Abstract
Although previous research with human and nonhuman primates has examined the neural correlates of performance monitoring, discrepancies in methodology have limited our ability to make cross-species generalizations. One major obstacle arises from the use of different behavioral responses and tasks across different primate species. Specifically, it is unknown whether performance-monitoring mechanisms rely on different neural circuitry in tasks requiring oculomotor vs. skeletomotor responses. Here, we show that the human error-related negativity (ERN) elicited by a saccadic eye-movement response relative to a manual response differs in several critical ways. The human saccadic ERN exhibits a prolonged duration, a broader frontomedial voltage distribution, and different neural source estimates than the manual ERN in exactly the same stop-signal task. The human saccadic error positivity (Pe) exhibited a frontomedial voltage distribution with estimated electrical sources in supplementary motor area and rostral anterior cingulate cortex for saccadic responses, whereas the manual Pe showed a posterior scalp distribution and potential origins in the superior parietal lobule. These findings constrain models of the cognitive mechanisms indexed by the ERN/Pe complex. Moreover, by paralleling work with nonhuman primates performing the same saccadic stop-signal task (Godlove et al. 2011), we demonstrate a cross-species homology of error event-related potentials (ERPs) and lay the groundwork for definitively localizing the neural sources of performance-monitoring ERPs.
Keywords: countermanding, error detection, error-related negativity, executive control, performance monitoring
neurophysiological studies of performance-monitoring mechanisms in humans and macaque monkeys have proven to be critical in shaping models of executive control (Alexander and Brown 2010). Although nonhuman primate studies indicate that areas in medial frontal cortex of macaque monkeys produce signals sufficient to monitor performance and exert executive control (e.g., Emeric et al. 2008, 2010; Ito et al. 2003; Nakamura et al. 2005; Stuphorn et al. 2000), a debate has arisen as to whether monkeys exhibit the same neural mechanisms of performance monitoring found in humans (Cole et al. 2009, 2010; but see Schall and Emeric 2010). Settling this question is made particularly difficult because neurophysiological studies of performance monitoring in humans and monkeys rely on different response modalities, tasks, and electrophysiological measurements. In the present study, we sought to determine how differences in response modality affect event-related potential (ERP) indexes of performance monitoring, thereby eliminating a crucial difference between studies of different primate species. This work then allowed us to directly compare the same ERP signals measured from humans and monkeys to determine whether homology exists, an approach that is surprisingly uncommon (for the other instance, see Arthur and Starr 1984).
To date, nonhuman primate studies of performance monitoring in the stop-signal task commonly require saccadic eye-movement responses, whereas human studies require manual button-press responses (Chen et al. 2010; Scangos and Stuphorn 2010). It is well known that motor control of the eyes and hands differs in multiple respects, including kinematic degrees of freedom, relative needs to account for gravity and collisions with physical objects, and their respective patterns of anatomic connectivity (Dum and Strick 2002). Thus the present study addresses the question of whether electrophysiological indexes of performance monitoring differ depending on whether eye-movement or manual responses are made in the same task.
It is possible that neural mechanisms of performance monitoring are instantiated differently in the oculomotor and skeletomotor domains. However, output dependence of the performance monitoring system would speak against the view that the ERP components elicited by errors (i.e., the error-related negativity, or ERN, and error positivity, or Pe) index a high-level executive mechanism that operates independently of response modality (Bernstein et al. 1995; Dehaene et al. 1994; Falkenstein et al. 1990, 1991, 2000; Hajcak et al. 2005; Holroyd et al. 1998; Luu et al. 2000; Miltner et al. 1997; Van't Ent and Apkarian 1999). This is a critical theoretical issue for providing an understanding of both the functional and mechanistic organization of performance monitoring, as well as the nature of prefrontal contributions to this system suggested by neuropsychological (Goldberg and Barr 1991; Turken and Swick 1999) and neurophysiological data (Brázdil et al. 2002, 2005; Emeric et al. 2008, 2010). This means that in addition to providing the findings necessary for comparative electrophysiology (i.e., measuring the same neurophysiological signals in the same experimental paradigm, analyzed with the procedures) between human and nonhuman primates, the present experiments address the nature of the cognitive mechanisms indexed by the ERP component elicited by errors.
We tested the hypothesis that the ERN/Pe complex elicited by errors relative to correct trials during saccadic (experiment 1) and manual response (experiment 2) versions of the same stop-signal task differ significantly in their amplitude, timing, voltage distributions, and source models. Such findings would suggest that different neural generators underlie the ERN and Pe when different effectors are used to respond in the same task. Given that the anterior cingulate cortex (ACC) has been proposed to be important for the generation of these ERP components, such findings would be consistent with previous work indicating that the ACC has a somatotopic organization in humans (Paus et al. 1993, 1998; Picard and Strick 1996) and monkeys (Morecraft and Van Hoesen 1992). However, there are reasons to expect that we would not observe differences in the ERN and Pe when saccadic vs. manual responses were made. Specifically, previous work has supported the hypothesis that the ERN and Pe index response-general performance-monitoring mechanisms that do not differ as a function of the effector used to respond (Holroyd et al. 1998). This previous work compared the ERN elicited by errors vs. correct responses made with either manual button presses or response levers contacted with the feet. With these two effectors, the ERPs were found to be similar. However, it is possible that the ERN and Pe are fundamentally different when different effectors are used to make responses but that the electrical field generated by foot and hand movements in the critical cortical regions do not appear to differ given the limited spatial resolution of the ERP technique. The present study examined this question of potential output dependence of the ERN and Pe, given the importance of the findings for understanding the nature of the mechanisms indexed by error ERPs, but focused on the contrast of manual vs. eye-movement responses.
To reduce sources of variance that would make between-effector comparisons problematic, we used within-subjects comparisons (i.e., experiment 1 vs. experiment 2). In addition, to determine the generality of the findings and integrate the present study with the largest body of existing work, we measured the characteristics of the ERN/Pe complex during multiple types of manual response tasks (i.e., experiment 2 vs. experiment 3, using unimanual and bimanual responses, respectively).
To foreshadow our findings, the properties of the ERN/Pe complex elicited during manual stop-signal tasks (experiments 2 and 3) conformed to those previously observed in the human literature under different task conditions but with similar manual response demands (Gehring et al. 1993, 2012; Kopp et al. 1996; Kopp and Rist 1999). However, when eye-movement responses were made by the same subjects in the same task, the ERN and Pe components differed significantly in their timing, distribution, and source models. Finally, the characteristics of the saccadic error ERPs mirrored those found in our parallel study of nonhuman primates performing the same task (Godlove et al. 2011).
MATERIALS AND METHODS
Participants.
Volunteers (18–32 yr of age) participated in exchange for monetary compensation. Thirteen subjects were used in experiments 1 (4 female), 2 (4 female), and 3 (5 female). To eliminate between-subjects variance in our comparisons of the ERPs from experiments 1 and 2, the same group of subjects performed both tasks in different sessions with order counterbalanced. Informed consent was obtained before any experimental procedures began. All procedures were approved by the Vanderbilt University Institutional Review Board. All subjects had normal color vision, no history of neurological problems, and normal or corrected-to- normal visual acuity.
Stimuli and task.
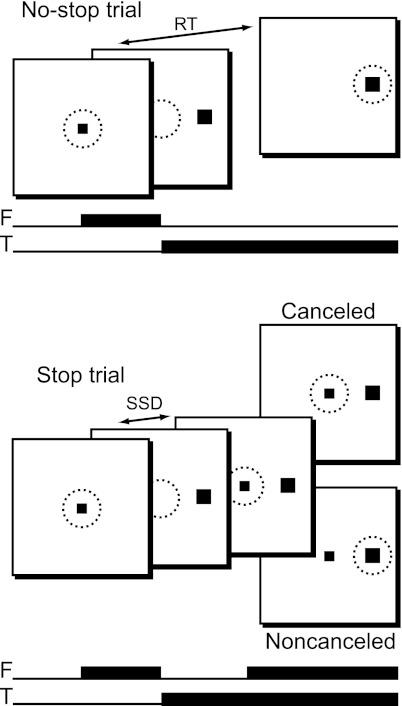
The stop-signal stimuli employed in experiments 1–3 were identical to what we have used in parallel experiments with nonhuman primates (e.g., Godlove et al. 2011). The task is illustrated in Fig. 1. Each trial began with the presentation of a 0.37° square fixation point (30 cd/m2). The fixation point remained on the screen for 800–1,200 ms. The fixation point was then extinguished, and a 1° × 1° target (10 cd/m2) appeared 10° from the center of screen along the horizontal meridian. The target appeared to the left of fixation on half of the trials and to the right of fixation on the remainder of the randomly interleaved trials. Two-thirds of trials were no-stop trials, and the target was extinguished after 700 ms. On the remaining one-third of trials, a stop signal appeared. The stop signal was a square box (30 cd/m2, subtending 0.66° with a line width of 0.08°) centered on the screen. Six stop signal delays (SSDs) were sampled with equal probability: 60, 120, 180, 240, 300, and 360 ms. After presentation of the stop signal, the stimuli remained on the screen until 700 ms had elapsed from the presentation of the target. The intertrial interval was 1,000–1,200 ms (randomly jittered with a rectangular distribution).
Fig. 1.
Schematic representation of the stop-signal (or countermanding) task. Black squares indicate stimulus location. No-stop trials began with a central fixation point. The fixation point was then extinguished, and a peripheral target was simultaneously presented at 1 of 2 possible locations. Subjects were instructed to fixate targets as quickly and accurately as possible. Stop trials began in the same way. However, after 1 of 6 possible time intervals following target onset, termed stop-signal delay (SSD), the fixation point was re-illuminated and subjects were instructed to withhold their response. Successful response inhibition resulted in canceled trials, whereas errant responses resulted in noncanceled trials. Dotted circles represent area of fixation. F, fixation point; T, target; RT, reaction time.
Subjects were instructed to respond to all target stimuli as quickly as possible, and to withhold their response if they saw the stop signal. In experiment 1, subjects responded with a saccadic eye movement. In experiment 2, subjects responded with a button press on a gamepad using the thumb of their right hand [also known as a simple reaction time (RT) or target detection task]. In experiment 3, each participant responded bimanually to targets appearing to the right of fixation with the index finger of their right hand and to targets appearing on the left with the index finger of their left hand [also known as a 2-alternative forced choice (2AFC) RT or target discrimination task]. We included the bimanual response task in experiment 3 to connect with the modal design of behavioral countermanding experiments in the literature (for reviews, see Logan 1994; Verbruggen and Logan 2008). For experiments 2 and 3, subjects were instructed to maintain fixation at the center of the computer display for the entire trial. In each experiment, 1,440 trials were completed by each participant, with a 30-s break every 100 trials and one subject-terminated break after half of the experimental trials. Subjects began and ended the experimental session of experiment 1 with a block of 40 no-stop trials. These trials were used to calibrate the algorithm that detected saccades offline for each participant.
Data acquisition.
Subjects were seated in an electrically shielded, sound-attenuated booth with the lights off. The electroencephalogram (EEG) was acquired (250 Hz sampling rate, 0.01- to 100-Hz bandpass filter) using an SA Instrumentation Amplifier from 21 tin electrodes, including 3 midline (Fz, Cz, Pz), 8 lateral pairs (F3/F4, C3/C4, P3/P4, PO3/PO4, T3/T4, T5/T6, O1/O2), and 2 nonstandard sites (OL, midway between O1 and T5; and OR, midway between O2 and T6), arrayed according to the International 10-20 System and embedded in an elastic cap (Electrocap International, Eaton, OH). The right mastoid electrode served as the online reference for these active electrode sites. Signals were re-referenced offline to the average of the left and the right mastoids (Nunez and Srinivasan 2006). Horizontal eye position was monitored by recording electrooculogram (EOG) from bipolar electrodes located at the outer canthi of each eye. Vertical eye position and blinks were similarly monitored with bipolar electrodes placed above and below the left eye. All electrode impedances were kept under 5 kΩ.
Data analysis.
A custom Matlab function (The MathWorks, Natick, MA) automatically identified saccade initiation and termination using the bipolar EOG signals. This function measured when the instantaneous saccadic velocity became elevated above 30°/s and then calculated the beginning and end of the monotonic change in eye position (Hanes and Schall 1995). If subjects successfully withheld their response on stop trials, the trial was labeled “canceled.” If they were unable to do so, the trial was labeled “noncanceled.” We adopted the method of Logan and Cowan (1984) as implemented by Hanes et al. (1998) to estimate stop-signal reaction time (SSRT; see also Godlove et al. 2011).
EEG epoching, baseline correcting, and truncating procedures were identical to those of our parallel study of ERPs from nonhuman primate (Godlove et al. 2011). Waveforms were baseline corrected to the interval from 150 to 50 ms before the response events we time locked to. The baseline interval ended 50 ms before the time-locking event onset to allow these analyses to reveal error-related activity beginning before response onset, where it might exist. Stop trials on which subjects responded before stop signals were presented were not included in error ERPs because subjects did not have the necessary information to deduce that an error had been committed. When constructing grand average ERP waveforms collapsed across left and right target locations, the number of trials presented at each location was matched in a given condition by excluding random trials from the more heavily represented target with the behavior of interest. Single-trial EEG signals were truncated 50 ms before the onset of the second, non-task-related saccade to eliminate artifacts arising from temporally smeared second-saccade activity. Epochs containing blink activity exceeding ±100 μV were excluded from the analyses. The latter was done, instead of artifact correction, to remain consistent with the methods of our study of nonhuman primates, which used an eye tracker that lost the pupil during blinks.
Saccade-locked grand average bipolar horizontal EOG waveforms are depicted in Fig. 2 for both rightward (positive voltage deflection) and leftward (negative voltage deflection) eye movements. These waveforms demonstrate that after truncation, saccade-related activity in experiment 1 did not differ between error and correct trials and, as a result, could not explain the ERN or Pe differences found between experiments during the measurement epochs. Because our averages of error and correct trials included an equal number of leftward and rightward responses, the eye-movement artifacts are averaged out in the grand average waveforms but apparent in averages separated by target location. That is, the ERPs elicited by erroneous saccades relative to correct saccades ride on top of these shifts in the corneoretinal potential before these horizontal eye-movement artifacts are averaged out. The vertical and horizontal EOG averages shown in Fig. 2 for all effector conditions also show the absence of oculomotor contamination of the scalp ERPs and verify the appropriateness of our artifact rejection criterion. Rare no-stop trials with no responses were excluded, and a minimum of 20 trials in any condition was required for study inclusion (Larson et al. 2010). An independent two-sample t-test revealed no significant difference across conditions (noncanceled and no-stop trials) in the total number of rejected trials due to artifacts or missed responses in experiments 1, 2, and 3 (P < 0.98, P < 0.97, and P < 0.97, respectively).
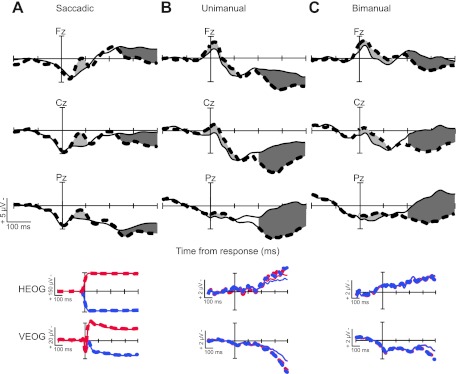
Fig. 2.
Event-related potentials (ERPs) from experiments 1–3. Response-aligned grand average correct no-stop (thin solid line) and errant noncanceled waveforms (thick broken line) at each midline scalp site (Fz, Cz, and Pz) are shown for saccadic (A), unimanual/simple RT (B), and bimanual/2-alternative forced choice (2AFC) RT (C) experiments. Shaded regions represent significant differences in the error-related negativity (ERN; light gray) and error positivity (Pe; dark gray) components. Response-aligned grand average bipolar horizontal (HEOG) and vertical electrooculogram (VEOG) waveforms for right (blue) and left (red) peripheral target locations and errant noncanceled and correct no-stop trials are illustrated for each experiment. Note that in this and all subsequent figures, negativity is plotted upwardly.
Analyses of variance (ANOVAs) were used for the majority of statistical tests, and P values were adjusted using the Greenhouse-Geisser epsilon correction for nonsphericity (Jennings and Wood 1976). ERP mean amplitude and peak latency values were extracted separately for the ERN (0–100 ms) and Pe time windows (250–400 ms). The same pattern of component latency values was found using fractional area latency measures as peak latency values reported below. The latter are reported because they are easier to directly compare with those of other species, because the local maxima or minima of the peak is a less subjective feature of the component to compute than a window bracketing the entire component of interest, across all electrodes of interest. The robustness of our statistical results was confirmed by testing multiple measurement windows (i.e., ±10, ±20, ±30, ±40, ±50 ms) centered on peak amplitude of the midline electrode showing maximal activity of each ERP component elicited by errors relative to correct trials with the same response, as well as numerous fixed measurement windows (e.g., −25–75 ms, 0–125 ms, for the ERN across experiments). The broad time intervals we used were sufficient to capture the time in which both components were present and provided a more conservative statistical approach than measuring peak amplitude (Luck 2005; Woodman 2010).
To compare findings between species, we also tested significant ERP differences following the method adopted by our parallel study of nonhuman primates (Godlove et al. 2011) based on Emeric et al. (2008). Briefly, a difference wave was calculated by subtracting no-stop trial ERPs from noncanceled ERPs. The variability of the difference wave was assessed by calculating the standard deviation (SD) during the baseline period (−150 to −50 ms, time-locked to the response). Significant epochs were defined as periods when the difference wave deviated from baseline by >2 SD for longer than 50 ms, provided it exceeded 3 SD in that interval. For presentation, ERPs were digitally filtered with a zero-phase shift 35-Hz low-pass hamming window (SD = 6 ms). All statistical analyses were carried out on unfiltered data.
We carried out distributed current density analyses using the same methods we used with the data from our nonhuman primates (Godlove et al. 2011). The interpolated boundary element method (BEM) model was derived from averaged magnetic resonance imaging (MRI) data from the Montreal Neurological Institute (MNI). It consisted of 9,300 triangular meshes overall, or 4,656 nodes, which describe the smoothed inner skull (2,286 nodes), the outer skull (1,305 nodes), and the outside of the skin (1,065 nodes). The mean triangle edge lengths (node distances) were 9 mm (skin), 6.8 mm (skull), and 5.1 mm (brain compartment). Standard conductivity values for the three compartments were set as follows: skin = 0.33 S/m, skull = 0.0042 S/m, and brain = 0.33 S/m. The interpolated BEM model was built using the onboard CURRY 6 MRI data set (Fuchs et al. 2002). The standardized low-resolution electromagnetic tomography weighted accurate minimum norm method (SWARM) was estimated using sensor positions based on the International 10-20 System and a cortical surface obtained from a segmentation of the CURRY 6 individual reference brain.
Error-related ERPs and analyses of subsequent behavioral effects.
To examine the electrophysiological correlates of post-error adjustments in behavior, we conducted statistical analysis of the association between the single-trial ERN and Pe amplitudes and the change in RT on trials following an error for experiments 1 and 2. This was achieved by first identifying erroneous noncanceled trials (trial n) that were followed by no-stop trials (trial n+1). We measured the maximum negative and positive deflections during the ERN and Pe windows on trial n and then determined the post-error behavioral adjustment, defined as ΔRT (RT on trial n+1 minus RT on trial n). We measured correlation coefficient (ρ) values for maximum ERN and Pe amplitude vs. ΔRT and subjected these distributions of ρ values to one-sample t-tests to determine if correlations tended to deviate from zero across the entire data set.
For a second RT adjustment analysis, each participant's single-trial EEGs were divided by a median split into two groups on the basis of post-error RT values and averaged to increase the signal-to-noise ratio. A two-sample t-test was applied to assess amplitude differences between the median split based on RT. As in the dipole and current source density analyses above, time windows for both single-trial and median split n+1 analyses were ±30 ms (ERN) and ±40 ms wide (Pe) centered on the peak amplitude of the difference wave on the midline electrode showing maximal ERN and Pe activity. All analysis procedures were consistent with those implemented by Godlove et al. (2011) reporting the ERP findings from macaque monkeys performing the same task.
Conflict analysis.
To test for relationships between the amplitude of the negativity underlying the ERN component and response conflict, the following procedure was implemented in an identical fashion across studies with human subjects and nonhuman primates. First, raw EEG traces were z-scored to removed incidental intersubject and interelectrode amplitude differences. Next, successfully canceled trials at each SSD were identified. According to findings of Hanes et al. (1998) and Paré and Hanes (2003), canceled trials are those containing the largest magnitude of neural response conflict in the stop-signal task (see also Stuphorn et al. 2000). We identified no-stop trials from each subject with RT > SSD + SSRT. These latency-matched trials are those that were slow enough to have been successfully canceled had stop-signals been presented and therefore provide an appropriate control for canceled trials (see Hanes et al. 1998 for a more elaborate description of this latency-matching method). ERPs were generated from trials in these two conditions at each SSD, and mean amplitudes on canceled trials and latency-matched no-stop trials in the window from −50 to +100 ms around SSRT were obtained. This window corresponds to the time of conflict-related neural modulation in the supplementary eye field conflict-related response (Emeric et al. 2010; Stuphorn et al. 2000). By subtracting mean no-stop voltage from mean canceled voltage, we obtained measurements of canceled-trial negativity. Finally, we tested this canceled-trial negativity against response conflict by assessing its correlation with SSD and the probability of committing an errant response in experiments 1 (saccadic responses) and 2 (unimanual responses).
RESULTS
Behavior.
One subject from each experiment succeeded on fewer than 45% of the stop trials and was excluded from further analysis. The performance of the remaining 12 subjects from experiment 1, 12 subjects from experiment 2, and 12 subjects from experiment 3 is summarized in Table 1.
Table 1.
Summary statistics for stop-signal task performancy
| Exp. 1: Saccadic | Exp. 2: Simple Unimanual | Exp. 3: 2AFC Bimanual | Exp. 1 vs. 2: P Value | Exp. 1 vs. 3: P Value | |
|---|---|---|---|---|---|
| Pr(response|no-stop trial) | 98.0 ± 1 | 97.1 ± 2 | 97.0 ± 2 | 0.41 | 0.47 |
| Pr(response|stop-signal) | 51.2 ± 3 | 54.7 ± 2 | 52.9 ± 2 | 0.33 | 0.37 |
| Mean no-stop RT, ms | 341 ± 78 | 525 ± 39 | 520 ± 33 | <0.01 | <0.01 |
| Mean noncanceled RT, ms | 271 ± 20 | 448 ± 37 | 441 ± 37 | <0.01 | <0.01 |
| Mean SSRT, ms | 132 ± 14 | 227 ± 17 | 230 ± 15 | <0.01 | <0.01 |
All values are means ± SD. Exp, experiment; 2AFC, 2-alternative forced choice; Pr(response|no-stop trial), probability of responding on no-stop trial; Pr(response|stop-signal), probability of responding on stop-signal trial; RT, reaction time; SSRT, stop-signal reaction time. P values are calculated from 2-sample t-tests.
Consistent with earlier findings (e.g., Logan et al. 1984), mean RT on no-stop trials was significantly slower than erroneous noncanceled RT on stop-signal trials across experiments (P < 0.01). Although saccadic responses had faster RTs than manual responses, subjects produced correct responses on ∼100% of no-stop trials and on ∼50% of the stop trials in all experiments. These behavioral findings show that the fixed SSDs we used approximated the patterns of behavior derived using dynamic tracking procedures, such as those used with electrophysiological studies of nonhuman primates (Godlove et al. 2011).
Mean SSRTs for experiments 1–3 were in the range of those values reported in previous studies using saccadic and manual stop-signal tasks (saccade: Asrress and Carpenter 2001; Boucher et al. 2007; Colonius et al. 2001; Hanes and Carpenter 1999; Logan and Irwin 2000; Özyurt et al. 2003; manual: Logan et al. 1997; Logan and Irwin 2000; Williams et al. 1999). Differences in SSRT between experiment 1 (saccadic responses) vs. experiment 2 (unimanual responses) and experiment 1 vs. experiment 3 (bimanual responses) were significant, as suggested by previous studies measuring only behavior (Boucher et al. 2007). In summary, the behavioral performance across experiments 1–3 conformed to the predictions of the Logan and Cowan (1984) race model of inhibition (for reviews, see Logan 1994; Verbruggen and Logan 2008), as well as similarities previously observed between stop-signal tasks using manual simple detection (i.e., unimanual) vs. discrimination responses (i.e., 2AFC bimanual; Logan et al. 1984).
ERPs.
Grand average response-locked ERPs, collapsed across left and right target locations and all subjects, are shown separately for each experiment in Fig. 2, A–C. The gray-shaded regions indicate the times at which waveforms from errant noncanceled trials (thick broken line) significantly diverged from correct no-stop trials (thin solid line) using the methods described above.
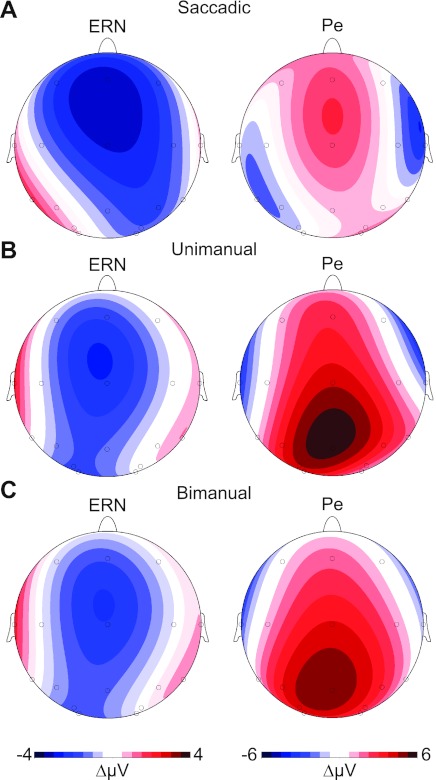
In Fig. 2, A–C, errors elicited a clear ERN followed by a Pe, with the distributions of these effects shown across the frontal, central, and parietal midline electrode sites. The scalp topographies of these effects are shown in Fig. 3, A–C, during the time range of the maximum amplitude differences between error and correct waveforms (saccadic: ERN from 48 to 108 ms, Pe from 320 to 400 ms; unimanual: ERN from 16 to 76 ms, Pe from 286 to 366 ms; bimanual: ERN from 32 to 92 ms, Pe from 286 to 366 ms). For the saccadic errors, the ERN and Pe components both had frontal maxima, whereas the manual response errors elicited ERNs with frontal maxima and Pe components with parietal maxima. This difference is particularly striking given that the same individuals participated in experiment 1 with saccadic responses and in experiment 2 with unimanual responses. It is also noteworthy that a correct-related negativity was present in the manual ERN waveforms only. Therefore, we considered both correct and error trial types separately when we generated the figures and performed the statistical analysis.
Fig. 3.
Scalp voltage topographies of the ERN and Pe from experiments 1–3. Voltage distribution across the scalp for the ERN and Pe elicited by saccadic (A), unimanual (simple RT; B), and bimanual responses (2AFC RT; C) in the same stop-signal task are shown. Scalp topographies were computed based on errant noncanceled minus correct no-stop grand average difference waves. Time windows ±30 ms wide (ERN) and ±40 ms wide (Pe) were centered on the peak amplitude of the difference wave of the midline electrode showing maximal activity (as in Godlove et al. 2011).
Analyses of the ERN across experiments 1–3 provided statistical support for the above observations, using an ANOVA with the factors of effector (saccadic vs. unimanual vs. bimanual), trial type (correct vs. error), and electrode site (Fz vs. Cz vs. Pz) on the amplitude of the waveforms during the ERN measurement window. This analysis yielded a significant main effect of trial type [F(1,35) = 11.92, P < 0.003]. Critically, there was a significant interaction of effector × electrode site [F(4,35) = 10.11, P < 0.004]. The source of this interaction was revealed by the follow-up ANOVAs described below, which demonstrate that the distribution of the ERNs following saccadic responses was broader compared with the frontomedial distribution following manual errors. ERN latency was assessed across experiments 1–3 using the same factors described above, yielding a significant interaction of effector × trial type [F(2,35) = 6.13, P < 0.007], driven by shorter latency ERNs observed during manual responses than those observed during saccadic responses (as described in detail below).
Our next subset of ANOVAs focused on the ERP amplitudes during the ERN measurement window between experiments 1 and 2 (i.e., saccadic responses compared with unimanual responses). The first ANOVA with the factors of effector (saccadic vs. unimanual), trial type (correct vs. error), and electrode site (Fz vs. Cz vs. Pz) yielded a main effect of trial type on ERN amplitude [F(1,23) = 4.91, P < 0.04] such that error trials elicited a more negative potential (i.e., the ERN). A higher order interaction of effector × trial type × electrode site [F(2,23) = 4.79, P < 0.04] was parsed with follow-up analyses within each experiment. These revealed significant main effects of trial type on ERN amplitude for each experiment [saccadic: F(1,11) = 5.75, P < 0.03; unimanual: F(1,11) = 4.98, P = 0.04], but the trial type × electrode site interaction was significant for only experiment 2 [F(2,11) = 7.14, P < 0.03]. The latter was driven by larger values in anterior vs. posterior electrode sites as single degree of freedom contrasts within subjects reached significance (Fz vs. Pz: P < 0.04, Cz vs. Pz: P < 0.05). To summarize, the saccadic ERN was more broadly distributed across the scalp than the unimanual ERN, which had a distribution skewed to frontal midline electrodes. The ERN peak latency analysis for experiments 1 and 2 revealed a significant interaction of effector × trial type [F(1,23) = 12.12, P < 0.008] such that ERN timing was earlier in experiment 2 (∼20 ms postresponse) compared with experiment 1 (∼76 ms postresponse). No other main effects or interactions were significant in the ERN latency analysis of experiments 1 and 2.
Our next set of ANOVAs examined the amplitude differences during the ERN window between experiments 2 and 3 (i.e., unimanual responses compared with the 2AFC bimanual responses). An ANOVA with the factors of manual response (unimanual vs. bimanual), trial type (correct vs. error), and electrode site (Fz vs. Cz vs. Pz) yielded a main effect of trial type on ERN amplitude [F(1,23) = 5.571, P < 0.04], indicating that there was a larger negativity on error trials (i.e., the ERN). A significant trial type × electrode site interaction on ERN amplitude [F(2,23) = 5.46, P < 0.04] was due to the known voltage distribution of the ERN across the scalp when erroneous manual responses are made (Gehring et al. 2012), namely, a frontocentral midline pattern in both experiments 2 and 3. ERN latency differences between experiments 2 and 3 did not reach significance.
As in the above-mentioned ERN analyses, Pe component differences were assessed across experiments 1–3 with an ANOVA using the factors of effector (saccadic vs. unimanual vs. bimanual), trial type (correct vs. error), and electrode site (Fz, Cz, and Pz). There was a significant main effect of trial type [F(1,35) = 16.40, P < 0.001] and two significant interactions of effector × trial type [F(2,35) = 4.69, P < 0.02] and effector × trial type × electrode site on Pe amplitude [F(4,35) = 7.71, P < 0.005]. The follow-up comparisons described below revealed the source of these interactions. The Pe omnibus ANOVA across experiments 1–3 yielded no significant effects of component latency.
Pe component differences were determined between experiments 1 and 2 (i.e., saccadic responses compared with unimanual responses) with an ANOVA using the factors of effector (saccadic vs. unimanual), trial type (correct vs. error), and electrode site (Fz vs. Cz vs. Pz). A significant effect of trial type on Pe amplitude [F(1,23) = 14.00, P < 0.003] showed that the waveform on error trials was more positive than on correct trials. A significant effector × trial type × electrode site interaction for Pe amplitude [F(2,23) = 7.81, P < 0.02] was parsed with additional within-experiment ANOVAs and revealed that the main effect of trial type on Pe amplitude was significant for each experiment [saccadic: F(1,11) = 5.90, P < 0.05; unimanual: F(1,11) = 9.02, P < 0.02]. An interaction of trial type × electrode site on Pe amplitude was also significant for each experiment [saccadic: F(2,11) = 5.77, P < 0.04; unimanual: F(2,11) = 6.21, P < 0.05]. These results confirmed the difference in scalp distribution we observed between the saccadic Pe with a frontocentral maximum and the unimanual Pe with a posterior parietal maximum.
Our next set of ANOVAs examined Pe component differences between experiments 2 and 3 (i.e., unimanual responses compared with 2AFC bimanual responses). An ANOVA with factors of manual response (unimanual vs. bimanual), trial type (correct vs. error), and electrode site (Fz vs. Cz vs. Pz) yielded a main effect of trial type on Pe amplitude [F(1,23) = 14.13, P < 0.004], demonstrating a larger Pe (i.e., more positive waveform) on error trials. A significant trial type × electrode site interaction on Pe amplitude [F(2,23) = 5.20, P < 0.04] resulted from a distribution with a posterior parietal maximum in both experiments 2 and 3. As was the case with the ERN, these results did not show any significant latency differences between experiments 2 and 3.
Current density.
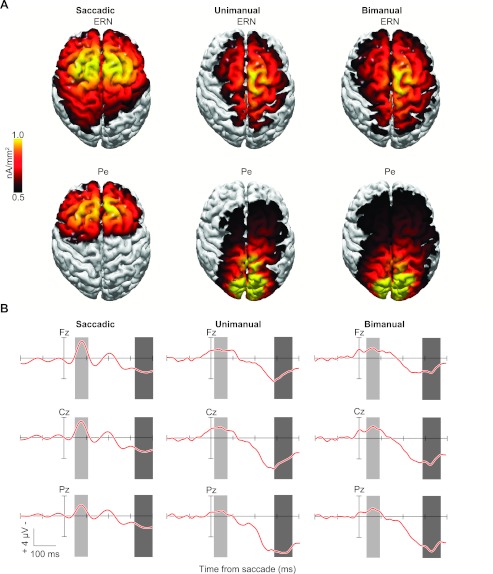
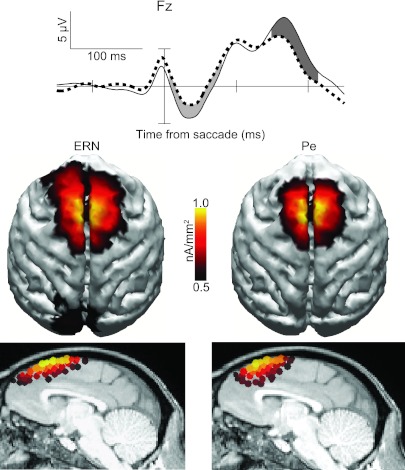
The cortical distribution of each error-elicited ERP component was assessed by estimating the underlying electrical current density at each point along the cortical surface in the time range of the maximum amplitude differences between error and correct waveforms (saccadic: ERN from 48 to 108 ms, Pe from 320 to 400 ms; unimanual: ERN from 16 to 76 ms, Pe from 286 to 366 ms; bimanual: ERN from 32 to 92 ms, Pe from 286 to 366 ms). Of note, we obtained comparable results from all current density analyses across a wide range of different measurement windows (identical to those tested in the statistical analyses described in materials and methods). Figure 4 shows current density distributions over the cortical surface (A) and the corresponding difference waveforms (B) for the ERN and Pe components from experiments 1–3. The SWARM solutions over the cortical surface in Fig. 4A depict the frontomedial distribution for the saccadic Pe (97% explained variance) and parietomedial distribution of the manual Pe (unimanual: 99%; bimanual: 98% explained variance).
Fig. 4.
Distributed current densities across the cortical surface of the ERN and Pe from experiments 1–3. A: distributed source model of the ERN and Pe elicited by saccadic, unimanual (simple RT), and bimanual (2AFC RT) responses in the same stop-signal task. The model was computed based on errant noncanceled minus correct no-stop grand average difference waves. Time windows ±30 ms wide (ERN) and ±40 ms wide (Pe) were centered on the peak amplitude of the difference wave of the midline electrode showing maximal activity (as in Godlove et al. 2011). B: grand average difference waves with ERN (light gray shading) and Pe (dark gray shading) time periods of peak activity for each experiment at midline scalp electrodes (Fz, Cz, Pz).
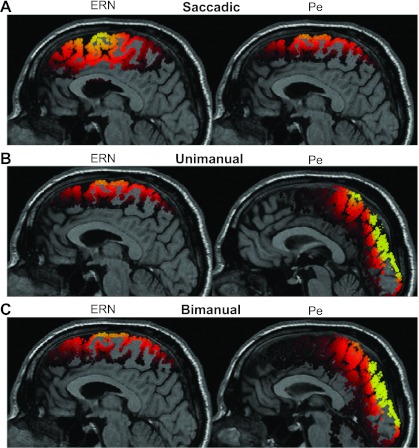
To identify potential neural generators and view the internal distribution of electrical current responsible for the observed electrical field distributions, the SWARM solutions for ERN and Pe from experiments 1–3 are also shown as an MRI midsaggital section (Fig. 5, A–C). The center of mass of SWARM activity is intended to represent a plausible spatial location of neural generation. The ERN exhibited a consistent frontomedial distribution across the cortical surface with a gravity center situated on the medial wall of posterior frontal cortex, including contributions from supplementary motor area (SMA) and dorsal ACC for saccadic (97% explained variance) and SMA for both manual tasks (unimanual: 95%; bimanual: 93% explained variance). The modeled electrical field was stronger at more anterior locations and more broadly distributed for the saccadic relative to the manual response ERN. Pe distributions illustrated in Fig. 5, A–C, reveal a concentration of high-magnitude SWARM activity in SMA and rostral ACC for saccadic responses and in the superior parietal lobule, hidden in the medial longitudinal fissure between the two cerebral hemispheres, for both types of manual responses. The projections of current densities across the cortical surface and within the dorsal bank of posterior frontal cortex from human saccadic ERN and Pe components show a high degree of correspondence with the monkey error-ERP distributions (see Fig. 6) reported in our parallel studies with macaque monkeys (Godlove et al. 2011). The relatively more caudal current densities in the human brain are consistent with what is known about the comparative neuroanatomy of the expanded prefrontal cortex of the human, which places homologous brain regions more posterior to those in the macaque (Kaas 2005; Petrides and Pandya 1999). Importantly, without conducting these studies in parallel, we would have been led to believe that the monkey ERN and Pe showed different distributions from those of humans, but these differences in topography appear to be effector, not species, specific.
Fig. 5.
Distributed current densities from a midsaggital MRI section of the ERN and Pe from experiments 1 and 2. Distributed source model of the ERN and Pe elicited by saccadic (A), unimanual (B), and bimanual responses (C) on the stop-signal task. The model was computed based on errant noncanceled minus correct no-stop grand average difference waves. Time windows ±30 ms wide (ERN) and ±40 ms wide (Pe) were centered on peak amplitude of the difference wave of the midline electrode showing maximal activity, consistent with method from our parallel study (Godlove et al. 2011).
Fig. 6.
Grand average ERPs and distributed current density topographies for ERN and Pe of the macaque monkeys performing the same task as in experiment 1 (from Godlove et al. 2011). Conventions and measurement windows are the same as in human data. [Reprinted with permission of the Society for Neuroscience.]
Error ERPs, RT adjustments, and response conflict.
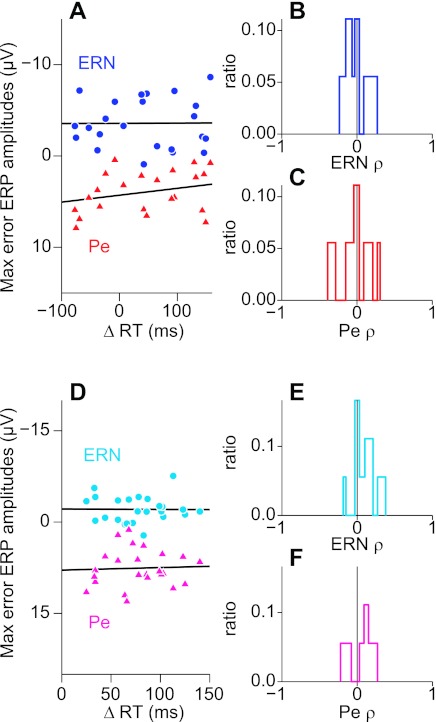
Recent studies suggest error ERPs do not reliably predict behavioral adjustments on the next trial of a task (Gehring and Fencsik 2001; Nunez Castellar et al. 2010; van Meel et al. 2007). However, the evidence regarding the impact of error ERPs on behavior has been decidedly mixed. Specifically, several other studies have suggested that human error-ERP components predict behavioral adjustments on subsequent trials (Debener et al. 2005; Gehring et al. 1993; Holroyd et al. 2005), and local field potentials recorded in medial frontal areas of macaque monkeys exhibit a measurable relationship to performance adjustments (Emeric et al. 2008, 2010). Our first assessment of the relationship between ERN or Pe amplitude and the RT adjustments on the subsequent trial was performed at the single-trial level. Figure 7A illustrates the correlations between the maximum ERN or Pe amplitude and ΔRT (RT on no-stop trial n+1 minus RT on noncanceled trial n) for a representative subject from experiment 1 (paralleling our analyses of error ERPs from nonhuman primates). Figure 7D illustrates correlations between maximum ERN/Pe amplitude measures and ΔRT for the same subject from experiment 2 (unimanual responses). In both response conditions, RT adjustments on subsequent trials were uncorrelated with the ERN peak negativity (saccadic: ρ = −0.01, P = 0.97, df = 21; unimanual: ρ = 0.01, P = 0.95, df = 22) or Pe peak positivity (saccadic: ρ = −0.34, P = 0.12, df = 21; unimanual: ρ = −0.06, P = 0.80, df = 21) following the errant response. The distributions of ρ values collapsed across all subjects are shown in Fig. 7, B and C, for experiment 1 (saccadic responses) and Fig. 7, E and F, for experiment 2 (unimanual responses). None of these distributions deviated significantly from zero (saccadic ERN: t = 0.21, P = 0.80, df = 11; unimanual ERN: t = 1.30, P = 0.22, df = 11; saccadic Pe: t = −0.13, P = 0.89, df = 11; unimanual Pe: t = 0.63, P = 0.69, df = 11).
Fig. 7.
Single-trial analysis of ERN or Pe amplitude and post-error RT adjustments from experiments 1 and 2. Correlations between maximum ERN or Pe amplitude and ΔRT (RT on no-stop trial n+1 minus RT on noncanceled trial n) from an example subject in experiment 1 (saccadic responses; A) and experiment 2 (unimanual responses; D). Neither the correlation between maximum ERN amplitude and ΔRT nor the correlation between maximum Pe amplitude and ΔRT reached significance for this subject in either experiment. Distribution of correlation coefficients (ρ) between maximum ERN amplitude and ΔRT across all subjects is shown separately for experiment 1 (A) and experiment 2 (E). Distribution of ρ between maximum Pe amplitude and ΔRT across all subjects is shown separately for experiment 1 (C) and experiment 2 (F). No distribution of ρ values deviated significantly from zero.
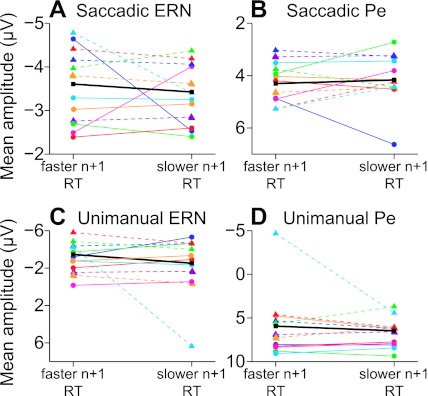
Next, we assessed the relationship between ERN or Pe amplitude and the RT adjustment on the subsequent trial using a median split of ΔRTs to increase the signal-to-noise ratio brought to bear in these analyses. Post-error RT adjustments were measured in the same way as described above, and median post-error ΔRT values were determined separately for each subject. ERPs were averaged using these values to determine median splits according to changes in post-error ΔRT and ERP amplitude. Figure 8, A and C, shows mean ERN amplitude between the fastest and slowest ΔRT trials separately for each subject (colors) and averaged across all subjects (black). Grand average ERN amplitude did not differ with post-error RT adjustments in either experiment 1 (t = 0.41, P = 0.72, df = 22) or experiment 2 (t = 0.59, P = 0.60, df = 22). Figure 8, B and D, displays mean Pe amplitude separated by subject, post-error RT median, and effector. As above, significant effects were not observed in the averaged waveforms from these analyses (saccadic Pe: t = −0.32, P = 0.77, df = 22; unimanual Pe: t = 0.51, P = 0.59, df = 22).1
Fig. 8.
Median split ERP analyses of ERN or Pe amplitude and post-error RT adjustments from experiments 1 and 2. Mean amplitude of the ERN (A) and Pe (B) followed by no-stop trials with faster RTs (left) or trials with slower RTs (right) is shown for each subject (colors) and as a grand average (black) from experiment 1 (saccadic responses). Mean amplitude of the ERN (C) and Pe (D) from experiment 2 (unimanual responses) are presented in the same format as in A. No comparisons reached statistical significance.
An influential theory posits that the ERN is produced by neural processing of response conflict (Botvinick et al. 2001; Yeung et al. 2004). The occurrence of response conflict is not restricted to error trials only but is hypothesized to occur with varying timing and magnitude on all trial types (Yeung et al. 2004). In the stop-signal task, subjects must choose between committing responses and canceling them. Thus, in the context of the stop-signal task, response conflict is engendered when subjects must choose between producing a response and not responding, with this occurring on every trial on which a stop-signal is present.
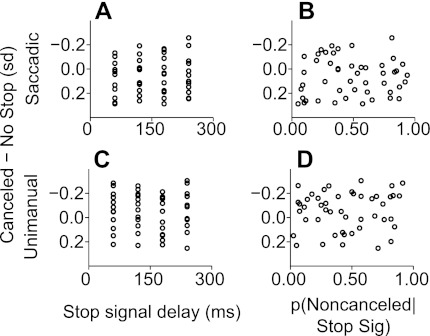
We tested for conflict-related activity in ERPs aligned to SSRT on canceled trials in experiment 1 (saccadic responses) and experiment 2 (unimanual responses) using the method of Stuphorn et al. (2000; see also Emeric et al. 2008, 2010; Ito et al. 2003) implemented in the same way as in our parallel study of nonhuman primate ERPs (Godlove et al. 2011). The results are shown in Fig. 9. The mean voltage differences between canceled and no-stop trials in the −50- to +100-ms time window around SSRT at each stop signal delay are displayed on the ordinate. In experiment 1 (saccadic responses), these voltage differences failed to show significant correlations with either SSD (ρ = −0.26, P = 0.30, df = 56) or the probability of committing errant noncanceled responses (ρ = −0.14, P = 0.63, df = 56). Similarly, voltage differences in experiment 2 (unimanual responses) did not show significant correlations with either SSD (ρ = −0.11, P = 0.60, df = 56) or error probability (ρ = −0.06, P = 0.72, df = 56).
Fig. 9.
Test for conflict-related activity in canceled ERP data in experiments 1 and 2. Normalized mean voltage difference between canceled trials and latency matched no-stop trials in the −50- to 100-ms time window around SSRT was plotted against SSD for experiment 1 (saccadic responses; A) and experiment 2 (unimanual responses; C). The same voltage data as in A and C were plotted against the probability of committing an errant noncanceled saccade at each SSD for experiment 1 (B) and experiment 2 (D). Significant correlations were not observed in either case (see results).
DISCUSSION
The ERN and Pe are electrophysiological indexes of performance monitoring used to study the neural mechanisms of executive control. In the present study, ERN and Pe were recorded during saccadic, unimanual, and bimanual response versions of the stop-signal task. The stimuli presented during these tasks were identical, and only response demands were manipulated across experiments. Despite these similarities, we observed substantial differences in amplitude, timing, voltage distribution, and current density models of the error ERPs across experiments. Because we used identical stimuli and within-subjects comparisons between experiment 1 (saccadic responses) and experiment 2 (unimanual responses), removing large sources of variability in ERP studies (Luck 2005; Woodman 2010), these findings are due to differences in response modality.
Our findings are consistent with the view that different neural circuits monitor eye and hand movements, and complement previous neuroimaging (Paus et al. 1993, 1998; Picard and Strick 1996) and primate neurophysiological evidence (Morecraft and Van Hoesen 1992) in support of a somatotopic organization of ACC. The property of output dependence serves to constrain models of learning and executive control that hinge on the nature of the ERP components we measured (e.g., Holroyd and Coles 2002; Yeung et al. 2004). Furthermore, these findings show that the ERP components of monkeys elicited by errors during a saccadic stop-signal task (Godlove et al. 2011) have similar timing, distributions, and possible generators as those found in humans performing the same task with the expected differences due to known neuroanatomical characteristics across species.
As expected, errors using finger movements elicited a large ERN, maximal at frontomedial electrode sites, with a peak amplitude ∼20 ms after the response. This result is consistent with classic work (e.g., Falkenstein et al. 1990, 1991; Gehring et al. 1993; Kopp et al. 1996; Kopp and Rist 1999) as well as studies specifically employing manual stop-signal tasks (Stahl and Gibbons 2007; van Boxtel et al. 2005). Errors on the same task requiring a saccadic eye movement also produced a large ERN; however, it peaked later, at ∼76 ms postresponse, comparable to the timing and morphology of polarization from a previous study utilizing an antisaccade task (Nieuwenhuis et al. 2001). Our findings are also supported by a previous study that sought to measure the ERN during a saccadic stop-signal task but did not compare ERPs on correct vs. error trials (Endrass et al. 2005). The precise ERN latency depends on the time-locking event. For example, a component will appear later in a waveform time-locked to the onset of electromyographic activity compared with a waveform time-locked to button-press closure (Gehring et al. 2012). However, in our study, aligning to the latest possible response event (saccade end) would only add at most 20 ms (see Fig. 1A, inset), leaving an ∼40-ms latency shift in ERNs aligned to the button-press closure vs. saccade. Thus output modality appears to alter the temporal characteristics of performance-monitoring processing, our first indication that hand and eye effector systems lead performance monitoring neural circuitry to operate in different ways.
To better determine whether error-elicited ERPs were specific to output modality, we estimated the spatial distribution and plausible neural generators for error-related components in each effector modality. We observed a broader frontomedial voltage distribution of the ERN across the scalp when an oculomotor response was required compared with a manual response. Although using scalp topography to infer neural generators is not a straightforward endeavor (e.g., Urbach and Kutas 2002), the different distributions observed across effectors are traditionally considered to be evidence for different neural sources. Consistent with this suggestion, current density models mapped the ERN onto separate but overlapping regions of posterior mediofrontal cortex, depending on output modality. Specifically, the manual ERN was centered on SMA, whereas saccadic ERN current density spanned SMA and dorsal portions of caudal ACC. These findings converge with several lines of evidence from previous human neuroimaging and monkey neurophysiology on the somatotopic organization of medial frontal cortex using different response modalities (Morecraft and Van Hoesen 1992; Paus et al. 1993, 1998; Picard and Strick 1996). Although there are no ERN source modeling studies using oculomotor response data, studies modeling the manual ERN have consistently implicated posterior mediofrontal cortex, including SMA (Dehaene et al. 1994; Herrmann et al. 2004; Miltner et al. 1997; Ridderinkhof et al. 2004), as well as regions of dorsal ACC thought to govern performance monitoring processes, such as conflict and error detection, and interference control (Dehaene et al. 1994; Luu et al. 2003; van Boxtel et al. 2005; van Veen and Carter 2002). Converging evidence for a link between the ACC and the ERN comes from patients with ACC lesions who show reduced error awareness (Turken and Swick 1999) and diminished or absent manual ERNs following errors (Stemmers et al. 2000). However, it seems clear that ERN generation is a product of combined activity from a network of areas working in concert with the ACC (e.g., Gehring and Knight 2000), not all of which produce electrical fields captured by the current density modeling.
Executive control is necessary to adapt behavior according to changing environmental demands so that one may optimize task performance. Error detection plays a vital role in this process by signaling the need to implement online adjustments. However, ERN and Pe amplitude in the present study were uncorrelated with post-error RT adjustments according to single- and average-trial analyses, irrespective of output modality. This result is in accord with findings from a number of recent studies (Gehring and Fencsik 2001; Nunez Castellar et al. 2010; van Meel et al. 2007) and provides another piece of evidence against the view that there is a consistently positive relationship between ERN amplitude and RT adjustments (Debener et al. 2005; Gehring et al. 1993; Holroyd et al. 2005).
The absence of a relationship between ERN amplitude and RT adjustments may be rendered clearer if we expand our view of the psychological terrain (Gehring et al. 2012). Specifically, slowing down processing after an error is not the only method for implementing strategic adjustments and avoiding future errors. Indeed, post-error slowing is not consistently observed in the stop-signal task and may not be the primary way that subjects adjust behavior in response to errors (Bissett and Logan 2011; Emeric et al. 2007; Nelson et al. 2010). Moreover, human neuropsychology studies have shown that prefrontal cortex lesions eliminate the ERN but have no effect on compensatory behavior such as slowing after errors or exerting less forceful responses on errors (Gehring and Knight 2000). Just as we observed here, neither single- nor average-trial error-ERP analyses lead to significant results in nonhuman primates performing the same saccadic stop-signal task (as shown in our parallel study, Godlove et al. 2011).
The relationship between the ERN and the cognitive processes that could underlie its generation are still being actively investigated. Some investigators argue that the ERN reflects an error-detection process (e.g., Bernstein et al. 1995; Falkenstein et al. 1990, 1991, 2000; Nieuwenhuis et al. 2001; Scheffers et al. 1996), whereas others contend the ERN reflects a more general detection of response conflict, rather than errors per se (e.g., Yeung et al. 2004). The former error-detection theory posits that the ERN represents the detection of a mismatch between representations of the actual and intended response. In contrast, the latter conflict monitoring theory suggests that the medial cortex, hypothesized to generate the ERN, monitors conflict during response selection and that errors are simply a specific type of response conflict that occurs between erroneous and correct responses. One of the more prominent mathematical models that explains amplitude variation of the ERN is the reinforcement learning theory of the ERN (Holroyd and Coles 2002). Based in part on a machine learning algorithm, called the method of temporal differences, the reinforcement learning theory postulates that midbrain dopaminergic cells send signals related to both the environment and self-generated behavior, which index ongoing events according to learned expectations (Barto 1995; Holroyd and Coles 2002; Houk et al. 1995; Schultz 2002). A more recent view, which accounts for motivational and individual differences, unlike existing models, holds that the ERN reflects a more emotionally or motivationally relevant response to errors (Bush et al. 2000; Gehring and Willoughby 2002; Pailing et al. 2002). A theoretical synthesis of the numerous models of ERN functional significance was recently proposed, called the prediction of response-outcome (PRO) theory (Alexander and Brown 2010). On this view, the brain predicts the probability of many possible previously experienced action outcomes and compares actual vs. expected outcomes at which point a discrepancy signal, such as the ERN, may be produced, resulting in an updating of the learned response-outcome predictions.
Our findings introduce new constraints on the theories of the functional relevance of the ERN described above by challenging the view that the ERN indexes a generic cognitive mechanism with no ties to output modality. Moreover, working in concert with our companion study using nonhuman primates, the present findings establish the existence of nonhuman primate homologs of the ERN and Pe that can be used to distinguish between these competing models using converging evidence from simultaneous measurements of behavior, ERPs, invasive microelectrode recordings, and causal manipulations of neural activity.
One goal of this study and our parallel studies with macaque monkeys (Godlove et al. 2011) was to relate error ERPs in humans to those of nonhuman primates while they performed the same tasks. Striking similarities in the electrophysiological indexes of performance monitoring were observed in the macaque monkeys and humans. Specifically, the timing and the current density activation foci obtained for the ERN and Pe components between species were remarkably similar. Although these findings do not preclude the existence of species specificity in error monitoring, they do indicate that previous conflicting interspecies results may have reflected fundamental differences between oculomotor and skeletomotor effector systems. Together, this previous work and our concurrent work with macaque monkeys (Godlove et al. 2011) establish that humans and nonhuman primates have homologous ERP components elicited by the commission of errors. These findings pave the way for intracranial recordings and inactivation studies with monkeys to localize and further characterize the generators of error ERPs.
GRANTS
This work was supported by National Institutes of Health Grants R01-EY019882, R01-EY-08890-S1, and P30-EY08126.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.M.R., N.B.C., and G.F.W. conception and design of research; R.M.R. and N.B.C. performed experiments; R.M.R., N.B.C., and M.-S.K. analyzed data; R.M.R., N.B.C., M.-S.K., and G.F.W. interpreted results of experiments; R.M.R. prepared figures; R.M.R. drafted manuscript; R.M.R., N.B.C., M.-S.K., and G.F.W. edited and revised manuscript; R.M.R., N.B.C., M.-S.K., and G.F.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank J. Haitas and M. Young for help with data analysis. G. Logan, D. Godlove, and J. Schall provided useful comments on the manuscript.
Footnotes
To account for the effects of nonstationarity on RT estimates (Nelson et al. 2010), both single-trial and median split average-trial post-error RT adjustment analyses were repeated using a second ΔRT metric (see Godlove et al. 2011 for a description of methods). Neither single-trial analysis (saccadic ERN: t = 0.45, P = 0.71, df = 11; unimanual ERN: t = 0.91, P = 0.49, df = 11; saccadic Pe: t = −0.07, P = 0.89, df = 11; unimanual Pe: t = 0.53, P = 0.57, df = 11) nor median split analysis yielded significant results (saccadic ERN: t = 0.39, P = 0.65, df = 22; unimanual ERN: t = 0.21, P = 0.75, df = 22; saccadic Pe: t = 0.24, P = 0.76, df = 22; unimanual Pe: t = 0.73, P = 0.31, df = 22).
REFERENCES
- Alexander and Brown, 2010. Alexander W, Brown J. Computational models of performance monitoring and cognitive control. Top Cogn Sci 2: 658–677, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur and Starr, 1984. Arthur DL, Starr A. Task-relevant late positive component of the auditory event-related potential in monkeys resembles P300 in humans. Science 223: 186–188, 1984 [DOI] [PubMed] [Google Scholar]
- Asrress and Carpenter, 2001. Asrress K, Carpenter R. Saccadic countermanding: a comparison of central and peripheral stop signals. Vision Res 41: 2645–2651, 2001 [DOI] [PubMed] [Google Scholar]
- Barto, 1995. Barto A. Adaptive critics and the basal ganglia. In: Models of Information Processing in the Basal Ganglia, edited by Houk J, Davis J, Beiser D. Cambridge, MA: MIT Press, 1995, p. 215–232 [Google Scholar]
- Bernstein et al., 1995. Bernstein P, Scheffers MK, Coles MG. Where did I go wrong? A psychophysiological analysis of error detection. J Exp Psychol Hum Percept Perform 21: 1312–1322, 1995 [DOI] [PubMed] [Google Scholar]
- Bissett and Logan, 2011. Bissett P, Logan GD. Balancing cognitive demands: control adjustments in the stop-signal paradigm. J Exp Psychol 37: 392–404, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick et al., 2001. Botvinick M, Braver T, Barch D, Carter C, Cohen J. Conflict monitoring and cognitive control. Psychol Rev 108: 624–652, 2001 [DOI] [PubMed] [Google Scholar]
- Boucher et al., 2007. Boucher L, Palmeri T, Logan G, Schall J. Inhibitory control in mind and brain: an interactive race model of countermanding saccades. Psychol Rev 114: 376–397, 2007 [DOI] [PubMed] [Google Scholar]
- Brázdil et al., 2005. Brázdil M, Roman R, Daniel P, Rektor I. Intracerebral error-related negativity in a simple go/nogo task. J Psychophysiol 19: 244–255, 2005 [Google Scholar]
- Brázdil et al., 2002. Brázdil M, Roman R, Falkenstein M, Hohnsbein J, Hoormann J, Blanke L, Daniel P, Jurak P, Rektor I. Error processing evidence from intracerebral ERP recordings. Exp Brain Res 146: 460–466, 2002 [DOI] [PubMed] [Google Scholar]
- Bush et al., 2000. Bush G, Luu P, Posner M. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222, 2000 [DOI] [PubMed] [Google Scholar]
- Chen et al., 2010. Chen X, Scangos KW, Stuphorn V. Supplementary motor area exerts proactive and reactive control of arm movements. J Neurosci 30: 14657–14675, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole et al., 2010. Cole M, Yeung N, Freiwald W, Botvinick M. Conflict over cingulate cortex: between-species differences in cingulate may support enhanced cognitive flexibility in humans. Brain Behav Evol 75: 239–240, 2010 [DOI] [PubMed] [Google Scholar]
- Cole et al., 2009. Cole MW, Yeung N, Freiwald WA, Botvinick M. Cingulate cortex: diverging data from humans and monkeys. Trends Neurosci 32: 566–574, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonius et al., 2001. Colonius H, Özyurt J, Arndt PA. Countermanding saccades with auditory stop signals: testing the race model. Vision Res 41: 1951–1968, 2001 [DOI] [PubMed] [Google Scholar]
- Debener et al., 2005. Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic imaging identifies the dynamics of performance monitoring. J Neurosci 25: 11730–11737, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene et al., 1994. Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error-detection and compensation. Psychol Sci 5: 303–305, 1994 [Google Scholar]
- Dum and Strick, 2002. Dum R, Strick PL. Motor areas in the frontal lobe of the primate. Physiol Behav 77: 677–682, 2002 [DOI] [PubMed] [Google Scholar]
- Emeric et al., 2007. Emeric EE, Brown JW, Boucher L, Carpenter RH, Hanes DP, Harris R, Logan GD, Mashru RN, Paré M, Pouget P, Stuphorn V, Taylor TL, Schall JD. Influence of history on saccade countermanding performance in humans and macaque monkeys. Vision Res 47: 35– 49, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeric et al., 2008. Emeric E, Brown JW, Leslie M, Pouget P, Stuphorn V, Schall JD. Performance monitoring local field potentials in the medial frontal cortex of primates: anterior cingulate cortex. J Neurophysiol 99: 759–772, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeric et al., 2010. Emeric E, Leslie M, Pouget P, Schall JD. Performance monitoring local field potentials in the medial frontal cortex of primates: supplementary eye field. J Neurophysiol 104: 1523–1537, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endrass et al., 2005. Endrass T, Cosima F, Norbert K. Error awareness in a saccade countermanding task. J Psychophysiol 19: 275–280, 2005 [Google Scholar]
- Falkenstein et al., 1991. Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol 78: 447–455, 1991 [DOI] [PubMed] [Google Scholar]
- Falkenstein et al., 1990. Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of errors in choice reaction tasks on the ERP under focused and divided attention. In: Psychophysiological Brain Research, edited by Brunia CH, Gaillard AW, Kok A. Tilburg, The Netherlands: Tilburg University Press, 1990, p. 192–195 [Google Scholar]
- Falkenstein et al., 2000. Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biol Psychol 51: 87–107, 2000 [DOI] [PubMed] [Google Scholar]
- Fuchs et al., 2002. Fuchs M, Kastner J, Wagner M, Hawes S, Ebersole JS. A standardized boundary element method volume conductor model. Clin Neurophysiol 113: 702–712, 2002 [DOI] [PubMed] [Google Scholar]
- Gehring and Fencsik, 2001. Gehring W, Fencsik DE. Functions of the medial frontal cortex in the processing of conflict and errors. J Neurosci 21: 9430–9437, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring et al., 1993. Gehring W, Goss B, Coles MG, Meyer DE, Donchin E. A neural system for error-detection and compensation. Psychol Sci 4: 385–390, 1993 [Google Scholar]
- Gehring and Knight, 2000. Gehring W, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nat Neurosci 3: 516–520, 2000 [DOI] [PubMed] [Google Scholar]
- Gehring et al., 2012. Gehring W, Liu Y, Orr JM, Carp J. The error-related negativity (ERN/Ne). In: Oxford Handbook of Event-Related Potential Components edited by Luck S, Kappenman E. New York: Oxford University Press, 2012, p. 231–291 [Google Scholar]
- Gehring and Willoughby, 2002. Gehring W, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science 295: 2279–2282, 2002 [DOI] [PubMed] [Google Scholar]
- Godlove et al., 2011. Godlove D, Emeric E, Segovis C, Young M, Schall J, Woodman G. Event-related potentials elicited by errors during the stop-signal task. I: Macaque monkeys. J Neurosci 31: 15640–15649, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg and Barr, 1991. Goldberg E, Barr W. Three Possible Mechanisms of Unawareness of Deficit. New York: Oxford University Press, 1991 [Google Scholar]
- Hajcak et al., 2005. Hajcak G, Moser J, Yeung N, Simons R. On the ERN and the significance of errors. Psychophysiology 42: 151–160, 2005 [DOI] [PubMed] [Google Scholar]
- Hanes and Carpenter, 1999. Hanes D, Carpenter RH. Countermanding saccades in humans. Vision Res 39: 2777–2791, 1999 [DOI] [PubMed] [Google Scholar]
- Hanes et al., 1998. Hanes D, Carpenter RH, Patterson WF, Schall JD. Role of frontal eye fields in countermanding saccades: visual, movement, and fixation activity. J Neurophysiol 79: 817–834, 1998 [DOI] [PubMed] [Google Scholar]
- Hanes and Schall, 1995. Hanes D, Schall JD. Countermanding saccades in macaque. Vis Neurosci 12: 929–937, 1995 [DOI] [PubMed] [Google Scholar]
- Herrmann et al., 2004. Herrmann M, Rommler J, Ehlis AC, Heidrich A, Fallgatter AJ. Source localization (LORETA) of the error-related-negativity (ERN/Ne) and positivity (Pe). Brain Res Cogn Brain Res 20: 294–299, 2004 [DOI] [PubMed] [Google Scholar]
- Holroyd and Coles, 2002. Holroyd C, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev 109: 679–709, 2002 [DOI] [PubMed] [Google Scholar]
- Holroyd et al., 1998. Holroyd C, Dien J, Coles M. Error-related scalp potentials elicited by hand and foot movements: evidence for an output-independent error-processing system in humans. Neurosci Lett 242: 65–68, 1998 [DOI] [PubMed] [Google Scholar]
- Holroyd et al., 2005. Holroyd C, Yeung N, Coles MG, Cohen JD. A mechanism for error detection in speeded response time tasks. J Exp Psychol Gen 134: 163–191, 2005 [DOI] [PubMed] [Google Scholar]
- Houk et al., 1995. Houk J, Adams JL, Barto AG. A model of how the basal ganglia generate and use neural signals that predict reinforcement. In: Models of Information Processing in the Basal Ganglia, edited by Houk J, Davis J, Beiser D. Cambridge, MA: MIT Press, 1995, p. 249–227 [Google Scholar]
- Ito et al., 2003. Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science 302: 120–122, 2003 [DOI] [PubMed] [Google Scholar]
- Jennings and Wood, 1976. Jennings J, Wood CC. Thee-adjustment procedure for repeated measures analyses of variance. Psychophysiology 13: 277–278, 1976 [DOI] [PubMed] [Google Scholar]
- Kaas, 2005. Kaas JH. The evolution of visual cortex in primates. In: The Primate Visual System, edited by Kremers J. Chichester, UK: Wiley & Sons, 2005, p. 267–283 [Google Scholar]
- Kopp and Rist, 1999. Kopp B, Rist F. An event-related brain potential substrate of disturbed response monitoring in paranoid schizophrenic patients. J Abnorm Psychol 108: 337–346, 1999 [DOI] [PubMed] [Google Scholar]
- Kopp et al., 1996. Kopp B, Rist F, Mattler U. N200 in the flanker task as a neurobehavioral tool for investigating executive control. Psychophysiology 33: 282–294, 1996 [DOI] [PubMed] [Google Scholar]
- Larson et al., 2010. Larson M, Baldwin SA, Good DA, Fair JE. Temporal stability of the error-related negativity (ERN) and post-error positivity (Pe): the role of number of trials. Psychophysiology 47: 1167–1171, 2010 [DOI] [PubMed] [Google Scholar]
- Logan, 1994. Logan G. On the ability to inhibit thought and action: a users' guide to the stop signal paradigm. In: Inhibitory Processes in Attention, Memory, and Language, edited by Dagenback D, Carr T. San Diego, CA: Academic, 1994, p. 189–239 [Google Scholar]
- Logan and Cowan, 1984. Logan G, Cowan WB. On the ability to inhibit thought and action—a theory of an act of control. Psychol Rev 91: 295–327, 1984 [DOI] [PubMed] [Google Scholar]
- Logan et al., 1984. Logan G, Cowan WB, Davis KA. On the ability to inhibit responses in simple and choice reaction time tasks: a model and a method. J Exp Psychol Hum Percept Perform 10: 276–291, 1984 [DOI] [PubMed] [Google Scholar]
- Logan and Irwin, 2000. Logan G, Irwin DE. Don't look! Don't touch! Inhibitory control of eye and hand movements. Psychon Bull Rev 7: 107–112, 2000 [DOI] [PubMed] [Google Scholar]
- Logan et al., 1997. Logan G, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychol Sci 8: 60–64, 1997 [Google Scholar]
- Luck, 2005. Luck S. An Introduction to the Event-Related Potential Technique. Cambridge, MA: MIT Press, 2005 [Google Scholar]
- Luu et al., 2000. Luu P, Flaisch T, Tucker D. Medial frontal cortex in action monitoring. J Neurosci 20: 464–469, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu et al., 2003. Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychol Sci 14: 47–53, 2003 [DOI] [PubMed] [Google Scholar]
- Miltner et al., 1997. Miltner W, Braun C, Coles M. Event-related brain potentials following incorrect feedback in a time-estimation task: evidence for a “generic” neural system for error detection. J Cogn Neurosci 9: 788–798, 1997 [DOI] [PubMed] [Google Scholar]
- Morecraft and Van Hoesen, 1992. Morecraft R, Van Hoesen GW. Cingulate input to the primary and supplementary motor cortices in the rhesus monkey: evidence for somatotopy in areas 24c and 23c. J Comp Neurol 322: 471–489, 1992 [DOI] [PubMed] [Google Scholar]
- Nakamura et al., 2005. Nakamura K, Roesch MR, Olson CR. Neuronal activity in macaque SEF and ACC during performance of tasks involving conflict. J Neurophysiol 93: 884–908, 2005 [DOI] [PubMed] [Google Scholar]
- Nelson et al., 2010. Nelson M, Boucher L, Logan GD, Palmeri TJ, Schall JD. Nonindependent, and nonstationary response times in stopping and stepping saccade tasks. Atten Percept Psychophys 72: 1913–1929, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis et al., 2001. Nieuwenhuis S, Ridderinkhof K, Blow J, Band G, Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology 38: 752–760, 2001 [PubMed] [Google Scholar]
- Nunez Castellar et al., 2010. Nunez Castellar E, Kühn S, Fias W, Notebaert W. Outcome expectancy and not accuracy determines posterror slowing: ERP support. Cogn Affect Behav Neurosci 10: 270–278, 2010 [DOI] [PubMed] [Google Scholar]
- Nunez and Srinivasan, 2006. Nunez P, Srinivasan R. Electric Fields of the Brain: the Neurophysics of EEG. Oxford: Oxford University Press, 2006 [Google Scholar]
- Özyurt et al., 2003. Özyurt J, Colonius H, Arndt P. Countermanding saccades: evidence against independent processing of go and stop signals. Percept Psychophys 65: 420–428, 2003 [DOI] [PubMed] [Google Scholar]
- Pailing et al., 2002. Pailing P, Segalowitz SJ, Dywan J, Davies PL. Error negativity and response control. Psychophysiology 39: 198–206, 2002 [DOI] [PubMed] [Google Scholar]
- Paré and Hanes, 2003. Paré M, Hanes D. Controlled movement processing: superior colliculus activity associated with countermanded saccades. J Neurosci 23: 6480–6489, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus et al., 1998. Paus T, Koski L, Caramanos Z, Westbury C. Regional differences in the effects of task difficulty, and motor output on blood flow response in the human anterior cingulate cortex: a review of 107 PET activation studies. Neuroreport 9: R37–R47, 1998 [DOI] [PubMed] [Google Scholar]
- Paus et al., 1993. Paus T, Petrides M, Evans AC, Meyer E. Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: a positron emission tomography study. J Neurophysiol 70: 453–469, 1993 [DOI] [PubMed] [Google Scholar]
- Petrides and Pandya, 1999. Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci 11: 1011–1036, 1999 [DOI] [PubMed] [Google Scholar]
- Picard and Strick, 1996. Picard N, Strick P. Motor areas of the medial wall: a review of their location, and functional activation. Cereb Cortex 6: 342–353, 1996 [DOI] [PubMed] [Google Scholar]
- Ridderinkhof et al., 2004. Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science 306: 443–447, 2004 [DOI] [PubMed] [Google Scholar]
- Scangos and Stuphorn, 2010. Scangos K, Stuphorn V. Medial frontal cortex motivates but does not control movement initiation in the countermanding task. J Neurosci 30: 1968–1982, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall and Emeric, 2010. Schall J, Emeric EE. Conflict in cingulate cortex function between humans, and macaque monkeys: more apparent than real. Brain Behav Evol 75: 237–238, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffers et al., 1996. Scheffers M, Coles M, Bernstein P, Gehring W, Donchin E. Event-related brain potentials and error-related processing: an analysis of incorrect responses to go and no-go stimuli. Psychophysiology 33: 42–53, 1996 [DOI] [PubMed] [Google Scholar]
- Schultz, 2002. Schultz W. Getting formal with dopamine and reward. Neuron 36: 241–263, 2002 [DOI] [PubMed] [Google Scholar]
- Stahl and Gibbons, 2007. Stahl J, Gibbons H. Dynamics of response-conflict monitoring and individual differences in response control and behavioral control: an electrophysiological investigation using a stop-signal task. Clin Neurophysiol 118: 581–596, 2007 [DOI] [PubMed] [Google Scholar]
- Stemmers et al., 2000. Stemmers B, Segalowitz SJ, Witzke W, Lacher S, Schönle PW. Do patients with damage to the anterior cingulate and adjacent regions produce an error-related negativity (ERN)? Psychophysiology 37: S95, 2000 [Google Scholar]
- Stuphorn et al., 2000. Stuphorn V, Taylor T, Schall J. Performance monitoring by the supplementary eye field. Nature 408: 857–860, 2000 [DOI] [PubMed] [Google Scholar]
- Turken and Swick, 1999. Turken A, Swick D. Response selection in the human anterior cingulate cortex. Nat Neurosci 2: 920–924, 1999 [DOI] [PubMed] [Google Scholar]
- Urbach and Kutas, 2002. Urbach T, Kutas M. he intractability of scaling scalp distributions to infer neuroelectric sources. Psychophysiology 39: 791–808, 2002 [DOI] [PubMed] [Google Scholar]
- van Boxtel et al., 2005. van Boxtel G, Van Der Molen MW, Jennings JR. Differential involvement of the anterior cingulate cortex in performance monitoring during a stop-signal task. J Psychophysiol 19: 1–10, 2005 [Google Scholar]
- van Meel et al., 2007. van Meel C, Heslenfeld DJ, Oosterlaan J, Sergeant JA. Adaptive control deficits in attention-deficit/hyperactivity disorder (ADHD): the role of error processing. Psychiatry Res 151: 211–220, 2007 [DOI] [PubMed] [Google Scholar]
- van Veen and Carter, 2002. van Veen V, Carter C. The timing of action-monitoring processes in the anterior cingulate cortex. J Cogn Neurosci 14: 593–602, 2002 [DOI] [PubMed] [Google Scholar]
- Van't Ent and Apkarian, 1999. Van't Ent D, Apkarian P. Motoric response inhibition in finger movement and saccadic eye movement: a comparative study. Clin Neurophysiol 110: 1058–1072, 1999 [DOI] [PubMed] [Google Scholar]
- Verbruggen and Logan, 2008. Verbruggen F, Logan G. Response inhibition in the stop-signal paradigm. Trends Cogn Sci 12: 418–424, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams et al., 1999. Williams B, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Dev Psychol 35: 205–213, 1999 [DOI] [PubMed] [Google Scholar]
- Woodman, 2010. Woodman G. A brief introduction to the use of event-related potentials (ERPs) in studies of perception and attention. Atten Percept Psychophys 72: 2031–2046, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung et al., 2004. Yeung N, Botvinick M, Cohen J. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev 111: 931–959, 2004 [DOI] [PubMed] [Google Scholar]