Abstract
Selective attention allows us to focus on particular sensory modalities and locations. Relatively little is known about how attention to a sensory modality may relate to selection of other features, such as spatial location, in terms of brain oscillations, although it has been proposed that low-frequency modulation (α- and β-bands) may be key. Here, we investigated how attention to space (left or right) and attention to modality (vision or touch) affect ongoing low-frequency oscillatory brain activity over human sensory cortex. Magnetoencephalography was recorded while participants performed a visual or tactile task. In different blocks, touch or vision was task-relevant, whereas spatial attention was cued to the left or right on each trial. Attending to one or other modality suppressed α-oscillations over the corresponding sensory cortex. Spatial attention led to reduced α-oscillations over both sensorimotor and occipital cortex contralateral to the attended location in the cue-target interval, when either modality was task-relevant. Even modality-selective sensors also showed spatial-attention effects for both modalities. The visual and sensorimotor results were generally highly convergent, yet, although attention effects in occipital cortex were dominant in the α-band, in sensorimotor cortex, these were also clearly present in the β-band. These results extend previous findings that spatial attention can operate in a multimodal fashion and indicate that attention to space and modality both rely on similar mechanisms that modulate low-frequency oscillations.
Keywords: multisensory, magnetoencephalography, oscillations, alpha, beta, visual, somatosensory, prestimulus
numerous recent studies have revealed modulations of oscillatory synchrony as a potential mechanism for attentional selection in sensory cortex (Jensen et al. 2007; Womelsdorf and Fries 2007). Most studies focused on the effects of spatial attention, including anticipatory effects that follow an attentional instruction or cue, in advance of the expected imperative stimuli. A common finding is that attention to a particular location in space suppresses low-frequency α-oscillations in the corresponding neural population, for instance contralateral visual cortex (Haegens et al. 2011; Siegel et al. 2008; Trenner et al. 2008; Worden et al. 2000). These modulations of α-oscillations are thought to reflect a gating mechanism that determines the readiness of neural populations to process afferent input (Foxe and Snyder 2011; Jensen and Mazaheri 2010). Importantly, this α-suppression mechanism emerges in advance of the anticipated stimulus to attend, and it has been shown that it can predict subsequent sensory performance (Haegens et al. 2011; Thut et al. 2006) and may play a causal role in attentional modulation (Romei et al. 2010). Several studies have also found spatial-attention effects in the β-band (Bauer et al. 2006; Siegel et al. 2008), although the neurophysiological mechanisms and the functional role of β-oscillations seem somewhat more complex (Donner et al. 2007; Gross et al. 2004; Ziegler et al. 2010).
Although most studies of selective attention consider a single sensory modality at a time, growing literature has studied possible cross-modal links in spatial attention (Macaluso and Driver 2005). Several studies with a variety of measures have indicated that when attention is directed to a particular location in one modality (e.g., touch), other modalities (e.g., vision) may be spatially affected in a corresponding manner, even when task-irrelevant (Eimer et al. 2002; Eimer and Driver 2001; Kennett et al. 2001; Spence and Driver 1998; Talsma et al. 2010). However, relatively few studies have addressed how cross-modal spatial attention may influence oscillatory activity, such as the low-frequency α-suppression mentioned above (Foxe and Snyder 2011).
Bauer et al. (2006) reported that spatially attended tactile stimuli can suppress stimulus-related low-frequency activity in occipital cortex, suggesting a possible cross-modal effect, whereas Banerjee et al. (2011) reported similar parieto-occipital spatial-attention effects in an auditory task as for a visual task. Jones et al. (2010), Haegens et al. (2011), and Van Ede et al. (2011) reported low-frequency changes over sensorimotor cortex for spatial attention in unimodal tactile studies (with respect to the target stimulus).
Relatively few studies have investigated oscillatory mechanisms of attention to one modality vs. another, as opposed to attention to one location vs. another. Some have either focused on synchrony in a nonfrequency-specific way (Steinmetz et al. 2000) or with an emphasis on phase-modulations in the low-frequency θ-band (Lakatos et al. 2008). Foxe et al. (1998) and Fu et al. (2001) investigated the impact of attention to modality on α-oscillations but only for occipital α-oscillations in visuoauditory tasks (Foxe and Snyder 2011). We are unaware of any study to date that has investigated the impact of modality selection on neural oscillatory activity for more than one modality (e.g., not just for visual cortex when attending to a different modality, but also for the latter modality when attending vision). Furthermore, we are not aware of any oscillatory studies that have as yet examined the impacts of both spatial attention and of attention to a modality, as we investigated here in a visual-tactile task.
We used magnetoencephalography (MEG) to assess low-frequency oscillations in relation to spatial attention and modality attention in a visuotactile oddball task. Participants had to attend to one of two modalities (vision or touch), with the currently task-relevant modality being blocked in a counterbalanced manner, whereas the relevant location was cued on a trial-by-trial basis. We hypothesized that spatial attention would suppress low-frequency activity in contralateral sensorimotor and occipital cortex when either modality was relevant (Macaluso and Driver 2005). We focused exclusively on oscillatory activity in the cue-target interval, as any effects in this period reflect pure top-down influences of anticipatory attention since they arise before any imperative target stimulus (and the central cue was not lateralized). We predicted that attention to modality (here, vision or touch) would operate by modulating low-frequency activity in the sensory cortices associated with the task-relevant (vs. irrelevant) modalities and hence via an analogous mechanism as for the spatial attention effects that we could study within the same experiment.
METHODS
Participants.
Thirteen right-handed, healthy, adult volunteers (aged 18–35 yr, 4 female) were acquired through a local subject database. Approval for this study was given by University College London's local research ethics committee, and all participants gave informed written consent.
Task.
Participants performed a visual or tactile task where they reported only rare deviant stimuli (see below) in the currently task-relevant modality if appearing at the cued location for that trial (either left or right at 25° eccentricity); see Eimer and colleagues (Eimer 2001; Eimer et al. 2002; Kennett et al. 2001) for similar tasks and stimuli but focusing on evoked potentials in EEG. The standard stimuli consisted of single-pulse light-emitting diode (LED) flashes in vision (duration 200 ms) or electrical (index) finger stimulation in touch for 0.5 ms, whereas deviants were double-pulse versions of the same stimuli. On different blocks of 88 trials, either the visual or the tactile modality was task-relevant, and subjects were verbally instructed which modality was relevant before the beginning of each block. Each trial started with a centrally presented arrow cue stimulus (100 ms) that instructed the participant to attend either the left or right stimulus location for that trial (see Fig. 1 for an illustration of experimental setup and task). The cue stimulus would be drawn pseudorandomly from one out of four different options (see below) and, importantly, was always presented bilaterally, with cue color being the relevant feature instructing participants which side to attend to. Regardless of this spatial cue, and independent of which modality was task-relevant, on each trial, just one peripheral stimulus was presented, either to the left or to the right equiprobably, and this could either be a visual or a tactile stimulus equiprobably. The task was to make a vocal response (saying “gap”) only if a deviant double-pulse stimulus was detected in the relevant modality at the cued location. Any deviant in the currently irrelevant modality required no response and likewise for any deviant in the relevant modality but on the uncued side.
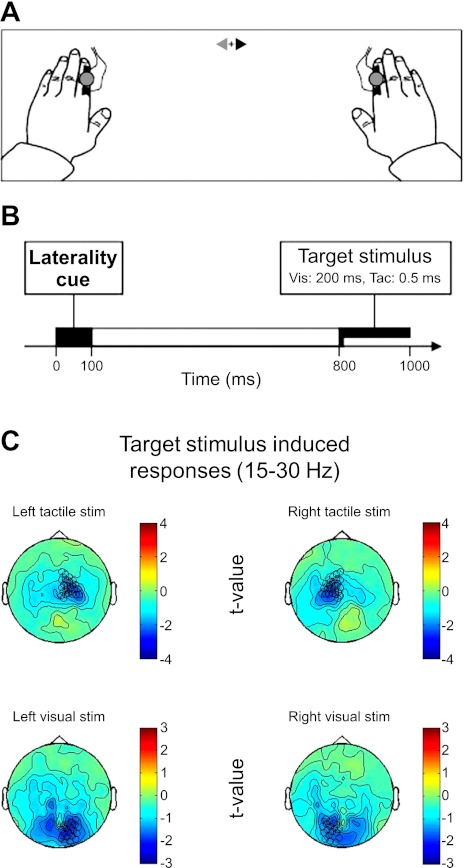
Fig. 1.
Task schematics. A: experimental task setup. Participants fixate centrally, peripheral stimuli are presented to left or right, with tactile (Tac.) and visual (Vic.) stimuli in close spatial proximity. B: timeline: each trial starts with the presentation of a central cue for 100 ms, followed by a 700-ms empty interval, then presentation of either a tactile stimulus (0.5-ms electrical shock) or visual stimulus [light-emitting diode (LED) 200 ms]. Note that our magnetoencephalography (MEG) analyses focus on the cue-target interval, before target stimulus, to highlight pure top-down effects of preparatory attention (in absence of peripheral target stimulus-related effects). C: mapping of the visual or tactile (target) stimulus-induced responses (β-band suppression, 15–30 Hz) on the planar gradients. Left tactile stimuli suppress β-activity in predominantly right sensorimotor channels, and right tactile stimuli in left sensorimotor channels. Visual stimuli suppress β-activity in bilateral occipital channels. Note the spatial specificity of these response patterns. These sensors are used and labeled as sensorimotor and occipital channels throughout Figs. 2–4.
The experiment comprised 10 experimental blocks for most participants (only 8 for 2 participants due to practical constraints). Attended modality followed an ABBA or BAAB pattern across blocks. Starting modality was counterbalanced across participants. For each block of 88 trials, on 64 trials, a standard stimulus was presented (either left or right, visual or somatosensory, with equal likelihood). Deviant (gap) stimuli were presented in the remaining, randomly intermingled 24 trials. Of these, 12 were on the cued side and in the attended modality (6 on the left and 6 on the right), 4 on the cued side but in the unattended modality (2 left and 2 right), and the remaining 8 were uncued and either visual or somatosensory on the left or right.
Two or more training blocks were performed before the experiment until performance was at or near ceiling. Practice blocks contained 32 trials: 16 standard stimuli (4 each side for each modality), 4 attended modality cued-side deviant stimuli, 4 ignored modality cued-side deviant stimuli, and 8 stimuli uncued-side deviant stimuli (4 visual and 4 somatosensory).
Stimulus details.
Participants' arms were placed palm down on a board so that their index fingers were 600 mm from the participant's eyes, 25° to left or right of straight ahead. A screen, 550 mm in front, received an image projected from outside (PRO xtraX LCD projector; Sanyo, Osaka, Japan) via a waveguide porthole and two mirrors. A central gray fixation cross (0.9°) was continuously present on the screen during the experiment 200 mm below horizontal straight ahead. The cue stimulus comprised two triangles, one red, one blue, pointing in opposite directions either side of the fixation cross (i.e., ▶+◀ or ◀+▶; Fig. 1, top). Equiprobably, either arrowhead could be red, with the other blue. Thus there were four equiprobable cue stimuli, all subtending 4.0 × 1.6°, and participants were verbally instructed (and reminded before each block) which color indicated the imperative cue for them. Eight analyzed participants were cued by the blue arrowhead, and the remainder by red.
A peripheral stimulus, either visual or tactile (electrical), was presented unilaterally after the cue-target interval (800 ms onset to onset). Visual stimuli were circles subtending 2.3°, 550 mm from the eyes, 25° to left or right of straight ahead (i.e., same as index fingers), and 10° below the level of the fixation cross. These visual stimuli were produced by external LEDs, with their light conveyed via fiber optic cables to produce the circle when lit. Standard (“nontarget”) visual stimuli comprised illumination of one circle for 200 ms. Rare deviant visual events, to be reported only for the cued side and only when vision was the attended modality, comprised one circle being flashed on-off-on for 85-30-85 ms (duration 200 ms).
Tactile (electrical) stimuli were presented to either hand using two Digitimer DS7A constant-current stimulators (Welwyn Garden City, United Kingdom), one connected to each index finger via a pair of gel Ag/AgCl electrodes (20 × 25 mm). Positive and negative electrodes were placed on the medial surface of the distal and proximal phalanx, respectively. Stimulators were set to deliver 0.5-ms square-wave pulses. Detection threshold of each participant's right index finger was determined using an informal staircase procedure. Experimental stimulus intensity was then 150% of this threshold (mean intensity 4.2 mA). Standard (nontarget) electrical stimuli comprised a single pulse, to one index finger, lasting 0.5 ms. Rare deviant electrical events, reported only when on the cued side and only when touch was attended, were two 0.5-ms pulses separated by a 70-ms gap presented to a single index finger.
Procedure.
Participants sat comfortably in the magnetically shielded room and were familiarized with the stimulus equipment. Three head localization coils were attached to anatomic landmarks (left and right preauricular points and nasion) to measure head position before and after each block. MEG data were subsequently recorded while subjects performed the task using 275 axial gradiometers (whole-head Omega 275 CTF MEG system; VSM MedTech, Vancouver, British Columbia, Canada) with a sampling rate of 240 Hz given our focus on low-frequency oscillations here (see Introduction).
Data processing.
Data were analyzed using the FieldTrip software package (http://www.ru.nl/fcdonders/fieldtrip; see Oostenveld et al. 2011), a MATLAB-based toolbox for analysis of electrophysiological data. A semiautomatic artifact-rejection toolbox was run over the data to search for eye blinks, muscle artifacts, as well as any sudden jumps in the signal caused by the electronic circuit controlling the SQUIDs (standard options in FieldTrip). The data were then screened visually to exclude trials or channels with excessive power using “rejectvisual.” Line noise was removed from 10-s periods around each trial of interest using a narrow-band notch filter. To correct for the interindividually varying head positions in the MEG sensor array (as traditionally the case with MEG recordings), the artifact- and response-free data were interpolated to a common sensor array template using a minimum-norm projection method (Knösche 2002) as implemented in FieldTrip (Oostenveld et al. 2011) and used with default parameters. This procedure projects the sensor data onto the individual participants' head surface and reprojects it to a sensor array that has positions (in this individual subjects-based coordinate system) corresponding to the sample-averaged head position in the MEG scanner. The minimum norm projection is not used here to make inferences about the source distributions but solely serves as a way to represent an optimal fit of the measured scalp topography in a head-centered coordinate system; the obtained distribution on the head surface has little impact for the projection to the average sensor array (for further details, see Knösche 2002 and Oostenveld et al. 2011 for the FieldTrip toolbox). Subsequently, planar gradients of the MEG field distribution were calculated using a nearest-neighbor method comparable with a method described elsewhere (Bastiaansen and Knösche 2000). This metric effectively calculates a 1st-order spatial derivative (in 2 planar dimensions later aggregated by the vector norm, see below). Since MEG is maximally sensitive to superficial tangential dipoles, and insensitive to deeper or radially oriented sources (Hämäläinen et al. 1993), the measured peak of activity can be expected to be in fairly close spatial proximity to its cortical generator. Thus, although planar gradients will attenuate signals originating from deeper sources (using the spatial derivative as here will emphasize superficial sources even more), they are considered to allow an approximate localization of the generators of the measured signals from topographies (Bastiaansen and Knösche 2000; Knösche 2002).
Spectral analysis.
In this study, we were interested a priori in low-frequency oscillations in the anticipatory cue-target interval (see Introduction), and spectral analysis was therefore conducted between 2.5 and 50 Hz. To this end, we used a Hanning taper of 400-ms window length, which was Fourier-transformed and multiplied with the Fourier-transformed data segments in the interval between −400 and 1,200 ms around cue onset (multiplication in the frequency domain). This procedure is equivalent to a wavelet analysis except that the window length was kept constant across frequencies and a Hanning window was used instead of a Gaussian for more effective tapering of the data at the edges. The resulting time-frequency data were then sampled at steps of 50 ms and finally collapsed across vertical and horizontal planar gradients by taking the resultant vector length (see Bauer et al. 2006). For statistics concerning the attentional effects, we used nonbaseline-corrected estimates of log power (from the vector length of planar gradients). For the display of the stimulus-induced effects presented in Fig. 1C (to show the mapping of planar gradients to occipital and sensorimotor regions), the pretarget baseline (in windows centered at −300 to −200 ms before target onset) was estimated and a t-test was calculated within subjects (with individual trials as repetitions) as a comparison of poststimulus power vs. baseline power, separately for each frequency bin and planar gradient sensor. The resultant t-statistics were then simply averaged across subjects (compare with Bauer et al. 2006 for a similar approach).
RESULTS
Behavior.
Participants were able to perform the task quickly and accurately; 93.5% of visual targets and 90.6% of tactile targets were correctly detected at mean reaction times of 545 and 524 ms, respectively. Only 1.4% of nontargets in vision primary blocks and 1.3% in touch primary blocks were erroneously responded to. No performance differences between vision primary and touch primary blocks approached significance by paired t-test (all t < 1.4, all P > 0.2).
MEG.
Here, we focused on attention-related changes in low-frequency oscillatory activity in the cue-target interval. Such top-down changes in low-frequency activity are known to arise primarily before and approaching stimulus onset, as shown in several previous studies assessing spatial-attention effects in one modality only (Haegens et al. 2011; Worden et al. 2000). Accordingly, for the attentional contrasts, which were the main focus of this study, we focused on anticipatory changes within the cue-target interval and before stimulus onset. This allowed us to conduct analyses independent of the target stimulus that was subsequently presented (tactile or a visual stimulus on the left or right). As one exception to this, we present in Fig. 1C the topographies of the visual and tactile stimulation effects (on β-band suppression, from 15 to 30 Hz during the posttarget interval) to familiarize the reader with the mapping of occipital and sensorimotor cortex that will become relevant in the subsequent figures on attentional modulation. Note that the planar gradient technique, a metric that represents local cortical activity underneath the sensors considered (Bastiaansen and Knösche 2000), nicely separates activity induced by visual stimuli (bottom row in Fig. 1C) and tactile stimuli (top row in Fig. 1C). The sensors marked in these topographies are chosen based on the MEG manufacturer's labeling (and correspond well with the shown lateralized stimulus-induced effects) and are used to delineate left and right sensorimotor and occipital cortex in the subsequent Figs. 2–4. In all subsequently presented analyses, however, we restricted the analysis to the pretarget stimulus period and could therefore isolate pure top-down attentional effects on low-frequency oscillations due solely to which modality was task-relevant (blocked) and which location was currently task-relevant (as cued at the start of each trial; see schematic timeline in Fig. 1B and methods for details on the cue stimulus). Figure 1A shows the experimental setup with electrical finger stimulators located proximally to LEDs, on the left or right index finger. These visual or tactile target stimuli were presented 800 ms after cue onset. Cue onset is denoted at 0 ms in all subsequent figures and results.
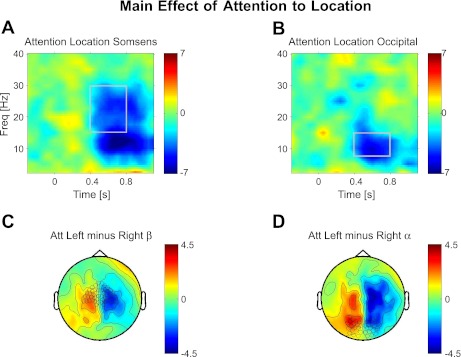
Fig. 2.
Attention to spatial location (main effect). Topographies and time-frequency (Freq) representations of the spatial-attention effect (regardless of modality judged). A and B: time-frequency plot of contralateral attention effects: (AttLeft−AttRight)left sensors minus (AttLeft−AttRight)right sensors. A: plots for the sensors over somatosensory cortex highlighted in C, whereas B shows this for the sensors over occipital cortex highlighted in D. Shown are the topography of t-values for attending left minus right in the β-band (C) of 15–30 Hz (A) and the α-band (D) of 7.5–15 Hz (B) over the time-frequency windows marked in A and B above, respectively. Topographies are averaged over the time-frequency windows as marked in A for C and B for D. Sensor selection is marked in C and D for time-frequency plots in A and B, respectively. All plots show t-values.
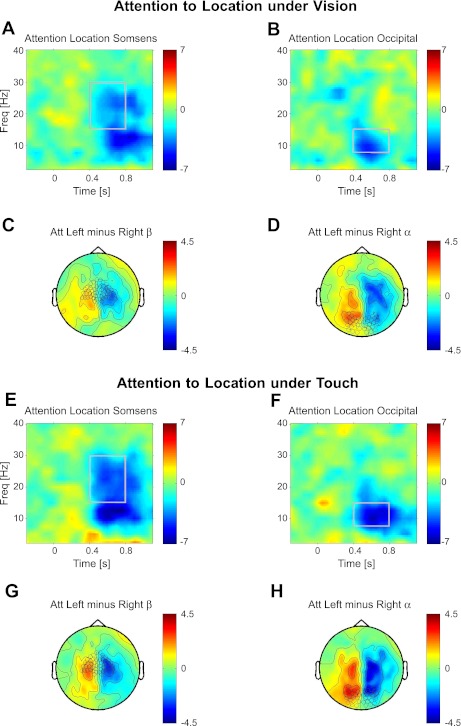
Fig. 3.
Attention to spatial location under vision and touch. Topographies and time-frequency representations of spatial-attention effects are shown analogously to Fig. 2 but now separately for vision or touch being task-relevant. A–D: spatial-attention effect when vision-relevant. A and B: time-frequency plot of contralateral attention effects: (AttLeft−AttRight)left sensors minus (AttLeft−AttRight)right sensors for sensorimotor (A) and occipital (B) sensors marked in C and D, respectively. C and D: topography of t-values attending left minus right for β- (C) and α- (D) activity, averaged over time-frequency windows marked in A and B, respectively. E–H: attention to space when touch-relevant. E and F: time-frequency plot of contralateral attention effects: (AttLeft−AttRight)left sensors minus (AttLeft−AttRight)right sensors for sensorimotor (E) and occipital (F) sensors marked in G and H, respectively. G and H: topography of t-values attending left minus right for β- (G) and α- (H) activity, averaged over time-frequency windows marked in E and F, respectively. All plots show t-values.
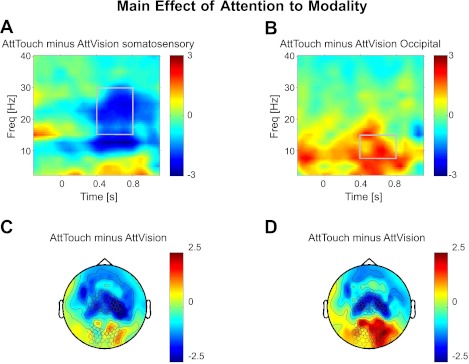
Fig. 4.
Attention to modality. Topographies and time-frequency representations of modality relevance effect (regardless of attended side). A and B: time-frequency plot of α- and β-suppression for somatosensory (A) and occipital (B) sensors; sensor selection is marked in C and D, respectively. All plots show t-values. C and D: topography of attending touch minus vision for β- (C) and α- (D) bands (B), averaged over time-frequency windows marked in A and B, respectively.
Attention to space.
To investigate the effects of attention to a spatial location (left or right hemifield or finger), we first calculated the main effect of log-transformed nonbaseline-corrected power (on synthetic planar MEG gradients) between the relevant conditions. Thus, for the effect of attention to space, we first calculated the comparison “Attend Left” minus “Attend Right” pooled across the two attended modality (“Vision” or “Touch”) conditions. Figure 2 shows time-frequency windows (Fig. 2, A and B) and topographies (Fig. 2, C and D) of this effect at sensors overlying sensorimotor and occipital cortex as indicated. We also calculated the double subtraction (AttLeft−AttRight)left sensors minus (AttLeft−AttRight)right sensors to combine the results of both hemispheres into one value, as justified by the observed symmetry of the contralateral spatial-attention results (but for separate analysis of the hemispheres, see below).
Two related time-frequency patterns are clearly observable (see Fig. 2, which plots t-values) for the impact of spatial attention in the cue-target interval. A relative suppression of broadband α- (7.5–15 Hz) and β-power (15–30 Hz) arises between 400 and up to ∼1,000 ms (we restricted our analysis to 800 ms to avoid containment of target stimulus-induced responses) following cue onset over sensorimotor cortex, contralateral to the attended side (relative suppression over right sensorimotor cortex and relative enhancement over left sensorimotor cortex in the comparison of Attend Left minus Attend Right; see Fig. 2, A and C), and a relative suppression of primarily α- (7.5–15 Hz) and to some extent β-power (approximately 15–30 Hz) over occipital cortex contralateral to the attended hemifield (relative suppression over right occipital cortex and relative enhancement over left occipital cortex; see Fig. 2, B and D). Although both sensorimotor and occipital cortex show evidence of lateralized relative suppression in both α- and β-bands, topographies of the β-band effect (Fig. 2A) and time-frequency-profile (compare Fig. 2, A and B) indicate a predominance over sensorimotor cortex for the higher β-band and relative predominance over occipital cortex for the α-band. For the time-frequency windows highlighted in Fig. 2 (which correspond to the windows for the topographies shown), the mean t-values for the spatial-attention effect at sensorimotor sensors were t = −3.6 (P < 0.005) for the β-band and t = −5.6 (P < 0.001) for the α-band. In sensors overlying occipital cortex, the spatial-attention effect was nearly significant for the β-band (t = −1.78, P = 0.0503) and highly significant for the α-band (t = −5.6, P < 0.001). Thus attention to a location in space suppresses the amplitudes of α-oscillations over occipital and sensorimotor cortex contralateral to the attended hemifield, extending prior work in unisensory studies (e.g., Bauer et al. 2006; Haegens et al. 2011; Jones et al. 2010; Siegel et al. 2008; van Ede et al. 2011; Worden et al. 2000), and clearly also modulates β-oscillations overlying sensorimotor regions (Jones et al. 2010) and with a strong trend for occipital cortex (Siegel et al. 2008).
In the next step, we investigated the effect of attention to space separately when either Vision was relevant (Fig. 3, A–D) or Touch was relevant (Fig. 3, E–H). Importantly, the result of this spatial-attention comparison is highly similar, regardless of which modality was relevant. When vision was relevant, the contralateral suppression of low-frequency oscillations did not only affect α-band activity over occipital cortex (Fig. 3, B and D; t = −3.8, P < 0.01 for α-, and t = −0.74, P > 0.1 for β-), but also α- and β-band activity over sensorimotor cortex (Fig. 3, A and C; t = −3.1, P < 0.005 for β-, and t = −4.7, P < 0.001 for α-). Conversely, when touch was relevant, the contralateral suppression also involved not only sensorimotor (Fig. 3, E and G; t = −3.9, P < 0.005 for β- and t = −5.4, P < 0.001 for α-), but also occipital sensors (Fig. 3, F and H; t = −2.86, P < 0.01 for β-, and t = −7.1, P < 0.001 for α-). Hence, α-band activity was consistently modulated in sensorimotor and occipital regions as a consequence of spatial attention, whereas β-band activity was modulated by spatial attention consistently in sensorimotor cortex but not significantly in occipital cortex when vision was relevant. These results provide an oscillatory analog of previous (nonoscillatory) reports that spatial attention in vision can affect sensorimotor cortex spatially, and vice versa (Macaluso and Driver 2005), thereby providing a new form of evidence that spatial attention can act in a multimodal fashion, with spatial attention for a task in one modality affecting low-frequency oscillations over sensory cortex ordinarily associated with the other modality.
Attention to modality.
Next, we tested the main effect of attention to modality by the contrast “Attend Touch” minus “Attend Vision.” The statistical results of this comparison are shown in Fig. 4. Analogously to the effect of spatial attention (but showing a distinct although partially overlapping topography), attention to modality leads to relative suppression of α- and β-band power over the sensory cortex corresponding to the task-relevant modality compared with when that modality is task-irrelevant. In sensors overlying sensorimotor cortex (Fig. 4, A and C), when touch was attended, α- and β-band power was suppressed in a time-frequency window from about 10 to 30 Hz and from around 400 to 800 ms following cue onset (t = −2.22, P < 0.05 for the β-band and t = −2.15, P < 0.05 for the α-band as in the highlighted windows and wide sensor selection as indicated in Fig. 4). The frequency distribution of this modality-selection effect is highly similar to that found for the spatial-attention effect in sensorimotor cortex (see above). Over occipital cortex (Fig. 4, B and D), when touch was attended, this resulted analogously in increased α- (t = 2.20, P < 0.01) but not increased β-power (t = 0.37, nonsignificant). This relative enhancement of occipital α-power in a condition when vision was irrelevant compared with when it was relevant mirrors the ipsilateral relative enhancement/contralateral relative suppression of α-/β-power for the case of spatial attention. Note that the reverse-contrast “Vision minus Touch” would lead to results with (numerically exact) inverted sign. That is, attention to vision would lead to a relative reduction for occipital α- and a relative increase for sensorimotor α- and β-oscillations.
Spatial-attention effects for sensors showing modality selectivity.
To rule out potential concerns that, due to the relatively wide sensor selection, spatial-attention effects would only appear to be prominent in modality-selective regions, our final step was to select those sensors that each show highly significant (at P < 0.01) effects of attention to modality and then to investigate whether these same modality-selective sensors would also show clear effects of spatial attention with either modality relevant, as we anticipated. Please note that these sensors were thus selected by a criterion (modality selectivity) that was independent of subsequent tests for spatial-attention effects and that by definition they show a modality selectivity (i.e., a significant preference for either touch or vision being the relevant modality). As expected, the modality-selection criterion highlighted sensors over parieto-occipital or sensorimotor cortex, respectively, for attending vision or touch (see highlighted sensors in Fig. 5E for the α-band). The key outcome is that despite this modality selectivity, the highlighted visual-preferring parieto-occipital sensors showed significant effects of spatial attention (Fig. 5, C and D) when either modality was task-relevant for the visual or tactile task and analogously for the touch-preferring sensorimotor sensors, which showed spatial-attention effects for the tactile or visual task (Fig. 5, A and B). Thus, in terms of pure top-down attentional effects in the cue-target interval, sensorimotor sensors showed relative low-frequency suppression when attending to touch and when attending contralaterally for either modality, whereas parieto-occipital sensors analogously showed low-frequency suppression when attending to vision and when attending contralaterally for either modality.
Fig. 5.
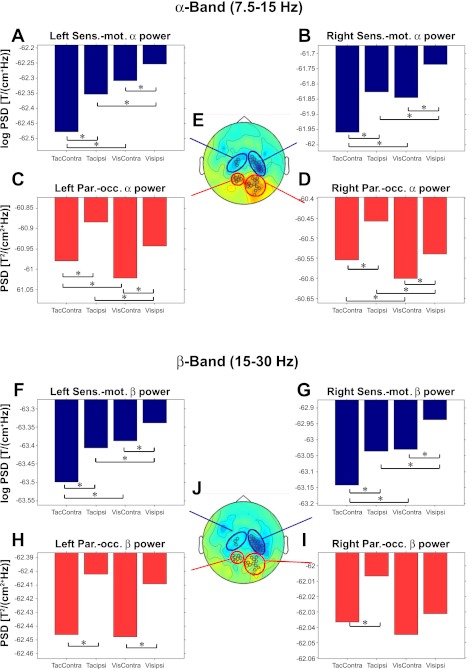
Attention to space and modality at modality-selective sensors. Activity profiles for α-activity (7.5–15 Hz, for 0.4–0.8 s into the cue-target interval; A–D) at sensors selected to show a preference (significance exceeding P < 0.01) either for touch being task-relevant, which arose at sensorimotor sensors (Sens.-mot.; A and B), or for vision being task-relevant, which arose at parieto-occipital sensors (Par.-occ.; C and D) as marked in E, the middle head view (occipital red and sensorimotor blue), which also depicts the topography for the contrast “Attend Touch” minus “Attend Vision” (in the α-band). The bar plots show the mean log-transformed α-power of magnetic induction in planar gradients (hence negative log values) for the 4 different attentional conditions (“Touch Left,” “Touch Right,” “Vision Left,” and “Vision Right”) in the preparatory cue-target interval. The conditions are plotted relative to the lateral sensor positions (Touch Right becomes “TacContra” for left sensors). Asterisks indicate significant (P < 0.05) attention to modality and location effects (by paired t-test, which are found for every case). In particular, note that spatial-attention effects are significant even in sensors that show a clear preference for the other modality. H–J: same as above (A–E) but now for the β-band (15–30 Hz, for 0.4–0.8 s into the cue-target interval). Whereas all contrast in sensorimotor sensors are also significant for the β-band, in parieto-occipital sensors, they were not. Since effects of modality selection in the β-band were not significant anywhere in sensors over parieto-occipital cortex, the sensor selection (J) here was therefore adopted from the contrast of attention to touch minus vision in the α-band (E). PSD, power spectral density; T, time; ipsi, ipsilateral; Contra, contralateral.
These results in the α-band were confirmed statistically by an ANOVA on the group data for which means are plotted in Fig. 5. An initial 2 × 2 × 2 ANOVA (hemisphere: left/right; spatial attention: contralateral/ipsilateral; attention to modality: Touch/Vision) found no interactions with factor hemisphere when recoding attended side in terms of contralateral or ipsilateral rather than left or right hemifield/finger (all P > 0.16). Accordingly, we next pooled the data across hemispheres, again with attended side coded as contralateral or ipsilateral, in a 2 × 2 ANOVA with factors of spatial attention and relevant modality. For α at sensorimotor sensors, this revealed main effects of spatial attention [lower α for contralateral attention, F(1,12) = 36.31, P < 0.001] and attended modality [lower α when touch was task-relevant, F(1,12) = 11.84, P < 0.01] and an interaction [F(1,12) = 6.39, P < 0.05] due to a somewhat stronger spatial-attention effect with touch relevant (although note that the spatial-attention effect remained significant here with vision task relevant also, at P < 0.01; see also Fig. 5, A and B).
Analogously, for α at occipital sensors, there were main effects of spatial attention [lower α for contralateral attention, F(1,12) = 22.50, P < 0.01] and of attended modality [lower α now when vision was task-relevant, F(1,12) = 15.60, P < 0.01] and a trend for an interaction [F(1,12) = 4.28, P = 0.06] that was not quite significant.
The β-band (Fig. 5, F–J) showed a similar but statistically less robust pattern than the α-band shown in Fig. 5, in line with β-frequencies being more prominent at sensorimotor than occipital sensors, whereas the α-band was implicated for both sites. The ANOVA results for the β-band (with the sensor selection as above, chosen by modality effects in the α-band, as there were no modality-selective sensors, e.g., in left parieto-occipital cortex in the β-band, yet the difference had a similar topology) were the following: in sensorimotor regions (as depicted in Fig. 5, collapsed over the hemispheres), both attention to location and modality effects were significant (F = 14.4, P < 0.01 and F = 8.9, P < 0.05, respectively) as was the interaction of both (F = 7.1, P < 0.05). In occipital sensors, the effect of attention to location was significant (F = 8.15, P < 0.05), and although the effect of attention to modality was not (F = 0.69, P > 0.1), the interaction was also significant here (F = 6.37, P < 0.05).
DISCUSSION
Our study adds to a growing literature that increasingly emphasizes the role of low-frequency oscillations (particularly α-band) for attentional modulations arising in an anticipatory interval before onset of a target stimulus (e.g., Foxe et al. 1998; Fu et al. 2001; Haegens et al. 2011; Lakatos et al. 2008; Thut et al. 2006; Trenner et al. 2008; van Ede et al. 2011; Worden et al. 2000). Such oscillations have been implicated in spatial attention, with most studies (although not all: Haegens et al. 2011; Jones et al. 2010; van Ede et al. 2011) focusing on α-band modulations for parietal-occipital cortex related to visual processing. α-Oscillations have also been implicated in modality selection (Foxe et al. 1998) for audiovisual cases, with a focus on parietal-occipital oscillations related to visual processing. In the present study, we jointly investigated spatial and modality selection effects simultaneously in the visual and tactile modality and report attention-related modulations in α- and β-bands over sensorimotor and occipital cortex as measured with planar gradient MEG sensors.
We found not only that attention toward or away from vision as a relevant modality modulates parieto-occipital low-frequency oscillations, as previous studies had shown, but also that attending toward or away from touch as a relevant modality analogously modulates sensorimotor α- and β-oscillations (Fig. 4). In this sense, modality selection behaved “symmetrically,” i.e., both occipital and sensorimotor regions were modulated, in a converse see-saw manner, suggesting that α-modulation related to modality selection is a more general phenomenon that does not only apply to parieto-occipital cortex.
We also found that spatial attention to one or other side led to contralateral/ipsilateral relative increases/decreases in low-frequency power, as expected. Importantly, we were able to show further that these preparatory spatial-attention effects were found in the present visual-tactile paradigm over both occipital and sensorimotor cortex when either modality was attended (Figs. 2 and 3). Finally, even when selecting planar gradient sensors that showed a significant preference for one or other modality being task-relevant (Fig. 5) and that had the expected corresponding localization over parieto-occipital (for vision-relevant) or sensorimotor (for touch-relevant) regions, we still found that spatial-attention effects arose for these sensors with either modality being attended.
Thus attention to space is not constrained to the currently selected modality but operates also on the task-irrelevant modality, and therefore attention to space and modality can operate in parallel.
This had been previously shown for (target) stimulus-related responses using behavioral, ERP, and fMRI measures (Eimer et al. 2002; Eimer and Driver 2001; Kennett et al. 2001; Spence and Driver 1998; Talsma et al. 2010), but here we show it also for the pure top-down effect manifested in low-frequency oscillations measured with MEG.
Sensorimotor and parieto-occipital regions thus seem to act analogously with respect to low-frequency modulation of activity due to spatial attention and modality selection. Nonetheless, there were two notable differences. First, whereas spatial-attention effects were pronounced in the α-band (7.5–15 Hz) for both sensorimotor and (parieto-) occipital sensors, effects in the former clearly extended further into the β-band (Fig. 2). For modality selection (Fig. 4), it was also the case that effects over sensorimotor cortex were more dominant in the β-band than in α-, although present for both. In occipital cortex, the results in the β-band were somewhat less consistent. Whereas in parieto-occipital regions (as selected post hoc by modality-selective effects) spatial attention yielded a significant modulatory effect and in more posterior sensors (selected a priori) there was a strong trend (P = 0.503), effects on modality selection were almost absent. These tentative differences for the spectral consequences of attentional modulation in occipital vs. sensorimotor cortex may relate to previous discussions about resonance frequencies in these different regions (Gaetz and Cheyne 2006; Hari and Salmelin 1997; Rosanova et al. 2009).
Recent studies (Buffalo et al. 2010; Maier et al. 2010) indicate that low-frequency activity (predominantly α-band) in visual cortex may predominantly originate from the deep (infragranular) layers innervated by feedback projections from higher areas (Felleman and Van Essen 1991). Such top-down influences would accord with α-modulations being prominent in the anticipatory period before target onset (Haegens et al. 2011; Worden et al. 2000), as found here. Jensen and Mazaheri (2010) propose that such modulations of low-frequency activity in sensory cortex are predominantly caused by inhibitory mechanisms, such that during the troughs of strong α-oscillations (when the membrane is hyperpolarized), an increase of α-oscillations leads to reduced excitability of the neuronal population with respect to incoming afferent information.
Although often α- and β-oscillations seem to behave in a correlated fashion with respect to bottom-up and top-down inputs (e.g., Hoogenboom et al. 2006; Jones et al. 2010; Siegel et al. 2008), there are also notable differences: despite the fact that β-oscillations are often suppressed following stimulus processing (Bauer et al. 2006; Hoogenboom et al. 2006), enhanced levels of β-activity in widespread networks are often correlated with enhanced task performance related to sensorimotor integration and decision making (Donner et al. 2007; Gross et al. 2004). Furthermore, on the neurophysiological level, Jensen et al. (2005) provided evidence for a dissociation between α- and β-rhythms in response to GABAergic pharmacological manipulation in sensorimotor cortex. Ziegler et al. (2010) argued that the sensorimotor β-rhythm may reflect a superposition of two different α-rhythms in sensorimotor cortex. Clearly, more research is needed to understand fully the differential nature of the neurogenesis as well as the more functional aspects of α- and β-oscillations in sensorimotor and occipital cortex.
Although there were strong commonalities for the effects of spatial attention (Fig. 2) and modality selection (Fig. 4) in terms of their spectral signature (especially in sensorimotor cortex) and the topographies, these effects were not necessarily identical. In particular, in occipital and parieto-occipital regions, attention to modality effects did not modulate β-power, but a mild effect of spatial attention on parieto-occipital β-oscillations was observed. However, since relevant modality was assigned on a block-by-block basis here, whereas attended side was cued on a trial-by-trial basis, future extensions of the current paradigm could investigate the possible basis of the potential topographical and spectral differences, for instance by reversing which factor is cued and which blocked.
Furthermore, the interaction effect of spatial attention and attention to modality, that showed a general tendency for stronger spatial-attention effects when touch was the relevant modality, compared with when vision was the relevant modality (in both sensorimotor and occipital cortex), may be attributable to slight differences in task difficulty or attentional load for the tactile vs. visual task. Although the behavioral results are not significantly different between these task, subjects tentatively responded slower and committed more errors in the tactile compared with the visual task (nonsignificant).
To conclude, this MEG study shows that spatial attention affects not only occipital, but also sensorimotor regions via top-down-modulation of low-frequency α- and β-oscillations when either modality is task-relevant in a visuotactile situation. At the same time, modality selection can likewise affect not only parieto-occipital α-oscillations related to vision, but also sensorimotor α- and β-oscillations. The results show further that the spectral concentration of these effects may reflect resonance properties in the affected sensory areas, predominantly α for occipital cortex but α and β for sensorimotor areas (Rosanova et al. 2009). This spectral nature was similarly distinctive here for either site in the case of both spatial-attention and modality-selection influences. Thus, for vision and touch alike, spatial attention and modality selection both involve top-down modulation of low-frequency oscillations, although the exact spectral patterns differ regionally.
GRANTS
This work was supported by the Medical Research Council (G0500784) and the Wellcome Trust (087756/Z/08/Z). J. Driver was a Royal Society Research Professor, and the Wellcome Trust Centre for Neuroimaging is supported by core funding from the Wellcome Trust (091593/Z/10/Z).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.B. analyzed data; M.B. and J.D. interpreted results of experiments; M.B. and S.K. prepared figures; M.B. drafted manuscript; M.B., S.K., and J.D. edited and revised manuscript; M.B., S.K., and J.D. approved final version of manuscript; S.K. and J.D. conception and design of research; S.K. performed experiments.
REFERENCES
- Banerjee S, Snyder AC, Molholm S, Foxe JJ. Oscillatory alpha-band mechanisms and the deployment of spatial attention to anticipated auditory and visual target locations: supramodel or sensory-specific control mechanisms? J Neurosci 31: 9923–9932, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaansen MC, Knösche TR. Tangential derivative mapping of axial MEG applied to event-related desynchronization research. Clin Neurophysiol 111: 1300–1305, 2000 [DOI] [PubMed] [Google Scholar]
- Bauer M, Oostenveld R, Peeters M, Fries P. Tactile spatial attention enhances gamma-band activity in somatosensory cortex and reduces low-frequency activity in parieto-occipital areas. J Neurosci 26: 490–501, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalo EA, Fries P, Landman R, Liang H, Desimone R. A backward progression of attentional effects in the ventral stream. Proc Natl Acad Sci USA 107: 361–365, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner TH, Siegel M, Oostenveld R, Fries P, Bauer M, Engel AK. Population activity in the human dorsal pathway predicts the accuracy of visual motion detection. J Neurophysiol 98: 345–359, 2007 [DOI] [PubMed] [Google Scholar]
- Eimer M. Crossmodal links in spatial attention between vision, audition, and touch: evidence from event-related brain potentials. Neuropsychologia 39: 1292–1303, 2001 [DOI] [PubMed] [Google Scholar]
- Eimer M, Driver J. Crossmodal links in endogenous and exogenous spatial attention: evidence from event-related brain potential studies. Neurosci Biobehav Rev 25: 497–511, 2001 [DOI] [PubMed] [Google Scholar]
- Eimer M, van-Velzen J, Driver J. Cross-modal interactions between audition, touch, and vision in endogenous spatial attention: ERP evidence on preparatory states and sensory modulations. J Cogn Neurosci 14: 254–271, 2002 [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1: 1–47, 1991 [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV, Ahlfors SP. Parieto-occipital approximately 10 Hz activity reflects anticipatory state of visual attention mechanisms. Neuroreport 9: 3929–3933, 1998 [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Snyder AC. The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Front Psychol 2: 154, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu KM, Foxe JJ, Murray MM, Higgins BA, Javitt DC, Schroeder CE. Attention-dependent suppression of distracter visual input can be cross-modally cued as indexed by anticipatory parieto-occipital alpha-band oscillations. Brain Res 12: 145–152, 2001 [DOI] [PubMed] [Google Scholar]
- Gaetz W, Cheyne D. Localization of sensorimotor cortical rhythms induced by tactile stimulation using spatially filtered MEG. Neuroimage 30: 899–908, 2006 [DOI] [PubMed] [Google Scholar]
- Gross J, Schmitz F, Schnitzler I, Kessler K, Shapiro K, Hommel B, Schnitzler A. Modulation of long-range neural synchrony reflects temporal limitations of visual attention in humans. Proc Natl Acad Sci USA 101: 13050–13055, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Handel BF, Jensen O. Top-down controlled alpha band activity in somatosensory areas determines behavioral performance in a discrimination task. J Neurosci 31: 5197–5204, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen M, Hari R, Ilmoniemi RJ, Knuutila J, Lounasmaa OV. Magnetoencephalography - theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev Modern Physics 65: 413–497, 1993 [Google Scholar]
- Hari R, Salmelin R. Human cortical oscillations: a neuromagnetic view through the skull. Trends Neurosci 20: 44–49, 1997 [DOI] [PubMed] [Google Scholar]
- Hoogenboom N, Schoffelen JM, Oostenveld R, Parkes LM, Fries P. Localizing human visual gamma-band activity in frequency, time and space. Neuroimage 29: 764–773, 2006 [DOI] [PubMed] [Google Scholar]
- Jensen O, Goel P, Kopell N, Pohja M, Hari R, Ermentrout B. On the human sensorimotor-cortex beta rhythm: sources and modeling. Neuroimage 26: 347–355, 2005 [DOI] [PubMed] [Google Scholar]
- Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci 30: 317–324, 2007 [DOI] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci 4: 186, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Kerr CE, Wan Q, Pritchett DL, Hämäläinen M, Moore CI. Cued spatial attention drives functionally relevant modulation of the mu rhythm in primary somatosensory cortex. J Neurosci 30: 13760–13765, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett S, Eimer M, Spence C, Driver J. Tactile-visual links in exogenous spatial attention under different postures: convergent evidence from psychophysics and ERPs. J Cogn Neurosci 13: 462–478, 2001 [DOI] [PubMed] [Google Scholar]
- Knösche TR. Transformation of whole-head MEG recordings between different sensor positions. Biomedizinische Technik 47: 59–62, 2002 [DOI] [PubMed] [Google Scholar]
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science 320: 110–113, 2008 [DOI] [PubMed] [Google Scholar]
- Linkenkaer-Hansen K, Nikulin VV, Palva S, Ilmoniemi RJ, Palva JM. Prestimulus oscillations enhance psychophysical performance in humans. J Neurosci 24: 10186–10190, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso E, Driver J. Multisensory spatial interactions: a window onto functional integration in the human brain. Trends Neurosci 28: 264–271, 2005 [DOI] [PubMed] [Google Scholar]
- Maier A, Adams GK, Aura C, Leopold DA. Distinct superficial and deep laminar domains of activity in the visual cortex during rest and stimulation. Front Syst Neurosci 4: pii: 31, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011: 156869, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei V, Gross J, Thut G. On the role of prestimulus alpha rhythms over occipito-parietal areas in visual input regulation: correlation or causation? J Neurosci 30: 8692–8697, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M. Natural frequencies of human corticothalamic circuits. J Neurosci 29: 7679–7685, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Oostenveld R, Fries P, Engel AK. Neuronal synchronization along the dorsal visual pathway reflects the focus of spatial attention. Neuron 60: 709–19, 2008 [DOI] [PubMed] [Google Scholar]
- Spence C, Driver J. Crossmodal Space and Crossmodal Attention. Oxford, UK: Oxford Univ. Press, 1998 [Google Scholar]
- Steinmetz PN, Roy A, Fitzgerald PJ, Hsiao SS, Johnson KO, Niebur E. Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature 404: 187–190, 2000 [DOI] [PubMed] [Google Scholar]
- Talsma D, Senkowski D, Soto-Faraco S, Woldorff MG. The multifaceted interplay between attention and multisensory integration. Trends Cogn Sci 14: 400–410, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci 26: 9494–9502, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenner MU, Heekeren HR, Bauer M, Rössner K, Wenzel R, Villringer A, Fahle M. What happens in between? Human oscillatory brain activity related to crossmodal spatial cueing. PLoS One 3: e1467, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk H, Schoffelen JM, Oostenveld R, Jensen O. Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J Neurosci 28: 1816–1823, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede F, de Lange F, Jensen O, Maris E. Orienting attention to an upcoming tactile event involves a spatially and temporally specific modulation of sensorimotor alpha- and beta-band oscillations. J Neurosci 31: 2016–2024, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Fries P. The role of neuronal synchronization in selective attention. Curr Opin Neurobiol 17: 154–160, 2007 [DOI] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. J Neurosci 20: RC63, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DA, Pritchett DL, Hosseini-Varnamkhasti P, Corkin S, Hämäläinen M, Moore CI, Jones SR. Transformations in oscillatory activity and evoked responses in primary somatosensory cortex in middle age: a combined computational neural modeling and MEG study. Neuroimage 52: 897–912, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]