Abstract
In severe pulmonary arterial hypertension (PAH), vascular lesions are composed of phenotypically altered vascular and inflammatory cells that form clusters or tumorlets. Because macrophages are found in increased numbers in intravascular and perivascular space in human PAH, here we address the question whether macrophages play a role in pulmonary vascular remodeling and whether accumulation of macrophages in the lung vasculature could be compromised by the immune system. We used the mouse macrophage cell line RAW 264.7 because these cells are resistant to apoptosis, have high proliferative capacity, and resemble cells in the plexiform lesions that tend to pile up instead of maintaining a monolayer. Cells were characterized by immunocytochemistry with cell surface markers (Lycopersicon Esculentum Lectin, CD117, CD133, FVIII, CD31, VEGFR-2, and S100). Activated, but not quiescent, T cells were able to suppress RAW 264.7 cell proliferative and migration activity in vitro. The carboxyfluorescein diacetate-labeled RAW 264.7 cells were injected into the naïve Sprague Dawley (SD) rat and athymic nude rat. Twelve days later, cells were found in the lung vasculature of athymic nude rats that lack functional T cells, contributing to vascular remodeling. No labeled RAW 264.7 cells were detected in the lungs of immune-competent SD rats. Our data demonstrate that T cells can inhibit in vitro migration and in vivo accumulation of macrophage-like cells.
Keywords: endothelium, pulmonary hypertension
severe pulmonary arterial hypertension (PAH) is a progressive, multifactorial vascular disease that is characterized by lung vascular remodeling, high pulmonary blood pressure, and right ventricular hypertrophy (2, 16, 38, 41). In addition, some forms of PAH are known to be associated with immune dysfunction (21, 24). The predominant feature of the lung remodeling in PAH is the development of vascular occlusive plexiform lesions as the result of exuberant and deregulated growth of endothelial cell accompanied by infiltration of inflammatory cells such as T cells, B and mast cells, and macrophages (2, 11, 39, 40). These findings demonstrate that the immune system plays an important role in pathogenesis of severe pulmonary hypertension. In an attempt to explain the pathobiology of small vessel disease in patients with severe PAH, Norbert Voelkel and his colleagues proposed a paradigm of plexiform lesion formation as a quasi-cancerous process (23). Indeed, the vascular lesions of patients with severe PAH exhibit some characteristics of malignant tumors. These lesions are angiogenic, have a lower population of apoptotic cells, and overexpress antiapoptotic proteins, all of which are cancer-like processes. However, whether a tumorigenic mechanism can fully explain the pathobiology of PAH remains controversial (25).
Macrophages play a crucial role in the inflammatory and immune responses and are major sources of inflammatory mediators. Together with dendritic cells, they compose the family of antigen-presenting cells in response to infection or inflammation (20). These cells have long been used as models to study pathogenic response to a wide variety of stimuli. Cells of monocyte/macrophage lineage are present in pulmonary vascular lesions in humans with PAH (40); however, their role in the pathogenesis of PAH is poorly understood. Under certain conditions these cell adhere on injured endothelium and can contribute to vascular lesion formation. Because macrophages are found in plexiform lesions of the patients with PAH and in animal models of severe PAH, here we ask the question whether macrophage-like cells can contribute to vascular remodeling in a rat model and/or exhibit an endothelial-like cell phenotype and whether their accumulation and migration can be modulated by T cells.
To address this question, we used Abelson leukemia virus-transformed macrophage cell line RAW 264.7 that, according to their pinocytotic, phagocytotic, secretory and adhesive properties, exhibit a typical macrophage phenotype. The RAW 264.7 cells are the most commonly used macrophage cell line in biomedical research.
MATERIALS AND METHODS
Animals.
Male Sprague Dawley (SD) and athymic nude (AN) rats (rnu/rnu) 6 wk of age were used in this study. AN rats have black and white hooded pigmentation and are occasionally Albino. During their life span, some animals exhibit intermittent periods of hair growth and loss. The T lymphocyte deficiency is reflected by the depleted cell populations in the peripheral lymphoid organs and thymic aplasia. The B-lymphocyte function in these animals is normal; however, there is an increased natural killer cell population. The Foxn1rnu autosomal recessive mutation is located on chromosome 10, and only homozygous mutants exhibit rnu phenotype. The Foxn1rnu/Foxn1+ heterozygotes animals are not T cell deficient.
The experimental protocol was approved by the Animal Care and Use Committee of the University of Colorado Anschutz Medical Center. Animals were divided into eight groups (n = 4 animals per group): Group 1: SD, injected with human umbilical vein endothelial cells (HUVEC); Group 2: SD, injected with HUVEC and exposed to hypobaric hypoxia (simulated 17,000-ft altitude) for 2 wk; Group 3: SD, injected with RAW 264.7 cells; Group 4: SD, injected with RAW 264.7 cells and exposed to hypobaric hypoxia; Group 5: AN, injected with HUVEC; Group 6: AN, injected with HUVEC plus hypoxia; Group 7: AN, injected with RAW 264.7 cells; Group 8: AN, RAW 264.7 cells and exposed to hypobaric hypoxia.
Human subjects.
Human tissue was obtained from University of Colorado Hospital patients with PAH at autopsy. Informed consent to use the tissue for research purposes had previously been obtained. Unused donor lungs without evidence of pulmonary disease served as controls. Five patients with idiopathic PAH and five control lungs were studied.
Cell culture.
RAW 264.7 cells (purchased from ATCC; no. TIB-71) are a macrophage-like, Abelson leukemia virus transformed cell line derived from BALB/c mice. Cells were maintained in endotoxin-free DMEM containing 10% FBS and 1% penicillin/streptomycin (P/S) at 37°C in a humidified atmosphere with 5% CO2.
Primary HUVEC were isolated in our laboratory following the protocol described by Bruneel et al. (1). Cells were grown on gelatin (type 1 porcine skin; Sigma Chemical, St. Louis, MO)-coated tissue culture flasks in 1:1 ISOCOVES/RPMI-1640 (Cellgro, Herndon, VA) medium with 10% fetal bovine serum (HyClone Laboratories, Logan, UT) in a 5% CO2 humidified atmosphere at 37°C. Media for primary cultures contained no heparin, except where indicated, and were supplemented with penicillin (100 U/ml) and streptomycin (100 mg/ml). All other studies were performed with 80%-confluent HUVEC cultures at the fourth or fifth passage.
Antibodies.
The following antibodies were used: CD117-FITC (c-kit, 1:50), FVIII (1:1,000) VEGFR2/KDR (1:50) (Abcam, Cambridge, MA); Lycopersicon Esculentum (tomato) lectin (1:100) (Vector Laboratories, Burlingame, CA); CD31 (1:400) (Dako, Carpinteria, CA); CD34 (1:50) (Miltenyi Biotech, Surrey, UK); anti-mouse IgG1-R-PE (1:250) (Invitrogen, Carlsbad, CA); anti-rabbit-Alexa-Fluor 555 (1:250) (Molecular Probes, Eugene, OR); anti-human CD68 (1:200) (DakoCytomation).
Cell proliferation assay.
Cell proliferation was assessed using the CyQuant Cell Proliferation Assay Kit (Invitrogen/Molecular Probes). Cells were plated at 1,000 cells/well on 96-well plates, and cell proliferation was measured at 48, 72, and 120 h. Fluorescence measurements were performed using a Victor 1420 (Perkin Elmer, Boston, MA) microplate reader with excitation at 480 nm and emission detection at 520 nm.
Immunocytochemistry.
Cells were seeded in eight-well glass slides in DMEM/10% FBS and cultivated for 24 h. Cells were washed with PBS and fixed in 2% paraformaldehyde/PBS for 10 min at room temperature. Cells were permeabilized in cold methanol (−20°C), washed with PBS, blocked with 2% donkey serum (in PBS) for 1 h and incubated with primary antibodies overnight at 4°C. After three washes with 2% serum (in PBS), cells were incubated with anti-rabbit Rhodamine Red-X-conjugated antibody or anti-mouse-FITC antibody for 45 min at room temperature. Slides were mounted using DAPI mounting media (Invitrogen) and visualized using a Zeiss microscope. Images were captured with an attached camera linked to a computer.
Cell labeling protocol.
RAW 264.7 and/or HUVEC cells (107 cells) were trypsinized and centrifuged (1,200 g, 5 min). Supernatant was aspirated, and a cell pellet was resuspended in 5 ml of warm PBS, containing 1 μM carboxyfluorescein diacetate succinimidyl ester (CFDA-SE, Invitrogen) and incubated for 15 min at 37°C. Labeled cells were centrifuged, resuspended in 5 ml of warm DMEM, and incubated for another 30 min. At the end cells were centrifuged (1,200 g, 10 min) and resuspended in 2 ml of warm PBS.
Cell migration assay.
Growth-arrested (DMEM without serum, 72 h) RAW 264.7 cells (2.0 × 105 cells/well) were plated in 24-well plates in 0.8 ml of serum-free DMEM. Activated (anti-CD3 Abs, 72 h) or quiescent T cells (3.0 × 105 cells/well) were added on permeable cell culture inserts (3.0 μM pore size; Costar, Milpitas, CA) precoated with 1% gelatin (Sigma). After 48 h in coculture, T cells from the top of the filter were wiped off, and cells on the bottom were fixed with methanol and stained with 0.2% crystal violet in 2% (vol/vol) ethanol for a minimum of 15 min. Cells were examined on a phase-contrast microscope at ×10 magnification. Alternatively, growth-arrested RAW 264.7 cells (2.0 × 105 cells/well) were plated in the permeable inserts (5.0 μM pore size), and activated (anti-CD3 Abs, 72 h) or quiescent T cells (3.0 × 105 cells/well) were added to the wells in 24-well plates. After 48 h, cells on the bottom of filters and on bottom of wells were stained and examined as described above.
Adoptive transfer procedure.
The CFDA-SE-labeled HUVEC or RAW 264.7 (10 × 106 cells in 250 μl PBS) was injected into the tail vein of SD and nude rats. Two weeks after the injection animals were killed, lungs were inflated with 0.5% low-melting agarose in PBS, fixed in formalin for 48 h, and paraffin embedded as previously described (34). Lung sections were processed (34) and examined for the accumulation of CFDA-labeled cells. The dose was chosen based on our earlier work with adoptive transfer of lymphocytes (35) and HUVEC cells (36).
Statistical analysis.
Data were analyzed using Prism 3.0 (GraphPad Software, San Diego, CA). All data are expressed as means ± SE unless otherwise noted. Statistical analysis of the data was performed using Student's unpaired t-test or one-way ANOVA followed by Bonferroni's multiple-comparison test. Differences were considered significant with P ≤ 0.05.
RESULTS
Presence of macrophages in the plexiform lesions in PAH.
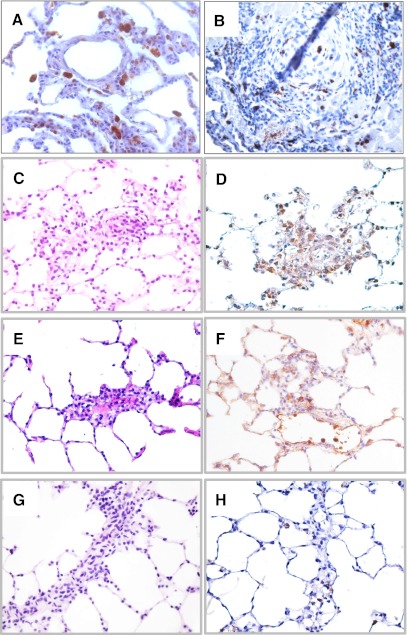
In severe PAH, vascular occlusive plexiform lesions comprise phenotypically altered vascular endothelial-like and smooth muscle cell, as well as inflammatory cells such as T cells, B and mast cells, and macrophages. Human lung tissue sections from normal and PAH patient lung were stained for macrophage marker CD68. Although, in the normal lung, macrophages were mainly found in the alveolar airspaces (Fig. 1A), there was an abundant presence of macrophages in the pulmonary lesion from patients with severe PAH (Fig. 1B). It appears that macrophages were randomly distributed within the lesion and as shown in Fig. 1B were present in the intima, media, and adventitia of the obliterated vessels. Presence of macrophages is also prominent in pulmonary lesions of experimental severe pulmonary hypertension models. Here we show presence of macrophages in vascular lesions of VEGF receptor inhibitor SU5416 plus hypoxia-induced PAH in SD rats (Fig. 1, C and D), in SU5416-induced PAH in AN rats (Fig. 1, E and F), and in hypoxia-induced PAH in AN rats (Fig. 1, G and H).
Fig. 1.
Immunohistochemistry for macrophage marker CD68 in human paraffin-embedded tissue sections from normal lung (A) and in the plexiform lesion from patients with pulmonary arterial hypertension (PAH) (B) and in experimental rat models of severe PAH. VEGF receptor inhibitor SU5416 plus hypoxia-induced PAH in Sprague Dawley (SD) rats (C and D). SU5416-induced PAH in athymic nude (AN) rats (E and F) and hypoxia-induced PAH in AN rats (G and H). Hematoxylin/eosin stain rat lung tissue (C, E, and G).
Proliferative capacity of macrophage-like cells.
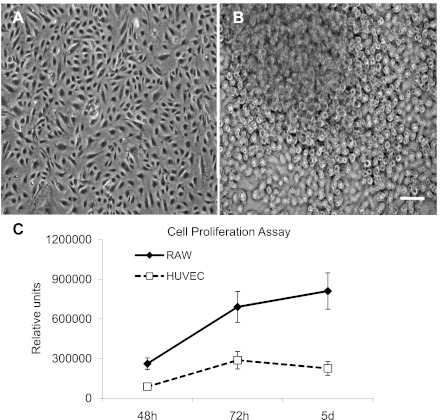
In contrast to primary lung macrophages that are difficult to retain in culture, RAW 264.7 cells are relatively easy to grow; they are well characterized and easily available. The semiadherent property of RAW 264.7 cells indicates that their adhesion to vascular cells can be modulated by local environmental factors. When cultured in DMEM/10% FBS medium, RAW 264.7 cells had high proliferative capacity and resembled cells in the plexiform lesions that tend to pile up instead of growing as a monolayer. Moreover, RAW 264.7 cells were resistant to apoptosis (as tested by active caspase-3 staining, data not shown) and (in contrast to HUVEC) could survive for an extended period of time (2–4 days) in highly acidic conditions (pH = 4.0; data not shown). As shown in Fig. 2, RAW 264.7 cells had much higher proliferative capacity than HUVEC.
Fig. 2.
Light microscopy images of confluent human umbilical vein endothelial cells (HUVEC) (A) and RAW 264.7 cell (B) cultures. Cell proliferation curves of HUVEC and RAW 264.7 cells from 3 independent experiments (C). Scale bar = 50 μm.
Cell surface marker expression.
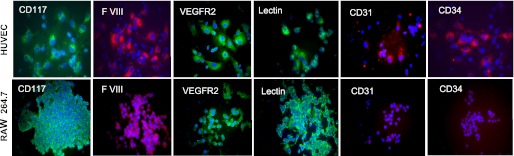
To characterize RAW 264 cells and HUVEC phenotype, we performed immunostaining for the following cell surface markers: CD117 - c-kit; endothelial cell antigen F VIII; VEGFR2, lectin (from Lycopersicon esculentum); CD31 - platelet endothelial cell adhesion molecule-1; CD34 - cell-cell adhesion factor. As shown in Fig. 3, macrophage-like RAW 264.1 cells, similar to HUVEC cells, stained positive for CD117, VEGFR2, F VIII, and tomato lectin. However, RAW 264.1 cells did not express cell adhesion molecules CD31 and CD34.
Fig. 3.
Immunofluorescent detection of cell surface markers, CD117 (c-kit), factor VIII (F VIII), VEGFR2, Lycopersicon esculentum lectin, CD31 (platelet endothelial cell adhesion molecule-1), and cell-cell adhesion glycoprotein CD34 in HUVEC and RAW 264.7 cells. Representative images from 3 independent experiments. Scale bar = 20 μm.
Effect of T cells on macrophage-like cell migration.
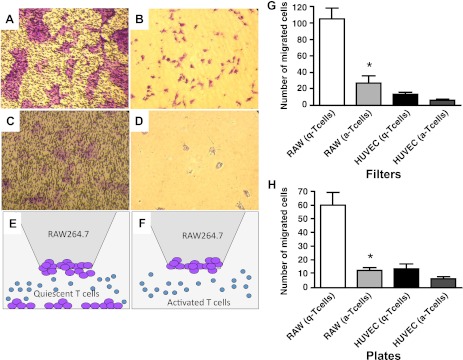
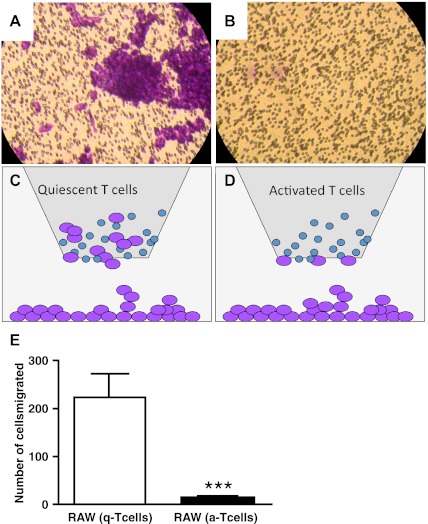
Earlier we have demonstrated that AN rats treated with VEGF receptor inhibitor SU5416 develop severe PAH at normoxic conditions, suggesting that T cells play a protective role against the development of PAH (35). Moreover, hypoxia induces T cell activation in vivo. There was a significant increase of number of T cells expressing T cell activation marker CD25 in SD rat lungs exposed to hypoxia for 3 wk. To examine whether activated T cells can modulate the angiogenic capability of RAW 264.1 cells, we performed Boyden chamber assays. RAW 264.7 or HUVEC cells were plated in the upper compartment of a Boyden chamber. CD3 antibody-treated activated T cells or quiescent T cells were plated in the lower compartment of a chamber. After 24 h of incubation at 37°C, 5% vol/vol CO2-transmigrated cells on the filter and on the bottom of the plate were counted. As shown in Fig. 4, RAW 264.7 cells migrated toward quiescent T cells and were found at the bottom of the filter and at the bottom of the plate, whereas activated T cells prevented RAW 264.7 cell migration. HUVEC migration was insignificant. The quantitative data are shown in Fig. 4B.
Fig. 4.
Activated T cells suppress migratory capacity of RAW 264.7 cells. RAW 264.7 cells incubated with quiescent (A and B) or activated (C and D) T cells. E and F: schematic representation of experiments presented in A–D. A and C: cells in the filters. B and D: cells on the bottom of the plate. G and H: quantitative analysis of cells on the filters (G) and on the bottom of the plate (H). *P < 0.01. Data from 3 independent experiments.
When T cells were plated in the upper chamber and RAW 264.7 cell or HUVEC cells were plated in the lower chamber, RAW 264.7 cells migrated toward quiescent T cells and were found piled up on the filters of the upper chamber, whereas activated T cells significantly suppressed this migration (Fig. 5). Under the same experimental conditions, HUVEC cells did not migrate toward T cells (data not shown).
Fig. 5.
Migratory properties of RAW 264.1 cells plated on the bottom of the chamber. After 24 h RAW 264.1 cells migrate toward quiescent (A) but not activated (B) T cells. C and D: schematic representation of the experiment. E: quantitative analysis of RAW 264.7 cells incubated with quiescent (A) or activated (B) T cells on the filters. ***P < 0.0001. Data from 3 independent experiments.
Absence of T cells facilitates RAW 264.7 cell homing to the lung.
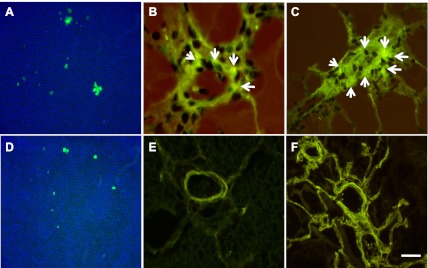
RAW 264.7 and HUVEC cells were labeled with CFDA-SE and injected into the naïve SD rat and AN rat. Twelve days after the injection, circulating RAW 264.7 cells were found in both AN (Fig. 6A) and SD (Fig. 6D) rats. Moreover, labeled RAW 264.7 cells were found in the vasculature of AN rat lungs in normoxic (Fig. 6B) and hypoxic condition (Fig. 6C). There were no labeled RAW 264.7 cells in the lung vasculature of the immune-competent SD rats either in normoxic (Fig. 6E) or hypoxic conditions (Fig. 6F).
Fig. 6.
Adoptive transfer of carboxyfluorescein diacetate succinimidyl ester (CFDA-SE)-labeled RAW 264.1 cells. A: circulating CFDA-labeled RAW 264.7 cells in AN rats 12 days after injection. B: CFDA-labeled RAW 264.7 cells in AN rat lung vasculature (arrows) 12 days after the injection. C: CFDA-labeled RAW 264.7 cells in the AN rat lung vasculature (arrows) 12 days after the injection and exposure to hypobaric hypoxia. D: circulating CFDA-labeled RAW 264.7 cells in SD rats 12 days after injection. E: CFDA-labeled RAW 264.7 cells in SD rat lung vasculature 12 days after the injection. F: CFDA-labeled RAW 264.7 cells in SD rat lung vasculature 12 days after the injection and hypoxic exposure. Scale bar = 50 μm.
Labeled HUVEC cells were undetectable in both SD and AN rat lungs under normoxic or hypoxic conditions. Thus our data show that macrophages can contribute to vascular remodeling and that functional T cells play a protective role by suppressing migratory/invasive potential of macrophage-like cells.
DISCUSSION
Macrophages are present in almost in all tissues. They differentiate from circulating peripheral-blood mononuclear cells, which migrate into tissue in the steady state or in response to inflammation (7). It is well established that macrophages play the major role in the removal of cellular debris that is generated during tissue remodeling and rapidly and efficiently clear local microenvironments from apoptotic cells (19, 22). Macrophages are highly heterogeneous cells that can rapidly change their function in response to local microenvironmental signals (20). Macrophages are classified to classically (M1) and alternatively (M2) activated macrophages. According to their function, they are described as host defense, wound healing, and immune regulatory. Presence of macrophages in PAH lesions has been reported (40), and macrophage-derived factors such as endothelin-1 and IL-6 have been implicated in pathogenesis of PAH (9, 11). Recently Graham and colleagues (8) reported that Schistosoma mansoni causes experimental PAH associated with pulmonary vascular remodeling in an IL-13-dependent manner. IL-13 is known to promote the development of wound-healing macrophages. Wound-healing macrophages can also be detrimental to the host when their matrix-enhancing activity is impaired, similarly to the dysregulated activity of classically activated macrophages in autoimmunity. The tissue fibrosis that occurs during chronic schistosomiasis has been attributed to the uncontrolled activation of wound-healing macrophages (10). Macrophages have remarkable plasticity that allows them to efficiently respond to environmental signals and change their phenotype and their physiology. It has been demonstrated that lipopolysaccharide treatment of macrophage cell line RAW 264.7 induces release of monocyte chemoattractant protein-1, IL-1β, IL-6, tumor necrosis factor-α, and IL-10 (14, 30) that are implicated in the pathogenesis of PAH. Macrophage derived-endothelin-1 has been suggested to contribute to a number of chronic lung diseases including PAH (32). Moreover, endothelin-1 expression was demonstrated in lung macrophages from controls and patients with idiopathic PAH and heritable PAH (32).
Macrophages play a key role in inflammation and tumor angiogenesis. In the cancer field there is increasing evidence that macrophages promote tumor progression by stimulating tumor vascularization, invasion, and metastasis (17). Macrophage infiltration has been reported in many human cancers and was associated with poor prognoses (3, 12). It was also reported that activated tumor-associated macrophages due to release of multiple proteases, growth factors (basic fibroblast growth factor, TGF-α, insulin-like growth factor-I, platelet-derived growth factor, VEGF, TGF-β) cytokines (IL-1, IL-6, IL-8), and other vasoactive molecules (substance P, prostaglandins, transfer/carrier protein-1) are actively involved in regulation of all steps of the angiogenic process (31, 37). Tumor-associated macrophages can suppress tumor-infiltrating T cells. Data in vitro suggest that hypoxia powerfully augments macrophage-mediated T cell suppression in a hypoxia-inducible factor-1a-dependent manner (5). Although T cells can inhibit tumor growth, often their function is suppressed in tumor microenvironment (13, 43). Recently Doedens and colleagues (5) reported that hypoxia powerfully augmented macrophage-mediated T-cell suppression in vitro in a manner dependent on macrophage expression of hypoxia-inducible factor-1α. Interestingly, tumor-associated macrophages show a remarkable degree of plasticity and functional heterogeneity, suggesting that during tumor progression macrophages undergo a phenotypic “switch”, eventually exhibiting the alternatively activate M2 phenotype, associated with immunosuppression, promotion of tumor angiogenesis, and metastasis. On the other hand, inflammasome activation and IL-1b production underlie ultimate activation of protective immunity (15). These recent findings suggest that targeting signaling pathways involved in the switch of macrophage polarization states in favor of a more anti-tumor phenotype (from M2 to a full M1 phenotype) might lead in development of new strategies to treat cancer (12, 28).
Our previous studies in a rat model of PAH demonstrated that VEGF inhibitor SU5416-induced plexiform lesions comprised of apoptosis-resistant highly proliferative endothelial-like cells (tumorlets). Although these endothelial-like cells still maintain some of the endothelial cell markers, they lost cell adhesion molecules. As the result of that, they do not form monolayer, instead they pile-up and occlude pulmonary vessels, forming vascular lesions that contain different cell types including macrophages.
Data presented in this study demonstrate that culturing of RAW 264.1 cells in growth media (DMEM/10% FBS) typically used for endothelial cell resulted in morphological changes in this cell line toward a mesenchymal/endothelial phenotype. RAW 264.1 possess high proliferative capacity and express CD117 (c-kit), VEGFR2, Factor VIII, and bind Lycopersicon Esculentum lectin. In addition, similar to endothelial-like cells in plexiform lesions, RAW 264.1 cells do not express cell adhesion molecules CD31 and CD34.
The proto-oncogene c-kit, otherwise known as CD117 antigen or stem cell factor receptor, is a 145-kDa type III transmembrane tyrosine kinase receptor. The c-kit gene encodes a transmembrane tyrosine kinase receptor, structurally similar to platelet-derived growth factor receptors A and B, as well as the colony stimulating factor 1 receptor. It is thought to play an important role in hematopoiesis, spermatogenesis, melanogenesis and several cancers (29). The c-kit protein is expressed on a variety of cells including many epithelial cell types, mast cells, and macrophages. Recently Montani and colleagues (18) reported an increased expression of c-kit mRNA in pulmonary arterial lesions in patients with idiopathic PAH. The c-kit expression was detected in perivascular cells, including bone marrow-derived progenitors, mast cells, and other inflammatory cells. Moreover, both c-kit1/CXCR41 cells and recruiting factors, such as CXCL12/SDF-1, were increased in vascular lesions. Authors suggested that vasa vasorum expansion might play a role in the recruitment of perivascular circulating progenitor cells associated with pulmonary arterial lesions. Earlier Kurt Stenmark's group reported that chronic hypoxia induced mobilization of c-kit+ cells from the bone marrow into the circulation and that there was an accumulation of c-kit+ cells in the remodeled blood vessels in an experimental model of pulmonary hypertension (4). As previously demonstrated, c-kit+ cells mobilized from the bone marrow cells are able to differentiate into vascular cells; moreover, hypoxia enhances differentiation of VEGFR21/c-kit1 cells into a-smooth muscle actin-1 cells (26, 27). In addition, c-kit+ cells can express inflammatory and proliferative factors that stimulate proliferation of other resident vascular wall cells, supporting the idea that c-kit-expressing cells could contribute both directly and indirectly to the vascular remodeling process (6). Here we show for the first time that c-kit+ macrophages can home to the lung vasculature and contribute to vascular lesion formation. These findings are supported by recent data from Mark Nicolls's laboratory (33) showing obliteration of the blood vessels with macrophages in the athymic rat model of SU5416-induced PAH. Our findings are also in agreement with previous reports (4, 18) suggesting a role of c-kit+ cells in pulmonary vascular remodeling.
As immune cells can modulate angiogenic properties of endothelial cells and macrophages, we tested this possibility in the in vitro migration experiments. We found that RAW 264.1 cells migrated to quiescent but not to anti-CD3 antibody-activated T cells. In fact, activated T cells attenuated macrophage-like cell migration, whereas quiescent T cells did not. To further demonstrate a protective role of T cells against invasive macrophage response, we performed the in vivo experiments in which CFDA-SE-labeled RAW 264.1 cells were injected into the naïve SD rat and AN rat. Our results clearly demonstrate that RAW 264.7 cells (but not HUVEC) can be found in the vasculature of AN nude rat lungs. These findings further provide evidence for a protective role of the immune system (T cell) in regulation of macrophage-mediated inflammatory angiogenesis in the lung. We also show that hypoxia-activated T cells and Treg cell numbers are increased in the lung tissue, suggesting a protective role of Treg cells against vascular lesion formation. Recent data from Mark Nicolls's laboratory show that adoptive transfer of Treg cells limited lung injury and prevented the formation of vascular lesions in the experimental SU5416/athymic rat model of PAH (33). Moreover, in the absence of T cells, hypoxia facilitated macrophage-like cell homing to the lung vasculature, suggesting that macrophages can contribute to the vascular lesion formation. Whether, as in cancer paradigm (5), lung tissue macrophages in the vascular lesions (possibly of the M2 phenotype) play a role in T cell suppression remains to be investigated.
Both macrophages and hyperproliferative endothelial-like cells apparently contribute to the plexiform lesion formation in PAH. Phenotypical plasticity and invasive capacity of macrophages confer their ability to interact with other cell types within the vascular wall. Recently it has been reported that macrophages overexpressing VEGF transdifferentiate into endothelial-like cells in vitro and in vivo (42). Moreover, in a model of myocardial infarction, macrophages overexpressing VEGF incorporated into blood vessels. Therefore, endothelial-like behavior of RAW 264.7 cells and their accumulation in the vessel wall of athymic rats that lack functional T cells represent physiologically relevant responses.
In summary, our data provide evidence that highly proliferative macrophage-like cells can contribute to vascular lesion formation. Our data demonstrate an abundant presence of macrophages in the pulmonary lesion from patients with severe PAH. This suggests a possibility that macrophages can be used as carriers for drug or gene delivery to the regions of tumor angiogenesis, plexiform lesions, and to the sites of tissue regeneration, yet more studies are needed to support this notion.
The understandable limitation of our work is that in the timeframe examined we did not show the formation of the true vascular lesions. Moreover, the functional status of the macrophages present in vascular lesions of human or experimental PAH currently remains unknown. The isolation and characterization of macrophages from vascular lesions (similar to tumor-associated macrophages) would be a reasonable approach. Studies regarding the role of macrophages and their interplay with other inflammatory cells in the formation of vascular lesions are just emerging. This opens an exciting chapter in the pathobiology of pulmonary hypertension.
The approach of directing the macrophage polarization from M2 to M1 might become a potential therapeutic approach. Future studies are necessary to investigate in more detail modulatory roles of both T cells and macrophages for antiangiogenic therapy in pulmonary vascular diseases.
GRANTS
This work was supported by grants from the American Heart Association SDG 0735388N, AHA 11GRNT 7520020, Flight Attendant Medical Research Institute CIA award 072053, Bixler Family Foundation, Emphysema Research Fund (L. Taraseviciene-Stewart), and NIH R01 HL086783 (E. Gerasimovskaya).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: E.V.G. and L.T.-S. conception and design of research; E.V.G., J.S., and L.T.-S. performed experiments; E.V.G., A.K., A.S., J.S., and L.T.-S. analyzed data; E.V.G., A.K., M.Z., and L.T.-S. interpreted results of experiments; E.V.G., A.S., and L.T.-S. prepared figures; E.V.G., A.K., J.S., M.Z., and L.T.-S. edited and revised manuscript; E.V.G., A.K., A.S., J.S., M.Z., and L.T.-S. approved final version of manuscript; L.T.-S. drafted manuscript.
ACKNOWLEDGMENTS
We thank Liudas Slepikas for technical assistance and Dr. John Stewart for helpful comments in preparing the manuscript.
REFERENCES
- 1. Bruneel A, Labas V, Mailloux A, Sharma S, Vinh J, Vaubourdolle M, Baudin B. Proteomic study of human umbilical vein endothelial cells in culture. Proteomics 3: 714, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Chan SY, Loscalzo J. Pathogenic mechanisms of pulmonary arterial hypertension. J Mol Cell Cardiol 44: 14, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coffelt SB, Hughes R, Lewis CE. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochim Biophys Acta 1796: 11–18, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Davie NJ, Crossno JT, Jr, Frid MG, Hofmeister SE, Reeves JT, Hyde DM, Carpenter TC, Brunetti JA, McNiece IK, Stenmark KR. Hypoxia-induced pulmonary artery adventitial remodeling and neovascularization: contribution of progenitor cells. Am J Physiol Lung Cell Mol Physiol 286: L668–L678, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, Coussens LM, Karin M, Goldrath AW, Johnson RS. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res 70: 7465–7475, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frid MG, Li M, Gnanasekharan M, Burke DL, Fragoso M, Strassheim D, Sylman JL, Stenmark KR. Sustained hypoxia leads to the emergence of cells with enhanced growth, migratory, and promitogenic potentials within the distal pulmonary artery wall. Am J Physiol Lung Cell Mol Physiol 297: L1059–L1072, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953–964, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Graham BB, Mentink-Kane MM, El-Haddad H, Purnell S, Zhang L, Zaiman A, Redente EF, Riches DW, Hassoun PM, Bandeira A, Champion HC, Butrous G, Wynn TA, Tuder RM. Schistosomiasis-induced experimental pulmonary hypertension: role of interleukin-13 signaling. Am J Pathol 177: 1549–1561, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 54: S10–S19, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, Cheever AW, Pearce EJ, Wynn TA. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J Immunol 167: 6533–6544, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 43: 13S, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, Yagita H, Takaoka A, Tahara H. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci USA 108: 12425–12430, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 450: 903–907, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Ma W, Dumont Y, Vercauteren F, Quirion R. Lipopolysaccharide induces calcitonin gene-related peptide in the RAW264.7 macrophage cell line. Immunology 130: 399–409, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol 22: 231–237, 2010 [DOI] [PubMed] [Google Scholar]
- 16. McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation 114: 1417–1431, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Mellor AL, Munn DH. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat Rev Immunol 8: 74–80, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Montani D, Perros F, Gambaryan N, Girerd B, Dorfmuller P, Price LC, Huertas A, Hammad H, Lambrecht B, Simonneau G, Launay JM, Cohen-Kaminsky S, Humbert M. C-kit-positive cells accumulate in remodeled vessels of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 184: 116–123, 2011 [DOI] [PubMed] [Google Scholar]
- 19. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8: 958–969, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11: 723–737, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nicolls MR, Taraseviciene-Stewart L, Rai PR, Badesch DB, Voelkel NF. Autoimmunity and pulmonary hypertension: a perspective. Eur Respir J 26: 1110, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Qualls JE, Murray PJ. Tumor macrophages protective and pathogenic roles in cancer development. Curr Top Dev Biol 94: 309–328, 2011 [DOI] [PubMed] [Google Scholar]
- 23. Rai PR, Cool CD, King JA, Stevens T, Burns N, Winn RA, Kasper M, Voelkel NF. The cancer paradigm of severe pulmonary arterial hypertension. Am J Respir Crit Care Med 178: 558, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rhodes CJ, Davidson A, Gibbs JS, Wharton J, Wilkins MR. Therapeutic targets in pulmonary arterial hypertension. Pharmacol Ther 121: 69–88, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Sakao S, Tatsumi K. Vascular remodeling in pulmonary arterial hypertension: Multiple cancer-like pathways and possible treatment modalities. Int J Cardiol, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med 8: 403–409, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Satoh K, Fukumoto Y, Nakano M, Sugimura K, Nawata J, Demachi J, Karibe A, Kagaya Y, Ishii N, Sugamura K, Shimokawa H. Statin ameliorates hypoxia-induced pulmonary hypertension associated with down-regulated stromal cell-derived factor-1. Cardiovasc Res 81: 226–234, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Sica A. Role of tumour-associated macrophages in cancer-related inflammation. Exp Oncol 32: 153–158, 2010 [PubMed] [Google Scholar]
- 29. Smithey BE, Pappo AS, Hill DA. C-kit expression in pediatric solid tumors: a comparative immunohistochemical study. Am J Surg Pathol 26: 486–492, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Stow JL, Low PC, Offenhauser C, Sangermani D. Cytokine secretion in macrophages and other cells: pathways and mediators. Immunobiology 214: 601–612, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukoc Biol 55: 410–422, 1994 [DOI] [PubMed] [Google Scholar]
- 32. Talati M, West J, Blackwell TR, Loyd JE, Meyrick B. BMPR2 mutation alters the lung macrophage endothelin-1 cascade in a mouse model and patients with heritable pulmonary artery hypertension. Am J Physiol Lung Cell Mol Physiol 299: L363–L373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tamosiuniene R, Tian W, Dhillon G, Wang L, Sung YK, Gera L, Patterson AJ, Agrawal R, Rabinovitch M, Ambler K, Long CS, Voelkel NF, Nicolls MR. Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension. Circ Res 109: 867–879, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc MG, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 15: 427, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Taraseviciene-Stewart L, Nicolls MR, Kraskauskas D, Scerbavicius R, Burns N, Cool C, Wood K, Parr JE, Boackle SA, Voelkel NF. Absence of T cells confers increased pulmonary arterial hypertension and vascular remodeling. Am J Respir Crit Care Med 175: 1280, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Taraseviciene-Stewart L, Scerbavicius R, Choe KH, Moore M, Sullivan A, Nicolls MR, Fontenot AP, Tuder RM, Voelkel NF. An animal model of autoimmune emphysema. Am J Respir Crit Care Med 171: 734, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Torisu H, Ono M, Kiryu H, Furue M, Ohmoto Y, Nakayama J, Nishioka Y, Sone S, Kuwano M. Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: possible involvement of TNFalpha and IL-1alpha. Int J Cancer 85: 182–188, 2000 [PubMed] [Google Scholar]
- 38. Tuder RM, Abman SH, Braun T, Capron F, Stevens T, Thistlethwaite PA, Haworth SG. Development and pathology of pulmonary hypertension. J Am Coll Cardiol 54: S3, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Tuder RM, Cool CD, Yeager M, Taraseviciene-Stewart L, Bull TM, Voelkel NF. The pathobiology of pulmonary hypertension. Endo Clin Chest Med 22: 405, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 144: 275, 1994 [PMC free article] [PubMed] [Google Scholar]
- 41. Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 114: 1883, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Yan D, Wang X, Li D, Qu Z, Ruan Q. Macrophages overexpressing VEGF, transdifferentiate into endothelial-like cells in vitro and in vivo. Biotechnol Lett 33: 1751–1758, 2011 [DOI] [PubMed] [Google Scholar]
- 43. Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J Clin Invest 118: 1991–2001, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]