Abstract
The estrogen receptor (E2R) from rabbit uterus has an enhanced affinity for BrdUrd-substituted DNA compared to unsubstituted DNA. Increasing levels of BrdUrd substitution (from 0 to 100%) in a DNA sample are associated with increasingly tighter binding of E2R to that DNA as measured by equilibrium competition experiments and by rates of dissociation (or receptor transfer experiments). Although the rates of dissociation of E2R-DNA complexes vary greatly, depending on the BrdUrd-substitution level in the DNA, no differences were detected between the rates of association of E2R with unsubstituted and fully BrdUrd-substituted DNA. The E2R-DNA complex dissociates more rapidly in 150 mM KCl than in 50 mM KCl; but, at both ionic strengths, BrdUrd substitution in the DNA confers enhanced stability on the complex. The demonstration that a specific mammalian regulatory protein has an enhanced affinity for BrdUrd-substituted DNA further strengthens the possibility that BrdUrd modulates gene expression through an altered binding of regulatory proteins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J., Scolnick E. M. Induction of murine C-type viruses from clonal lines of virus-free BALB-3T3 cells. Science. 1971 Oct 8;174(4005):157–159. doi: 10.1126/science.174.4005.157. [DOI] [PubMed] [Google Scholar]
- Andre J., Rochefort H. In vitro binding of the estrogen receptor to DNA: absence of saturation at equilibrium. FEBS Lett. 1975 Feb 15;50(3):319–323. doi: 10.1016/0014-5793(75)80519-9. [DOI] [PubMed] [Google Scholar]
- Bick M. D., Davidson R. L. Total substitution of bromodeoxyuridine for thymidine in the DNA of a bromodeoxyuridine-dependent cell line. Proc Natl Acad Sci U S A. 1974 May;71(5):2082–2086. doi: 10.1073/pnas.71.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick M. D., Devine E. A. Interaction of chromosomal proteins with BrdU substituted DNA as determined by chromatin-DNA competition. Nucleic Acids Res. 1977 Nov;4(11):3687–3700. doi: 10.1093/nar/4.11.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R., Holtzer H. Inhibition of myoblast fusion after one round of DNA synthesis in 5-bromodeoxyuridine. J Cell Biol. 1970 Jan;44(1):134–150. doi: 10.1083/jcb.44.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas D. K., Lyons J., Tashjian A. H., Jr Induction of prolactin synthesis in rat pituitary tumor cells by 5-bromodeoxyuridine. Cell. 1977 Jun;11(2):431–439. doi: 10.1016/0092-8674(77)90061-7. [DOI] [PubMed] [Google Scholar]
- Bourgeois S., Pfahl M. Repressors. Adv Protein Chem. 1976;30:1–99. doi: 10.1016/s0065-3233(08)60478-7. [DOI] [PubMed] [Google Scholar]
- Bresloff J. L., Crothers D. M. DNA-ethidium reaction kinetics: demonstration of direct ligand transfer between DNA binding sites. J Mol Biol. 1975 Jun 15;95(1):103–123. doi: 10.1016/0022-2836(75)90339-3. [DOI] [PubMed] [Google Scholar]
- Buller R. E., O'Malley B. W. The biology and mechanism of steroid hormone receptor interaction with the eukaryotic nucleus. Biochem Pharmacol. 1976 Jan;25(1):1–12. doi: 10.1016/0006-2952(76)90164-7. [DOI] [PubMed] [Google Scholar]
- Cullen B. R., Bick M. D. Bromodeoxyuridine induction of deoxycytidine deaminase activity in a hamster cell line. Biochim Biophys Acta. 1978 Jan 26;517(1):158–168. doi: 10.1016/0005-2787(78)90043-6. [DOI] [PubMed] [Google Scholar]
- Dannies P. S., Yen P. M., Tashijian A. H., Jr Anti-estrogenic compounds increase prolactin and growth hormone synthesis in clonal strains of rat pituitary cells. Endocrinology. 1977 Oct;101(4):1151–1156. doi: 10.1210/endo-101-4-1151. [DOI] [PubMed] [Google Scholar]
- Davidson R. L., Bick M. D. Bromodeoxyuridine dependence--a new mutation in mammalian cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):138–142. doi: 10.1073/pnas.70.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSombre E. R., Mohla S., Jensen E. V. Estrogen-independent activation of the receptor protein of calf uterine cytosol. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1601–1608. doi: 10.1016/0006-291x(72)90897-2. [DOI] [PubMed] [Google Scholar]
- Gorski J., Gannon F. Current models of steroid hormone action: a critique. Annu Rev Physiol. 1976;38:425–450. doi: 10.1146/annurev.ph.38.030176.002233. [DOI] [PubMed] [Google Scholar]
- Hinkle D. C., Chamberlin M. J. Studies of the binding of Escherichia coli RNA polymerase to DNA. I. The role of sigma subunit in site selection. J Mol Biol. 1972 Sep 28;70(2):157–185. doi: 10.1016/0022-2836(72)90531-1. [DOI] [PubMed] [Google Scholar]
- Jensen E. V., DeSombre E. R. Mechanism of action of the female sex hormones. Annu Rev Biochem. 1972;41:203–230. doi: 10.1146/annurev.bi.41.070172.001223. [DOI] [PubMed] [Google Scholar]
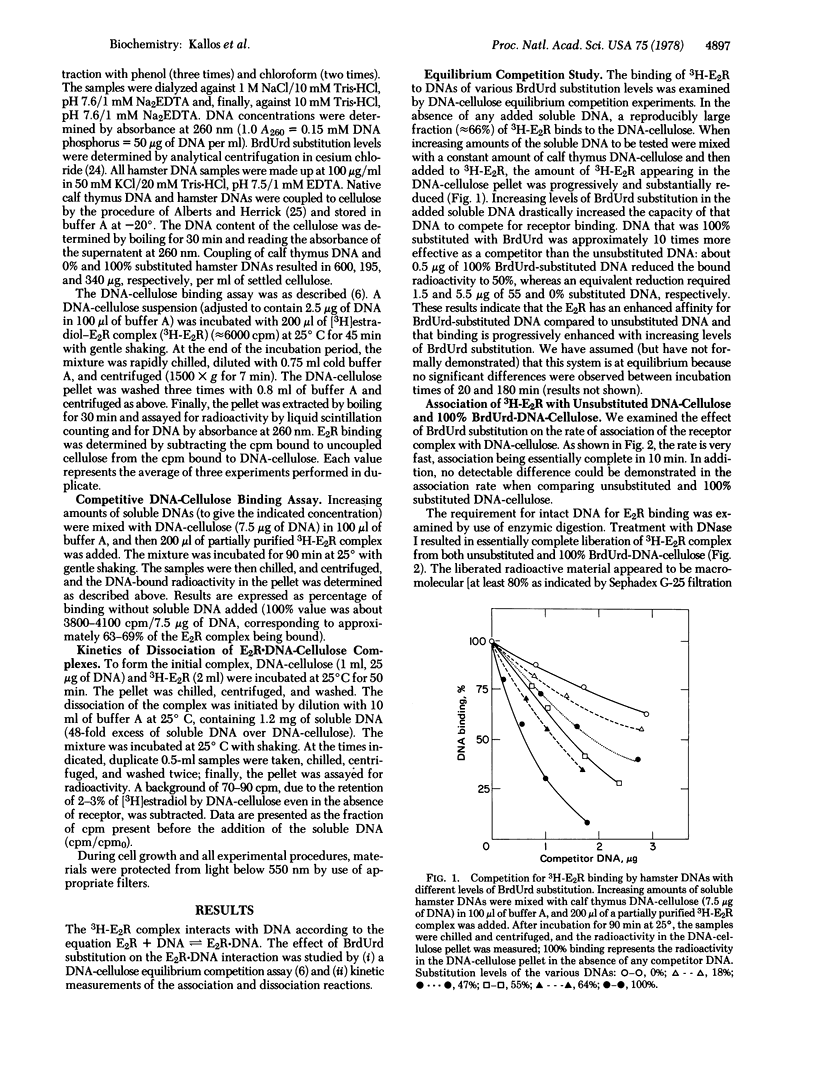
- Kallos J., Hollander V. P. Assessment of specificity of oestrogen receptor-DNA interaction by a competitive assay. Nature. 1978 Mar 9;272(5649):177–179. doi: 10.1038/272177a0. [DOI] [PubMed] [Google Scholar]
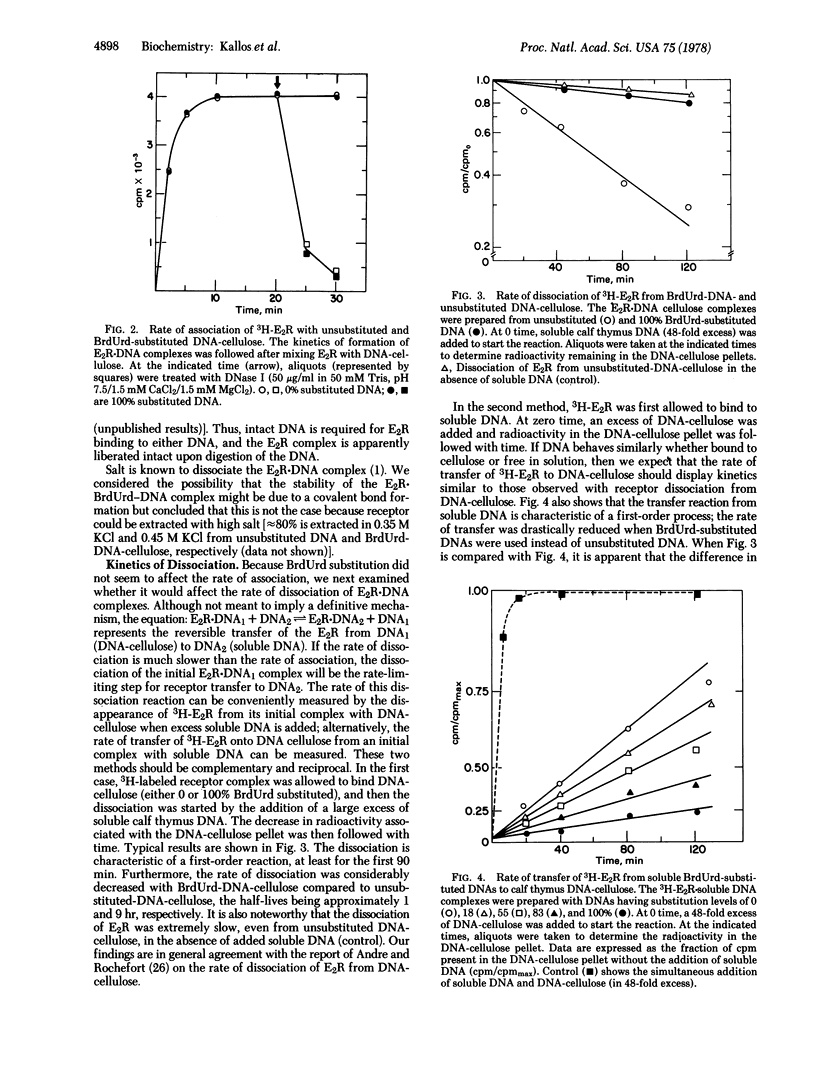
- Koyama H., Ono T. Further studies on the induction of alkaline phosphatase by 5-bromodeoxyuridine in a hybrid line between mouse and Chinese hamster in culture. Biochim Biophys Acta. 1972 May 16;264(3):497–507. doi: 10.1016/0304-4165(72)90013-x. [DOI] [PubMed] [Google Scholar]
- Latt S. A. Localization of sister chromatid exchanges in human chromosomes. Science. 1974 Jul 5;185(4145):74–76. doi: 10.1126/science.185.4145.74. [DOI] [PubMed] [Google Scholar]
- Leavitt W. W., Kimmel G. L., Friend J. P. Steroid hormone uptake by anterior pituitary cell suspensions. Endocrinology. 1973 Jan;92(1):94–103. doi: 10.1210/endo-92-1-94. [DOI] [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. Lac operator analogues: bromodeoxyuridine substitution in the lac operator affects the rate of dissociation of the lac repressor. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2574–2576. doi: 10.1073/pnas.69.9.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
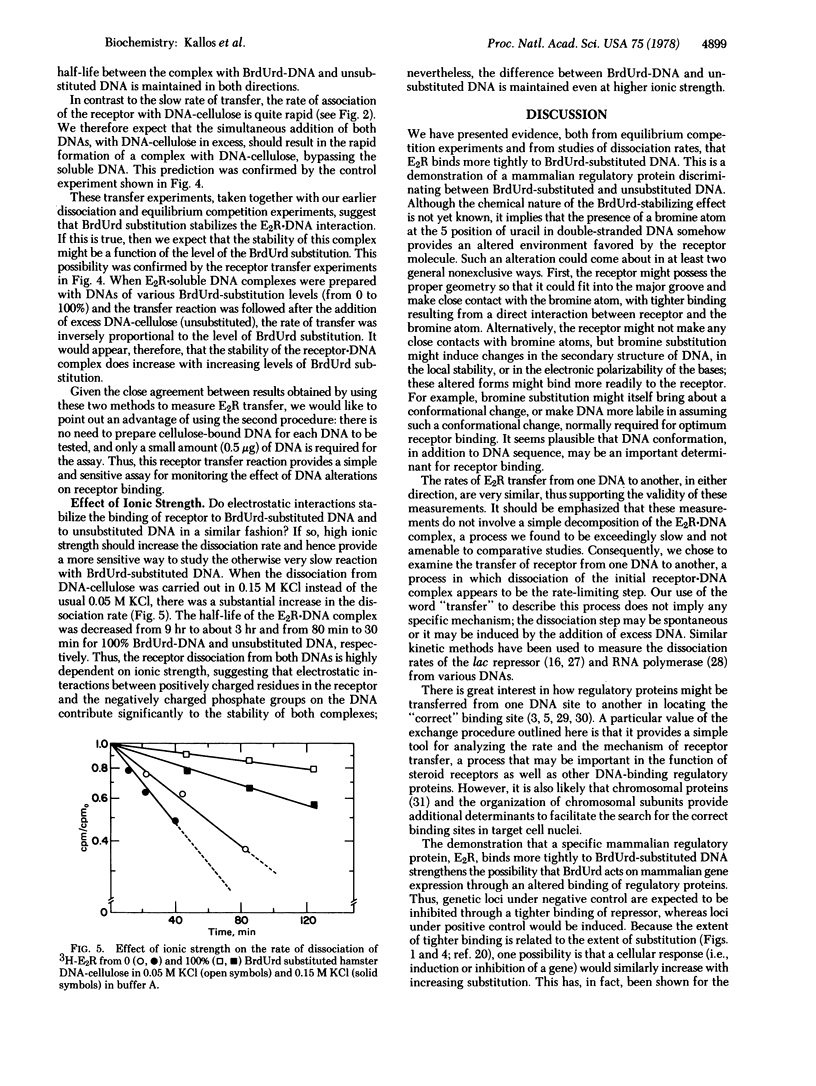
- Lin S. Y., Riggs A. D. The binding of lac repressor and the catabolite gene activator protein to halogen-substituted analogues of poly[d(A-T)]. Biochim Biophys Acta. 1976 May 3;432(2):185–191. doi: 10.1016/0005-2787(76)90160-x. [DOI] [PubMed] [Google Scholar]
- Lin S., Lin D., Riggs A. D. Histones bind more tightly to bromodeoxyuridine-substituted DNA than to normal DNA. Nucleic Acids Res. 1976 Sep;3(9):2183–2191. doi: 10.1093/nar/3.9.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- Luk D. C., Bick M. D. Determination of 5'-bromodeoxyuridine in DNA by buoyant density. Anal Biochem. 1977 Feb;77(2):346–349. doi: 10.1016/0003-2697(77)90247-0. [DOI] [PubMed] [Google Scholar]
- Notides A. C. The binding affinity and specificity of the estrogen receptor of the rat uterus and anterior pituitary. Endocrinology. 1970 Nov;87(5):987–992. doi: 10.1210/endo-87-5-987. [DOI] [PubMed] [Google Scholar]
- Scher W., Preisler H. D., Friend C. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro. 3. Effects of 5-bromo-2'-deoxyuridine, dimethylformamide and dimethylsulfoxide. J Cell Physiol. 1973 Feb;81(1):63–70. doi: 10.1002/jcp.1040810108. [DOI] [PubMed] [Google Scholar]
- Schwartz S. A. Rat embryo nonhistone chromosomal proteins: interaction in vitro with normal and bromodeoxyuridine-substituted DNA. Biochemistry. 1977 Sep 6;16(18):4101–4108. doi: 10.1021/bi00637a025. [DOI] [PubMed] [Google Scholar]
- Silagi S., Bruce S. A. Suppression of malignancy and differentiation in melanotic melanoma cells. Proc Natl Acad Sci U S A. 1970 May;66(1):72–78. doi: 10.1073/pnas.66.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen R. H., Tomkins G. M. Preferential inhibition by 5-bromodeoxyuridine of the synthesis of tyrosine aminotransferase in hepatoma cell cultures. J Mol Biol. 1971 Feb 28;56(1):167–182. doi: 10.1016/0022-2836(71)90092-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M. Steroid receptors: elements for modulation of eukaryotic transcription. Annu Rev Biochem. 1976;45:721–746. doi: 10.1146/annurev.bi.45.070176.003445. [DOI] [PubMed] [Google Scholar]



