Abstract
Background:
The Gruppo Oncologico Italia Meridionale 9902 trial compared four cycles of high-dose epirubicin plus cyclophosphamide (EC) with four cycles of docetaxel (Taxotere, D) followed by four cycles of EC as adjuvant treatment of node-positive breast cancer.
Patients and methods:
Patients were randomly assigned to EC (E 120 mg/m2, C 600 mg/m2, arm A) for four cycles or four cycles of D (100 mg/m2) followed by four cycles of EC (arm B), both regimens every 21 days. Hormone receptor-positive patients were given hormonal therapy for 5 years. Primary end point was 5-year disease-free survival (DFS). Secondary objectives were overall survival (OS) and safety.
Results:
There were 750 patients enrolled. With a median follow-up of 64 months, 5-year DFS was 73.4% in both arms, and 5-year OS was 89.5% versus 90.7% in arm A and B [hazard ratio was 0.99 (95% confidence interval for DFS 0.75–1.31; P = 0.95)], respectively. Grade 3–4 toxicity was more common in arm B.
Conclusions:
This study did not show advantages from the addition of docetaxel to high-dose EC as adjuvant chemotherapy in node-positive breast cancer. The small sample size and low number of DFS events may have limited the ability to observe statistically significant difference between the two arms.
Keywords: adjuvant chemotherapy, docetaxel, early breast cancer
introduction
Adjuvant chemotherapy has an important role in early-stage breast cancer. In the late 1990s, combination chemotherapy with cyclophosphamide, methotrexate and fluorouracil-like regimens were known to reduce the annual odds of recurrence and death by 24% and 14%, respectively, compared with no treatment; the Early Breast Cancer Trialists' Collaborative Group meta-analysis in 1998 showed a reduction of recurrences and mortality with adjuvant anthracycline-containing regimens in comparison with CMF-like regimens [1]. A National Surgical Adjuvant Breast and Bowel Project randomized trial demonstrated that four cycles of adjuvant doxorubicin and cyclophosphamide (AC) were as effective as six cycles of classical CMF in node-positive breast cancer patients [2], and consequently AC regimen for four cycles became widely used throughout the world. Mainly in Europe, doxorubicin had been frequently replaced by epirubicin, known to be as active as the parent compound but with lower toxicity [3–5]. The shape of the dose–response curve for epirubicin above 60 mg/m2, and the optimal dose remained a topic controversy in the late 1990s, many trials in advanced diseases suggesting a clear dose-related effect [6, 7], so there was an urgent need for further dose-response data in adjuvant setting.
Docetaxel is probably the most active single agent in breast cancer, and results in advanced disease supported the development of trials including both paclitaxel or docetaxel, in combination or in sequence with anthracyclines, in the adjuvant setting [8]. The most commonly employed sequence is an anthracycline followed by a taxane; the reverse sequence was rarely used, mostly in small phase II trials in metastatic disease, suggesting activity and perhaps lower toxicity [9–14].
A number of ‘first-generation’ adjuvant randomized phase III trials have been designed at the end of the 1990s and the early 2000s comparing an anthracycline-containing arm with a concurrent or sequential anthracycline plus taxane-containing arm; the preliminary data showed encouraging results in favor of the taxane arms [15–18].
Based on the early results of a previous our experience in adjuvant setting with a regimen of high-dose epirubicin in combination with cyclophosphamide, showing activity and manageable toxicity [19], we designed a multicenter phase III prospective randomized trial with four cycles of high-dose epirubicin plus cyclophosphamide (EC) as the standard comparator arm versus four cycles of docetaxel (D) followed by four cycles of high-dose EC in node-positive operable breast cancer patients.
patients and methods
study population
From April 1999 to October 2005, 750 surgery-treated node-positive breast cancer patients entered the trial. Main eligibility criteria were age 18–70 years; definite primary surgery (tumorectomy, quadrantectomy, or modified radical mastectomy) plus axillary dissection for operable (T1–T3) breast cancer within 6 weeks; histologically proven axillary lymph node involvement (at least five nodes removed); World Health Organization performance status less than 2; adequate hematologic, hepatic, renal, and cardiac function [baseline left ventricular ejection fraction (LVEF) >50%]. Exclusion criteria included pregnancy, locally advanced or metastatic breast cancer, previous chemotherapy, hormonal therapy, radiotherapy, previous other cancers (except treated basal cell skin carcinoma or in situ cervical cancer) or contralateral breast cancer, documented history of cardiac disease contraindicating anthracyclines, preexistent neuropathy or any other severe illness or medical condition.
Eligible patients underwent a complete staging workup within 8 weeks before registration, including bilateral mammography, bone scan, chest X-ray, abdominal ultrasound, blood count, and chemistry, and signed a written informed consent form before randomization; the protocol was reviewed and approved by the ethic committee/institutional review boards of all participant centers and was carried out according to the European Good Clinical Practice requirements and the Helsinki Declaration.
study design and treatment
This was a multicenter, prospective, randomized phase III trial. Patients were stratified according to center, number of metastatic axillary nodes (1–3, 4–9, ≥10), age (<50 years; ≥50 years), hormonal receptor status [estrogen receptor (ER) and/or progesterone receptor (PgR) positive; ER and PgR negative]. Randomization procedures were computer generated, centralized at the Regina Elena National Cancer Institute, which was the coordinator center, and patients assigned according to the minimization technique [20]. Eligible patients were randomly allocated to one of the following arms: A: four cycles of EC (epirubicin 120 mg/m2 and cyclophosphamide 600 mg/m2 i.v., on day 1, every 21 days); B: four cycles of D [docetaxel (Taxotere), 100 mg/m2 i.v. as 1-h infusion, on day 1, every 21 days], followed by four cycles of EC as above. At the end of chemotherapy, patients with positive hormonal receptors (ER, PgR, or both) were given hormonal treatment (tamoxifen) for 5 years; starting January 2003, postmenopausal women were given anastrozole for 5 years; radiotherapy was administered in case of conservative surgery, and in case of four or more positive nodes.
Antiemetics (5-HT3 receptor antagonists and corticosteroids) were given before each cycle; premedication with corticosteroids (100 mg/day of prednisolone), starting 24 h before and ending 30 h after docetaxel infusion, was delivered to all the patients. Primary prophylaxis with granulocyte colony-stimulating factors (G-CSF) was not allowed.
Treatment was delayed for a maximum of 2 weeks in case of granulocyte count of <1.5 × 109/l and/or a platelet count <100 × 109/l on day 21, and G-CSF was prescribed for subsequent cycles. In the event of G4 febrile neutropenia, G-CSF and antibiotic were administered, and prophylactic G-CSF was added in the subsequent cycles. In case of a second episode of G4 febrile neutropenia, drugs doses were reduced by 25%. Epirubicin dose reduction was carried out also in case of bilirubin >50% upper limit of normal (UNL), whereas the drug was discontinued for values ≥3 × UNL. In case of signs or symptoms of cardiotoxicity, EC regimen was discontinued. Docetaxel dose was reduced by 25% also for transaminases or bilirubin >2.5 × UNL, or for G2 peripheral neurotoxicity or fluid retention; in case of G3 neurotoxicity, docetaxel was discontinued and EC regimen was started. Discontinuation of treatment was required for disease progression, patient refusal, unacceptable toxicity, and any other severe toxicity at the discretion of the investigators.
ER and PgR, analyzed immunohistochemically in an automated autostainer, were considered positive when at least 10% of the neoplastic cells showed distinct nuclear immunoreactivity. HER-2 protein overexpression was retrospectively evaluated with the DAKO Hercept Test kit and scored by intensity and pattern of membrane staining; HER-2 gene amplification was evaluated by FISH.
Radiotherapy (two tangential photon fields to 50 Gy in 25 fractions plus 10 Gy boost on tumor bed) was started within 5 weeks after the last cycle of chemotherapy in all the patients who had undergone breast conservative surgery and, starting March 2004, in all the patients with four or more involved axillary lymph nodes (chest wall and supraclavicular fossa). Minor deviations from this technique according to local institutional guidelines were allowed.
Toxicity was evaluated in each cycle and graded according to National Cancer Institute Common Toxicity Criteria (version 3.0) criteria. Hematologic toxicity was evaluated at nadir and on day 21 of each cycle. LVEF was evaluated, by Multi Gated Acquisition Scan scan or echocardiography, at baseline, after four EC cycles, and during the follow-up period. Imaging studies (chest X-ray, liver ultrasound) were carried out every 6 months for 5 years and yearly thereafter. Mammography and bone scan were carried out yearly.
statistical analysis
According to protocol, the primary end point of the study was 5-year disease-free survival (DFS), defined according to the STEEP system [21] as the time from randomization to the time of first relapse (local, regional, distant), contralateral breast cancer, or death from any cause. The trial was designed to detect a 10% difference in DFS, with 80% power and two-sided type I error of 5%. Assuming a 5-year DFS of 60% in the control arm (EC) and a hazard ratio (HR) of 0.70, 252 total DFS events were needed; this hypothesis required to enroll 740 patients (370 in each arm) in 3 years of accrual, a median follow-up of 5 years, with a drop out of 5%. No interim analyses were carried out. Secondary end points were overall survival (OS), defined as the time from randomization to the time of death from any cause, and safety. The DFS and OS were estimated by the Kaplan–Meier method and groups were compared by use of the log-rank test. HRs were obtained from Cox proportional hazards regression models. Univariate analyses tested the influence of the baseline covariates on the DFS and OS and multivariate analyses adjusted the effect of covariates in the presence of the other covariates. The analysis was carried out on all randomized patients on an intention-to-treat (ITT) basis while a secondary analysis was done on the subgroup of patients receiving the entire planned chemotherapy regimen (per-protocol population). Exploratory analyses on subgroups of patients were done, without any adjustment for multiple testing, reporting HRs and their 95% confidence intervals (CIs).
All the patients treated with at least one cycle were considered assessable for toxicity. The proportion of patients with grade 3 or 4 toxicity in the two treatment arms was compared using the chi-square test.
All the analyses have been carried out by the coordinator center. SPSS 17.0 was used to analyze the database. Fixed effects meta-analysis using data extracted from published reports of relevant adjuvant and neoadjuvant taxane clinical trials was carried out.
Because the accrual rate was slower than expected, and DFS events were lower than calculated, the actual power of the sample, revised according the 198 disease-free related events observed, decreases to 70%; on the other hand, recalculating sample size with a 5-year DFS of 73% in the control group, 198 events give an 80% power of identifying a difference of 8% between the two arms.
results
patient characteristics
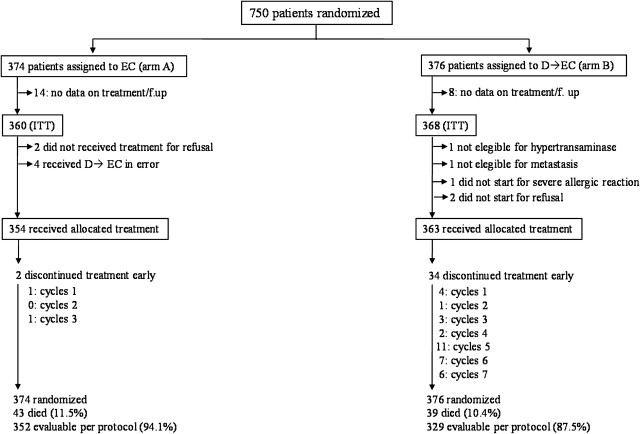
Figure 1 shows the CONSORT diagram. From April 1999 to October 2005, 750 patients entered the trial, 374 in arm A (EC) and 376 in arm B (D→EC), from 20 Italian oncologic centers. Data on treatment and follow-up were completely lacking from 14 (arm A) and 8 (arm B) patients. The ITT analysis was carried out on 360 (arm A) and 368 (arm B) patients; 354 and 363 patients received the allocated treatment, respectively; 352 (94.1%) and 329 (87.5%) patients received the complete protocol-specified treatment, respectively. Four patients randomized to arm A received in error treatment B and were analyzed for efficacy as ITT in arm A.
Figure 1.
CONSORT diagram. D, docetaxel; EC, epirubicin plus cyclophosphamide; ITT, intention to treat.
Patient characteristics are shown in Table 1. In general, the two treatment arms were well balanced in terms of demographics and tumor characteristics; the majority of the patients, 94.2% (arm A) and 94.5% (arm B), had one to three positive nodes; 4.5% (arm A) and 4.0% (arm B) had four to nine positive nodes, respectively; and 1.3% of the patients in each arm had more than nine positive nodes. Negative hormonal receptor status was found in 23.3% (arm A) and 23.1% (arm B) of the patients. HER-2, retrospectively evaluated in 167 (arm A) and 165 (arm B) patients, was overexpressed or amplified in 29.4% and 27.3% of the cases, respectively. Adjuvant radiotherapy was administered to 57.8% (arm A) and 56.4% (arm B) of the patients. Hormonal therapy (tamoxifen 450 patients, anastrozole 69 patients) for 5 years was given to 69.5% and 68.9% of the patients in the arms A and B, respectively.
Table 1.
Patient and tumor characteristics
| Characteristics | EC (n = 374) | D→EC (n = 376) |
| No. (%) | No. (%) | |
| Median age (25th–75th percentiles), years | 51 (44–60) | 50 (43–59) |
| ≤50 | 187 (50.0) | 196 (52.1) |
| ≥50 | 187 (50.0) | 180 (47.9) |
| Menopausal status | ||
| Premenopausal | 170 (45.4) | 179 (47.6) |
| Postmenopausal | 185 (48.1) | 183 (48.6) |
| WHO performance status | ||
| 0 | 367 (98.1) | 369 (98.1) |
| 1 | 7 (1.9) | 7 (1.9) |
| Tumor size | ||
| T1 | 146 (39.1) | 159 (42.4) |
| T2 | 203 (54.4) | 192 (51.2) |
| T3 | 24 (6.4) | 24 (6.4) |
| Number of involved nodes | ||
| 1–3 | 352 (94.2) | 356 (94.7) |
| 4–9 | 17 (4.5) | 15 (4.0) |
| ≥10 | 5 (1.3) | 5 (1.3) |
| Hormonal receptor status | ||
| Positive (ER and/or PgR) | 287 (76.7) | 289 (76.9) |
| Negative (ER and PgR) | 87 (23.3) | 87 (23.1) |
| Histopathological grade | ||
| G1 | 20 (6.0) | 19 (5.6) |
| G2 | 164 (49.5) | 199 (58.4) |
| G3 | 145 (43.8) | 123 (36.1) |
| HER-2 status | ||
| Negative | 118 (70.6) | 120 (72.7) |
| Positive | 49 (29.4) | 45 (27.3) |
| Conservative surgery | 206 (55.1) | 204 (54.3) |
| Adjuvant therapy | ||
| Radiotherapy | ||
| Complementary | 206 (55.1) | 204 (54.3) |
| Postmastectomy | 10 (2.7) | 8 (2.1) |
| Hormonal therapy | 260 (69.5) | 259 (68.9) |
D, docetaxel; EC, epirubicin plus cyclophosphamide; ER, estrogen receptor; PgR, progesterone receptor; WHO, World Health Organization. Missing values: T size EC group 1, D→EC group 1; Histopathological grade EC group 45, D→EC group 35; HER-2 status EC group 207, D→EC group 211; Menopausal status EC group 19, D→EC group 14.
efficacy results
In the current analysis, with a median follow-up of 64 months (IQR 41–84, range 1–130 months), no evidence was found of a difference in DFS between the control (EC) and the experimental (D→EC) arms (overall HR for D→EC versus EC 0.99, 95% CI 0.75–1.31; P = 0.95); 5-year DFS were 73.4% for both arms. Figure 2A shows Kaplan–Meier curves for DFS in the two arms (log-rank test P = 0.95). Events related to DFS and OS are reported on Table 2; no significant differences were observed between the two arms for type of relapse; distant 5-year DFS was 82.8% (arm A) and 80.7% (arm B), P = 0.74. Even evaluating 5-year recurrence-free interval according to STEEP system, no significant differences were observed between the two arms, being 76.7% and 76.3% in arms A and B, respectively (P = 0.95, HR = 0.99, 95% CI 0.73–1.34).
Figure 2.
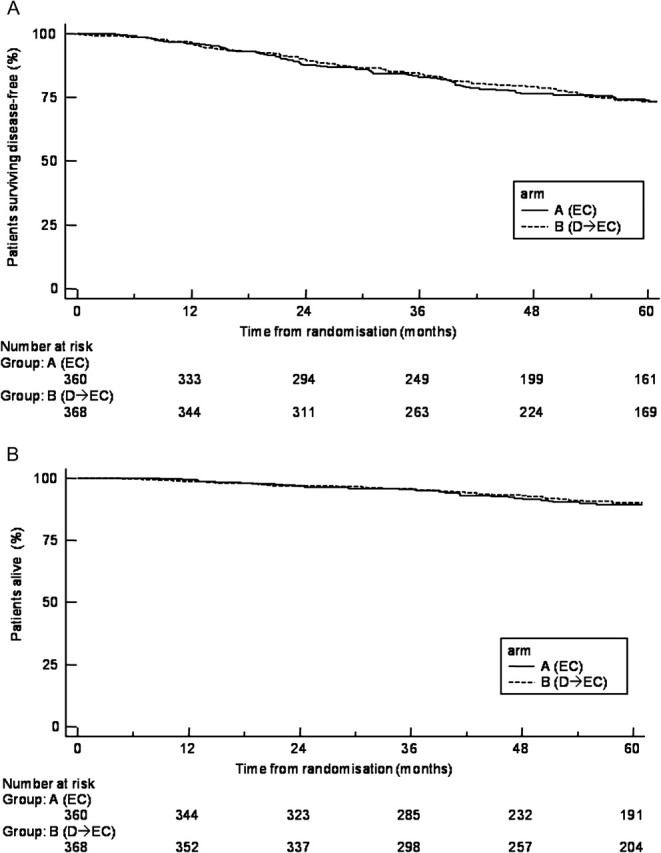
Disease-free survival (A) and overall survival (B) by treatment. D, docetaxel; EC, epirubicin plus cyclophosphamide.
Table 2.
Events contributing to disease-free survival and number of distant relapses, second cancers, and deaths
| EC (n = 374) | D→EC (n = 376) | |
| Number of patients with event contributing to disease-free survival analysis | 93 | 96 |
| Locoregional recurrence | 24 | 21 |
| Distant recurrence | 60 | 66 |
| New breast diseasea | 9 | 7 |
| Death from any cause (no recurrence) | 0 | 2 |
| Distant relapse ever reported | 91 | 89 |
| New breast disease ever reporteda | 13 | 8 |
| All non-breast cancer primary tumorsb | 5b | 4c |
| All deaths | 43 | 39 |
| Breast cancer | 40 | 37 |
| Death from other causes (without recurrence) | 0 | 2d |
| Cancer (non-breast) | 3 | 0 |
| Treatment toxicitye | 0 | 0 |
D, docetaxel; EC, epirubicin plus cyclophosphamide.
Includes contralateral breast cancer recurrences and new contralateral and ipsilateral breast second primary tumors.
Lymphoma 1, pancreas 1, thyroid 1, endometrium 2.
Skin 1, ovary 1, peritoneum 1, colon-rectum 1.
Car accident.
Deaths occurring during chemotherapy or with 30 days of chemotherapy completion.
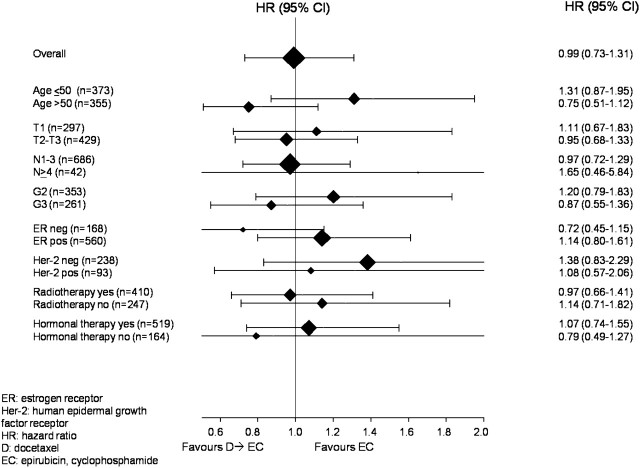
Cox proportional hazards analysis was carried out to evaluate the effects of patient and tumor characteristics on DFS (Table 3). Tumor size, histopathologic grade, hormonal receptor status, and HER-2 status significantly correlate with prognosis; at multivariate analysis, only hormonal receptor status and tumor size maintained their significance. Figure 3 shows the effect of treatment on DFS within subgroups of baseline characteristics. Considering two subgroups at good and poor prognosis created according to multivariate analysis (T2–3/ER negative versus T1/ER positive in the two treatment arms), no differences in DFS between the two arms were observed; even evaluating together ER and HER-2 status, and considering HER-2-positive/ER-negative versus HER-2-negative/ER-positive patients in the two treatment arms, no differences in DFS between the two arms were observed.
Table 3.
Cox regression model analysis for disease-free survival in the intent-to-treat population
| Univariate |
Multivariate |
|||
| Characteristics | HR (95% CI) | P | HR (95% CI) | P |
| Treatment | ||||
| D→EC versus EC | 0.99 (0.73–1.31) | 0.95 | ||
| Age | ||||
| >50 versus ≤50 years | 1.11 (0.84–1.46) | 0.47 | ||
| T size | ||||
| T2–T3 versus T1 | 1.84 (1.36–2.49) | <0.0001 | 1.80 (1.33–2.44) | <0.0001 |
| N involvement | ||||
| ≥4 versus 1–3 | 1.15 (0.61–2.17) | 0.67 | ||
| Histopathological grade | ||||
| G3 versus G2 | 1.41 (1.04–1.91) | 0.01 | ||
| Hormonal receptor status | ||||
| Positive versus negative | 0.48 (0.36–0.64) | <0.0001 | 0.49 (0.36–0.66) | <0.0001 |
| HER-2 status | ||||
| Negative versus positive | 0.54 (0.36–0.81) | 0.004 | ||
| Adjuvant radiotherapy | ||||
| Yes versus no | 1.00 (0.74-–1.35) | 0.98 | ||
CI, confidence interval; D, docetaxel; EC, epirubicin plus cyclophosphamide; HR, hazard ratio; N, node; T, tumor.
Figure 3.
Hazard ratios for disease-free survival by patient and tumor characteristics.
To date, 43 (arm A) and 39 (arm B) randomized patients have died. Figure 2B shows OS Kaplan–Meier curves for each treatment arm, which demonstrated 89.5% survival rate at 5 years for EC arm and 90.7% for the D→EC arm, with no significant differences between the two arms (HR for D→EC versus EC 0.84; 95% CI 0.54–1.31; P = 0.45).
toxicity
Main toxic effects are reported on Table 4. In arm A and arm B, 354 and 363 patients were assessable for toxicity, respectively. Adverse events were assessed after every cycle in each patient and reported as maximum grade. There was a higher incidence of G3–G4 neutropenia in arm B (D→EC) compared with the EC arm, 64.2% versus 54.2% (P = 0.007). Neutropenic fever was observed in 2% and 6.6% of the patients of arms A and B, respectively (P = 0.02). Hypersensitivity reactions were observed in 1 (arm A) and 19 (arm B) patients, respectively (P < 0.0001), and in 13 among 19 patients prompted docetaxel discontinuation. There were six cases of reversible cardiotoxicity, all in the follow-up period, one in arm A and five in arm B. Grade 3 alopecia was universal in both arms. No cases of secondary leukemia or myelodisplastic syndrome were observed; one case of non-Hodgkin lymphoma was observed in arm A.
Table 4.
Main toxic effects
| G3–G4 toxic effect | EC (n = 354), N (%) | D→EC (n = 363), N (%) | P |
| Neutropenia | 192 (54.2) | 233 (64.2) | 0.007 |
| Neutropenic fever | 10 (2.8) | 24 (6.6) | 0.02 |
| Anemia | 9 (2.5) | 7 (1.9) | 0.76 |
| Thrombocytopenia | 2 (0.6) | 4 (1.1) | 0.70 |
| Nausea-vomiting | 21 (5.9) | 21 (5.8) | 0.94 |
| Mucositis | 9 (2.5) | 18 (5.0) | 0.13 |
| Diarrhea | 1 (0.3) | 12 (3.3) | 0.006 |
| Hepatic | 1 (0.3) | 4 (1.1) | 0.38 |
| Neurological | 0 | 12 (3.3) | <0.0001 |
| Cutaneous | 0 | 6 (1.6) | 0.03 |
| Cardiac | 1 (0.3) | 5 (1.4) | 0.23 |
| Hypersensitivity | 1 (0.3) | 19 (5.2) | <0.0001 |
D, docetaxel; EC, epirubicin plus cyclophosphamide.
In groups A and B, 20/374 (5.3%) and 13/376 (3.5%) patients did not receive planned chemotherapy (P = 0.28) (Figure 1); among 363 treated patients of arm B, reasons of discontinuating allocated treatment were allergic reactions in 13, progressive disease in 2, refusal in 6, various toxic effects in 7, and other reasons in 6 patients; among 354 treated patients of arm A, reasons of discontinuating EC allocated regimen were one refusal and one allergic reaction.
The median relative dose intensity of EC in arm A was 100%; in arm B it was 100% for docetaxel and 98% for EC.
discussion
With a median follow-up of 64 months, the Gruppo Oncologico Italia Meridionale (GOIM) 9902 trial did not show a statistically significant difference in 5-year DFS between the two arms, being 73.4% in both arms, nor in 5-year OS (89.5% and 90.7%). As expected, the rate of grade 3–4 neutropenia was higher in the arm B (P = 0.007); moreover, hypersensivity reactions, neurological toxicity, cutaneous toxicity, and mild diarrhea were more frequently observed in arm B. No other relevant differences in adverse events were observed between the two arms, and no life-threatening toxic effects were reported, being toxic effects usually manageable.
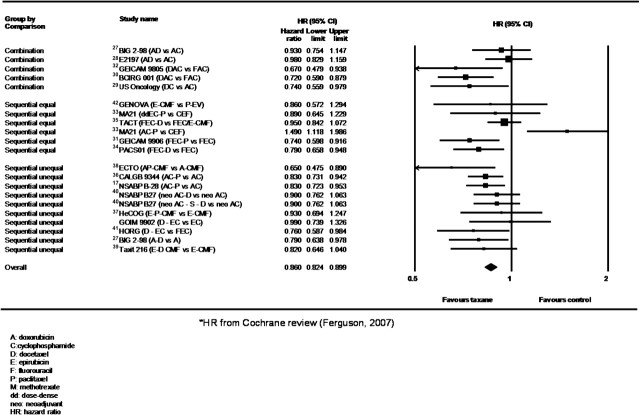
To date, several phase III clinical trials have evaluated taxanes in adjuvant setting; definite and detailed conclusions about taxanes benefit are difficult to draw from individual trials because studies are differently powered, include biologically heterogeneous patients and tumors, have employed different taxanes schedules, control anthracycline arms that are dissimilar and often of unequal duration, and results are reported at various follow-up periods. Overall, anthracycline-containing regimens are associated with a small reduction in the risk of recurrence and death compared with CMF, with an absolute benefit of 3% at 5 years and 4% at 10 years, and taxanes provided a clear adjunctive advantage, as confirmed by several meta-analyses [22–25]. Recently, a large meta-analysis including 13 studies and >22 000 patients showed a pooled HR estimated of 0.83 and 0.85 for DFS and OS, respectively, with an absolute improvement in 5-year DFS and OS of 5% and 3%, respectively, over non-taxane regimens [26]. The meta-analysis shown on Figure 4 reports more recent results on main first-generation taxane neoadjuvant/adjuvant trials confirming an advantage in DFS for the taxane arms of 3.2%, (95% CI 2.3% to 4.2%), with an HR of 0.86 (95% CI 0.82–0.90).
Figure 4.
Meta-analysis on disease-free survival of main taxane first-generation adjuvant/neoadjuvant trials.
Some potential limitations of the present study, including the relative small sample size and the lower number of events than expected, should be taken in account. First, our results are to be interpreted in the context of the size of the difference the trial was powered to detect; more than 94% of the enrolled patients had one to three positive nodes with a presumably reduced and delayed relapse risk and a long life-expectancy, and this may have contributed to the lower rate of events than expected; we cannot exclude that with a higher number of patients and longer follow-up differences in 5-year DFS lower than 10% between the two arms might have been found. Second, in the GOIM 9902 trial, the comparator EC arm was probably more active than other comparator anthracycline-containing arms (see supplementary data, available at Annals of Oncology online), even if no direct comparison is available from literature data, and the benefit of adding a taxane may have been attenuated; when the trial was designed, EC was considered among the ‘standard’ regimens in adjuvant setting, but epirubicin dose was usually lower, and clear evidence on the importance of anthracycline dose in adjuvant setting came later from trials and retrospective analyses [43–45]. Third, the different duration of chemotherapy in the two arms, not uncommon in the first-generation adjuvant taxane trials; longer treatments may be more efficacious but have the potential disadvantage of possible reduced treatment compliance over time, with higher risk of dose reductions/delays/discontinuations; analyzing our results on 352 and 329 assessable per-protocol patients, we observed 5-year DFS of 73.8% (arm A) and 74.2% (arm B), respectively (P = 0.86). Fourth, the ‘sequence’ employed in the trial, taxane→anthracycline, based on the Gompertzian kinetic model proposed by Norton and Simon, is considered feasible and effective in advanced setting; in the adjuvant setting, few trials employed the reverse sequence [41, 42, 46–49], with favorable results, for reduced toxicity [47–49], sometimes for higher activity [41], and, recently, a significant advantage in pathological complete responses was reported also in neoadjuvant setting [50, 51]. To date, a number of ongoing randomized adjuvant trials are investigating chemotherapy sequencing and, based on the above reported literature results, it is unlikely that the sequence order could have negatively influenced our results.
Overall, since the magnitude of anthracycline and taxane advantage in DFS and OS over non-anthracycline and taxane-containing adjuvant regimens is relatively small, the selection of patients who are more likely to respond according to tumor biology would be mandatory. Several studies, mostly retrospectives, suggested anthracycline and/or taxane benefit related to HER-2 iperexpression or topoisomerase II amplification, or to negative hormonal receptor status, but results are conflicting [52, 53]. In our trial, no significant differences have been observed in the two arms according to HER-2 status, even if numbers are very small, and there was a trend toward higher activity of docetaxel arm in ER-negative tumors (Figure 3).
Other taxane-based randomized adjuvant trials did not demonstrate significant advantage in DFS over non-taxane comparator adjuvant regimens; among the main first-generation trials, there are seven other ‘negative’ studies, possibly for underpowered design [33, 37, 40, 42], intensified control arm [33, 35, 37], lower chemotherapy doses [28, 32], and shorter chemotherapy duration in the experimental arm [54]. In conclusion, the GOIM 9902 is a negative trial, but we should consider the limited power of the study due to the small sample size, and to the lower number of DFS events than originally planned; we cannot exclude that, considering the relative favorable prognosis of our patient population, some differences could emerge with longer follow-up.
funding
Gruppo Oncologico Italia Meridionale 9902 study has been funded by Sanofi-Aventis and the Gruppo Oncologico Italia Meridionale. The funders did not interfere in any way in the interpretation of data or in the content of the manuscript.
disclosure
The authors declare no conflicts of interest.
Supplementary Material
Acknowledgments
The findings of this study have been in part presented as an abstract at the 42nd ASCO Annual Meeting (2006).
References
- 1.Early Breast Cancer Trialists’ Collaborative Group. Polychemotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;352:930–942. [PubMed] [Google Scholar]
- 2.Fisher B, Brown AM, Dimitrov NV, et al. Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: results from the National Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol. 1990;8:1483–1496. doi: 10.1200/JCO.1990.8.9.1483. [DOI] [PubMed] [Google Scholar]
- 3.Jain KK, Casper ES, Geller NL, et al. A prospective randomized comparison of epirubicin and doxorubicin in patients with advanced breast cancer. J Clin Oncol. 1985;3:818–826. doi: 10.1200/JCO.1985.3.6.818. [DOI] [PubMed] [Google Scholar]
- 4.French Epirubicin Study Group. A prospective randomized phase III trial comparing combination chemotherapy with cyclophosphamide, fluorouracil, and either doxorubicin or epirubicin. French Epirubicin Study Group. J Clin Oncol. 1988;6:679–688. doi: 10.1200/JCO.1988.6.4.679. [DOI] [PubMed] [Google Scholar]
- 5.Medical Department, Farmitalia Carlo Erba, Milano, Italy. Phase III randomized study of fluorouracil, epirubicin, and cyclophosphamide v fluorouracil, doxorubicin, and cyclophosphamide in advanced breast cancer: an Italian multicentre trial. Italian Multicentre Breast Study with Epirubicin. J Clin Oncol. 1988;6:976–982. doi: 10.1200/JCO.1988.6.6.976. [DOI] [PubMed] [Google Scholar]
- 6.Lopez M, Vici P, Di Lauro L, et al. Randomized prospective clinical trial of high-dose epirubicin and dexrazoxane in patients with advanced breast cancer and soft tissue sarcomas. J Clin Oncol. 1998;16:86–92. doi: 10.1200/JCO.1998.16.1.86. [DOI] [PubMed] [Google Scholar]
- 7.Bastholt L, Dalmark M, Gjedde SB, et al. Dose-response relationship of epirubicin in the treatment of postmenopausal patients with metastatic breast cancer: a randomized study of epirubicin at four different dose levels performed by the Danish Breast Cancer Cooperative Group. J Clin Oncol. 1996;14:1146–1155. doi: 10.1200/JCO.1996.14.4.1146. [DOI] [PubMed] [Google Scholar]
- 8.López-Tarruella S, Martìn M. Advances in adjuvant systemic chemotherapy of early breast cancer. Breast Cancer Res. 2009;11:204. doi: 10.1186/bcr2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antón A, Hornedo J, Lluch A, et al. A phase II study of sequential docetaxel followed by doxorubicin/cyclophosphamide as first-line chemotherapy for metastatic breast cancer. Clin Breast Cancer. 2003;4:286–291. doi: 10.3816/cbc.2003.n.034. [DOI] [PubMed] [Google Scholar]
- 10.Khayat D, Chollet P, Antoine EC, et al. Phase II study of sequential administration of docetaxel followed by doxorubicin and cyclophosphamide as first-line chemotherapy in metastatic breast cancer. J Clin Oncol. 2001;19:3367–3375. doi: 10.1200/JCO.2001.19.14.3367. [DOI] [PubMed] [Google Scholar]
- 11.Spielmann M, Tubiana-Hulin M, Namer M, et al. Sequential or alternating administration of docetaxel (Taxotere) combined with FEC in metastatic breast cancer: a randomised phase II trial. Br J Cancer. 2002;86:692–697. doi: 10.1038/sj.bjc.6600165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cresta S, Grasselli G, Mansutti M, et al. A randomized phase II study of combination, alternating and sequential regimens of doxorubicin and docetaxel as first-line chemotherapy for women with metastatic breast cancer. Ann Oncol. 2004;15:433–439. doi: 10.1093/annonc/mdh107. [DOI] [PubMed] [Google Scholar]
- 13.Paridaens R, Van Aelst F, Georgoulias V, et al. A randomized phase II study of alternating and sequential regimens of docetaxel and doxorubicin as first-line chemotherapy for metastatic breast cancer. Ann Oncol. 2003;14:433–440. doi: 10.1093/annonc/mdg111. [DOI] [PubMed] [Google Scholar]
- 14.Perez EA, Geeraerts L, Suman VJ, et al. A randomized phase II study of sequential docetaxel and doxorubicin/cyclophosphamide in patients with metastatic breast cancer. Ann Oncol. 2002;13:1225–1235. doi: 10.1093/annonc/mdf222. [DOI] [PubMed] [Google Scholar]
- 15.Demetri GD, Berry D, Norton L, et al. Clinical outcomes of node-positive breast cancer patients treated with dose-intensified Adriamycin/cyclophosphamide (AC) followed by Taxol (T) as adjuvant systemic chemotherapy (CALGB 9141) Proc Am Soc Clin Oncol. 1997 (Abstr 503) [Google Scholar]
- 16.Hudis C, Seidman A, Baselga J, et al. Sequential dose-dense doxorubicin, paclitaxel, and cyclophosphamide for resectable high-risk breast cancer: feasibility and efficacy. J Clin Oncol. 1999;17:93–100. doi: 10.1200/JCO.1999.17.1.93. [DOI] [PubMed] [Google Scholar]
- 17.Mamounas EP, Bryant J, Lembersky BC, et al. Paclitaxel (T) following doxorubicin/cyclophosphamide (AC) as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. Proc Am Soc Clin Oncol. 2003;22 doi: 10.1200/JCO.2005.10.517. (Abstr 12) [DOI] [PubMed] [Google Scholar]
- 18.Henderson IC, Berry D, Demetri G, et al. Improved disease-free (DFS) and overall survival (OS) from the addition of sequential paclitaxel (T) but not from the escalation of doxorubicin (A) dose level in the adjuvant chemotherapy of patients (PTS) with node-positive primary breast cancer (BC) Proc Am Soc Clin Oncol. 1998 (Abstr 390) [Google Scholar]
- 19.Papaldo P, Lopez M, Cortesi E, et al. Addition of either lonidamine or granulocyte colony-stimulating factor does not improve survival in early breast cancer patients treated with high-dose epirubicin and cyclophosphamide. J Clin Oncol. 2003;21:3462–3468. doi: 10.1200/JCO.2003.03.034. [DOI] [PubMed] [Google Scholar]
- 20.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 21.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25:2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 22.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 23. Peto R. The worldwide overview: new result for sistemic adjuvant therapy. Plenary lecture. 30th Annual San Antonio Breast Cancer Symposium, San Antonio; 2007. [Google Scholar]
- 24.Bria E, Nistico C, Cuppone F, et al. Benefit of taxanes as adjuvant chemotherapy for early breast cancer: pooled analysis of 15,500 patients. Cancer. 2006;106:2337–2344. doi: 10.1002/cncr.21886. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson T, Wilcken N, Vagg R, et al. Taxanes for adjuvant treatment of early breast cancer. Cochrane Database Syst Rev. 2007;(4) doi: 10.1002/14651858.CD004421.pub2. CD004421. [DOI] [PubMed] [Google Scholar]
- 26.De Laurentiis M, Cancello G, D'Agostino D, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol. 2008;26:44–53. doi: 10.1200/JCO.2007.11.3787. [DOI] [PubMed] [Google Scholar]
- 27.Francis P, Crown J, Di Leo A, et al. Adjuvant chemotherapy with sequential or concurrent anthracycline and docetaxel: Breast International Group 02-98 randomized trial. J Natl Cancer Inst. 2008;100:121–133. doi: 10.1093/jnci/djm287. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein LJ, O’Neill A, Sparano JA, et al. Concurrent doxorubicin plus docetaxel is not more effective than concurrent doxorubicin plus cyclophosphamide in operable breast cancer with 0 to 3 positive axillary nodes: North American Breast Cancer Intergroup Trial E 2197. J Clin Oncol. 2008;26:4092–4099. doi: 10.1200/JCO.2008.16.7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones S, Holmes FA, O’Shaughnessy J, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol. 2009;27:1177–1183. doi: 10.1200/JCO.2008.18.4028. [DOI] [PubMed] [Google Scholar]
- 30.Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 31.Martìn M, Rodrìguez-Lescure A, Ruiz A, et al. Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by paclitaxel fo early breast cancer. J Natl Cancer Inst. 2008;100:805–814. doi: 10.1093/jnci/djn151. [DOI] [PubMed] [Google Scholar]
- 32.Martín M, Lluch A, Segui MA, et al. TAC versus FAC as adjuvant chemotherapy for high-risk node-negative breast cancer: results of the GEICAM 9805 trial. Ann Oncol 2008; 19 (Suppl 8): viii77–viii88 (Abstr 1830) [Google Scholar]
- 33.Burnell M, Levine MN, Chapman JA, et al. Cyclophosphamide, epirubicin, and fluorouracil versus dose-dense epirubicin and cyclophosphamide followed by paclitaxel versus doxorubicin and cyclophosphamide followed by paclitaxel in node-positive or high-risk node-negative breast cancer. J Clin Oncol. 2010;8:77–82. doi: 10.1200/JCO.2009.22.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roche H, Fumoleau P, Spielmann M, et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 Trial. J Clin Oncol. 2006;24:5664–5671. doi: 10.1200/JCO.2006.07.3916. [DOI] [PubMed] [Google Scholar]
- 35.Ellis P, Barrett-Lee P, Johnson L, et al. Sequential docetaxel as adjuvant chemotherapy for early breast cancer (TACT): an open-label, phase III, randomised controlled trial. Lancet. 2009;373:1681–1692. doi: 10.1016/S0140-6736(09)60740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 37.Fountzilas G, Skarlos D, Dafni U, et al. Postoperative dose-dense sequential chemotherapy with epirubicin, followed by CMF with or without paclitaxel, in patients with high-risk operable breast cancer: a randomized phase III study conducted by the Hellenic Cooperative Oncology Group. Ann Oncol. 2005;16:1762–1771. doi: 10.1093/annonc/mdi366. [DOI] [PubMed] [Google Scholar]
- 38.Gianni L, Baselga J, Eiermann W, et al. Phase III trial evaluating the addition of paclitaxel to doxorubicin followed by cyclophosphamide, methotrexate, and fluorouracil, as adjuvant or primary systemic therapy: European Cooperative Trial in Operable Breast Cancer. J Clin Oncol. 2009;27:2474–2481. doi: 10.1200/JCO.2008.19.2567. [DOI] [PubMed] [Google Scholar]
- 39.Cognetti F, De Laurentiis M, De Matteis A, et al. Docetaxel added to anthracycline regimens improves outcomes in early breast cancer. Proc Eur Soc Med Oncol. 2008 (Abstr 1820) [Google Scholar]
- 40.Bear HD, Anderson S, Smith RE, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006;24:2019–2027. doi: 10.1200/JCO.2005.04.1665. [DOI] [PubMed] [Google Scholar]
- 41.Polyzos A. FEC versus sequential docetaxel followed by epirubicin/ cyclophosphamide as adjuvant chemotherapy in women with axillary nodepositive early breast cancer: a randomized study of the Hellenic Oncology Research Group (HORG) Breast Cancer Res Treat. 2010;119:95–104. doi: 10.1007/s10549-009-0468-0. [DOI] [PubMed] [Google Scholar]
- 42.Boccardo F, Amadori D, Guglielmini P, et al. Epirubicin followed by cyclophosphamide, methotrexate and 5-fluorouracil versus paclitaxel followed by epirubicin and vinorelbine in patients with high-risk operable breast cancer. Oncology. 2010;78:274–281. doi: 10.1159/000315735. [DOI] [PubMed] [Google Scholar]
- 43.de Azambuja E, Paesmans M, Beauduin M, et al. Long-term benefit of high-dose epirubicin in adjuvant chemotherapy for node-positive breast cancer: 15-year efficacy results of the Belgian multicentre study. J Clin Oncol. 2009;27:720–725. doi: 10.1200/JCO.2008.17.2155. [DOI] [PubMed] [Google Scholar]
- 44.Bonneterre J, Bercez C, Bonneterre ME, et al. Cost-effectiveness analysis of breast cancer adjuvant treatment: FEC 50 versus FEC 100 (FASG05 study) Ann Oncol. 2005;16:915–922. doi: 10.1093/annonc/mdi195. [DOI] [PubMed] [Google Scholar]
- 45.Chirivella I, Bermejo B, Insa A, et al. Optimal delivery of anthracycline-based chemotherapy in the adjuvant setting improves outcome of breast cancer patients. Breast Cancer Res Treat. 2009;114:479–484. doi: 10.1007/s10549-008-0018-1. [DOI] [PubMed] [Google Scholar]
- 46.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 47.Piedbois P, Serin D, Priou F, et al. Dose-dense adjuvant chemotherapy in nodepositive reast cancer: docetaxel followed by epirubicin/cyclophosphamide (T/EC), or the reverse sequence (EC/T), every 2 weeks, versus docetaxel, epirubicin and cyclophosphamide (TEC) every 3 weeks. AERO B03 randomized phase II study. Ann Oncol. 2007;18:52–57. doi: 10.1093/annonc/mdl355. [DOI] [PubMed] [Google Scholar]
- 48.Puhalla S, Mrozek E, Young D, et al. Randomized phase II adjuvant trial of dosedense docetaxel before or after doxorubicin plus cyclophosphamide in axillary node-positive breast cancer. J Clin Oncol. 2008;26:1691–1697. doi: 10.1200/JCO.2007.14.3941. [DOI] [PubMed] [Google Scholar]
- 49.Wildiers H, Dirix L, Neven P, et al. Delivery of adjuvant sequential dose-dense FEC-Doc to patients with breast cancer is feasible, but dose reductions and toxicity are dependent on treatment sequence. Breast Cancer Res Treat. 2009;114:103–112. doi: 10.1007/s10549-008-9970-z. [DOI] [PubMed] [Google Scholar]
- 50.Earl HM, Vallier A, Hiller L, et al. Neo-tAnGo: a neoadjuvant randomized phase III trial of epirubicin/cyclophosphamide and paclitaxel 6 gemcitabine in the treatment of women with high-risk early breast cancer (EBC): first report of the primary endpoint, pathological complete response (pCR) Proc Am Soc Clin Oncol. 2009;27:15s. (Abstr 522) [Google Scholar]
- 51.Alvarez RH, Bianchini G, Hsu L, et al. The effect of different sequencing regimens of taxanes and anthracyclines in the primary systemic treatment (PST) of breast cancer (BC) patients (pts): M. D. Anderson Cancer Center retrospective analysis. J Clin Oncol. 2010;28:15s. (Abstr 548) [Google Scholar]
- 52.Tang SC. Taxanes in the adjuvant treatment of early breast cancer, emerging consensus and unanswered questions. Cancer Invest. 2009;27:489–495. doi: 10.1080/07357900802427943. [DOI] [PubMed] [Google Scholar]
- 53.André F, Broglio K, Roche H, et al. Estrogen receptor expression and efficacy of docetaxel-containing adjuvant chemotherapy in patients with node-positive breast cancer: results from a pooled analysis. J Clin Oncol. 2008;26:2636–2643. doi: 10.1200/JCO.2007.14.9146. [DOI] [PubMed] [Google Scholar]
- 54.Del Mastro L, Costantini M, Durando A, et al. Cyclophosphamide, epirubicin and 5-fluorouracile versus epirubicin plus paclitaxel in node-positive early breast cancer patients: A randomized, phase III study of Gruppo Oncologico Nord-Ovest Mammella Intergruppo Group. Proc Am Soc Clin Oncol. 2008;26:10s. (Abstr 516) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.