Abstract
Neurons in the ventral tegmental area (VTA) synthesize several major neurotransmitters, including dopamine (DA), GABA, and glutamate. To classify VTA single-unit neural activity from freely moving rats, we used hierarchical agglomerative clustering and probability distributions as quantitative methods. After many parameters were examined, a firing rate of 10 Hz emerged as a transition frequency between clusters of low-firing and high-firing neurons. To form a subgroup identified as high-firing neurons with GABAergic characteristics, the high-firing classification was sorted by spike duration. To form a subgroup identified as putative DA neurons, the low-firing classification was sorted by DA D2-type receptor pharmacological responses to quinpirole and eticlopride. Putative DA neurons were inhibited by the D2-type receptor agonist quinpirole and returned to near-baseline firing rates or higher following the D2-type receptor antagonist eticlopride. Other unit types showed different responses to these D2-type receptor drugs. A multidimensional comparison of neural properties indicated that these subgroups often clustered independently of each other with minimal overlap. Firing pattern variability reliably distinguished putative DA neurons from other unit types. A combination of phasic burst properties and a low skew in the interspike interval distribution produced a neural population that was comparable to the one sorted by D2 pharmacology. These findings provide a quantitative statistical approach for the classification of VTA neurons in unanesthetized animals.
Keywords: dopamine, γ-aminobutyric acid, spike sorting, quinpirole, eticlopride
the ventral tegmental area (VTA) is a heterogeneous structure that serves a central role in incentive-based behavior, motivation, and cognition (Berridge 2007; Schultz 2007). Abnormalities in the function of VTA dopamine (DA) neurons are implicated in neural pathologies, such as schizophrenia, attention deficit hyperactivity disorder, and drug dependence (Genro et al. 2010; Grace et al. 2007; Heinz and Schlagenhauf 2010). The majority of neurons in the VTA are dopaminergic, but the exact percentage can vary between subregions (Nair-Roberts et al. 2008; Swanson 1982; Yamaguchi et al. 2007). The VTA also consists of fast-firing GABA neurons that project to several forebrain regions and can form local circuit connections with other neurons through their axon collaterals (Carr and Sesack 2000; Omelchenko and Sesack 2009; Steffensen et al. 1998; Van Bockstaele and Pickel 1995). Another neuronal group synthesizes glutamate, and these are found predominately in the more medial and rostral aspects of the VTA, along with a subset of neurons that corelease DA and glutamate (Chuhma et al. 2009; Hnasko et al. 2010; Nair-Roberts et al. 2008; Stuber et al. 2010; Tecuapetla et al. 2010; Yamaguchi et al. 2011).
Immunohistochemical identification in combination with electrophysiological and pharmacological markers remains the most reliable method for distinguishing VTA neurons. Electrophysiological techniques, such as antidromic activation and juxtacellular labeling, have proven valuable for identifying DA and non-DA neurons in the anesthetized preparation (Chiodo et al. 1984; Grace and Bunney 1983; Luo et al. 2008; Steffensen et al. 1998; Ungless et al. 2004; Wang 1981) but are not practical in a freely moving animal. Therefore, studies of VTA neurons in awake animals have relied primarily on indirect electrophysiological (e.g., spike waveform characteristics, basal firing rate, and burst activity) and pharmacological (e.g., DA D2 receptor pharmacology) criteria for the identification of the DA phenotype (Anstrom and Woodward 2005; Dahan et al. 2007; Freeman and Bunney 1987; Hyland et al. 2002; Morris et al. 2004; Pan et al. 2008; Robinson et al. 2004; Roesch et al. 2007; Satoh et al. 2003; Schultz 1986; Zhang et al. 2009).
In this study, probability-based quantitative approaches were used to classify VTA neurons with the aid of known neurotransmitter phenotypes for dopaminergic and GABAergic neurons. Neural units that consistently grouped together across a multidimensional space were hypothesized to be of the same neuronal class. Recent studies demonstrated the basic utility of this approach on a more limited scale by using action potential waveform characteristics to distinguish VTA DA neurons from non-DA neurons (Pan et al. 2008; Roesch et al. 2007). To codify putative neuron types, we used hierarchical clustering and probability distributions to group single-unit recordings from freely moving rats, using a range of common VTA neuron parameters, including spontaneous action potential properties, waveform characteristics, and D2-type receptor pharmacological responses. Although no single parameter alone was sufficient to define a classified unit, in combination the parameters reliably separated at least three neural populations.
MATERIALS AND METHODS
Animal care and surgical procedures.
Male Long-Evans rats (300–450 g) were housed individually and kept on a 12:12-h light-dark cycle with food and water available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine.
Rats were initially injected with pentobarbital sodium (40 mg/kg) and then maintained with isoflurane anesthesia (2–3% in 100% oxygen, 1 l/min) for the duration of the surgery. Body temperature was maintained with a thermostatically controlled heating blanket. To avoid handling-related stress during the experiments, the animals were implanted with either an intraperitoneal catheter or an intravenous jugular catheter (Dong et al. 2010; Howard et al. 2008). Different procedures were performed because this study combined baseline data from two independently conducted sets of experiments. The intravenous catheters were constructed with Silastic tubing (0.30-mm ID, 0.64-mm OD; Dow Corning, Midland, MI) with one end modified with a cannula (22 gauge; Plastics One). The other end of the tubing was inserted into the jugular vein above the right atrium, and the cannula was advanced subcutaneously to exit above the skull. The procedure for the intraperitoneal catheters was similar except that a larger Tygon tubing (0.7-mm ID, 2.4-mm OD; Saint-Gobain, Paris, France) was inserted into the intraperitoneal space. Using a stereotaxic apparatus, we implanted a custom-made microdrive (≈20 g) with 12–18 independently movable tetrodes above the surface of the brain (coordinates in mm relative to bregma: −5.6 anterior-posterior, 1.0–2.0 medial-lateral). Some microdrives were implanted at a 7–12° angle to avoid blood vessels on the surface of the brain. The drive and the catheters were fixed to the skull using stainless steel screws and dental cement. The cannulas were flushed with saline daily after implantation to maintain patency and to habituate the animals to the injections.
In vivo tetrode recordings from midbrain neurons.
Tetrodes were constructed by twisting together four strands of insulated nickel-chromium wire (12-μm OD per strand) (H.P. Reid, Palm Coast, FL) and gold-plating each lead to reduce its impedance to 150–400 kΩ. The tetrodes were slowly positioned into the midbrain DA area (7.5–9.0 mm beneath the surface of the cortex) over several days, and the experiments were conducted within a 3- to 5-wk period. During recording (Cheetah system; Neuralynx, Tucson, AZ), the animals were free to move within a circular platform (35 cm in diameter).
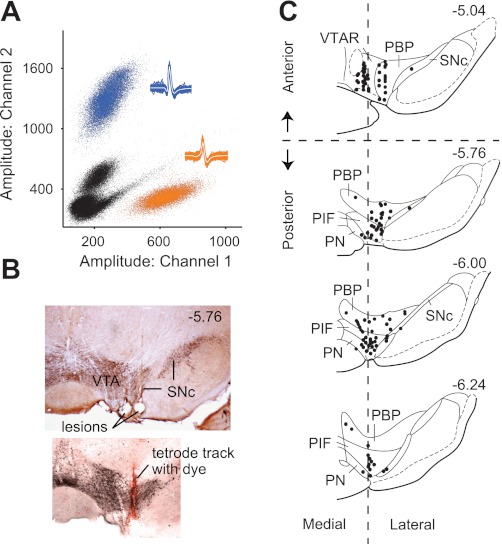
The recordings were sampled gap free at 26.5 or 40 kHz with variable high-pass and low-pass filtering (600–6,000 or 50–9,000 Hz). A higher sampling rate (40 kHz) was used in some cases to determine if additional waveform characteristics were present, but no meaningful differences were found. Spikes were identified by their large signal-to-noise ratios and extracted off-line in Matlab (Xtractor by Krause M and Li W). The size of the sampling window was 2 ms by default, but was increased if the waveforms were longer. Individual unit isolation was achieved by using the relative amplitudes of the action potentials on each tetrode channel in combination with spike waveform parameters (action potential duration, waveform shape). This spike-sorting procedure was accomplished using in-house software and software developed by Dr. A. David Redish (MClust). We sorted the clusters (of single-unit activity) to ensure that they were well separated from the background noise and from other clusters (Fig. 1A), using measures such as Mahalanobis distance to quantify cluster quality (Harris et al. 2001; Schmitzer-Torbert et al. 2005).
Fig. 1.
Anatomical locations of tetrodes for in vivo unit recordings from rat ventral tegmental area (VTA). A: isolation of single-unit activity was achieved by comparing the relative signal (e.g., spike amplitude) recorded on each tetrode channel. Only units that were well separated from the background noise (black dots) and separated from other clusters were accepted for analysis. Insets show the spike waveforms that correspond to the clusters of identified units (blue, orange). B: tetrode placements were confirmed by electrolytic lesions and fluorescent dyes in VTA regions stained positively for tyrosine hydroxylase. The lesions represent the last position of the tetrode as it is moved downward (ventrally) after successive days of recording. SNc: substantia nigra pars compacta. C: recordings were dispersed throughout the VTA with a majority in the parabrachial pigmented nucleus (PBP) and the paraintrafascicular nucleus (PIF). Each black circle may represent more than 1 recorded neuron. For certain post hoc analyses, the VTA was subdivided into anterior and posterior regions and medial and lateral regions based on coordinates estimated from previous studies (Ikemoto 2007). Numerals denote the position (in mm) relative to bregma. PN, paranigral nucleus; VTAR, rostral VTA. Diagram is based on illustrations in Paxinos and Watson (2007).
The final location of the tetrodes in the midbrain was marked by electrolytic lesions (50 μA, 15 s), and in many cases a fluorescent dye (Fig. 1B) was used to label the tetrode track (DiI or DiO; Sigma-Aldrich, St. Louis, MO) (Zhang et al. 2009). The dye was applied to the surface of some tetrodes (but not to the gold-plated tip) before implantation of the microdrive. To aid in tetrode identification, adjacent tetrodes were always marked with a different dye or not marked at all. Postmortem analysis using immunohistochemistry confirmed that the recordings were from tetrodes located in tyrosine hydroxylase-positive regions of the midbrain (Fig. 1, B and C).
Analysis of neural firing parameters.
The analysis of parameters was applied to 207 units from 17 animals. We focused on action potential firing properties and dopamine receptor pharmacology.
To examine basal firing properties, the mean firing rate was calculated by dividing the total number of spikes by the total baseline recording period (10–20 min) before any drug administration. The mean percentage of spikes occurring within bursts (%SWB) was calculated using two different burst definitions: 1) the classic burst criteria of Grace and Bunney (Grace and Bunney 1984a) was used for low-firing cells (<10 Hz), where the onset of a burst is defined as two spikes having an interspike interval (ISI) <80 ms, and the termination of the burst occurs when the ISI exceeds 160 ms; 2) the burst definition described by Kaneoke and Vitek (1996) is not dependent on firing rate and allows for a combined analysis of fast-firing cells (>10 Hz) and low-firing cells (Luo et al. 2008). The coefficient of variation (CV) of the ISI distribution was calculated by dividing the standard deviation (SD) of the ISI by its mean (×100%). Skewness (s) of the ISI distribution was calculated using an established formula:
where n is sample size, x̄ is the sample mean, and xi is the sample.
Aspects of the action potential duration were examined by considering the spike width. Action potential duration was calculated using different intervals of time: full duration from the first inflection of the waveform to the return to baseline, half-action potential duration from the first inflection of the waveform to the peak amplitude (Ungless et al. 2004), or the width at 50% peak amplitude (Margolis et al. 2006). We chose to display the half-action potential duration since it is often used to distinguish VTA neurons in vivo. High-pass filtering does alter the shape particularly of long-duration action potentials. However, the two high-pass filter settings (i.e., 50 or 600 Hz) used in this study produced no apparent trends in the duration distribution histograms using either the 50- to 9,000-Hz or 600- to 6,000-Hz filter settings. Thus the two filter settings likely caused similar influences over the waveform, enabling us to combine these data to create Fig. 2B.
Fig. 2.
Comparison of frequency distributions of all recorded midbrain units using multiple electrophysiological and pharmacological parameters. A: percentage of spikes occurring within bursts (%SWB) using a rate-independent burst definition (Kaneoke and Vitek 1996). B: half-action potential (AP) duration (inset, dotted line). C and D: dopamine (DA) D2-type receptor pharmacology showing the percentage of basal firing response to either quinpirole (Quin; C) or eticlopride (Etic; D) administration. E: average basal firing rate. F: coefficient of variation (CV) in the interspike interval (ISI) histogram. G and H: most parameters, including ISI skew (G) and basal firing rate (H), were log-transformed to determine whether additional patterns in the distribution would emerge. Log transformation of firing rate and, to a lesser extent, ISI skew revealed a bimodal distribution. Not all frequency distribution plots are shown due to space limitations.
DA D2-type receptor pharmacology was examined using the D2-type agonist quinpirole and the D2-type antagonist eticlopride (Sigma-Aldrich) (Levant et al. 1992; Martelle and Nader 2008). To assess the involvement of the D2 receptor in mediating the neural responses, quinpirole and eticlopride (in sterile saline) were administered consecutively with a 15- to 20-min recording period following each drug infusion. The maximum pharmacological effect was determined by computing the maximum change in the firing rate after drug administration relative to baseline. The maximum pharmacological response was taken as the average of the last 2–5 bins (3-min bins) of each recording period depending on the approach to stable firing rates after drug application. This long time frame and bin size are sufficient to minimize transient fluctuations in the firing rate during the averaging. To determine whether eticlopride “reversed” the neural responses to quinpirole, we examined those units whose firing rate either increased or decreased relative to baseline (by more than 20%). If the difference between the response to eticlopride and the response to the quinpirole was >50% of the baseline firing, then we concluded that the antagonist (eticlopride) “reversed” the action of the agonist (quinpirole), which was administered first.
The quinpirole and eticlopride were administered by either intraperitoneal (1 mg/kg) or intravenous catheter (0.25 mg/kg) no more than once per day (Hyland et al. 2002; Marinelli et al. 2003). Although the rate of the pharmacological effect was influenced by the administration route, the maximum pharmacological responses were similar between these conditions and at these doses (see results, Additional support).
Repeated administration of quinpirole and eticlopride over several weeks could potentially have long-term physiological effects on midbrain neuron function. Despite this possibility, the mean basal firing rates and the responses to these D2-type receptor drugs were not significantly different when we compared the first injection with the injections given weeks later at the end of an experiment for any given animal.
Hierarchical clustering.
Unsupervised hierarchical clustering was performed in Matlab (The MathWorks, Natick, MA) and provides an objective method for sorting single-unit parameters. Hierarchical agglomerative clustering treats an individual data object as a cluster and then continues to merge pairs of clusters until all data objects converge into a single cluster. The resulting dendrogram is derived from the (squared Euclidean) linkage distance between data objects. The greater the distance between the branch points, the more likely it is that the branch represents a discrete cluster. Inherent divisions in the data set were quantified by the largest inconsistency coefficient (Y) among the top four links in the cluster tree. This Matlab function normalizes the linkage distance by taking the difference between the relative height of one link and the average height of the links below it and dividing the difference by the SD. Hierarchical clustering was performed on a single parameter at a time to determine the optimal starting point in the classification process. We adopted a top-down classification approach in which large clusters of unit data (identified by hierarchical clustering) were then sorted into successively smaller clusters.
Correlation coefficient and cross-covariance analysis.
To quantify significant interactions between two spike trains, we computed the correlation coefficient (de la Rocha et al. 2007) as well as the cross-covariance (Siapas et al. 2005) for 78 pairs of neurons recorded simultaneously on the same tetrode or on different tetrodes. For correlation coefficients, peristimulus time histograms for each neuron were divided into 5-ms bins and smoothed with a Gaussian filter (SD = 50 ms). The correlation coefficient was calculated as the covariance of these histograms divided by the product of their SD values. The correlation coefficient can vary between −1 and +1 (with +1 being a perfect positive correlation).
To determine significant temporal changes in correlations, we computed the cross-covariance Qij(μ) (Siapas et al. 2005) over the time lag μ between −250 and +250 ms using 5-ms bins. The null hypothesis of independent firing activity was assumed if Qij(μ) was below a 99.95% confidence limit. To identify a significant increase in Qij(μ) and to avoid false correlations caused by noise, we required three consecutive bins to be above the 99.95% confidence limit before rejecting the null hypothesis, corresponding to 3.3 SD from the mean. Cross-correlations were measured from the last 5–10 min of the baseline period depending on the stability of the cell's firing rate. Because of significant differences in firing pattern variability, which violated the assumptions of a stationary time series, correlations between putative DA neurons and other neural classifications were not determined.
Statistical analysis.
Depending on the distribution of the frequency histograms, outlying data were identified based on the mean ± SD or the median ± median absolute deviation (MAD) of the data set. Median was used in the case of a skewed distribution. Certain parameters were log-transformed if their distributions were skewed or irregular. Because bin size can alter the overall shape of the frequency distribution, we computed the histograms using different bin sizes to determine the optimal representation of the data. After classification, the parameters of a given population were no longer skewed in their distributions. Therefore, mean comparisons between neuron populations were assessed by two-tailed t-tests assuming equal variance.
RESULTS
Anatomy.
Chronically implanted tetrodes were used to isolate individual neural units. The isolated units used in this study were well separated from the background. The amplitudes of the action potentials from exemplary neural units and representative waveforms are shown in Fig. 1A. Recordings were located within the VTA (199 units) or, in a few cases, the substantia nigra pars compacta (SNc; 8 units) for comparison. The precise location of the recording tetrode was determined by electrolytic lesions after the final day of recording, and in many cases a fluorescent dye applied to the tetrode wire also labeled the recording track (Fig. 1B) (Zhang et al. 2009). Most recordings were located within the paraintrafascicular nucleus (PIF) and the parabrachial pigmented nucleus (PBP), ranging from the anterior to the posterior VTA as well as from the medial to lateral regions of the VTA (Fig. 1C).
Frequency distribution analysis.
Neurons that share similar cellular properties are more likely to arise from the same phenotypic class than neurons that do not have common features. To identify units that separate into classes on the basis of their physiological parameters, we plotted frequency distributions of the units using several VTA neuron parameters (Fig. 2): the percentage of spikes occurring within bursts (Kaneoke and Vitek 1996), action potential duration, D2-type receptor pharmacology, basal firing rate, and firing pattern activity (e.g., ISI, CV, and skewness). Because of their skewed distributions, many features (e.g., basal firing rate, quinpirole-induced firing rate, ISI skewness, and CV) were also log-transformed to better observe the separation of units into groups (Fig. 2, G and H). Compared with other parameters, the (log transformed) mean basal firing rate exhibited the most apparent bimodal frequency distribution (Fig. 2H), suggesting at least two underlying populations.
Hierarchical clustering.
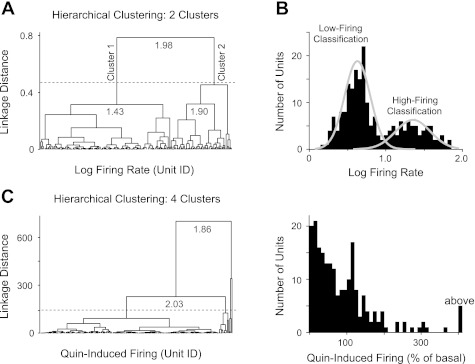
The first step in our classification of units applied hierarchical agglomerative clustering to the log-transformed basal firing rates. The hierarchical clustering produced a dendrogram with two distinguishable clusters of units with a boundary at 10-Hz firing frequency (Fig. 3, A and B). The two major clusters were justified by the largest inconsistency coefficient (Y = 1.98) among the top links in the hierarchical tree (Fig. 3A, gray numerals), suggesting a natural separation. Links at the bottom of the hierarchical tree have Y values close to zero. A separately determined double Gaussian distribution in the firing rates (Fig. 3B) further supported the identification of two principal clusters. These two clusters were designated as “low-firing” and “high-firing” clusters for subsequent analyses.
Fig. 3.
Unsupervised hierarchical clustering was applied to different neural parameters shown in Fig. 2. A: hierarchical clustering of the log-transformed basal firing rates. The resulting dendrogram shows the linkage distance between groups of data points. Data were grouped into 2 clusters based on the largest inconsistency coefficients (gray numerals) for the top 4 links in the hierarchical tree. B: analysis produced 2 disparate clusters demarcated at 10-Hz firing rate (1.0 on log scale) and represented by the double Gaussian function. These clusters were labeled as low-firing and high-firing classifications for subsequent analyses. C: alternatively, hierarchical clustering of other parameters, such as the Quin-induced firing rates, resulted in multiple clusters of data (indicated by dotted line). To maintain a conservative classification scheme, neural parameters defined by multiple clusters were avoided at this step of the analysis.
The hierarchical clustering approach enabled two large classifications (low-firing and high-firing clusters) to be further subdivided into successively smaller classes. Sorting units from larger classes into smaller classes (i.e., top-down) is a conservative classification method for analyzing an undefined data set. Firing rate splits into two large clusters, but other parameters can split into multiple clusters, as shown in Fig. 3C, where the largest inconsistency coefficient suggests three or four clusters. Multiple hierarchical clusters create unwarranted complexity as more clusters arise during subsequent rounds of classification. Therefore, one limitation of this clustering algorithm is that, initially, the process benefits from a small number of large classifications.
Sorting low-firing units by D2-type receptor pharmacology.
In vivo studies have consistently shown that major populations of midbrain DA neurons fire between 1 and 10 Hz (Bunney et al. 1973; Chiodo et al. 1984; Grace and Bunney 1984b; Guyenet and Aghajanian 1978; Luo et al. 2008; Wang 1981; White and Wang 1984; Yim and Mogenson 1980). Therefore, we hypothesized that the low-firing classification (<10 Hz) contained mainly DA neurons. Other parameters, such as ISI skew, could be selected to further sort the low-firing and high-firing classifications, but this option results in additional subdivisions in the data set. We limited the number of subdivisions to maintain conservative classifications that are supported by recognized VTA neuron phenotypes.
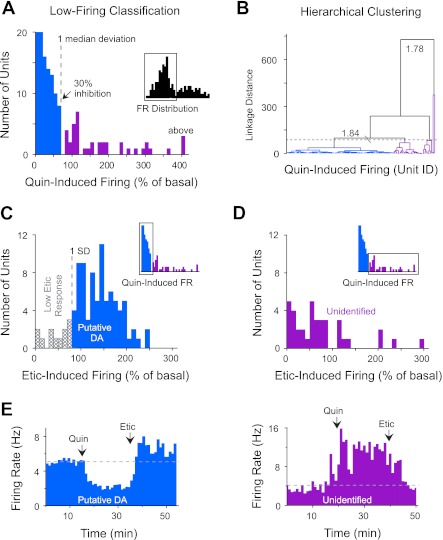
For example, DA D2-type receptor agonists, such as apomorphine and quinpirole, inhibit the firing rate of nearly all VTA DA neurons identified by antidromic activation or neurochemical methods (Chiodo et al. 1984; Clark and Chiodo 1988; Gariano et al. 1989; Lammel et al. 2008; Luo et al. 2008; Wang 1981; Yim and Mogenson 1980). As expected, quinpirole (D2-type agonist) administration strongly inhibited the firing rate of most neurons in the low-firing classification (Fig. 4A, blue bars). Because of a skewed distribution, statistical variance was measured by MAD. The units were divided according to their sensitivity to quinpirole, with the cutoff point at one absolute deviation from the median. The units inhibited by quinpirole showed on average a 70 ± 2% decrease in firing rate (n = 102), ranging from 30 to 100% inhibition. The remaining units (n = 40) either increased their firing rate in response to quinpirole or in some cases showed no significant change from baseline (Fig. 4A, purple bars). The division of the low-firing classification into two groups was also confirmed by a separate hierarchical clustering analysis of these data (Fig. 4B).
Fig. 4.
DA D2-type receptor pharmacology of low-firing units (<10 Hz). A: administration of the D2-type agonist quinpirole strongly inhibited the action potential firing of most low-firing neurons. Inset shows distribution of firing rate for the units from Fig. 3 that were analyzed for their response to quinpirole. Purple bars represent outlying units that were more than 1 absolute deviation from the median (MAD). These units were classified into a separate group of unidentified VTA neurons. FR, firing rate. B: the population of low-firing units inhibited by 30% or more (blue links) was also confirmed by hierarchical clustering (inconsistency coefficients indicated by gray numerals). The dotted line indicates the presence of 5 clusters. These additional clusters (in purple) were not distinguishable by any single feature. C: administration of the D2 receptor antagonist eticlopride increased the firing rate of most low-firing units following quinpirole exposure (inset). To maximize the probability of DA neuron selection, we excluded units that did not return to near-baseline firing (low Etic response) based on them being more than 1 SD from the mean (1-tailed analysis; gray crosshatched bars). D: eticlopride-induced firing rates for the unidentified units, which were low firing and not inhibited by quinpirole (inset; purple bars). 100% = baseline firing rate. E: peristimulus time histograms (1-min bins) showing changes in the firing rate of a representative putative DA neuron and an unidentified neuron following the intravenous infusion of quinpirole and eticlopride.
By contrast, DA D2-type receptor antagonists, such as haloperidol and eticlopride, enhance the firing rate of identified VTA DA neurons (Chiodo et al. 1984; Clark and Chiodo 1988; Mereu et al. 1985; Wang 1981). Analysis of our quinpirole-inhibited units indicated that, for the large majority, eticlopride reversed their firing rates back to or beyond baseline levels (Fig. 4C, blue bars), confirming a D2-type receptor- mediated response. Low-firing units that responded in this way to both quinpirole and eticlopride were identified as putative DA neurons. Twelve units were inhibited by quinpirole but did not rebound after eticlopride (Fig. 4C, gray crosshatched bars). This weak pharmacological response to eticlopride suggests a lack of tonic DA input to the D2 receptor and could reflect the activity of a non-DA neuron (Luo et al. 2008). These outlying units, termed “low eticlopride response” neurons, were separated from the group based on being 1 SD from the mean.
For those low-firing units (<10 Hz) that were not inhibited by quinpirole, eticlopride generally decreased their firing rate relative to quinpirole-induced activity (Fig. 4D, purple bars). Of those units that were excited by quinpirole, 79% displayed a pharmacological reversal in firing rate in response to eticlopride. These units were simply designated as “unidentified” because their properties did not match with those of more commonly identified VTA neurons. The time dependence of the firing rate for representative neural responses to quinpirole and eticlopride administration is shown for a putative DA neuron and an unidentified neuron (Fig. 4E).
D2-type receptor pharmacology of high-firing units.
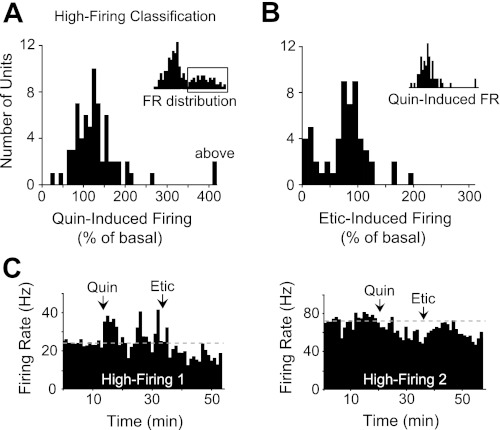
By comparison, the units in the high-firing (>10 Hz) classification (n = 66) showed a normal distribution of responses to quinpirole (Fig. 5A). Quinpirole induced an increase or a decrease in the baseline firing rate (over 20% change) in 62% of these units, whereas the remaining ones showed no significant change from baseline. Eticlopride induced different responses in the high-firing population with no clear trend (Fig. 5B). Of the units excited by quinpirole, only 38% displayed a pharmacological reversal of quinpirole-induced activity by eticlopride (Fig. 5C, high-firing 1). All 13 units that were inhibited by quinpirole were not fully reversed by eticlopride (Fig. 5C, high-firing 2). Because of these variable D2 pharmacological responses and a lack of established information on D2-mediated responses in fast-firing VTA neurons, we did not use D2 pharmacology to group the units in the high-firing classification.
Fig. 5.
DA D2-type pharmacology of high-firing units (>10 Hz). A: high-firing units (inset) displayed a normal distribution of responses to quinpirole administration with no obvious trend. B: eticlopride administration also produced a variety of neural responses in the high-firing population. These units were typically less sensitive to eticlopride compared with the low-firing population. We did not separate these units on the basis of their D2 pharmacological responses. 100% = baseline firing activity. C: peristimulus time histograms (1-min bins) showing changes in the firing rate of 2 types of high-firing neurons following the infusion of quinpirole and eticlopride. Approximately 38% showed responses similar to neuron 1 (left), and ∼23% showed responses similar to neuron 2 (right).
Sorting high-firing units by spike duration.
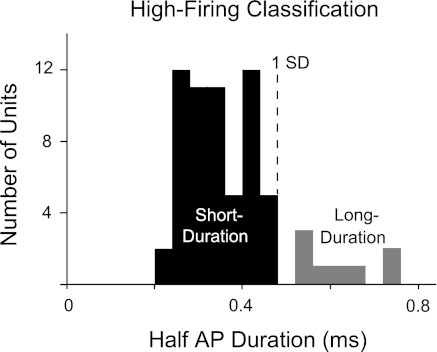
Previous studies have indicated that verified VTA GABA neurons possessing short-duration action potentials reliably fire at frequencies above 10 Hz, suggesting that GABA neurons mainly reside in the high-firing classification (Grace and Onn 1989; Luo et al. 2008; Steffensen et al. 1998, 2008; Stobbs et al. 2004). Taking a conservative approach based on the expectation of short durations, we excluded eight units from the high-firing classification because their spike durations were more than 1 SD from the mean (Fig. 6, gray bars). The remaining majority with half-action potential durations of <0.5 ms were labeled as “high-firing/short-duration” neurons with GABAergic characteristics. Because of considerable overlap in the action potential durations between DA and non-DA populations (Hyland et al. 2002; Kiyatkin and Rebec 2001; Pan et al. 2008; Ungless et al. 2004), spike duration was not used to sort and identify possible DA neurons (see Fig. 2B). At least some of this overlap in spike duration among classes may be a consequence of the substantial low-cut (high-pass) filtering that we and others apply to the recordings.
Fig. 6.
Shorter spike durations characterize a population of rapid-firing VTA neurons with GABA characteristics (Steffensen et al. 1998). To increase the probability of GABA neuron selection, we excluded units with half-AP durations >0.5 ms (gray bars) to form a group identified as high-firing/short-duration neurons, using 1 SD as a cutoff between short- and long-duration units.
Multidimensional comparisons.
Hierarchical clustering of single-unit activity, followed by sorting via D2-type receptor pharmacology and spike duration, produced at least three distinct neural classifications that we designated as putative DA, high-firing/short-duration, and unidentified. To assess the within-group homogeneity of each of these three general populations, we analyzed these units across a multidimensional vector space. Individual units were analyzed by tracking their position across one- and two-dimensional scatter plots to determine their dispersion as well as their overlap between groups (Figs. 7 and 8).
Fig. 7.
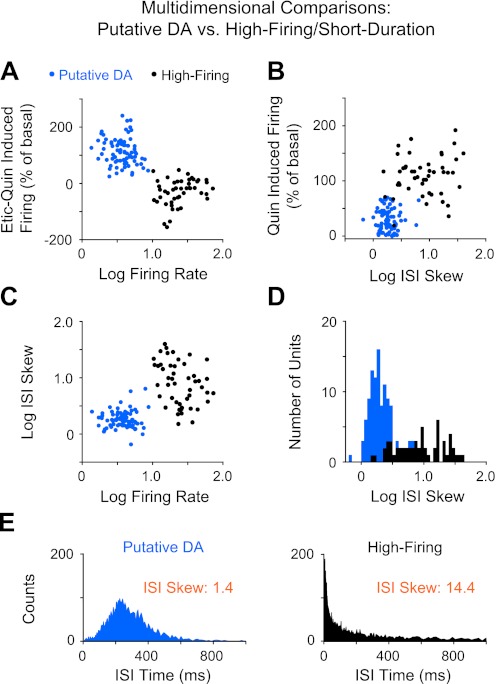
Multidimensional comparison of putative DA units and high-firing/short-duration units. A: log-transformed basal firing rate vs. D2 pharmacological responses. Etic-Quin is defined as the difference between eticlopride-induced firing and quinpirole-induced firing normalized as a percentage of the baseline firing. A positive value indicates that the eticlopride response relative to basal was greater than the quinpirole response, and vice versa for a negative value. B: log-transformed ISI skew vs. %basal response to quinpirole. One unit in the high-firing population with an outlying response to quinpirole (760% above basal) is not shown. C: log-transformed basal firing rate vs. log-transformed ISI skew. Overlap between the clusters (in B and C) disappeared when viewed across different dimensions. D: frequency distribution plot of the log-transformed ISI skew for putative DA and high-firing neurons shows a high degree of natural separation. E: ISI histogram distributions for a representative putative DA neuron and a high-firing neuron with their ISI skew values shown in orange.
Fig. 8.
Multidimensional comparison of putative DA units and unidentified units in the low-firing classification. A: log-transformed basal firing rate vs. D2 pharmacological responses. High-firing neuron responses are shown for comparison. B: log-transformed ISI skew vs. D2 pharmacological responses. A linear regression performed on the data pooled from all low-firing units shows a significant negative correlation between ISI skew and D2 pharmacological responses (P < 0.01). The low-firing units with a weak response to eticlopride (low Etic response, Fig. 4C) were included in the regression but are not shown on the plot to avoid confusion. Three outlying unidentified units with D2 pharmacological responses more than 5 SD from the mean are not shown. C: frequency distribution plot of the log-transformed ISI skew for putative DA units and unidentified units. One absolute deviation from the median (log ISI skew = 0.5) served as a boundary between low-skew and high-skew units in the low-firing classification. D: frequency distribution plot of the intraburst firing rate for units with a low ISI skew (log value <0.5). One absolute deviation from the median (28 Hz) served to identify outliers within the low-skew population, resulting in the separation of 2 unidentified neurons (red arrows). E: ISI histogram distributions for a representative putative DA neuron and an unidentified neuron with their ISI skew values shown in orange.
The baseline firing rate of putative DA neurons was 4.0 ± 0.2 Hz (n = 90) compared with 27.3 ± 2.1 Hz (n = 57) for the high-firing/short-duration neurons. A subtraction of the maximum pharmacological response induced by eticlopride (measured as %basal firing) from the maximum response induced by quinpirole showed that this difference was positive for DA neurons (+114 ± 5%) (Fig. 7A, Etic–Quin-induced firing), indicating an inhibitory response to quinpirole (29 ± 2%) and an excitatory response to eticlopride (143 ± 5%) relative to basal. For most high-firing/short-duration neurons, which were not sorted by D2 pharmacology, the difference between the firing rates induced by eticlopride and quinpirole was negative (−43 ± 15%), indicating an excitatory response to quinpirole (+123 ± 15%). Twelve high-firing/short-duration neurons were inhibited by quinpirole but did not return to baseline activity following eticlopride administration, as putative DA neurons were required to do.
Measures of firing pattern variability were quantified by calculating ISI skew and ISI CV, which were not used to classify these two populations. The mean ISI skew was 1.9 ± 0.1 for putative DA neurons and 12.4 ± 1.3 for the high-firing neurons (P < 0.01). The mean ISI CV was 75 ± 3% for DA neurons and 207 ± 25% for the high-firing neurons (P < 0.01). ISI skew produced minimal overlap between the putative DA neurons and the high-firing/short-duration neurons (Fig. 7, B–D). The relationship between the ISI histogram distribution and the ISI skew for a representative neuron in the putative DA classification and the high-firing/short-duration classification is shown in Fig. 7E.
Putative DA neurons were distinguished from the low-firing unidentified units primarily by D2 pharmacology and ISI skewness (Fig. 8, A–C). The mean basal firing rate for unidentified neurons was 4.7 ± 0.3 Hz (n = 40). The difference between the eticlopride-induced firing rate and the quinpirole-induced firing rate (measured as %basal) for putative DA neurons was +114 ± 5% compared with −137 ± 35% in unidentified neurons. Compared with putative DA neurons, unidentified units showed significantly higher ISI skew (DA, 1.9 ± 0.1; unidentified, 9.1 ± 1.2; P < 0.01) and ISI CV (DA, 75 ± 3%; unidentified, 273 ± 32%). A regression analysis of all low-firing units (<10 Hz) indicated a significant negative correlation (r = 0.73; P < 0.01) between the (log transformed) ISI skew and D2 pharmacological responses (Fig. 8B, Etic–Quin-induced firing). A statistical analysis of the frequency distribution of the ISI skew indicated that putative DA neurons and unidentified neurons formed largely separate populations as indicated in Fig. 8C.
The burst properties of the low-firing units were also examined using the classic burst definition of Grace and Bunny (1984a). All burst properties, including the percentage of spikes occurring within bursts (DA, 27 ± 2%; unidentified, 65 ± 4%), burst length (DA, 3.0 ± 0.1 spikes/burst; unidentified, 8.4 ± 0.9 spikes/burst), and intraburst firing rate (DA, 19.7 ± 0.3 Hz; unidentified, 27.3 ± 2.3 Hz) were statistically different between the putative DA and unidentified classifications (P < 0.01 for all tests).
These combined results suggest that the putative DA population can be classified similarly using either D2 pharmacology or firing pattern characteristics, such as burst properties and ISI skew. To increase the probability of identifying a putative DA neuron without the use of D2 pharmacology, we projected the low-skew neurons (defined as log ISI skew ≤ 0.5 as in Fig. 8C) onto their burst properties (i.e., %SWB, burst length, and intraburst firing rate). Two of five unidentified neurons could be excluded from the low-skew population on the basis of their high intraburst firing rates (red arrows, Fig. 8D). The relationship between the ISI histogram distribution and the ISI skew for a representative neuron in the putative DA classification and the unidentified classification is shown in Fig. 8E.
We excluded a small percentage of units that remained unclassified (Fig. 9A). Twelve low-firing units were inhibited by quinpirole but did not rebound to baseline after eticlopride (low Etic response; see Fig. 4C) and were separated from the putative DA population (6%, crosshatched region in Fig. 9A). These units fell within the range of the putative DA neurons in terms of their ISI skewness, ISI coefficient of variation, burst activity, burst length, and intraburst firing rate but were different in their eticlopride response. Likewise, eight fast-firing neurons with longer spike durations were excluded from the high-firing neuron group (see Fig. 6 and 4%, gray region in Fig. 9A). These eight units consistently overlapped with the majority of high-firing neurons in all other dimensions, including basal firing rate, ISI skewness, and D2 pharmacological responses. The properties of these excluded units suggest an underlying homogeneity with either the putative DA neurons (for the low eticlopride response) or the high-firing/short-duration neurons (for the high-firing/long-duration), respectively. A summary of properties from identified neuron classes is shown in Fig. 9, including the putative DA neurons, the high-firing/short-duration neurons, and the unidentified neurons.
Fig. 9.
Summary of VTA neuron classifications and their properties. A: percentages of all units sorted by their respective group. In addition to the 3 main groups, a minority of unclassified units were excluded on the basis of a low response to eticlopride (low Etic response) or a long AP duration (for more details, see Fig. 6). B: electrophysiological and pharmacological properties of putative DA, high-firing/short-duration, and unidentified neurons, including basal firing rate, ISI skewness, Etic-Quin-induced firing (relative to baseline), half-AP duration, burst length (spikes per burst), and intraburst firing rate. All values are means ± SE. *P < 0.01 (2-tailed t-test).
Additional support.
This clustering analysis was performed on two independent data sets collected by different investigators (i.e., W. Li and W. M. Doyon), recorded on separate acquisition systems and from different animals. To further validate the analysis, we compared the firing properties of the putative DA and the high-firing/short-duration units separately from each of the two data sets. Post hoc t-tests found no significant difference between putative DA neurons from each data set (P > 0.05; n = 59, 31) in terms of mean basal firing rate (data set 1, 3.8 ± 0.2 Hz; data set 2, 4.2 ± 0.2 Hz), mean ISI skewness (data set 1, 1.9 ± 0.1; data set 2, 2.0 ± 0.1), mean ISI CV (data set 1, 78 ± 3%; data set 2, 70 ± 6%), and mean percentage of spikes within bursts (data set 1, 27 ± 2%; data set 2, 24 ± 3%). The %basal difference between eticlopride-induced firing and quinpirole-induced firing was also similar (data set 1, 112 ± 6%; data set 2, 120 ± 11%). Similarly, high-firing neurons (n = 45 and 12, respectively) did not show any differences in their mean basal firing rate (data set 1, 27.3 ± 2.2 Hz; data set 2, 29.2 ± 5.8 Hz), mean ISI skewness (data set 1, 11.3 ± 1.5; data set 2, 16.8 ± 3.2), and mean ISI CV (data set 1, 201 ± 0.29 Hz; data set 2, 230 ± 50 Hz).
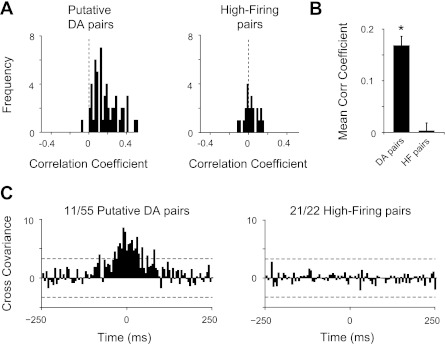
Indirect support for the putative DA classification was also obtained from a cross-correlation analysis performed using pairs of neurons recorded simultaneously at baseline (Fig. 10). Previous studies have indicated that 20–27% of presumed DA neurons show correlated firing activity (Hyland et al. 2002; Morris et al. 2004; Wilson et al. 1977). These studies predicted few, if any, monosynaptic inhibitory interactions (i.e., negative correlations) between DA neurons. Using the correlation coefficient as a measure of correlated spike activity (de la Rocha et al. 2007), we found that nearly all putative DA neuron pairs (n = 55) showed positive correlation coefficients (Fig. 10, A and B), suggesting a tendency to fire together. In contrast, pairs of high-firing neurons (n = 22) exhibited correlation coefficients distributed more narrowly above and below zero (Fig. 10, A and B).
Fig. 10.
Frequency distribution histograms of correlation coefficients for pairs of neurons recorded at the same time (i.e., obtained from a single recording session). Neuron pairs were recorded simultaneously either on the same tetrode or on different tetrodes. A: correlation coefficients for pairs of putative DA neurons (n = 55) were skewed to right of the frequency plot, indicating a tendency for cross-correlated firing activity among these neurons. Correlation coefficients for pairs of high-firing/short-duration neurons (n = 22) were distributed normally at approximately zero (dotted black line). B: mean ± SE of correlation coefficients for each type of neuron pair. *P < 0.01, significantly different from mean correlation coefficient of high-firing neurons (2-tailed t-test). C: cross-correlograms for pairs of putative DA neurons and pairs of high-firing neurons: 20% of putative DA neurons showed significant cross-covariance above a 99.95% confidence interval (dotted black lines). High-firing neuron pairs did not show significant positive interactions.
The significance of these correlations was defined when the cross-covariance between neuron pairs (for 3 consecutive bins) rose above a 99.95% confidence limit (Siapas et al. 2005). By this measure, synchronous firing activity was present in 20% of the putative DA neuron pairs, whereas cross-covariance values for pairs of high-firing neurons did not show significant positive correlations (Fig. 10C). One high-firing neuron pair exhibited a significant negative cross-correlation, indicating an inhibitory interaction, which was not present in any putative DA neuron pair.
Anatomical variations in firing activity.
To examine whether single-unit activity displayed any easily distinguishable anatomical heterogeneity, we divided the putative DA and the high-firing units on the basis of their respective locations in the medial and lateral VTA and in the anterior and posterior VTA (see Fig. 1C). These anatomical boundaries were taken directly from the stereotaxic estimates described previously (Ikemoto 2007). It should be noted that these analyses were limited by an insufficient number of recordings in the very medial regions of the VTA. For this reason the unidentified population was not analyzed here.
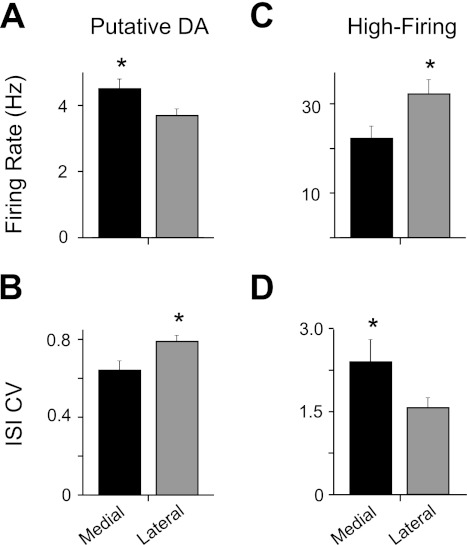
Putative DA neurons showed a small but significant difference in basal firing rate between the medial VTA (4.5 ± 0.3 Hz; n = 30) and lateral VTA (3.7 ± 0.2 Hz; n = 59) (Fig. 11A). In addition, the medial VTA DA neurons showed a lower ISI CV (64 ± 5%) than the lateral VTA DA neurons (79 ± 3%) (Fig. 11B). By contrast, the high-firing neurons showed higher firing rates in the lateral VTA (31.5 ± 3.0 Hz; n = 33) than in the medial VTA (22.5 ± 2.7 Hz; n = 24) (Fig. 11C), whereas lateral high-firing neurons had a lower ISI CV (158 ± 18%) than medial high-firing neurons (241 ± 41%) (Fig. 11D). Other measures, such as burst activity (%SWB), ISI skewness, and spike duration, were not significantly different between regions for either the putative DA or the high-firing neuron groups. It should be noted, however, that our low-cut/high-pass filtering will alter spike duration and waveform. Putative DA or high-firing neurons did not show significant differences in basal firing properties between the anterior and posterior VTA in this data set.
Fig. 11.
Comparison of medial and lateral VTA neuron properties. A: putative DA neurons in the medial VTA showed moderately higher basal firing rates compared with those in the lateral VTA. B: lateral VTA DA neurons showed a higher CV in their ISI than medial VTA DA neurons. C: high-firing/short-duration neurons showed the opposite effects. Lateral VTA high-firing neurons fired faster. D: lateral high-firing neurons had a lower CV than medial VTA high-firing neurons. All values are means ± SE. *P < 0.05 (2-tailed t-test).
Finally, attempts were made to find patterns in the distribution of the putative DA, the high-firing, and the unidentified neurons in subregions of the VTA. The only apparent tendency was for the unidentified population to be located in the more ventral aspects of the midbrain. 75% of the unidentified neurons were recorded in the PIF, the paranigral nucleus, and the ventral SNc. In addition to the VTA, five putative DA neurons were recorded in the SNc. The firing properties of these SNc DA neurons, including their basal firing rate, ISI skewness, percentage of spikes within bursts, and D2-type pharmacological responses, were entirely within the range of the VTA putative DA neurons.
DISCUSSION
The ability to classify VTA neuron populations in freely moving animals is critical for understanding the basic function of the VTA as it relates to broader behavioral and pathological activity. Several studies have used clustering analyses to classify subpopulations of neurons for a single cell type based on multiple electrophysiological parameters (Cauli et al. 2000; Garrido-Sanabria et al. 2007; Halabisky et al. 2006; Lammel et al. 2008). Sorting more than one cell type, as we did in the VTA, is complex because in most cases there is not a single cellular feature that is exclusive to one neuron type, and one neuron type may consist of several subclasses (Nowak et al. 2003).
We sorted single-unit activity according to its ability to cluster together across several dimensions. The following steps summarize this process (Fig. 12). 1) We selected a single parameter that demonstrated modality in its frequency distribution (i.e., log-transformed firing rate). This attribute suggests at least two underlying neuron populations. 2) Unsupervised hierarchical clustering was then applied to group that distribution. 3) On the basis of known VTA neuron properties, the low-firing and high-firing units were then projected onto their D2 pharmacological responses or their spike duration to create three main classifications: putative DA neurons, high-firing neurons with GABAergic characteristics, and unidentified neurons. 4) Putative DA neurons were further refined through multidimensional comparisons. ISI skewness emerged as an orthogonal parameter for distinguishing putative DA neurons from other unit types. Although not directly identified, these classifications were based in part on select VTA neuron parameters established previously by rigorous identification methods, such as antidromic activation and juxtacellular labeling (Chiodo et al. 1984; Grace and Bunney 1983; Luo et al. 2008; Steffensen et al. 1998; Ungless et al. 2004; Wang 1981).
Fig. 12.
Flow chart summarizing the procedures involved in the clustering of VTA single-unit activity. See description of steps 1–4 in the text.
Putative DA neurons.
A unique combination of features distinguished putative DA neurons from the other units (Figs. 7–10), including 1) a low basal firing rate (1–7 Hz), 2) a low ISI skewness (0.7–3.5), and 3) a large (positive) difference between their eticlopride-induced firing rates and their quinpirole-induced firing rates (25–241%). Other properties, such as percentage of spikes within bursts (2–67%), intraburst firing rate (15–30 Hz), burst length (2–4 spikes), and ISI CV (0.1–1.4%) were comparable with results from presumed VTA DA neurons in freely moving rats (Anstrom and Woodward 2005; Dahan et al. 2007; Dong et al. 2010; Freeman and Bunney 1987; Hyland et al. 2002; Zhang et al. 2009). These properties in general are consistent with previous in vivo and in vitro results from antidromically and histochemically identified VTA DA neurons (Brischoux et al. 2009; Clark and Chiodo 1988; Luo et al. 2008; Margolis et al. 2006; Mereu et al. 1985; Ungless et al. 2004; Wang 1981; Yim and Mogenson 1980). The positive correlation coefficients and significant cross- covariance values between putative DA neuron pairs (Hyland et al. 2002; Morris et al. 2004; Wilson et al. 1977), and lack thereof in high-firing neuron pairs, lends indirect support to the DA classification.
Importantly, a low ISI skew (<3.2) predicted the inclusion of a unit into the putative DA neuron classification. That is, neurons that were both inhibited by quinpirole and then excited by eticlopride displayed consistently lower ISI skew values and nominal overlap with the ISI skew of other unit classifications (Figs. 7 and 8). With the use of this criterion, in combination with firing rate or burst properties, only 6% of all recorded units could not be distinguished from the DA classification. Measures of firing pattern variability, such as ISI skew, may provide an alternative criterion for the selection of putative DA neurons in vivo that is comparable to results obtained by the use of D2-type receptor pharmacology and other classical criteria. These results may help researchers to avoid excessive use of D2-receptor agents for pharmacologically sensitive experiments.
It was previously shown in vitro that ISI skew values overlap for DA and some non-DA neurons in brain slices (Margolis et al. 2006). Because VTA neurons in brain slices lack many intact afferent synaptic connections and lack environmental input available in freely moving animals, normal burst firing activity in slices is compromised. Without intact mechanisms to regulate firing patterns, it is likely that the range of ISI skew values measured in vitro will not necessarily match those seen under in vivo conditions. Therefore, we anticipate different results when applying the ISI skew analysis under different experimental conditions, i.e., the in vitro slice preparation, in vivo recording from an anesthetized animal, or in vivo recording from a freely moving animal (used in the present study). Therefore, caution must be applied when classifying neurons on the basis of ISI skew under these distinct experimental conditions.
Based on the putative DA classification, certain single-parameter criteria that are sometimes used to identify DA neurons may not be optimal. DA neurons are often identified by their strong inhibitory responses (>50%) to D2-type receptor agonists (Guyenet and Aghajanian 1978; Hyland et al. 2002; Luo et al. 2008; Pan et al. 2008; Wang 1981; Zhang et al. 2009). In the present study, quinpirole inhibited 13 putative DA neurons by 30–50% (Fig. 4A), which would be considered weak inhibition by previous standards. The fact that eticlopride caused these 13 units to rebound well above baseline (131 ± 9%) indicates a clear D2-mediated pharmacological response. Previous studies have also shown that some (antidromically identified) DA neurons show a weak inhibition (20–30%) to D2 activation (Chiodo et al. 1984; Shepard and German 1988; White and Wang 1984), consistent with these findings.
High-firing neurons.
The features of the high-firing neurons, including short spike durations and fast basal firing rates (10–72 Hz), are consistent with the properties of antidromically and neurochemically identified VTA GABA neurons in vivo (Luo et al. 2008; Steffensen et al. 1998, 2008; Stobbs et al. 2004). Consistent with our low- and high-firing classification (Fig. 3), previous studies indicated that 10 Hz (firing rate) is the transition point for distinguishing DA neurons from faster firing non-DA neurons (Wang 1981; Yim and Mogenson 1980). Our observation of an abrupt increase in ISI skewness above 10 Hz (Fig. 7C) supports this criterion.
Many high-firing neurons increased their firing rate in response to quinpirole administration (D2 activation) or showed a minimal pharmacological response (Fig. 5). Comparable effects were reported by Steffensen and colleagues in antidromically identified GABA neurons (Lassen et al. 2007; Steffensen et al. 2008; Stobbs et al. 2004). On the other hand, some of our high-firing neurons were also inhibited by quinpirole (Fig. 7B), which has also been reported in some non-DA neurons (Luo et al. 2008; Yim and Mogenson 1980). It is important to note, however, that none of the high-firing neurons that were inhibited by quinpirole returned to baseline activity after eticlopride exposure, suggesting a lack of tonic DA input to these cells. This finding may provide a pharmacological marker that distinguishes some fast-firing, possibly GABAergic, VTA neurons (>10 Hz) from putative DA neurons. That said, the diverse D2-type pharmacological responses of the high-firing neuron population, as well as their ISI skew variability, suggest that this group is not necessarily homogenous. Luo et al. (2008) identified a minority of fast-firing neurons that were positive for tyrosine hydroxylase. It remains to be determined whether these fast-firing, putative DA neurons are sensitive to D2-selective drugs, as shown by many fast-firing units in our data set.
Unidentified neurons.
The classification of unidentified neurons showed significant variability in firing patterns, as measured by ISI skewness, ISI CV, and burst activity, similar to the high-firing population. The unidentified neurons and putative DA neurons were also characterized by varying degrees of overlap when viewed in certain dimensions, including basal firing rate, burst activity, and action potential duration. However, orthogonal parameters that were not used to sort units, such as ISI skewness (Fig. 8, B and C) and ISI CV, provided a robust alternative measure for distinguishing these groups. These data suggest that differences in firing pattern activity, along with D2 pharmacological responses, may distinguish some low-firing VTA neurons that otherwise have similar electrophysiological properties.
The unidentified neurons showed relatively less group coherence when viewed in two dimensions (Fig. 8), which suggests a heterogeneous cell population. Considering the known cellular diversity of the VTA, this cluster probably consists of a mixture of neuron types, most likely including those neurons that release glutamate (Chuhma et al. 2009; Hnasko et al. 2010; Stuber et al. 2010; Tecuapetla et al. 2010; Yamaguchi et al. 2011) or perhaps outliers of the DA classification. The exact percentage of glutamate neurons in the VTA remains unclear with estimates ranging from 3 to 60% depending on the VTA subregion (Nair-Roberts et al. 2008; Yamaguchi et al. 2007, 2011). The relative density of glutamatergic neurons (including those that corelease DA) is high in the medial VTA but decreases significantly in the lateral direction (Hnasko et al. 2010; Yamaguchi et al. 2011), which may explain why estimates can vary. In any case, our finding that unidentified neurons comprise 21% of the total neuron population is within the range of these observations.
Because our method for sorting putative DA neurons relies partly on their D2-type receptor pharmacological responses, we are likely excluding certain mesocortical projecting DA neurons that can show little or no response to D2 activation (Chiodo et al. 1984; Lammel et al. 2008; White and Wang 1984). The spontaneous firing rate of mesocortical DA neurons is generally below 10 Hz in adult rodents (Chiodo et al. 1984; Gariano et al. 1989; White and Wang 1984). Therefore, it is possible that the unidentified units with quinpirole responses close to baseline or just below baseline (−80 to +110%) represent some of these mesocortical DA neurons. Support for this hypothesis comes from the fact that these units displayed significantly higher intraburst firing rates (23.4 ± 2.7 Hz) compared with the putative DA neurons (19.6 ± 0.3 Hz), as suggested previously (Lammel et al. 2008).
Basal firing properties vary between medial and lateral VTA.
Accumulating evidence indicates that DA neurons show differences in spontaneous and evoked action potential properties that vary according to their location in the VTA (Brischoux et al. 2009; Chiodo et al. 1984; Lammel et al. 2008; Margolis et al. 2008; Zhao-Shea et al. 2011). Consistent with those results, the present study shows that putative DA neurons in the medial VTA fire moderately faster but less variably than those in the lateral VTA. The fact that high-firing (GABAergic-like) neurons showed the opposite pattern of firing activity between the medial and lateral VTA is intriguing. To further substantiate these results and to reveal their functional significance, further studies with larger data sets from additional VTA subregions and in behaving animals are required.
In summary, these results provide a quantitative and systematic method for classifying VTA neurons in freely behaving rats on the basis of their electrophysiological and pharmacological properties. This probability-based clustering analysis distinguished two major neuron classifications that possessed attributes consistent with previously identified populations of DA and GABA neurons. These groups were not distinguished by any single feature, but in combination they were separable from a third, unidentified neuron population. The uniformity within the groups, particularly for putative DA neurons, makes this methodology useful for isolating major subsets of VTA neurons in awake rats in the absence of molecular markers. However, to confirm these results and to reveal additional subclassifications, further studies are required with more diverse pharmacological approaches applied to neurochemically identified VTA neurons from awake animals.
GRANTS
This work was supported by National Institutes of Health Grants DA09411 and NS21229, the Cancer Prevention and Research Institute of Texas, and the Alliance for Nanohealth Research Project W81XWH-09-2-0139. We also acknowledge joint participation by the Diana Helis Henry Medical Research Foundation through its direct engagement in the continuous active conduct of medical research in conjunction with Baylor College of Medicine and the “Genomic, Neural, Preclinical Analysis for Smoking Cessation” Project for the Cancer Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. Andreas Tolias for expert advice on cluster analysis and Drs. John I. Broussard, Andon N. Placzek, and Michael Krause for valuable comments on the manuscript.
REFERENCES
- Anstrom and Woodward, 2005. Anstrom KK, Woodward DJ. Restraint increases dopaminergic burst firing in awake rats. Neuropsychopharmacology 30: 1832–1840, 2005 [DOI] [PubMed] [Google Scholar]
- Berridge, 2007. Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 191: 391–431, 2007 [DOI] [PubMed] [Google Scholar]
- Brischoux et al., 2009. Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci USA 106: 4894–4899, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney et al., 1973. Bunney BS, Walters JR, Roth RH, Aghajanian GK. Dopaminergic neurons: effect of antipsychotic drugs and amphetamine on single cell activity. J Pharmacol Exp Ther 185: 560–571, 1973 [PubMed] [Google Scholar]
- Carr and Sesack, 2000. Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci 20: 3864–3873, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli et al., 2000. Cauli B, Porter JT, Tsuzuki K, Lambolez B, Rossier J, Quenet B, Audinat E. Classification of fusiform neocortical interneurons based on unsupervised clustering. Proc Natl Acad Sci USA 97: 6144–6149, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo et al., 1984. Chiodo LA, Bannon MJ, Grace AA, Roth RH, Bunney BS. Evidence for the absence of impulse-regulating somatodendritic and synthesis-modulating nerve terminal autoreceptors on subpopulations of mesocortical dopamine neurons. Neuroscience 12: 1–16, 1984 [DOI] [PubMed] [Google Scholar]
- Chuhma et al., 2009. Chuhma N, Choi WY, Mingote S, Rayport S. Dopamine neuron glutamate cotransmission: frequency-dependent modulation in the mesoventromedial projection. Neuroscience 164: 1068–1083, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark and Chiodo, 1988. Clark D, Chiodo LA. Electrophysiological and pharmacological characterization of identified nigrostriatal and mesoaccumbens dopamine neurons in the rat. Synapse 2: 474–485, 1988 [DOI] [PubMed] [Google Scholar]
- Dahan et al., 2007. Dahan L, Astier B, Vautrelle N, Urbain N, Kocsis B, Chouvet G. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology 32: 1232–1241, 2007 [DOI] [PubMed] [Google Scholar]
- de la Rocha et al., 2007. de la Rocha J, Doiron B, Shea-Brown E, Josic K, Reyes A. Correlation between neural spike trains increases with firing rate. Nature 448: 802–806, 2007 [DOI] [PubMed] [Google Scholar]
- Dong et al., 2010. Dong Y, Zhang T, Li W, Doyon WM, Dani JA. Route of nicotine administration influences in vivo dopamine neuron activity: habituation, needle injection, and cannula infusion. J Mol Neurosci 40: 164–171, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman and Bunney, 1987. Freeman AS, Bunney BS. Activity of A9 and A10 dopaminergic neurons in unrestrained rats: further characterization and effects of apomorphine and cholecystokinin. Brain Res 405: 46–55, 1987 [DOI] [PubMed] [Google Scholar]
- Gariano et al., 1989. Gariano RF, Tepper JM, Sawyer SF, Young SJ, Groves PM. Mesocortical dopaminergic neurons. 1. Electrophysiological properties and evidence for soma-dendritic autoreceptors. Brain Res Bull 22: 511–516, 1989 [DOI] [PubMed] [Google Scholar]
- Garrido-Sanabria et al., 2007. Garrido-Sanabria ER, Perez MG, Banuelos C, Reyna T, Hernandez S, Castaneda MT, Colom LV. Electrophysiological and morphological heterogeneity of slow firing neurons in medial septal/diagonal band complex as revealed by cluster analysis. Neuroscience 146: 931–945, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genro et al., 2010. Genro JP, Kieling C, Rohde LA, Hutz MH. Attention-deficit/hyperactivity disorder and the dopaminergic hypotheses. Expert Rev Neurother 10: 587–601, 2010 [DOI] [PubMed] [Google Scholar]
- Grace and Bunney, 1984a. Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci 4: 2877–2890, 1984a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace and Bunney, 1984b. Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci 4: 2866–2876, 1984b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace and Bunney, 1983. Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons—1. Identification and characterization. Neuroscience 10: 301–315, 1983 [DOI] [PubMed] [Google Scholar]
- Grace et al., 2007. Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci 30: 220–227, 2007 [DOI] [PubMed] [Google Scholar]
- Grace and Onn, 1989. Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci 9: 3463–3481, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet and Aghajanian, 1978. Guyenet PG, Aghajanian GK. Antidromic identification of dopaminergic and other output neurons of the rat substantia nigra. Brain Res 150: 69–84, 1978 [DOI] [PubMed] [Google Scholar]
- Halabisky et al., 2006. Halabisky B, Shen F, Huguenard JR, Prince DA. Electrophysiological classification of somatostatin-positive interneurons in mouse sensorimotor cortex. J Neurophysiol 96: 834–845, 2006 [DOI] [PubMed] [Google Scholar]
- Harris et al., 2001. Harris KD, Hirase H, Leinekugel X, Henze DA, Buzsaki G. Temporal interaction between single spikes and complex spike bursts in hippocampal pyramidal cells. Neuron 32: 141–149, 2001 [DOI] [PubMed] [Google Scholar]
- Heinz and Schlagenhauf, 2010. Heinz A, Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr Bull 36: 472–485, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko et al., 2010. Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S, Edwards RH. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron 65: 643–656, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard et al., 2008. Howard EC, Schier CJ, Wetzel JS, Duvauchelle CL, Gonzales RA. The shell of the nucleus accumbens has a higher dopamine response compared with the core after non-contingent intravenous ethanol administration. Neuroscience 154: 1042–1053, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland et al., 2002. Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience 114: 475–492, 2002 [DOI] [PubMed] [Google Scholar]
- Ikemoto, 2007. Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev 56: 27–78, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneoke and Vitek, 1996. Kaneoke Y, Vitek JL. Burst and oscillation as disparate neuronal properties. J Neurosci Methods 68: 211–223, 1996 [DOI] [PubMed] [Google Scholar]
- Kiyatkin and Rebec, 2001. Kiyatkin EA, Rebec GV. Impulse activity of ventral tegmental area neurons during heroin self-administration in rats. Neuroscience 102: 565–580, 2001 [DOI] [PubMed] [Google Scholar]
- Lammel et al., 2008. Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron 57: 760–773, 2008 [DOI] [PubMed] [Google Scholar]
- Lassen et al., 2007. Lassen MB, Brown JE, Stobbs SH, Gunderson SH, Maes L, Valenzuela CF, Ray AP, Henriksen SJ, Steffensen SC. Brain stimulation reward is integrated by a network of electrically coupled GABA neurons. Brain Res 1156: 46–58, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levant et al., 1992. Levant B, Grigoriadis DE, DeSouza EB. Characterization of [3H]quinpirole binding to D2-like dopamine receptors in rat brain. J Pharmacol Exp Ther 262: 929–935, 1992 [PubMed] [Google Scholar]
- Luo et al., 2008. Luo AH, Georges FE, Aston-Jones GS. Novel neurons in ventral tegmental area fire selectively during the active phase of the diurnal cycle. Eur J Neurosci 27: 408–422, 2008 [DOI] [PubMed] [Google Scholar]
- Margolis et al., 2006. Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol 577: 907–924, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis et al., 2008. Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D2 receptor inhibition. J Neurosci 28: 8908–8913, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli et al., 2003. Marinelli M, Cooper DC, Baker LK, White FJ. Impulse activity of midbrain dopamine neurons modulates drug-seeking behavior. Psychopharmacology (Berl) 168: 84–98, 2003 [DOI] [PubMed] [Google Scholar]
- Martelle and Nader, 2008. Martelle JL, Nader MA. A review of the discovery, pharmacological characterization, and behavioral effects of the dopamine D2-like receptor antagonist eticlopride. CNS Neurosci Ther 14: 248–262, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereu et al., 1985. Mereu G, Westfall TC, Wang RY. Modulation of terminal excitability of mesolimbic dopaminergic neurons by d-amphetamine and haloperidol. Brain Res 359: 88–96, 1985 [DOI] [PubMed] [Google Scholar]
- Morris et al., 2004. Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron 43: 133–143, 2004 [DOI] [PubMed] [Google Scholar]
- Nair-Roberts et al., 2008. Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience 152: 1024–1031, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak et al., 2003. Nowak LG, Azouz R, Sanchez-Vives MV, Gray CM, McCormick DA. Electrophysiological classes of cat primary visual cortical neurons in vivo as revealed by quantitative analyses. J Neurophysiol 89: 1541–1566, 2003 [DOI] [PubMed] [Google Scholar]
- Omelchenko and Sesack, 2009. Omelchenko N, Sesack SR. Ultrastructural analysis of local collaterals of rat ventral tegmental area neurons: GABA phenotype and synapses onto dopamine and GABA cells. Synapse 63: 895–906, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan et al., 2008. Pan WX, Schmidt R, Wickens JR, Hyland BI. Tripartite mechanism of extinction suggested by dopamine neuron activity and temporal difference model. J Neurosci 28: 9619–9631, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos and Watson, 2007. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Burlington, MA: Academic, 2007 [Google Scholar]
- Robinson et al., 2004. Robinson S, Smith DM, Mizumori SJ, Palmiter RD. Firing properties of dopamine neurons in freely moving dopamine-deficient mice: effects of dopamine receptor activation and anesthesia. Proc Natl Acad Sci USA 101: 13329–13334, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch et al., 2007. Roesch MR, Calu DJ, Schoenbaum G. Dopamine neurons encode the better option in rats deciding between differently delayed or sized rewards. Nat Neurosci 10: 1615–1624, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh et al., 2003. Satoh T, Nakai S, Sato T, Kimura M. Correlated coding of motivation and outcome of decision by dopamine neurons. J Neurosci 23: 9913–9923, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitzer-Torbert et al., 2005. Schmitzer-Torbert N, Jackson J, Henze D, Harris K, Redish AD. Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience 131: 1–11, 2005 [DOI] [PubMed] [Google Scholar]
- Schultz, 2007. Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci 30: 259–288, 2007 [DOI] [PubMed] [Google Scholar]
- Schultz, 1986. Schultz W. Responses of midbrain dopamine neurons to behavioral trigger stimuli in the monkey. J Neurophysiol 56: 1439–1461, 1986 [DOI] [PubMed] [Google Scholar]
- Shepard and German, 1988. Shepard PD, German DC. Electrophysiological and pharmacological evidence for the existence of distinct subpopulations of nigrostriatal dopaminergic neuron in the rat. Neuroscience 27: 537–546, 1988 [DOI] [PubMed] [Google Scholar]
- Siapas et al., 2005. Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron 46: 141–151, 2005 [DOI] [PubMed] [Google Scholar]
- Steffensen et al., 1998. Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci 18: 8003–8015, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen et al., 2008. Steffensen SC, Taylor SR, Horton ML, Barber EN, Lyle LT, Stobbs SH, Allison DW. Cocaine disinhibits dopamine neurons in the ventral tegmental area via use-dependent blockade of GABA neuron voltage-sensitive sodium channels. Eur J Neurosci 28: 2028–2040, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobbs et al., 2004. Stobbs SH, Ohran AJ, Lassen MB, Allison DW, Brown JE, Steffensen SC. Ethanol suppression of ventral tegmental area GABA neuron electrical transmission involves N-methyl-d-aspartate receptors. J Pharmacol Exp Ther 311: 282–289, 2004 [DOI] [PubMed] [Google Scholar]
- Stuber et al., 2010. Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci 30: 8229–8233, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, 1982. Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull 9: 321–353, 1982 [DOI] [PubMed] [Google Scholar]
- Tecuapetla et al., 2010. Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, Koos T. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci 30: 7105–7110, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless et al., 2004. Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science 303: 2040–2042, 2004 [DOI] [PubMed] [Google Scholar]
- Van Bockstaele and Pickel, 1995. Van Bockstaele EJ, Pickel VM. GABA-containing neurons in the ventral tegmental area project to the nucleus accumbens in rat brain. Brain Res 682: 215–221, 1995 [DOI] [PubMed] [Google Scholar]
- Wang, 1981. Wang RY. Dopaminergic neurons in the rat ventral tegmental area. I. Identification and characterization. Brain Res Rev 3: 123–140, 1981 [Google Scholar]
- White and Wang, 1984. White FJ, Wang RY. Pharmacological characterization of dopamine autoreceptors in the rat ventral tegmental area: microiontophoretic studies. J Pharmacol Exp Ther 231: 275–280, 1984 [PubMed] [Google Scholar]
- Wilson et al., 1977. Wilson CJ, Young SJ, Groves PM. Statistical properties of neuronal spike trains in the substantia nigra: cell types and their interactions. Brain Res 136: 243–260, 1977 [DOI] [PubMed] [Google Scholar]
- Yamaguchi et al., 2007. Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci 25: 106–118, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi et al., 2011. Yamaguchi T, Wang HL, Li X, Ng TH, Morales M. Mesocorticolimbic glutamatergic pathway. J Neurosci 31: 8476–8490, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim and Mogenson, 1980. Yim CY, Mogenson GJ. Electrophysiological studies of neurons in the ventral tegmental area of Tsai. Brain Res 181: 301–313, 1980 [DOI] [PubMed] [Google Scholar]
- Zhang et al., 2009. Zhang T, Zhang L, Liang Y, Siapas AG, Zhou FM, Dani JA. Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine. J Neurosci 29: 4035–4043, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao-Shea et al., 2011. Zhao-Shea R, Liu L, Soll LG, Improgo MR, Meyers EE, McIntosh JM, Grady SR, Marks MJ, Gardner PD, Tapper AR. Nicotine-mediated activation of dopaminergic neurons in distinct regions of the ventral tegmental area. Neuropsychopharmacology 36: 1021–1032, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]