Abstract
Exposure to chronic hypoxia (CH) causes pulmonary hypertension. The vasoconstrictor endothelin-1 (ET-1) is thought to play a role in the development of hypoxic pulmonary hypertension. In pulmonary arterial smooth muscle cells (PASMCs) from chronically hypoxic rats, ET-1 signaling is altered, with the ET-1-induced change in intracellular calcium concentration (Δ[Ca2+]i) occurring through activation of voltage-dependent Ca2+ channels (VDCC) even though ET-1-induced depolarization via inhibition of K+ channels is lost. The mechanism underlying this response is unclear. We hypothesized that activation of VDCCs by ET-1 following CH might be mediated by protein kinase C (PKC) and/or Rho kinase, both of which have been shown to phosphorylate and activate VDCCs. To test this hypothesis, we examined the effects of PKC and Rho kinase inhibitors on the ET-1-induced Δ[Ca2+]i in PASMCs from rats exposed to CH (10% O2, 3 wk) using the Ca2+-sensitive dye fura 2-AM and fluorescent microscopy techniques. We found that staurosporine and GF109203X, inhibitors of PKC, and Y-27632 and HA 1077, Rho kinase inhibitors, reduced the ET-1-induced Δ[Ca2+]i by >70%. Inhibition of tyrosine kinases (TKs) with genistein or tyrphostin A23, or combined inhibition of PKC, TKs, and Rho kinase, reduced the Δ[Ca2+]i to a similar extent as inhibition of either PKC or Rho kinase alone. The ability of PKC or Rho kinase to activate VDCCs in our cells was verified using phorbol 12-myristate 13-acetate and GTP-γ-S. These results suggest that following CH, the ET-1-induced Δ[Ca2+]i in PASMCs occurs via Ca2+ influx through VDCCs mediated primarily by PKC, TKs, and Rho kinase.
Keywords: vascular smooth muscle, protein kinase C, Rho kinase, intracellular calcium concentration, endothelin-1
prolonged exposure to hypoxic conditions, as can occur with many chronic lung diseases, leads to several changes in the pulmonary vasculature, including contraction and growth of pulmonary arterial smooth muscle cells (PASMCs). Both sustained active vasoconstriction and remodeling of the small pulmonary arteries during chronic hypoxia (CH) contribute to the development of pulmonary hypertension. In recent years, evidence demonstrating the ability of Rho kinase inhibitors to acutely normalize pulmonary arterial pressures in a variety of rodent models of pulmonary hypertension (37, 38) suggests that active contraction of the pulmonary vessels may play a greater role in maintaining elevated pulmonary arterial pressures than previously thought.
The mechanisms controlling sustained pulmonary vasoconstriction during CH are still incompletely understood. Much attention has been focused on the endothelial-derived vasoactive factor endothelin-1 (ET-1), a potent vasoconstrictor, as a mediator of hypoxia-induced pulmonary hypertension. Under normoxic conditions, low levels of ET-1 are synthesized primarily in endothelial cells and act locally on pulmonary arteries. With exposure to hypoxia, both ET-1 gene expression and protein secretion increase (15, 31). Moreover, in animal models of hypoxic pulmonary hypertension, ET-1 receptor antagonists prevented and, in some cases reversed, the elevation in pulmonary arterial pressure and vascular remodeling (5, 11, 16).
Work from our laboratory and others (2, 22, 43, 49–51, 58, 59, 71) has characterized the mechanisms by which ET-1 causes contraction in PASMCs under normoxic conditions. ET-1 has been shown to increase intracellular calcium concentration ([Ca2+]i) in PASMCs, causing Ca2+ release from intracellular stores and inhibiting K+ channels, which results in membrane depolarization, subsequent activation of voltage-dependent Ca2+ channels (VDCCs), and influx of extracellular Ca2+. However, we (55, 57) have also demonstrated that exposure to CH causes changes in ET-1 signaling mechanisms; in particular, ET-1 neither causes release from intracellular stores nor inhibits K+ channels but still increases [Ca2+]i in PASMCs from chronically hypoxic rats. The attenuated change in [Ca2+]i is entirely due to extracellular Ca2+ influx through L-type VDCCs; however, the activation of VDCCs under these conditions appears to occur independent of changes in membrane potential (Em) in chronically hypoxic PASMCs (55, 57). The mechanisms responsible for the Em-independent activation of VDCCs are unclear. Studies (9, 10, 40, 45, 61, 70) have shown that activation could be achieved through phosphorylation of the channel, possibly by Rho kinase and protein kinase C (PKC), both of which can be activated by ET-1.
Based on these considerations, in this study, we tested the hypothesis that activation of VDCCs by ET-1 in PASMCs from chronically hypoxic rats requires activation of PKC and/or Rho kinase. We used pharmacologic activators and inhibitors of PKC and Rho kinase to further explore the cellular pathway involved in activation of the VDCCs by ET-1 after exposure to CH.
METHODS
Hypoxic exposure.
All protocols were approved by the Johns Hopkins Animal Care and Use Committee. Adult male Wistar rats (250–350 g) were placed in a hypoxic chamber for 3 wk as previously described (55). The chamber was continuously flushed with a mixture of room air and 100% nitrogen to maintain oxygen at 10 ± 0.5% and carbon dioxide concentrations <0.5%. Oxygen levels inside the chamber were continuously monitored and a servo-controlled system used to inject nitrogen as needed (PRO-OX; RCI Hudson, Anaheim, CA). Animals were allowed free access to food and water and were removed from the chamber for <5 min twice a week to replenish food and water supplies and to clean the cages. Normoxic animals were kept in room air next to the hypoxic chamber.
Isolation of PASMCs.
The methods for obtaining primary cultures of rat PASMCs have been previously described (55). After anesthesia with sodium pentobarbital (130 mg/kg ip), the heart and lungs were removed en bloc. Intrapulmonary arteries (200–600 μm od) were dissected from the lung in ice cold HEPES-buffered saline solution (HBSS) containing the following (in mM): 130 NaCl, 5 KCl, 1.2 MgCl2, 10 HEPES, and 10 glucose with pH adjusted to 7.2 with 5 M NaOH. Extraneous connective tissue was removed, and the arteries were opened and the lumen gently scraped with a cotton-tipped swab to remove endothelial cells. The arteries were allowed to recover for 30 min in cold (4° C) HBSS, followed by a 20-min recovery in reduced-Ca2+ HBSS (20 μM CaCl2) at room temperature. The tissue was enzymatically digested for 20–25 min at 37°C in reduced-Ca2+ HBSS containing collagenase (type I; 1750 U/ml), papain (9.5 U/ml), bovine serum albumin (2 mg/ml), and dithiothreitol (1 mM). After digestion, single smooth muscle cells were dispersed by gentle trituration in Ca2+-free HBSS and were placed on 25-mm glass coverslips. PASMCs were cultured for 24–48 h in Ham's F-12 media supplemented with 0.5% fetal calf serum and 1% penicillin/streptomycin.
Measurement of [Ca2+]i.
[Ca2+]i was measured in rat PASMCs as previously described (69). Cells were incubated for 60 min at 37° C with 5 μM fura 2-AM, a membrane permanent (acetoxymethyl ester) form of fura 2, under an atmosphere of 16% O2-5% CO2. Afterincubation, cells were placed in a laminar flow cell chamber and perfused with modified Krebs solution containing the following (in mM):118.3 NaCl, 4.7 KCl, 1.2 MgSO4, 25 NaHCO3, 10 glucose, 1.2 KH2PO4, and 2.5 CaCl2, and gassed with 16% O2-5% CO2 to maintain a pH of 7.4. The cells were washed for 15 min at 37° C to remove extracellular dye and allow complete deesterification of cytosolic dye. Ratiometric measurement of fura 2 fluorescence was performed on a workstation consisting of a Nikon TES 100 Ellipse inverted microscope with epi-fluoresence attachments. PASMCs were exposed to the light beam from a xenon arc lamp (filtered at 340 and 380 nm) via a ×20 fluorescence objective (Super Flour 20; Nikon). Light emitted from the cell was returned through the objective and detected by a cooled charged-coupled device imaging camera. To minimize photobleaching, an electronic shutter was used to prevent excessive light exposure during the experiment. Protocols were executed and data collected online with InCyte software (Intracellular Imaging, Cincinnati, OH).
Measurement of Rho kinase activity.
PASMCs were lysed and sonicated in extraction buffer containing 50 mM Tris·HCl (pH 8.0), 0.1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 0.2 mM PMSF, 2 mM Na3VO4, 10 mM β-mercaptoethanol, and protease inhibitor cocktail (Complete tablets; Roche). Samples were centrifuged and the supernatants collected for use in the assay following the manufacturer's instructions (Rho-kinase assay kit; CycLex, Nagano, Japan). Ten microliters of supernatants were aliquoted per microtiter well along with 80–90 μl of kinase reaction buffer, containing 2.5 mM ATP, and incubated plate for 30 min at room temperature. Wells were washed five times with wash buffer before addition of 100 μl of horseradish peroxidase-conjugated detection antibody AF20 to each well and incubation for 60 min. Wells were washed an additional five times with wash buffer, and 100 μl of substrate reagent were added to each well and incubated for 1 h at room temperature before addition of 100 μl of stop solution. All reactions were performed in duplicate, and absorbance was measured at dual wavelengths (420/540 nm) using a microplate reader.
Drugs and solutions.
ET-1, BQ-123, and BQ-788 were obtained from American Peptides (Sunnyvale, CA). Nifedipine, Y-27632, and GF 102903X were obtained from Calbiochem (La Jolla, CA). All other reagents were obtained from Sigma-Aldrich (St. Louis, MO). BQ-123 (10 mM in deionized H2O), BQ-788 (10 mM in deionized H2O), and ET-1 (10−5 M in deionized H2O) were made up in a stock solution, divided into aliquots, and stored at −20°C until used. Stock solutions of Y-27632 (10 mM in deionized H2O), genistein (Gen; 100 mM in DMSO), phorbol 12-myristate 13-acetate (PMA; 0.1 mM in deionized H2O), and guanosine 5′-[γ-thio]triphosphate (GTP-γ-S; 10 mM in deionized H2O) were made, aliquotted, and stored at 0°C until used. HA-1077 (10 mM in deionized H2O), tyrphosin A23 (TA23; 100 mM in DMSO), GF 109203X (GFX; 10 mM in DMSO), and staurosporine (Stauro; 10 mM in DMSO) were made up in a stock solution and stored at 4°C. A stock solution of nifedipine (10 mM in ethanol) was made and used on the day of the experiment. All stock solutions were diluted to working concentrations in perfusate. To depolarize PASMCs, cells were exposed to a solution in which KCl was substituted for NaCl to increase the K+ concentration to 60 mM. The solution was made fresh daily and contained the following (in mM): 59 NaCl, 60 KCl, 1.2 MgSO4, 25 NaHCO3, 10 glucose, 1.2 KH2PO4, and 2.5 CaCl2.
Data analysis.
All values are expressed as means ± SE. In each experiment, data was collected from up to 30 cells, and the values were averaged to obtain a single value for each experiment. Cells isolated from different animals were used in each experiment; thus, n refers to both the number of experiments as well as number of animals from which cells were derived. Change in [Ca2+]i (Δ[Ca2+]i) was computed by subtracting the average basal [Ca2+]i, determined from 1 min of data collected immediately before beginning challenge, from the average of five data points at the peak of the response. For each agonist (KCl, ET-1, PMA, and GTP-γ-S), all data were compared against a single control group as a single analysis using a one-way ANOVA with a Dunnett's method post hoc test to determine differences between groups. In some cases, a one-sample t-test was used to determine whether the residual change in [Ca2+]i observed after treatment was statistically different from zero (i.e., value for the Δ[Ca2+]i is significantly different from the test value of 0) or if an inhibitor or activator caused a significant change in Rho kinase activity. A P value <0.05 was accepted as statistically significant.
RESULTS
Effect of ET-1 on [Ca2+]i.
ET-1 (10−8 M) caused a significant increase in [Ca2+]i in PASMCs isolated from chronically hypoxic rats (Fig. 1A). The change in [Ca2+]i (Δ[Ca2+]i; 368.2 ± 79.4 nM; n = 11 experiments on 186 cells) was similar in magnitude to the increase we previously observed in these cells (55). Removal of extracellular Ca2+ (Fig. 1B) or pretreatment with the VDCC inhibitor nifedipine (10 μM; Fig. 1C) for 5 min before challenge with ET-1 prevented the increase in [Ca2+]i. In cells perfused with Ca2+-free Krebs solution containing 1 mM EGTA, the Δ[Ca2+]i was reduced to 5.2 ± 5.6 nM (n = 5 experiments on 121 cells). In the presence of nifedipine, the ET-1-induced Δ[Ca2+]i was reduced to 23.3 ± 6.9 nM (n = 4 experiments on 75 cells). To determine which ET receptor subtype was responsible for the action of ET-1, we pretreated cells with BQ-123 (10 μM), a selective ETA receptor antagonist, or BQ-788 (10 μM), a selective ETB receptor antagonist. BQ-123 prevented the ET-1-induced increase in [Ca2+]i (Δ[Ca2+]i = 50.0 ± 10.4 nM ; n = 4 experiments on 79 cells; Fig. 2A). Pretreatment with BQ-788 also significantly attenuated the increase in [Ca2+]i in response to ET-1 (Δ[Ca2+]i = 81.2 ± 47.7 nM ; n = 4 experiments on 98 cells; Fig. 2B). Since BQ-788 at a concentration of 10 μM almost completely inhibited the ET-1-induced increase in [Ca2+]i, we tested whether the effect of BQ-788 could possibly be attributed to nonselective effects against the ETA receptor by using a lower (1 μM), more selective concentration of BQ-788. Even at the lower concentration of BQ-788, the ET-1-induced increase in [Ca2+]i was almost completely prevented (Δ[Ca2+]i = 41.9 ± 29.8 nM; n = 4 experiments on 76 cells; Fig. 2C).
Fig. 1.
Representative traces demonstrating the effect of endothelin-1 (ET-1) on intracellular calcium concentration ([Ca2+]i) in untreated cells (A) and after removal of extracellular Ca2+ (B) or treatment with nifedipine (C). D: bar graph representing means ± SE change in [Ca2+]i (Δ[Ca2+]i) in response to ET-1 under control conditions, after removal of extracellular Ca2+ (Ca2+-free) and in the presence of nifedipine. *Significant difference from control value.
Fig. 2.
Representative traces illustrating the effect of BQ-123 (A) and BQ-788 (B and C) on ET-1-induced Δ[Ca2+]i. D: bar graph showing means ± SE Δ[Ca2+]i in response to ET-1 in the absence (control) and presence of ET receptor inhibitors. *Significant difference from control value.
Effect of PKC inhibition on ET-1-induced Ca2+ responses.
The role of PKC in mediating the increase in [Ca2+]i induced by ET-1 was tested by pretreating cells for 5 min with Stauro (50 nM), a nonselective PKC inhibitor, before beginning the ET-1 challenge. Stauro markedly reduced the Δ[Ca2+]i induced by ET-1 to 43.7 ± 9.7 nM (n = 5 experiments on 109 cells; Fig. 3A). This concentration of Stauro was previously found to completely abolish ET-1-induced inhibition of KV channels (56, 58), and higher concentrations of Stauro produced no further reduction in the Δ[Ca2+]i induced by ET-1 (data not shown). Similarly, pretreatment with GFX (30 nM), a putative selective inhibitor PKC thought to be more potent against Ca2+-dependent (classical) PKC isoforms (35), also reduced the rise in [Ca2+]i in response to ET-1 to 76.5 ± 33.6 nM (n = 4 experiments on 61 cells; Fig. 3B).
Fig. 3.
A and B: representative traces showing effect of PKC inhibition on Δ[Ca2+]i induced by ET-1. C: bar graph shows means ± SE Δ[Ca2+]i in untreated cells (control) and cells treated with staurosporine (Stauro; 50 nM) or GF109203X (30 nM). *Significant difference from control value; †significant difference from zero.
Role of Rho kinase in ET-1-induced Ca2+ responses.
We next tested whether activation of Rho kinase was required for ET-1-induced Ca2+ mobilization by pretreating cells for 5 min with either Y-27632 (10 μM; Fig. 4A) or HA 1077 (10 μM; Fig. 4B), two structurally unrelated Rho kinase inhibitors. Both drugs markedly reduced the ET-1-induced Δ[Ca2+]i to a similar extent (Δ[Ca2+]i = 98.9 ± 27.3 nM; n = 6 experiments on 78 cells for Y-27632 and 94.1 ± 27.2; n = 4 experiments on 73 cells for HA 1077). Increasing the concentration of Y-27632 to 50 μM had no further inhibitory effect (data not shown).
Fig. 4.
Representative traces represent the ET-1-induced Δ[Ca2+]i in cells treated with the Rho kinase inhibitors Y-27632 (A; 10−5 M) and HA 1077 (B; 10−5 M) or with a combination of Stauro (50 nM) and Y-27632 (C). D: bar graph represents means ± SE Δ[Ca2+]i in the absence (control) and presence of inhibitors. *Significant difference from control value; †significant difference from zero.
Since a residual increase in [Ca2+]i was observed in the presence of either PKC or Rho kinase inhibitors, we tested whether these kinases might be acting along the same pathway or through additive pathways. Cells were pretreated with a combination of Stauro (50 nM) and Y-27632 (10 μM) before ET-1 challenge (Fig. 4C). In the presence of both inhibitors, a small increase in [Ca2+]i was still observed in response to ET-1 that was similar in magnitude to the change observed in the presence of either inhibitor alone (Δ[Ca2+]i = 77.9 ± 19.6 nM; n = 6 experiments on 75 cells). Similar results were observed in cells pretreated with GFX and Y-27632 (data not shown).
Effect of tyrosine kinase inhibition on ET-1-induced Ca2+ responses.
The results using simultaneous inhibition of PKC and Rho kinase suggested the involvement of another mechanism in the activation of VDCC by ET-1 in these cells. Since ET-1 has also been shown to activate tyrosine kinases, including the Src family of tyrosine kinases, we treated cells with Gen (100 μM; Fig. 5A), a relatively nonspecific tyrosine kinase inhibitor, and TA23 (100 μM; Fig. 5B), a more selective, structurally unrelated tyrosine kinase inhibitor. Both Gen and TA23 caused a similar reduction in the ET-1-induced increase in [Ca2+]i (Δ[Ca2+]i = 93.6 ± 35.7 nM; n = 10 experiments on 144 cells for Gen and 54.2 ± 17.7 nM; n = 5 experiments on 70 cells for TA23), suggesting that tyrosine kinase activation was involved in the ET-1-induced activation of VDCCs. To determine whether activation of tyrosine kinases could be responsible for the residual increase in [Ca2+]i observed during simultaneous inhibition of PKC and Rho kinase, we pretreated cells with a combination of Stauro, Y-27632, and Gen (Fig. 5C). Addition of Gen had no effect on the residual increase in [Ca2+]i (Δ[Ca2+]i = 119.8 ± 20.3 nM; n = 5 experiments on 66 cells).
Fig. 5.
Representative traces illustrating the effect of tyrosine kinase inhibition with genistein (Gen; A; 100 μM) and tyrphostin A23 (TA23; B; 100 μM) or combined blockade of PKC, Rho kinase, and tyrosine kinases (C) on ET-1-induced Δ[Ca2+]i. D: bar graph shows means ± SE Δ[Ca2+]i in control cells and cells treated with tyrosine kinase inhibitors and with a combination of Gen, Stauro (50 nM), and Y-27632 (10−5 M). *Significant difference from control value; †significant difference from zero.
Effect of kinase inhibitors on depolarization-driven VDCC activation.
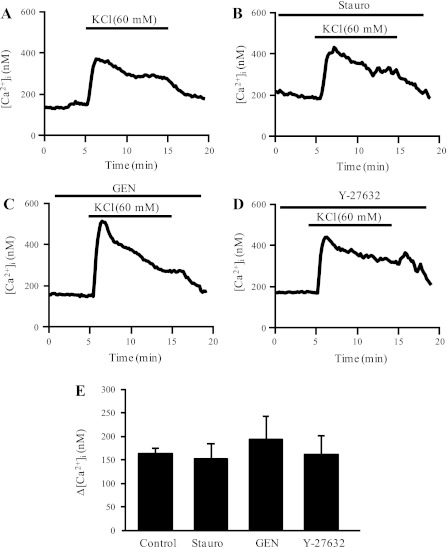
To test the specificity of kinase inhibitors for ET-1-induced activation of VDCC, we also examined whether these inhibitors altered the increase in [Ca2+]i induced by exposure to high external K+. During perfusion with extracellular solution in which NaCl was substituted with KCl to achieve an external [K+] of 60 mM (Fig. 6A), cells exhibited a rapid, reversible increase in [Ca2+]i (Δ[Ca2+]i = 163.6 ± 11.1; n = 5 experiments on 85 cells). Pretreatment with Stauro (10 nM), Y-27632 (10 μM), or Gen (100 μM) had no effect on the KCl-induced increase in [Ca2+]i (Fig. 6, B-D).
Fig. 6.
Representative traces illustrating the effect of KCl (60 mM) on [Ca2+]i in the absence (A) and presence of kinase inhibition with Stauro (B; 50 nM), Gen (C; 100 μM), and Y-27632 (D; 10−5 M). E: bar graph shows means ± SE Δ[Ca2+]i in control cells and cells treated with kinase inhibitors.
Ability of PKC to activate VDCC.
That inhibition of PKC caused a marked reduction in the nifedipine-sensitive increase in [Ca2+]i induced by ET-1 suggested that activation of PKC was responsible for opening VDCC. To confirm this possibility, we challenged cells with PMA (500 nM), a PKC activator (Fig. 7A). We found that PMA induced a rapid, reversible increase in [Ca2+]i that was qualitatively similar to that observed in response to ET-1 (Δ[Ca2+]i = 252.2 ± 73.7 nM; n = 8 experiments on 120 cells). The PMA-induced increase in [Ca2+]i was completely prevented in the presence of nifedipine (Δ[Ca2+]i = 20.6 ± 13.4 nM; n = 5 experiments on 98 cells; Fig. 7B), indicating that the response was due to activation of VDCC. As expected, the increase in [Ca2+]i was prevented by pretreatment with Stauro (Δ[Ca2+]i = −3.3 ± 7.2 nM; n = 3 experiments on 62 cells; Fig. 7C). Surprisingly, pretreatment with either Y-27632 (Fig. 7D) or Gen (Fig. 7E) also completely blocked the PMA-induced increase in [Ca2+]i (Δ[Ca2+]i = 1.6 ± 8.1 nM; n = 3 experiments on 49 cells for Y-27632 and 9.1 ± 2.2 nM; n = 3 experiments on 69 cells for Gen), suggesting that activation of these kinases may be downstream of PKC.
Fig. 7.
Representative traces illustrating the effect of activating PKC with PMA (500 nM) on [Ca2+]i in untreated cells (A) and in the presence of nifedipine (B; 10−5 M), Stauro (C; 50 nM), Y-27632 (D; 10−5 M), and Gen (E; 100 μM). F: bar graph shows means ± SE Δ[Ca2+]i in the absence (control) and presence of inhibitors. *Significant difference from control value.
Effect of GTP-γ-S on [Ca2+]i.
To determine whether activation of Rho kinase could play a role in the ET-1-induced Ca2+ influx through VDCC, cells were treated with GTP-γ-S, a nonhydrolyzable form of GTP (Fig. 8A). GTP-γ-S caused an increase in [Ca2+]i (Δ[Ca2+]i = 335.8 ± 153.7 nM; n = 7 experiments in 115 cells) that was similar in magnitude to the increase observed with either ET-1 or PMA. Pretreatment with either nifedipine (Fig. 8B) or Y-27632 (Fig. 8C) prevented the change in [Ca2+]i induced by GTP-γ-S (Δ[Ca2+]i = 3.2 ± 14.2 nM; n = 3 experiments on 56 cells for nifedipine and 29.6 ± 12.1 nM; n = 5 experiments on 61 cells for Y-27632). In cells pretreated with Stauro (Fig. 8D), the GTP-γ-S -induced Δ[Ca2+]i was significantly reduced to 71.9 ± 23.8 nM (n = 5 experiments on 96 cells) but was not eliminated. Inhibition of tyrosine kinases with Gen reduced, but did not prevent, the increase in [Ca2+]i in response to GTP-γ-S (Δ[Ca2+]i = 83.9 ± 27.7 nM; n = 8 experiments on 152 cells; Fig. 8E).
Fig. 8.
Representative traces illustrating the effect of GTP-γ-S on [Ca2+]i in untreated cells (A) and in the presence of nifedipine (B; 10−5 M), Y-27632 (C; 10−5 M), Stauro (D; 50 nM), and Gen (E; 100 μM). F: bar graph shows means ± SE Δ[Ca2+]i in the absence (control) and presence of inhibitors. *Significant difference from control value; †significant difference from zero.
Effect of PKC activation on Rho kinase activity.
That Y-27632 could prevent the effects of PMA on [Ca2+]i suggested that PKC activation could lead to activation of Rho kinase. To test this possibility, we measured the effect of PMA on Rho kinase activity. Addition of PMA (500 nM) to PASMC extracts from chronically hypoxic rats caused a doubling of Rho kinase activity (Fig. 9). Pretreatment with Y-27632 (10 μM), but not Stauro (10 nM) or Gen (100 μM), reduced basal Rho kinase activity, while all three inhibitors prevented the increase in Rho kinase activity observed in response to PMA.
Fig. 9.
Effect of PKC activation on Rho kinase activity. Bar graph shows means ± SE values for Rho kinase activity expressed as percentage of control. Basal measurements were compared in cells treated with Y-27632 (10 μM), Stauro (30 nM), and Gen (100 μM). Effect of PMA (500 nM) was measured in control cells, as well as in cells pretreated with Y-27632, Stauro, or Gen; n = 3 for each experiment. *Significant difference from control value; †significant difference from PMA.
DISCUSSION
Contraction of pulmonary vascular smooth muscle from chronically hypoxic rats in response to ET-1 can be separated into two components: a Ca2+-independent component that is mediated by an increase in Ca2+-sensitivity of the contractile apparatus and a Ca2+-dependent component that can be blocked by inhibitors of L-type Ca2+ channels (67). We (55) have previously determined that the increase in [Ca2+]i induced by ET-1 in PASMCs from chronically hypoxic animals was due to activation of L-type Ca2+ channels but did not require depolarization. In the current study, we found that VDCC activity can be modulated by PKC, Rho kinase, and tyrosine kinases and that all of these kinases appear to be involved in mediating the activation of VDCC by ET-1 in PASMCs from chronically hypoxic animals.
During normoxia, ET-1-induced Ca2+ mobilization requires release and influx from several sources. ET-1 causes depolarization of the resting membrane potential, leading to influx through VDCCs (50, 51, 58). In addition, Ca2+ is released from intracellular stores via both ryanodine and inositol triphosphate receptors (47, 59, 63, 71). ET-1 also causes influx through nonselective cation channels, either secondary to receptor activation (receptor-operated Ca2+ channels) or Ca2+ release (store-operated Ca2+ channels; Ref. 30). In PASMCs from main pulmonary arteries, CH has no effect on ET-1-induced Ca2+-signaling, which appears to occur primarily via Ca2+ release (6). In contrast, following exposure to CH, ET-1-induced Ca2+ signaling in PASMCs isolated from more distal pulmonary arteries is markedly altered with the absence of Ca2+ release (55). The peak increase in [Ca2+]i induced by ET-1 in PASMCs from chronically hypoxic rats measured in the current study was on the same order as, but slightly higher than, that reported previously, likely resulting from differences in the methods used to measure [Ca2+]i (i.e., different indicator, differences in calibration, one cell/experiment vs. 10–30 cells/experiment, etc.). Nonetheless, consistent with our previously reported results, in PASMCs isolated from intralobar pulmonary arteries obtained from chronically hypoxic animals, the ET-1-induced increase in [Ca2+]i was eliminated in the absence of external Ca2+ and nearly abolished in the presence of VDCC inhibitors, indicating the response is due primarily to Ca2+ influx through VDCCs.
PASMCs express both ETA and ETB receptors, although many studies find that these receptors mediate separate effects (17, 30, 52, 63, 68). While the fact that blockade of ETA receptors almost completely blocked the ET-1-induced increase in [Ca2+]i was not unexpected, the fact that BQ-788 was nearly as effective in reducing the effect of ET-1 was somewhat surprising. The level of inhibition by either receptor antagonist was >75%, suggesting that activation of both receptors is required for the response. We have observed a similar ability of either receptor to mediate ET-1-induced downregulation of KV channel expression (69) and certain other effects of ET-1 also appear to be ablated by inhibiting either receptor (28, 54, 66). The antagonists, at the concentrations used in this study, have been shown to be receptor subtype selective (23, 24); therefore, it is unlikely, although not impossible, that our results are due to some cross-reactivity of the inhibitors. Another possibility could be that the ETA and ETB receptors are coupled to separate pathways, both of which are required for the response. Further experiments will be required to determine whether this is indeed the case.
Under basal normoxic conditions, the negative resting Em renders most PASMC VDCCs inactive, resulting in low [Ca2+]i. As the name implies, VDCCs are activated by depolarization, resulting in rapid Ca2+ influx. While this is the most commonly described mode of activation, voltage-independent activation of VDCCs has also been demonstrated under a variety of conditions. That the PKC-dependent inhibition of voltage-gated K+ channels, and resulting depolarization, were absent in PASMCs from chronically hypoxic animals (57) while Ca2+ influx through VDCCs was still evident (55) suggested voltage-independent modulation of VDCCs was occurring in these cells. Precedent for voltage-independent activation of VDCCs is abundant in studies conducted in cardiomyocytes and smooth muscle from systemic vascular beds (9, 13, 18–20, 29, 32, 61) and appears to be mediated primarily by phosphorylation of VDCCs, leading to changes in channel activity independent of Em. Rho kinase (70), PKC (9, 13, 20, 40, 61), and/or tyrosine kinases, including Src (14, 27, 32, 41), have been shown to activate VDCCs, although PKC- and tyrosine kinase-dependent inhibition of channel activity has also been reported (53, 65). We found that inhibition of each of these kinases could inhibit the ET-1-induced change in [Ca2+]i, suggesting that all were involved in the response. To minimize the possibility that these effects were due to nonspecific actions, we inhibited each kinase using at least two structurally unrelated compounds at concentrations shown previously to have the desired effects. Furthermore, we showed that these kinase inhibitors had no effect on the Ca2+ influx via VDCCs induced by depolarization with KCl, ruling out nonspecific effects of these inhibitors on the channels. Although others (12) have found that inhibition of Rho kinase did not impact the Ca2+ response to ET-1 in PASMCs, there are several differences that could explain this discrepancy. For example, the previous study used human PASMCs derived from proximal pulmonary arteries, rather than the rat distal arteries used in our study and the human PASMCs were from normal subjects and not exposed to CH. Whether the different role of Rho kinase in mediating ET-1-induced Ca2+ responses observed in our study is due to species, location in the vascular tree, or the effect of hypoxia remains to be determined.
After verifying that the kinase inhibitors blocked the ET-1-induced activation of VDCCs, we next used activators of the kinases to 1) demonstrate that these kinases are indeed capable of modulating VDCC activity, and 2) verify the effectiveness of our inhibitors. PMA, a phorbol ester that is a potent PKC activator, increased [Ca2+]i via activation of VDCCs in a manner qualitatively and quantitatively similar to ET-1. Surprisingly, inhibition of either Rho kinase or tyrosine kinases prevented the PMA-induced increase in [Ca2+]i, suggesting that PKC activation leads to activation of Rho kinase. Consistent with these results, PMA was able to increase Rho kinase activity in PASMCs from chronically hypoxic animals, which could be attenuated by blockade of PKC or tyrosine kinases. On the other hand, unlike the Rho kinase inhibitor, these inhibitors had no effect on basal Rho kinase activity, indicating that there was little contribution of PKC to Rho kinase activation at baseline.
GTP-γ-S, a Rho kinase activator, also induced Ca2+ influx via VDCC. Interestingly, inhibition of either PKC or tyrosine kinases was also able to cause a significant inhibition of, but not entirely eliminate, the GTP-γ-S response. Since G-protein activation can stimulate PLC, it would not be surprising that GTP-γ-S activated PKC in our cells. Indeed, in certain instances, GTP-γ-S has been found to activate PKC in other cell types (1, 33, 34, 46, 48).
Several studies indicate that there are interactions between Rho kinase and PKC, although the nature of the interaction remains in debate and may be cell type or agonist specific. Although Rho may be upstream of PKC activation in endothelial cells (21), in smooth muscle PKC has been shown to be upstream of Rho kinase activation (7, 26, 60). These findings were confirmed by in vitro kinase assays, which revealed that PKC is required for Rho activity (62). Similar patterns of PKC-dependent Rho/Rho kinase activation were observed in neuroblastoma cells (4, 64), endothelial cells (3, 36), cardiomyocytes (42), and neurons (44). In addition, tyrosine kinase activity, which may also be downstream of PKC activation (8), has been shown to be required for translocation of Rho kinase to the membrane and Rho kinase activity (39). Consistent with these other studies, our results indicated that while basal Rho kinase activity is not influenced by PKC or tyrosine kinase inhibition, activation of PKC resulted in increased Rho kinase activity that could be reduced by inhibition of either PKC or tyrosine kinase.
Even in the presence of combined PKC, Rho kinase, and tyrosine kinase inhibitors, a small residual rise in [Ca2+]i was observed in response to ET-1 challenge. This was clearly due to Ca2+ influx, since no change in [Ca2+]i was observed in the absence of extracellular Ca2+. A small increase in [Ca2+]i was also observed in the presence of nifedipine. This suggests either the residual response occurs via influx through other channels (i.e., receptor-operated Ca2+ channels) or that the concentrations of the inhibitors were not maximal. However, since the same concentrations of nifedipine was sufficient to completely block Ca2+ influx induced by both PMA and GTP-γ-S, we speculate that the former possibility is likely to be true, although the exact mechanism responsible remains to be determined.
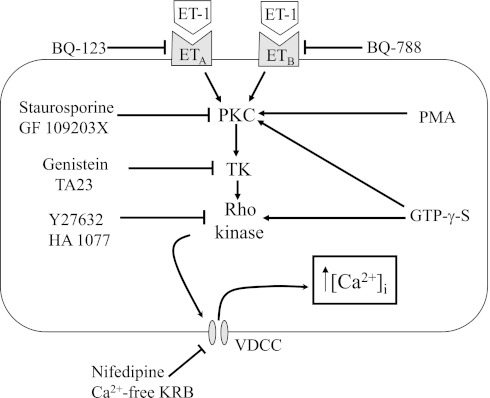
In summary, our data suggest that initiation of Ca2+ influx through VDCCs by ET-1 in PASMCs from chronically hypoxic rats requires activation of PKC, Rho kinase and tyrosine kinases (Fig. 10). Together with our previous work (67) and work from other laboratories (25), our results indicate that full contraction in response to ET-1 in pulmonary arteries following exposure to CH requires kinase mediated-changes in both Ca2+ influx and Ca2+ sensitivity of the contractile apparatus. Given that ET-1 levels are markedly increased during CH and pulmonary arteries from chronically hypoxic animals exhibit enhanced vasoreactivity to ET-1, it is tempting to speculate that the ability of Rho kinase inhibitors to normalize pulmonary arterial pressure in CH rats may be due, at least in part, to alleviation of ET-1-induced contraction.
Fig. 10.
Schematic demonstrating proposed mechanism of ET-1-induced Ca2+ influx in chronically hypoxic pulmonary arterial smooth muscle cells. ET-1 binds to either ETA or ETB receptors, leading to activation of protein kinase C (PKC), with resulting activation of tyrosine kinases (TK) and Rho kinase. Activation of Rho kinase then causes activation of voltage-dependent Ca2+ channels (VDCC) and an increase in intracellular calcium concentration ([Ca2+]i). PMA, phorbol 12-myristate 13-acetate; GTP-γ-S, guanosine 5′-[γ-thio]triphosphate; TA23, tyrphosin A23; KRB, Krebs solution.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-67191, HL-75113, and HL-07963.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.L., J.T.S., and L.A.S. conception and design of research; T.L., J.M., and C.U. performed experiments; T.L., J.M., C.U., and L.A.S. analyzed data; T.L., J.T.S., and L.A.S. interpreted results of experiments; T.L. and L.A.S. prepared figures; T.L., J.M., and L.A.S. drafted manuscript; T.L., J.T.S., and L.A.S. edited and revised manuscript; T.L., J.M., C.U., J.T.S., and L.A.S. approved final version of manuscript.
REFERENCES
- 1. Baker MD. Protein kinase C mediates up-regulation of tetrodotoxin-resistant, persistent Na+ current in rat and mouse sensory neurones. J Physiol 567: 851–867, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bakhramov A, Hartley SA, Salter KJ, Kozlowski RZ. Contractile agonists preferentially activate Cl− over K+ currents in arterial myocytes. Biochem Biophys Res Commun 227: 168–175, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Barandier C, Ming XF, Rusconi S, Yang Z. PKC is required for activation of ROCK by RhoA in human endothelial cells. Biochem Biophys Res Commun 304: 714–719, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Bellon A, Ortiz-Lopez L, Ramirez-Rodriguez G, Anton-Tay F, Benitez-King G. Melatonin induces neuritogenesis at early stages in N1E-115 cells through actin rearrangements via activation of protein kinase C and Rho-associated kinase. J Pineal Res 42: 214–221, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Bialecki RA, Fisher CS, Abbott BM, Barthlow HG, Caccese RG, Stow RB, Rumsey J, Rumsey W. ZD1611, an orally active endothelin-A receptor antagonist, prevents chronic hypoxia-induced pulmonary hypertension in the rat. Pulm Pharmacol Ther 12: 303–312, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Bonnet S, Belus A, Hyvelin JM, Roux E, Marthan R, Savineau JP. Effect of chronic hypoxia on agonist-induced tone and calcium signaling in rat pulmonary artery. Am J Physiol Lung Cell Mol Physiol 281: L193–L201, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Brandt D, Gimona M, Hillmann M, Haller H, Mischak H. Protein kinase C induces actin reorganization via a Src- and Rho-dependent pathway. J Biol Chem 277: 20903–20910, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Brandt DT, Goerke A, Heuer M, Gimona M, Leitges M, Kremmer E, Lammers R, Haller H, Mischak H. Protein kinase C delta induces Src kinase activity via activation of the protein tyrosine phosphatase PTP alpha. J Biol Chem 278: 34073–34078, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Callaghan B, Zhong J, Keef KD. Signaling pathway underlying stimulation of L-type Ca2+ channels in rabbit portal vein myocytes by recombinant Gβγ subunits. Am J Physiol Heart Circ Physiol 291: H2541–H2546, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol 16: 521–555, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Chen SJ, Chen YF, Opgenorth TJ, Wessale JL, Meng QC, Durand J, DiCarlo VS, Oparil S. The orally active nonpeptide endothelin A-receptor antagonist A-127722 prevents and reverses hypoxia-induced pulmonary hypertension and pulmonary vascular remodeling in Sprague-Dawley rats. J Cardiovasc Pharmacol 29: 713–725, 1997 [DOI] [PubMed] [Google Scholar]
- 12. de Frutos S, Diaz JM, Nitta CH, Sherpa ML, Bosc LV. Endothelin-1 contributes to increased NFATc3 activation by chronic hypoxia in pulmonary arteries. Am J Physiol Cell Physiol 301: C441–C450, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dosemeci A, Dhallan RS, Cohen NM, Lederer WJ, Rogers TB. Phorbol ester increases calcium current and simulates the effects of angiotensin II on cultured neonatal rat heart myocytes. Circ Res 62: 347–357, 1988 [DOI] [PubMed] [Google Scholar]
- 14. Dubuis E, Rockliffe N, Hussain M, Boyett M, Wray D, Gawler D. Evidence for multiple Src binding sites on the alpha1c L-type Ca2+ channel and their roles in activity regulation. Cardiovasc Res 69: 391–401, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Earley S, Nelin LD, Chicoine LG, Walker BR. Hypoxia-induced pulmonary endothelin-1 expression is unaltered by nitric oxide. J Appl Physiol 92: 1152–1158, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Eddahibi S, Raffestin B, Clozel M, Levame M, Adnot S. Protection from pulmonary hypertension with an orally active endothelin receptor antagonist in hypoxic rats. Am J Physiol Heart Circ Physiol 268: H828–H835, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Evans AM, Cobban HJ, Nixon GF. ET(A) receptors are the primary mediators of myofilament calcium sensitization induced by ET-1 in rat pulmonary artery smooth muscle: a tyrosine kinase independent pathway. Br J Pharmacol 127: 153–160, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fish RD, Sperti G, Colucci WS, Clapham DE. Phorbol ester increases the dihydropyridine-sensitive calcium conductance in a vascular smooth muscle cell line. Circ Res 62: 1049–1054, 1988 [DOI] [PubMed] [Google Scholar]
- 19. Goto K, Kasuya Y, Matsuki N, Takuwa Y, Kurihara H, Ishikawa T, Kimura S, Yanagisawa M, Masaki T. Endothelin activates the dihydropyridine-sensitive, voltage-dependent Ca2+ channel in vascular smooth muscle. Proc Natl Acad Sci USA 86: 3915–3918, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. He JQ, Pi Y, Walker JW, Kamp TJ. Endothelin-1 and photoreleased diacylglycerol increase L-type Ca2+ current by activation of protein kinase C in rat ventricular myocytes. J Physiol 524: 807–820, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hippenstiel S, Kratz T, Krull M, Seybold J, von Eichel-Streiber C, Suttorp N. Rho protein inhibition blocks protein kinase C translocation and activation. Biochem Biophys Res Commun 245: 830–834, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Hyvelin JM, Guibert C, Marthan R, Savineau JP. Cellular mechanisms and role of endothelin-1-induced calcium oscillations in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 275: L269–L282, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Ihara M, Ishikawa K, Fukuroda T, Saeki T, Funabashi K, Fukami T, Suda H, Yano M. In vitro biological profile of a highly potent novel endothelin (ET) antagonist BQ-123 selective for the ETA receptor. J Cardiovasc Pharmacol 20, Suppl 12: S11–14, 1992 [DOI] [PubMed] [Google Scholar]
- 24. Ishikawa K, Ihara M, Noguchi K, Mase T, Mino N, Saeki T, Fukuroda T, Fukami T, Ozaki S, Nagase T, Nishikibe M, Yano M. Biochemical and pharmacological profile of a potent and selective endothelin B-receptor antagonist, BQ-788. Proc Natl Acad Sci USA 91: 4892–4896, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jernigan NL, Walker BR, Resta TC. Reactive oxygen species mediate RhoA/Rho kinase-induced Ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 295: L515–L529, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kandabashi T, Shimokawa H, Miyata K, Kunihiro I, Eto Y, Morishige K, Matsumoto Y, Obara K, Nakayama K, Takahashi S, Takeshita A. Evidence for protein kinase C-mediated activation of Rho-kinase in a porcine model of coronary artery spasm. Arterioscler Thromb Vasc Biol 23: 2209–2214, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Kang M, Ross GR, Akbarali HI. COOH-terminal association of human smooth muscle calcium channel Cav1.2b with c-Src kinase protein binding domains: effect of nitrotyrosylation. Am J Physiol Cell Physiol 293: C1983–C1990, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Khimenko PL, Moore TM, Taylor AE. Blocked ETA receptors prevent ischemia and reperfusion injury in rat lungs. J Appl Physiol 80: 203–207, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Le Blanc C, Mironneau C, Barbot C, Henaff M, Bondeva T, Wetzker R, Macrez N. Regulation of vascular L-type Ca2+ channels by phosphatidylinositol 3,4,5-trisphosphate. Circ Res 95: 300–307, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Lee HA, Baek EB, Park KS, Jung HJ, Kim JI, Kim SJ, Earm YE. Mechanosensitive nonselective cation channel facilitation by endothelin-1 is regulated by protein kinase C in arterial myocytes. Cardiovasc Res 76: 224–235, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Li H, Elton TS, Chen YF, Oparil S. Increased endothelin receptor gene expression in hypoxic rat lung. Am J Physiol Lung Cell Mol Physiol 266: L553–L560, 1994 [DOI] [PubMed] [Google Scholar]
- 32. Liu H, Sperelakis N. Tyrosine kinases modulate the activity of single L-type calcium channels in vascular smooth muscle cells from rat portal vein. Can J Physiol Pharmacol 75: 1063–1068, 1997 [PubMed] [Google Scholar]
- 33. Lowe GM, Slupsky JR, Galvani DW, Edwards SW. GTPgammaS-stimulated phospholipase D activation in human neutrophils occurs by protein kinase C-dependent and -independent pathways but not tyrosine kinases. Biochem Biophys Res Commun 220: 484–490, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Mansfield PJ, Carey SS, Hinkovska-Galcheva V, Shayman JA, Boxer LA. Ceramide inhibition of phospholipase D and its relationship to RhoA and ARF1 translocation in GTP gamma S-stimulated polymorphonuclear leukocytes. Blood 103: 2363–2368, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem 268: 9194–9197, 1993 [PubMed] [Google Scholar]
- 36. Mehta D, Rahman A, Malik AB. Protein kinase C-alpha signals rho-guanine nucleotide dissociation inhibitor phosphorylation and rho activation and regulates the endothelial cell barrier function. J Biol Chem 276: 22614–22620, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Nagaoka T, Fagan KA, Gebb SA, Morris KG, Suzuki T, Shimokawa H, McMurtry IF, Oka M. Inhaled Rho kinase inhibitors are potent and selective vasodilators in rat pulmonary hypertension. Am J Respir Crit Care Med 171: 494–499, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Nagaoka T, Morio Y, Casanova N, Bauer N, Gebb S, McMurtry I, Oka M. Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 287: L665–L672, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Nakao F, Kobayashi S, Mogami K, Mizukami Y, Shirao S, Miwa S, Todoroki-Ikeda N, Ito M, Matsuzaki M. Involvement of Src family protein tyrosine kinases in Ca2+ sensitization of coronary artery contraction mediated by a sphingosylphosphorylcholine-Rho-kinase pathway. Circ Res 91: 953–960, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Obejero-Paz CA, Auslender M, Scarpa A. PKC activity modulates availability and long openings of L-type Ca2+ channels in A7r5 cells. Am J Physiol Cell Physiol 275: C535–C543, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Ogura T, Shuba LM, McDonald TF. L-type Ca2+ current in guinea pig ventricular myocytes treated with modulators of tyrosine phosphorylation. Am J Physiol Heart Circ Physiol 276: H1724–H1733, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Pan J, Singh US, Takahashi T, Oka Y, Palm-Leis A, Herbelin BS, Baker KM. PKC mediates cyclic stretch-induced cardiac hypertrophy through Rho family GTPases and mitogen-activated protein kinases in cardiomyocytes. J Cell Physiol 202: 536–553, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Peng W, Michael JR, Hoidal JR, Karwande SV, Farrukh IS. ET-1 modulates KCa-channel activity and arterial tension in normoxic and hypoxic human pulmonary vasculature. Am J Physiol Lung Cell Mol Physiol 275: L729–L739, 1998 [DOI] [PubMed] [Google Scholar]
- 44. Pilpel Y, Segal M. Activation of PKC induces rapid morphological plasticity in dendrites of hippocampal neurons via Rac and Rho-dependent mechanisms. Eur J Neurosci 19: 3151–3164, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Puri TS, Gerhardstein BL, Zhao XL, Ladner MB, Hosey MM. Differential effects of subunit interactions on protein kinase A- and C-mediated phosphorylation of L-type calcium channels. Biochemistry 36: 9605–9615, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Regazzi R, Li G, Ullrich S, Jaggi C, Wollheim CB. Different requirements for protein kinase C activation and Ca2+-independent insulin secretion in response to guanine nucleotides. Endogenously generated diacylglycerol requires elevated Ca2+ for kinase C insertion into membranes. J Biol Chem 264: 9939–9944, 1989 [PubMed] [Google Scholar]
- 47. Remillard CV, Zhang WM, Shimoda LA, Sham JS. Physiological properties and functions of Ca2+ sparks in rat intrapulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 283: L433–L444, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Saab CY, Cummins TR, Waxman SG. GTP gamma S increases Nav1.8 current in small-diameter dorsal root ganglia neurons. Exp Brain Res 152: 415–419, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Salter KJ, Kozlowski RZ. Differential electrophysiological actions of endothelin-1 on Cl− and K+ currents in myocytes isolated from aorta, basilar and pulmonary artery. J Pharmacol Exp Ther 284: 1122–1131, 1998 [PubMed] [Google Scholar]
- 50. Salter KJ, Kozlowski RZ. Endothelin receptor coupling to potassium and chloride channels in isolated rat pulmonary arterial myocytes. J Pharmacol Exp Ther 279: 1053–1062, 1996 [PubMed] [Google Scholar]
- 51. Salter KJ, Turner JL, Albarwani S, Clapp LH, Kozlowski RZ. Ca2+-activated Cl− and K+ channels and their modulation by endothelin-1 in rat pulmonary arterial smooth muscle cells. Exp Physiol 80: 815–824, 1995 [DOI] [PubMed] [Google Scholar]
- 52. Schmeck J, Gluth H, Mihaljevic N, Born M, Wendel-Wellner M, Krafft P. ET-1-induced pulmonary vasoconstriction shifts from ETA- to ETB-receptor-mediated reaction after preconstriction. J Appl Physiol 87: 2284–2289, 1999 [DOI] [PubMed] [Google Scholar]
- 53. Schroder F, Klein G, Frank T, Bastein M, Indris S, Karck M, Drexler H, Wollert KC. Src family tyrosine kinases inhibit single L-type: Ca2+ channel activity in human atrial myocytes. J Mol Cell Cardiol 37: 735–745, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Shi-Wen X, Rodriguez-Pascual F, Lamas S, Holmes A, Howat S, Pearson JD, Dashwood MR, du Bois RM, Denton CP, Black CM, Abraham DJ, Leask A. Constitutive ALK5-independent c-Jun N-terminal kinase activation contributes to endothelin-1 overexpression in pulmonary fibrosis: evidence of an autocrine endothelin loop operating through the endothelin A and B receptors. Mol Cell Biol 26: 5518–5527, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shimoda LA, Sham JS, Shimoda TH, Sylvester JT. L-type Ca2+ channels, resting [Ca2+]i, and ET-1-induced responses in chronically hypoxic pulmonary myocytes. Am J Physiol Lung Cell Mol Physiol 279: L884–L894, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Shimoda LA, Sylvester JT, Booth GM, Shimoda TH, Meeker S, Undem BJ, Sham JS. Inhibition of voltage-gated K+ currents by endothelin-1 in human pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 281: L1115–L1122, 2001 [DOI] [PubMed] [Google Scholar]
- 57. Shimoda LA, Sylvester JT, Sham JS. Chronic hypoxia alters effects of endothelin and angiotensin on K+ currents in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 277: L431–L439, 1999 [DOI] [PubMed] [Google Scholar]
- 58. Shimoda LA, Sylvester JT, Sham JS. Inhibition of voltage-gated K+ current in rat intrapulmonary arterial myocytes by endothelin-1. Am J Physiol Lung Cell Mol Physiol 274: L842–L853, 1998 [DOI] [PubMed] [Google Scholar]
- 59. Shimoda LA, Sylvester JT, Sham JS. Mobilization of intracellular Ca2+ by endothelin-1 in rat intrapulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 278: L157–L164, 2000 [DOI] [PubMed] [Google Scholar]
- 60. Shimomura E, Shiraishi M, Iwanaga T, Seto M, Sasaki Y, Ikeda M, Ito K. Inhibition of protein kinase C-mediated contraction by Rho kinase inhibitor fasudil in rabbit aorta. Naunyn Schmiedebergs Arch Pharmacol 370: 414–422, 2004 [DOI] [PubMed] [Google Scholar]
- 61. Shistik E, Ivanina T, Blumenstein Y, Dascal N. Crucial role of N terminus in function of cardiac L-type Ca2+ channel and its modulation by protein kinase C. J Biol Chem 273: 17901–17909, 1998 [DOI] [PubMed] [Google Scholar]
- 62. Slater SJ, Seiz JL, Stagliano BA, Stubbs CD. Interaction of protein kinase C isozymes with Rho GTPases. Biochemistry 40: 4437–4445, 2001 [DOI] [PubMed] [Google Scholar]
- 63. Sudjarwo SA, Hori M, Tanaka T, Matsuda Y, Karaki H. Coupling of the endothelin ETA and ETB receptors to Ca2+ mobilization and Ca2+ sensitization in vascular smooth muscle. Eur J Pharmacol 289: 197–204, 1995 [DOI] [PubMed] [Google Scholar]
- 64. Tanabe A, Kamisuki Y, Hidaka H, Suzuki M, Negishi M, Takuwa Y. PKC phosphorylates MARCKS Ser159 not only directly but also through RhoA/ROCK. Biochem Biophys Res Commun 345: 156–161, 2006 [DOI] [PubMed] [Google Scholar]
- 65. Toselli M, Taglietti V. L-type calcium channel gating is modulated by bradykinin with a PKC-dependent mechanism in NG108–15 cells. Eur Biophys J 34: 217–229, 2005 [DOI] [PubMed] [Google Scholar]
- 66. Uhlig S, von Bethmann AN, Featherstone RL, Wendel A. Pharmacologic characterization of endothelin receptor responses in the isolated perfused rat lung. Am J Respir Crit Care Med 152: 1449–1460, 1995 [DOI] [PubMed] [Google Scholar]
- 67. Weigand LA, Sylvester JT, Shimoda LA. Mechanisms of endothelin-1-induced contraction in pulmonary arteries from chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 290: L284–L290, 2006 [DOI] [PubMed] [Google Scholar]
- 68. White DG, Cannon TR, Garratt H, Mundin JW, Sumner MJ, Watts IS. Endothelin ETA and ETB receptors mediate vascular smooth-muscle contraction. J Cardiovasc Pharmacol 22, Suppl 8: S144–148, 1993 [DOI] [PubMed] [Google Scholar]
- 69. Whitman EM, Pisarcik S, Luke T, Fallon M, Wang J, Sylvester JT, Semenza GL, Shimoda LA. Endothelin-1 mediates hypoxia-induced inhibition of voltage-gated K+ channel expression in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 294: L309–L318, 2008 [DOI] [PubMed] [Google Scholar]
- 70. Yatani A, Irie K, Otani T, Abdellatif M, Wei L. RhoA GTPase regulates L-type Ca2+ currents in cardiac myocytes. Am J Physiol Heart Circ Physiol 288: H650–H659, 2005 [DOI] [PubMed] [Google Scholar]
- 71. Zhang WM, Yip KP, Lin MJ, Shimoda LA, Li WH, Sham JS. ET-1 activates Ca2+ sparks in PASMC: local Ca2+ signaling between inositol trisphosphate and ryanodine receptors. Am J Physiol Lung Cell Mol Physiol 285: L680–L690, 2003 [DOI] [PubMed] [Google Scholar]