Abstract
Limb movements can be driven by muscle contractions, external forces, or intrinsic passive forces. For lightweight limbs like those of insects or small vertebrates, passive forces can be large enough to overcome the effects of gravity and may even generate limb movements in the absence of active muscle contractions. Understanding the sources and actions of such forces is therefore important in understanding motor control. We describe passive properties of the femur-tibia joint of the locust hind leg. The resting angle is determined primarily by passive properties of the relatively large extensor tibiae muscle and is influenced by the history of activation of the fast extensor tibiae motor neuron. The resting angle is therefore better described as a history-dependent resting state. We selectively stimulated different flexor tibiae motor neurons to generate a range of isometric contractions of the flexor tibiae muscle and then stimulated the fast extensor tibiae motor neuron to elicit active tibial extensions. Residual forces in the flexor muscle have only a small effect on subsequent active extensions, but the effect is larger for distal than for proximal flexor motor neurons and varies with the strength of flexor activation. We conclude that passive properties of a lightweight limb make substantial and complex contributions to the resting state of the limb that must be taken into account in the patterning of neuronal control signals driving its active movements. Low variability in the effects of the passive forces may permit the nervous system to accurately predict their contributions to behavior.
Keywords: biomechanics, motor control, phase change, locomotion, aimed limb movement, locust
to understand how animals generate coordinated movements, it is necessary to account not only for the neuronal signals that drive muscles but also for biomechanical properties of the body. In animals with relatively heavy limbs, humans, for example, limb posture is influenced strongly by gravity so that in the absence of active muscle contractions, the limbs hang downward to the extent permitted by the constraints of joint rotations. The situation can be very different for small vertebrates and insects, however, where passive forces intrinsic to the very light limbs can be larger than the force exerted by gravity (Hooper et al. 2009). In the absence of active muscular contractions, such limbs can assume a gravity-independent resting position governed by these internal passive forces. A biomechanical model of the femur-tibia (FT) joint of a locust metathoracic (hind) leg (Zakotnik et al. 2006) predicted that passive forces acting at this joint could be as large as active forces generated by muscular contractions, and in locusts carrying out aimed scratches, some limb movements are indeed driven solely by such passive forces (Page et al. 2008). The actions of passive forces acting on a limb joint are defined by the joint anatomy and by the biomechanical properties of the structures giving rise to the forces, which may include muscles, tendons, surrounding soft tissues, and the cuticle of the joint itself.
We have analyzed the actions of passive forces on the FT joint of a locust hindlimb, which is a particularly well-characterized invertebrate model system for movement control (review: Burrows 1996). We sought to determine how the resting state of this limb joint is affected by prior stretch and compression of the two antagonistic muscles acting on it and how the resting state can be shifted by prior motor neuron activity. Moreover, we sought to determine whether the different walking postures of isolated solitarious locusts and gregarious (swarming) locusts (Blackburn et al. 2010) are driven by a change in the resting state of the limb.
The tibia of the locust hind leg is articulated to the femur at a hinge joint (the FT joint), which rotates in a single plane. The tibia is extended and flexed about the joint by contractions of the two antagonistic muscles: the extensor and the flexor tibiae muscles. Both have a pinnate structure, but the extensor tibiae muscle has a cross-sectional area that is 88% larger than that of the flexor tibiae muscle, and it has five times the mass (Bennet-Clark, 1975). As a consequence, the extensor peak force (measured at the tendon) is 17–33 times larger than that of the flexor tibiae muscle. The muscle tendons insert onto the tibia in such a way that the lever arms transmitting force from the muscles to the tibia are asymmetric, and they vary nonlinearly with joint angle. The flexor tendon also passes over a ridge in the femur (Heitler's lump: Heitler 1974) that alters the angle of pull in a further nonlinearity. The result of these properties is that the smaller and weaker flexor muscle gains a mechanical advantage over the more powerful extensor muscle at flexed FT angles (Heitler 1974). This is particularly important in the generation of kicking and jumping behaviors for which the large hind leg is specialized (Burrows 1996) but necessarily affects all movements of the tibia, including those made during walking and scratching, for example. These mechanical properties of the joint and tendons must also affect how passive forces originating in the muscles are transferred to the tibia.
Locusts show remarkable plasticity in many aspects of morphology and behavior in their transition from solitarious individuals to gregarious swarming pests (e.g., Pener and Simpson 2009). One aspect of this is a change in walking posture in which the hind leg tibia is characteristically held more flexed in solitarious than in gregarious locusts. This is driven in part by changes in motor activity (Blackburn et al. 2010), but it is not known whether there are also changes in muscles, tendons, or limb joints, or in the passive forces acting on them.
The extensor and flexor tibiae muscles are innervated by excitatory and inhibitory motor neurons, as well as modulatory dorsal unpaired median (DUM) neurons. The cell bodies and input branches of all these cells lie within the metathoracic ganglion of the ventral nerve cord (Burrows 1996), and their axons reach the leg muscles through leg nerves 3 or 5 (Hoyle 1955; Sasaki and Burrows 1998).
The extensor tibiae muscle is innervated by only one fast motor neuron, the fast extensor tibiae (FETi), and one slow motor neuron, the slow extensor tibiae (SETi), as well as one common inhibitory neuron (CI1; Hoyle 1955, 1978b). Spikes in FETi generate large excitatory junction potentials in fast extensor tibiae muscle fibers and evoke strong muscle contractions. A single FETi spike is thus sufficient to elicit a large and rapid extension of the tibia. SETi spikes, on the other hand, elicit relatively small excitatory junction potentials in slow muscle fibers and thus evoke smaller and slower contractions (Hoyle 1955; Burns and Usherwood 1979). Single SETi spikes do not generally elicit visible tibial movements, but trains of SETi activity at frequencies of 10 spikes/s or more produce smoothly graded slow extensions (Hoyle 1955). CI1 modulates and inhibits the effects of SETi (Usherwood and Grundfest 1965), increases the relaxation rate of the extensor tibiae muscle so that there is less residual tension, and thus promotes faster cyclic movements (Pearson 1973; Wolf 1990). In addition to neurogenic contractions driven by SETi or FETi, particular extensor tibiae muscle fibers also express an intrinsic myogenic rhythm that leads to slow, small-amplitude tibial movements in the absence of motor activity (Hoyle 1978a; Voskresenskaya 1959).
The flexor tibiae muscle is smaller and weaker than the antagonistic extensor tibiae muscle in the legs of many Orthoptera, and yet it is innervated by more motor neurons (Debrodt and Bässler 1989; Dresden and Nijenhuis 1958; Phillips 1980; Sasaki and Burrows 1998; Storrer et al. 1986). In the locust Schistocerca gregaria, it is innervated by at least nine excitatory motor neurons that have been categorized as slow, intermediate, or fast according to their different properties, as well as two common inhibitors (CI2 and CI3). Different combinations of flexor tibiae motor neurons, and thus muscle fibers, can be recruited in different behaviors (Bässler and Stein 1996; Duch and Pflüger 1995; Hoyle 1964; Nishino 2003; Page et al. 2008).
When animals perform cyclic limb movements like those underlying scratching, walking, or stridulating, antagonistic muscles acting on the limb segments are generally activated by alternating patterns of activity. In insects, it is possible to record much of the underlying motor activity using electromyographic (EMG) recording techniques in behaving animals (e.g., Cruse and Schmitz 1983; Elsner 1975; Page et al. 2008; Rosenbaum et al. 2010). Even so, it is difficult to determine the influence of residual muscle forces on subsequent movements in this situation because 1) it is not always possible to be sure that all motor activity is recorded; 2) antagonistic motor activity may overlap; 3) the movements may be complex in three-dimensional space, thus giving rise to complex interactions with gravity as well as joint interaction torques; and 4) the limbs may make contact with external objects. To overcome these limitations, we selectively stimulated individual flexor tibiae motor neurons or particular subsets of them in denervated legs in which we had control over all motor activity. We focused on the planar movements of the FT joint in legs oriented so as to avoid the influence of gravity or contact with other objects.
We show that the resting posture is dominated by passive forces in the large extensor muscle and that there is no difference between the resting postures of solitarious and gregarious locusts. Activity in FETi that elicited large twitches of the tibia shifted very consistently, but did not reset, the resting state. Instead, we suggest that the myogenic rhythm might provide a resetting mechanism. Residual flexor tibiae muscle forces influence subsequent active extensions in a predictable and reliable fashion, which is remarkably similar across animals. Activation of different flexor motor neurons has different effects on subsequent extension movements, thus providing a mechanism that could tune joint stiffness. We conclude that the neuronal control of locust limb movements must take into account the substantial and time-varying passive forces and residual muscle forces acting on the joints. This may be assisted by the highly predictable nature of the effects we demonstrate.
METHODS
Adult desert locusts (S. gregaria) with intact hind legs were taken from a crowded laboratory culture at the University of Leicester. The animals were kept under a 12:12-h light-dark cycle with 36°C daytime and 25°C nighttime temperatures. Experiments were conducted at room temperature, between 23 and 25°C. Some experiments were repeated with solitarious locusts obtained from a culture at the University of Cambridge (Rogers et al. 2010). Before experiments, solitarious locusts were kept isolated under the same light and temperature conditions as described above for gregarious animals.
Measurements of Resting Angle of the FT Joint in Isolated Hind Legs
The hind legs of 17 adult locusts (4 male and 3 female gregarious; 5 male and 5 female solitarious) were cut off at the midcoxa level, and the resting angle of the FT joint was measured under different conditions. The leg was mounted medial side down in modeling clay so that the tibia was free to move in the horizontal plane, thus removing the effects of gravity, and was photographed from above using a Basler A602fc-2 camera (Basler, Ahrensburg, Germany). The images (656 × 490 pixels) were saved in bitmap format and opened in Corel Draw. The main axis of the femur was defined as the line connecting the middle of the widest proximal part of the femur with the middle of the thinnest distal part of the femur. The main axis of the tibia was defined as the midline of the relatively thin and cylindrical tibia. The outer angle between the two axes was measured to the nearest degree in Corel Draw. For every animal, the resting angle of the FT joint was measured 10 s after a manually imposed full extension of the tibia and again 10 s after a manually imposed full flexion. The fully extended or fully flexed position was held for a maximum of 1 s in all cases, but this duration was not recorded. After that, either the flexor tibiae or the extensor tibiae muscle tendon was cut at the point of attachment to the tibia so that the remaining stump did not drag against other tissues of the femur, and the measurement was repeated. Both legs of each animal were used, with the flexor muscle tendon being ablated in one leg and the extensor muscle tendon ablated in the other. Each leg thus yielded only two measurements, one of the resting angle under control conditions and the other following the ablation of a muscle.
Stimulating FETi and Flexor Tibiae Motor Neurons in the Muscle
Adult female locusts were fixed ventral side up on a modeling clay platform; the right hind leg was glued medial side down onto a metal platform underlying the distal femur using quick-setting glue (5 min epoxy, Araldite; Huntsman Advanced Materials, The Woodlands, TX). The three proximal leg joints were fixed to the thorax with Araldite. The tibia protruded over the edge of the platform so that it could move freely in a horizontal plane. Distally, the tibia was attached to a small metal hook, which in turn was attached to a force transducer (300C; Aurora Scientific Aurora, ON, Canada). The axon of FETi runs through nerve 5, whereas the axons of SETi and CI1 run through nerve 3 (Burns and Usherwood 1979; Hoyle 1955, 1978b). All of the excitatory flexor tibiae motor neurons have axons in nerve 5 (Hale and Burrows 1985; Philips 1980; Sasaki and Burrows 1998). A small flap was cut in the ventral thoracic cuticle, and nerve 3 was cut to remove SETi and CI1 motor innervation of the extensor tibiae muscle. Nerve 5 was lifted onto a bipolar silver hook electrode and crushed proximal to the electrode to block normal FETi and flexor tibiae motor innervation. Thus the tibia could not be actively moved by the animal.
A pair of minutien pin electrodes was inserted through the cuticle of the femur over the 4th to 6th proximal extensor tibiae muscle bundles. These EMG electrodes were used to stimulate FETi directly in the leg. Preliminary experiments in nine animals showed that this stimulation reliably excited the fast extensor tibiae motor neuron without activating the slow extensor tibiae or flexor tibiae motor neurons (Ache 2010). The forces of tibial movements elicited by extensor tibiae muscle stimulation could thus be measured. Stimulation was driven by a pulse generator (Master 8; A.M.P.I., Jerusalem, Israel). The data were recorded and analyzed using an analog-to-digital converter (micro 1401 mk 2) and the software Spike 2 (both from Cambridge Electronic Design, Cambridge, UK).
Flexor Tibiae Motor Neuron Stimulation
To characterize the effects on tibial force production of activating different groups of flexor tibiae motor neurons, and to determine how many different flexor tibiae motor neurons could be stimulated, nerve 5 recordings were made together with force recordings while different flexor tibiae muscle bundles were stimulated using EMG wires. Locusts were prepared as described above for FETi stimulation experiments. The tibia was fully extended before it was firmly attached to the force transducer. A bipolar silver hook electrode was used to record from nerve 5 in the thorax, and the signals were amplified 1,000 times and bandpass filtered (500 Hz to 2 kHz) using a custom-built amplifier (University of Cambridge). Signals were captured at 10 kHz in Spike 2. The position of flexor muscle stimulation was recorded by reference to the closest extensor tibiae muscle bundles, which provide clear landmarks (see Fig. 5). Stimuli were applied as single pulses, and the resultant maximal force amplitudes were used to further quantify how many different motor neurons were activated at different stimulus sites and stimulus voltages. The amplitudes of all twitches that were elicited during a series of 20 single-pulse stimulations at an interstimulus interval of 2 s were measured as the difference between the mean force in a 100-ms window ending 1 ms before stimulus onset and the peak force recorded during the 100 ms following the stimulus. The peak force was averaged over a range from 2 ms before to 2 ms after the maximum. These data for the maximal force produced at all stimulus amplitudes at every stimulus site were imported into Origin, and histograms counting the number of twitches with different maximal force levels were generated. The bin size was 0.01 (in arbitrary units: absolute force values may differ between animals due to variation in muscle lever arms or the point of attachment of the transducer to the tibia). The number of peaks in the force-amplitude histogram was used as an estimate of the number of different motor neurons stimulated at different sites in the flexor muscle. Finally, the complexity of the compound antidromic potential recorded from nerve 5 during stimulation of flexor tibiae motor neurons was analyzed. The nerve 5 recording was averaged over all trials at a given stimulus voltage and position, using the stimulus artifact as a trigger.
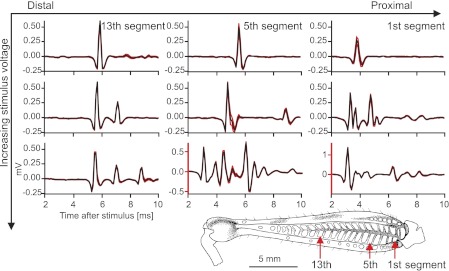
Fig. 5.
Stimulation of more proximal flexor muscle bundles or using higher voltages evoked more motor neuron spikes than did distal or lower voltage stimulation. The curves show the mean (black) ± SE (red) antidromic spike waveform recorded from nerve 5 in the thorax. The flexor tibiae muscle was stimulated at different positions, which are annotated by reference to the closest extensor tibiae muscle bundle in the top row (see inset illustration of femur). Each column represents recordings of potentials evoked at the same stimulus site at 3 different stimulus amplitudes. The top row corresponds to stimuli just above threshold to elicit twitches; the middle row corresponds to trials with stimuli of medium voltage; and the bottom row to trials with stimuli at high stimulus voltages. The goal of these experiments was to sequentially recruit different motor neurons: the absolute or relative levels of stimulation required to do so are not important for the analyses. n = 20 or 21 sweeps were used per average. Note that the bottom center and right recordings are scaled differently from all other recordings (red axes). All data are from 1 continuous experiment.
Motion Capture
To study controlled tibial movements in a motion capture setup, locusts were fixed ventral side up onto an experimental platform using modeling clay and dental glue (Protemp II; 3M ESPE, Seefeld, Germany). All legs were fixed down onto the platform, except for the right metathoracic leg. The femur of the right metathoracic leg was fixed with dental glue at two points: the coxa, trochanter, and femur were fixed to the thorax, and distally the femur was fixed to the platform just proximal to the FT joint. Great care was taken to ensure that the tibia could move freely in both directions. A flap was cut in the cuticle of the ventral metathorax, and all the leg nerves cut to completely denervate the leg. The tracheae and air supply were kept intact. The thoracic cavity was filled with saline solution (Usherwood and Grundfest 1965), and the flap was replaced to prevent evaporation. FETi spikes were elicited in the extensor tibiae muscle using muscle stimulation as described above. The stimulator driving these electrodes simultaneously triggered a light-emitting diode (LED) that was visible in the camera's field of view, thus indicating the timing of the stimulation in the video recordings of the resultant tibial movements. Animals could be stimulated over several hours without detectable changes in the response.
Movements were recorded (VirtualDub, version 1.8.8; by Avery Lee) at 100-Hz temporal resolution with a digital video camera (Basler A602fc-2) mounted 45 cm above the animal. The image size was 656 × 490 pixels. Videos were converted and analyzed with custom-written VideoTrack motion capture software (Jure Zakotnik) as described by Dürr and Matheson (2003). Three 1-mm (diameter) disks made of retroreflective tape (3M ESPE) served as high-contrast markers for the motion capturing. One marker on the proximal femur and one on the distal femur, directly at the FT joint, made it possible to measure the midline of the femur. A third marker was fixed on the distal tibia. The line joining the second and the third marker gave the midline of the tibia. The FT angle could be measured with a temporal resolution of 10 ms and an angular resolution of ∼0.7° (depending on leg size and the distance of the camera), as determined by measuring the variability of repeated FT angle measurements made when a leg was in a fixed position. FT angles measured directly from isolated limbs as described in the first paragraph of the methods section were larger than those measured using the motion capture system. These differences may result in part from different patterns of prior muscle activity in the two cases. In addition, because the marker on the distal femur used for motion capture did not lie exactly on the FT joint pivot point, the motion capture system yielded resting angles that could be up to 10° more extended than corresponding directly measured values.
The effect of isometric flexor tibiae muscle activation on subsequent isotonic extensions driven by FETi spikes was analyzed in five animals. Flexor tibiae motor neurons and FETi were stimulated using EMG electrodes as described above. Flexor tibiae stimulation was set to the minimum voltage at which reliable twitches could be elicited (unless noted otherwise). The flexor tibiae motor neurons were always stimulated prior to FETi. Three variables were altered during the stimulation: the number of pulses used to stimulate flexor tibiae motor neurons, the delay between the last flexor tibiae stimulus and FETi stimulation, and the interval between two flexor tibiae stimuli. These variables were altered for two different stimulus sites, one at the level of the 2nd/3rd extensor tibiae muscle bundle (proximal) and one at the level of the 12th/13th extensor tibiae muscle bundle (distal).
Conventions and Statistics
Unless noted otherwise, N refers to the number of animals and n to the number of trials. The significance level for all statistical tests was set to α = 0.05. Values are means ± SD unless noted otherwise. The FT angle is always given as the external angle, with a straight line extending beyond the femur defined as 0° (see Fig. 1, inset). Wilcoxon signed rank tests were used for comparisons of the data presented in Fig. 1 because the sample sizes were relatively small in some cases and some subsets of data were not normally distributed. The effect of flexor tibiae motor neuron activity on subsequent extension movements was analyzed using one-way repeated-measures ANOVA on the pooled data of all animals for distal stimulation. If sphericity could not be assumed, Greenhouse-Geisser corrected values are reported. For all comparisons, data were randomly sampled from all animals tested at a given stimulus to yield a balanced design. t-Tests were applied to compare the effects of proximal and distal flexor tibiae motor neuron stimulation. Data analyses were carried out using OriginPro (version 8.50; OriginLab, Northampton, MA), Spike2 (version 6; Cambridge Electronic Design), MATLAB (version 7.9; The MathWorks, Natick, MA), and custom-written software. All graphs were plotted in Origin and edited in Corel Draw Graphics Suite (version X4; Corel, Ottawa, ON, Canada).
Fig. 1.
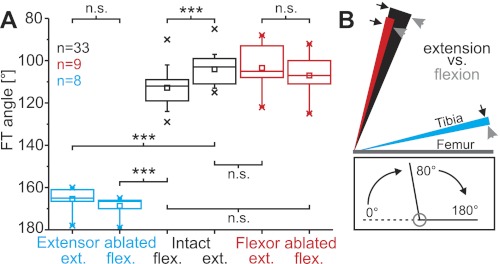
A: the hind leg femorotibial (FT) resting angle of intact legs (black) was significantly more flexed after release from full flexion (intact flexed) than after release from full extension (intact extended). Ablation of the extensor tibiae muscle led to a significant increase of the resting angle (tibial flexion) following release from either full extension or full flexion (blue), whereas ablation of the flexor muscle had no significant effect (red). The boxes indicate the quartiles; whiskers show the 90 and 10 percentiles, respectively; crosses indicate minima and maxima of the distributions; and squares show the respective mean angle. Ext., extended; flex., flexed; n.s., not significant. ***P < 0.001. B: the history dependence of the FT resting angle. The inner border of each colored area (arrowheads) represents the posture of the tibia after release from full flexion, and the outer border (arrows) represents the posture after full extension (mean positions). The difference between these angles (areas of solid color) therefore indicates the history dependency of the resting angle. Black, red, and blue correspond to the same-colored experimental conditions as in A. Lines representing the tibiae are drawn with different lengths for clarity of illustration; n = no. of legs for A and B, N = 17 animals: 10 solitarious, 7 gregarious, data pooled. Inset shows the angle conventions used throughout this report.
RESULTS
Resting Angle of the FT Joint in Isolated Hind Legs
In the absence of motor activity, the FT joint of a locust hind leg assumes a characteristic resting position near the midpoint of its range. Perturbations away from this position are followed by passive return movements. The resting angle of the FT joint was measured following either 1) a brief full extension or 2) full flexion of the tibia in intact but isolated legs, and following ablation of either the flexor tibiae or the extensor tibiae muscle (6 conditions in total).
In all 17 animals tested, the resting FT angles of intact isolated legs were close to 100° in the absence of muscle activity (Fig. 1, A and B, black; see inset for angular measurement convention). The FT angle following extension was 104.1 ± 7.2° (mean ± SD), whereas that following flexion was 112.8 ± 8.8°. This difference was highly significant (Wilcoxon signed rank test, P < 0.001), as all legs were more flexed after flexion than they were after extension. Ablation of the extensor tibiae tendon caused the tibia to flex further so that the mean resting angle was 165.4 ± 5.7° after full extension and 168.6 ± 4.7° after full flexion (Fig. 1, A and B, blue). The effects of extensor tibiae muscle ablation were statistically highly significant after both full extension and full flexion (Wilcoxon signed rank test, n = 8 legs from N = 8 animals, both P values <0.001). In contrast to the large effect of extensor tibiae tendon ablation, ablating the flexor tibiae tendon had no significant effect on the FT resting angle following either extension or flexion (Fig. 1, A and B, red; Wilcoxon signed rank test, both P values >0.1, n = 9, N = 9). There were no significant differences between the resting angles of intact legs of solitarious (n = 20) and gregarious (n = 13) locusts after either full flexion (gregarious: 110 ± 9.1°, solitarious: 114.6 ± 8.2°; P = 0.14, 2-sample t-test) or full extension (gregarious: 102.8 ± 9.0°, solitarious: 104.9 ± 5.9°; P = 0.42, 2-sample t-test).
Resting Angle of the FT Joint in Intact Animals
In intact, behaving locusts, tibial extensions and flexions are often driven by motor activity. We therefore measured the effect of motor activity on the resting angle of the FT joint. The fast extensor tibiae motor neuron (FETi) was selectively and reliably stimulated by inserting EMG electrodes through the cuticle into the 4th to 6th extensor tibiae muscle bundles (see Fig. 5, inset).
Resting angle of the FT joint depends on the history of FETi activation and tibial movement.
The resting angle is defined by passive forces of the flexor tibiae and extensor tibiae muscles as demonstrated in the preceding section, by joint friction and possibly other joint forces, and by the presence or absence of an ongoing myogenic rhythm (see following section). Here we show that the resting FT angle is also dependent on the pattern of prior activity in FETi. Starting positions were not imposed manually during these experiments, but instead reflected the resting angle attained following a previous movement.
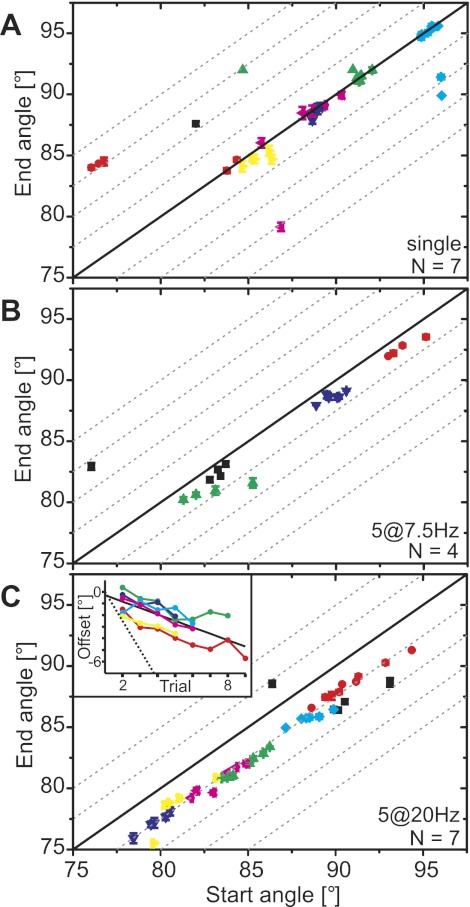
The resting FT angle prior to an active movement elicited by controlled stimulation of FETi (“start angle”) predicted very reliably the resting angle measured 10 s after the end of stimulation (“end angle”), regardless of stimulation protocol or animal (Fig. 2). When FETi was stimulated with a single pulse, the tibia extended actively and then returned passively to within 2° of the starting position in 34 trials from 7 animals (Figs. 2A and 3). In 8 trials, the tibia returned to positions that were extended or flexed by more than 2° from the starting position. This resulted in an offset of −0.07 ± 0.56° [median ± interquartile range (IQR); Fig. 3].
Fig. 2.
Resting angle (start angle) prior to an active movement generated by stimulation of the fast extensor tibiae motor neuron (FETi) predicts reliably the resting angle reached 10 s after the movement (end angle). A: the end angle was equal to the start angle for single twitches. Data from N = 7 different animals are shown in different colors. Symbols show means ± SD of single trials; the angles were averaged over 5 frames. In most cases the SD is smaller than the symbol size. Dashed gray lines indicate offsets in steps of 2.5° from the solid black line, which indicates end angle = start angle. Trials in A: 42, n = 5–10. There are 16 partially overlaid data points falling between starting angles of 88° and 90°, including 4 from 1 animal (black). B: the end angle depended directly on the start angle and had an offset of approximately −1° after movements generated by stimulation at 7.5 Hz. Trials in B: 19, n = 4–5. C: the end angle depended directly on the start angle and had an offset of approximately −2.5° after movements generated by stimulation at 20 Hz. Trials in C: 44, n = 5–9. Inset: repeated stimulation of FETi shifts the resting angle to more extended angles. The start angle of the first trial was subtracted from all trials such that the data indicate the offset of the start angle with respect to the first trial. The trial number had a significant effect on the resting angle (solid black line: Y = −0.55X − 0.29, R = −0.783, P < 0.001). The linear fit was applied to all individual trials of all animals. The dotted black line follows Y = −2.5 × (trial − 1) and thus gives the relation expected if the end angle of one trial was the start angle of the following trial. The data set shown in black in C was excluded from the inset because the first trial was an outlier.
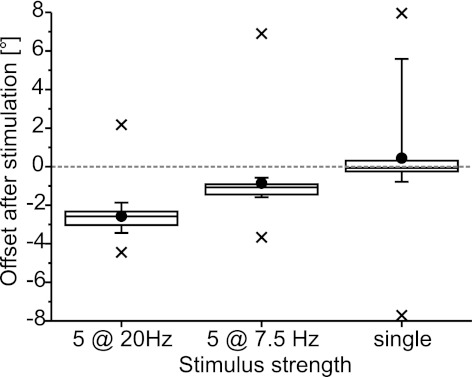
Fig. 3.
Strength of stimulation of FETi influenced the offset of the resting angle. The data for all stimuli were pooled across all animals; sample sizes are as given in Fig. 2. Negative values indicate more extended angles. The boxes indicate the quartiles; filled circles show the mean offset; whiskers indicate 10% and 90% intervals; and crosses indicate minimum and maximum values. The offset increased with the number of pulses applied, as shown by the difference between the offset after single-pulse stimulation and the other 2 stimulus types, in which 5 spikes were elicited in the FETi motor neuron. The offset also increased with increasing stimulus frequency, which manifests in the difference in offset after 7.5- and 20-Hz stimulation. These effects were significant (1-way repeated-measures ANOVA, P = 4 × 10−7, Tukey's test: all P values <0.002).
Stimulating FETi with 5 pulses at 7.5 Hz caused a stronger active extension (not shown) and led to an offset in the end angle (Figs. 2B and 3). The offset was small (median = −1.07) but consistent (IQR = 0.54°) across the four animals tested. Seventeen of 19 trials fell between 0° offset and −2.5° offset.
Stimulating FETi with 5 pulses at 20 Hz led to a larger and very consistent offset in the end angle relative to the start angle (−2.58 ± 0.75°; Figs. 2C and 3). Even small differences in the start angle between trials from a single animal were reflected in corresponding offsets in the end angle (e.g., animals shown in blue, green and red in Fig. 2C). Across all animals, the offset varied by <0.5° between trials even though the start angle spanned 16°.
In addition to the immediate effect of FETi stimulation on the resting angle, there was a long-term effect of repeated FETi stimulation on the resting angle, as Fig. 2C, inset, shows for 20-Hz FETi stimulation. The resting angle depended linearly on the trial number (Fig. 2C, inset, solid black line). The slope of this curve was −0.55, which is only 22% of the slope expected if the end angle of one trial was the start angle of the next (indicated by the dotted black line, Fig. 2C, inset). Moreover, only two curves (yellow and magenta) were continuous; in all other animals, at least one trial had a more flexed start angle than the trial before. All of this indicates that there was another effect, apart from the offset in end angle, that reduced the long-term history dependency and could lead to more flexed start angles during subsequent stimulation.
For statistical analysis, the maxima and minima of the three distributions shown in Fig. 3 were excluded as outliers (because they were likely influenced by myogenic contractions, as described in the next section) and the 7.5- and 20-Hz stimulation data set was truncated by randomly excluding trials until all three data sets contained n = 17 trials. A one-way repeated-measures ANOVA showed that the effect of the stimulus strength on the offset after FETi stimulation was highly significant (P < 0.001). Tukey's test yielded P values <0.002 for the cross-comparison of the three truncated distributions. The effect of stimulus strength on the offset was still significant if the outliers (minima and maxima) were included (P < 0.001), but this was only due to the differences between single-pulse and 20-Hz stimulation. The analysis in which the outliers were excluded, however, showed that both the number of spikes elicited in FETi and the frequency of the spike trains affected the offset of the FT angle after a movement.
The motion capture experiments thus showed that the resting angle is shifted by activation of the extensor tibiae muscle and that the shift is highly predictable both within and across animals.
An intrinsic myogenic rhythm of the extensor tibiae muscle can reset the resting state of the FT joint.
As shown in the preceding section, the resting state was not reset by FETi activation. Nevertheless, consecutive trials did not always start from progressively more extended angles as might be expected from the consistent offset in end angle.
The completely denervated hind legs of all animals showed slow, rhythmic tibial extension-flexion movements (Fig. 4). These spontaneous myogenic movements generally occurred at intervals of longer than 90 s. They had an amplitude of 11.7 ± 1.5° (mean ± SD, N = 3). When the tibia was extended by a myogenic contraction, it fell back to a position that was not directly related to, and could be more flexed than, the initial position. Myogenic movements that occurred between FETi stimulation trials could therefore reset the resting FT angle.
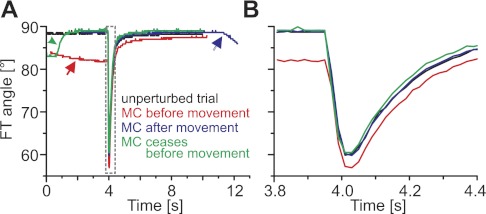
Fig. 4.
Active movements (rapid downward deflections at 4 s) were modified by slower sporadic myogenic contractions (MC). A: the 4 traces show movements of the same tibia, each driven by a single spike in the FETi motor neuron. One of the movements was unperturbed (black), one was elicited during an ongoing MC (red line, red arrow indicates the MC), one was followed by an MC (blue line and arrow), and one was preceded by an MC (green line, green arrowhead indicates the passive return following the myogenic contraction). MCs were slower and of lower amplitude than either FETi-driven or passive movements. B: the active, FETi-driven extensions on an expanded timescale as indicated by the dashed gray box in A. The movement elicited during the ongoing MC (red line) had the largest overall amplitude.
Myogenic contractions that occurred during FETi stimulation trials (e.g., Fig. 4) probably gave rise to the few outlying data points in Fig. 2. The movement curve shown in red in Fig. 4 started from an angle that was 7° more extended than the other three movements shown, as FETi was stimulated during the rising phase of a myogenic rhythm. Since the myogenic rhythm ceased during the FETi-driven movement, the tibia returned to a more flexed angle than the one it started from. The start and the end angles therefore differ significantly in such trials, and the corresponding data points in Fig. 2 are shifted toward the upper left. The movement curve shown in blue (Fig. 4) gives another example of how the start-to-end angle ratio could be biased by the myogenic rhythm. Here, the myogenic rhythm started after the FETi-driven movement had ceased, which led to an end angle that was more extended than the start angle. All outliers in Fig. 2 lie within the amplitude range of movements driven by the myogenic rhythm.
Influence of Flexor Tibiae Motor Activity on Subsequent Extensions Driven by FETi
In a behaving animal, the flexor and extensor tibiae muscles are usually activated alternately. We therefore measured the effect of residual flexor tibiae muscle forces on subsequent tibial extensions.
Differential stimulation of flexor tibiae motor neurons using EMG wires.
To determine whether flexor tibiae motor neurons could be stimulated selectively using EMG electrodes, the flexor tibiae muscle was stimulated at different locations and with different stimulus amplitudes. The antidromic motor spikes were recorded in nerve 5 along with the force produced by the consequent tibial twitches.
Stimulating the flexor tibiae muscle reliably elicited single or compound motor spikes in nerve 5 in all animals tested. The number and amplitude of the spikes depended on the stimulus amplitude and the stimulus site (Fig. 5).
Single antidromic motor spikes were evoked at all stimulus sites at a voltage just above the threshold to elicit visible twitches of the tibia (Fig. 5, top row). Increasing the stimulus voltage recruited more motor neurons at all stimulus sites (Fig. 5).When the flexor tibiae muscle was stimulated at the level of the 13th extensor tibiae muscle block, a single flexor tibiae motor neuron was activated at low voltages, a second one was recruited at higher voltages, and a third one was added at the highest voltages used (Fig. 5, left column, top to bottom). There was low variability in the waveform of the antidromic spikes (Fig. 5, red error curves). Motor neurons activated at higher stimulus amplitudes generally had longer delays (slower conduction velocities) and lower spike amplitudes than those activated at lower stimulus amplitudes.
Progressively stronger stimulation at more proximal sites in the flexor tibiae muscle (Fig. 5, center and right columns) led to increasingly complex patterns of activity recorded in nerve 5, presumably reflecting superposition of multiple antidromic spikes at the recording site. At both proximal stimulation sites, some larger amplitude motor spikes with shorter delays were recorded at the highest stimulus amplitudes, suggesting that large, fast-conducting neurons were being activated at a larger distance from the electrodes.
Sequential recruitment of flexor tibiae motor neurons can generate a wide range of flexion forces.
The selective stimulation of different flexor tibiae motor neurons demonstrated in the previous section provided a means to measure the forces produced by particular subsets of the motor neuron pool for this muscle. The forces differed in amplitude between motor neurons, and the total peak force changed as a function of the motor neurons activated (Fig. 6).
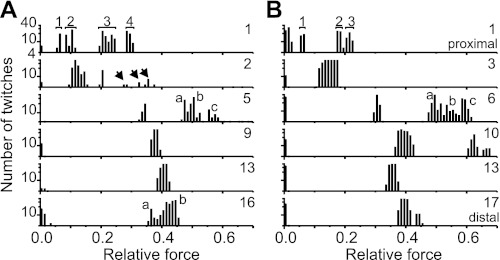
Fig. 6.
Different sets of motor neurons could be stimulated at different sites in the flexor tibiae muscle. A and B show data from 2 different animals; data in A were recorded from the same animal as that used for Fig. 5. The stimulus site used for each set of trials is indicated by the number at top right of each row, as described in the legend to Fig. 5. The numbers of twitches with a particular force X were counted and binned for each stimulus position, independently of the stimulus voltage used to elicit them, so all motor units that could be stimulated at each position were included. Force is comparable within but not directly between animals. All of the Y-axes are log-scaled and span 4–40 as shown at top left. Each number in the top row indicates a force peak generated by 1 particular set of neurons. Arrows in the second row in A mark a set of medium-force peaks. The letters a, b, and c in the third row mark 3 distinct force peaks generated that were similar in both animals (A and B). a and b in the bottom row in A mark 2 distinct peaks in the force distribution. Very low amplitude forces (<0.05 relative force) reflect noise in the measurement.
The peak forces of all twitches elicited by stimulation at each site were measured and binned across all stimulus amplitudes (Fig. 6). Each peak in the distribution represents the forces generated by one distinct set of motor neurons (e.g., peak forces 1–4 in Fig. 6A). The number and distribution of these distinct peaks in each graph provides a minimal estimate of the number of motor neurons innervating that region of the flexor tibiae muscle and describes the full range of forces that their combined activity could generate. The patterns of the distributions were remarkably similar in different animals (Fig. 6, compare A and B).
Stimulation of the flexor tibiae muscle at different voltages at the level of the 1st extensor tibiae muscle bundle (see Fig. 5, inset) led to at least four different peak force values in one animal (Fig. 6A) and at least three peaks in a second animal (Fig. 6B). The maximal forces generated by the proximal motor neurons were relatively small in both animals, whereas stimulation at medial or distal sites resulted in larger peak forces (compare peak forces for segments 1–3 with those for segments 5–17 in Fig. 6, A and B). The distribution of peak forces corresponded well with the number of motor neuron spikes evident in a simultaneous nerve 5 recording (4 spikes evident in Fig. 5, right column).
Stimulating at the level of the 2nd extensor tibiae muscle bundle in the animal shown in Fig. 6A elicited low forces in most trials but also, in a few cases, medium forces (arrows). Stimulation at the level of the 3rd extensor tibiae muscle bundle in the second animal resulted in only one, broad peak of forces.
Stimulation at the level of the 5th and 6th extensor tibiae muscle bundles generated four peaks in both animals. The two patterns were similar in both animals, with one distinct peak at medium force values and a continuous distribution with three more peaks at higher force values (a, b, and c in Fig. 6, third row). Stimulating at the level of the 9th extensor tibiae muscle bundle in one animal elicited contractions of medium force. In the second animal, stimulation at the level of the 10th extensor tibiae bundle elicited contractions with three distinct amplitudes, one of which was the strongest observed.
Stimulation of more distal flexor motor neurons at the level of the 13th extensor tibiae muscle bundle generated peak forces of medium strength in both animals (Fig. 6, fifth row). (This stimulus site was used for the flexor tibiae muscle stimulation in motion capture experiments described in later sections). Stimulation of the flexor tibiae muscle at the level of the 16th or 17th extensor bundle generated at least two peaks of force in both animals (a and b in Fig. 6A, sixth row).
Flexor Tibiae Influence Depends on the Type of Recruited Muscle Fibers and the Strength of Flexor Tibiae Contraction
To characterize the influence of residual flexor tibiae muscle forces on subsequent extension movements, different flexor tibiae motor neurons were stimulated and the resulting effects on extensions driven by FETi studied in five animals.
Velocity and amplitude of FETi-driven extensions decline with an increasing number of preceding flexor tibiae motor spikes.
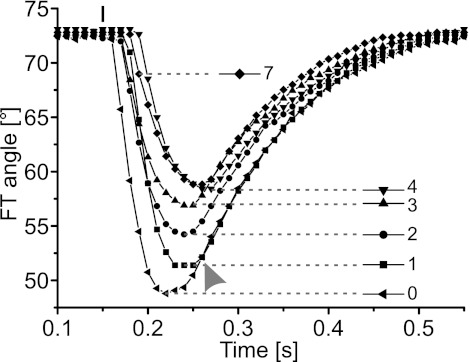
Stimulating flexor tibiae motor neurons with even a single pulse 10 ms before FETi stimulation was sufficient to reduce the amplitude of the subsequent extension movement and delay both its onset and peak (Fig. 7). These effects became more pronounced as the number of flexor spikes was increased (Fig. 7). In contrast, flexor stimulation had little effect on the passive return flexion that followed each extension (arrowhead in Fig. 7).
Fig. 7.
Contractions of the flexor tibiae muscle modified subsequent active extensions of the tibia generated by stimulation of FETi. The amplitude of the extension movement decreased with increasing strength of flexor stimulation: the onset of movement was increasingly delayed, and the movements were slower. Each curve shows the time course of a single active extension movement after the flexor tibiae was stimulated with a different number of spikes (see key). The small vertical bar at top indicates the point of extensor stimulation. When multiple stimuli were applied to the flexor tibiae muscle, the interval between pulses was 10 ms. The delay between the last flexor tibiae stimulus and extensor stimulation was constant at 10 ms. Flexor stimulation was isometric, so the starting angle for subsequent isotonic extension movements was constant. The passive return movements after pure extensor stimulation and extensor stimulation following a single flexor spike were similar (gray arrowhead). Prior stimulation of the flexor tibiae muscle with 4 and 7 spikes resulted in similar extension movements.
The effects on extension movements of prior stimulation of distal flexor tibiae motor neurons were analyzed in detail in four animals. The effects of stimulating proximal motor neurons were analyzed in one of these animals and in one additional animal (Fig. 8).
Fig. 8.
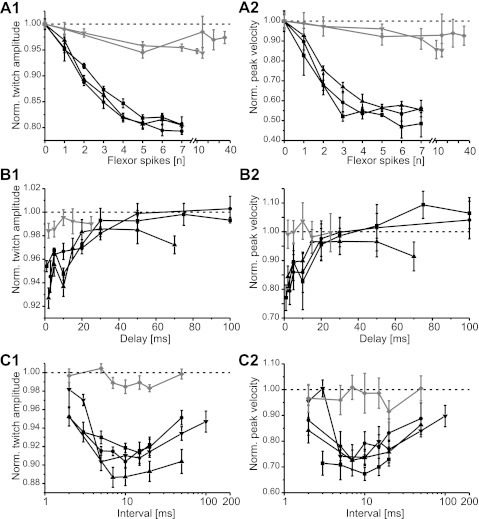
Velocity and amplitude of active extensions change with the number of preceding spikes in flexor tibiae motor neurons (A), the delay between flexor and extensor tibiae activation (B), and the rate of preceding flexor motor neuron stimulation (C). Left column shows the amplitude, and right column shows the peak velocity, of active extension movements following isometric flexor tibiae contractions. The extension twitch amplitude and peak velocity were normalized by the mean values of the 2 measures for trials without flexor tibiae activation for each animal. Black symbols show results for stimulation of distal flexor tibiae motor neurons (N = 3–4 animals; n = 4–7 trials); gray symbols show results for proximal stimulation (N = 1–2 animals; n = 4–7 trials). Note that the X-axis is broken in A and log-scaled in C. Norm., normalized.
Single spikes in the distal flexor tibiae motor neurons reduced the amplitude of a subsequent active extension movement by 5% and the peak velocity by 10% (Fig. 8, A and B, black symbols). Additional spikes further reduced the amplitude by as much as 20% and the velocity by 50% in all three animals. The reduction of the amplitude saturated at six spikes (Fig. 8A1), and the reduction in velocity saturated after three or four spikes (Fig. 8A2). The number of spikes elicited in the distal flexor tibiae had a significant influence on the amplitude [F(7, 77) = 192.33, P < 0.001] and velocity [F(2.91, 31.97) = 503.48, P < 0.001] of the extension movement.
Stimulation of proximal flexor tibiae motor neurons had a similar but much weaker effect on both amplitude (t = 31.70, P < 0.001) and velocity (t = 29.12, P < 0.001). [Comparison was made for the case of 5 flexor spikes: n (distal) = 12, n (proximal) = 11.] The amplitude of extension movements was reduced by up to 5% at the proximal stimulation site (saturating at 5 and 15 spikes, respectively; Fig. 8A1). Extension velocity also decreased consistently with the number of spikes in both animals tested, saturating at 5 and 10 spikes, respectively (Fig. 8A2, gray symbols). The peak velocity was reduced by 15% in one and by 9% in the other animal (Fig. 8A2).
Influence of residual flexor tibiae muscle force declines over time.
FETi was stimulated at different delays after flexor tibiae stimulation, and the velocity and amplitude of the extension movements were analyzed. When distal flexor tibiae motor neurons were stimulated with single pulses, the amplitude of the subsequent extension movement driven by FETi stimulation decreased by up to 7% if the delay was 1–2 ms (Fig. 8B1, black symbols). With increasing delay, the amplitude of the extension movements increased and reached the control value at delays of 50 ms in two animals. In a third animal, control values were not reached within the range of delays studied. Delays of 10 ms had a larger effect than expected from a steadily rising function (Fig. 8B). Two of the curves show an inflection at 10 ms, and the third curve plateaus at 10 ms.
The peak velocity curves followed a similar pattern (Fig. 8B2, black symbols). The velocity was decreased by a maximum of 22.5% at the shortest delay of 1 ms. The curves rose steadily as the delay increased, except for an inflection at 10 ms. At delays of 30 ms or more, the peak velocity was the same as, or even greater than, control values (Fig. 8B2). The delay between flexor tibiae motor neuron stimulation and FETi stimulation had a highly significant effect on the extension movement amplitude [F(2.16, 17.30) = 22.68, P < 0.001] and velocity [F(5, 40) = 18.81, P < 0.001].
Single-pulse stimulation of proximal flexor tibiae motor neurons had very little effect on subsequent extension movements (Fig. 8B, gray symbols in both graphs). Proximal and distal flexor tibiae stimulation had significantly different effects on both the amplitude (t = 5.69, P < 0.001) and the velocity (t = 5.48, P < 0.001) of the movement, determined at a delay of 5 ms [n (distal) = 9; n (proximal) = 6]. The amplitude of the extension movements decreased by 1.5% at 2- and 3-ms delay but was close to the control value for larger delays. There was no marked effect on the peak velocity of the movement at any delay (Fig. 8B2, gray symbols). The extension amplitude was least impaired at a delay of 10 ms. The mean peak velocity of an extension was 4% larger following proximal flexor tibiae motor neuron stimulation than during control trials at 10-ms delay (Fig. 8B2, gray symbols).
Two spikes in flexor tibiae motor neurons are most effective when evoked at an interval of 10 ms.
Flexor tibiae motor neurons were stimulated with two spikes at varying intervals, and the impact on subsequent active extension movements was analyzed in four animals (Fig. 8C). At the shortest interval of 2 ms (500-Hz frequency), stimulation of distal motor neurons decreased the amplitude of a subsequent active extension by 2–5% (Fig. 8C1, black symbols) and decreased the velocity by 5–15% (Fig. 8C2, black symbols). These intervals are likely to fall within the partial refractory period of the flexor motor neurons. As the flexor stimulation interval increased toward 10 ms, subsequent extension movements became slower and smaller in amplitude. At a stimulation interval of 10 ms, the amplitude was reduced by 8.5–11% and the peak velocity was decreased by 26–32%. At intervals longer than 10 ms, the extensions became faster and reached higher amplitudes again. At intervals of 50 ms, movement amplitudes were still decreased by at least 5% and velocities by 10%. This corresponds to the effect of single flexor tibiae spikes shown in Fig. 8A. The flexor motor neuron interspike interval had a significant influence on the extension movement amplitude [F(6, 66) = 17.48, P < 0.001] and velocity [F(6, 66) = 8.54, P < 0.001].
Stimulation of proximal flexor tibiae motor neurons (Fig. 8C, gray symbols) had only a small effect on the amplitude of subsequent extension movements driven by FETi stimulation (Fig. 8C1) and a negligible effect on the velocity (Fig. 8C2), regardless of the interstimulus interval. The differences between proximal and distal flexor tibiae motor neuron stimulation were highly significant at an interval of 10 ms [amplitude: t = 12.82, P < 0.001; velocity: t = 6.43, P < 0.001; n (distal) = 12, n (proximal) = 7]. The amplitude decreased by at most 1.5% at stimulus intervals of 7–20 ms.
DISCUSSION
The FT Joint has a History-Dependent Resting State that is Dominated by the Extensor Tibiae Muscle
In the absence of motor activity and external loading, the locust hind leg FT joint assumes a history-dependent resting state, rather than a particular resting angle. This is in marked contrast to the larger and heavier limbs of many vertebrates, which come to rest in positions primarily governed by the actions of gravity. The resting state of the locust FT joint is dominated by the large extensor tibiae muscle. Ablation of the extensor tibiae muscle led to almost full flexion of the resting FT angle, whereas ablation of the smaller flexor tibiae muscle had no significant effect. The resting state was also altered by passive stretch of the extensor and flexor tibiae muscles induced by imposed prior extensions or flexions of the tibia. The tibiae of stick insects and cockroaches also assume characteristic resting postures driven in part by passive muscle forces. Examination of the data presented by Hooper et al. 2009 (their Fig. 2) indicates that the resting angles of stick insect legs are, like those of the locust hind leg, subject to the influence of prior passive extensions or flexions. In stick insects, passive forces originating in structures other than the extensor and flexor tibiae muscles make a larger contribution to passive extensions than to passive flexions (Hooper et al. 2009). Some of the variability in our data might be due to varying holding times at the fully extended and fully flexed angles, which were not controlled for as the movements were imposed manually.
In locusts the resting state was also shifted by prior active extension movements driven by activity of the fast extensor tibiae motor neuron (FETi). Individual FETi spikes (at <0.02 spikes/s) did not influence the resting angle, but increases in FETi spike frequency and thus extensor tibiae muscle force caused progressively larger shifts in resting position. These shifts were highly consistent both within and across animals, indicating that there is low variability of the relative forces in the FT joint. The resting state was determined before onset of the movement, and it was not reset by stimulating FETi and eliciting fast extensions of the tibia. A resetting mechanism would uncouple the resting angles before and after movement.
The strength of FETi activation had a significant effect on the shift in resting angle, with both larger numbers of spikes and higher spike frequencies leading to larger offsets. If these parameters are varied, several parameters of the extension movements change (Ache 2010). Briefly, the variability in movements driven by the same stimulus in the same individual was small. A larger number of spikes led to a larger amplitude of movement. In our case, single FETi spikes generated movements of ∼30° amplitude, whereas five FETi spikes led to full extension of the tibia, with an amplitude of ∼60° (both amplitudes depended on the start angle). The difference in offset between single- and five-pulse stimulation could thus be due to a difference in extension amplitude, which could lead to stronger passive stretch of the flexor tibiae muscle. However, both five-pulse stimulation protocols led to a full extension of the tibia, but the offset was larger after 20-Hz stimulation. The 20-Hz stimulation elicited a smooth, full extension of the tibia which was then held fully extended for up to 500 ms, whereas the 7.5-Hz stimulation generated a series of 5 extension twitches with only the last twitch reaching full extension. The total amplitudes of the movements were therefore similar between the two five-pulse stimulation protocols. However, the total duration of movement was longer during 7.5-Hz stimulation, but the time held at full extension was longer during 20-Hz stimulation. It therefore seems likely that the total amplitude and the duration the tibia is held fully extended are important factors determining the offset in resting angle, whereas the overall duration of extension movements below full extension seems to play a minor role.
The shift in FT resting angle caused by FETi activation could have two sources. First, extensor tibiae muscle fibers innervated by FETi produce residual forces that outlast the FETi activation. Under isometric conditions, such fibers have relaxation times of 120 ms following single-pulse stimulation of FETi, whereas tonic extensor tibiae muscle fibers innervated by the slow extensor tibiae motor neuron (SETi) have relaxation times of up to 3 s (Cochrane et al. 1972). In our experiments, however, the extensor tibiae muscle shortened isotonically during FETi activation, and the flexor tibiae muscle was stretched as the tibia extended. These changes in muscle length could provide a second mechanism leading to a shift in the resting angle. For example, passive stretch of the extensor tibiae muscle in the locust can lead to changes in passive forces for the next 100 s (Clare et al. 2009). We did not distinguish between the effects of passive stretch and residual extensor tibiae muscle force, since active movements always had both components.
FETi activation led to highly consistent extension biases in the resting state, and yet sequential activations did not result in a strong, longer term gradual shift to progressively more extended resting angles. The long-term shift that was observed was small compared with the short-term effect. Therefore, an additional mechanism has to be present, preventing the resting angle from shifting to extreme angles by resetting it to more flexed angles between active extensions. The “resetting” of the resting state required to avoid such cumulative effects over timescales of tens of seconds is achieved, at least in part, by an intrinsic myogenic rhythm of particular extensor tibiae muscle fibers.
A Myogenic Rhythm Resets the Resting State
Slow tibial extensions were observed in denervated hind legs, and occasionally in isolated hind legs, in the absence of motor neuron activity. These extensions were followed by slightly faster flexions. Such extension movements are driven by myogenic contractions of a specialized bundle of muscle fibers in the extensor tibiae muscle (Burns and Usherwood 1978; Evans and O'Shea 1978; Hoyle and O'Shea 1974; Voskresenskaya 1959). The return flexions are passive. Since myogenic contractions that occurred between measurement trials seemed to reset the resting state, it is possible that the resting state is dominated primarily by maintained forces in only those extensor tibiae muscle fibers of the proximal bundles that show myogenic activity (Evans and O'Shea 1978; Hoyle and O'Shea 1974). Stretch of the extensor or flexor tibiae muscles or activation of FETi may simply add or subtract a constant force to these forces determining the resting state, rather than modulating them directly. Only a new myogenic contraction would provide the necessary “reset.” In our experiments, FETi stimulation sometimes paced the frequency of the myogenic rhythm, and in in situ preparations (Burns and Usherwood 1978), motor neuronal inputs to the myogenic fibers influenced the amplitude, time course, and frequency of their myogenic depolarizations. Such influences could tune the resting properties of the FT joint to patterns of extensor tibiae muscle contraction such that FT movements are likely to start from a narrow resting range, rather than an extended position reached after strong FETi activation. This would reduce the variability in the FETi spike to movement transfer by keeping the variability in start angle small.
The myogenic rhythm was responsible for the outlying data points in the analysis of the history dependency of the resting state (Fig. 2). For example, the starting FT angles of ∼77° for the three leftmost data points in Fig. 2A (red symbols) were imposed by myogenic contractions of ∼10° in this animal (cf. 2 unaffected trials with starting angles of ∼84° in the same animal; red symbols in Fig. 2A). Trials in which the end angle was more extended than the start angle can be explained by myogenic contractions that started while the tibia was being actively extended by FETi activity.
Selective Stimulation of Flexor Tibiae Motor Neurons
The flexor tibiae muscle is innervated by at least nine excitatory motor neurons (Sasaki and Burrows 1998). To investigate interactions between flexor and extensor forces in the control of tibial movements we selectively stimulated single motor neurons or sets of motor neurons in a controlled way (Fig. 5). By adjusting the stimulation strength, we reliably elicited spikes in a single distal flexor tibiae motor neuron (Fig. 5), suggesting that the same motor neuron was activated in all animals. Having demonstrated that our distal stimulation reliably activated a single motor neuron, we went on to use this technique to characterize the effects of the contractions generated by this specific motor neuron on subsequent FETi-driven extensions. These data are shown in Figs. 6 and 7 and are discussed in Role of the Flexor Tibiae Muscle During Active Extensions below.
We also searched for differences in the roles of flexor tibiae motor neurons innervating different regions of the muscle. Stimulation of proximal motor neurons generated flexion forces that were approximately one-half as strong as the forces generated by stimulation of distal motor neurons, but more importantly, the effects on subsequent extensions were very much smaller. For example, a train of 5 spikes in the single distal motor neuron caused a 20% reduction in the amplitude and a 50% reduction in the velocity of subsequent active extensions, but 5 spikes in proximal motor neurons caused no more than a 5% reduction in either amplitude or velocity of subsequent tibial extensions, even when we activated 4–5 flexor motor neurons together at higher stimulation levels (Fig. 8).
Activity in different combinations of flexor tibiae motor neurons generated a wide range of different peak forces as expected (Fig. 6), but the number of distinct peaks in force was not always equal to the number of motor neurons stimulated (compare Figs. 5 and 6). Broader peaks presumably resulted from the summation of forces generated by simultaneous spikes in two or more motor neurons. A second possibility is that our stimulation activated the common inhibitory motor neurons CI2 and CI3 that innervate flexor tibiae muscle fibers, leading to additional spikes in the nerve 5 recording. CI2 and CI3 axons have small diameters (Hale and Burrows 1985) and therefore have high thresholds for extracellular stimulation, so this could only have occurred at the highest stimulation strengths. During walking, CI2 and CI3 exert their effects on the flexor muscle by firing at over 48 spikes/s (Wolf 1990). In our experiments, even if CI2 and CI3 were stimulated alongside flexor tibiae motor neurons at the highest stimulation levels, they would not have exerted marked effects on the subsequent FETi-driven extension movements that we analyzed because our stimulation elicited at most only single spikes at low frequency.
Role of the Flexor Tibiae Muscle During Active Extensions
Residual flexor tibiae muscle force shapes active extensions.
During walking or scratching in locusts, the extensor and flexor tibiae muscles generally exhibit reciprocal patterns of activity (Burns and Usherwood 1979; Page et al. 2008). We therefore examined the effects of residual flexor tibiae muscle forces on subsequent active extension movements. The history of flexor tibiae activation had a direct and graded effect on subsequent extension of the tibia. The velocity of extension movements was reduced more markedly than the amplitude, and activation of different flexor tibiae motor neurons led to markedly different effects on subsequent extension movements, thus providing a mechanism for fine control over joint stiffness.
Activation of distal flexor tibiae motor neurons had relatively large effects on subsequent active tibial extensions, whereas activation of proximal flexor tibiae motor neurons had relatively small effects, even if they were stimulated strongly. This suggests that the residual force produced by proximal flexor tibiae muscle bundles was much smaller than that produced by more distal bundles. This is consistent with the observation that proximal flexor tibiae muscle fibers are predominantly phasic, i.e., they contract and relax rapidly, whereas distal fibers are more tonic, so they contract and relax more slowly (Philips 1981). Such regional differentiation is not uncommon: proximal fibers of the extensor tibiae muscle of both locust and stick insect hind legs are predominantly innervated by FETi, whereas distal fibers are predominantly innervated by SETi (Bässler and Storrer 1980; Bässler et al. 1996; Burns and Usherwood 1979).
The extent to which flexor tibiae contractions affected subsequent active tibial extensions depended on three additional parameters: the number of flexor tibiae motor spikes (Fig. 8A), the delay between flexor tibiae and extensor tibiae muscle activation (Fig. 8B), and the spike frequency (Fig. 8C). Tibial extensions were considerably slower, and had smaller amplitudes, if FETi fired within 30 ms of preceding activity in distal flexor tibiae motor neurons.
Some muscle fibers of both the extensor and flexor tibiae muscles, particularly those innervated by tonically firing slow motor neurons, are also innervated by common inhibitory motor neurons (Hale and Burrows 1985; Usherwood and Grundfest 1965). Activity in the inhibitors often accompanies activity in the excitatory motor neurons to the same muscle and through both pre- and postsynaptic mechanisms can reduce or prevent the activation of the fibers that they innervate (Rathmayer and Erxleben 1983). Moreover, the subsequent relaxation rate can be enhanced (Pearson 1973) so that residual tension in the fibers diminishes more quickly following a contraction. This can permit more rapid cyclical movements (Wolf 1990). In our experiments on denervated legs there was no inhibitory activity, so our data pertain to the noninhibited state of the extensor and flexor muscles. In behaving locusts, the actions of the common inhibitory motor neurons would add another variable layer of complexity to the interactions we describe. In locusts, CI2 and CI3 innervate the flexor tibiae muscle, but their specific actions on it have not been described.
Webb (2004) has suggested that insects use forward models to control their movements; i.e., that they use information about the current state of the limb to predict the sensory consequences of a movement and that this prediction in turn modulates the activity of sensory neurons that are activated when the movement occurs. Since the velocity and amplitude of tibial extension movements were affected by prior flexor tibiae muscle activity, it would not be sufficient for the nervous system to measure the FT angle and to then generate a motor pattern to bring the tibia to a certain position. The activation history of the flexor tibiae muscle would also need to be taken into account, since it has a strong and long-lasting influence on the spike-movement transfer of FETi. But even this information would be insufficient for forward control, since the effect of different flexor tibiae motor neurons on subsequent extensions differed markedly. Exact information about which motor neurons were activated in the flexor tibiae would therefore be needed to generate a precisely targeted movement. If locusts use precise forward models to plan leg movements, then it seems likely that the models must take into account the history of activity in a number of motor neurons over periods of tens of milliseconds.
Why does the flexor tibiae muscle have a more complex innervation than the extensor tibiae muscle?
The simple innervation of the extensor tibiae muscle is conserved between different pairs of locust legs (Wilson 1979) and across different Orthopteran families (e.g., Diapheromeridae: Godden 1972; Acrididae, Tettigoniidae: Theophilidis 1983). The flexor tibiae muscle innervation, in contrast, differs between the leg pairs of the locust and across different species. The mesothoracic flexor tibiae muscle of the locust is innervated by 12 excitatory motor neurons (Theophilidis and Burns 1983), in contrast to the 9 found in the metathoracic leg. The mesothoracic flexor tibiae muscle of the stick insect is innervated by up to 25 excitatory motor neurons (Debrodt and Bässler 1989; Goldammer et al. 2012), and that of the metathoracic leg of the cricket is innervated by 19–21 different excitatory motor neurons (Nishino 2003). The complexity of the flexor innervation arising from innervation by motor neurons with different properties (Hoyle and Burrows 1973) and patterns of synaptic input (Burrows 1996; Field and Burrows 1982; Newland and Emptage 1996) permits the differential recruitment of particular muscle fibers in different behaviors (Bässler and Stein 1996; Duch and Pflüger 1995; Hoyle 1964; Nishino 2003; Page et al. 2008). In kicking and jumping of locusts, however, many or perhaps all flexor motor neurons show similar patterns of activity (Burrows 1995). All of this suggests that flexor tibiae muscle innervation is subject to adaptation for specializations of legs and different behaviors across species. The marked difference in the size of the extensor and flexor tibiae motor pools may reflect developmental and evolutionary differences between the muscles. The flexor muscle develops from more muscle pioneer cells than does the extensor, suggesting that it may have its evolutionary origins in several separate muscles, with a consequent oversupply of motor neurons (Ball and Goodman 1985a, 1985b; Sasaki and Burrows 1998).
We have demonstrated that activation of proximal flexor tibiae muscle fibers has a smaller effect on subsequent extensions than does activation of distal fibers. In behaviors such as walking or scratching that might require more subtle control of extension movements than can be provided by the extensor tibia muscle's simple innervation, the differential recruitment of flexor tibiae fibers with different properties could be used to modulate the spike to movement transfer of FETi and SETi, and thus shape extensions as well as flexions. In other words, the flexor tibiae muscle could regulate tibial extensions by acting as a variable damper. Coactivation of extensor and flexor tibiae motor neurons can occur during walking (Burns and Usherwood 1978; Duch and Pflüger 1995) and grooming of the ear (Berkowitz and Laurent 1996) in locusts and during walking in cockroaches (Krauthamer and Fourtner 1978; Larsen et al. 1995), but it rarely occurs during wing scratching in the locust (Page et al. 2008). In the slowly walking stick insect Cuniculina impigra, there is often a distinct gap between alternating extensor and flexor tibiae motor bursts (Fischer et al. 2001). Even in the latter two cases, however, the slow relaxation of force following muscle activation (Clare et al. 2009; Guschlbauer et al. 2007) means that there is likely to be considerable co-contraction of the antagonistic muscles (Zakotnik et al. 2006).
Co-contraction increases FT joint stiffness, which is important for load compensation during scratching in the locust (Zakotnik et al. 2006) and running in the cockroach (Dickinson et al. 2000). All of this adds to the mounting evidence (e.g., Ahn and Full 2002; Dickinson et al. 2000) that muscles need to be considered not only as motors generating movements but also as brakes.
We also have shown that selective activation of different flexor tibiae motor neurons can generate overall flexor tibiae forces spanning a large range at a fine scale. Flexor tibiae motor neurons with small- or large-amplitude spikes (presumably slow and fast types, respectively) are activated differentially during scratching, as are proximal and distal flexor tibiae motor neurons (Page et al. 2008). The data presented by Page et al. (2008) do not permit a comparison of the exact timing of activity in SETi and different groups of flexor tibiae motor neurons while taking the direction of movement into account. If locusts make use of the different properties of proximal and distal flexor tibiae muscle fibers, flexions immediately preceding extensions should be driven primarily by proximal muscle fibers, to weaken the impact on the following extension, whereas flexions following extensions should be driven primarily by distal muscle fibers and fast motor neurons, to enhance the change of movement direction.
Resting Properties of the FT Joint are Similar in Gregarious and Solitarious Locusts
Solitarious phase locusts keep their hind tibiae more flexed during walking than do gregarious phase locusts, which contributes to their creeping gait (Blackburn et al. 2010). Blackburn et al. show that this can, in part, be explained by different rates of tonic SETi activity in solitarious and gregarious locusts. An energy-efficient way to support this behavioral difference could be to shift the resting state of the joint to more flexed angles. We have shown that this does not occur: the resting properties were similar in solitarious and gregarious animals.
We furthermore compared the FETi spike to movement transfer of solitarious and gregarious locusts and found no obvious differences. The metathoracic FETi drives fast, large-amplitude movements during kicking and jumping (Burrows 1996; Burrows and Morris 2003) and can be recruited during scratching (Page et al. 2008). These behaviors are all likely to be conspicuous to predators and are suppressed in solitarious locusts (Simpson et al. 1999). A reduction in the spike-to-movement transfer of FETi would impair the animal's ability to carry out critical escape jumping and kicking, which may be very disadvantageous. Instead, it seems likely that the reduced occurrence of walking, jumping, and scratching in solitarious locusts is achieved by central modulation of excitability in the pathways controlling these behaviors, leaving the peripheral consequences of FETi spikes unchanged.
We have shown that the resting state of the FT joint varies over time. Moreover, the spike-to-movement transfer function depends on joint angle (Ache 2010), so generation of accurate movements must rely on proprioceptive signals that encode the current joint state. Artificial modification of signals from the femorotibial chordotonal organ systematically biases aimed scratching movements (Page and Matheson 2009), indicating that this limb joint proprioceptor contributes to such feedback. Our data indicate that, in addition to this, the history of antagonistic muscle activation needs to be taken into account when movements are being generated.
GRANTS
This work was funded by a stipend from the Heinrich Hertz Stiftung, Ministry of Innovation, Science and Research of the German State of North Rhine-Westphalia to J. M. Ache, and by Biotechnology and Biological Sciences Research Council Grants BB/H014047/1 and BB/I019065/1 and Royal Society Grant IJP 2006/R3 to T. Matheson.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.M.A. and T.M. conception and design of research; J.M.A. performed experiments; J.M.A. analyzed data; J.M.A. and T.M. interpreted results of experiments; J.M.A. prepared figures; J.M.A. drafted manuscript; J.M.A. and T.M. edited and revised manuscript; T.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Rachel Lockley for maintaining the locust colony, Volker Dürr and Ansgar Büschges for support throughout the project, Christoph Guschlbauer and Ansgar Büschges for comments on a draft of the manuscript, and Tom A. Nielsen for helpful discussions.
Present address of J. M. Ache: Department of Biological Cybernetics, Faculty of Biology, Bielefeld University, Universitätsstr. 25, D-33615 Bielefeld, Germany.
REFERENCES
- Ache, 2010. Ache JM. From Spike to Movement—Biomechanics and Passive Forces in an Insect Joint (MSc thesis). Cologne, Germany: University of Cologne, 2010 [Google Scholar]
- Ahn and Full, 2002. Ahn AN, Full RJ. A motor and a brake: two leg extensor muscles acting at the same joint manage energy differently in a running insect. J Exp Biol 205: 379–389, 2002 [DOI] [PubMed] [Google Scholar]
- Ball and Goodman, 1985a. Ball EE, Goodman CS. Muscle development in the grasshopper embryo. II. Syncytial origin of the extensor tibiae muscle pioneers. Dev Biol 111: 399–416, 1985a [DOI] [PubMed] [Google Scholar]
- Ball and Goodman, 1985b. Ball EE, Goodman CS. Muscle development in the grasshopper embryo. III. Sequential origin of the flexor tibiae muscle pioneers. Dev Biol 111: 417–424, 1985b [DOI] [PubMed] [Google Scholar]
- Bässler et al., 1996. Bässler D, Büschges A, Meditz S, Bässler U. Correlation between muscle structure and filter characteristics of the muscle-joint system in three orthopteran insect species. J Exp Biol 199: 2169–2183, 1996 [DOI] [PubMed] [Google Scholar]
- Bässler and Stein, 1996. Bässler U, Stein W. Contributions of structure and innervation pattern of the stick insect extensor tibiae muscle to the filter characteristics of the muscle-joint system. J Exp Biol 199: 2185–2198, 1996 [DOI] [PubMed] [Google Scholar]
- Bässler and Storrer, 1980. Bässler U, Storrer J. The neural basis of the femur-tibia-control-system in the stick insect Carausius morosus. Biol Cybern 38: 107–114, 1980 [Google Scholar]
- Bennet-Clark, 1975. Bennet-Clark HC. The energetics of the jump of the locust Schistocerca gregaria. J Exp Biol 63: 53–83, 1975 [DOI] [PubMed] [Google Scholar]
- Berkowitz and Laurent, 1996. Berkowitz A, Laurent G. Central generation of grooming motor patterns and interlimb coordination in locusts. J Neurosci 16: 8079–8091, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn et al., 2010. Blackburn LM, Ott SR, Matheson T, Burrows M, Rogers SM. Motor neurone responses during a postural reflex in solitarious and gregarious desert locusts. J Insect Physiol 56: 902–910, 2010 [DOI] [PubMed] [Google Scholar]
- Burns and Usherwood, 1978. Burns MD, Usherwood PN. Mechanical properties of locust extensor tibiae muscles. Comp Biochem Physiol A 61: 85–95, 1978 [Google Scholar]
- Burns and Usherwood, 1979. Burns MD, Usherwood PN. The control of walking in Orthoptera. II. Motor neurone activity in normal free-walking animals. J Exp Biol 79:69–98, 1979 [Google Scholar]
- Burrows, 1996. Burrows M. The Neurobiology of an Insect Brain. Oxford, UK: Oxford University Press, 1996 [Google Scholar]
- Burrows, 1995. Burrows M. Motor patterns during kicking movements in the locust. J Comp Physiol A 176: 289–305, 1995 [DOI] [PubMed] [Google Scholar]
- Burrows and Morris, 2003. Burrows M, Morris O. Jumping and kicking in bush crickets. J Exp Biol 206: 1035–1049, 2003 [DOI] [PubMed] [Google Scholar]
- Clare et al., 2009. Clare AJ, Sutton G, Burrows M, Matheson T. Physiological characterisation of a simply innervated insect muscle. Proceedings of the 32nd Göttingen Neurobiology Conference, T21-11B, 2009 [Google Scholar]
- Cochrane et al., 1972. Cochrane DG, Elder HY, Usherwood PN. Physiology and ultrastructure of phasic and tonic skeletal muscle fibres in the locust, Schistocerca gregaria. J Cell Sci 10: 419–441, 1972 [DOI] [PubMed] [Google Scholar]
- Cruse and Schmitz, 1983. Cruse H, Schmitz J. The control system of the femur-tibia joint in the standing leg of a walking stick insect Carausius morosus. J Exp Biol 102: 175–185, 1983 [Google Scholar]
- Debrodt and Bässler, 1989. Debrodt B, Bässler U. Motor neurones of the flexor tibiae muscle in phasmids. Zool Jahrb Physiol 94: 481–494, 1989 [Google Scholar]
- Dickinson et al., 2000. Dickinson MH, Farley CT, Full RJ, Koehl MA, Kram R, Lehman S. How animals move: an integrative view. Science 288:100–106, 2000 [DOI] [PubMed] [Google Scholar]
- Dresden and Nijenhuis, 1958. Dresden D, Nijenhuis ED. Fiber analysis of the nerves of the second thoracic leg in Periplaneta americana. Proc K Ned Akad Wet C 61: 213–223, 1958 [Google Scholar]
- Duch and Pflüger, 1995. Duch C, Pflüger HJ. Motor patterns for horizontal and upside-down walking and vertical climbing in the locust. J Exp Biol 198: 1963–1976, 1995 [DOI] [PubMed] [Google Scholar]
- Dürr and Matheson, 2003. Dürr V, Matheson T. Graded limb targeting in an insect is caused by the shift of a single movement pattern. J Neurophysiol 90: 1754–1765, 2003 [DOI] [PubMed] [Google Scholar]
- Elsner, 1975. Elsner N. Neuroethology of sound production in gomphocerine grasshoppers (Orthoptera: Acrididae). II. Neuromuscular activity underlying stridulation. J Comp Physiol 97: 315, 1975 [Google Scholar]
- Evans and O'Shea, 1978. Evans PD, O'Shea M. The identification of an octopaminergic neurone and the modulation of a myogenic rhythm in the locust. J Exp Biol 73: 235–260, 1978 [DOI] [PubMed] [Google Scholar]
- Field and Burrows, 1982. Field LH, Burrows M. Reflex effects of the femoral chordotonal organ upon leg motor neurones of the locust. J Exp Biol 101: 265–285, 1982 [Google Scholar]
- Fischer et al., 2001. Fischer H, Schmidt J, Haas R, Büschges A. Pattern generation for walking and searching movements of a stick insect leg. I. Coordination of motor activity. J Neurophysiol 85: 341–353, 2001 [DOI] [PubMed] [Google Scholar]
- Godden, 1972. Godden DH. The motor innervation of the leg musculature and motor output during thanatosis in the stick insect Carausius morosus Br. J Comp Physiol A 80: 201–225, 1972 [Google Scholar]
- Goldammer et al., 2012. Goldammer J, Büschges A, Schmidt J. Motoneurons, DUM cells, and sensory neurons in an insect thoracic ganglion: a tracing study in the stick insect Carausius morosus. J Comp Neurol 520: 230–257, 2012 [DOI] [PubMed] [Google Scholar]
- Guschlbauer et al., 2007. Guschlbauer C, Scharstein H, Büschges A. The extensor tibiae muscle of the stick insect: biomechanical properties of an insect walking leg muscle. J Exp Biol 210: 1092–1108, 2007 [DOI] [PubMed] [Google Scholar]
- Hale and Burrows, 1985. Hale JP, Burrows M. Innervation patterns of inhibitory motor neurones in the thorax of the locust. J Exp Biol 117: 401–413, 1985 [DOI] [PubMed] [Google Scholar]
- Heitler, 1974. Heitler WJ. The locust jump. J Comp Physiol A 89: 93–104, 1974 [Google Scholar]
- Hooper et al., 2009. Hooper SL, Guschlbauer C, Blümel M, Rosenbaum P, Gruhn M, Akay T, Büschges A. Neural control of unloaded leg posture and of leg swing in stick insect, cockroach, and mouse differs from that in larger animals. J Neurosci 29: 4109–4119, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle, 1964. Hoyle G. Exploration of neuronal mechanisms underlying behavior in insects. In: Neural Theory and Modeling, edited by Reiss RF. Stanford, CA: Stanford University Press, 1964, p. 346–376 [Google Scholar]
- Hoyle, 1955. Hoyle G. The anatomy and innervation of locust skeletal muscle. Proc R Soc Lond B Biol Sci 143: 281–292, 1955 [DOI] [PubMed] [Google Scholar]
- Hoyle, 1978a. Hoyle G. Intrinsic rhythm and basic tonus in insect skeletal muscle. J Exp Biol 73: 173–203, 1978a [DOI] [PubMed] [Google Scholar]
- Hoyle, 1978b. Hoyle G. Distributions of nerve and muscle fibre types in locust jumping muscle. J Exp Biol 73: 205–233, 1978b [DOI] [PubMed] [Google Scholar]
- Hoyle and Burrows, 1973. Hoyle G, Burrows M. Neural mechanisms underlying behavior in the locust Schistocerca gregaria. I. Physiology of identified motorneurons in the metathoracic ganglion. J Neurobiol 4: 3–41, 1973 [DOI] [PubMed] [Google Scholar]
- Hoyle and O'Shea, 1974. Hoyle G, O'Shea M. Intrinsic rhythmic contractions in insect skeletal muscle. J Exp Zool 189: 407–412, 1974 [DOI] [PubMed] [Google Scholar]
- Krauthamer and Fourtner, 1978. Krauthamer V, Fourtner CR. Locomotory activity in the extensor and flexor tibiae of the cockroach, Periplaneta americana. J Insect Physiol 24: 813–819, 1978 [Google Scholar]
- Larsen et al., 1995. Larsen GS, Frazier SF, Fish SE, Zill SN. Effects of load inversion in cockroach walking. J Comp Physiol A 176: 229–238, 1995 [DOI] [PubMed] [Google Scholar]
- Newland and Emptage, 1996. Newland PL, Emptage NJ. The central connections and actions during walking of tibial campaniform sensilla in the locust. J Comp Physiol A 178: 749–762, 1996 [DOI] [PubMed] [Google Scholar]
- Nishino, 2003. Nishino H. Local innervation patterns of the metathoracic flexor and extensor tibiae motor neurons in the cricket Gryllus bimaculatus. Zoolog Sci 20: 697–707, 2003 [DOI] [PubMed] [Google Scholar]
- Page and Matheson, 2009. Page KL, Matheson T. Functional recovery of aimed scratching movements after a graded proprioceptive manipulation. J Neurosci 12: 3897–3907, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page et al., 2008. Page KL, Zakotnik J, Dürr V, Matheson T. Motor control of aimed limb movements in an insect. J Neurophysiol 99: 484–499, 2008 [DOI] [PubMed] [Google Scholar]
- Pearson, 1973. Pearson KG. Function of peripheral inhibitory axons in insects. Am Zool 13: 321–330, 1973 [Google Scholar]
- Pener and Simpson, 2009. Pener MP, Simpson SJ. Locust phase polyphenism: an update. Adv Insect Physiol 36: 1–286, 2009 [Google Scholar]
- Philips, 1980. Philips CE. An arthropod muscle innervated by nine excitatory motor neurones. J Exp Biol 88: 249–258, 1980 [Google Scholar]
- Phillips, 1981. Phillips CE. Organization of motor neurons to a multiply innervated insect muscle. J Neurobiol 12: 269–280, 1981 [DOI] [PubMed] [Google Scholar]
- Rathmayer and Erxleben, 1983. Rathmayer W, Erxleben C. Identified muscle fibers in a crab. I. Characteristics of excitatory and inhibitory neuromuscular transmission. J Comp Physiol 152: 411–420, 1983 [Google Scholar]
- Rogers et al., 2010. Rogers SM, Harston GW, Kilburn-Toppin F, Matheson T, Burrows M, Gabbiani F, Krapp HG. Spatiotemporal receptive field properties of a looming-sensitive neuron in solitarious and gregarious phases of the desert locust. J Neurophysiol 103: 779–792, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum et al., 2010. Rosenbaum P, Wosnitza A, Büschges A, Gruhn M. Activity patterns and timing of muscle activity in the forward walking and backward walking stick insect Carausius morosus. J Neurophysiol 104: 1681–1695, 2010 [DOI] [PubMed] [Google Scholar]
- Sasaki and Burrows, 1998. Sasaki K, Burrows M. Innervation pattern of a pool of nine excitatory motor neurons in the flexor tibiae muscle of a locust hind leg. J Exp Biol 201: 1885–1893, 1998 [DOI] [PubMed] [Google Scholar]
- Simpson et al., 1999. Simpson SJ, McCaffery AR, Hägele BF. A behavioural analysis of phase change in the desert locust. Biol Rev 74: 461–480, 1999 [Google Scholar]
- Storrer et al., 1986. Storrer J, Bässler U, Mayer S. Motoneurone im Meso- und Metathorakalganglion der Stabheuschrecke Carausius morosus. Zool Jahrb Physiol 90: 359–374, 1986 [Google Scholar]
- Theophilidis, 1983. Theophilidis G. A comparative study of the anatomy and innervation of the metathoracic extensor tibia muscle in three orthopteran species. Comp Biochem Physiol A 75: 285–292, 1983 [Google Scholar]
- Theophilidis and Burns, 1983. Theophilidis G, Burns MD. The innervation of the mesothoracic flexor tibiae muscle of the locust. J Exp Biol 105: 373–388, 1983 [Google Scholar]
- Usherwood and Grundfest, 1965. Usherwood PN, Grundfest H. Peripheral inhibition in skeletal muscle of insects. J Neurophysiol 28: 497–518, 1965 [DOI] [PubMed] [Google Scholar]
- Voskresenskaya, 1959. Voskresenskaya AK. Neuromuscular Function in Insects, translated by Travers RE. and edited by Horridge GA. Moscow: Acad. Sci. USSR, 1959 [Google Scholar]
- Webb, 2004. Webb B. Neural mechanisms for prediction: do insects have forward models? Trends Neurosci 27: 278–282, 2004 [DOI] [PubMed] [Google Scholar]
- Wilson, 1979. Wilson JA. The structure and function of serially homologous leg motor neurons in the locust. I. Anatomy. J Neurobiol 10: 41–65, 1979 [DOI] [PubMed] [Google Scholar]
- Wolf, 1990. Wolf H. Activity patterns of inhibitory motoneurones and their impact on leg movement in tethered walking locusts. J Exp Biol 152: 281–304, 1990 [Google Scholar]
- Zakotnik et al., 2006. Zakotnik J, Matheson T, Dürr V. Co-contraction and passive forces facilitate load compensation of aimed limb movements. J Neurosci 26: 4995–5007, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]