubMed] [Google Scholar]
- Lagerlund TD, Sharbrough FW, Jack CR, Jr, Erickson BJ, Strelow DC, Cicora KM, Busacker NE. Determination of 10–20 system electrode locations using magnetic resonance image scanning with markers. Electroencephalogr Clin Neurophysiol 86: 7–14, 1993 [DOI] [PubMed] [Google Scholar]
- Lam T, Anderschitz M, Dietz V. Contribution of feedback and feedforward strategies to locomotor adaptations. J Neurophysiol 95: 766–773, 2006 [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain 91: 15–36, 1968 [DOI] [PubMed] [Google Scholar]
- Liu X, Robertson E, Miall RC. Neuronal activity related to the visual representation of arm movements in the lateral cerebellar cortex. J Neurophysiol 89: 1223–1237, 2003 [DOI] [PubMed] [Google Scholar]
- Mahmoudi H, Borhani Haghighi A, Petramfar P, Jahanshahi S, Salehi Z, Fregni F. Transcranial direct current stimulation: electrode montage in stroke. Disabil Rehabil 33: 1383–1388, 2011 [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Mori F, Nakajima K, Drew T, Aoki M, Mori S. Locomotor role of the corticoreticular-reticulospinal-spinal interneuronal system. Prog Brain Res 143: 239–249, 2004 [DOI] [PubMed] [Google Scholar]
- Miranda PC, Faria P, Hallett M. What does the ratio of injected current to electrode area tell us about current density in the brain during tDCS? Clin Neurophysiol 120: 1183–1187, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda PC, Lomarev M, Hallett M. Modeling the current distribution during transcranial direct current stimulation. Clin Neurophysiol 117: 1623–1629, 2006 [DOI] [PubMed] [Google Scholar]
- Mitz AR, Godschalk M, Wise SP. Learning-dependent neuronal activity in the premotor cortex: activity during the acquisition of conditional motor associations. J Neurosci 11: 1855–1872, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci 26: 9107–9116, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Early consolidation in human primary motor cortex. Nature 415: 640–644, 2002 [DOI] [PubMed] [Google Scholar]
- Newell KM. Motor skill acquisition. Annu Rev Psychol 42: 213–237, 1991 [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57: 1899–1901, 2001 [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, Tergau F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci 15: 619–626, 2003 [DOI] [PubMed] [Google Scholar]
- Nutt JG, Marsden CD, Thompson PD. Human walking and higher-level gait disorders, particularly in the elderly. Neurology 43: 268–279, 1993 [DOI] [PubMed] [Google Scholar]
- Ohtsuka K, Enoki T. Transcranial magnetic stimulation over the posterior cerebellum during smooth pursuit eye movements in man. Brain 121: 429–435, 1998 [DOI] [PubMed] [Google Scholar]
- Okamoto M, Dan H, Sakamoto K, Takeo K, Shimizu K, Kohno S, Oda I, Isobe S, Suzuki T, Kohyama K, Dan I. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage 21: 99–111, 2004 [DOI] [PubMed] [Google Scholar]
- Orban de Xivry JJ, Criscimagna-Hemminger SE, Shadmehr R. Contributions of the motor cortex to adaptive control of reaching depend on the perturbation schedule. Cereb Cortex 21: 1475–1484, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Li CS, Bizzi E. Neuronal correlates of kinematics-to-dynamics transformation in the supplementary motor area. Neuron 36: 751–765, 2002 [DOI] [PubMed] [Google Scholar]
- Parazzini M, Fiocchi S, Rossi E, Paglialonga A, Ravazzani P. Transcranial direct current stimulation: estimation of the electric field and of the current density in an anatomical human head model. IEEE Trans Biomed Eng 58: 1773–1780, 2011 [DOI] [PubMed] [Google Scholar]
- Reis J, Fritsch B. Modulation of motor performance and motor learning by transcranial direct current stimulation. Curr Opin Neurol 24: 590–596, 2011 [DOI] [PubMed] [Google Scholar]
- Reynolds RF, Bronstein AM. The broken escalator phenomenon. Aftereffect of walking onto a moving platform. Exp Brain Res 151: 301–308, 2003 [DOI] [PubMed] [Google Scholar]
- Reynolds RF, Bronstein AM. The moving platform aftereffect: limited generalization of a locomotor adaptation. J Neurophysiol 91: 92–100, 2004 [DOI] [PubMed] [Google Scholar]
- Richardson AG, Overduin SA, Valero-Cabre A, Padoa-Schioppa C, Pascual-Leone A, Bizzi E, Press DZ. Disruption of primary motor cortex before learning impairs memory of movement dynamics. J Neurosci 26: 12466–12470, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EM, Press DZ, Pascual-Leone A. Off-line learning and the primary motor cortex. J Neurosci 25: 6372–6378, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche N, Lackmy A, Achache V, Bussel B, Katz R. Effects of anodal transcranial direct current stimulation over the leg motor area on lumbar spinal network excitability in healthy subjects. J Physiol 589: 2813–2826, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche N, Lackmy A, Achache V, Bussel B, Katz R. Impact of transcranial direct current stimulation on spinal network excitability in humans. J Physiol 587: 5653–5664, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush S, Driscoll DA. EEG electrode sensitivity–an application of reciprocity. IEEE Trans Biomed Eng 16: 15–22, 1969 [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Holcomb HH. Neural correlates of motor memory consolidation. Science 277: 821–825, 1997 [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res 185: 359–381, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Alexander GE. Preferential representation of instructed target location versus limb trajectory in dorsal premotor area. J Neurophysiol 77: 1195–1212, 1997 [DOI] [PubMed] [Google Scholar]
- Sommer M, Grafman J, Clark K, Hallett M. Learning in Parkinson's disease: eyeblink conditioning, declarative learning, and procedural learning. J Neurol Neurosurg Psychiatry 67: 27–34, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Jayaram G, Pastor D, Kincses ZT, Matthews PM, Johansen-Berg H. Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia 49: 800–804, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner P, Nachev P, Morris P, Peters AM, Jackson SR, Kennard C, Husain M. Human medial frontal cortex mediates unconscious inhibition of voluntary action. Neuron 54: 697–711, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji J, Shima K. Role for supplementary motor area cells in planning several movements ahead. Nature 371: 413–416, 1994 [DOI] [PubMed] [Google Scholar]
- Teo JT, van de Warrenburg BP, Schneider SA, Rothwell JC, Bhatia KP. Neurophysiological evidence for cerebellar dysfunction in primary focal dystonia. J Neurol Neurosurg Psychiatry 80: 80–83, 2009 [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Oliveri M, Gangitano M, Pascual-Leone A. Modulation of spinal cord excitability by subthreshold repetitive transcranial magnetic stimulation of the primary motor cortex in humans. Neuroreport 12: 3845–3848, 2001 [DOI] [PubMed] [Google Scholar]
- Vatta F, Bruno P, Inchingolo P. Multiregion bicentric-spheres models of the head for the simulation of bioelectric phenomena. IEEE Trans Biomed Eng 52: 384–389, 2005 [DOI] [PubMed] [Google Scholar]
- Wagner T, Fregni F, Fecteau S, Grodzinsky A, Zahn M, Pascual-Leone A. Transcranial direct current stimulation: a computer-based human model study. Neuroimage 35: 1113–1124, 2007 [DOI] [PubMed] [Google Scholar]
- Wang C, Wai Y, Kuo B, Yeh YY, Wang J. Cortical control of gait in healthy humans: an fMRI study. J Neural Transm 115: 1149–1158, 2008 [DOI] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci 20: 25–42, 1997 [DOI] [PubMed] [Google Scholar]
- Wise SP, Moody SL, Blomstrom KJ, Mitz AR. Changes in motor cortical activity during visuomotor adaptation. Exp Brain Res 121: 285–299, 1998 [DOI] [PubMed] [Google Scholar]
- Wolpert DM. Probabilistic models in human sensorimotor control. Hum Mov Sci 26: 511–524, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Padoa-Schioppa C, Bizzi E. Neuronal correlates of movement dynamics in the dorsal and ventral premotor area in the monkey. Exp Brain Res 168: 106–119, 2006 [DOI] [PubMed] [Google Scholar]