Abstract
The nervous system frequently integrates parallel streams of information to encode a broad range of stimulus strengths. In mammalian retina it is generally believed that signals generated by rod and cone photoreceptors converge onto cone bipolar cells prior to reaching the retinal output, the ganglion cells. Near absolute visual threshold a specialized mammalian retinal circuit, the rod bipolar pathway, pools signals from many rods and converges on depolarizing (AII) amacrine cells. However, whether subsequent signal flow to OFF ganglion cells requires OFF cone bipolar cells near visual threshold remains unclear. Glycinergic synapses between AII amacrine cells and OFF cone bipolar cells are believed to relay subsequently rod-driven signals to OFF ganglion cells. However, AII amacrine cells also make glycinergic synapses directly with OFF ganglion cells. To determine the route for signal flow near visual threshold, we measured the effect of the glycine receptor antagonist strychnine on response threshold in fully dark-adapted retinal cells. As shown previously, we found that response threshold for OFF ganglion cells was elevated by strychnine. Surprisingly, strychnine did not elevate response threshold in any subclass of OFF cone bipolar cell. Instead, in every OFF cone bipolar subclass strychnine suppressed tonic glycinergic inhibition without altering response threshold. Consistent with this lack of influence of strychnine, we found that the dominant input to OFF cone bipolar cells in darkness was excitatory and the response threshold of the excitatory input varied by subclass. Thus, in the dark-adapted mouse retina, the high absolute sensitivity of OFF ganglion cells cannot be explained by signal transmission through OFF cone bipolar cells.
Keywords: rod circuitry, amacrine cell, glycine receptor, scotopic vision
the mammalian visual system can respond to light that varies in intensity by 12 orders of magnitude, a feature originating in two classes of photoreceptor cells (rods and cones) and several parallel pathways from photoreceptors to ganglion cells that function at different mean light levels (Völgyi et al. 2004). Near absolute visual threshold, when photons are scarce, all mammals use a specialized retinal circuit, the rod bipolar pathway, that pools the output of thousands of rods. In this pathway many rods converge onto rod ON bipolar cells, which subsequently converge on narrow-field depolarizing AII amacrine cells (Dacheux and Raviola 1986; Strettoi et al. 1990).
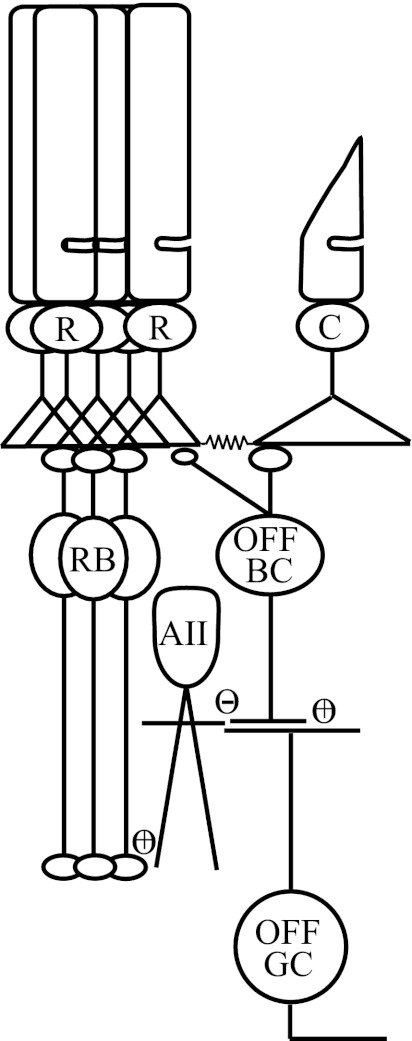
While it is commonly held that the principal route for light-evoked signal flow from AII amacrine cells is to ON and OFF cone bipolar cells as depicted in Fig. 1 (reviewed by Bloomfield and Dacheux 2001), in reality signals from AII amacrine cells to OFF ganglion cells may take two potential routes: 1) As above, AII amacrine cell lobular appendages make glycinergic inhibitory synapses on OFF cone bipolar cell synaptic terminals, synapses whose functional properties are poorly defined, which in turn relay excitatory signals to OFF ganglion cells (Dacheux and Raviola 1986; Famiglietti and Kolb 1975; Strettoi et al. 1990), or 2) AII amacrine cells make direct glycinergic inhibitory synapses on the dendrites of OFF ganglion cells (Chun et al. 1993; Famiglietti and Kolb 1975; Kolb and Nelson 1993; Strettoi et al. 1992). More recent studies in the mouse retina indicate that inhibitory synapses from AII amacrine cells may provide the dominant input to OFF ganglion cells (Margolis and Detwiler 2007; Murphy and Rieke 2006, 2008; van Wyk et al. 2009). However, the relative contributions of AII amacrine cells and OFF cone bipolar cells to setting the sensitivity of OFF ganglion cells remain unknown at any light level.
Fig. 1.
Rod pathways in the mouse retina: identified pathways by which rod (R)-generated signals can traverse the retinal circuitry en route to ganglion cells. In the rod bipolar pathway rods converge on rod ON bipolar cells (RB), which in turn provide glutamatergic input (+) to AII amacrine cells. AII amacrine cells make glycinergic inhibitory (−) synapses with both OFF ganglion cells (OFF GC) and OFF cone bipolar cells (OFF BC). OFF cone bipolar cells in turn relay glutamatergic (+) input to OFF ganglion cells. In the secondary rod pathways, OFF cone bipolar cells receive excitatory input from the cone photoreceptor (C) pedicles, or directly from rod spherules, depending on their subclass (see discussion).
To determine which of these two pathways relays light-evoked signals near visual threshold, we made physiological recordings from OFF ganglion cells and OFF cone bipolar cells in the dark-adapted mouse retina. These recordings revealed that glycinergic transmission directly to OFF ganglion cells played a greater functional role in setting response threshold than glycinergic transmission through OFF cone bipolar cells. Consistent with classical studies, pharmacological blockade of glycine receptors (GlyRs) by strychnine impaired the threshold for light-evoked responses in OFF ganglion cells (Müller et al. 1988) but, surprisingly, did not influence response threshold in OFF cone bipolar cells. Recordings from each subclass of OFF cone bipolar cell instead showed that strychnine suppressed tonic inhibitory postsynaptic potentials, thereby depolarizing the resting membrane potential. However, strychnine did not increase the OFF cone bipolar response threshold. Furthermore, voltage-clamp recordings from OFF cone bipolar cells revealed that their dominant input in darkness was excitatory, and response threshold was dependent on the subclass of OFF cone bipolar cell. Collectively these results indicate that low response thresholds in OFF ganglion cells cannot be explained by glycinergic transmission between AII amacrine cells and OFF cone bipolar synaptic terminals.
MATERIALS AND METHODS
Mice and preparation.
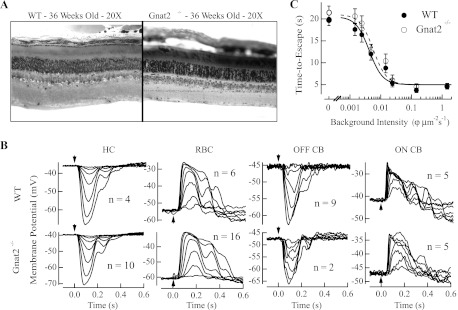
To characterize the properties of rod-driven signals in the mouse retina, we used a line of mice lacking cone photoresponses. Gnat2−/−, or Gnat2cpfl3/cpfl3, mice were bred for over five generations into a C57BL/6 background and provided by Dr. Bo Chang (The Jackson Laboratory). Mice were dark-adapted overnight and killed according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Southern California (Protocol no. 10890). These mice lack the α-subunit of the heterotrimeric G protein cone transducin. Despite the lack of cone phototransduction, cone cells remain with little retinal degeneration up to 15 wk of age (Chang et al. 2006). We further compared Gnat2−/− mice with wild-type (WT) mice to determine whether the deletion of cone transducin influenced the functional connectivity of the retinal circuitry. Epoxy resin sections (Chen et al. 2006) from Gnat2−/− and WT mice did not document retinal degeneration up to 36 wk of age (Fig. 2A). Furthermore, physiological recordings performed in 8- to 12-wk-old Gnat2−/− mice displayed robust rod-driven light responses whose sensitivity and temporal properties were statistically indistinguishable from WT in rod bipolar cells, ON and OFF cone bipolar cells, and horizontal cells (Fig. 2B). Finally, visual threshold measured in Gnat2−/− mice with a water maze task (Okawa et al. 2010; Sampath et al. 2005) indicated that threshold was similar to that in WT mice (Fig. 2C).
Fig. 2.
Morphological characteristics, response properties of retinal cells, and behavioral threshold of Gnat2−/− mice. A: comparison of retinal morphology in wild-type (WT) and Gnat2−/− retinas. Epoxy resin sections (Chen et al. 2006) from 36-wk-old WT and Gnat2−/− retinas reveal no loss in retinal thickness or rod outer segment length. B: comparison of response properties in horizontal cells (HC), rod bipolar cells (RBC), OFF cone bipolar cells (OFF CB), and ON cone bipolar cells (ON CB) from dark-adapted WT and Gnat2−/− retinas. Representative flash families in current clamp were collected after the delivery of a 10-ms flash of light (arrowhead). Derived response characteristics are shown in Table 1, including the response threshold (see materials and methods), resting membrane potential, and time-to-peak of the dim flash responses for the number of cells shown next to the respective responses. We found no statistical difference in the response characteristics of any of these classes of cells between WT and Gnat2−/− mice. C: behavioral threshold was estimated in WT and Gnat2−/− mice based on their ability to escape from a water maze task (Okawa et al. 2010; Sampath et al. 2005). Briefly, mice were trained in room light to escape from a 6-walled water maze task, and we characterized the time they required to exit the maze as background light levels were progressively lowered to darkness. Behavioral data are plotted as time to find the escape platform vs. background light intensity. Data reflect the behavioral performance of 4 WT and 4 Gnat2−/− mice, both bred into a C57BL/6 background. Each data point reflects the average of trials collected from 4 mice over 3 days with 3 trials/day (i.e., 36 samples). Data at every background light intensity are plotted as means ± SE. Behavioral data were fit with a Hill equation whose inflection points were 0.0048 and 0.0053 φ·μm−2·s−1 for WT (solid line) and Gnat2−/− (dashed line) mice, respectively. These data indicate that behavioral thresholds in Gnat2−/− and WT mice were similar and occurred at light levels that rely on single-photon responses traversing the retinal circuitry.
Electrophysiological recordings.
Procedures used for the handling of retinal tissue and electrophysiological recordings from bipolar cells, amacrine cells, and ganglion cells were similar to those used previously (Okawa et al. 2010; Sampath et al. 2005). Briefly, animals were dark-adapted overnight, eyes were enucleated under infrared illumination, the lens and cornea were removed, and the resulting eyecups were stored at 32°C in Ames medium equilibrated with 5% CO2-95% O2. All subsequent manipulations of the retinal tissue were done under infrared illumination (>950 nm) with infrared image converting goggles. For electrophysiological recordings the retinas were prepared as described below and superfused with Ames medium heated to 35–37°C. To block glycinergic transmission, the GlyR antagonist strychnine was used (Bolz et al. 1985). Strychnine-HCl (Sigma) was dissolved in dH2O to prepare a single stock solution of 1 mM that was used for all subsequent experiments. The stock solution was diluted in Ames medium to produce final concentrations of 1 μM and 20 μM.
Whole cell recordings were made from ON and OFF ganglion cells in dark-adapted whole mount preparations and from OFF cone bipolar cells and AII amacrine cells in dark-adapted retinal slices. The internal solution for ganglion cell recordings consisted of (in mM) 110 CsCH3SO3, 12 TEA-Cl, 10 HEPES, 10 EGTA, 2 QX-314, 4 ATP-Mg, 0.5 GTP-Tris, and 0.5 MgCl2; pH was adjusted to ∼7.3 with CsOH, and osmolarity was adjusted to ∼280 mosM. This solution prevented ganglion cells from firing action potentials, thereby allowing the synaptic currents or potentials to be isolated, but will alter the normal resting membrane potential of the cell. OFF ganglion cells with highly sensitive responses tended to have large oval-shaped cell bodies, while ON ganglion cells had large polygon-shaped cell bodies, consistent with anatomical characterizations of α-ganglion cells (Peichl and Wässle 1981). With the exception of ganglion cells in Fig. 9, A and B (OFF sustained), no attempt was made to further characterize these ganglion cells as displaying sustained or transient responses to light stimuli, although both classes are known to receive predominantly inhibitory input (cf. Margolis and Detwiler 2007; Murphy and Rieke 2006, 2008; van Wyk et al. 2009). It should be noted that the limited number of OFF ganglion cells studied do not reflect the full diversity of OFF ganglion cells, including perhaps highly sensitive OFF ganglion cells that receive input only from the rod bipolar pathway (Völgyi et al. 2004).
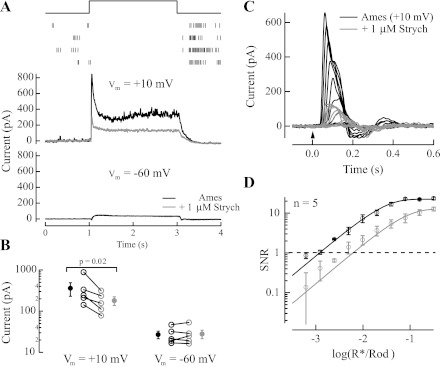
Fig. 9.
Inhibitory input to OFF ganglion cells sets response threshold. A: light-evoked synaptic currents from 1 OFF ganglion cell. Top: raster plot generated from an on-cell recording of action potentials generated in response to a step of light that delivered ∼32 R*·rod−1·s−1 in 4 subsequent trials. After the whole cell recording was established in this cell, inhibitory currents were isolated at +10 mV (middle) and excitatory currents were isolated at −60 mV (bottom) in Ames medium (black). Application of Ames medium with 1 μM strychnine (gray) reduced inhibitory currents but did not influence substantially the suppression of excitatory currents. B: maximum inhibitory and excitatory currents plotted for 5 OFF ganglion cells (based on cell body size, response characteristics, and susceptibility of the inhibitory input to strychnine, these are likely OFF sustained α-ganglion cells; Murphy and Rieke 2011) before and after application of 1 μM strychnine. A line connects currents associated with the same cell before and after the application of strychnine. Strychnine reduced the maximum inhibitory current on average by ∼2-fold (1.9 ± 0.27-fold, mean ± SE; n = 5) but did not influence the maximum excitatory current. C: voltage-clamp recordings of light-evoked responses to a flash from an OFF ganglion cell (Vm = + 10 mV) in the absence (black) and presence (gray) of 1 μM strychnine. D: collected data from 5 OFF ganglion cells (Vm = +10 mV) indicates that response threshold shifted ∼6-fold in the presence of 1 μM strychnine, from 0.0013 ± 0.00027 R*/Rod in Ames medium to 0.0081 ± 0.0024 R*/Rod in Ames medium with strychnine (P = 0.041).
The internal solution for the OFF bipolar cell and AII amacrine cell recordings consisted of (in mM) 125 K-aspartate, 10 KCl, 10 HEPES, 5 NMG-HEDTA, 0.5 CaCl2, 1 ATP-Mg, and 0.2 GTP-Mg; pH was adjusted to ∼7.3 with NMG-OH, and osmolarity was adjusted to ∼280 mosM. Identification of OFF bipolar cells was determined by the location of their cell bodies and their hyperpolarizing response to the onset of the light stimulus. In some recordings the morphology of the OFF bipolar cell was documented to determine its subclass (Ghosh et al. 2004; Pignatelli and Strettoi 2004). In these whole cell recordings, Lucifer yellow (Invitrogen) was included in the electrode internal solution and pictures were taken immediately after the recordings or the tissue section was fixed overnight in 4% paraformaldehyde, mounted on a microscope slide, and imaged with a Zeiss Axio Imager Z1 with an Apotome slider module. OFF cone bipolar cell subclass was then classified based on the branching pattern of the primary dendrite and the ramification of the axon terminal in the OFF sublamina of the inner plexiform layer.
Flash families were measured in response to a 10-ms flash from a blue LED [λmax ∼470 nm, full width at half-maximum (FWHM) ∼30 nm] whose strength varied from generating a just-measurable response and increased by factors of 2. Membrane currents were low-pass filtered at 300 Hz by an eight-pole Bessel filter and digitized at 1 kHz. Series and input resistances from these cells were monitored at regular intervals to ensure stability.
Light calibration.
In all recordings light was focused on the retinal tissue with a ×20 0.75 NA (Nikon) objective lens. Light intensities were calculated for all recordings as an effective photon flux at the peak wavelength of spectral sensitivity for mouse rhodopsin (λmax ∼501 nm) to facilitate comparison across cell types. In each instance the effective number of activated rhodopsins (R*) per rod (R*/Rod) was determined by convolving the power-scaled spectral output of the light source with the normalized spectral sensitivity curve for mouse rhodopsin, correcting for added neutral density in the light path. The R*/Rod was calculated based on the effective collecting area of our recording setup (Cao et al. 2008; Okawa et al. 2010). For whole mount experiments light calibrations were also corrected for the loss of light through the filter paper on which the retina was mounted.
Analysis and estimating response threshold.
To establish the sensitivity of retinal cells we defined response threshold as the flash strength where the signal-to-noise ratio (SNR) was equal to 1, the stimulus strength at which the light-evoked signal became distinguishable from cellular noise. Thus for every cell type the flash strengths used to determine response threshold will vary. To determine this flash strength we generated distributions of noise and response amplitudes from flash families. The noise variance was determined from the baseline membrane potential in the 0.2 s prior to the flash, and the size of the light-evoked potential was determined from the best fit of each individual trial to the mean response at each flash strength. From these distributions we estimated the SNR for each cell at each flash strength with the relation
where μs and μn are the mean of the distributions of the extracted signals and the noise, respectively, and σs and σn are the standard deviations of distributions of the signal and the noise. This definition of SNR is typically referred to as the unequal variance model of d′ or da (Swets et al. 1961; Swets 1961). Calculating the SNR at each flash strength generated a profile for each cell, which was well described by a saturating exponential:
Threshold was defined as the flash strength where this function equaled 1, and SNR versus flash strength is plotted in Figs. 3–6, 8, and 9 on a log-log scale to observe where this function crossed 1.
Fig. 3.
Strychnine increased response threshold in OFF, but not ON, ganglion cells. A: current-clamp flash-response families from a dark-adapted Gnat2−/− OFF ganglion cell in the absence (black) and presence (gray) of 1 μM strychnine. Flashes were delivered at the time indicated by the downward arrow. B: signal-to-noise ratios (SNR) of flash responses vs. flash strength plotted on a log-log scale for the cell shown in A were fit with a saturating exponential function, and threshold was estimated where SNR = 1 (see materials and methods). C: collected data from 4 OFF ganglion cells indicate that response threshold shifted ∼4-fold in the presence of strychnine, from 0.0015 ± 0.00016 R*/Rod in Ames medium to 0.0062 ± 0.0013 R*/Rod in Ames medium with strychnine (P = 0.045). D: current-clamp flash response families from a dark-adapted Gnat2−/− ON ganglion cell in the absence (black) and presence (gray) of 1 μM strychnine. Flashes were delivered at the time indicated by the downward arrow for the same flash strengths as the OFF ganglion cell in A. E: SNR of flash responses plotted vs. flash strength for the cell shown in D were fit with a saturating exponential function, and threshold was estimated. F: collected data from 5 ON ganglion cells indicate that response threshold was unaffected by the presence of strychnine and was 0.0015 ± 0.00042 R*/Rod in Ames medium and 0.0012 ± 0.00026 R*/Rod in Ames medium with strychnine (P = 0.344).
Fig. 4.
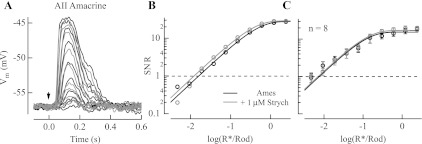
Strychnine did not influence the response threshold for AII amacrine cells. A: current-clamp flash response families from a dark-adapted Gnat2−/− AII amacrine cell in the absence (black) and presence (gray) of 1 μM strychnine. Flashes were delivered at the time indicated by the downward arrow. B: SNR of flash responses plotted vs. flash strength for the cell shown in A were fit with a saturating exponential function from which response threshold was determined (see materials and methods). C: collected data from 8 AII amacrine cells indicate that response threshold was unaffected by the presence of strychnine and was 0.012 ± 0.002 R*/Rod in Ames medium and 0.015 ± 0.0022 R*/Rod in Ames medium with strychnine (P = 0.624).
Fig. 5.
Strychnine depolarized the resting membrane potential and suppressed spontaneous inhibitory noise in OFF cone bipolar cells. A: typical current-clamp recording from an OFF cone bipolar cell where the membrane potential was monitored in the absence (black) and presence (gray) of 1 μM strychnine in darkness and after a flash delivering ∼30 R*/Rod, whose timing is denoted by the downward arrowhead. B: current-clamp recordings of flash families in the absence (black) and presence (gray) of 1 μM strychnine. C: SNR plotted as a function of flash strength for the flash families in the absence (black) and presence (gray) of 1 μM strychnine as shown in B were fit with a saturating exponential function from which response threshold was determined (see materials and methods). D: average SNR plotted as a function of flash strength across 10 OFF cone bipolar cells, with response thresholds of 0.41 ± 0.082 R*/Rod in Ames medium and 0.38 ± 0.096 R*/Rod in Ames medium with strychnine (P = 0.578).
Fig. 6.
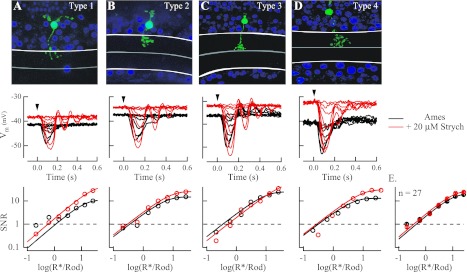
Strychnine did not influence response threshold in any of the 4 subclasses of OFF cone bipolar cells. A–D, top: type 1–4 OFF cone bipolar cells in Gnat2−/− retinas were identified on the basis of stratification of the axon terminal in the inner plexiform layer and the width of the dendritic arbor. White lines denote the limits of the inner plexiform layer, with the midpoint separating OFF from ON sublamina marked with a gray line. Middle: current-clamp recordings of flash families in the absence (black) and presence (red) of 20 μM strychnine for the photographed cells at top. Application of strychnine in all 27 OFF cone bipolar cells led to a depolarization of the cell's resting membrane potential (Vm), from −42.8 ± 1.3 mV in Ames medium to −37.6 ± 1.2 mV in Ames medium with strychnine (P = 0.0013). Bottom: SNR of flash responses plotted vs. flash strength for the cells shown at middle were fit with a saturating exponential function from which response threshold was determined (see materials and methods). E: collected data from 27 OFF cone bipolar cells across all subclasses indicate that despite the depolarization of the resting membrane potential response threshold was unaffected by the presence of strychnine and was 0.012 ± 0.002 R*/Rod in Ames medium and 0.015 ± 0.0022 R*/Rod in Ames medium with strychnine (P = 0.624).
Fig. 8.
Input to OFF cone bipolar cells was mainly excitatory and varied in response threshold based on subclass. A: type 1–4 OFF cone bipolar cells in Gnat2−/− retinas were identified based on the stratification of the axon terminal in the inner plexiform layer and branching on the primary dendrite (see materials and methods). B: voltage-clamp flash families are shown for each subclass of OFF cone bipolar cell at Vm = −60 mV (black) and Vm = +10 mV (blue). C: average SNR of excitatory flash responses (Vm = −60 mV) plotted vs. flash strength for each subclass of OFF cone bipolar cell. Number of recorded cells in each subclass is also noted. D: response threshold for excitatory input, or flash strength where the SNR = 1 (see materials and methods), plotted on a log10 scale for each recorded cell (○) in each subclass of OFF cone bipolar cell. The average and SE response threshold (●) was 0.23 ± 0.073 R*/Rod for type 1 cells (n = 7), 0.21 ± 0.047 R*/Rod for type 2 cells (n = 9), 0.53 ± 0.057 R*/Rod for type 3 cells (n = 22), and 0.62 ± 0.07 R*/Rod for type 4 cells (n = 10). Note that response threshold was ∼3-fold higher in type 3 and 4 cells compared with type 1 and 2 cells, a significant difference (P = 0.00001).
Table 1.
Response properties of retinal cells in WT and Gnat2−/− mice
| Horizontal Cells |
Rod Bipolar Cells |
OFF Cone Bipolar Cells |
ON Cone Bipolar Cells |
|||||
|---|---|---|---|---|---|---|---|---|
| WT | Gnat2−/− | WT | Gnat2−/− | WT | Gnat2−/− | WT | Gnat2−/− | |
| Response threshold, R*/Rod | 0.13 ± 0.01 | 0.15 ± 0.03 | 0.10 ± 0.03 | 0.11 ± 0.01 | 0.41 ± 0.06 | 0.42 ± 0.04 | 0.06 ± 0.01 | 0.08 ± 0.03 |
| Resting membrane potential, mV | −41 ± 3.2 | −45 ± 2.5 | −61 ± 2.5 | −58 ± 2.5 | −36 ± 4.5 | −42 ± 1.3 | −47 ± 2.7 | −41 ± 3.8 |
| Time-to-peak dim flash, ms | 140 ± 18 | 150 ± 10 | 160 ± 10 | 160 ± 6 | 100 ± 22 | 110 ± 10 | 120 ± 19 | 130 ± 16 |
Values are means ± SE. WT, wild type; R
/Rod, effective number of activated rhodopsins per rod.
Two-tailed Student t-tests were used to determine statistical significance for the difference between distributions; all averaged data are presented as means ± SE, with P values listed.
RESULTS
Strychnine increases threshold for light-evoked responses in dark-adapted OFF ganglion cells.
Previous work demonstrated that glycinergic transmission is a primary contributor to the light responses of dark-adapted mammalian OFF ganglion cells (Müller et al. 1988). To determine how the GlyRs set response threshold in these cells, we assessed the ability of the GlyR antagonist strychnine to alter the threshold for rod-driven signals in the retinas of mice lacking cone light-evoked responses (see materials and methods; Fig. 2). As shown in Fig. 3A, current-clamp recordings from OFF ganglion cells in the presence of 1 μM strychnine reduced the amplitude of light-evoked hyperpolarization at every flash strength. We quantified the influence of strychnine on the response threshold for light-evoked signals by plotting the SNR of the light-evoked response as a function of the flash strength (Fig. 3B; see materials and methods). Collected results from four OFF ganglion cells (Fig. 3C; see materials and methods for criteria in establishing subclass) demonstrate that application of strychnine resulted in an approximately fourfold increase in the threshold for rod responses, with response threshold increasing from 0.0015 ± 0.00017 R*/Rod in Ames medium to 0.0062 ± 0.0013 R*/Rod in the presence of strychnine (P = 0.045). Similar effects of strychnine were not observed in current-clamp recordings from ON ganglion cells (Müller et al. 1988), where light-evoked potentials remained unaltered at all flash strengths (Fig. 3, D–F). Figure 3F shows that application of 1 μM strychnine in five ON ganglion cells did not alter the response threshold (0.0015 ± 0.00042 R*/Rod in Ames medium to 0.0012 ± 0.00026 R*/Rod with strychnine; P = 0.34).
The specificity of strychnine's action to OFF ganglion cells, but not ON ganglion cells, has classically been attributed to the blockade of glycinergic synapses between AII amacrine cells and OFF cone bipolar synaptic terminals (Famiglietti and Kolb 1975; Strettoi et al. 1990), which in turn make excitatory synapses on OFF ganglion cells. However, strychnine's effect on OFF ganglion cells may also originate from direct glycinergic synapses from AII amacrine cells (Chun et al. 1993; Famiglietti and Kolb 1975; Kolb and Nelson 1993; Strettoi et al. 1992) or within AII amacrine cells themselves, which are known to express GlyRs (Zhou and Dacheux 2004). Thus, in principle, strychnine's effect on response threshold in OFF ganglion cells may result from any upstream action in their efferent connections originating in AII amacrine cells or OFF cone bipolar cells.
Strychnine does not affect rod response threshold in AII amacrine cells.
We assessed the effect of strychnine on the light-evoked responses of dark-adapted AII amacrine cells. Current-clamp recordings from AII amacrine cells revealed that addition of 1 μM strychnine produced little change in light-evoked responses (Fig. 4A) and did not alter the threshold for rod-driven responses (Fig. 4B). In eight AII amacrine cells, threshold was 0.023 ± 0.008 R*/Rod in Ames medium and 0.024 ± 0.009 R*/Rod with strychnine (Fig. 4C; P = 0.85). Strychnine's lack of effect on the AII amacrine cell light-evoked response rules out both the AII amacrine cells and their upstream rod bipolar cells, which also express functional GlyRs on their axon terminals (Gillette and Dacheux 1995; Suzuki et al. 1990) and receive inhibitory input via amacrine cells (Kolb and Nelson 1981; Kolb et al. 1981; Nelson and Kolb 1985), as the source of the approximately fourfold shift in response threshold observed in the OFF ganglion cells. Furthermore, these results are consistent with strychnine's lack of effect in ON ganglion cells (Fig. 3, D–F), which receive input from ON cone bipolar synaptic terminals that are coupled by gap junctions with AII amacrine cells (Strettoi et al. 1990, 1992; Veruki and Hartveit 2002). Thus the strychnine-mediated shift in the response threshold of OFF ganglion cells must be attributable to the AII amacrine cell's afferent connections either to the OFF bipolar synaptic terminal or directly to OFF ganglion cells (see Fig. 1). We sought to determine which of these connections is primarily responsible for setting the dark-adapted OFF ganglion cell response threshold.
Strychnine blocks tonic inhibition to OFF cone bipolar cells but does not affect dark-adapted response threshold.
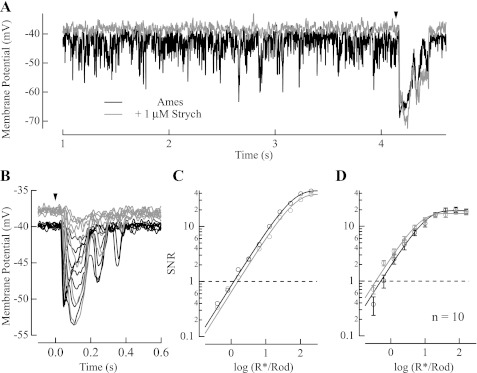
Anatomical studies in several mammalian species indicate that 50% or more of the lobular appendage output of AII amacrine cells is to OFF cone bipolar cell synaptic terminals (Chun et al. 1993; Famiglietti and Kolb 1975; Strettoi et al. 1990). To determine the extent to which these synapses contribute to setting the dark-adapted response threshold of OFF ganglion cells, we recorded light-evoked potentials from OFF cone bipolar cells in retinal slices. In every OFF cone bipolar cell tested, the application of 1 μM strychnine resulted in a depolarization of the cell's resting membrane potential and a suppression of spontaneous inhibitory potentials, without affecting the magnitude of the light-evoked potential (Fig. 5A). This effect presumably results from blockade of GlyRs in the axon terminal, the primary location of GlyR subunit in the mouse retina (Heinze et al. 2007). On average from 10 OFF cone bipolar cells, strychnine caused an ∼3-mV depolarization in resting membrane potential (from −38.0 ± 1.8 mV in Ames medium to −35.0 ± 1.1 mV in Ames medium with strychnine; P = 0.043).
Estimates of the SNR from flash families measured in the absence and presence of 1 μM strychnine reveal that the blockade of GlyRs did not degrade response threshold (Fig. 5, B and C), and in fact maximal responses became larger. The larger size of the response in strychnine is consistent with an increase in input resistance of OFF cone bipolar cells when GlyRs are blocked. In 10 OFF cone bipolar cells response threshold was 0.41 ± 0.082 R*/Rod in Ames medium and 0.38 ± 0.096 R*/Rod with 1 μM strychnine (P = 0.58). Thus strychnine's lack of influence on the response magnitude or response threshold suggests that GlyR inhibition in darkness originates from a different source than the light-driven input. Such lack of effect of strychnine on response threshold is surprising given the extent of AII amacrine cell synaptic contact with OFF cone bipolar cell axon terminals.
To provide a more rigorous evaluation of the role played by inhibitory input in OFF cone bipolar cell terminals on response threshold, we studied the influence of strychnine on the light-evoked potentials in the identified subclasses of OFF cone bipolar cells (as determined by Ghosh et al. 2004; see materials and methods). Such analysis would account for studies showing that the output of AII amacrine cells may be limited to certain OFF cone bipolar cell subclasses in the mouse retina (Tsukamoto et al. 2001). Given the lack of influence of 1 μM strychnine on responses (Fig. 5), we increased the concentration to 20 μM (Fig. 6)—it should be noted that at 20 μM strychnine also blocks GABAA receptors (O'Brien and Berger 1999). Figure 6 shows that light-evoked responses were not attenuated during the application of 20 μM strychnine in any of the four OFF cone bipolar subclasses. Response families in the absence and presence of strychnine instead showed an increase in magnitude of the light-evoked hyperpolarization to brighter flashes, just as for the application of 1 μM strychnine (Fig. 5). The application of 20 μM strychnine also produced a depolarization of the resting membrane potential of ∼5 mV (shifting from −42.8 ± 1.3 mV in Ames medium to −37.6 ± 1.2 mV with strychnine; n = 27; P = 0.0013) but did not influence the threshold for rod-driven responses in OFF cone bipolar cells (Fig. 6E; response threshold was 0.46 ± 0.043 R*/Rod in Ames medium and 0.39 ± 0.039 R*/Rod with strychnine; n = 27; P = 0.055). The similar depolarization in membrane potential and lack of influence on response threshold with 1 μM and 20 μM strychnine collectively indicate that the tonic inhibition of OFF cone bipolar cells in darkness is largely glycinergic.
OFF ganglion cell threshold was selectively elevated by strychnine.
We compared the influence of strychnine on the response threshold in AII amacrine cells, cone OFF bipolar cells, and OFF ganglion cells. Figure 7 plots on a cell-by-cell basis the response threshold for light-evoked signals for cells bathed in Ames medium versus threshold when cells were moved to Ames medium containing strychnine. A line with unity slope is included that reflects no change in response threshold between these conditions. Figure 7 highlights two important findings: 1) Response threshold for rod-driven signals in dark-adapted OFF cone bipolar cells was ∼20-fold higher than in AII amacrine cells and ∼270-fold higher than OFF ganglion cells, suggesting that OFF bipolar cells cannot receive a significant contribution from AII amacrine cells near their response threshold. Otherwise, OFF bipolar cell response thresholds would be expected to be comparable to the AII amacrine cell thresholds. 2) Dark-adapted OFF ganglion cell response thresholds, but not response thresholds in either AII amacrine cells or OFF cone bipolar cells, were increased significantly in the presence of strychnine as indicated by the clustering of cells below the line of unity slope. Thus the primary influence of strychnine on elevating the response threshold of OFF ganglion cells is on the OFF ganglion cells themselves.
Fig. 7.
Strychnine influenced response threshold only in OFF ganglion cells. Collected data from current-clamp recordings of OFF ganglion cells (OFFGC), AII amacrine cells (AIIAC), and OFF cone bipolar cells (OFFBC) (independent of subclasses) is shown on a cell-by-cell basis as the threshold in Ames medium plotted against the threshold in Ames medium with 1 μM strychnine and similar data collected from OFF cone bipolar cells in the absence and presence of 20 μM strychnine. A line with unity slope is included to compare changes in response threshold between recording conditions.
Input to OFF cone bipolar cells in darkness was primarily excitatory, with response threshold varying by subclass.
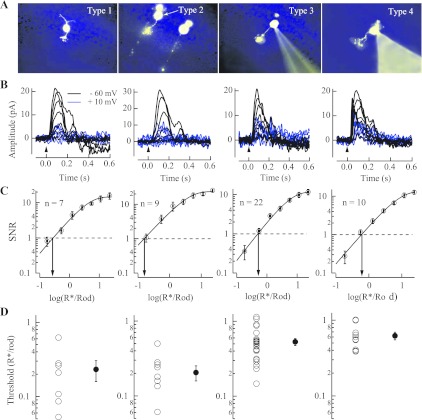
The lack of influence of strychnine on the response threshold of OFF cone bipolar cells is peculiar given previous anatomical evidence (Chun et al. 1993; Strettoi et al. 1992; Tsukamoto et al. 2001). We sought to characterize the relative contributions of inhibitory versus excitatory input to OFF cone bipolar cells in darkness to establish the physiological process that dominates their light-evoked response under dark-adapted conditions. Figure 8B shows families of light-evoked responses in each identified class of OFF cone bipolar cell (Ghosh et al. 2004; see also materials and methods) when the membrane potential was held at either −60 mV (isolates excitatory nonselective cationic current while holding the membrane potential near ECl) or +10 mV (isolates the inhibitory anionic current while holding the membrane potential near ECat). The rod-driven excitatory current under these conditions (arising from glutamate release from cone pedicles or directly from rod spherules; Hack et al. 1999; Soucy et al. 1998) was always greater than the inhibitory current. The magnitudes of the maximal excitatory and inhibitory currents indicate that near the resting membrane potential of dark-adapted OFF cone bipolar cells (∼ −40 mV; Figs. 5 and 6) ∼75% of the input was excitatory (assuming ECl = −60 mV, ECat = +10 mV, and Imax,Cat/Imax,Cl = 1.7 ± 0.26; n = 22).
The largely excitatory input to OFF cone bipolar cells in darkness arises from glutamate release from the rod and cone photoreceptors. We characterized the response threshold for this excitatory input in each subclass of OFF cone bipolar cell as shown in Fig. 8, C and D. Voltage-clamp recordings (Vm = −60 mV) revealed that response thresholds were lowest in type 1 and type 2 OFF cone bipolar cells [type 1 was 0.23 ± 0.073 R*/Rod (n = 7); type 2 was 0.21 ± 0.047 R*/Rod (n = 9)] and were approximately threefold higher in type 3 and type 4 cells [type 3 was 0.53 ± 0.057 R*/Rod (n = 22); type 4 was 0.62 ± 0.07 R*/Rod (n = 10)]. The OFF cone bipolar cells thus appear to separate rod-driven signals into distinct channels with varying sensitivities (DeVries 2000; see also discussion).
Response threshold in OFF ganglion cells was set by inhibitory input and was elevated by strychnine.
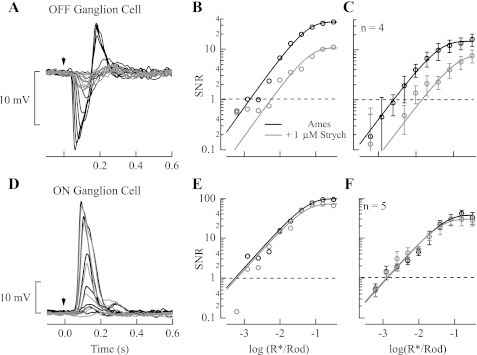
The lack of influence of strychnine on the response thresholds for all subclasses of OFF cone bipolar cells suggests that these cells do not contribute substantially to setting the high dark-adapted sensitivity of OFF ganglion cells. We studied the relative contribution of excitatory and inhibitory input to OFF ganglion cell responses (Fig. 9A). These experiments focused on a subclass of OFF ganglion cells with highly sensitive light-evoked responses (see materials and methods). Recordings revealed that a step of light produced a strong inhibitory input when the membrane potential was held at +10 mV and suppression of weaker excitatory input when the membrane potential was held at −60 mV; the magnitude of the inhibitory input was ∼10-fold greater than the excitatory input, consistent with previous work (Margolis and Detwiler 2007; Murphy and Rieke 2006, 2008; van Wyk et al. 2009). The strong inhibition onto OFF ganglion cells is suggestive that direct AII amacrine cell input is responsible primarily for setting the response threshold, with OFF bipolar input from the rod bipolar pathway playing little or no role at all. To test this hypothesis we evaluated the effect of strychnine on both inhibitory and excitatory input to OFF ganglion cells. The application of 1 μM strychnine had a strong effect on the maximum inhibitory input to the OFF ganglion cells studied, reducing the current by approximately twofold (Fig. 9B). However, strychnine had little effect on the maximum excitatory input. Thus, consistent with the lack of influence of strychnine on response threshold in dark-adapted OFF cone bipolar cells, we also observed little influence of strychnine on the excitatory input to dark-adapted OFF ganglion cells.
We determined the role of inhibitory input in setting response threshold by measuring light-evoked inhibitory currents while the OFF ganglion cell membrane potential was held at +10 mV in the absence and presence of 1 μM strychnine (Fig. 9C). As shown in Fig. 9D, response threshold for light-evoked inhibition was increased by approximately sixfold in the presence of strychnine (threshold was 0.0013 ± 0.0003 R*/Rod in Ames medium and 0.0081 ± 0.0024 R*/Rod in Ames medium with strychnine; n = 5; P = 0.041). Thus direct glycinergic inhibition can account largely for the increase in response threshold observed for dark-adapted OFF ganglion cells in the presence of strychnine (Fig. 3).
DISCUSSION
Anatomical convergence and synaptic specializations have been recognized as the hallmarks of the rod bipolar pathway that underlie its high sensitivity (reviewed by Bloomfield and Dacheux 2001; Field et al. 2005; Pahlberg and Sampath 2011; Singer 2007). AII amacrine cells provide a key junction point in this circuit where rod-driven signals move into the cone circuitry and can be subsequently relayed to both ON and OFF ganglion cells. Anatomically defined synapses between AII amacrine cells and OFF cone bipolar cells have traditionally been considered the main pathway for relaying the most sensitive rod signals to OFF ganglion cells, but little is known about the functional properties of these connections near visual threshold. Here we have characterized the properties of rod-driven signals as they move from AII amacrine cells to OFF ganglion cells in the dark-adapted retina of mice lacking cone photoresponses (see materials and methods). Consistent with previous studies we found that the dark-adapted responsiveness of a subclass of high-sensitivity OFF ganglion cells was attenuated during the application of strychnine (Müller et al. 1988), but we found that this sensitivity to strychnine does not originate in the synapses between AII amacrine cells and OFF cone bipolar cells. Instead strychnine's effect appears to manifest in the afferent connections of the AII amacrine cell, providing evidence against the idea that AII amacrine cell to OFF cone bipolar synapses may carry rod-driven signals near visual threshold in the mouse retina.
OFF cone bipolar cells do not contribute to OFF ganglion cell photoresponses at low light levels.
Perhaps our most surprising finding was that the response threshold of dark-adapted OFF cone bipolar cells was unaffected during the application of strychnine (leading to our increase in strychnine concentration from 1 μM up to 20 μM; see Figs. 5 and 6). Anatomical studies of mammalian retina all share in common the finding that a large fraction of the output of the lobular appendages of AII amacrine cells is to OFF cone bipolar cells, with the fraction varying from 50% of the output in cat (Kolb and Nelson 1993) and rat (Chun et al. 1993) to as much as 95% of the output in rabbit (Strettoi et al. 1992). The latter of these studies has led to the assumption that these synapses must be important for the processing of light-evoked signals for the rod bipolar pathway, whose function as a low light level detection pathway has long been appreciated.
Physiological studies of inhibition in OFF cone bipolar cells have focused on characterizing the postsynaptic receptors responsible. However, studies that puff glycine directly onto OFF bipolar axon terminals (Ivanova et al. 2006) make it difficult to draw inferences about their functional properties, particularly as light intensity varies. More recent studies in the mouse retina have characterized the inhibitory light-evoked response of OFF cone bipolar cells in dark-adapted retinas to bright flashes (Eggers and Lukasiewicz 2010; Eggers et al. 2007) but do not define the features of the signal or noise in the light-evoked response or synaptic transmission that would be critical for defining the response threshold. In sum, these studies unequivocally identify GlyRs on OFF cone bipolar synaptic terminals but do not necessarily provide insights into signal transmission at low light levels.
We characterized the role of GlyRs in setting the response threshold of dark-adapted OFF cone bipolar cells by measuring how threshold changes during application of strychnine. Given the potential heterogeneity of contacts between AII amacrine cells and the various OFF cone bipolar cell subclasses (Tsukamoto et al. 2001), we endeavored to measure the influence of strychnine on light-evoked responses of the identified classes of these cells (Ghosh et al. 2004). The common theme among all OFF cone bipolar subclasses (Figs. 5 and 6) was that voltage responses to flashes in darkness were not reduced by strychnine, as might be expected if they resulted from glycinergic transmission from AII amacrine cells, but instead they were frequently larger in amplitude. Furthermore, 1 μM strychnine appears to suppress spontaneous inhibitory potentials but does not influence the response threshold or maximal amplitude of the flash response (Fig. 4A). These results, along with a similar effect of 1 μM and 20 μM strychnine on resting membrane potential and response threshold, indicate that all OFF bipolar cells receive tonic inhibition in darkness, largely glycinergic, whose origin is distinct from that which generates the light-evoked response. Thus the properties of signal transmission between AII amacrine cells and OFF cone bipolar cells cannot explain the high sensitivity of glycinergic signaling to OFF ganglion cells under dark-adapted conditions.
The apparent lack of sensitivity of glycinergic synapses between AII amacrine cells and OFF cone bipolar cells (compared with OFF ganglion cells) remains curious. The high sensitivity of OFF ganglion cells suggests that glycine release from AII lobular appendages is not impaired at low light levels. Perhaps these differences in glycinergic sensitivity can be partially attributable to differences in GlyR subunit expression. For instance, OFF cone bipolar cells are known to express selectively the GlyR α1-subunit, as glycinergic responses are eliminated in Glra1−/− mice (Ivanova et al. 2006). OFF ganglion cells, however, express the GlyR α1-, α2-, and α3-subunits (Haverkamp et al. 2003; Heinze et al. 2007; Ivanova et al. 2006). The slower response kinetics of GlyR α2- and α3-subunits may allow for more sensitive light-evoked responses (see also Wässle et al. 2009). Thus determination of the light intensities under which OFF cone bipolar cells use input from the rod bipolar pathway to modulate the activity of OFF ganglion cells remains fundamental and unresolved.
OFF cone bipolar cell subclasses display distinct response thresholds for rod-driven signals.
The functional consequence of cellular diversity in any neural tissue is poorly understood, and highlights one of the most important questions in neurobiology. Diversity in bipolar cells presumably reflects differences in their ability to encode information from the photoreceptors or in the circuits they impinge on downstream (see Wässle 2004). Of the 10 different anatomically identified subclasses of bipolar cell in the mouse retina, we evaluated the dark-adapted response threshold of the 4 subclasses of OFF cone bipolar cells (Ghosh et al. 2004). Voltage-clamp recordings of the excitatory (i.e., photoreceptor) input to these cells reveals variations in sensitivity, with type 1 and 2 cells exhibiting approximately threefold lower response threshold than type 3 and 4 cells (Fig. 8D). These differences in sensitivity must be due to differences in the photoreceptor cell types these OFF cone bipolar cells contact, the distance of their dendrites from the site of glutamate release (DeVries et al. 2006), and/or the properties of their glutamate receptors. Indeed, anatomical (Tsukamoto et al. 2001) and physiological (Breuninger et al. 2011; Pang et al. 2012) studies of the mouse retina indicate that these subclasses receive distinct sets of contacts from photoreceptors, although some discrepancy exists in these studies. The higher sensitivity of type 1 and 2 cells we observe is broadly consistent with results from Pang and colleagues (2012), who suggest that these cells might contact rods directly. It should be noted that a limited number of anatomical reconstructions show that putative type 1 and 2 cells receive input from cones selectively, which in turn receive rod-driven signals through gap junctions (Tsukamoto et al. 2001). The reality is that we are approaching a complete characterization of bipolar cell subclasses (Wässle et al. 2009). Reconciling these studies will be of great importance in determining how specificity in wiring influences the sensitivity of subclasses of retinal cells. Nonetheless, the diversity of cone bipolar cells might ultimately allow rod-driven signals to be divided into many parallel streams that vary in how they sample the rod spherule and cone pedicle. Such an organization could allow intensity to be represented seamlessly as light levels increase in the scotopic range (see Sharpe and Stockman 1999).
GRANTS
This work was supported by National Eye Institute Grant EY-17606, the McKnight Endowment Fund for Neurosciences, and a research grant from the Karl Kirschgessner Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.C.A. and A.P.S. conception and design of research; A.C.A. and A.P.S. performed experiments; A.C.A. and A.P.S. analyzed data; A.C.A. and A.P.S. interpreted results of experiments; A.C.A. and A.P.S. prepared figures; A.C.A. and A.P.S. drafted manuscript; A.C.A. and A.P.S. edited and revised manuscript; A.C.A. and A.P.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Bo Chang from the Jackson Laboratory for providing Gnat2−/− bred into a C57BL/6 background and Jeannie Chen for assistance with morphological studies. We also thank Drs. Erica Eggers, David Marshak, Gabe Murphy, Dutch Ratliff, and Fred Rieke for thoughtful comments on the manuscript.
REFERENCES
- Bloomfield and Dacheux, 2001. Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res 20: 351–384, 2001 [DOI] [PubMed] [Google Scholar]
- Bolz et al., 1985. Bolz J, Thier P, Voigt T, Wässle H. Action and localization of glycine and taurine in the cat retina. J Physiol 362: 395–413, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuninger et al., 2011. Breuninger T, Puller C, Haverkamp S, Euler T. Chromatic bipolar cell pathways in the mouse retina. J Neurosci 31: 6504–6517, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao et al., 2008. Cao Y, Song H, Okawa H, Sampath AP, Sokolov M, Martemyanov KA. Targeting of RGS7/Gbeta5 to the dendritic tips of ON-bipolar cells is independent of its association with membrane anchor R7BP. J Neurosci 28: 10443–10449, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang et al., 2006. Chang B, Dacey MS, Hawes NL, Hitchcock PF, Milam AH, Atmaca-Sonmez P, Nusinowitz S, Heckenlively JR. Cone photoreceptor function loss-3, a novel mouse model of achromatopsia due to a mutation in Gnat2. Invest Ophthalmol Vis Sci 47: 5017–5021, 2006 [DOI] [PubMed] [Google Scholar]
- Chen et al., 2006. Chen J, Shi G, Concepcion FA, Xie G, Oprian D. Stable rhodopsin/arrestin complex leads to retinal degeneration in a transgenic mouse model of autosomal dominant retinitis pigmentosa. J Neurosci 26: 11929–11937, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun et al., 1993. Chun MH, Han SH, Chung JW, Wässle H. Electron microscopic analysis of the rod pathway of the rat retina. J Comp Neurol 332: 421–432, 1993 [DOI] [PubMed] [Google Scholar]
- Dacheux and Raviola, 1986. Dacheux RF, Raviola E. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. J Neurosci 6: 331–345, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries, 2000. DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron 28: 847–856, 2000 [DOI] [PubMed] [Google Scholar]
- DeVries et al., 2006. DeVries SH, Li W, Saszik S. Parallel processing in two transmitter microenvironments at the cone photoreceptor synapse. Neuron 50: 735–748, 2006 [DOI] [PubMed] [Google Scholar]
- Eggers and Lukasiewicz, 2010. Eggers ED, Lukasiewicz PD. Interneuron circuits tune inhibition in retinal bipolar cells. J Neurophysiol 103: 25–37, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers et al., 2007. Eggers ED, McCall MA, Lukasiewicz PD. Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J Physiol 582: 569–582, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti and Kolb, 1975. Famiglietti EV, Jr, Kolb H. A bistratified amacrine cell and synaptic circuitry in the inner plexiform layer of the retina. Brain Res 84: 293–300, 1975 [DOI] [PubMed] [Google Scholar]
- Field et al., 2005. Field GD, Sampath AP, Rieke F. Retinal processing near absolute threshold: from behavior to mechanism. Annu Rev Physiol 67: 491–514, 2005 [DOI] [PubMed] [Google Scholar]
- Ghosh et al., 2004. Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wässle H. Types of bipolar cells in the mouse retina. J Comp Neurol 469: 70–82, 2004 [DOI] [PubMed] [Google Scholar]
- Gillette and Dacheux, 1995. Gillette MA, Dacheux RF. GABA- and glycine-activated currents in the rod bipolar cell of the rabbit retina. J Neurophysiol 74: 856–875, 1995 [DOI] [PubMed] [Google Scholar]
- Hack et al., 1999. Hack I, Peichl L, Brandstatter JH. An alternative pathway for rod signals in the rodent retina: rod photoreceptors, cone bipolar cells, and the localization of glutamate receptors. Proc Natl Acad Sci USA 96: 14130–14135, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp et al., 2003. Haverkamp S, Müller U, Harvey K, Harvey RJ, Betz H, Wässle H. Diversity of glycine receptors in the mouse retina: localization of the alpha3 subunit. J Comp Neurol 465: 524–539, 2003 [DOI] [PubMed] [Google Scholar]
- Heinze et al., 2007. Heinze L, Harvey RJ, Haverkamp S, Wässle H. Diversity of glycine receptors in the mouse retina: localization of the alpha4 subunit. J Comp Neurol 500: 693–707, 2007 [DOI] [PubMed] [Google Scholar]
- Ivanova et al., 2006. Ivanova E, Müller U, Wässle H. Characterization of the glycinergic input to bipolar cells of the mouse retina. Eur J Neurosci 23: 350–364, 2006 [DOI] [PubMed] [Google Scholar]
- Kolb and Nelson, 1981. Kolb H, Nelson R. Amacrine cells of the cat retina. Vision Res 21: 1625–1633, 1981 [DOI] [PubMed] [Google Scholar]
- Kolb and Nelson, 1993. Kolb H, Nelson R. OFF-alpha and OFF-beta ganglion cells in cat retina. II. Neural circuitry as revealed by electron microscopy of HRP stains. J Comp Neurol 329: 85–110, 1993 [DOI] [PubMed] [Google Scholar]
- Kolb et al., 1981. Kolb H, Nelson R, Mariani A. Amacrine cells, bipolar cells and ganglion cells of the cat retina: a Golgi study. Vision Res 21: 1081–1114, 1981 [DOI] [PubMed] [Google Scholar]
- Margolis and Detwiler, 2007. Margolis DJ, Detwiler PB. Different mechanisms generate maintained activity in ON and OFF retinal ganglion cells. J Neurosci 27: 5994–6005, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller et al., 1988. Müller F, Wässle H, Voigt T. Pharmacological modulation of the rod pathway in the cat retina. J Neurophysiol 59: 1657–1672, 1988 [DOI] [PubMed] [Google Scholar]
- Murphy and Rieke, 2011. Murphy GJ, Rieke F. Electrical synaptic input to ganglion cells underlies differences in the output and absolute sensitivity of parallel retinal circuits. J Neurosci 31: 12218–12228, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy and Rieke, 2006. Murphy GJ, Rieke F. Network variability limits stimulus-evoked spike timing precision in retinal ganglion cells. Neuron 52: 511–524, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy and Rieke, 2008. Murphy GJ, Rieke F. Signals and noise in an inhibitory interneuron diverge to control activity in nearby retinal ganglion cells. Nat Neurosci 11: 318–326, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson and Kolb, 1985. Nelson R, Kolb H. A17: a broad-field amacrine cell in the rod system of the cat retina. J Neurophysiol 54: 592–614, 1985 [DOI] [PubMed] [Google Scholar]
- O'Brien and Berger, 1999. O'Brien JA, Berger AJ. Cotransmission of GABA and glycine to brain stem motoneurons. J Neurophysiol 82: 1638–1641, 1999 [DOI] [PubMed] [Google Scholar]
- Okawa et al., 2010. Okawa H, Miyagishima KJ, Arman AC, Hurley JB, Field GD, Sampath AP. Optimal processing of photoreceptor signals is required to maximize behavioural sensitivity. J Physiol 588: 1947–1960, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahlberg and Sampath, 2011. Pahlberg J, Sampath AP. Visual threshold is set by linear and nonlinear mechanisms in the retina that mitigate noise: how neural circuits in the retina improve the signal-to-noise ratio of the single-photon response. Bioessays 33: 438–447, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang et al., 2012. Pang JJ, Gao F, Paul DL, Wu SM. Rod, M-cone and M/S-cone inputs to hyperpolarizing bipolar cells in the mouse retina. J Physiol 590: 845–854, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl and Wässle, 1981. Peichl L, Wässle H. Morphological identification of on- and off-centre brisk transient (Y) cells in the cat retina. Proc R Soc Lond B Biol Sci 212: 139–153, 1981 [DOI] [PubMed] [Google Scholar]
- Pignatelli and Strettoi, 2004. Pignatelli V, Strettoi E. Bipolar cells of the mouse retina: a gene gun, morphological study. J Comp Neurol 476: 254–266, 2004 [DOI] [PubMed] [Google Scholar]
- Sampath et al., 2005. Sampath AP, Strissel KJ, Elias R, Arshavsky VY, McGinnis JF, Chen J, Kawamura S, Rieke F, Hurley JB. Recoverin improves rod-mediated vision by enhancing signal transmission in the mouse retina. Neuron 46: 413–420, 2005 [DOI] [PubMed] [Google Scholar]
- Sharpe and Stockman, 1999. Sharpe LT, Stockman A. Rod pathways: the importance of seeing nothing. Trends Neurosci 22: 497–504, 1999 [DOI] [PubMed] [Google Scholar]
- Singer, 2007. Singer JH. Multivesicular release and saturation of glutamatergic signalling at retinal ribbon synapses. J Physiol 580: 23–29, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy et al., 1998. Soucy E, Wang Y, Nirenberg S, Nathans J, Meister M. A novel signaling pathway from rod photoreceptors to ganglion cells in mammalian retina. Neuron 21: 481–493, 1998 [DOI] [PubMed] [Google Scholar]
- Strettoi et al., 1990. Strettoi E, Dacheux RF, Raviola E. Synaptic connections of rod bipolar cells in the inner plexiform layer of the rabbit retina. J Comp Neurol 295: 449–466, 1990 [DOI] [PubMed] [Google Scholar]
- Strettoi et al., 1992. Strettoi E, Raviola E, Dacheux RF. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J Comp Neurol 325: 152–168, 1992 [DOI] [PubMed] [Google Scholar]
- Suzuki et al., 1990. Suzuki S, Tachibana M, Kaneko A. Effects of glycine and GABA on isolated bipolar cells of the mouse retina. J Physiol 421: 645–662, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swets et al., 1961. Swets J, Tanner WP, Jr, Birdsall TG. Decision processes in perception. Psychol Rev 68: 301–340, 1961 [PubMed] [Google Scholar]
- Swets, 1961. Swets JA. Detection theory and psychophysics: a review. Psychometrika 26: 49–63, 1961 [DOI] [PubMed] [Google Scholar]
- Tsukamoto et al., 2001. Tsukamoto Y, Morigiwa K, Ueda M, Sterling P. Microcircuits for night vision in mouse retina. J Neurosci 21: 8616–8623, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyk et al., 2009. van Wyk M, Wässle H, Taylor WR. Receptive field properties of ON- and OFF-ganglion cells in the mouse retina. Vis Neurosci 26: 297–308, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veruki and Hartveit, 2002. Veruki ML, Hartveit E. Electrical synapses mediate signal transmission in the rod pathway of the mammalian retina. J Neurosci 22: 10558–10566, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völgyi et al., 2004. Völgyi B, Deans MR, Paul DL, Bloomfield SA. Convergence and segregation of the multiple rod pathways in mammalian retina. J Neurosci 24: 11182–11192, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle, 2004. Wässle H. Parallel processing in the mammalian retina. Nat Rev Neurosci 5: 747–757, 2004 [DOI] [PubMed] [Google Scholar]
- Wässle et al., 2009. Wässle H, Heinze L, Ivanova E, Majumdar S, Weiss J, Harvey RJ, Haverkamp S. Glycinergic transmission in the mammalian retina. Front Mol Neurosci 2: 6, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou and Dacheux, 2004. Zhou C, Dacheux RF. All amacrine cells in the rabbit retina possess AMPA-, NMDA-, GABA-, and glycine-activated currents. Vis Neurosci 21: 181–188, 2004 [DOI] [PubMed] [Google Scholar]