Abstract
Connexin channels mediate electrical synaptic transmission when assembled as cell-to-cell pores at gap junctions and can mediate transmembrane currents when expressed in plasma membranes as hemichannels. They are widely expressed in the vertebrate retina where in electrical synapses they are critical for transmission of visual signals. While the roles of connexins in electrical synapses are well-studied, the function and roles of connexin hemichannels in the nervous system are less well understood. Genetic deletion in zebrafish of connexin (Cx) 55.5 alters horizontal cell feedback to cones, spectral responses, and visual behavior. Here, we have characterized the properties of hemichannel currents in zebrafish retinal horizontal cells and examined the roles of two connexin isoforms, Cx55.5 and Cx52.6, that are coexpressed in these cells. We report that zebrafish horizontal cells express hemichannel currents that conduct inward current at physiological negative potentials and Ca2+ levels. Manipulation of Cx55.5 and Cx52.6 gene expression in horizontal cells of adult zebrafish revealed that both Cx55.5 and Cx52.6 contribute to hemichannel currents; however, Cx55.5 expression is necessary for high-amplitude currents. Similarly, coexpression of Cx55.5 with Cx52.6 in oocytes increased hemichannel currents in a supra-additive manner. Taken together these results demonstrate that zebrafish horizontal cell hemichannel currents exhibit the functional characteristics necessary to contribute to synaptic feedback at the first visual synapse, that both Cx55.5 and Cx52.6 contribute to hemichannel currents, and that Cx55.5 may have an additional regulatory function enhancing the amplitude of hemichannel currents.
Keywords: zebrafish, oocyte, horizontal cells, connexin
the vertebrate retina is a highly ordered laminar structure composed of multiple types of neurons and synapses. Many retinal neurons have been shown to express electrical synapses, which consists of a collection of connexin proteins. These connexin protein channels play a prominent role in retinal circuitry, mediating electrical synaptic communication at retinal gap junctions (Mills and Massey, 1995; Guldenagel et al., 2000; Massey et al., 2003; Bloomfield and Volgyi, 2009). Thus, the vertebrate retina offers a model system to study the diverse properties and roles of connexin channels in neural function. Whereas the roles of connexin-mediated electrical synapses in retinal function are well studied, the roles of connexin-mediated plasma membrane currents due to connexin hemichannels are less well understood.
As the structural subunits of gap junctions and hemichannels, connexin proteins are encoded by a multigene family. Twenty connexin gene isoforms have been identified in humans and 19 in the mouse (Sohl and Willecke, 2004), whereas, in the zebrafish, an expanded family of 37 connexin genes has been revealed, and more than 10 connexin genes are expressed in the retina (Zoidl et al., 2008). Cell-to-cell connexin pores at gap junctions can be formed from a single connexin isoform contributed by both cells (homotypic) or by two different connexin isoforms, one contributed by each cell across the synapse (heterotypic). Similarly, connexin hemichannels can be formed by a single isoform (homomeric) or by incorporating different connexin isoforms (heteromeric) into the hexameric structure that forms each hemichannel, a connexon. Connexin composition influences the properties of voltage and chemical gating, size and charge selectivity, pore conductance, permeability, and regulation of connexin-mediated currents (Ebihara et al., 1995; Dermietzel et al., 2000; Beahm and Hall, 2002).
The primary function proposed for connexin hemichannels in the retina to date is mediation of horizontal cell feedback to cones via an ephaptic mechanism dependent on inward hemichannel currents generated in horizontal cells during hyperpolarizing light responses (Kamermans et al., 2001; Fahrenfort et al., 2009). In zebrafish, the connexin channel genes connexin (Cx) 55.5 and Cx52.6 are specifically expressed in retinal horizontal cells and are capable of forming functional hemichannels in heterologous expression systems (Dermietzel et al., 2000; Zoidl et al., 2004; Shields et al., 2007). Recently, functional deletion in zebrafish of Cx55.5 has been shown to alter many aspects of retinal physiology and behavior, including horizontal cell feedback to cones, spectral opponent horizontal cell light responses, Cx52.6 expression and horizontal cell hemichannel currents, and behaviorally measured constrast sensitivity (Klaassen et at., 2011). Those results provide genetic evidence for a role for connexins in horizontal cell feedback and engender questions regarding the functional characteristics of the native hemichannel currents of zebrafish horizontal cells that allow them to contribute to horizontal cell function and feedback and to what degree the physiological and molecular effects of Cx55.5 knockout could be mediated by developmental or genetic compensation.
Here we have further characterized the hemichannel currents of wild-type zebrafish retinal horizontal cells and examined the molecular roles of Cx55.5 and Cx52.6 using patch-clamp recording combined with acute morpholino-mediated gene knockdown in the adult retina, as well as heterologous expression in oocytes. Our data show that the hemichannel currents of horizontal cells maintain an inward component that is active at physiological negative potentials and at physiological extracellular Ca2+ levels, appropriate for mediating inward currents during hyperpolarizing light responses in situ.
METHODS
Cell culture.
All experiments were conducted in accordance with the National Institutes of Health guidelines for animal use and with the approval of the Vanderbilt University Institutional Animal Care and Use Committee. Wild-type adult zebrafish (Danio rerio) were dark-adapted and killed, and then the eyes were enucleated and hemisected under dim red light. Retinas were extracted and incubated in L-15 medium (GIBCO) for 10 min, containing 20 U/ml papain (Worthington) and 0.3 mg/ml cysteine. After being rinsed with six changes of fresh L-15 medium, retinas were dissociated by repeated passage through a trimmed 1-ml pipette tip. Cultures were maintained at 20°C.
Solutions and chemicals.
The normal Ca2+ bath solution contained (in mM): 137 NaCl, 2.5 KCl, 2.5 MgCl2, 2.5 CaCl2, 10 HEPES, 10 glucose, and 1 mg/ml BSA (Fraction VII; Sigma). The Ca2+-free bath solution contained (in mM): 114.5 NaCl, 2.5 KCl, 1 MgCl2, 10 HEPES, 30 CsCl, 1 sodium pyruvate, 10 glucose, and 1 mg/ml BSA. Both whole cell and outside-out single channel recordings used the same pipette solution. To block potassium channels, K+ in the normal pipette solution was replaced by Cs+ and tetraethylammonium chloride (TEA). The pipette solution contained (in mM): 124 CsCl, 1 CaCl2, 11 EGTA, 10 HEPES, 1 Mg-ATP, 0.1 Na-GTP, and 10 TEA, pH to 7.5 with CsOH. The external solution switch surrounding the target cell was completed within 30 s using a perfusion system.
Patch-clamp recording.
Recordings from solitary horizontal cells were performed using the conventional whole cell patch-clamp configuration. Patch pipettes were pulled from Corning 7052 glass (AM Systems) and fire-polished to resistances of 6–10 MΩ. The pipette series resistance and capacitance were compensated by 80%. The offset potential between the pipette and bath solutions was zeroed before seal formation. For outside-out single channel recording, recording pipettes were fire polished with resistance of 10–20 MΩ and were coated with silicone elastomer to reduce pipette capacitance and noise. Single channel currents were filtered at 2 kHz (−3 dB Bessel filter) and digitally sampled at 20 kHz. Currents were recorded using an Axopatch one-dimensional amplifier (Axon Instruments) in voltage-clamp mode. Morpholino-transduced horizontal cells were identified for recording by the red fluorescence of the lissamine tag incorporated into the oligos (Cy3 filter; Chroma).
Data analysis.
The amplitude of macroscopic hemichannel currents referred to in this work was read as steady-state currents in the last 10 ms of the voltage steps. Single channel currents were analyzed using pCLAMP 9.0 software. All of the recordings were further digitally filtered at 500 Hz (lowpass 8-pole Bessel) in the analysis software. Single channel amplitudes were binned and displayed as an all-point amplitude histogram and fit by the sum of two Gaussians. The distributions of channel opening and shutting duration were plotted as a histogram fit by a single decaying exponential curve. The single channel open probability (Po) was determined from the ratio of the time in the open state to the duration of recording (T): NPo = (t1 + t2 + t3 … tn)/T, where t is the amount of time that N channels are open, and N is the number of channel levels observed in the patch (1 for most).
The results are presented as means ± SE. P values stated were calculated using either the t or the paired t-test. Curve fitting and statistical analyses were done with Sigmaplot 10.0.
Intraocular injection and electroporation of morpholinos in adult zebrafish retina.
Dark-adapted adult wild-type zebrafish were anesthetized in 2-phenoxyethanol dilute solution (1:1,000). The outer cornea layer was removed, and a small incision was made in the cornea adjacent to the iris. Around 0.5 μl lissamine-tagged morpholino solutions (3 mM) were injected into the vitreous space of the left eye through the incision while the right eye was not injected, retaining it as a control.
After the intraocular injection, fish were transferred to a petri dish filled with anesthesic and individually received the electroporation event. A 3-mm-diameter platinum plate electrode (CUY 650-P3 Tweezers; Protech International) was applied to direct the morpholinos into the retina. We typically target the dorsal part of the retina by gently pressing the ventral half of the eye with the positive electrode. Because the morpholinos are lissamine tagged, they are easily visualized as a red fluorescence. Electroporation of the left eye was performed using a CUY 21 Edit Square Wave Electroporator (Protech Internatioinal) through two consecutive 50-ms pulses at 75 volts with a 999-ms pause between pulses. The fish were then revived in fresh fish water within 5 min. Four or five days following the electroporation, the retina was removed for further experiments.
Morpholino oligonucleotides.
Four different lissamine-tagged morpholino oligonucleotides were used in this study (Gene Tools, Philomath, OR): 1) standard control morpholino (5′-CCTCTTACCTCAGTTACAATTTATA-3′), which is targeted to a splice site mutant of human β-gobin and has no complementary sequence in the zebrafish genome; 2) anti-Cx55.5 morpholino (5′-CACCAAGAAAGTTCCAGTCTCCCAT-3′) targets the first 25 bases of the Cx55.5 gene coding sequence and is designed to inhibit translation of the Cx55.5 RNA into protein; 3) anti-Cx52.6 morpholino (5′-TCACATGGCCTAAAACTGGAAGAGT-3′) targets the 25 bases of the 5′-untranslated region (UTR) of Cx52.6 gene proximal to the transcription start site and is designed to inhibit translation of the Cx52.6 RNA into protein; and 4) anti-Cx55.5 morpholino II (5′-TACTCGGGCTTTTTTGTGTCTGTAA-3′) targets the 25 bases of the 5′-UTR region of Cx55.5 gene proximal to the transcription start site and is designed to inhibit translation of the Cx55.5 RNA into protein. Each was resuspended in distilled water to make a stock solution of 3 mM concentration and stored at 4°C.
Immunocytochemistry.
The primary antibodies used here against Cx52.6 and Cx55.5 were previously confirmed as specific for these connexins in zebrafish retinal neurons based on controls with preimmune sera, preabsorption with antigenic peptide, and Western blotting (Shields et al., 2007). For our experiments, cultured isolated cells were fixed at room temperature with 4% paraformaldehyde in 0.1 M pH 6.5 phosphate buffer for 10 min and then fixed with 4% paraformaldehyde in 0.1 M pH 10.4 sodium carbonate buffers for another 10 min. After the samples were rinsed with 0.1 M pH 7.4 PBS for 4 × 10 min, they were blocked at room temperature with 2% normal goat serum (NGS; Jackson Immunoresearch, West Grove, PA) in 0.1 M pH 7.4 PBS for 1 h. Next, the cell samples were incubated with primary antibodies for 2 h at room temperature and then overnight at 4°C. Primary antibodies were dissolved in 0.1 M pH 7.4 PBS containing 0.3% Triton X-100, 0.02% sodium azide, and 5% NGS. Primary antibodies of rabbit anti-Cx52.6 and rabbit anti-Cx55.5 (Shields et al., 2007) were diluted 1:800 and 1:4,000 respectively. After being rinsed, the samples were incubated in secondary antibodies for 2 h. The secondary antibodies (1:500) were Alexa Fluor 488 goat anti-rabbit IgG (Molecular Probes, Eugene, OR).
Confocal imaging.
Specimens were visualized using a Zeiss LSM5 PASCAL confocal microscopy (Carl Zeiss, Thornwood, NY) at the excitation wavelength of 488 nm for Alexa 488. Lissamine-tagged morpholinos were visualized via a 543-nm laser excitation (excitation peak: 575 nm) and a 560-nm long pass emission filter (emission peak: 593 nm) in confocal microscopy. Horizontal cells were z-scanned at a thickness of 0.8 μm, digitized, and projected using software from the LSM5. The contrast and brightness of sampled images were adjusted using either confocal software (Pascal) or Adobe Photoshop. All settings were the same for each set of experiments.
Oocytes.
Xenopus laevis oocytes were obtained from EcoCyte Bioscience (Castrop-Rauxel, Germany). With the use of a nanoject II (Drummond, Broomall, PA), oocytes were injected with 46 nl of a solution containing a total of 100 ng/μl RNA coding for the indicated constructs and 20 ng/μl of an antisense oligonucleotide against Cx38 mRNA in diethylpyrocarbonate-treated water. Oocytes injected with 46 nl of a solution containing only the oligonucleotide served as controls. The cells were incubated at 18°C for ∼72 h, after which they were kept at 4°C for at most 60 h. Medium was first refreshed after 72 h and then after each subsequent 24 h. Cells were incubated in a Modified Barth's solution containing (in mM): 88 NaCl, 1.0 KCl, 0.4 CaCl, 2.4 NaHCO3, 0.33 Ca(NO3)2, 0.82 MgSO4, 5.0 C6H12O6, and 15.0 HEPES, adjusted to pH 7.6 with 10 M NaOH.
Oocyte electrophysiology.
Perfusion solutions contained (in mM): 110 NaCl, 1.3 KCl, 3.0 NaHCO3, 0.9 MgSO4, and 19.0 HEPES, adjusted to pH 7.6 with 10 M NaOH. Two CaCl2 concentrations were used: 0.1 and 10 mM. The 10 mM condition was considered as the condition where hemichannels were closed. Oocytes were placed in a OPC-1 oocyte perfusion chamber (Automate Scientific, Berkeley, CA). A gravity-driven perfusion system was used in combination with a ValveLink8.2 controller (Automate Scientific). Electrodes were pulled on a Sutter PC87 puller (Sutter) from GC150TF-10 capillaries with an inner diameter of 1.17 mm and an outer diameter of 1.50 mm (Harvard Apparatus, Kent, UK) to a resistance of 0.2–1 MΩ and filled with 3 M KCl, 10 mM EGTA, and 10 mM HEPES in water, adjusted to pH 7.4 with NaOH. Electrodes were connected to an OC-725C Oocyte Clamp (Warner Instruments, Hamden, CT) and a CED1401 mkII (Cambridge Electronic Devises, Cambridge, UK). Data acquisition was done with a personal computer running Signal 3.0 (Cambridge Electronic Design, Cambridge, UK). Cells were kept at a holding potential of either −60 mV and stepped for 10 s to potentials varying between −100 and 20 mV. Experiments were performed at room temperature. Data analysis was performed using Matlab (MathWorks, Natick, USA), Excel (Microsoft, Redmond, WA), and Origin Pro 8.0 (OriginLab, Northampton, PA). Current-voltage relations were constructed by averaging over 20 ms around the indicated times in the trace. From these, conductances were calculated and plotted. Means and SE were calculated for the indicated number of cells.
RESULTS
Cx55.5 and Cx52.6 are expressed in cultured zebrafish retinal horizontal cells.
Cx55.5 and Cx52.6 proteins are expressed specifically in horizontal cells of the zebrafish intact retina (Shields et al., 2007). In the present study, we first tested by immunocytochemistry whether both of these connexins remain expressed in cultured horizontal cells after isolation. Horizontal cells were readily identified in culture due to their relatively large cell bodies with multiple processes (Fig. 1A). Cells were fixed and stained with antibodies against Cx55.5 (Fig. 1B, left) or Cx52.6 (Fig. 1B, right), and both antibodies stained cells with bright puncta on the cell body and dendrites.
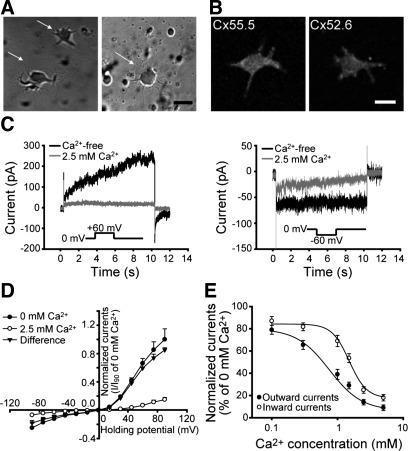
Fig. 1.
Voltage-gating and Ca2+-gating properties of low Ca2+-evoked hemichannel currents in cultured zebrafish retinal horizontal cells (HCs). A: photomicrographs of solitary HCs in primary cell culture (indicated by arrows) immediately after isolation (left) and 1 day later in culture (right). Scale bar = 10 μm. B: immunohistochemistry for connexin (Cx) 55.5 and Cx52.6 proteins in cultured solitary HCs. Both Cx55.5 (left) and Cx52.6 (right) antibodies produce intense and bright punctate staining on the somata and dendrites of cultured HCs. Scale bar = 5 μm. C: both outward (left) and inward (right) components of hemichannel currents were substantially blocked in 2.5 mM Ca2+ medium (gray traces), whereas they were greatly increased after perfusion with Ca2+-free medium (black traces). D: normalized current-voltage (I–V) curves in Ca2+-free medium (●) and in 2.5 mM Ca2+ medium (○). Net currents mediated by hemichannels were shown as the difference (▴) (n = 5, mean ± SE). E: normalized Ca2+ inhibitory curves for outward (●) and inward (○) components of hemichannel currents. Each point is an average from 4–6 cells, and smooth curves were fitted with the Hill equation. The half-maximal inhibitory concentration (IC50) is 0.69 mM for the outward component and 1.58 mM for the inward component.
Hemichannel currents of zebrafish horizontal cells.
We then assayed for the presence of functional hemichannels in cultured zebrafish horizontal cells using whole cell patch-clamp recording. Hemichannels of retinal horizontal cells have previously been shown to be unblocked by lowered extracellular Ca2+ concentration and to be outwardly rectifying (DeVries and Schwartz, 1992; Zhang and McMahon, 2001). To block K+ channels and to eliminate their potential contamination of the hemichannel current, K+ was replaced with Cs+, and the K+ channel blocker TEA was added to the intracellular pipette solution as described previously (Zhang and McMahon, 2001). Horizontal cells were voltage clamped at 0 mV and the voltage stepped to +60 mV or −60 mV to elicit hemichannel currents. An example recording is shown in Fig. 1C. At +60 mV (Fig. 1C, left), this cell exhibited an outward current of 23 pA in the elevated Ca2+ (2.5 mM) solution. This outward current was increased to 247 pA in Ca2+-free medium. Similarly, at −60 mV (Fig. 1C, right) this cell exhibited an inward current of 9 pA in the 2.5 mM Ca2+ medium, which increased to 54 pA in the Ca2+-free medium. The increase of both outward and inward hemichannel currents observed upon perfusion with Ca2+-free medium was reversible upon return to 2.5 mM Ca2+. In Ca2+-free medium, the outward current at +60 mV and the inward current at −60 mV averaged 182 ± 21 and 40 ± 5 pA, respectively (n = 40 cells).
To further confirm that these currents were mediated by hemichannels, we tested their response to low-pH solutions. When switching extracellular pH from 7.5 to 5.5, outward currents were reduced to 10 ± 2% of control (P < 0.001, N = 5), and inward currents were reduced to 28 ± 5% of control (P < 0.001, N = 5), consistent with reported hemichannel properties (Verselis et al., 2000; Beahm and Hall, 2002). In addition, 100 μM Co2+ significantly blocked the currents in zebrafish horizontal cells recorded under these conditions (Klaassen et al., 2011), as expected of hemichannel currents (Ripps et al., 2004; Fahrenfort et al., 2004). The fact that these currents are sensitive to changes in extracellular Ca2+, pH, and to Co2+, together with the connexin gene knockdown experiments reported below, demonstrates that connexin-mediated hemichannel currents can be elicited in zebrafish retinal horizontal cells.
To test the voltage-gating properties of hemichannel currents, a series of 10-s voltage steps from −90 to +90 mV with 15-mV increments was applied to cultured horizontal cells from a holding potential of 0 mV in Ca2+-free medium. The resulting steady-state hemichannel currents at different potentials were normalized and plotted in Fig. 1D. Hemichannel currents showed outward rectification with outward currents at positive potentials being larger in amplitude than inward current at negative potentials with both polarities of current increasing as the holding potential was moved away from zero. Switching to 2.5 mM high-Ca2+ medium suppressed hemichannel currents by >90%.
To test the effects of extracellular Ca2+ concentration on the amplitude of hemichannel currents, outward and inward currents at +60 and −60 mV were recorded in media in which Ca2+ concentration varied from 0.1 to 5 mM. As shown in Fig. 1E, the outward and inward components of the hemichannel current exhibited different sensitivities to extracellular Ca2+ concentration, with the inward current at negative potentials being substantially less sensitive to calcium blockade. The half-maximal inhibitory concentrations (IC50) were 0.69 mM at +60 mV and 1.58 mM at −60 mV. Interestingly, at 1–2 mM Ca2+, which is comparable to the bulk physiological Ca2+ concentration in the fish retina (Dearry and Burnside, 1984), the majority of the inward current at negative potentials remained active, whereas the majority of the outward current was suppressed. Of note, the concentration of Ca2+ may be lower in the synaptic cleft between horizontal cells and photoreceptors due to depletion during synaptic transmission (Rabl and Thoreson, 2002), which would further enhance the inward hemichannel current.
To test for additional differences in the inward and outward aspects of the hemichannel current, we applied quinine, which has been shown to exert differential effects on hemichannels consisting of different connexin proteins (Dixon et al., 1996; Al-Ubaidi et al., 2000; Srinivas et al., 2001; Ripps et al., 2004). Quinine (200 μM) significantly enhanced the outward currents to 294 ± 62% of control (P < 0.05, N = 5), leaving inward currents unchanged at 105 ± 8% of control (P > 0.05, N = 5).
Single channel hemichannel currents.
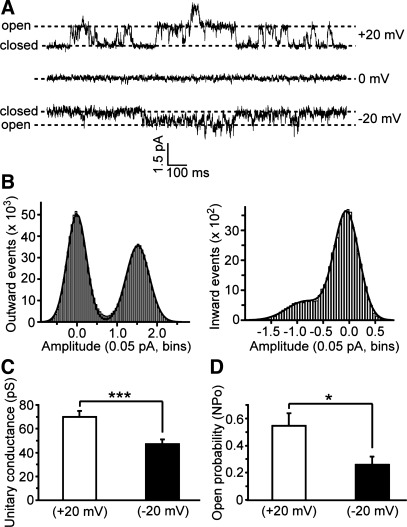
To further understand the biophysical properties of these currents, we assayed them at the single channel level using outside-out patch-clamp recording in which potassium channels were blocked as in the whole cell experiments. As shown in Fig. 2A, both outward and inward single channel currents were elicited in Ca2+-free medium by stepping the membrane potential from 0 mM to +20 and to −20 mV, respectively. Consistent with macroscopic hemichannel currents, both outward and inward single channel currents were blocked substantially by external 2.5 mM Ca2+. All-points amplitude histograms at +20- and −20-mV holding potentials revealed that both the number and amplitude of nonzero events were lower at −20 mV compared with +20 mV (Fig. 2B). However, although the amplitude of channel events differed across positive and negative holding potentials, only a single nonzero amplitude peak was observed at each holding potential (Fig. 2B). The unitary channel events carrying outward current at positive potentials averaged 70 ± 5 pS, whereas those carrying inward current at negative potentials averaged 47 ± 4 pS (P < 0.001, n = 5) (Fig. 2C). Open-time kinetics also differed across holding potentials. The open probability (NPo: the ratio of the time in the open state to the duration of recording) averaged 0.54 ± 0.08 for single channel currents at +20 mV vs. 0.26 ± 0.06 for single channel currents at −20 mV (P < 0.05, n = 5) (Fig. 2D). The larger unitary conductance and greater open time of single hemichannels at positive potentials are consistent with the observed outward rectification of whole cell hemichannel currents in horizontal cells.
Fig. 2.
Single channel events of outward and inward components of hemichannel currents. A: single channel traces of outward (top) and inward (bottom) components of hemichannel currents elicited from holding potentials of 0 mV (middle) to +20 and −20 mV, respectively. Time and amplitude are indicated as the scale bar. B: all-points histogram of outward and inward current arms from A. Distribution of the digitized current values was plotted as a histogram, and data points were fit with Gaussian curves. Peaks shown in histogram indicate currents corresponding to open and closed channels. C: average unitary conductance of outward (70 ± 5 pS, open bar) and inward (47 ± 4 pS, filled bar) hemichannel current arms. ***P < 0.001 (n = 5, mean ± SE). D: averaged overall open probability of outward (0.54 ± 0.08, open bar) and inward (0.26 ± 0.06, filled bar) hemichannel current arms. *P < 0.05 (n = 5, mean ± SE).
Morpholino oligonucleotide knockdown of Cx55.5 and Cx52.6.
To test the roles of Cx55.5 and Cx52.6 in zebrafish horizontal cell hemichannel currents, the expression of Cx55.5 and Cx52.6 in horizontal cells was suppressed by intraocular injection and electroporation of connexin isoform-specific antisense morpholino oligonucleotides into adult zebrafish (Thummel et al., 2008).
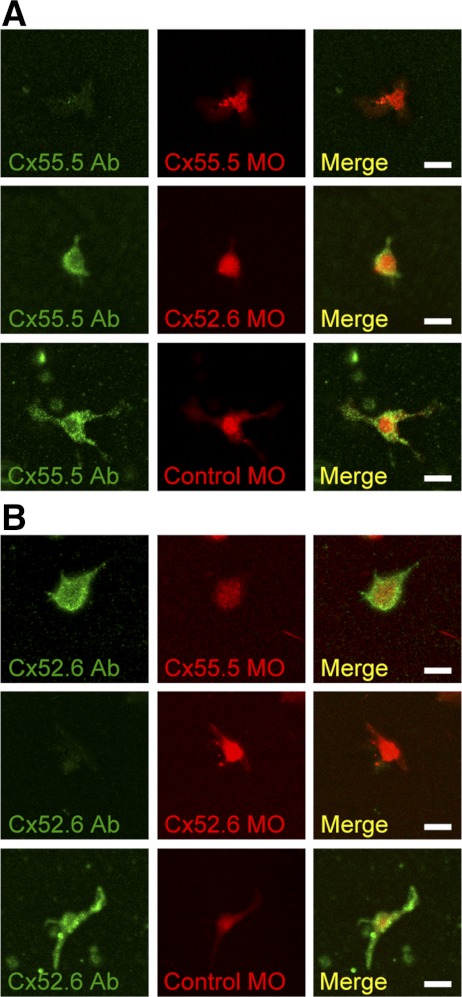
To confirm connexin isoform protein knockdown and specificity, horizontal cells were isolated from zebrafish retinas 4 days postelectroporation and then stained with antibodies against either Cx55.5 or Cx52.6 (Fig. 3). Horizontal cells transduced with morpholinos were identified in culture by a red fluorescent lissamine tag incorporated into the morpholino oligonucleotides. Horizontal cells transduced with the anti-Cx55.5 morpholino showed a dramatic decrease in Cx55.5 staining, but normal Cx52.6 expression, whereas horizontal cells targeted with the anti-Cx52.6 morpholino exhibited normal Cx55.5 expression but a significant decrease in Cx52.6 staining. To test for potential nonspecific effects, a control morpholino that has no complementary sequence in the zebrafish genome (methods) was introduced into the cells. This construct had no effect on either Cx55.5 or Cx52.6 protein expression.
Fig. 3.
Anti-Cx55.5 and anti-Cx52.6 morpholinos (MOs) inhibited the protein expression of Cx55.5 and Cx52.6 isoforms in the cultured HCs, respectively. A: immunostaining of Cx55.5 proteins (green) in anti-Cx55.5 MO-treated HC (top), anti-Cx52.6 MO-treated HC (middle), and control MO-treated HC (bottom). Lissamine-tagged MOs are visualized as red fluorescence. Merged images indicate that the expression of Cx55.5 protein in HCs was suppressed 4 days after the introduction of anti-Cx55.5 MO, whereas the Cx52.6 protein expression was normal. Scale bar = 10 μm. B: immunostaining of Cx52.6 proteins (green) in anti-Cx55.5 MO-treated HC (top), anti-Cx52.6 MO-treated HC (middle), and control MO-treated HC (bottom). Merged images indicate that the expression of Cx52.6 protein in HCs was suppressed 4 days after the introduction of anti-Cx52.6 MOs, whereas the Cx55.5 protein expression was normal. Scale bar = 10 μm.
Morpholino effects on hemichannel currents.
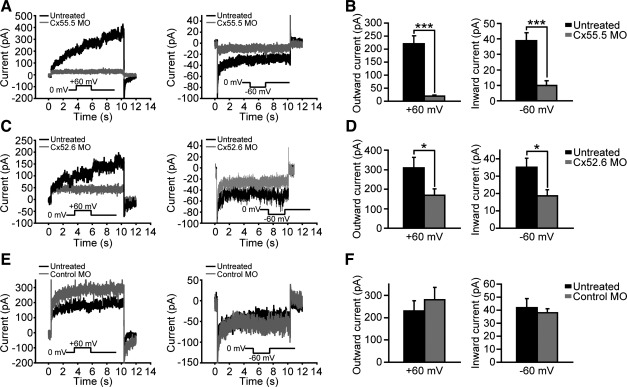
Hemichannel currents were recorded from morpholino-transduced horizontal cells isolated from morpholino-treated zebrafish retinas 4 days postelectroporation and then targeted for recording by lissamine red fluorescence. An example recording from an anti-Cx55.5 morpholino-transduced horizontal cell is shown in Fig. 4A. This cell showed markedly reduced hemichannel currents with 40 pA of outward current (Fig. 4A, left) and 9 pA of inward current (Fig. 4A, right) elicited in Ca2+-free medium (+60 and −60 mV, respectively). These currents were significantly less than the 374 pA of outward current (Fig. 4A, left) and 38 pA of inward current (Fig. 4A, right) recorded in an example untreated cell under the same conditions. Consistent results were obtained from three independent morpholino transductions, and the statistical results are shown in Fig. 4B. In untreated cells, the outward current at +60 mV averaged 221 ± 30 pA, and the inward current at −60 mV averaged 39 ± 5 pA (n = 10), whereas, in anti-Cx55.5 morpholino-treated cells, these currents were reduced by ∼90 and 75% to 20 ± 3 and 10 ± 2 pA, respectively (P < 0.001, n = 5). Similar results were observed with a second anti-Cx55.5 morpholino (anti-Cx55.5 morpholino II, methods), which targeted the Cx55.5 gene in a different region. After treatment with anti-Cx55.5 morpholino II, outward currents decreased from 290 ± 57 to 34 ± 7 pA (P < 0.001, N = 8), and inward currents decreased from 34 ± 3 to 8 ± 2 pA (P < 0.001, N = 8).
Fig. 4.
Anti-Cx55.5 MOs suppressed both outward and inward current arms, whereas anti-Cx52.6 MOs selectively inhibited the outward current arm. A: compared with untreated HCs (black trace), both outward (left) and inward (right) hemichannel current arms were substantially decreased in anti-Cx55.5 MO-treated HCs (gray). B: averaged outward (left) and inward (right) current arms between untreated HCs (black bar) and anti-Cx55.5 MO-treated HCs (gray bar). ***P < 0.001 (n = 5). C: compared with untreated HCs (black trace), the outward current arm (left) was substantially decreased in anti-Cx52.6 MO-treated HCs (gray trace), as was the inward current (right). D: averaged outward (left) and inward (right) current arms between untreated HCs (black bar) and anti-Cx52.6 MO-treated HCs (gray bar). *P < 0.05 (untreated n = 9; Cx52.6 MO treated n = 12). E: compared with untreated HCs (black trace), both outward (left) and inward (right) current arms were sustained in control MO-treated HCs (gray trace). F: averaged outward (left) and inward (right) current arms between untreated HCs (black bar) and control MO-treated HCs (gray bar). There is no significant difference between these two groups (P > 0.05, n = 5).
Horizontal cells treated with anti-Cx52.6 morpholino also showed significant reductions in hemichannel currents at both positive and negative potentials, but to a lesser degree than anti-Cx55.5-treated cells. Individual cell traces are shown in Fig. 4C, and summed data are shown in Fig. 4D. On average, in eight independent transductions, both outward current at +60 mV and inward current at −60 mV were reduced by ∼50%, with outward current decreased from 309 ± 52 pA in untreated horizontal cells to 169 ± 32 pA in the anti-Cx52.6 morpholino-transduced horizontal cells (P < 0.05, untreated n = 9; Cx52.6 morpholino treated n = 12) (Fig. 4D, left) and inward current reduced from 35 ± 5 pA in untreated to 18 ± 3 pA in anti-Cx52.6 morpholino-transduced cells (P < 0.05, untreated n = 9; Cx52.6 morpholino treated n = 12) (Fig. 4D, right). Consistent with the immunocytochemical results (Fig. 3), the control morpholino did not affect hemichannel currents (P > 0.05, n = 6; Fig. 4, E and F).
Heterologous expression in oocytes.
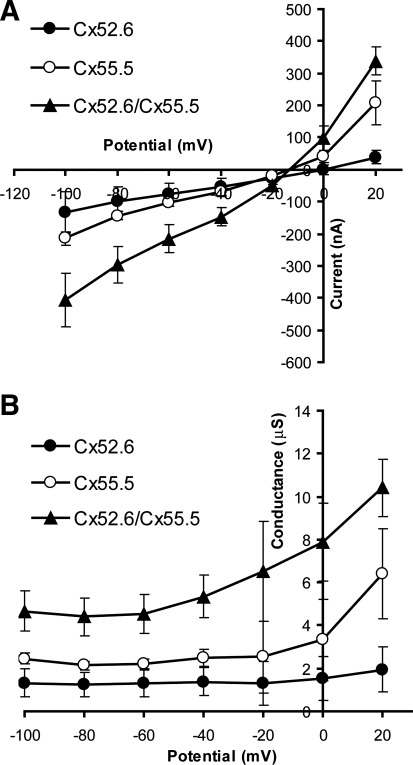
While the morpholino experiments suppressed individual connexins in the background of coexpression of Cx55.5 and Cx52.6, we also performed the complementary experiment of expressing each of these connexins individually and then coexpressing them in a heterologous system. mRNAs for zebrafish Cx55.5, zebrafish Cx52.6, and a mixture of the two, identical in total RNA content to the individual gene cases, were injected into Xenopus oocytes and hemichannel currents and then analyzed in low Ca2+ medium by two-electrode voltage clamp. Steady-state current-voltage relationships were recorded from −100 to +20 mV (Fig. 5A), with the positive holding potential being limited by current passing capacity, and steady-state conductances were derived for each condition (Fig. 5B). The current-voltage relations for Cx52.6 alone, Cx55.5 alone, and Cx55.5/Cx52.6 coexpression all showed some degree of outward rectification. As with the morpholino experiments above, expression of Cx52.6 without expression of Cx55.5 produced low-amplitude hemichannel currents (cf. Cx55.5 morpholino), whereas expression of Cx55.5 without Cx52.6 produced somewhat higher-amplitude currents (cf. Cx52.6 morpholino), and coexpression of Cx55.5 and Cx52.6 produced current amplitudes that exceeded the simple mean of the Cx55.5 and Cx52.6 individual currents as would have been expected from the injection of the same amount of total RNA.
Fig. 5.
Characterization of Cx52.6, Cx55.5, and Cx52.6/Cx55.5 hemichannel currents. A: current-voltage (I–V) relations of hemichannel currents recorded from Xenopus laevis oocytes. Mean current from 190 to 210 ms after pulse onset. All I–V relations show outward rectifying behavior with larger currents at positive potentials and smaller currents at negative potentials. B: I–V based on the I–V relations in A. Both the Cx55.5 and Cx52.6/Cx55.5 currents show an increase in conductance at negative potentials. Cx52.6 has the lowest conductance, whereas Cx52.6/Cx55.5 has the highest conductance.
DISCUSSION
In the present study, we analyzed the physiological properties of native hemichannel currents in cultured zebrafish retinal horizontal cells and investigated the contribution of two connexin channel genes, Cx55.5 and Cx52.6, to overall hemichannel current using morpholino knockdown techniques and heterologous expression. Our findings reveal that zebrafish horizontal cell hemichannel currents possess an inward component that is active at membrane potentials and extracellular Ca2+ levels in the physiological range, that both Cx55.5 and Cx52.6 contribute to these hemichannel currents, and that Cx55.5 expression is necessary for high-amplitude hemichannel currents. These results indicate that zebrafish hemichannel currents exhibit the requisite functional characteristics to participate in ephaptic feedback at the first visual synapse and that Cx55.5 may have both structural and regulatory roles in the expression of hemichannel currents.
Zebrafish horizontal cells express both Cx55.5 and Cx52.6 in culture, as they do in the intact retina (Shields et al., 2007), and the hemichannel currents of these cells are outwardly rectifying. The large outward hemichannel current component induced at positive potentials is similar in characteristics to hemichannel currents previously reported in fish horizontal cells (DeVries and Schwartz, 1992; Zhang and McMahon, 2000; Zhang and McMahon, 2001), but the inward component, which is active at physiological potentials for horizontal cells (−30 to −80 mV), has not been previously characterized. Given the modest amplitude of the inward component, it may have been missed or misattributed to nonspecific leak in previous studies of horizontal cell hemichannel currents. However, our data clearly demonstrate that this steady-state current, recorded in the presence of K+ channel blockers, is Ca2+-sensitive (with shifted IC50), pH-sensitive, and Co2+-sensitive, as expected of a hemichannel current. In addition, the inward component of the horizontal cell hemichannel current is significantly blunted by specific knockdown of Cx55.5 and Cx52.6 protein expression. This inward component of the hemichannel current is relatively insensitive to external Ca2+ and remains largely active in the 1 to 2 mM extracellular concentration range that is estimated to represent the physiological levels of Ca2+ in fish retina (Dearry and Burnside, 1994). Taken together, these findings indicate that the inward component of the horizontal cell hemichannel current is a connexin-mediated current that is active at physiological potentials and Ca2+ levels.
The properties of the hemichannel currents we have described here are consistent with the potential for connexin hemichannels to contribute to physiological function of horizontal cells by mediating an inward nonselective current that increases in amplitude at more negative potentials. A prediction of our results is that both the magnitude of the inward hemichannel current and its contribution to the overall horizontal cell membrane current would be greatest during the hyperpolarizing light responses of horizontal cells (−60 to −80 mV) brought about by the cessation of glutamate release from photoreceptors and closure of the horizontal cell glutamate conductance. In these circumstances, the inward hemichannel current would act to partially depolarize horizontal cells during light responses. In addition, modeling of the cone-horizontal cell synapse indicates that inward hemichannel currents induced by horizontal cell hyperpolarization during light responses can provide an ephaptic feedback signal to modulate the voltage-dependent Ca2+ current and transmitter release in cone terminals through hyperpolarization of the synaptic cleft (Kamermans et al., 2001; Fahrenfort et al., 2009). Our results show, for the first time, native horizontal cell hemichannel currents with voltage and Ca2+-gating characteristics consistent with such a mechanism.
Single channel analysis revealed voltage dependence of both the conductance and gating kinetics of zebrafish horizontal cell hemichannels that underlies the outward rectification of whole cell currents. Both unitary conductance and channel open time were increased at positive potentials compared with negative potentials. Based on the voltage dependence of Cx52.6 and Cx55.5 homomeric hemichannel currents in expression systems (Zoidl et al., 2004; Shields et al., 2007; Fig. 5), and on sequence divergence in the M1 segment of these proteins (Dermietel et al., 2000; Zoidl et al., 2004), which influences channel conductance (Hu et al., 2006), we would expect that Cx52.6 and Cx55.5 homomeric channels would have distinct voltage-gating properties, with Cx55.5 being more active at negative potentials, and have distinct unitary conductances. However, whereas channel characteristics differed across potentials, within each polarity, only one characteristic unitary conductance peak was observed, consistent with a single species of channel with voltage-dependent characteristics. This supports, but does not demonstrate, the possibility that zebrafish horizontal cell hemichannel currents are carried by Cx52.6/Cx55.5 heteromeric channels. Channel characteristics of connexin hemichannels vary widely across cell type and species, but, compared with other characterized zebrafish and horizontal cell hemichannels, the single channel currents we have observed are similar in magnitude. Zebrafish Cx35 hemichannels also show voltage rectification of their conductance, with values in the 72- to 24-pS range (Valiunas et al., 2004), whereas hybrid bass horizontal cell hemichannels have single channel conductances of ∼40 pS (Zhang and McMahon, 2000) and mouse horizontal cell Cx57 hemichannels of 57 pS (Palacios-Prado et al., 2009). In contrast, pannexin-1 protein hemichannels, which may also be expressed in zebrafish horizontal cells, have unitary conductances near 400 pS (Prochnow et al., 2009).
The average amplitude of the inward hemichannel conductance in isolated horizontal cells at negative potentials and physiological Ca2+ levels is ∼500 pS/cell, as calculated from our hemichannel current measurements. Based on the unitary conductance and open probability of hemichannels at −20 mV, we can estimate that the inward whole cell currents we observed were carried by ∼10 open channels and a total active channel pool of ∼40 channels. However, because these experiments were performed on isolated cells in which the number of channels may have been altered by dissociation, especially feedback-associated channels expressed in horizontal cell dendritic tips in situ, it is not possible to accurately predict the amplitude of inward hemichannel currents in in situ horizontal cells based on our in vitro results. Thus, although the currents we have described have the requisite physiological characteristics to participate in feedback, the present experiments do not allow an estimation of the number of hemichannels in situ that contribute to feedback at the first visual synapse. Furthermore, when combined with the genetic evidence that Cx55.5 mutation leads to a specific decrease in the inward hemichannel current at negative potentials in horizontal cells and concomitantly to alterations in horizontal cell feedback to cones (Klaassen et al., 2011), our results strongly implicate horizontal cell connexin hemichannel currents as contributing to the feedback mechanisms in the outer retina of zebrafish.
A variety of feedback mechanisms have been proposed that may contribute to horizontal cell photoreceptor feedback in a variety of vertebrate species, including glutamate receptor-mediated ephaptic feedback (Fahrenfort et al., 2009), synaptic pH change (DeVries, 2001; Vessey et al., 2005), and non-Ca2+-dependent γ-aminobutyric acid release (Schwartz, 1987; Schwartz, 2002). Such feedback is critical for regulating the gain of the first visual synapse (Van leeuwen et al., 2009), establishing receptive fields in cones and for chromatic feedback in horizontal cells (Kamermans and Spekreijse, 1999). Whether ephaptic feedback through horizontal cell connexin hemichannels also plays a role in photoreceptor feedback in the mammalian retina is not clear. Deletion of Cx57, the known horizontal cell connexin in the mouse, does not negate the roll back of horizontal cell responses (Shelly et al., 2006). However, results with Cx55.5 mutation in zebrafish retina show that lack of effect on the rollback response alone is insufficient to exclude the possibility of a contribution to feedback (Klaasen et al., 2011). Thus, a more refined analysis of feedback mechanisms in mammals is necessary to establish any role for hemichannels.
Using morpholino oligonucleotide knockdown of connexins in adult zebrafish horizontal cells and heterologous expression in oocytes, we found that both Cx55.5 and Cx52.6 contribute to the overall hemichannel current. Knockdown of either connexin in horizontal cells significantly reduced hemichannel currents at both positive and negative potentials. However, in both native cells and in oocytes, expression of Cx52.6 without coexpression of Cx55.5 resulted in strikingly lower amplitude currents compared with expression of Cx55.5 without Cx52.6. In both systems, coexpression of the two connexins produced the highest amplitude currents, which were greater than the average of the currents resulting from Cx55.5 and Cx52.6 alone. These results suggest that, although each of these connexin gene products is capable of forming hemichannels, Cx55.5 may play an additional regulatory role in enhancing the amplitude of hemichannel currents when coexpressed with Cx52.6. This effect could be mediated by enhancing channel number, by enhancing expression or assembly of channels, or by regulation of the conductance of existing channels. Cx55.5 is a genetic regulator of Cx52.6, since Cx55.5 mutant fish lack both Cx55.5 and Cx52.6 protein expression (Klaassen, et al. 2011). However, acute morpholino suppression of Cx55.5 protein does not qualitatively affect Cx52.6 protein expression yet strikingly suppresses total hemichannel current. Thus, genetic knockout, acute knockdown, and heterologous expression all suggest that Cx55.5 expression is necessary for high-amplitude hemichannel currents in horizontal cells.
Our results also revealed significant differences in the physiological and pharmacological properties of the zebrafish horizontal cell hemichannel currents over different voltage ranges. The inward hemichannel current at negative potentials is relatively insensitive to extracellular Ca2+, insensitive to quinine, and is carried by low-amplitude, low open probability unitary events, whereas the outward arm of the hemichannel current at positive potentials is relatively sensitive to Ca2+ and quinine, and is carried by high-amplitude, high open probability unitary events. These results could be explained by a single species of heteromeric channels containing both Cx52.6 and Cx55.5 subunits that adopts different operating states and pharmacological sensitivities at positive and negative potentials, or by distinct populations of homomeric Cx 52.6 and Cx55.5 channels that operate at positive and negative potentials, respectively. These possibilities cannot be rigorously distinguished with our current data. Cx55.5 expression is much more abundant than Cx52.6 on the horizontal cell dendritic tips by antibody staining (Shields et al., 2007; Klaassen et al., 2011), suggesting the possibility that Cx55.5 homomeric channels could exist there, and previous work on horizontal cell gap junctions has elucidated connexin currents with distinct pharmacologies (Lu et al., 1999). However, the simplest explanation consistent with our results is that Cx55.5 and Cx52.6 form heteromeric hemichannels that carry the hemichannel current and adopt different functional states at positive and negative transmembrane voltages. In this case, the unequal distribution of Cx55.5 vs. Cx52.6 antibody staining in dendritic tips vs. cell body could be the result of different Cx55.5-to-Cx52.6 ratios in heteromeric connexons or differences in antibody sensitivity. Recently, an additional connexin, Cx52.9, and pannexin-1, another hemichannel-forming protein, were shown to be present on zebrafish retinal horizontal cells (Klaassen et al., 2011; Prochnow et al., 2009), and it is possible that the low-amplitude hemichannel currents that persist following oligonucleotide knockdown of Cx52.6 or Cx55.5 could be carried by channels formed from these proteins.
The connexin family of channel genes is widely expressed in neurons and glia of the central nervous system. They have been extensively studied as the structural basis of cell-to-cell pores that mediate electrical and metabolic coupling at gap junctional points of cellular contact, but are also expressed unpaired in the plasma membranes of many cell types, where their function as hemichannels is less clear. As hemichannels, connexins have been shown to contribute to developmental and pathological processes in the nervous system, such as cell migration and adhesion, as well as ischemic damage and neurodegeneration (Thompson et al., 2006; Cotrina et al., 2008; Liu et al., 2010). Our results, combined with the genetic evidence, expand the functional roles of connexin hemichannels to include synaptic signaling in a novel feedback mechanism that shapes visual processing at the first visual synapse.
GRANTS
This work was supported by National Eye Institute Grant R01 EY-09256 to D. G. McMahon, by the Vanderbilt Vision Research Core Grant P30 EY-008126, by European Commission FP7 Grant RETICIRC HEALTH-F2-2009-223156 to M. Kamermans, and by a ZonMW-Top grant to M. Kamermans.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Z.S. and D.G.M. conception and design of research; Z.S., M.L.R., J.B.v.A., and D.-Q.Z. performed experiments; Z.S., M.L.R., and D.-Q.Z. analyzed data; Z.S., M.L.R., and D.G.M. interpreted results of experiments; Z.S. and M.L.R. prepared figures; Z.S., M.L.R., M.K., and D.G.M. drafted manuscript; Z.S., M.K., and D.G.M. edited and revised manuscript; Z.S., M.K., and D.G.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank David R. Hyde and Ryan Thummel from the University of Notre Dame for help in establishing the intraocular injection and electroporation techniques.
Present address for D.-Q. Zhang: Eye Research Institute, Oakland University, 423 Dodge Hall, Rochester, MI 48309.
REFERENCES
- Al-Ubaidi et al., 2000. Al-Ubaidi MR, White TW, Ripps H, Poras I, Avner P, Gomes D, Bruzzone R. Functional properties, developmental regulation, and chromosomal localization of murine connexin36, a gap-junctional protein expressed preferentially in retina and brain. J Neurosci Res 59: 813–826, 2000 [DOI] [PubMed] [Google Scholar]
- Beahm and Hall, 2002. Beahm DL, Hall JE. Hemichannel and junctional properties of connexin 50. Biophys J 82: 2016–2031, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield and Volgyi, 2009. Bloomfield SA, Volgyi B. The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat Rev Neurosci 10: 495–506, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina et al., 2008. Cotrina ML, Lin JH, Nedergaard M. Adhesive properties of connexin hemichannels. Glia 56: 1791–1798, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearry and Burnside, 1984. Dearry A, Burnside B. Effects of extracellular Ca++, K+, and Na+ on cone and retinal pigment epithelium retinomotor movements in isolated teleost retinas. J Gen Physiol 83: 589–611, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel et al., 2000. Dermietzel R, Kremer M, Paputsoglu G, Stang A, Skerrett IM, Gomes D, Srinivas M, Janssen-Bienhold U, Weiler R, Nicholson BJ, Bruzzone R, Spray DC. Molecular and functional diversity of neural connexins in the retina. J Neurosci 20: 8331–8343, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries, 2001. DeVries SH. Exocytosed protons feedback to suppress the Ca2+ current in mammalian cone photoreceptors. Neuron 32: 1107–1117, 2001 [DOI] [PubMed] [Google Scholar]
- DeVries and Schwartz, 1992. DeVries SH, Schwartz EA. Hemi-gap-junction channels in solitary horizontal cells of the catfish retina. J Physiol 445: 201–230, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon et al., 1996. Dixon DB, Takahashi K, Bieda M, Copenhagen DR. Quinine, intracellular pH and modulation of hemi-gap junctions in catfish horizontal cells. Vision Res 36: 3925–3931, 1996 [DOI] [PubMed] [Google Scholar]
- Ebihara et al., 1995. Ebihara L, Berthoud VM, Beyer EC. Distinct behavior of connexin56 and connexin46 gap junctional channels can be predicted from the behavior of their hemi-gap-junctional channels. Biophys J 68: 1796–1803, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenfort et al., 2009. Fahrenfort I, Steijaert M, Sjoerdsma T, Vickers E, Ripps H, van Asselt J. Hemichannel-mediatedand pH-based feedback from horizontal cells to cones in the vertebrate retina. PLoS One 30: e6090, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenfort et al., 2004. Fahrenfort I, Sjoerdsma T, Ripps H, Kamermans M. Cobalt ions inhibit negative feedback in the outer retina by blocking hemichannels on horizontal cells. Vis Neurosci 21: 501–511, 2004 [DOI] [PubMed] [Google Scholar]
- Guldenagel et al., 2000. Guldenagel M, Sohl G, Plum A, Traub O, Teubner B, Weiler R, Willecke K. Expression patterns of connexin genes in mouse retina. J Comp Neurol 425: 193–201, 2000 [PubMed] [Google Scholar]
- Kamermans et al., 2001. Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma T, Weiler R. Hemichannel-mediated inhibition in the outer retina. Science 292: 1178–1180, 2001 [DOI] [PubMed] [Google Scholar]
- Kamermans and Spekreijse, 1999. Kamermans M, Spekreijse H. The feedback pathway from horizontal cells to cones. A mini review with a look ahead Vision Res 39: 2449–2468, 1999 [DOI] [PubMed] [Google Scholar]
- Klaassen et al., 2011. Klaassen LJ, Sun Z, Steijaert MN, Bolte P, Fahrenfort I, Sjoerdsma T. Synaptic transmission from horizontal cells to cones is impaired by loss of connexin hemichannels. PLoS 9: e1001107, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al., 2010. Liu X, Hashimoto-Torii K, Torri M, Ding C, Rakic P. Gap junctions/hemichannels modulate interkinetic nuclear migration in the forebrain precursors. J Neurosci 30: 4197–209, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu et al., 1999. Lu C, Zhang DQ, McMahon DG. Electrical coupling of retinal horizontal cells mediated by distinct voltage-independent junctions. Vis Neurosci 16: 811–818, 1999 [DOI] [PubMed] [Google Scholar]
- Massey et al., 2003. Massey SC, O'Brien JJ, Trexler EB, Li W, Keung JW, Mills SL, O'Brien JJ. Multiple neuronal connexins in the mammalian retina. Cell Commun Adhes 10: 425–430, 2003 [DOI] [PubMed] [Google Scholar]
- Mills and Massey, 1995. Mills SL, Massey SC. Differential properties of two gap junctional pathways made by AII amacrine cells. Nature 377: 734–737, 1995 [DOI] [PubMed] [Google Scholar]
- Rabl and Thoreson, 2002. Rabl K, Thoreson WB. Calcium-dependent inavtivation and depletion of synaptic cleft calcium ions combine to redulate rod calcium currents under physiological conditions. Eur J Neurosci 16: 2070–2077, 2002 [DOI] [PubMed] [Google Scholar]
- Ripps et al., 2004. Ripps H, Qian H, Zakevicius J. Properties of connexin26 hemichannels expressed in Xenopus oocytes. Cell Mol Neurobiol 24: 647–665, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, 1987. Schwartz EA. Depolarization without calcium can release gamma-aminobutyric acid from a retinal neuron. Science 238: 350–355, 1987 [DOI] [PubMed] [Google Scholar]
- Schwartz, 2002. Schwartz EA. Transport-mediated synapses in the retina. Physiol Rev 82: 875–891, 2002 [DOI] [PubMed] [Google Scholar]
- Shields et al., 2007. Shields CR, Klooster J, Claassen Y, Ul-Hussain M, Zoidl G, Dermietzel R, Kamermans M. Retinal horizontal cell-specific promoter activity and protein expression of zebrafish connexin 52.6 and connexin 555 J Comp Neurol 501: 765–779, 2007 [DOI] [PubMed] [Google Scholar]
- Sohl and Willecke, 2004. Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res 62: 228–232, 2004 [DOI] [PubMed] [Google Scholar]
- Srinivas et al., 2001. Srinivas M, Hopperstad MG, Spray DC. Quinine blocks specific gap junction channel subtypes. Proc Natl Acad Sci USA 98: 10942–10947, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson et al., 2006. Thompson RJ, Zhou N, MacVicar BN. Ischemia opens neuronal gap junction hemichannels. Science 312: 924–947, 2006 [DOI] [PubMed] [Google Scholar]
- Thummel et al., 2008. Thummel R, Kassen SC, Montgomery JE, Enright JM, Hyde DR. Inhibition of Muller glial cell division blocks regeneration of the light-damaged zebrafish retina. Dev Neurobiol 68: 392–408, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leeuwen et al., 2009. Van Leeuwen M, Fahrenfort I, Sjoerdsma T, Numan R, Kamermans M. Lateral gain control in the outer retina leads to potentiation of center responses of retinal neurons. J Neurosci 29: 6358–6366, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verselis et al., 2000. Verselis VK, Trexler EB, Bukauskas FF. Connexin hemichannels and cell-cell channels: comparison of properties. Braz J Med Biol Res 33: 379–389, 2000 [DOI] [PubMed] [Google Scholar]
- Vessey et al., 2005. Vessey JP, Stratis AK, Daniels BA, Da Silva N, Jonz MG, Lalonde MR, Baldridge WH, Barnes S. Proton-mediated feedback inhibition of presynaptic calcium channels at the cone photoreceptor synapse. J Neurosci 25: 4108–4117, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang and McMahon, 2000. Zhang DQ, McMahon DG. Direct gating by retinoic acid of retinal electrical synapses. Proc Natl Acad Sci USA 97: 14754–14759, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang and McMahon, 2001. Zhang DQ, McMahon DG. Gating of retinal horizontal cell hemi gap junction channels by voltage, Ca2+, and retinoic acid. Mol Vis 7: 247–252, 2001 [PubMed] [Google Scholar]
- Zoidl et al., 2008. Zoidl G, Kremer M, Zoidl C, Bunse S, Dermietzel R. Molecular diversity of connexin and pannexin genes in the retina of the zebrafish Danio rerio. Cell Commun Adhes 15: 169–183, 2008 [DOI] [PubMed] [Google Scholar]
- Zoidl et al., 2004. Zoidl G, Bruzzone R, Wickert S, Kremer M, Zoidl C, Mitropoulou G. Molecular cloning and functional expression of zfCx52.6: a novel connexin with hemichannel-forming properties expressed in horizontal cells of the zebrafish retina. J Biol Chem 279: 2913–2921, 2004 [DOI] [PubMed] [Google Scholar]