Abstract
Adenosine has been proposed as an endogenous homeostatic sleep factor that accumulates during waking and inhibits wake-active neurons to promote sleep. It has been specifically hypothesized that adenosine decreases wakefulness and promotes sleep recovery by directly inhibiting wake-active neurons of the basal forebrain (BF), particularly BF cholinergic neurons. We previously showed that adenosine directly inhibits BF cholinergic neurons. Here, we investigated 1) how adenosine modulates glutamatergic input to BF cholinergic neurons and 2) how adenosine uptake and adenosine metabolism are involved in regulating extracellular levels of adenosine. Our experiments were conducted using whole cell patch-clamp recordings in mouse brain slices. We found that in BF cholinergic neurons, adenosine reduced the amplitude of AMPA-mediated evoked glutamatergic excitatory postsynaptic currents (EPSCs) and decreased the frequency of spontaneous and miniature EPSCs through presynaptic A1 receptors. Thus we have demonstrated that in addition to directly inhibiting BF cholinergic neurons, adenosine depresses excitatory inputs to these neurons. It is therefore possible that both direct and indirect inhibition may synergistically contribute to the sleep-promoting effects of adenosine in the BF. We also found that blocking the influx of adenosine through the equilibrative nucleoside transporters or inhibiting adenosine kinase and adenosine deaminase increased endogenous adenosine inhibitory tone, suggesting a possible mechanism through which adenosine extracellular levels in the basal forebrain are regulated.
Keywords: magnocellular preoptic nucleus, substantia innominata, adenosine A1 receptors, electrophysiology, excitatory postsynaptic currents, in vitro, mice
in the last eighty years, a large number of studies have been directed toward identifying the endogenous sleep factors that could build during the waking period to drive the need for sleep and then be dissipated by sleep itself (Achermann and Borbely 2003; Borbely and Tobler 1985). Among several endogenous sleep factors, one of the best candidates to serve as a homeostatic sleep-promoting signal is adenosine (Basheer et al. 2004; Stenberg 2007). Adenosine is a purine nucleoside; it is a by-product of ATP hydrolysis and might represent a cellular signal for energy demand in the brain (Benington and Heller 1995; Chagoya de Sanchez et al. 1993). Adenosine has been shown to promote slow-wave sleep and rapid eye movement (REM) sleep in cats and rats (Basheer et al. 2004; Porkka-Heiskanen et al. 2002; Radulovacki 1995; Radulovacki et al. 1984) and more recently in mice (Coleman et al. 2006; Oishi et al. 2008; Urade et al. 2003; Van Dort et al. 2009). In the early 1990s it was hypothesized that adenosine accumulates in the extracellular space during waking where, on reaching sufficiently high extracellular levels, it could produce sleep through inhibition of wake-active neurons, in particular those of the basal forebrain (BF) (Rainnie et al. 1994). Since then, several studies have shown that extracellular adenosine levels rise in the BF during prolonged wakefulness and decline during recovery sleep and that adenosine can inhibit BF wake-active neurons through A1 receptors (Alam et al. 1999; Arrigoni et al. 2006; Kalinchuk et al. 2003; Porkka-Heiskanen et al. 1997; Thakkar et al. 2003a). In addition, accumulation of adenosine in the BF is correlated with both increased electroencephalographic (EEG) slow-wave activity and sleep amount and has been shown to contribute to homeostatic sleep drive following sleep deprivation (McCarley 2007; Porkka-Heiskanen et al. 1997; Portas et al. 1997; Stenberg 2007; Strecker et al. 2000; Thakkar et al. 2003b).
The BF contains a heterogeneous population of neurons including corticopetal cholinergic, GABAergic, glutamatergic, and peptidergic neurons as well as interneurons (Gritti et al. 1993, 2006; Hur and Zaborszky 2005; Schwaber et al. 1987; Zaborszky et al. 1999, 2005; Zaborszky and Duque 2000, 2003). BF cholinergic neurons provide the major cholinergic innervation of the cerebral cortex (Gritti et al. 1997; Manns et al. 2001; Saper 1984; Woolf 1991); they fire in association with cortical activation during wakefulness and REM sleep, which is when cortical acetylcholine release is maximal, and their firing rate positively correlates with gamma EEG activity across the sleep-wake cycle (Hassani et al. 2009; Jasper and Tessier 1971; Jones 2004; Lee et al. 2005; Marrosu et al. 1995). More importantly, activation of BF cholinergic neurons is sufficient to promote cortical activation and increases wakefulness and REM sleep (Cape et al. 2000).
BF cholinergic neurons also receive robust glutamatergic inputs (Hur et al. 2009). These projections appear to arise from multiple sources and include the lateral hypothalamus, the amygdala, the intralaminar and midline nuclei of the thalamus, and the brain stem (Carnes et al. 1990; Espana et al. 2005; Fadel et al. 2005; Jolkkonen et al. 2002; Materi et al. 2000; Rasmusson et al. 1994; Wu et al. 2004; Zaborszky et al. 1984, 1991). As such, glutamatergic inputs from these predominately wake-promoting structures could promote arousal through activation of BF cholinergic neurons.
We have previously reported that adenosine directly inhibits BF cholinergic neurons through A1 receptors and the subsequent activation of an inwardly rectifying potassium conductance (Arrigoni et al. 2006). It is also possible, however, that adenosine may inhibit BF cholinergic neurons indirectly by reducing glutamatergic input to the BF. We therefore examined in the present study 1) how adenosine modulates glutamatergic input to BF cholinergic neurons and 2) which enzymes control the level of extracellular adenosine in the BF.
MATERIAL AND METHODS
Animals.
In this study we used male and female (5–20 days old) C57BL/6 mice and an additional cohort of six mature (6 wk old) C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME). Mice were housed in a pathogen-free barrier animal research facility and maintained on a 12:12-h light-dark cycle (lights on at 7:00 AM) at 22°C ambient temperature and with ad libitum access to food and water. Care of the mice met the National Institutes of Health standards, as set forth in the Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the Beth Israel Deaconess Medical Center and Harvard Medical School Institutional Animal Care and Use Committees.
Prelabeling of BF cholinergic neurons.
Under isoflurane anesthesia, mice were injected into the lateral cerebroventricle [intracerebroventricular (icv) injections] with indocarbocyanine (Cy3)-coupled antibodies raised against mouse p75 neurotrophin receptors (Cy3-p75NTR-IgG; Advanced Targeting Systems, San Diego, CA). Cy3-p75NTR-IgGs (100–150 nl, 0.4 mg/ml) were slowly (over 5 min) air pressure-injected using a silane-coated glass pipette (25- to 30-μm tip diameter) (Scammell et al. 1998). Coordinates for the left lateral cerebroventricle for the mice < 21 days old were anterioposterior (AP) = −0.1 mm, dorsoventral (DV) = −0.8 mm, and mediolateral (ML) = −1.4 mm, and those for the 6-wk-old mice were AP = −0.2 mm, DV = −2 mm, and ML = −1 mm (Franklin and Paxinos 1997). One to 3 days after the surgery, brain slices were prepared from mice for in vitro recordings. Labeled neurons were found ipsi- and contralateral to the injection (Hartig et al. 1998), and recordings were made from both hemispheres.
Slice preparation and whole cell patch-clamp recordings.
Brain slices from immature mice (<21 days old) were prepared as follows. The animals were anesthetized (isoflurane inhalation) to the point of respiratory arrest and then decapitated. Coronal brain slices (300-μm thickness) were cut with a vibrating microtome (VT1000; Leica, Bannockburn, IL) in ice-cold artificial cerebrospinal fluid (ACSF; HEPES-buffered solution) oxygenated with 100% O2. Brain slices from the cohort of 6-wk-old mice were prepared as follows (Peca et al. 2011; Zhao et al. 2011). The animals were deeply anesthetized by intraperitoneal injection of a ketamine-xylazine mixture (150 mg/kg ketamine and 15 mg/kg xylazine) and then transcardially perfused with ice-cold cutting ACSF [N-methyl-d-glucamine (NMDG)-based solution, carbogenated with 95% O2 and 5% CO2]. Mice were then decapitated, and coronal brain slices (300-μm thickness) were cut in ice-cold cutting ACSF (NMDG-based). Slices containing the BF were kept for 20 min at 36°C in the cutting ACSF and then maintained in the holding chamber at room temperature in oxygenated recording ACSF (HEPES-buffered solution) until transferred to the recording chamber.
Slices were recorded at room temperature, submerged, and perfused (2 ml/min) with oxygenated ACSF (HEPES-buffered solution), and recordings were made from Cy3-p75NTR-IgG-labeled neurons of the magnocellular preoptic nucleus and substantia innominata (MCPO/SI). Whole cell recordings were guided by combined fluorescence and infrared differential interference contrast (IR-DIC) video microscopy using a fixed-stage upright microscope (Axioscope 2FS; Carl Zeiss MicroImaging, Thornwood, NY) equipped with a Normarki water-immersion lens (×40, 0.8 NA). IR-DIC and fluorescence images were detected with an IR-sensitive charge-coupled device camera (ORCA-ER; Hamamatsu, Bridgewater, NJ) and displayed on a computer screen in real time using AxioVision software (Carl Zeiss MicroImaging). Whole cell recordings were made using a Multiclamp 700B amplifier, a Digidata 1322A interface, and Clampex 9 software (Molecular Devices, Foster City, CA).
Evoked excitatory postsynaptic currents (evEPSCs) were induced by local stimulation with a bipolar electrode (Frederick Haer, Bowdoinham, ME) placed within a 200-μm radius of the recorded cell. Current pulses (0.1- to 0.6-ms duration, repeated every 20 s) were delivered with a constant-current source (Stimulus Isolator A360; World Precision Instruments, Sarasota, FL), triggered by Clampex software (Molecular Devices), and the stimulus strength was adjusted (0.1–0.6 mA) to give maximum evEPSC amplitude. Evoked EPSCs were displayed as an average of 5–10 consecutive traces, and trace baselines were superimposed. Recordings in which the evEPSCs displayed multiple peaks or were unstable over time, or in which the stimulations evoked antidromic or orthodromic action potentials, were discarded. The paired-pulse facilitation (PPF) test was delivered using two identical electrical stimulating pulses (40-ms interval, repeated every 20 s). Paired-pulse ratio (PPR) was calculated as the average of six consecutive traces by dividing the evEPSC peak amplitude of the second pulse by the evEPSC amplitude of the first pulse. The effects of adenosine on the PPF were represented by scaling the traces during adenosine application so that the evEPSC (1st pulse) matches the control evEPSC (1st pulse) (see Fig. 3B).
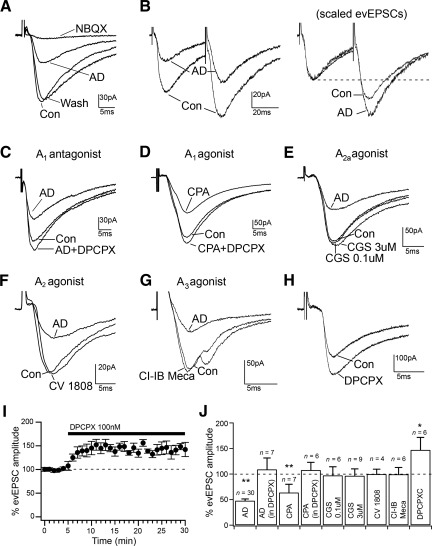
Fig. 3.
Adenosine inhibits the evoked glutamatergic input to MCPO/SI cholinergic neurons through presynaptic A1 receptors. A: adenosine (100 μM) inhibits glutamatergic AMPA-receptor-mediated evoked excitatory postsynaptic currents (evEPSCs); 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX) was used at 10 μM. AD, adenosine. B: adenosine increases paired-pulse facilitation (PPF; paired-pulses test using 40-ms interstimulus interval). At right, scaled traces to match evEPSC (1st pulses) are represented to show the increased PPF by adenosine. C and D: adenosine inhibition of evEPSCs is blocked by the A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX; 100 nM) and is mimicked by the A1 agonist N6-cyclopentyladenosine (CPA; 100 nM). E–G: evoked glutamatergic EPSCs are unaffected by the A2A receptor agonist CGS-216800 (0.1–3 μM), the A2 receptor agonist CV-1808 (250 nM), or the A3 receptor agonist Cl-IB-MECA (100 nM). H and I: DPCPX (100 nM) increases the amplitude of evEPSCs by blocking endogenous adenosine. J: summary graph showing the averaged effects of adenosine, adenosine in the presence of DPCPX, CPA, CPA in the presence of DPCPX, CGS-21680 (0.1 and 3 μM), CV-1808, Cl-IB-MECA, and DPCPX on evEPSC amplitude. *P < 0.05;**P < 0.01, paired t-test, comparing evEPSC amplitude in control artificial cerebrospinal fluid (ACSF) and during drug applications. All evEPSCs were recorded at Vh = −70 mV.
Reagents and solutions.
Composition of the ACSF used for recording and for preparation of the brain slices of immature mice was (in mM) 140 NaCl, 3 KCl, 1.3 MgSO4, 2.4 CaCl2, 1.4 NaH2PO4, 11 glucose, and 5 HEPES (pH 7.2 with NaOH, 315–320 mosmol/l). Composition of the cutting ACSF (NMDG-based solution) used for the preparation of brain slices from 6-wk-old mice was (in mM) 119 NMDG-Cl, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 25 glucose, 2 thiourea, 5 l-ascorbic acid, 3 Na-pyruvate, 0.5 CaCl2, and 10 MgSO4 (pH 7.3 with HCl when carbogenated with 95% O2 and 5% CO2). Composition of the pipette solution was (in mM) 120 K-gluconate, 10 KCl, 3 MgCl2, 10 HEPES, 2.5 K-ATP, 0.5 Na-GTP, and Lucifer yellow CH-ammonium salt (0.1%) (pH 7.2 adjusted with KOH, 280 mosmol/l). Miniature EPSCs (mEPSCs) were recorded in tetrodotoxin (TTX; 1 μM), bicuculline methiodide (10 μM), and a pipette solution containing (in mM) 110 d-gluconic acid, 10 CsCl, 10 HEPES, 3 MgCl2, 1 CaCl2, 11 EGTA, 20 d-sorbitol, 2 MgATP, 0.3 NaGTP, and Lucifer yellow CH-ammonium salt (0.1%) (pH 7.2 adjusted with CsOH; 280 mosmol/l). Bicuculline methiodide, 6,7-dinitroquinoxaline-2,3-dione (DNQX) disodium salt, 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX) disodium salt, 4-aminopyridine (4-AP), TTX, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), N6-cyclopentyladenosine (CPA), CGS-21680, 2-Cl-IB-MECA, 2-phenylaminoadenosine (CV-1808), dipyridamole (DIPY), 5-iodotubercidin (5-IT), and erythro-9-(2-hydroxy-3-nonyl)adenine hydrochloride (EHNA) were purchased from Tocris Bioscience (Ellisville, MO). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO). Stock solutions of 4-AP, bicuculline methiodide, DNQX, NBQX, and EHNA were prepared in H2O. Stock solutions of adenosine, DPCPX, CPA, CGS-21680, CV-1808, 2-Cl-IB-MECA, S-(4-nitrobenzyl)-6-thioinosine (NBTI), DIPY, and 5-IT were prepared in dimethyl sulfoxide (DMSO). The final concentration of DMSO in the ACSF was <0.1%.
Data analysis and statistics.
Data were analyzed using Clampfit 9 (Molecular Devices), IGOR Pro 6 (WaveMetrics, Lake Oswego, OR), Mini Analysis 6 (Synaptosoft, Leonia, NJ), Origin (Microcal, Northampton, MA), and StatView software (SAS Institute, Cary, NC). The A-current was isolated using a standard two-step voltage protocol [holding potential (Vh) = −90 and −40 mV] (Burdakov et al. 2004). The peak A-type conductance (gA) was calculated using EK = 96.8 mV (based on the Nernst K+ equilibrium potential for our solutions). The voltage-dependence of A-current activation was fit using the sigmoid function f(Vm) = 1/{1 + exp[(Vm + V50)/k]}, where Vm is the test pulse potential and V50 is the test pulse potential that gives half-maximal activation. Curve fitting was performed using Origin (Microcal). Evoked EPSC peak amplitudes were normalized by dividing the values of control and treatment samples by the mean of the control sample. Such normalization conserves the distribution and the relative variance of the samples, allowing the subsequent use of a t-test (Valcu and Valcu 2011). Data were compared using paired t-tests. Spontaneous EPSCs (sEPSCs) and mEPSCs were semiautomatically analyzed off-line using Mini Analysis software. Traces (5 min in control, last 5 min of 10-min adenosine application, and last 5 min of 15-min washout) were visually examined and erroneous events discarded. Synaptic events for each condition were ranked by amplitude and interevent interval for preparation of cumulative probability distribution. Frequency and amplitude values of sEPSCs and mEPSCs were normalized by dividing the values of control and treatment samples by the mean of the control sample. For the experiments in DPCPX, data were normalized by dividing the values of DPCPX and DPCPX + adenosine samples by the mean of the DPCPX sample. Data were compared using one-way ANOVA (with repeated measures) followed by Fisher's protected least significant difference tests. An α value <0.05 was considered significant. Results are means ± SE, and n refers to the number of cells.
Choline acetyltransferase immunohistochemistry, cell counting, and antibody characterization.
After recordings were made, slices (300 μm) were fixed overnight in a 10% buffered formalin solution (Fisher Scientific, Pittsburg, PA) and then processed for choline acetyltransferase (ChAT) and Lucifer yellow immunoreactivities. Recorded slices were incubated overnight in goat anti-ChAT primary antibody (1:1,000; Chemicon International/Millipore, Temecula, CA) and rabbit anti-Lucifer yellow primary antibodies (1:1,000; Molecular Probe/Invitrogen, Gaithersburg, MD) in phosphate-buffered saline (PBS) containing 0.3% Triton X-100 (PBT). The next day, the slices were incubated (2 h) in PBT containing donkey Cy3-anti-goat secondary antibody (1:500; Jackson ImmunoResearch, West Grove, PA) and donkey Alexa Fluor488 anti-rabbit secondary antibodies (1:200; Molecular Probes/Invitrogen). Recorded slices were examined under fluorescence to determine the location of the recorded neurons with respect to the MCPO/SI area and whether they were positive for ChAT immunoreactivity (Arrigoni et al. 2010).
To determine whether Cy3-p75NTR-IgG was internalized by only cholinergic neurons within the BF, we used seven icv Cy3-p75NTR-IgG-injected mice for ChAT double immunolabeling studies. One day after the icv injections the mice were deeply anesthetized with isoflurane and perfused transcardially with 20 ml of PBS followed by 20 ml of 10% formalin. Brains were removed, postfixed overnight in 10% formalin, equilibrated in 20% sucrose and 0.02% sodium azide in PBS, and then cut into 40-μm sections on a freezing microtome. We found that the Cy3-p75NTR-IgG labeling was mostly lost following overnight treatment in 0.3% Triton X-100; therefore, for these experiments, the sections were pretreated in PBT for only 1 h and then incubated overnight in ChAT primary antibodies (1:1,000; Chemicon International/Millipore) in PBS. The next day, the sections were incubated for 2 h in donkey Alexa Fluor488 anti-goat secondary antibodies (1:500; Jackson ImmunoResearch) in PBS. The sections were mounted on gelatin-coated slices and coverslipped.
Cy3-p75NTR-IgG-positive cells and ChAT-positive cells were counted bilaterally in 3 adjacent 40-μm sections using a ×10 objective lens. Cell counting was done using rectangular counting boxes (1.4 × 1 mm) placed in the medial septum (MS; AP = 0.68 mm from bregma), the horizontal limb of the diagonal band (hDB; AP = 0.62 mm), the magnocellular preoptic nucleus (MCPO; AP = 0.14 mm), and the substantia innominata (SI; AP = −0.1 mm) (Franklin and Paxinos 1997).
The goat polyclonal antibody against ChAT used in this study was purchased from Chemicon International/Millipore (AB144; lot no. JC1618187) (Saito et al. 2009). The rabbit polyclonal antibody against the Lucifer yellow dye was purchased from Molecular Probes/Invitrogen (A-5750; lot no. 764816). It was raised against Lucifer yellow, and the specificity of immunostaining for Lucifer yellow was indicated by the lack of detectable immunostaining in unrecorded slices. For all secondary antibody immunohistochemical controls, the primary antibodies were omitted and the tissue showed no immunoreactivity above background.
RESULTS
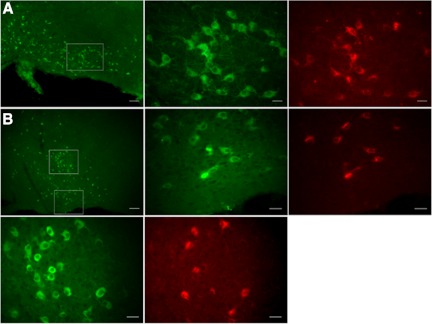
In the BF, the neurotrophin receptor p75 (p75NTR) is expressed almost exclusively on cholinergic neurons across species, including rats and mice (Rossner et al. 2000; Springer et al. 1987; Tremere et al. 2000). In recent years, a number of studies have used fluorescent conjugated anti-rat p75NTR antibodies (192IgG) to label in vivo BF cholinergic neurons in rats (Arrigoni et al. 2006; Hartig et al. 1998; Wu et al. 2000). More recently, a new polyclonal fluorescent antibody against murine p75NTR (Cy3-p75NTR-IgG; Advanced Targeting Systems) has become available. To determine whether this antibody specifically labels the cholinergic population within the BF and can therefore be used for in vitro electrophysiological recordings in mice, we injected seven mice in the lateral cerebroventricle with anti-murine Cy3-p75NTR-IgG and used them for a ChAT-immunohistochemical double-label study. From the MS to the SI region, we found that ∼50% of ChAT-positive neurons were labeled with Cy3-p75NTR-IgG (Table 1 and Fig. 1). More importantly we found that Cy3-p75NTR-IgGs were internalized almost exclusively by ChAT-positive neurons. Only 3–5% of the Cy3-p75NTR-IgG-labeled neurons in BF were not ChAT positive (Table 1), indicating that similar to what has been reported for the fluorescent 192IgG in rats (Hartig et al. 1998), the fluorescent antibody against murine p75NTRs is a useful tool to label BF cholinergic neurons for in vitro electrophysiological recordings in mice.
Table 1.
Cell counting in BF nuclei of neurons labeled by Cy3-p75NTR-IgG and positive for ChAT immunoreactivity
| %ChAT(+) Cells Labeled by Cy3-p75NTR-IgG | %Cy3-p75NTR-IgG(+) Cells ChAT(−) | No. of ChAT(+) Cells | No. of Cy3-p75NTR-IgG(+) Cells | |
|---|---|---|---|---|
| MS ipsilateral | 57.7 ± 4.5 | 5.5 ± 0.9 | 982 | 639 |
| MS contralateral | 58.8 ± 4.6 | 4.5 ± 1.0 | 871 | 575 |
| hDB ipsilateral | 53.6 ± 5.9 | 4.7 ± 1.8 | 1422 | 866 |
| hDB contralateral | 58.3 ± 5.0 | 3.8 ± 0.8 | 1848 | 1172 |
| MCPO ipsilateral | 50.2 ± 6.8 | 3.3 ± 1.5 | 1357 | 774 |
| MCPO contralateral | 51.7 ± 6.9 | 3.4 ± 1.3 | 1605 | 882 |
| SI ipsilateral | 44.6 ± 4.8 | 4.5 ± 1.6 | 485 | 237 |
| SI contralateral | 43.1 ± 5.1 | 4.0 ± 1.9 | 640 | 279 |
Percentage values are means ± SE from 7 mice, indicating the percentage of choline acetyltransferase-positive [ChAT(+)] neurons labeled with indocarbocyanine-coupled antibodies raised against mouse p75 neutrophin receptors (Cy3-p75NTR-IgG) and the percentage of neurons labeled with Cy3-p75NTR-IgG that are ChAT(−). Cell counts were performed in the medial septum (MS), horizontal limb of the diagonal band (hDB), magnocellular preoptic nucleus (MCPO), and substantia innominata (SI) ipsilateral and contralateral to the Cy3-p75NTR-IgG intracerebroventricular injections.
Fig. 1.
Cy3-coupled antibodies raised against mouse p75 neurotrophin receptors (Cy3-p75NTR-IgGs) specifically label cholinergic neurons in the basal forebrain (BF). Photographs show neurons of the horizontal limb of the diagonal band (hDB), magnocellular preoptic nucleus (MCPO), and substantia innominata (SI) nuclei. p75NTR-IgG label is shown in red and choline acetyltransferase (ChAT) immunoreactivity in green. A: ChAT immunostaining in the hDB shown at low magnification (left; scale bar = 100 μm). ChAT-positive neurons and p75NTR-IgG-positive neurons (boxed area) are shown at higher magnification at middle and right, respectively (scale bars = 25 μm). B: ChAT immunostaining in the MCPO and SI at the level of the crossing fibers of the anterior commissure is shown at low magnification (top row, left; scale bar = 200 μm). ChAT-positive neurons and p75NTR-IgG-positive neurons of the MCPO (bottom boxed area) and of the SI (top boxed area) are displayed at higher magnification at top row, middle and right, for MCPO and at bottom row, left and middle, for SI (scale bars = 25 μm).
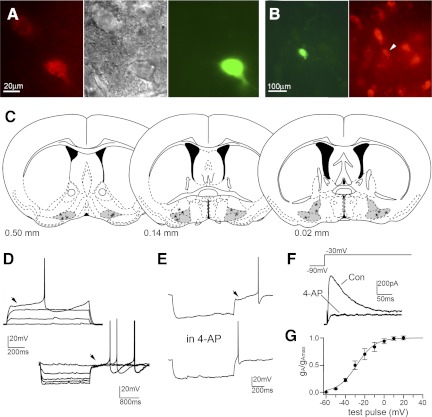
In this study, we identified MCPO/SI cholinergic neurons based on the presence of internalized Cy3-p75NTR-IgG and/or by post hoc immunoreactivity for ChAT (Fig. 2). We found that mouse MCPO/SI cholinergic neurons were mostly silent (resting membrane potential = −54.4 ± 1.1 mV; input resistance = 419.3 ± 41.6 MΩ; n = 17), and they had a distinctive delayed rebound firing on recovery from hyperpolarizing current pulses that was abolished by the A-channel blocker 4-AP (5 mM; n = 8; Fig. 2D). There was a small increase in the input resistance during the application of 4-AP (5.6 ± 10.6%; n = 8; at resting membrane potential), but it was not statistically significant (P = 0.737, paired t-test). Voltage steps to −40 mV from a holding potential of −90 mV evoked a transient outward current with rapid activation and slower inactivation, characteristic of an A-type current (Connor and Stevens 1971; Jackson and Bean 2007), that was blocked by 4-AP (n = 4). The activation threshold of the A-current was between −60 and −50 mV, and activation was half-maximal at −27.3 ± 2.7 mV (n = 9) and complete at potentials positive to +20 mV (Fig. 2G). In addition, these neurons respond to negative current pulses with a voltage-dependent rectification compatible with the presence of an inwardly rectifying potassium conductance. Collectively, these data indicate that MCPO/SI cholinergic neurons of mice have firing patterns very similar to those of the BF cholinergic neurons previously described in rats and guinea pigs (Arrigoni et al. 2006; Khateb et al. 1995; Wu et al. 2000).
Fig. 2.
A: in vivo labeling of MCPO/SI cholinergic neurons using fluorescent anti-murine p75NTR-IgGs. Two MCPO neurons were labeled with Cy3-p75NTR-IgG (left) and visualized under infrared differential interference contrast (middle), and the lower cell was filled with Lucifer yellow from the recording pipette (right). B: the recorded/Lucifer yellow-filled neuron (in green) is positive for ChAT immunoreactivity (in red). C: coronal diagrams (modified from Franklin and Paxinos 1997) represent the distribution of 17 recorded cholinergic neurons. hDB, MCPO, and SI nuclei are highlighted in gray. The number at the bottom of each diagram indicates mm anterior to bregma. D and E: firing properties of MCPO/SI neurons during depolarizing (+40 pA, from −83 mV; top traces) and hyperpolarizing current pulses (−20 pA, from −50 mV; bottom traces) showing no Ih-mediated depolarizing sag, a voltage-dependent rectification compatible with the presence of an inwardly rectifying K+ current, and delayed rebound firing on recovery from hyperpolarizing pulses due to activation of an A-type current (arrowheads) that is abolished by 5 mM 4-aminopyridine (4-AP). F: voltage-clamp recordings (1 μM TTX) of the A-type current in MCPO/SI cholinergic neurons shown as a transient outward current that is abolished by 4-AP. G: activation curve for A-type conductance (gA; see materials and methods) expressed as a fraction of maximal conductance (gAmax) and plotted against the test pulse potential (holding potential Vh = −90 mV; n = 9). The curve is the best fit of a sigmoidal function (V50 = −28.3 mV and k = 9.59 mV; χ2 < 0.001). Con, control.
Adenosine inhibits the evoked glutamatergic input to MCPO/SI cholinergic neurons through presynaptic adenosine A1 receptors.
To investigate the effects of adenosine on the excitatory input to the MCPO/SI cholinergic neurons, we placed a bipolar stimulating electrode in the MCPO/SI region within a 200-μm radius of the recorded cell. Focal electrical stimulation of 100- to 600-μs duration produced glutamatergic AMPA receptor-mediated evEPSCs (Vh = −70 mV, in 10 μM bicuculline) that were completely blocked by the AMPA receptor antagonists DNQX (20 μM; n = 4) and NBQX (10 μM; n = 3). Adenosine (100 μM) reduced the amplitude of glutamatergic evEPSCs to 47.6 ± 6.0% (n = 30; P < 0.001, paired t-test), and this effect was reversible by washout of adenosine (Fig. 3A). Additional recordings were conducted on a cohort of 6-wk-old mice. Similar to the effect of adenosine in the immature mice, adenosine reduced the amplitude of glutamatergic evEPSCs (to 52.9 ± 4.1%; n = 5; P = 0.001, paired t-test) in the 6-wk-old mice.
To determine whether adenosine acted presynaptically, we used a paired-pulse test in which two evEPSCs were evoked in rapid sequence (40-ms interstimulus time) by two identical stimulating pulses. The ratio of the evEPSC amplitude in response to the second pulse to that of the first pulse, the so-called PPR, depends on the probability of vesicular release at the synapse, and changes in PPR have been used as a measure of changes in the release probability (Debanne et al. 1996; Dobrunz and Stevens 1997). We found that adenosine (100 μM) increased PPR from 1.26 ± 0.07 (in control) to 1.53 ± 0.11 (in adenosine; n = 7; P = 0.007, paired t-test, compared with PPR in control), and PPR returned to 1.38 ± 0.11 with adenosine washout, indicating that adenosine inhibited the glutamatergic input to the MCPO/SI neurons by reducing the probability of glutamate release at the synaptic terminals (Fig. 3B).
We next asked which adenosine receptor subtypes were responsible for this effect. We found that the A1 receptor antagonist DPCPX (100 nM) abolished the effect of adenosine on the evEPSC amplitude (DPCPX + adenosine: 108.9 ± 22.5%; n = 7; P = 0.19, paired t-test, compared with evEPSC amplitude in DPCPX), whereas the A1 agonist CPA (100 nM) mimicked the adenosine effects (CPA: 63.6 ± 16.4%; n = 7; P = 0.0013; CPA + DPCPX: 107.6 ± 12.3%; n = 6; P = 0.875, paired t-test, compared with evEPSC amplitude in control and DPCPX), indicating that adenosine inhibited the glutamatergic transmission to the MCPO/SI cholinergic neurons through A1 receptors (Fig. 3, C and D). Similar effects were also found in the 6-wk-old mice in which the effect of adenosine was completely blocked by DPCPX (DPCPX + adenosine: 98.6 ± 11.8%; n = 5; P = 0.51, paired t-test, compared with evEPSC amplitude in DPCPX).
We found no effects of the A2A receptor agonist CGS-21680 (0.1–3 μM), the dual A2A and A2B receptor agonist CV-1808 (250 nM), and the A3 receptor agonist Cl-IB-MECA (100 nM) on the evEPSC amplitude [CGS-2168 (100 nM): 97.2 ± 17.1%; n = 6; P = 0.51; CGS-2168 (3 μM): 96.5 ± 13.6%; n = 9; P = 0.58; CV-1808: 99.8 ± 9.8%; n = 4; P = 0.76; and IB-MECA: 99.4 ± 13.4%; n = 6; P = 0.95, paired t-test, compared with evEPSC amplitude in control], which suggests that there is no involvement of the A2 and A3 receptors in the inhibition of glutamatergic input to MCPO/SI cholinergic neurons (Fig. 3, E–G). In addition, we found that DPCPX alone increased the evEPSC amplitude to 147.1 ± 24.5% (n = 6; P = 0.034, paired t-test, compared with evEPSC in control), indicating that the glutamatergic input was under an inhibitory tone by endogenous adenosine (Fig. 3, H and I).
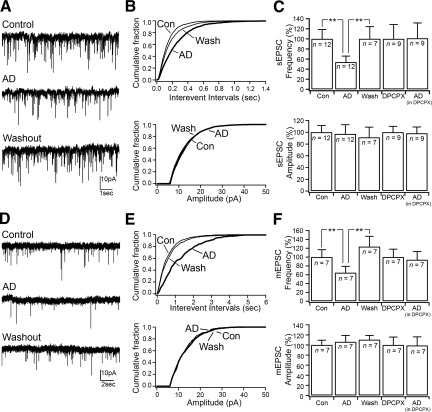
Adenosine inhibits spontaneous glutamatergic input to MCPO/SI cholinergic neurons through presynaptic adenosine A1 receptors.
We next examined the effects of adenosine on spontaneous glutamatergic input to MCPO/SI cholinergic neurons. Glutamatergic sEPSCs were recorded at a holding potential of −70 mV in bicuculline (10 μM) and were completely blocked by DNQX (20 μM; n = 4). We found that adenosine (100 μM) reduced the frequency of the glutamatergic sEPSCs and that this effect was reversed after 20 min in washout (Fig. 4). Adenosine reduced the sEPSC frequency to 53.9 ± 11.9% (control: 3.58 ± 0.67 Hz; adenosine: 1.93 ± 0.43 Hz; n = 12; P < 0.001, paired t-test; washout: 3.58 ± 0.86 Hz) but had no effects on the sEPSC amplitude (control: 12.71 ± 1.27 pA; adenosine: 11.98 ± 1.87 pA; n = 12; P = 0.41, paired t-test; washout: 11.72 ± 1.64 pA). Application of DPCPX (100 nM) blocked the effects of adenosine on sEPSC frequency (DPCPX: 4.91 ± 1.40 Hz; DPCPX + adenosine: 4.97 ± 1.50 Hz; n = 9; P = 0.88, paired t-test), indicating that the inhibition of the glutamatergic input was mediated by adenosine A1 receptors (Fig. 4).
Fig. 4.
Adenosine inhibits spontaneous glutamatergic input through presynaptic A1 receptors. A: effects of adenosine (100 μM) on the glutamatergic spontaneous EPSC (sEPSC). B: cumulative distribution plots of the sEPSC interevent intervals and amplitude (number of sEPSCs: control = 2,118; AD = 1,180; and wash = 1,830) of the cell represented in A. C: bar graphs showing the mean effects of adenosine applied alone and in the presence of DPCPX on sEPSC frequency and amplitude. In control ACSF: adenosine effects on sEPSC frequency [F(6,2) = 6.662, P = 0.011, 1-way ANOVA (**P < 0.01, Fisher's PLSD)] and on sEPSC amplitude [F(6,2) = 1.103, P = 0.363, 1-way ANOVA]. In the presence of DPCPX: adenosine effects on sEPSC frequency [F(8,1) = 0.022, P = 0.886, 1-way ANOVA] and on sEPSC amplitude [F(8,1) = 0.346, P = 0.573, 1-way ANOVA]. D: effects of adenosine on glutamatergic miniature EPSCs (mEPSCs) recorded in TTX (1 μM). E: cumulative distribution plots of mEPSC interevent intervals and amplitude (number of mEPSCs: control = 531; AD = 245; and wash = 432) of the cell represented in D. F: bar graphs showing the mean effects of adenosine applied alone and in the presence of DPCPX on mEPSC frequency and amplitude. In control ACSF: adenosine effects on mEPSC frequency [F(5,2) = 11.157, P = 0.003, 1-way ANOVA (**P < 0.01, Fisher's PLSD)] and on mEPSC amplitude [F(5,2) = 0.901, P = 0.437, 1-way ANOVA]. In the presence of DPCPX: adenosine effects on mEPSC frequency [F(8,1) = 0.022, P = 0.886, 1-way ANOVA] and on sEPSC amplitude [F(6,1) = 0.744, P = 0.421, 1-way ANOVA]. sEPSCs and mEPSCs were recorded at Vh = −70 mV.
To determine whether adenosine acted on the presynaptic glutamatergic terminals, we tested the effects of adenosine on glutamatergic mEPSCs recorded in the presence of TTX (1 μM; Vh = −70 mV). We found that adenosine decreased the frequency of glutamatergic mEPSCs to 64.6 ± 14.5% (control: 2.20 ± 0.37 Hz; adenosine: 1.42 ± 0.32 Hz; n = 7; P = 0.002, paired t-test; washout: 2.72 ± 0.52 Hz) without affecting the mEPSC amplitude (control: 16.26 ± 1.54 pA, adenosine: 17.26 ± 2.09 pA; P = 0.32, paired t-test; washout: 17.95 ± 1.41 pA), suggesting a presynaptic effect. Application of DPCPX (100 nM) abolished adenosine effects on mEPSC frequency (DPCPX: 1.72 ± 0.31 Hz; DPCPX + adenosine: 1.61 ± 0.32 Hz; n = 7; P = 0.42, paired t-test), indicating that the presynaptic adenosine receptors were A1 receptors (Fig. 4).
Endogenous extracellular adenosine.
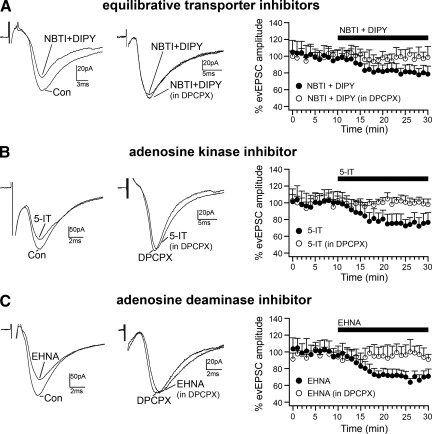
Extracellular adenosine is cleared through two main mechanisms. Adenosine is first taken up by neurons and astrocytes through the equilibrative nucleoside transporters and concentrative transporters, and it is then either phosphorylated to AMP by adenosine kinase or converted to inosine by adenosine deaminase. In addition, adenosine can be metabolized by adenosine deaminase in the extracellular matrix (Baldwin et al. 2004; Dunwiddie and Masino 2001; Fredholm et al. 2005; Noji et al. 2004). To determine the involvement of adenosine uptake (by the equilibrative nucleoside transporters: ENT1 and ENT2), adenosine phosphorylation (by adenosine kinase), and adenosine metabolism (by adenosine deaminase) in the regulation of extracellular adenosine in BF, we tested the effects of specific blockers for these enzymes on the amplitude of the evEPSC amplitude in MCPO/SI cholinergic neurons. We found that blocking the equilibrative nucleoside transporters ENT1 and ENT2 with NBTI (5 μM) + DIPY (10 μM) (Dunwiddie and Diao 1994; Frenguelli et al. 2007; Wall et al. 2007) reduced the amplitude of the evEPSCs to 79.7 ± 8.4% (n = 9; P = 0.003, paired t-test, compared with evEPSC amplitude in control). The effect of NBTI + DIPY was completely blocked by 500 nM DPCPX (DPCPX + NBTI + DIPY: 98.7 ± 9.5%; n = 5; P = 0.902, paired t-test, compared with evEPSC amplitude in DPCPX), indicating the presence of an influx of adenosine through the equilibrative nucleoside transporters (Fig. 5A).
Fig. 5.
Regulation of endogenous adenosine tone. A: the equilibrative nucleoside transporter inhibitors S-(4-nitrobenzyl)-6-thioinosine (NBTI; 5 μM) + dipyridamole (DIPY; 10 μM) reduce the amplitude of evEPSCs in BF cholinergic neurons, and this effect is blocked in the presence of DPCPX (500 nM). B: effect of the adenosine kinase inhibitor 5-iodotubercidin (5-IT; 7 μM) on evEPSC amplitude. C: effect of the adenosine deaminase inhibitor erythro-9-(2-hydroxy-3-nonyl)adenine hydrochloride (EHNA; 10 μM) on evEPSC amplitude. All evEPSCs were recorded at Vh = −70 mV. Time courses of the effects of NBTI + DIPY, 5-IT, and EHNA applied in control ACSF (●) and in the presence of DPCPX (○) are shown in graphs at right.
We next tested the role of adenosine kinase and adenosine deaminase in the regulation of extracellular adenosine. We found that both the adenosine kinase inhibitor 5-IT and the adenosine deaminase inhibitor EHNA reduced the evEPSC amplitude, and their effects were blocked by DPCPX (Fig. 5, B and C). Application of 5-IT (7 μM) reduced the evEPSC amplitude to 74.3 ± 10.1% (n = 9; P = 0.0014, paired t-test, compared with evEPSC amplitude in control), and the effect of 5-IT was blocked by DPCPX (DPCPX + 5-IT: 99.1 ± 4.6%; n = 7; P = 0.892, paired t-test, compared with evEPSC amplitude in DPCPX). Application of EHNA (10 μM) reduced the evEPSC amplitude to 68.2 ± 6.3% (n = 10; P = 0.001, paired t-test, compared with evEPSC amplitude in control), and this effect was blocked by DPCPX (DPCPX + EHNA: 96.0 ± 8.4%; n = 8; P = 0.499, paired t-test, compared with evEPSC amplitude in DPCPX). These results indicate that adenosine kinase and adenosine deaminase are both important in controlling extracellular adenosine levels and hence in the regulation of the inhibitory tone within the BF by adenosine.
DISCUSSION
In the present study we found that adenosine inhibits glutamatergic inputs to BF cholinergic neurons through the activation of presynaptic A1 receptors and that this glutamatergic input is under tonic inhibition by endogenous adenosine. We also found that extracellular adenosine levels are regulated by the combined activity of the equilibrative nucleoside transporters adenosine kinase and adenosine deaminase.
Technical considerations on the in vivo labeling of BF cholinergic neurons.
In the BF, the p75NTR is exclusively expressed on cholinergic neurons, and this is true in a number of mammalian species (Hefti et al. 1986; Maclean et al. 1997; Rossner et al. 2000; Springer et al. 1987; Tremere et al. 1998). The function of this receptor in the BF is still not fully understood, although there is increasing evidence that it might regulate axonal outgrowth and retraction (Coulson et al. 2009). Antibodies against the p75NTR conjugated with either the ribosome-inactivating protein saporin or a fluorescent dye have been used previously to both lesion and label the BF cholinergic neurons, respectively (Arrigoni et al. 2006; Fuller et al. 2011; Hartig et al. 1998; Kalinchuk et al. 2008; Kaur et al. 2008; Wu et al. 2000). An anti-murine p75NTR antibody (Advanced Targeting Systems) that recognizes the extracellular domain of murine p75NTR (Huber and Chao 1995; Rao and Anderson 1997) is now commercially available in the saporin- and Cy3-conjugated forms (Berger-Sweeney et al. 2001; Moreau et al. 2008). The aim of the double-labeling work in the present study was to determine whether this anti-murine Cy3-p75NTR-IgG could specifically label BF cholinergic neurons and whether it could be used for in vitro electrophysiological recordings in mouse brain slices. Our results show that the anti-murine Cy3-p75NTR-IgG is highly selective for BF cholinergic neurons and that about 50% of the BF cholinergic neurons, ipsi- and contralateral to the injection site, internalized the Cy3-p75NTR-IgG. There was a slightly higher percentage of cholinergic neurons labeled for Cy3-p75NTR-IgG in the rostromedial region of BF, consistent with the report that the cholinergic neurons that project to the amygdala, which are located in the caudolateral region, are devoid of p75NTR (Heckers et al. 1994). We also found a lower percentage of cholinergic neurons labeled with Cy3-p75NTR-IgG compared with previous reports (Hartig et al. 1998; Kordower et al. 1988; Rossner et al. 2000). This discrepancy may, however, be due to the detection limits of our methods. In most of the previous studies, the p75NTR-IgG-positive cells were determined by p75NTR immunoreactivity, whereas in our study we quantified the double-labeled cholinergic neurons based on the native fluorescence of the internalized Cy3-p75NTR-IgG.
It is also important to note that, as a labeling tool, this method has two distinct disadvantages: 1) it requires stereotaxic icv injections; and 2) the label is not permanent. The highest number of labeled cells was found between 24 and 48 h post-icv injection, and by days 4 and 5 the number of labeled cells and the intensity of the labeling were both significantly reduced. On the other hand, this method does offer the advantage that it can be used in wild-type and knockout mice, whereas a transgenic approach would require the crossing of two or more mouse lines (Rossi et al. 2011). Taken together, our results demonstrate that the anti-murine Cy3-p75NTR-IgG is highly selective and labels a large number, if not all, of the BF cholinergic neurons, and it is therefore a suitable marker for the in vivo labeling of BF cholinergic neurons in mice. This becomes particularly useful when recording from the SI region, where the cholinergic neurons are less densely distributed.
Adenosine inhibits the excitatory input to BF cholinergic neurons by presynaptic A1 receptors.
Previous electrophysiological studies in BF have investigated spontaneous and evoked glutamatergic EPSCs in cholinergic neurons (Momiyama et al. 1996; Sim and Griffith 1996; Zhang and Trussell 1994). The glutamate release at these synapses is controlled by multiple types of presynaptic Ca2+ channels (P/Q- and N-types), and these channels undergo postnatal developmental changes in which the contribution of the P/Q-type increases with age at the expense of the N-type Ca2+ channels (Momiyama 2010; Momiyama and Fukazawa 2007; Sim and Griffith 1996). Furthermore, the glutamatergic transmission to the BF cholinergic neurons has been shown to be inhibited by muscarinic M2, dopamine D1, and somatostatin SST1/4 presynaptic receptors, but unlike dopamine and somatostatin, which only act presynaptically, acetylcholine and adenosine, as shown in the present study, have both pre- and postsynaptic effects (Arrigoni et al. 2006; Momiyama 2010; Momiyama and Fukazawa 2007; Momiyama et al. 1996; Momiyama and Zaborszky 2006; Sim and Griffith 1996).
Previous studies have shown that adenosine, through its action on A1 receptors, inhibits the discharge of BF wake-active neurons, reduces acetylcholine release in the cortex, and directly inhibits cholinergic MCPO/SI neurons (Alam et al. 1999; Arrigoni et al. 2006; Materi et al. 2000; Thakkar et al. 2003a; Van Dort et al. 2009). In this study, we demonstrate that adenosine reduces the excitatory drive to BF cholinergic neurons, a finding that provides an additional putative inhibitory mechanism for adenosine on BF cholinergic neurons. Adenosine induces membrane hyperpolarization and inhibits glutamatergic transmission in several other brain regions, and as we have found in BF cholinergic neurons, often the pre- and postsynaptic effects of adenosine are synergistic (Arrigoni et al. 2001; Greene and Haas 1985; Kuwahata 2004; Liu and Gao 2007; Proctor and Dunwiddie 1987; Rainnie et al. 1994; Wu and Saggau 1994).
We also found that adenosine inhibits both evoked and spontaneous glutamatergic transmission to BF cholinergic neurons through a presynaptic effect. Adenosine increases evEPSC PPF, indicating that adenosine decreases glutamate release probability (Debanne et al. 1996; Dobrunz and Stevens 1997), and in the presence of TTX, adenosine decreases the frequency of the mEPSCs without affecting their amplitude, indicating that adenosine acts at the synaptic terminals (Redman 1990). This finding is consistent with our previous data showing that adenosine inhibits mEPSC frequency but not mEPSC amplitude in neurons of the brain stem cholinergic nuclei (Arrigoni et al. 2001). It remains unknown how adenosine affects glutamate release at the synaptic terminals. Adenosine could reduce the presynaptic Ca2+ influx through either the inhibition of the presynaptic voltage-dependent Ca2+ channels and/or the activation of an inwardly rectifying K+ current, which leads to the hyperpolarization of the synaptic terminal (Bean 1989; Dolphin et al. 1986; Greene and Haas 1991; Wu and Saggau 1994). In addition, adenosine could also inhibit glutamate release downstream of Ca2+ influx (Brambilla et al. 2005; Scanziani et al. 1992; Scholz and Miller 1992). In BF, for instance, dopamine inhibits the GABA release to cholinergic neurons by a presynaptic Ca2+-independent mechanism (Momiyama and Sim 1996).
Adenosine activates four subtypes of G protein-coupled adenosine receptors (A1, A2A, A2B, and A3) (Fredholm et al. 2001). MCPO/SI nuclei express A1 receptors, whereas A2A, A2B, and A3 receptor mRNAs and proteins are undetectable in the BF (Allen-Brain-Atlas 2007; Basheer et al. 2001). Consistent with this receptor distribution, MCPO/SI neurons respond to A1 but not to A2 receptor agonists (Alam et al. 1999; Arrigoni et al. 2006; Thakkar et al. 2003a). However, since glutamatergic inputs to BF cholinergic neurons can originate from many sources, it remains possible that the effects of adenosine on these afferents could be mediated by any of the four adenosine receptor subtypes. Taken from tests on a series of specific adenosine receptor agonists and antagonists, our results are consistent with adenosine signaling being mediated solely through A1 receptors, suggesting that the neuronal sources of glutamatergic input to BF cholinergic neurons express only A1 receptors or that their terminals contacting BF cholinergic neurons only express A1 receptors.
Possible sources of the glutamatergic input to BF cholinergic neurons.
BF cholinergic neurons respond to glutamate, and they are innervated by a robust glutamatergic input that represents >50% of their afference (Hur et al. 2009; Khateb et al. 1995; Momiyama and Fukazawa 2007; Sim and Griffith 1996). This input appears to arise from multiple sources, including the lateral hypothalamus, the amygdala, the intralaminar and midline nuclei of the thalamus, and the brain stem, but not the cortex, which sends glutamatergic connections back to the BF, but only to noncholinergic neurons (Carnes et al. 1990; Fadel et al. 2005; Jolkkonen et al. 2002; Zaborszky et al. 1984, 1991, 1997). It remains unclear, however, which of these regions serves as the source of the excitatory glutamatergic input to BF cholinergic neurons. Orexin neurons of the lateral hypothalamus, which also colocalize glutamate, are in direct contact with BF cholinergic neurons and also express adenosine A1 receptors, and so are a possible source of glutamatergic input to BF cholinergic neurons that would also be depressed by adenosine (Abrahamson et al. 2001; Liu and Gao 2007; Thakkar et al. 2002; Wu et al. 2004). Moreover, because orexin and glutamate localize at the same terminals, it is possible that adenosine could inhibit the release of both orexin and glutamate to BF cholinergic neurons (Torrealba et al. 2003). It is also the case that the pedunculopontine tegmentum (PPT) region could provide a wake-promoting input via the BF and that this input could be glutamatergic (Materi et al. 2000; Rasmusson et al. 1994). The cholinoceptive neurons of the medial pontine reticular formation (mPRF), which are implicated in the generation of REM sleep and wakefulness, could be another possible source of glutamatergic input to the BF (Jones 1995; Semba et al. 1988a; Steriade and McCarley 2005). These neurons are under the control of adenosine, although indirectly, through an A2- and A1-mediated modulation of the acetylcholine release (Coleman et al. 2006; Marks et al. 2003; Tanase et al. 2003). Whether mPRF neurons or their terminals respond directly to adenosine is a possibility of considerable interest. In addition, the brain stem parabrachial (PB) nucleus contains glutamatergic neurons that project to the BF (Fuller et al. 2011; Hur and Zaborszky 2005). Lesions of the PB significantly reduce wakefulness, suggesting that the PB might provide an ascending excitatory drive that activates BF cholinergic neurons to promote EEG and behavioral arousal (Fuller et al. 2011). Finally, glutamatergic BF neurons themselves could be a source of the glutamatergic input to the BF cholinergic neurons that are depressed by adenosine. These neurons in fact constitute more than half of the BF neuronal population, and about 60% of glutamatergic BF neurons fire in correlation with cortical EEG activation (Gritti et al. 2006; Hassani et al. 2009). Although the primary activating effects of the glutamatergic BF neurons on the cortex are likely mediated by direct projections to the cortex, it is also possible that these activating effects may be in part attributed to collaterals to local BF cholinergic neurons (Hajszan et al. 2004; Henny and Jones 2008; Hur et al. 2009; Hur and Zaborszky 2005). Indeed, corticopetal neurons in the BF have local axon collaterals displaying numerous boutons (Semba et al. 1987), and furthermore, noncholinergic BF neurons project to the contralateral BF (Semba et al. 1988b), but the nature of these projections, as well as the neurotransmitter content of the neurons receiving these local and commissural inputs, has not been identified. The local synaptic connection of BF is complex and probably involves multiple neuronal types including cholinergic, GABAergic, peptidergic, and glutamatergic neurons (see Zaborszky and Duque 2000 for a review on BF local circuitry). A number of studies using light and electron microcopy as well as studies combining juxtacellular recordings with immunostaining techniques have started to unravel some of the BF circuitry, but the architectural organization of the synaptic connections within the BF still remains for the most part obscured (Hur et al. 2009; Pang et al. 1998; Semba et al. 1987; Zaborszky and Duque 2000). Thus there are several as yet undefined possible sources of glutamatergic input that may promote cortical arousal through the activation of BF cholinergic neurons.
In addition to the cholinergic population, about one-third of the MCPO/SI cortically projecting neurons are GABAergic. Some of them are active during cortical arousal, whereas a second group discharges in association with EEG slow-wave activity (Duque et al. 2000; Gritti et al. 1997; Manns et al. 2000; Modirrousta et al. 2004). Although adenosine might inhibit the GABAergic wake-active neurons, it might not directly affect the sleep-active population, but as shown for the sleep-promoting neurons of the ventrolateral preoptic area (VLPO), adenosine could reduce the inhibitory drive to BF sleep-active neurons and in turn promote EEG slow-wave activity (Chamberlin et al. 2003). We previously reported that one population of noncholinergic putative GABAergic neurons was directly inhibited by adenosine while a second population was unaffected, but it remains to be determined whether adenosine could have a disinhibitory effect on these neurons (Arrigoni et al. 2006).
On the basis of the results from the present study, we hypothesize that accumulation of adenosine during prolonged wakefulness can inhibit the excitatory drive to BF cholinergic neurons and in turn reduce EEG arousal and promote the homeostatic sleep responses that follow sleep deprivation (McCarley 2007; Stenberg 2007). In addition, BF cholinergic neurons are known to modulate complex functions of vigilance including sensory processing and cognitive performances, involving both attentional and learning processes (McGaughy et al. 1996; Sarter and Bruno 1997; Sarter et al. 1999; Semba 2000). Therefore, by inhibiting cholinergic neurons, adenosine could also contribute to the progressive impairment in cognitive performance reported with sleep deprivation (Durmer and Dinges 2005).
Extracellular adenosine inhibitory tone.
Adenosine A1 receptor antagonists have shown to increase the excitability of BF cholinergic neurons (Arrigoni et al. 2006; Rainnie et al. 1994). We extend this finding in the present study by showing that DPCPX increases the amplitude of the evEPSCs in BF cholinergic neurons, indicating that glutamatergic synaptic transmission is tonically inhibited by endogenous adenosine in BF slices. Similar results have been reported in cerebellum and hippocampal slices in vitro (Dunwiddie and Diao 1994; Wall et al. 2007). We further used specific blockers to investigate the role of equilibrative nucleoside transporters, adenosine kinase and adenosine deaminase in the regulation of extracellular adenosine in the BF. The equilibrative nucleoside transporters facilitate the movement of adenosine across the cell membrane along its concentration gradient, and therefore blocking the transporters can inhibit either adenosine release or adenosine uptake depending on the direction of the adenosine gradient across the membrane (Gu et al. 1995; Latini and Pedata 2001). In several regions of the central nervous system, including the BF, blockade of the adenosine transporters increases extracellular adenosine levels (Ackley et al. 2003; Dunwiddie and Diao 1994; Porkka-Heiskanen et al. 1997; Safiulina et al. 2005; Wall et al. 2007). In our study we found that blocking adenosine equilibrative transporters with NBTI and DIPY depresses glutamatergic input and that this effect was blocked by prior application of an adenosine antagonist, indicating an influx of adenosine and a buildup of extracellular adenosine following the inhibition of its uptake.
We next investigated the involvement of adenosine kinase and adenosine deaminase in the clearance of adenosine. Adenosine kinase is thought to play a primary role in adenosine metabolism, whereas adenosine deaminase may be more important under pathophysiological conditions when adenosine levels are higher. This assumption is based mainly on the higher affinity of adenosine kinase for adenosine (2–3 orders of magnitudes higher than adenosine deaminase) and on reports that inhibitors for adenosine kinase are more effective than adenosine deaminase inhibitors at increasing extracellular levels (Latini and Pedata 2001). Interestingly, we found that the adenosine kinase inhibitor 5-IT and the adenosine deaminase inhibitor EHNA were equally effective at depressing glutamatergic input to BF cholinergic neurons, suggesting that basal extracellular levels of adenosine in the BF are likely regulated by the activity of both enzymes. The role of adenosine kinase and adenosine deaminase in controlling adenosine basal levels might vary among brain regions or might depend on the postnatal developmental age. In contrast with our results in the BF, in the hippocampus or cerebellum adenosine kinase appears to have a greater relevance than adenosine deaminase (Lloyd and Fredholm 1995; Wall et al. 2007), but whereas these studies were conducted in postweaning age animals, our work was done in immature mice. Postnatal developmental changes in the enzymes involved in the metabolism of adenosine metabolism cannot be excluded and should be addressed by future studies.
Evidence supporting a role for adenosine deaminase in sleep regulation is provided by a recent study showing that genetic variability of the adenosine deaminase gene is linked to the duration and intensity of sleep in humans (Retey et al. 2005). Furthermore, adenosine deaminase is highly expressed in the leptomeninges, where infusion of its inhibitors increases extracellular adenosine levels and promotes slow-wave sleep (Okada et al. 2003). In the brain, its highest levels are found in posterior hypothalamus and in particular in the neurons of the tuberomammillary nucleus (TMN) (Geiger and Nagy 1986; Wada et al. 1991). Because these neurons are important for the maintenance of behavioral vigilance (Lin 2000; Staines et al. 1986; Takahashi et al. 2006), the TMN is expected to be particularly sensitive to the sleep-promoting effects of adenosine deaminase inhibitors, but future studies are needed to address this possibility. Furthermore, a moderate number of adenosine deaminase-immunoreactive neurons and levels of enzymatic activity have been found in BF and other sleep regulatory nuclei, supporting the concept that its role in sleep regulation might extend beyond the TMN and the leptomeninges (Geiger and Nagy 1986; Mackiewicz et al. 2000, 2003; Staines et al. 1988). Of particular note is the finding that the ectoform of adenosine deaminase is functionally associated with the A1 receptors, and therefore it is strategically placed where the extracellular adenosine is sensed (Ciruela et al. 1996; Torvinen et al. 2002). This could mean that if coupled to the presynaptic A1 receptors in BF, adenosine deaminase could be effective in gauging the excitatory drive onto BF cholinergic neurons.
In conclusion, our findings demonstrate that adenosine inhibits glutamatergic input to BF cholinergic neurons through activation of presynaptic A1 receptors. This effect provides an additional putative mechanism by which the adenosine that accumulates during prolonged periods of wakefulness can progressively reduce the excitability of BF wake-active neurons and thereby promote sleep. The clearance of extracellular adenosine is mediated mainly by the combined effects of uptake and metabolism. Both adenosine kinase and adenosine deaminase appear to be important for maintaining the extracellular adenosine inhibitory tone within the BF.
GRANTS
This study was supported by National Institutes of Health (NIH) Grant 1R01 NS061863 and NIH Administrative Supplement Utilizing Recovery Act Funds 3R01 NS061863-01A1S3 and 1P01 HL095491-01A1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.M.H., L.L.F., S.A.K., and E.A. performed experiments; J.M.H., L.L.F., and E.A. analyzed data; J.M.H., L.L.F., and E.A. interpreted results of experiments; J.M.H., L.L.F., and E.A. prepared figures; J.M.H., L.L.F., S.A.K., and E.A. approved final version of manuscript; E.A. conception and design of research; E.A. drafted manuscript; E.A. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Sofia Z. Iqbal for helping with the animal surgeries, Dr. Patrick M. Fuller for helpful comments on this work, and Dr. Clifford B. Saper for support and advice.
REFERENCES
- Abrahamson et al., 2001. Abrahamson EE, Leak RK, Moore RY. The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport 12: 435–440, 2001 [DOI] [PubMed] [Google Scholar]
- Achermann and Borbely, 2003. Achermann P, Borbely AA. Mathematical models of sleep regulation. Front Biosci 8: s683–s693, 2003 [DOI] [PubMed] [Google Scholar]
- Ackley et al., 2003. Ackley MA, Governo RJ, Cass CE, Young JD, Baldwin SA, King AE. Control of glutamatergic neurotransmission in the rat spinal dorsal horn by the nucleoside transporter ENT1. J Physiol 548: 507–517, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam et al., 1999. Alam MN, Szymusiak R, Gong H, King J, McGinty D. Adenosinergic modulation of rat basal forebrain neurons during sleep and waking: neuronal recording with microdialysis. J Physiol 521: 679–690, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen Institute for Brain Science.Allen Institute for Brain Science. Allen Brain Atlas. (Online). http://www.brain-map.org.
- Arrigoni et al., 2006. Arrigoni E, Chamberlin NL, Saper CB, McCarley RW. Adenosine inhibits basal forebrain cholinergic and noncholinergic neurons in vitro. Neuroscience 140: 403–413, 2006 [DOI] [PubMed] [Google Scholar]
- Arrigoni et al., 2010. Arrigoni E, Mochizuki T, Scammell TE. Activation of the basal forebrain by the orexin/hypocretin neurones. Acta Physiol (Oxf) 198: 223–235, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigoni et al., 2001. Arrigoni E, Rainnie DG, McCarley RW, Greene RW. Adenosine-mediated presynaptic modulation of glutamatergic transmission in the laterodorsal tegmentum. J Neurosci 21: 1076–1085, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin et al., 2004. Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflügers Arch 447: 735–743, 2004 [DOI] [PubMed] [Google Scholar]
- Basheer et al., 2001. Basheer R, Halldner L, Alanko L, McCarley RW, Fredholm BB, Porkka-Heiskanen T. Opposite changes in adenosine A1 and A2A receptor mRNA in the rat following sleep deprivation. Neuroreport 12: 1577–1580, 2001 [DOI] [PubMed] [Google Scholar]
- Basheer et al., 2004. Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol 73: 379–396, 2004 [DOI] [PubMed] [Google Scholar]
- Bean, 1989. Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature 340: 153–156, 1989 [DOI] [PubMed] [Google Scholar]
- Benington and Heller, 1995. Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol 45: 347–360, 1995 [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney et al., 2001. Berger-Sweeney J, Stearns NA, Murg SL, Floerke-Nashner LR, Lappi DA, Baxter MG. Selective immunolesions of cholinergic neurons in mice: effects on neuroanatomy, neurochemistry, and behavior. J Neurosci 21: 8164–8173, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbely and Tobler, 1985. Borbely AA, Tobler I. Homeostatic and circadian principles in sleep regulation in the rats In: Brain Mechanisms of Sleep, edited by McGinty D. New York: Raven, 1985, p. 35–44 [Google Scholar]
- Brambilla et al., 2005. Brambilla D, Chapman D, Greene R. Adenosine mediation of presynaptic feedback inhibition of glutamate release. Neuron 46: 275–283, 2005 [DOI] [PubMed] [Google Scholar]
- Burdakov et al., 2004. Burdakov D, Alexopoulos H, Vincent A, Ashcroft FM. Low-voltage-activated A-current controls the firing dynamics of mouse hypothalamic orexin neurons. Eur J Neurosci 20: 3281–3285, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cape et al., 2000. Cape EG, Manns ID, Alonso A, Beaudet A, Jones BE. Neurotensin-induced bursting of cholinergic basal forebrain neurons promotes gamma and theta cortical activity together with waking and paradoxical sleep. J Neurosci 20: 8452–8461, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnes et al., 1990. Carnes KM, Fuller TA, Price JL. Sources of presumptive glutamatergic/aspartatergic afferents to the magnocellular basal forebrain in the rat. J Comp Neurol 302: 824–852, 1990 [DOI] [PubMed] [Google Scholar]
- Chagoya de Sanchez et al., 1993. Chagoya de Sanchez V, Hernandez Munoz R, Suarez J, Vidrio S, Yanez L, Diaz Munoz M. Day-night variations of adenosine and its metabolizing enzymes in the brain cortex of the rat—possible physiological significance for the energetic homeostasis and the sleep-wake cycle. Brain Res 612: 115–121, 1993 [DOI] [PubMed] [Google Scholar]
- Chamberlin et al., 2003. Chamberlin NL, Arrigoni E, Chou TC, Scammell TE, Greene RW, Saper CB. Effects of adenosine on GABAergic synaptic inputs to identified ventrolateral preoptic neurons. Neuroscience 119: 913–918, 2003 [DOI] [PubMed] [Google Scholar]
- Ciruela et al., 1996. Ciruela F, Saura C, Canela EI, Mallol J, Lluis C, Franco R. Adenosine deaminase affects ligand-induced signalling by interacting with cell surface adenosine receptors. FEBS Lett 380: 219–223, 1996 [DOI] [PubMed] [Google Scholar]
- Coleman et al., 2006. Coleman CG, Baghdoyan HA, Lydic R. Dialysis delivery of an adenosine A2A agonist into the pontine reticular formation of C57BL/6J mouse increases pontine acetylcholine release and sleep. J Neurochem 96: 1750–1759, 2006 [DOI] [PubMed] [Google Scholar]
- Connor and Stevens, 1971. Connor JA, Stevens CF. Prediction of repetitive firing behaviour from voltage clamp data on an isolated neurone soma. J Physiol 213: 31–53, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson et al., 2009. Coulson EJ, May LM, Sykes AM, Hamlin AS. The role of the p75 neurotrophin receptor in cholinergic dysfunction in Alzheimer's disease. Neuroscientist 15: 317–323, 2009 [DOI] [PubMed] [Google Scholar]
- Debanne et al., 1996. Debanne D, Guerineau NC, Gahwiler BH, Thompson SM. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J Physiol 491: 163–176, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz and Stevens, 1997. Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron 18: 995–1008, 1997 [DOI] [PubMed] [Google Scholar]
- Dolphin et al., 1986. Dolphin AC, Forda SR, Scott RH. Calcium-dependent currents in cultured rat dorsal root ganglion neurones are inhibited by an adenosine analogue. J Physiol 373: 47–61, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie and Diao, 1994. Dunwiddie TV, Diao L. Extracellular adenosine concentrations in hippocampal brain slices and the tonic inhibitory modulation of evoked excitatory responses. J Pharmacol Exp Ther 268: 537–545, 1994 [PubMed] [Google Scholar]
- Dunwiddie and Masino, 2001. Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci 24: 31–55, 2001 [DOI] [PubMed] [Google Scholar]
- Duque et al., 2000. Duque A, Balatoni B, Detari L, Zaborszky L. EEG correlation of the discharge properties of identified neurons in the basal forebrain. J Neurophysiol 84: 1627–1635, 2000 [DOI] [PubMed] [Google Scholar]
- Durmer and Dinges, 2005. Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol 25: 117–129, 2005 [DOI] [PubMed] [Google Scholar]
- Espana et al., 2005. Espana RA, Reis KM, Valentino RJ, Berridge CW. Organization of hypocretin/orexin efferents to locus coeruleus and basal forebrain arousal-related structures. J Comp Neurol 481: 160–178, 2005 [DOI] [PubMed] [Google Scholar]
- Fadel et al., 2005. Fadel J, Pasumarthi R, Reznikov LR. Stimulation of cortical acetylcholine release by orexin A. Neuroscience 130: 541–547, 2005 [DOI] [PubMed] [Google Scholar]
- Franklin and Paxinos, 1997. Franklin FBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 1997 [Google Scholar]
- Fredholm et al., 2001. Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53: 527–552, 2001 [PMC free article] [PubMed] [Google Scholar]
- Fredholm et al., 2005. Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol 63: 191–270, 2005 [DOI] [PubMed] [Google Scholar]
- Frenguelli et al., 2007. Frenguelli BG, Wigmore G, Llaudet E, Dale N. Temporal and mechanistic dissociation of ATP and adenosine release during ischaemia in the mammalian hippocampus. J Neurochem 101: 1400–1413, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller et al., 2011. Fuller P, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol 519: 933–956, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger and Nagy, 1986. Geiger JD, Nagy JI. Distribution of adenosine deaminase activity in rat brain and spinal cord. J Neurosci 6: 2707–2714, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene and Haas, 1985. Greene RW, Haas HL. Adenosine actions on CA1 pyramidal neurones in rat hippocampal slices. J Physiol 366: 119–127, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene and Haas, 1991. Greene RW, Haas HL. The electrophysiology of adenosine in the mammalian central nervous system. Prog Neurobiol 36: 329–341, 1991 [DOI] [PubMed] [Google Scholar]
- Gritti et al., 2006. Gritti I, Henny P, Galloni F, Mainville L, Mariotti M, Jones BE. Stereological estimates of the basal forebrain cell population in the rat, including neurons containing choline acetyltransferase, glutamic acid decarboxylase or phosphate-activated glutaminase and colocalizing vesicular glutamate transporters. Neuroscience 143: 1051–1064, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti et al., 1993. Gritti I, Mainville L, Jones BE. Codistribution of GABA- with acetylcholine-synthesizing neurons in the basal forebrain of the rat. J Comp Neurol 329: 438–457, 1993 [DOI] [PubMed] [Google Scholar]
- Gritti et al., 1997. Gritti I, Mainville L, Mancia M, Jones BE. GABAergic and other noncholinergic basal forebrain neurons, together with cholinergic neurons, project to the mesocortex and isocortex in the rat. J Comp Neurol 383: 163–177, 1997 [PubMed] [Google Scholar]
- Gu et al., 1995. Gu JG, Foga IO, Parkinson FE, Geiger JD. Involvement of bidirectional adenosine transporters in the release of l-[3H]adenosine from rat brain synaptosomal preparations. J Neurochem 64: 2105–2110, 1995 [DOI] [PubMed] [Google Scholar]
- Hajszan et al., 2004. Hajszan T, Alreja M, Leranth C. Intrinsic vesicular glutamate transporter 2-immunoreactive input to septohippocampal parvalbumin-containing neurons: novel glutamatergic local circuit cells. Hippocampus 14: 499–509, 2004 [DOI] [PubMed] [Google Scholar]
- Hartig et al., 1998. Hartig W, Seeger J, Naumann T, Brauer K, Bruckner G. Selective in vivo fluorescence labelling of cholinergic neurons containing p75(NTR) in the rat basal forebrain. Brain Res 808: 155–165, 1998 [DOI] [PubMed] [Google Scholar]
- Hassani et al., 2009. Hassani OK, Lee MG, Henny P, Jones BE. Discharge profiles of identified GABAergic in comparison to cholinergic and putative glutamatergic basal forebrain neurons across the sleep-wake cycle. J Neurosci 29: 11828–11840, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers et al., 1994. Heckers S, Ohtake T, Wiley RG, Lappi DA, Geula C, Mesulam MM. Complete and selective cholinergic denervation of rat neocortex and hippocampus but not amygdala by an immunotoxin against the p75 NGF receptor. J Neurosci 14: 1271–1289, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti et al., 1986. Hefti F, Hartikka J, Salvatierra A, Weiner WJ, Mash DC. Localization of nerve growth factor receptors in cholinergic neurons of the human basal forebrain. Neurosci Lett 69: 37–41, 1986 [DOI] [PubMed] [Google Scholar]
- Henny and Jones, 2008. Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci 27: 654–670, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber and Chao, 1995. Huber LJ, Chao MV. Mesenchymal and neuronal cell expression of the p75 neurotrophin receptor gene occur by different mechanisms. Dev Biol 167: 227–238, 1995 [DOI] [PubMed] [Google Scholar]
- Hur et al., 2009. Hur EE, Edwards RH, Rommer E, Zaborszky L. Vesicular glutamate transporter 1 and vesicular glutamate transporter 2 synapses on cholinergic neurons in the sublenticular gray of the rat basal forebrain: a double-label electron microscopic study. Neuroscience 164: 1721–1731, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur and Zaborszky, 2005. Hur EE, Zaborszky L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization study. J Comp Neurol 483: 351–373, 2005. [Erratum. J Comp Neurol 486 (May): 98–99, 2005.] [DOI] [PubMed] [Google Scholar]
- Jackson and Bean, 2007. Jackson AC, Bean BP. State-dependent enhancement of subthreshold A-type potassium current by 4-aminopyridine in tuberomammillary nucleus neurons. J Neurosci 27: 10785–10796, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper and Tessier, 1971. Jasper HH, Tessier J. Acetylcholine liberation from cerebral cortex during paradoxical (REM) sleep. Science 172: 601–602, 1971 [DOI] [PubMed] [Google Scholar]
- Jolkkonen et al., 2002. Jolkkonen E, Miettinen R, Pikkarainen M, Pitkanen A. Projections from the amygdaloid complex to the magnocellular cholinergic basal forebrain in rat. Neuroscience 111: 133–149, 2002 [DOI] [PubMed] [Google Scholar]
- Jones, 1995. Jones B. Reticular formation: cytoarchitecture, transmitters, projections. In: The Rat Nervous System, edited by Paxinos G. New York: Academic, 1995, p. 155–182 [Google Scholar]
- Jones, 2004. Jones BE. Activity, modulation and role of basal forebrain cholinergic neurons innervating the cerebral cortex. Prog Brain Res 145: 157–169, 2004 [DOI] [PubMed] [Google Scholar]
- Kalinchuk et al., 2008. Kalinchuk AV, McCarley RW, Stenberg D, Porkka-Heiskanen T, Basheer R. The role of cholinergic basal forebrain neurons in adenosine-mediated homeostatic control of sleep: lessons from 192 IgG-saporin lesions. Neuroscience 157: 238–253, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinchuk et al., 2003. Kalinchuk AV, Urrila AS, Alanko L, Heiskanen S, Wigren HK, Suomela M, Stenberg D, Porkka-Heiskanen T. Local energy depletion in the basal forebrain increases sleep. Eur J Neurosci 17: 863–869, 2003 [DOI] [PubMed] [Google Scholar]
- Kaur et al., 2008. Kaur S, Junek A, Black MA, Semba K. Effects of ibotenate and 192IgG-saporin lesions of the nucleus basalis magnocellularis/substantia innominata on spontaneous sleep and wake states and on recovery sleep after sleep deprivation in rats. J Neurosci 28: 491–504, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khateb et al., 1995. Khateb A, Fort P, Serafin M, Jones BE, Muhlethaler M. Rhythmical bursts induced by NMDA in guinea-pig cholinergic nucleus basalis neurones in vitro. J Physiol 487: 623–638, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower et al., 1988. Kordower JH, Bartus RT, Bothwell M, Schatteman G, Gash DM. Nerve growth factor receptor immunoreactivity in the nonhuman primate (Cebus apella): distribution, morphology, and colocalization with cholinergic enzymes. J Comp Neurol 277: 465–486, 1988 [DOI] [PubMed] [Google Scholar]
- Kuwahata, 2004. Kuwahata T. Effects of adenosine and ATP on the membrane potential and synaptic transmission in neurons of the rat locus coeruleus. Kurume Med J 51: 109–123, 2004 [DOI] [PubMed] [Google Scholar]
- Latini and Pedata, 2001. Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem 79: 463–484, 2001 [DOI] [PubMed] [Google Scholar]
- Lee et al., 2005. Lee MG, Hassani OK, Alonso A, Jones BE. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J Neurosci 25: 4365–4369, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, 2000. Lin JS. Brain structures and mechanisms involved in the control of cortical activation and wakefulness, with emphasis on the posterior hypothalamus and histaminergic neurons. Sleep Med Rev 4: 471–503, 2000 [DOI] [PubMed] [Google Scholar]
- Liu and Gao, 2007. Liu ZW, Gao XB. Adenosine inhibits activity of hypocretin/orexin neurons by the A1 receptor in the lateral hypothalamus: a possible sleep-promoting effect. J Neurophysiol 97: 837–848, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd and Fredholm, 1995. Lloyd HG, Fredholm BB. Involvement of adenosine deaminase and adenosine kinase in regulating extracellular adenosine concentration in rat hippocampal slices. Neurochem Int 26: 387–395, 1995 [DOI] [PubMed] [Google Scholar]
- Mackiewicz et al., 2000. Mackiewicz M, Nikonova EV, Bell CC, Galante RJ, Zhang L, Geiger JD, Pack AI. Activity of adenosine deaminase in the sleep regulatory areas of the rat CNS. Brain Res Mol Brain Res 80: 252–255, 2000 [DOI] [PubMed] [Google Scholar]
- Mackiewicz et al., 2003. Mackiewicz M, Nikonova EV, Zimmerman JE, Galante RJ, Zhang L, Cater JR, Geiger JD, Pack AI. Enzymes of adenosine metabolism in the brain: diurnal rhythm and the effect of sleep deprivation. J Neurochem 85: 348–357, 2003 [DOI] [PubMed] [Google Scholar]
- Maclean et al., 1997. Maclean CJ, Baker HF, Fine A, Ridley RM. The distribution of p75 neurotrophin receptor-immunoreactive cells in the forebrain of the common marmoset (Callithrix jacchus). Brain Res Bull 43: 197–208, 1997 [DOI] [PubMed] [Google Scholar]
- Manns et al., 2000. Manns ID, Alonso A, Jones BE. Discharge profiles of juxtacellularly labeled and immunohistochemically identified GABAergic basal forebrain neurons recorded in association with the electroencephalogram in anesthetized rats. J Neurosci 20: 9252–9263, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns et al., 2001. Manns ID, Mainville L, Jones BE. Evidence for glutamate, in addition to acetylcholine and GABA, neurotransmitter synthesis in basal forebrain neurons projecting to the entorhinal cortex. Neuroscience 107: 249–263, 2001 [DOI] [PubMed] [Google Scholar]
- Marks et al., 2003. Marks GA, Shaffery JP, Speciale SG, Birabil CG. Enhancement of rapid eye movement sleep in the rat by actions at A1 and A2a adenosine receptor subtypes with a differential sensitivity to atropine. Neuroscience 116: 913–920, 2003 [DOI] [PubMed] [Google Scholar]
- Marrosu et al., 1995. Marrosu F, Portas C, Mascia MS, Casu MA, Fa M, Giagheddu M, Imperato A, Gessa GL. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res 671: 329–332, 1995 [DOI] [PubMed] [Google Scholar]
- Materi et al., 2000. Materi LM, Rasmusson DD, Semba K. Inhibition of synaptically evoked cortical acetylcholine release by adenosine: an in vivo microdialysis study in the rat. Neuroscience 97: 219–226, 2000 [DOI] [PubMed] [Google Scholar]
- McCarley, 2007. McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med 8: 302–330, 2007 [DOI] [PubMed] [Google Scholar]
- McGaughy et al., 1996. McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav Neurosci 110: 247–265, 1996 [DOI] [PubMed] [Google Scholar]
- Modirrousta et al., 2004. Modirrousta M, Mainville L, Jones BE. GABAergic neurons with alpha2-adrenergic receptors in basal forebrain and preoptic area express c-Fos during sleep. Neuroscience 129: 803–810, 2004 [DOI] [PubMed] [Google Scholar]
- Momiyama, 2010. Momiyama T. Developmental increase in D1-like dopamine receptor-mediated inhibition of glutamatergic transmission through P/Q-type channel regulation in the basal forebrain of rats. Eur J Neurosci 32: 579–590, 2010 [DOI] [PubMed] [Google Scholar]
- Momiyama and Fukazawa, 2007. Momiyama T, Fukazawa Y. D1-like dopamine receptors selectively block P/Q-type calcium channels to reduce glutamate release onto cholinergic basal forebrain neurones of immature rats. J Physiol 580: 103–117, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momiyama and Sim, 1996. Momiyama T, Sim JA. Modulation of inhibitory transmission by dopamine in rat basal forebrain nuclei: activation of presynaptic D1-like dopaminergic receptors. J Neurosci 16: 7505–7512, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momiyama et al., 1996. Momiyama T, Sim JA, Brown DA. Dopamine D1-like receptor-mediated presynaptic inhibition of excitatory transmission onto rat magnocellular basal forebrain neurones. J Physiol 495: 97–106, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momiyama and Zaborszky, 2006. Momiyama T, Zaborszky L. Somatostatin presynaptically inhibits both GABA and glutamate release onto rat basal forebrain cholinergic neurons. J Neurophysiol 96: 686–694, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau et al., 2008. Moreau PH, Cosquer B, Jeltsch H, Cassel JC, Mathis C. Neuroanatomical and behavioral effects of a novel version of the cholinergic immunotoxin mu p75-saporin in mice. Hippocampus 18: 610–622, 2008 [DOI] [PubMed] [Google Scholar]
- Noji et al., 2004. Noji T, Karasawa A, Kusaka H. Adenosine uptake inhibitors. Eur J Pharmacol 495: 1–16, 2004 [DOI] [PubMed] [Google Scholar]
- Oishi et al., 2008. Oishi Y, Huang ZL, Fredholm BB, Urade Y, Hayaishi O. Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via A1 receptors and promotes non-rapid eye movement sleep. Proc Natl Acad Sci USA 105: 19992–19997, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada et al., 2003. Okada T, Mochizuki T, Huang ZL, Eguchi N, Sugita Y, Urade Y, Hayaishi O. Dominant localization of adenosine deaminase in leptomeninges and involvement of the enzyme in sleep. Biochem Biophys Res Commun 312: 29–34, 2003 [DOI] [PubMed] [Google Scholar]
- Pang et al., 1998. Pang K, Tepper JM, Zaborszky L. Morphological and electrophysiological characteristics of noncholinergic basal forebrain neurons. J Comp Neurol 394: 186–204, 1998 [DOI] [PubMed] [Google Scholar]
- Peca et al., 2011. Peca J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 472: 437–442, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen et al., 2002. Porkka-Heiskanen T, Alanko L, Kalinchuk A, Stenberg D. Adenosine and sleep. Sleep Med Rev 6: 321–332, 2002 [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen et al., 1997. Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science 276: 1265–1268, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portas et al., 1997. Portas CM, Thakkar M, Rainnie DG, Greene RW, McCarley RW. Role of adenosine in behavioral state modulation: a microdialysis study in the freely moving cat. Neuroscience 79: 225–235, 1997 [DOI] [PubMed] [Google Scholar]
- Proctor and Dunwiddie, 1987. Proctor WR, Dunwiddie TV. Pre- and postsynaptic actions of adenosine in the in vitro rat hippocampus. Brain Res 426: 187–190, 1987 [DOI] [PubMed] [Google Scholar]
- Radulovacki, 1995. Radulovacki M. The Pharmacology of the adenosine system. In: The Pharmacology of Sleep, edited by Kales A. Heidelberg, Germany: Springer, 1995, p. 307–319 [Google Scholar]
- Radulovacki et al., 1984. Radulovacki M, Virus RM, Djuricic-Nedelson M, Green RD. Adenosine analogs and sleep in rats. J Pharmacol Exp Ther 228: 268–274, 1984 [PubMed] [Google Scholar]
- Rainnie et al., 1994. Rainnie DG, Grunze HC, McCarley RW, Greene RW. Adenosine inhibition of mesopontine cholinergic neurons: implications for EEG arousal. Science 263: 689–692, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao and Anderson, 1997. Rao MS, Anderson DJ. Immortalization and controlled in vitro differentiation of murine multipotent neural crest stem cells. J Neurobiol 32: 722–746, 1997 [DOI] [PubMed] [Google Scholar]
- Rasmusson et al., 1994. Rasmusson DD, Clow K, Szerb JC. Modification of neocortical acetylcholine release and electroencephalogram desynchronization due to brainstem stimulation by drugs applied to the basal forebrain. Neuroscience 60: 665–677, 1994 [DOI] [PubMed] [Google Scholar]
- Redman, 1990. Redman S. Quantal analysis of synaptic potentials in neurons of the central nervous system. Physiol Rev 70: 165–198, 1990 [DOI] [PubMed] [Google Scholar]
- Retey et al., 2005. Retey JV, Adam M, Honegger E, Khatami R, Luhmann UF, Jung HH, Berger W, Landolt HP. A functional genetic variation of adenosine deaminase affects the duration and intensity of deep sleep in humans. Proc Natl Acad Sci USA 102: 15676–15681, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi et al., 2011. Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab 13: 195–204, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossner et al., 2000. Rossner S, Schliebs R, Bigl V. Intracerebroventricular infusion of CHO5, a rat monoclonal antibody directed against mouse low-affinity nerve growth factor receptor (p75NTR), specifically labels basal forebrain cholinergic neurons in mouse brain. Metab Brain Dis 15: 17–27, 2000 [DOI] [PubMed] [Google Scholar]
- Safiulina et al., 2005. Safiulina VF, Kasyanov AM, Giniatullin R, Cherubini E. Adenosine down-regulates giant depolarizing potentials in the developing rat hippocampus by exerting a negative control on glutamatergic inputs. J Neurophysiol 94: 2797–2804, 2005 [DOI] [PubMed] [Google Scholar]
- Saito et al., 2009. Saito A, Sato T, Okano H, Toyoda K, Bamba H, Kimura S, Bellier JP, Matsuo A, Kimura H, Hisa Y, Tooyama I. Axotomy alters alternative splicing of choline acetyltransferase in the rat dorsal motor nucleus of the vagus nerve. J Comp Neurol 513: 237–248, 2009 [DOI] [PubMed] [Google Scholar]
- Saper, 1984. Saper CB. Organization of cerebral cortical afferent systems in the rat. II. Magnocellular basal nucleus. J Comp Neurol 222: 313–342, 1984 [DOI] [PubMed] [Google Scholar]
- Sarter and Bruno, 1997. Sarter M, Bruno JP. Cognitive functions of cortical acetylcholine: toward a unifying hypothesis. Brain Res Brain Res Rev 23: 28–46, 1997 [DOI] [PubMed] [Google Scholar]
- Sarter et al., 1999. Sarter M, Bruno JP, Turchi J. Basal forebrain afferent projections modulating cortical acetylcholine, attention, and implications for neuropsychiatric disorders. Ann NY Acad Sci 877: 368–382, 1999 [DOI] [PubMed] [Google Scholar]
- Scammell et al., 1998. Scammell TE, Griffin JD, Elmquist JK, Saper CB. Microinjection of a cyclooxygenase inhibitor into the anteroventral preoptic region attenuates LPS fever. Am J Physiol Regul Integr Comp Physiol 274: R783–R789, 1998 [DOI] [PubMed] [Google Scholar]
- Scanziani et al., 1992. Scanziani M, Capogna M, Gahwiler BH, Thompson SM. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron 9: 919–927, 1992 [DOI] [PubMed] [Google Scholar]
- Scholz and Miller, 1992. Scholz KP, Miller RJ. Inhibition of quantal transmitter release in the absence of calcium influx by a G protein-linked adenosine receptor at hippocampal synapses. Neuron 8: 1139–1150, 1992 [DOI] [PubMed] [Google Scholar]
- Schwaber et al., 1987. Schwaber JS, Rogers WT, Satoh K, Fibiger HC. Distribution and organization of cholinergic neurons in the rat forebrain demonstrated by computer-aided data acquisition and three-dimensional reconstruction. J Comp Neurol 263: 309–325, 1987 [DOI] [PubMed] [Google Scholar]
- Semba, 2000. Semba K. Multiple output pathways of the basal forebrain: organization, chemical heterogeneity, and roles in vigilance. Behav Brain Res 115: 117–141, 2000 [DOI] [PubMed] [Google Scholar]
- Semba et al., 1988a. Semba K, Reiner PB, McGeer EG, Fibiger HC. Brainstem afferents to the magnocellular basal forebrain studied by axonal transport, immunohistochemistry, and electrophysiology in the rat. J Comp Neurol 267: 433–453, 1988a [DOI] [PubMed] [Google Scholar]
- Semba et al., 1987. Semba K, Reiner PB, McGeer EG, Fibiger HC. Morphology of cortically projecting basal forebrain neurons in the rat as revealed by intracellular iontophoresis of horseradish peroxidase. Neuroscience 20: 637–651, 1987 [DOI] [PubMed] [Google Scholar]
- Semba et al., 1988b. Semba K, Reiner PB, McGeer EG, Fibiger HC. Non-cholinergic basal forebrain neurons project to the contralateral basal forebrain in the rat. Neurosci Lett 84: 23–28, 1988b [DOI] [PubMed] [Google Scholar]
- Sim and Griffith, 1996. Sim JA, Griffith WH. Muscarinic inhibition of glutamatergic transmissions onto rat magnocellular basal forebrain neurons in a thin-slice preparation. Eur J Neurosci 8: 880–891, 1996 [DOI] [PubMed] [Google Scholar]
- Springer et al., 1987. Springer JE, Koh S, Tayrien MW, Loy R. Basal forebrain magnocellular neurons stain for nerve growth factor receptor: correlation with cholinergic cell bodies and effects of axotomy. J Neurosci Res 17: 111–118, 1987 [DOI] [PubMed] [Google Scholar]
- Staines et al., 1986. Staines WA, Yamamoto T, Daddona PE, Nagy JI. Neuronal colocalization of adenosine deaminase, monoamine oxidase, galanin and 5-hydroxytryptophan uptake in the tuberomammillary nucleus of the rat. Brain Res Bull 17: 351–365, 1986 [DOI] [PubMed] [Google Scholar]
- Staines et al., 1988. Staines WA, Yamamoto T, Dewar KM, Daddona PE, Geiger JD, Nagy JI. Distribution, morphology and habenular projections of adenosine deaminase-containing neurons in the septal area of rat. Brain Res 455: 72–87, 1988 [DOI] [PubMed] [Google Scholar]
- Stenberg, 2007. Stenberg D. Neuroanatomy and neurochemistry of sleep. Cell Mol Life Sci 64: 1187–1204, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade and McCarley, 2005. Steriade M, McCarley RW. Brain Control of Wakefulness and Sleep. New York: Kluwer Academic/Plenum, 2005 [Google Scholar]
- Strecker et al., 2000. Strecker RE, Morairty S, Thakkar MM, Porkka-Heiskanen T, Basheer R, Dauphin LJ, Rainnie DG, Portas CM, Greene RW, McCarley RW. Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behav Brain Res 115: 183–204, 2000 [DOI] [PubMed] [Google Scholar]
- Takahashi et al., 2006. Takahashi K, Lin JS, Sakai K. Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J Neurosci 26: 10292–10298, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanase et al., 2003. Tanase D, Baghdoyan HA, Lydic R. Dialysis delivery of an adenosine A1 receptor agonist to the pontine reticular formation decreases acetylcholine release and increases anesthesia recovery time. Anesthesiology 98: 912–920, 2003 [DOI] [PubMed] [Google Scholar]
- Thakkar et al., 2003a. Thakkar MM, Delgiacco RA, Strecker RE, McCarley RW. Adenosinergic inhibition of basal forebrain wakefulness-active neurons: a simultaneous unit recording and microdialysis study in freely behaving cats. Neuroscience 122: 1107–1113, 2003a [DOI] [PubMed] [Google Scholar]
- Thakkar et al., 2003b. Thakkar MM, Winston S, McCarley RW. A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: effects of antisense to the A1 receptor in the cholinergic basal forebrain. J Neurosci 23: 4278–4287, 2003b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar et al., 2002. Thakkar MM, Winston S, McCarley RW. Orexin neurons of the hypothalamus express adenosine A1 receptors. Brain Res 944: 190–194, 2002 [DOI] [PubMed] [Google Scholar]
- Torrealba et al., 2003. Torrealba F, Yanagisawa M, Saper CB. Colocalization of orexin a and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neuroscience 119: 1033–1044, 2003 [DOI] [PubMed] [Google Scholar]
- Torvinen et al., 2002. Torvinen M, Gines S, Hillion J, Latini S, Canals M, Ciruela F, Bordoni F, Staines W, Pedata F, Agnati LF, Lluis C, Franco R, Ferre S, Fuxe K. Interactions among adenosine deaminase, adenosine A1 receptors and dopamine D1 receptors in stably cotransfected fibroblast cells and neurons. Neuroscience 113: 709–719, 2002 [DOI] [PubMed] [Google Scholar]
- Tremere et al., 1998. Tremere L, Bruckner G, Brauer K, Rasmusson DD, Poethke R, Hartig W. Co-expression of p75NTR- and calbindin-immunoreactivity in cholinergic neurons of the raccoon basal forebrain. Brain Res 797: 351–356, 1998 [DOI] [PubMed] [Google Scholar]
- Tremere et al., 2000. Tremere LA, Pinaud R, Grosche J, Hartig W, Rasmusson DD. Antibody for human p75 LNTR identifies cholinergic basal forebrain of non-primate species. Neuroreport 11: 2177–2183, 2000 [DOI] [PubMed] [Google Scholar]
- Urade et al., 2003. Urade Y, Eguchi N, Qu WM, Sakata M, Huang ZL, Chen JF, Schwarzschild MA, Fink JS, Hayaishi O. Sleep regulation in adenosine A2A receptor-deficient mice. Neurology 61: S94–96, 2003 [DOI] [PubMed] [Google Scholar]
- Valcu and Valcu, 2011. Valcu M, Valcu CM. Data transformation practices in biomedical sciences. Nat Methods 8: 104–105, 2011 [DOI] [PubMed] [Google Scholar]
- Van Dort et al., 2009. Van Dort CJ, Baghdoyan HA, Lydic R. Adenosine A1 and A2A receptors in mouse prefrontal cortex modulate acetylcholine release and behavioral arousal. J Neurosci 29: 871–881, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada et al., 1991. Wada H, Inagaki N, Yamatodani A, Watanabe T. Is the histaminergic neuron system a regulatory center for whole-brain activity? Trends Neurosci 14: 415–418, 1991 [DOI] [PubMed] [Google Scholar]
- Wall et al., 2007. Wall MJ, Atterbury A, Dale N. Control of basal extracellular adenosine concentration in rat cerebellum. J Physiol 582: 137–151, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf, 1991. Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol 37: 475–524, 1991 [DOI] [PubMed] [Google Scholar]
- Wu and Saggau, 1994. Wu LG, Saggau P. Adenosine inhibits evoked synaptic transmission primarily by reducing presynaptic calcium influx in area CA1 of hippocampus. Neuron 12: 1139–1148, 1994 [DOI] [PubMed] [Google Scholar]
- Wu et al., 2000. Wu M, Shanabrough M, Leranth C, Alreja M. Cholinergic excitation of septohippocampal GABA but not cholinergic neurons: implications for learning and memory. J Neurosci 20: 3900–3908, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al., 2004. Wu M, Zaborszky L, Hajszan T, van den Pol AN, Alreja M. Hypocretin/orexin innervation and excitation of identified septohippocampal cholinergic neurons. J Neurosci 24: 3527–3536, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky et al., 2005. Zaborszky L, Buhl DL, Pobalashingham S, Bjaalie JG, Nadasdy Z. Three-dimensional chemoarchitecture of the basal forebrain: spatially specific association of cholinergic and calcium binding protein-containing neurons. Neuroscience 136: 697–713, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky et al., 1991. Zaborszky L, Cullinan WE, Braun A. Afferents to basal forebrain cholinergic projection neurons: an update. Adv Exp Med Biol 295: 43–100, 1991 [DOI] [PubMed] [Google Scholar]
- Zaborszky and Duque, 2000. Zaborszky L, Duque A. Local synaptic connections of basal forebrain neurons. Behav Brain Res 115: 143–158, 2000 [DOI] [PubMed] [Google Scholar]
- Zaborszky and Duque, 2003. Zaborszky L, Duque A. Sleep-wake mechanisms and basal forebrain circuitry. Front Biosci 8: d1146–d1169, 2003 [DOI] [PubMed] [Google Scholar]
- Zaborszky et al., 1997. Zaborszky L, Gaykema RP, Swanson DJ, Cullinan WE. Cortical input to the basal forebrain. Neuroscience 79: 1051–1078, 1997 [DOI] [PubMed] [Google Scholar]
- Zaborszky et al., 1984. Zaborszky L, Leranth C, Heimer L. Ultrastructural evidence of amygdalofugal axons terminating on cholinergic cells of the rostral forebrain. Neurosci Lett 52: 219–225, 1984 [DOI] [PubMed] [Google Scholar]
- Zaborszky et al., 1999. Zaborszky L, Pang K, Somogyi J, Nadasdy Z, Kallo I. The basal forebrain corticopetal system revisited. Ann NY Acad Sci 877: 339–367, 1999 [DOI] [PubMed] [Google Scholar]
- Zhang and Trussell, 1994. Zhang S, Trussell LO. Voltage clamp analysis of excitatory synaptic transmission in the avian nucleus magnocellularis. J Physiol 480: 123–136, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao et al., 2011. Zhao S, Ting JT, Atallah HE, Qiu L, Tan J, Gloss B, Augustine GJ, Deisseroth K, Luo M, Graybiel AM, Feng G. Cell type-specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat Methods 8: 745–752, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]