Abstract
While near-infrared (NIR) spectroscopy has been increasingly used to detect stimulated brain activities with an advantage of dissociating regional oxy- and deoxyhemoglobin concentrations simultaneously, it has not been utilized much in pain research. Here, we investigated and demonstrated the feasibility of using this technique to obtain whole brain hemodynamics in rats and speculated on the functional relevance of the NIR-based hemodynamic signals during pain processing. NIR signals were emitted and collected using a 26-optodes array on rat's dorsal skull surface after the removal of skin. Following the subcutaneous injection of formalin (50 μl, 3%) into a hindpaw, several isolable brain regions showed hemodynamic changes, including the anterior cingulate cortex, primary/secondary somatosensory cortexes, thalamus, and periaqueductal gray (n = 6). Time courses of hemodynamic changes in respective regions matched with the well-documented biphasic excitatory response. Surprisingly, an atypical pattern (i.e., a decrease in oxyhemoglobin concentration with a concomitant increase in deoxyhemoglobin concentration) was seen in phase II. In a separate group of rats with innocuous brush and noxious pinch of the same area (n = 11), results confirmed that the atypical pattern occurred more likely in the presence of nociception than nonpainful stimulation, suggesting it as a physiological substrate when the brain processes pain. In conclusion, the NIR whole brain imaging provides a useful alternative to study pain in vivo using small-animal models. Our results support the notion that neurovascular response patterns depend on stimuli, bringing attention to the interpretation of vascular-based neuroimaging data in studies of pain.
Keywords: formalin, neurovascular coupling, oxygenation, hemoglobin, nociception
pain perception is a result of a set of complicated processes involving a cerebral network: the primary/secondary somatosensory cortexes (SI/II), anterior cingulate cortex (ACC), thalamus, and midbrain periaqueductal gray (PAG). Modern neuroimaging technologies, such as positron emission tomography and functional magnetic resonance imaging (fMRI), allow us to detect activities at multiple brain sites in a parallel and noninvasive manner, which have profoundly advanced our understanding of neurophysiology of pain perception in the past decade (Kuo and Yen 2005; Lowe et al. 2007; Morrow et al. 1998; Seifert and Maihofner 2009; Tracey and Mantyh 2007).
However, there are two limitations of these technologies. First, they do not directly measure the neuronal electrical signal; instead, they detect only neighboring vascular dynamics. The physiological substrate of the vascular dynamics during neuron functioning (often referred as to neurovascular coupling) is not clearly established, so in some cases the interpretation of neuroimaging data will run into difficulties. For example, fMRI uses the blood oxygen level-dependent (BOLD) signal to quantify brain activity (Ogawa et al. 1990). In general, there is a tight neural correlate of BOLD signal as demonstrated using simultaneous extracellular recording techniques (Goense and Logothetis 2008; Logothetis et al. 2001). However, a paradoxical negative BOLD signal is pervasively found in the brain during functional activation (Lui et al. 2008; Mohr et al. 2005). Contradictory findings indicate that a negative BOLD is associated with both inhibition (Pasley et al. 2007; Shmuel et al. 2006) and excitation of neural activities (Schridde et al. 2008). Second, the low sampling rate is another weakness (in the order of a couple of seconds). The poor temporal resolution hinders the capability of determining the onset and temporal dynamics of activity at each brain site (in relation to pain processing for instance), which limits these techniques in studies to reveal how the brain network functions at a system level.
To overcome these limitations and boost our understanding of the mechanism of pain perception, some novel neuroimaging techniques may need to be explored, such as the functional near-infrared imaging (fNIRI). In contrast to other methods, fNIRI provides a relatively high sampling rate for assessing the cerebrovascular oxygen dynamics by measuring oxy- ([HbO]), deoxy- ([Hb]), and total hemoglobin concentration ([HbT]) changes (Bartocci et al. 2006; Slater et al. 2006). It is well known that physiologically [HbT] is equal to the summation of [HbO] and [Hb]. These hemodynamic parameters are often correlated well to the local field potential (Horovitz and Gore 2004; Rovati et al. 2007; Takeuchi et al. 2009) and the BOLD signal in the human brain (Huppert et al. 2006; Strangman et al. 2002; Toronov et al. 2007). This technique has already been broadly used in a variety of studies in the area of neuroscience, but with very limited applications to pain research (Bartocci et al. 2006; Becerra et al. 2008; Slater et al. 2006), particularly in animal studies using a well-established animal model. It is noteworthy that fNIRI is mainly used to detect cortical activities due to the restriction that near-infrared (NIR) light can penetrate only a few centimeters of the tissue because the largest source-detector (S-D) separation cannot be beyond 3–4 cm. In a small-animal model, this constraint becomes less critical since the largest S-D separation required for an animal head is usually in the range of 1.5–2.5 cm, and thus hemodynamic responses from both superficial and deep brain areas can be detected.
The objective of our current work was to determine whether an fNIRI-based whole brain imaging method can be used for studies of pain in rats. Particularly, our focus was on whether the fNIRI-based hemodynamic parameters are good indicators of the brain activation during pain processing. Two groups of rats were used in the study. In Group A, formalin was injected to a hindpaw as a long-lasting noxious stimulus. In Group B, mechanical brushing and pinching were applied to the same area as a nonpainful control and a brief noxious stimulus, respectively. Analyses on spatial and temporal profiles of hemodynamic brain images in [HbO], [Hb], and [HbT] (relative change; in arbitrary unit) were performed to reveal any pain-related hemodynamic pattern in comparisons among different stimulation modalities.
MATERIALS AND METHODS
Animal preparation.
In the first experiment with formalin injection (Group A), six male Sprague-Dawley rats were used. Their mean age was 118.5 ± 23.4 days (mean ± SE), and mean weight was 365 ± 27.1 g. In the second experiment with mechanical stimulations (Group B), 11 male Sprague-Dawley rats were used with a mean age of 96 ± 1.3 days and a mean weight of 358 ± 7.7 g. All animals were initially anesthetized by intraperitoneal injection of pentobarbital sodium solution (50 mg/kg). A PE10 tubing was inserted into the jugular vein for continuous intravenous administration of pentobarbital sodium (5 mg/ml) at a fixed rate of 0.02 ml/min to maintain anesthesia during data acquisition (Peng et al. 1996a). Accumulated evidence indicates that rats under this type of anesthesia show steady neural responses, heart rate, and peripheral cutaneous blood flow for hours (Hagains et al. 2011; He et al. 2010; He et al. 2011). It is thereby suggested that the rat's cardiovascular and nervous systems are physiologically stable. An incision was made along the midline of the scalp, and the skin flaps were reflected to expose the dorsal part of the skull. The rat head was positioned onto a stereotaxic frame for preventing motion. An electrical cautery pen (Bovie, Aaron Medical) was used to cauterize bleeding, if necessary. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas at Arlington and followed the guidelines written by the Committee for Research and Ethical Issues of IASP (Zimmermann 1983).
Instrumentation.
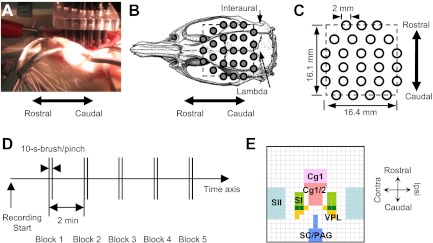
A commercialized continuous-wave NIRI (DYNOT, NIRx Medical Technologies) was used to continuously measure hemodynamics of the rat brain. Twenty-six bifurcated optodes (2 mm in diameter) were utilized at two wavelengths of light (760 and 830 nm). They were arranged by a plastic frame above the skull after the removal of skin (Fig. 1, A–C). Placement of the optode-array matched natural landmarks, i.e., sutures. Detailed descriptions on optode arrangements and placements can be found in the Supplemental Supporting Information (The online version of this article contains supplemental data). The sampling rate was 2 Hz.
Fig. 1.
Instrumentation and experiment procedures. A: photo presentation of optode placement on a rat dorsal skull after the scalp was removed. B: the locations of 26 optodes on the rat skull (Paxinos and Watson, 1998). C: size of a reconstructed image (noted by dash line). D: a 5-block design for 10-s brush and pinch. E: segmentation of regions of interest (ROIs) in a 21-by-21-pixel tomographic image based on a rat atlas (Paxinos and Watson 1998). Image orientations: up, rostral; bottom, caudal; right, ipsilateral; left, contralateral. Abbreviation: Cg1, dorsal cingulate cortex area 1; Cg2, ventral cingulate cortex; SI, primary somatosensory cortex for the hindlimb; SII, secondary somatosensory cortex; VPL, ventral posterolateral nucleus of thalamus; SC, superior colliculus; PAG, periaqueductal gray.
Formalin injection.
In Group A, after a 5-min baseline measurement, formalin solution (50 μl, 3%) was injected subcutaneously into the center of the plantar area of a rat's hindpaw unilaterally. The injection side was selected pseudorandomly: three rats were injected on the right side, and three on the left side.
Mechanical brushes and pinches.
In Group B, brush and pinch were applied on the plantar surface of a rat's hindpaw unilaterally. Innocuous brush was briefly sweeping a camel hair bush in a rhythmic fashion for 10 s, whereas noxious pinch was a constant pressure for 10 s using a straight arterial bulldog clamp (Ativanichayaphong et al. 2008; Peng et al. 1996b). Both stimulations were conducted in a block design (Fig. 1D). Five consecutive brushes or pinches were applied on the same side of the paw with an interval of 2 min. When all brushes were completed, a 5-min baseline was established before pinches were applied on the same hindpaw. The manipulation side was selected pseudorandomly: six rats received stimuli on the left, and five on the right.
Image reconstruction.
Two distinct methods, tomography and topography, were used to reconstruct brain images from optical signals. Tomography provides a series of two-dimensional (2D) images at lateral layers (i.e., in axial view) of the rat's brain by taking into account entire data sets from all S-D pairs, which include the adjacent (∼3 mm in separation), far apart (∼18.8 mm in separation), and every other optode pairs in between. In principle, it produces a 2D reconstructed image of the rat's brain at a selected depth, where the optical signals are most sensitive. To create the tomography, it usually requires a mathematical model (e.g., a diffusion theory) and complex computations (Arridge 1999; Tian et al. 2009a). In our study, tomography was produced for both Groups A and B by using an open-source software HOMER (PMI; http://www.nmr.mgh.harvard.edu/PMI/resources/homer/home.htm) under Matlab (MathWorks). A 2D tomographic image was composed of 21 horizontal by 21 vertical pixels. In contrast, topography provides an overall response pattern from the area underneath a limited number of S-D pairs. Topography requires relatively simple calculations without use of the diffusion theory. Topography was generated only for Group A (using Matlab). The details of procedures for both methods can be found in the Supplemental Supporting Information.
Identification and segmentation of regions of interest.
For Group A, after NIR tomography was created, areas showing the regional maximum changes in a continuous fashion over time were identified as regions of interest (ROIs; see Statistical analyses for details). It is noteworthy that depths of the 2D tomographic images used for statistical assessments were determined by locations of ROIs and the prior knowledge on the anatomy of well-established brain networks that are associated with pain and/or innocuous somatosensory processing (see Table 1 in results).
Table 1.
Statistics of the hemodynamics over time in [HbO], [Hb], and [HbT] from five ROIs
| ROI | Area | [HbO] | [Hb] | [HbT] | Image Depth, mm | Center Location |
|---|---|---|---|---|---|---|
| 1 | Anterior midline | F(10,50) = 12.2 | F(10, 50) = 7.63 | F(10,50) = 0.97 | 3 | A maximal increase in [HbO] at 1 min |
| P < 0.001* | P < 0.001* | P = 0.48 | ||||
| 2 | Contralateral | F(10,50) = 5.42 | F(10,50) = 3.62 | F(10,50) = 3.0 | 2 | A maximal decrease in [HbO] at 10 min |
| P < 0.001* | P = 0.001* | P = 0.98 | ||||
| 3 | Ipsilateral | F(10,40) = 17.9 | F(10,40) = 11.6 | F(10,40) = 5.84 | 6 | A maximal increase in [HbO] at 1 min |
| P < 0.001* | P < 0.001* | P < 0.001* | ||||
| 4 | Central midline | F(10,50) = 11.4 | F(10,50) = 0.99 | F(10,50) = 1.77 | 6 | A maximal decrease in [Hb] at 10 min |
| P < 0.001* | P = 0.46 | P = 0.091 | ||||
| 5 | Posterior midline | F(10,50) = 13.8 | F(10,50) = 9.30 | F(10,50) = 0.62 | 6 | A maximal increase in [HbO] at 1 min |
| P < 0.001* | P < 0.001* | P = 0.79 |
[HbO], [Hb], and [HbT]: oxy-, deoxy-, and total hemoglobin concentrations, respectively. One-way within-subject ANOVA was utilized to assess each hemodynamic over 11 time levels, including a 5-s baseline, and 5- to 50-min periods postinjection in 5-min bins. The depths of tomography for each region of interest (ROI) are shown, along with brief explanations of the criteria to determine the ROIs. Considering that the combined thickness of a rat skull, other soft tissues, and space is ∼1 mm, the selected depths were 2 mm for primary somatosensory cortex (SI), 3 mm for anterior cingulated cortex (ACC), and 6 mm for secondary somatosensory cortex (SII), ventral posterolateral nucleus (VPL), and periaqueductal gray (PAG).
Significant difference.
For Group B, due to the short duration of regional hemodynamic responses to brush or pinch (if they exist), the previous criteria (for Group A) of using the temporal continuum and local maximum change to identify a ROI may not be proper. Considering the noisylike perturbation over time and the interference from neighboring area showing a strong response, using only few consecutive time points simply gives less power to attribute a local maximum change to the activation of a brain area. To help ascertain what brain areas were involved, we created a handful of segments in the 2D tomographic image based on a rat atlas (Paxinos and Watson 1998). These segments correspond to the brain areas in tight relation to pain and/or nonpainful somatosensory processing and to the brain areas that showed responses in Group A (Fig. 1E). In particular, nine nuclei in both ipsilateral and contralateral hemispheres were segmented. In addition to the ACC, SI/II, and thalamus, the superior colliculus (SC) was included for brush, because the SC is believed to be a critical region involved in integrating multiple innocuous somatosensory inputs from the periphery, e.g., hindlimbs (Abrahams et al. 1988; Clemo and Stein 1991; Wallace et al. 1996). As it lies dorsally to the PAG, the brush-induced hemodynamics in the posterior midline area should be attributed to the SC, but not the PAG. Within the thalamus, the ventral posterolateral nucleus (VPL) was targeted, because it serves as a critical relay center to convey somatosensory input from the spinothalamic tract to cortex (Boivie 1971; Dykes et al. 1988; Harris 1978; Harris 1980).
Thus in the data analysis for Group B, the ROIs were predetermined based on findings from Group A and the usage of segmentation. We no longer had adequate power to declaim the spatial specificity of a regional hemodynamic response. However, because we sampled a large number of areas (9 in total) that were constrained with the spatial specificity individually, we gained more power to reveal complete temporal characteristics of the brain hemodynamics during functional activation.
Statistical analyses.
For Group A, to investigate spatial profiles of hemodynamic responses for identification of ROIs, one-sample t-tests were used for each pixel in tomographic images (as well as in each subdivision of topographic images) to assess statistical significances of hemodynamic changes at several given time points with one-tail assumption (Matlab). A mean-value image was generated in consideration of statistical significances on a pixel-by-pixel basis over all animals (Matlab). It tells where responses occur and how strongly they respond. Then a 3 × 3-pixel square (where the center pixel was determined by the regional maximum) in the mean-value image was defined as the ROI for Group A, and an average over the 9-pixel area was the dependent variable for further analyses on temporal characteristics of hemodynamics in [HbO], [Hb] and [HbT], respectively. For Group B, after spatially averaging within each segment (i.e., averaging over all pixels within each segment), the maximum response in the initial 20-s window [maximum (Max) value over time] and integrated response in the initial 30-s window [area under the curve over time (Area)] were obtained for each segment (i.e., a predetermined ROI). One-sample t-tests were used to assess any significant changes in Max and Area from all nine ROIs in three hemodynamic parameters using SAS.
To investigate temporal profiles of hemodynamic responses during functional activation, two different methods were used for Groups A and B, respectively. For Group A, one-way within-subject ANOVAs with 10 temporal levels (i.e., 5-s baseline and 5–50 min postinjection with a bin size of 5 min) were utilized to assess any statistically significant change as a function of time. Post hoc multiple comparisons between baseline and all the other time levels were performed with Bonferroni correction, if a main effect occurred. For Group B, the linear correlation between [HbO] and [Hb] changes and nonparametric chi-square tests were utilized to assess how often a pair of directional regional [HbO] and [Hb] changes (i.e., a dyad) occurred in response to brush or pinch as a function of time, and whether there was any difference between two stimuli. ANOVAs and post hoc tests were performed using SAS. Correlation calculations and chi-square tests were performed with SPSS 17.0. Alpha = 0.05. All data were expressed as means ± SE.
RESULTS
Comparisons between topography and tomography.
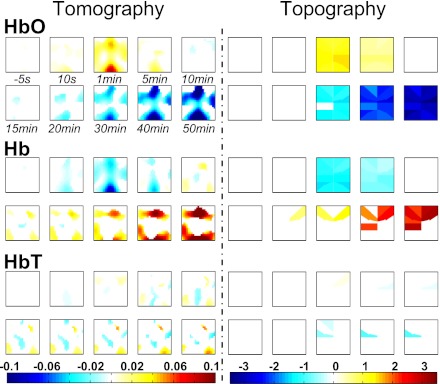
Hemodynamic changes of the rat's brain following formalin injection are presented in tomography (Fig. 2, left) and topography (Fig. 2, right). Side-by-side comparisons between two reconstruction methods indicate that 1) a short-term increase in [HbO] tended to occur within the first 5 min postinjection, followed by a long-term monotonic decrease over the whole brain (Fig. 2, top row); 2) a short-term decrease in [Hb] tended to occur within the first 5 min postinjection, followed by a long-term monotonic increase over nearly the entire brain (Fig. 2, middle row); and, finally, 3) a decrease in [HbT] tended to occur at some isolated areas after 5 min postinjection (Fig. 2, bottom row). Because the tomography used 100% of total S-D pairs (676) and used a mathematical model of the diffuse light, whereas the topography only used 1.8% (12 out of 676; see Supplemental Supporting Information for details), it is expected that the spatial resolution of the tomography was better than that of the topography. More importantly, comparisons demonstrate the validity of the mathematical model used for the tomography in our experimental settings. Based on the tomography, five ROIs in the anterior midline, lateral, central midline, and posterior midline areas were identified for further analyses on their temporal profiles (see criteria of finding the regional maximums in the last column of Table 1).
Fig. 2.
Comparisons between tomography (left; at a depth of 5 mm below the optode-array) and topography (right). On each panel, there are 3 rows: top (oxyhemoglobin concentration [HbO]), middle (deoxyhemoglobin concentration [Hb]), and bottom (total hemoglobin concentration [HbT]), each of which has 10 images at selected time points. The intensity of hemodynamic response is color-coded only if it reached a statistically significant level: red indicates a significant increase; blue a decrease; white nonsignificance (n = 6). Color map was generated in a linear manner as a function of the intensity of change. Image orientations: right, ipsilateral side; left, contralateral; top, rostral; bottom, caudal.
Temporal profiles of five ROIs associated with formalin injection.
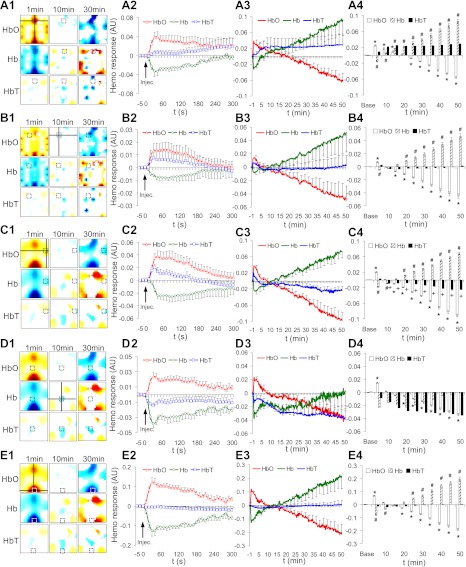
To demonstrate time courses of regional hemodynamic responses in relation to formalin-induced nociception and spatial specificity, five ROIs were analyzed separately. For each ROI, the spatial location is highlighted by a cross line (Fig. 3, A1–E1). The initial 300-s (Fig. 3, A2–E2) and the entire 50-min (Fig. 3, A3–E3) hemodynamic responses are presented, respectively. For a better visualization, error bars are shown at only a fraction of total time points. After grouping time points into 11 bins (Fig. 3, A4–E4), ANOVAs (Table 1) and post hoc analyses with Bonferroni error correction were performed.
Fig. 3.
Temporal profiles of hemodynamic changes in five ROIs: the anterior midline (A), contralateral (B), ipsilateral (C), central midline (D), and posterior midline (E) areas (ipsilateral: n = 5; all other: n = 6). Dependent variables were hemodynamic changes in average over 3-by-3-pixel ROI in [HbO], [Hb], and [HbT] (shown in first column, A1–E1). See Table 1 for the definition of each ROI. Time courses are plotted within the first 300 s (second column, A2–E2), the full 50 min (third column, A3–E3), and another full 50 min in 5-min bin (fourth column, A4–E4). AU, arbitrary units. Post hoc significant differences (P < 0.05) against baseline are noted: *[HbO], #[Hb], and + [HbT].
In general, there were significant changes in [HbO] over time at all five ROIs. Specifically, a significant initial increase was found at 5 min postinjection (open bars in Fig. 3, A4–E4). This effect returned to the baseline level between 10 and 20 min. Immediately after that, a continuous monotonic decrease persisted up to 50 min. Significant changes in [Hb] over time were also found at all ROIs, except the central midline area (Table 1). Particularly, significant initial decreases were observed at 5 min postinjection (hatched bars in Fig. 3, A4–C4 and E4). Similar to [HbO], this effect returned to the baseline level between 10 and 20 min, followed by a continuous monotonic increase up to 50 min. Unlike the [HbO] or [Hb], the regional [HbT] failed to show any significant changes in all ROIs, except in the ipsilateral area, where a decrease occurred at 10 min and sustained up to 50 min (solid bars in Fig. 3, C4). It is noteworthy that one animal was excluded in the analysis on the ipsilateral ROI, because a sudden stepwise regional increase in [HbT] was observed at 10 min, and later bleeding was found at the same site after removal of the optode array at the end of the experiment.
Spatial implications of ROIs associated with formalin injection.
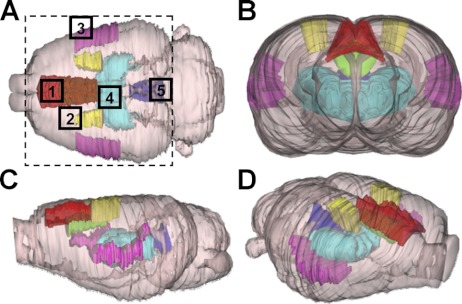
To help ascertain what brain regions are the anatomical substrates of ROIs, a three-dimensional rat brain atlas was used (Atlas3D, NeSys, Oslo, Norway). As shown in Fig. 4, five well-known pain processing-related brain nuclei are highlighted in different colors at various angles. There are substantial overlaps between the pain-processing network and the ROIs that were identified by fNIRI following formalin injection.
Fig. 4.
Spatial implications of ROIs based on a three-dimensional rat atlas (Atlas3D, NeSys, Oslo, Norway) in the dorsal-ventral (A), coronal (B), sagittal (C), and a dorsolateral (D) orientation. Several pain processing-related brain regions are labeled in colors: red for the dorsal ACC (Cg1); green for the ventral ACC (Cg2); yellow for the SI; pink for the SII; light blue for the thalamus; and blue for the PAG. Size of the tomography is shown by a dashed rectangle. Areas 1–5: the anterior midline, contralateral, ipsilateral, central midline, and posterior midline ROIs, respectively.
First, the anterior midline ROI (region 1 in Fig. 4A) completely overlaps the anterior portion of the Cg1 (in red), and some part of the Cg2 (in green; beneath the Cg1; visible in Fig. 4, B and C). Second, the contralateral ROI (region 2) overlaps the anterior tip of the SI (in yellow). Third, the ipsilateral ROI (region 3) moderately overlaps the anterior portion of the SII (in pink). As shown in Fig. 2, several tomographic images in [HbO] and [Hb] indicate simultaneous responses in a contralateral region (a symmetrical area of region 3). This area is located more laterally than region 2 in the same hemisphere and partially overlaps the anterior contralateral SII. Fourth, the central midline ROI (region 4) completely overlaps the thalamus (in light blue). Finally, the posterior midline ROI (region 5) heavily overlaps the posterior port of the PAG (in blue).
Spatial and temporal profiles of ROIs associated with brush and pinch.
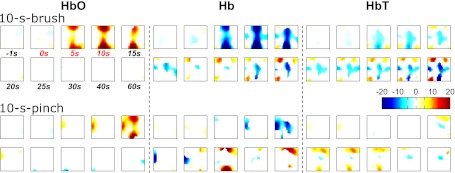
To determine a pain-related hemodynamic pattern, short-term mechanical stimuli were applied to a new animal group. Hemodynamic changes of the rat's brain associated with the innocuous brush (Fig. 5, top) and noxious pinch (Fig. 5, bottom) are presented in tomography. In general, both stimuli tended to produce short-term regional hemodynamics; the brush-induced hemodynamics tended to be larger in brain area than the pinch-induced hemodynamics. In view of the ROIs identified by the formalin protocol, to further investigate the role of each specific brain region in pain processing, we used a segmentation method. Time courses of regional responses from all regions are illustrated in Figs. 6 and 7 for brush and pinch, respectively. It is noteworthy that 1 rat out of 11 was removed for brush, because of an irregular (>1 min) global decrease in [HbO] and an increase in [Hb]. The magnitudes of such responses at some regions were as great as 8.19-fold of standard deviation over all other subjects. Thus the sample size for brush was 10, and the size for pinch remained 11.
Fig. 5.
Hemodynamic responses to innocuous 10-s brush (top row; n = 10) and noxious 10-s pinch (bottom row; n = 11) in tomography. [HbO], [Hb], and [HbT] changes are in AU. The reconstruction depth was 5 mm. Color map and image orientation are the same as in Fig. 2.
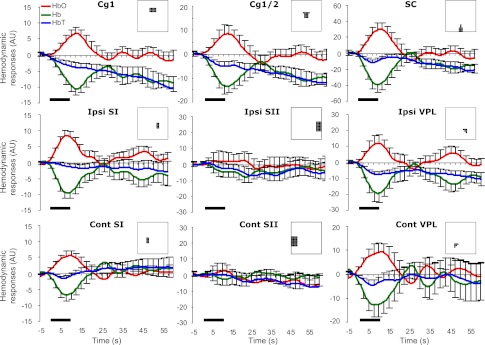
Fig. 6.
Temporal profiles of hemodynamic responses to brush from nine ROIs. Sampling rate was 2 Hz. The error bars (i.e., SE) are shown every 2.5 s for better visualization. The bold line indicates 10-s brush. The inset in each subfigure describes the segmentation corresponding to a ROI.
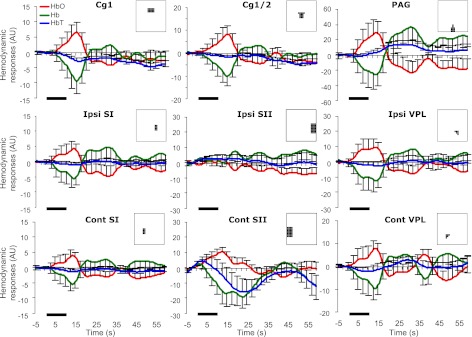
Fig. 7.
Temporal profiles of hemodynamic responses to pinch from nine ROIs. The bold line indicates 10-s pinch. The inset in each subfigure describes the segmentation corresponding to a ROI.
According to statistics, brush tended to elicit a handful of regional hemodynamic responses, which were prominently located on the midline. A typical increase in [HbO] with a decrease in [Hb] was found in the ACC, bilateral SI, and SC, but not in the SII (Table 2). In the VPL, an increase in [HbO] was indicated only in the contralateral hemisphere, whereas a decrease in [Hb] was shown bilaterally. A decrease in [HbT] was found in the ACC, ipsilateral VPL, and SC. Moreover, lateralization was found in the SI {pairwised t-test; Area in [Hb]: t(9) = −1.91, P = 0.044}, indicating that the ipsilateral SI showed a greater decrease in [Hb] than the contralateral SI.
Table 2.
A summary of [HbO], [Hb], and [HbT] changes from nine ROIs in response to brush
| HbO |
Hb |
HbT |
||||
|---|---|---|---|---|---|---|
| Region | Max | Area | Max | Area | Max | Area |
| Cg1 | 7 ± 3.1* | 147.8 ± 70.3* | −12.5 ± 2.2* | −374 ± 73* | −4.7 ± 1.7* | −226.2 ± 81.2* |
| Cg1/2 | 10.3 ± 5* | 172.7 ± 123.4 | −17.8 ± 3* | −475.8 ± 101.6* | −7.8 ± 2.1* | −303.3 ± 109.8* |
| SI | 10.2 ± 1.3* (I) | 212.3 ± 74.5* (I) | −11.2 ± 2.2* (I) | −286.7 ± 84.2* (I) | −2.4 ± 1.5 (I) | −74.6 ± 69.3 (I) |
| 5.9 ± 2.6* (C) | 126.1 ± 61.2* (C) | −6.3 ± 3* (C) | −114 ± 61.1* (C) | −0.4 ± 1.6 (C) | 12.1 ± 59.4 (C) | |
| SII | 4.5 ± 7.3 (I) | 52.4 ± 259.6 (I) | −7.2 ± 5.1 (I) | −255.4 ± 167.4 (I) | −5.6 ± 4.3 (I) | −203 ± 156.6 (I) |
| −0.5 ± 5.9 (C) | −58.9 ± 190.2 (C) | −3.2 ± 6.5 (C) | −30.3 ± 232.8 (C) | −4.9 ± 5.5 (C) | −89.2 ± 178 (C) | |
| VPL | 11.8 ± 6.9 (I) | 225 ± 199.8 (I) | −20.9 ± 6.6* (I) | −564.9 ± 237* (I) | −10.3 ± 2.3* (I) | −339.9 ± 103.6* (I) |
| 12.2 ± 6.4* (C) | 215.4 ± 139.6 (C) | −17.3 ± 6.2* (C) | −349.9 ± 203.6 (C) | −4.9 ± 4.6 (C) | −134.8 ± 188.4 (C) | |
| SC | 33.2 ± 12.7* | 693.2 ± 318.7* | −46.5 ± 10.2* | −1125.6 ± 308.1* | −12.1 ± 4.1* | −432.4 ± 201.1* |
Numerical values are shown as means ± SE in arbitrary units (n = 10). Note: Cg1, dorsal cingulated cortex area; Cg1/2, ventral cingulated cortex; Max, the maximum between 0 and 20 s; Area, the integrated area between 0 and 30 s; I, ipsilateral; C, contralateral.
P < 0.05.
In contrast, pinch tended to elicit hemodynamic responses in the contralateral SII (Table 3). Similar to brush, a decrease in [HbT] was found in the Cg1 and contralateral SI. Lateralization was found in the SI {Max in [HbT]: t(10) = 1.87, P = 0.045} and SII {Area in [HbO]: t(10) = −2.50, P = 0.016}. To investigate a pain-related response pattern from the regions showing significant hemodynamics during both stimuli, pairwise comparisons were made. However, no difference was found in the Cg1 (Max in [HbT]: P = 0.22; Area in [HbT]: P = 0.12), or in the contralateral SI (Max in [HbT]: P = 0.25; Area in [HbT]: P = 0.21). Nevertheless, it is noted that a delayed response tended to occur in the PAG between 20 and 35 s (Fig. 7). In such a time window, a significant increase was confirmed [Max: 17.9 ± 5.6 arbitrary units (AU), t(10) = 3.18, P = 0.005; Area: 360.6 ± 155.1 AU, t(10) = 2.32, P = 0.02]. This finding suggests a region-dependent temporal pattern of [HbT] change in response to the noxious pinch. To control errors in statistical analyses, we tried to avoid systemic selections of multiple time windows to process the same data, and, therefore, no further manipulation on time window was made.
Table 3.
Summary of [HbO], [Hb], and [HbT] changes from nine ROIs in response to pinch
| HbO |
Hb |
HbT |
||||
|---|---|---|---|---|---|---|
| Region | Max | Area | Max | Area | Max | Area |
| Cg1 | 2.7 ± 4.5 | 51.3 ± 118.1 | −4.4 ± 5.4 | −138.5 ± 143 | −2.7 ± 1* | −87 ± 33* |
| Cg1/2 | 3.9 ± 5.3 | 88.3 ± 136.1 | −4.7 ± 5.8 | −144.1 ± 152.7 | −1.4 ± 1 | −55.6 ± 36.6 |
| SI | 0.7 ± 4.5 (I) | −5.7 ± 99.7 (I) | −1.5 ± 5.2 (I) | −32.8 ± 124.6 (I) | −0.8 ± 0.9 (I) | −27.2 ± 35.7 (I) |
| 0.7 ± 3.6 (C) | 5.3 ± 79.8 (C) | −1.5 ± 4.3 (C) | −48 ± 105.8 (C) | −1.6 ± 0.8* (C) | −42.7 ± 34.2 (C) | |
| SII | −0.4 ± 5.8 (I) | −45.3 ± 161.9 (I) | 4.6 ± 6.4 (I) | 150 ± 203.1 (I) | 2.7 ± 3.5 (I) | 104.7 ± 115.2 (I) |
| 7.3 ± 4.7 (C) | 313.7 ± 146.2* (C) | −17.5 ± 8.8* (C) | −662.1 ± 318.3* (C) | −7.2 ± 9.8 (C) | −348.3 ± 354.2 (C) | |
| VPL | 9 ± 8.8 (I) | 136.2 ± 178.8 (I) | −10 ± 11 (I) | −195.5 ± 268.7 (I) | −1.6 ± 3.4 (I) | −59.3 ± 134.1 (I) |
| −4.3 ± 7.3 (C) | 49.5 ± 175.7 (C) | −1.9 ± 8.4 (C) | −77.1 ± 185.8 (C) | −0.7 ± 2.7 (C) | −27.7 ± 87.6 (C) | |
| PAG | 13.9 ± 23.3 | 328.8 ± 646.3 | −10.6 ± 25.8 | −23.9 ± 686.8 | 7.5 ± 5.7 | 305 ± 190.4 |
Numerical values are in means ± SE in arbitrary units (n = 11).
P < 0.05.
A reliable index of brain functioning: the linearity between [HbO] and [Hb] changes.
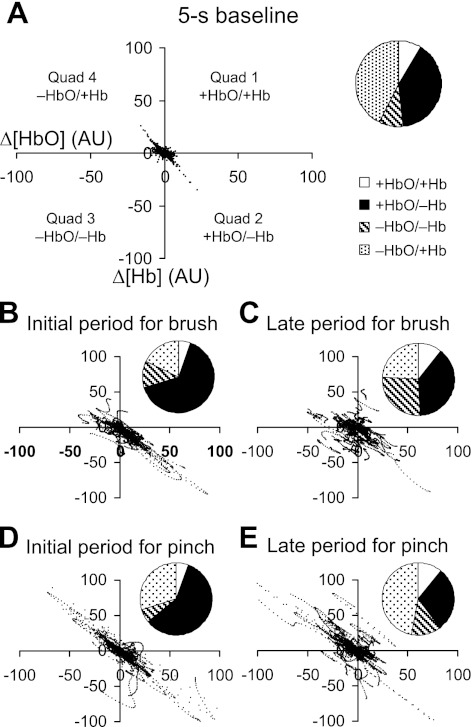
Unexpectedly, the pinch did not elicit a significant [HbO] or [Hb] change in the ACC, SI, and thalamus, where both the brush and formalin did. To find an explanation on such discrepancy, we investigated the relation between [HbO] and [Hb] changes. Regional [HbO] and [Hb] changes were paired as dyads, which were categorized into four kinds: increases in both components [+HbO/+Hb; quadrant 1 (Q1) in Fig. 8A], an increase in [HbO] with a decrease in [Hb] (+HbO/−Hb; Q2), decreases in both components (−HbO/−Hb; Q3), and a decrease in [HbO] with an increase in [Hb] (−HbO/+Hb; Q4). Five temporal levels were defined: a 5-s baseline (Fig. 8A) and an initial (0–20 s) and late periods (40–60 s) for the brush (Fig. 8, B and C) and pinch (Fig. 8, D and E), which indicate different functional statuses of the brain.
Fig. 8.
Distributions of the HbO and Hb dyads at five functionally distinct time levels. They include a baseline (−5–0.5 s), initial (0–20 s), and late (40–60 s) periods for brush and pinch. Each dyad indicates a pair of simultaneous [HbO] and [Hb] changes from one of nine ROIs. A pie chart demonstrates the percentages of the four kinds of dyads at each time level. Note: Q1, open area; Q2, dark area; Q3, hatched area; Q4, dotted area.
During the baseline and initial periods for the brush and pinch, more than 80% of total dyads were either +HbO/−Hb (Q2) or −HbO/+Hb (Q4; see column 5 in Table 4). A significant linear relationship between [HbO] and [Hb] changes is seen and confirmed by the Pearson correlation coefficient and the slope (columns 6 and 7 in Table 4). Particularly, the negative slope indicates that [HbO] and [Hb] changes were constantly opposite to each other. On the contrary, when the brain was not engaged in a functional task, e.g., during the late periods for the brush and pinch, there were more incidences of +HbO/+Hb (Q1) and −HbO/−Hb (Q3) over the brain. Also, the linearity between [HbO] and [Hb] changes suffered. Therefore, the substantial negative linear relationship between [HbO] and [Hb] tends to be a possible cerebrovascular signature of brain functioning.
Table 4.
Distributions of the HbO and Hb dyads and the linearity between [HbO] and [Hb] changes
| Quad 1: ΔHbO >0, ΔHb >0, % | Quad 2: ΔHbO >0, ΔHb <0, % | Quad 3: ΔHbO <0, ΔHb <0, % | Quad 4: ΔHbO <0, ΔHb >0, % | Quads 2 and 4, % | Pearson Correlation | Slope (Mean ± SE) | |
|---|---|---|---|---|---|---|---|
| Baseline (−5 to –0.5 s) | 8.6 | 39.7 | 9.23 | 42.43 | 82.1 | −0.79 | −0.91 ± 0.01 |
| Brush (0–20 s) | 5.2 | 65.2 | 12 | 17.7 | 82.9 | −0.87 | −0.91 ± 0.01 |
| Brush (40–60 s) | 10.7 | 38.5 | 26.7 | 24.1 | 62.6 | −0.39 | −0.46 ± 0.02 |
| Pinch (0–20 s) | 5.5 | 58.8 | 5.7 | 30 | 88.8 | −0.91 | −1.04 ± 0.01 |
| Pinch (40–60 s) | 10.8 | 29 | 13.1 | 47.1 | 76.1 | −0.75 | −0.88 ± 0.01 |
Δ, Change. Columns 1-4 illustrate the percentages of 4 kinds of dyads at different time levels. Column 5 shows the percentages of the combination of the +HbO/−Hb and −HbO/+Hb dyads. Columns 6 and 7 describe the linear relationship between [HbO] and [Hb] changes in terms of the Pearson correlation coefficient and slope.
Occurrence of −HbO/+Hb in favor of pain processing.
Both +HbO/−Hb and −HbO/+Hb dyads accounted for the negative linear relationship between [HbO] and [Hb] changes during functional activation. +HbO/−Hb appeared to be predominant. However, the noxious pinch induced more occurrences of −HbO/+Hb than the innocuous brush did.
Regardless of which stimulus was applied, the +HbO/−Hb dyads (Q2) constantly outnumbered the −HbO/+Hb (Q4) during functional activation, e.g., the initial periods for brush [65.2 vs. 17.7% of total dyads; χ2(1) = 1006.9, P < 0.0001] and pinch [58.8 vs. 30% of total dyads; χ2(1) = 378.3, P < 0.0001]. On the contrary, during baseline, such discrepancy disappeared [39.7 vs. 42.43% of total dyads; χ2(1) = 3.3, p > 0.05]. It is suggested that more +HbO/−Hb (Q2) tends to occur than −HbO/+Hb (Q4) in the presence of brain functioning.
In comparison with brush, pinch produced more −HbO/+Hb (Q4), as indicated in both the initial [30 vs. 17.7% of total dyads; χ2(1) = 426.4, p < 0.0001] and late periods [47.1 vs. 24.1% of total dyads; χ2(1) = 1168.4, P < 0.0001]. It is suggested that −HbO/+Hb (Q4) tends to occur more saliently (Fig. 8, D and E) and frequently in the presence of nociception than nonpainful stimulation. Because the +HbO/−Hb (Q2) component still counted for much during brain activation (e.g., ∼60% of total dyads in initial periods), the −HbO/+Hb (Q4) component (i.e., the opposite of +HbO/−Hb) could not reach to a statistically significant level. As it is, during the noxious pinch, no statistical significant changes were found over a broad area of the rat's brain, where changes were seen reliably during the innocuous brush (Fig. 5).
DISCUSSION
We have shown that the fNIRI provides whole brain hemodynamics with decent spatial and temporal resolutions in an in vivo rodent model. The significant regional hemodynamic responses from the ACC, SI/II, PAG/SC, and thalamus are in accordance with the well-established pain and nonpain somatosensory processing networks (Bornhovd et al. 2002; Chen et al. 2002; Maihofner and Kaltenhauser 2009; Peyron et al. 1999; Timmermann et al. 2001).
Particularly, in the nonpain somatosensory processing network, when action potentials are generated in the periphery, they are transmitted to the spinal cord via the primary afferents. The spinal cord further conveys sensory signals to various supraspinal structures via spinothalamic, spinomesencephalic, and other tracts. Within the thalamus, the VPL dominantly receives sensory input from the spinothalamic tract (Dykes et al. 1988). Within the midbrain, the mesencephalic SC contains multisensory neurons that are believed to process and integrate various sensory modalities from the spinal cord (Abrahams et al. 1988; Wallace et al. 1996). The sensory aspect of a stimulus (e.g., brush) is perceived when the SI receives inputs from the VPL (Dykes 1978). Meanwhile, as the mediodorsal and other subnuclei of thalamus deliver sensory signals to the prefrontal cortex (Berendse and Groenewegen 1991; Groenewegen et al. 1997; Groenewegen and Witter 2004; Rose and Woolsey 1948), the ACC and other prefrontal regions are activated, which are believed to initiate cognition, such as attention (Bornhovd et al. 2002; Buchel et al. 2002; Peyron et al. 1999).
In the pain processing network, at cortical level, the SII is believed to play an important role of discriminating pain, because the magnitude of its activity is positively correlated to the pain intensity (Bornhovd et al. 2002; Chen et al. 2002; Maihofner and Kaltenhauser 2009; Peyron et al. 2000; Timmermann et al. 2001). Similarly, our results indicate the activation of the SII in response to both noxious stimuli, e.g., mechanical pinch and formalin injection, but not to the innocuous brush. Furthermore, the temporal profile of hemodynamics from the SII, as well as from other ROIs, was correlated to the well-known biphasic response following formalin injection: an immediate short-term (phase I; within the first 5 min) excitatory period, followed by a quiescent period (10–15 min), and a later long-term (phase II; usually lasting for >1 h) excitatory period. This pattern was first described by behavioral measurements (e.g., licking and paw elevation) in conscious animals (Alreja et al. 1984; Dubuisson and Dennis 1977) and was later quantified by single-unit electrophysiological recordings of the spinal dorsal horn neurons in anesthetized animals (Dickenson and Sullivan 1987; Pitcher and Henry 2002). Our results demonstrate two periods of significant hemodynamic responses compared with the baseline (Fig. 3), which are in good agreement with the temporal profile of the biphasic excitatory response measured by behavioral and electrophysiological techniques.
The neurophysiological relevance of hemodynamics.
Historically, an increase in [HbO] with a concomitant decrease in [Hb] was often observed to be a typical hemodynamic signature of brain functioning (Villringer et al. 1993). fNIRI studies have provided converging evidence on such a hemodynamic pattern in related cortexes with visual (Schroeter et al. 2006; Seiyama et al. 2004), somatosensory (Becerra et al. 2008; Seiyama et al. 2004), and motor (Huppert et al. 2006) stimulations in human. It is not surprising that our results demonstrate a regional increase in [HbO] coupled with a decrease in [Hb] from a group of widely distributed and yet associated areas during the innocuous brush and the initial period postformalin injection. However, findings from recent studies have reported that the hemodynamic pattern of brain functioning tends to be brain region and task dependent. Instead of the conventional pattern, a reversed pattern {i.e., a decrease in [HbO] coupled with an increase or little change (Δ) in [Hb]} is found in human prefrontal cortex when subjects were performing anagram tasks (Tian et al. 2009b). A failure of finding the conventional pattern in human frontal area is also reported when participants were stimulated with thermal pain (Becerra et al. 2008). Those findings may share a similar neurovascular substrate with our results during pinch and the late period of postformalin injection, where the reversed pattern was shown substantially over the brain. The atypical reversed pattern was originally reported in human brain during functional activation and was hypothesized to be aging related (Villringer et al. 1993). Results of an age-matched study with young and elderly subjects fail to support this hypothesis (Mehagnoul-Schipper et al. 2002). On the other hand, Devor et al. (2007) utilized multimodal optical techniques on rat's somatosensory area and electrically stimulated the rat's forepaw. This study shows that both increase and decrease in Δ[HbO] can take place, depending on the locations of the measurement with respect to the stimulation site. Specifically, an increase in Δ[HbO] can occur near the center of stimulation, while a negative Δ[HbO] may take place at a distance of 4 mm from the stimulation site. Devor's study also utilized two photon microscopy to quantify changes in arterial diameter and observed vasodilation during Δ[HbO] increase and vasoconstriction during Δ[HbO] decrease. In the meantime, voltage-sensitive fluorescence dye measurements of neuronal activity were taken, showing an increased blood flow (higher activation) at the stimulation site due to depolarization, while hyperpolarization was observed at a distance of 4 mm from the same site. These results given by Devor et al. demonstrate clearly that specific neurotransmitter and neuropeptide release due to synaptic transmission gives rise to both arterial dilation and constriction, where the neuronal activity can be differentially engaged in the stimulation site and in the surrounding cortical columns. Their multimodal measurements show that an evoked decrease in Δ[HbO] could possibly be due to arterial constriction and inhibitory polysynaptic activity.
While the knowledge learned from Devor's study helps us understand the origin and possibility of having increases and decreases of Δ[HbO] during functional stimulations, the specific interpretation of the reversed hemodynamic response patterns seen during pinch and the late period of postformalin injection remain not completely clear. Since fNIRI has been employed with very limited applications to pain research (Bartocci et al. 2006; Becerra et al. 2008; Slater et al. 2006), particularly in animal studies, further studies are very much needed to interpret our observations of reversed hemodynamic response patterns. However, our present study does reveal an important and essential feature of cerebrovascular response to painful stimulation, namely, a decrease in [HbO] and increase in [Hb]. This feature is different from the conventional hemodynamic response patterns and possibly can serve as pain indicator or marker for future clinical applications.
Hypothetically, when the brain receives peripheral inputs (e.g., noxious formalin injection), neurons in associated brain areas (e.g., the thalamus, SI/II, ACC, and PAG) are activated with an increasing demand of oxygen. Local vasculatures respond to it by a series of potential actions: providing more HbO through arterial vasodilatation, increasing the speed of releasing oxygen from HbO, moving Hb to venules, and accelerating the transportation of Hb away from the site. As a result, an increase in [HbO] occurs with a decrease in [Hb]. If the cutaneous stimulation is relatively brief (e.g., in response to brush), the initial vascular responses may pump enough fuel for neural activities in the brain. Once the stimulus is removed, the local vascular response returns to normal. However, the formalin-induced response lasts for hours. As there is a constant demand for oxygen, the local vascular bed will reach to its limit and not be able to dilate any further. At this point, an initial increase in [HbO] cannot be maintained by vasodilatation and arterial blood influx. [HbO] decreases as the neuronal consumption of oxygen remains at high level. Meanwhile, the local vasculature cannot remove the accumulated Hb efficiently as its capability reaches to the ceiling level, resulting in an increase in [Hb]. Noxious input tends to activate more brain areas than innocuous input does, because there is an urgent need of coding the intensity of pain, initiating emotion arousals, and activating endogenous descending networks. To further test the proposed mechanism of the pain-induced neurovascular response patterns (e.g., in response to pinch and formalin), a simultaneous electrophysiological recording is needed at each associated area to confirm that both hemodynamic patterns are concurrent with neural activations.
Comparison between the results of fNIRI-based hemodynamics and BOLD fMRI signal.
The BOLD signal is influenced by numerous vascular parameters, including the cerebral blood flow, blood volume, and oxygen-related metabolic rate (Ogawa et al. 1990). These parameters are also correlated to hemodynamic components, such as [HbO], [Hb], and [HbT]. Human subject studies with simultaneous fMRI and fNIRI measures reveal a strong relationship between a positive BOLD response and a decrease in [Hb] with visual, somatic sensory stimulations, and motor tasks (Huppert et al. 2006; Mehagnoul-Schipper et al. 2002; Sakatani et al. 2007). A decrease in [Hb] from associated areas during noxious pinch and the initial period of postformalin injection are in line with the fMRI evidence. An enhanced BOLD signal is detected in the same regions in response to formalin injection (Shah et al. 2005) or other noxious stimuli (Hess et al. 2007; Malisza et al. 2003) in anesthetized rats. It is interesting to note that a fMRI study reports a monotonic increase in BOLD signal in a handful of associated areas in rats (Shah et al. 2005), which is contradictory to the curvilinear manner in our study (Fig. 3) and to the well-documented biphasic response pattern depicted by other methods. A possible explanation is that Shah et al. used a different anesthetic (halothane), while we used pentobarbital, similar to other groups in studying electrophysiological response to formalin injection. Future studies in pentobarbital-anesthetized rats with simultaneous measures by fNIRI, fMRI, and electrophysiological recording are needed to advance our understanding of the functional relationship between the BOLD and fNIRI signals during a long-lasting period of pain.
The relations among Δ[HbO], Δ[Hb], and Δ[HbT].
The conventional responses in fNIRI to functional stimulations are expected to have an increase in Δ[HbO] and Δ[HbT], with a decrease in Δ[Hb]. In fact, a transient regional increase in [HbT] was found in three areas (e.g., the ACC, SI, and SII) following an intense chemical stimulation, namely, the formalin injection into a hindpaw (Fig. 3). Apparently, our method is able to detect a regional blood volume change as other imaging techniques do, which often use visible light-based imaging methods (e.g., laser Doppler imager, intrinsic optical imaging, etc.) and report a reliable increase in blood volume/flow in SI associated with peripheral stimulations. However, we did not observe such a response pattern of an increase in Δ[HbT] during the mechanical stimulations (e.g., Figs. 6 and 7); there might be possible reasons, given several noticeable differences between our method and the others:
As mentioned earlier, the study by Devor et al. (2007) shows that an increase in Δ[HbO] can occur near the center of stimulation, while a negative Δ[HbO] may take place at a distance of 4 mm from the stimulation site. Along the same principle, Δ[HbT] shows an increase at the stimulation site and a decrease a few millimeters away from the same site.
Since the penetration depth of visible light is much less than that of the NIR light, the increase in cerebral blood volume or flow measured by visible light-based methods (laser Doppler and intrinsic optical imaging) is most likely to reflect the superficial layer of cortical areas. On the other hand, the local response to mechanical stimuli in SI to be detected by fNIRI may be a few millimeters deeper.
Given these two facts, it may be possible that the sensitive depths interrogated by fNIRI due to mechanical stimulations happen to be within the regions where Δ[HbT] have small increases, while Δ[HbO] still have detectable changes. To completely understand the mechanisms of [HbT] change patterns, we acknowledge that further studies are required to confirm our findings and to obtain more convincing explanations.
Coregistration between fNIRI-based ROIs and anatomical structures of the rat head.
Since a rat head is much smaller than a human head, it is surely reasonable to use much shorter S-D separations to measure the animal's hemodynamic changes. However, the most sensitive depth to be detected is still directly associated the S-D separations, with an approximated relation of detected depth equals one-third of the S-D separation. So, for the case in this study, the topographical reconstruction depth should be ∼33% of 6.5–8.8 mm, namely, 2.2–2.9 mm. At this depth, the detected signals should come from the animal's brain.
While we did not perform anatomical imaging of the animal head to coregister the ROIs selected from the fNIRI measurements with the anatomical structures shown in Fig. 4, we performed an approximate match between the optical ROIs and actual anatomy in the lateral (x–y) plane by using the physical dimensions measured with possible lateral-location errors in a few millimeters. Specifically, the identification of each ROI is based on the spatial profile on the x–y plane and the prior knowledge of neuroanatomy of sensory processing. In many cases, responses at various ROIs were nicely isolated on the x–y plane that gives adequate confidence for identification. It is of importance to note that ROIs were not “selected” arbitrarily (in Group A), but were suggested by statistical assessments with minimal bias.
Effect of anesthetic.
Given the depressive effect of general anesthesia on the central nervous system and the fact that anesthetics acts profoundly on microcirculatory system (Baez and Orkin 1963; Longnecker and Harris 1980), the anesthetic (i.e., pentobarbital) used in our study should have influence on the observed hemodynamics. It is less likely that hemodynamic responses to noxious stimulation in conscious or freely moving animals will be exactly the same as what we obtained. However, it is unlikely that the atypical response pattern (i.e., −HbO/+Hb; during pinch and the late period postformalin injection) is mainly attributed to the anesthesia, but not to the neural activity. There are three pieces of evidence. First, the typical hemodynamic response pattern (i.e., +HbO/−Hb) was observed in all stimulation modalities: brush, pinch (from a contralateral area; probably SII), and formalin injection (in the initial period). In view of the fact that rats were under deep anesthesia, it is evident that the sedative tends not to alter the typical pattern, which is well documented in conscious human subjects. Second, the occurrences of the atypical response pattern were event driven, as pinch is more likely to elicit the atypical pattern than brush (Fig. 8). Third, it has been shown that dorsal horn sensory neurons demonstrate a biphasic excitatory response in pentobarbital-anesthetized rats (Pitcher and Henry 2002). It is thereby expected that at least some brain regions that directly receive spinal sensory inputs should reflect such biphasic pattern. The temporal and spatial profiles of our results indeed confirm this expectation. In short, the observed regional hemodynamics are believed to be a valid index of neural activation.
While the knowledge gained from anesthetized animals regarding pain neuroanatomy is limited compared with anesthesia-free human pain imaging studies, the focus of this paper is to examine the feasibility of using fNIRI to obtain whole brain hemodynamics in rats and to investigate the functional relevance of the NIR-based hemodynamic signals during pain processing. If our present study shows meaningful changes in respective brain regions induced by pain, it will provide essence and confidence for other researchers who may wish to implant the fiber-probe array in a free-moving animal's head for anesthesia-free fNIRI measurements to investigate pain using an existing animal model to advance our knowledge on pain-associated neuroanatomy and cerebrovascular function.
Given that the mechanical protocol only lasted for 15–20 min and formalin protocol lasted for a little more than 1 h (including the baseline measure), we believe that any prompt functional change right after either the mechanical stimulus or formalin injection should be predominantly attributed to neural responses to the stimuli. It is known that basal physiological parameters, such as the blood flow, heart rate, breath rate, and oxygen saturation level, vary with animals (e.g., rats), even under the same amount of anesthetic. Furthermore, in the same animal, these parameters often fluctuate at a certain level, while in a long period of run they tend to stay within a relatively constant range. Of course, they contribute to vascular responses, which naturally cause the trial-by-trial and the between-subject differences. Since the standard errors of hemodynamic changes are quite small (Fig. 3) over six animals, the changes are considered very robust.
Conclusions.
The whole brain fNIRI provides us with an alternative neuroimaging means of detecting a handful of areas in response to nociception in an in vivo rodent model. Both spatial and temporal profiles of hemodynamic responses can be used as quantitative measures to investigate the pain-processing network. This technique also benefits our understanding on the nature of neurovascular coupling during functional activation.
GRANTS
This study was supported by a start up fund from University of Texas at Arlington.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.-W.H. and Y.B.P. conception and design of research; J.-W.H. performed experiments; J.-W.H. and F.T. analyzed data; J.-W.H., H.L., and Y.B.P. interpreted results of experiments; J.-W.H. prepared figures; J.-W.H. drafted manuscript; J.-W.H., H.L., and Y.B.P. edited and revised manuscript; Y.B.P. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the assistance in data acquisition by Christopher Hagains, Vijayalakshmi Chinta, Sweta Narvenkar, Hanan Elfallal, and Nimit Patel. Special thank goes to Ted Huppert for generous help on data processing software, HOMER. We also greatly appreciate the Neural Systems and Graphic Computing Laboratory at the University of Oslo in Norway for sharing 3D rat brain atlas software package.
REFERENCES
- Abrahams et al., 1988. Abrahams VC, Clinton RJ, Downey D. Somatosensory projections to the superior colliculus of the anaesthetized cat. J Physiol 396: 563–580, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alreja et al., 1984. Alreja M, Mutalik P, Nayar U, Manchanda SK. The formalin test: a tonic pain model in the primate. Pain 20: 97–105, 1984 [DOI] [PubMed] [Google Scholar]
- Arridge, 1999. Arridge SR. Optical tomography in medical imaging. Inverse Probl 15: R41–R93, 1999 [Google Scholar]
- Ativanichayaphong et al., 2008. Ativanichayaphong T, He JW, Hagains CE, Peng YB, Chiao JC. A combined wireless neural stimulating and recording system for study of pain processing. J Neurosci Methods 170: 25–34, 2008 [DOI] [PubMed] [Google Scholar]
- Baez and Orkin, 1963. Baez S, Orkin LR. Effects of anesthetics on the response of the microcirculation to circulating humors. Anesthesiology 24: 568–579, 1963 [DOI] [PubMed] [Google Scholar]
- Bartocci et al., 2006. Bartocci M, Bergqvist LL, Lagercrantz H, Anand KJ. Pain activates cortical areas in the preterm newborn brain. Pain 122: 109–117, 2006 [DOI] [PubMed] [Google Scholar]
- Becerra et al., 2008. Becerra L, Harris W, Joseph D, Huppert T, Boas DA, Borsook D. Diffuse optical tomography of pain and tactile stimulation: activation in cortical sensory and emotional systems. Neuroimage 41: 252–259, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendse and Groenewegen, 1991. Berendse HW, Groenewegen HJ. Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience 42: 73–102, 1991 [DOI] [PubMed] [Google Scholar]
- Boivie, 1971. Boivie J. The termination of the spinothalamic tract in the cat. An experimental study with silver impregnation methods. Exp Brain Res 112: 331–353, 1971 [DOI] [PubMed] [Google Scholar]
- Bornhovd et al., 2002. Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, Buchel C. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single-trial fMRI study. Brain 125: 1326–1336, 2002 [DOI] [PubMed] [Google Scholar]
- Buchel et al., 2002. Buchel C, Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C. Dissociable neural responses related to pain intensity, stimulus intensity, and stimulus awareness within the anterior cingulate cortex: a parametric single-trial laser functional magnetic resonance imaging study. J Neurosci 22: 970–976, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al., 2002. Chen JI, Ha B, Bushnell MC, Pike B, Duncan GH. Differentiating noxious- and innocuous-related activation of human somatosensory cortices using temporal analysis of fMRI. J Neurophysiol 88: 464–474, 2002 [DOI] [PubMed] [Google Scholar]
- Clemo and Stein, 1991. Clemo HR, Stein BE. Receptive field properties of somatosensory neurons in the cat superior colliculus. J Comp Neurol 314: 534–544, 1991 [DOI] [PubMed] [Google Scholar]
- Devor et al., 2007. Devor A, Tian P, Nishimura N, Teng IC, Hillman EM, Narayanan SN, Ulbert I, Boas DA, Kleinfeld D, Dale AM. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci 27: 4452–4459, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson and Sullivan, 1987. Dickenson AH, Sullivan AF. Peripheral origins and central modulation of subcutaneous formalin-induced activity of rat dorsal horn neurones. Neurosci Lett 83: 207–211, 1987 [DOI] [PubMed] [Google Scholar]
- Dubuisson and Dennis, 1977. Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 4: 161–174, 1977 [DOI] [PubMed] [Google Scholar]
- Dykes et al., 1988. Dykes RW, Landry P, Hicks TP, Diadori P, Metherate R. Specificity of connections in the ventroposterior nuclei of the thalamus. Prog Neurobiol 30: 87–103, 1988 [DOI] [PubMed] [Google Scholar]
- Goense and Logothetis, 2008. Goense JB, Logothetis NK. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr Biol 18: 631–640, 2008 [DOI] [PubMed] [Google Scholar]
- Groenewegen et al., 1997. Groenewegen HJ, Wright CI, Uylings HB. The anatomical relationships of the prefrontal cortex with limbic structures and the basal ganglia. J Psychopharmacol (Oxf) 11: 99–106, 1997 [DOI] [PubMed] [Google Scholar]
- Groenewegen and Witter, 2004. Groenewegen HJ, Witter MP. Thalamus. In: The Rat Nervous System (3rd Ed.), edited by Paxinos G. San Diego, CA: Academic, 2004, chapt. 17, p. 407–453 [Google Scholar]
- Hagains et al., 2011. Hagains CE, He JW, Chiao JC, Peng YB. Septal stimulation inhibits spinal cord dorsal horn neuronal activity. Brain Res 1382: 189–197, 2011 [DOI] [PubMed] [Google Scholar]
- Harris, 1978. Harris FA. Functional subsets of neurons in somatosensory thalamus of the cat. Exp Neurol 58: 149–170, 1978 [DOI] [PubMed] [Google Scholar]
- Harris, 1980. Harris FA. Wide-field neurons in somatosensory thalamus of domestic cats under barbiturate anesthesia. Exp Neurol 68: 27–49, 1980 [DOI] [PubMed] [Google Scholar]
- He et al., 2010. He JW, Herath PM, Peng YB. Biphasic effects of the anterior cingulate cortex stimulation on glabrous skin blood flow in rats. Brain Res 1356: 32–43, 2010 [DOI] [PubMed] [Google Scholar]
- He et al., 2011. He JW, Kashyap D, Trevino LA, Liu H, Peng YB. Simultaneous absolute measures of glabrous skin hemodynamic and light-scattering change in response to formalin injection in rats. Neurosci Lett 492: 59–63, 2011 [DOI] [PubMed] [Google Scholar]
- Hess et al., 2007. Hess A, Sergejeva M, Budinsky L, Zeilhofer HU, Brune K. Imaging of hyperalgesia in rats by functional MRI. Eur J Pain 11: 109–119, 2007 [DOI] [PubMed] [Google Scholar]
- Horovitz and Gore, 2004. Horovitz SG, Gore JC. Simultaneous event-related potential and near-infrared spectroscopic studies of semantic processing. Hum Brain Mapp 22: 110–115, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert et al., 2006. Huppert TJ, Hoge RD, Diamond SG, Franceschini MA, Boas DA. A temporal comparison of BOLD, ASL, and NIRS hemodynamic responses to motor stimuli in adult humans. Neuroimage 29: 368–382, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo and Yen, 2005. Kuo CC, Yen CT. Comparison of anterior cingulate and primary somatosensory neuronal responses to noxious laser-heat stimuli in conscious, behaving rats. J Neurophysiol 94: 1825–1836, 2005 [DOI] [PubMed] [Google Scholar]
- Logothetis et al., 2001. Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157, 2001 [DOI] [PubMed] [Google Scholar]
- Longnecker and Harris, 1980. Longnecker DE, Harris PD. Microcirculatory actions of general anesthetics. Fed Proc 39: 1580–1583, 1980 [PubMed] [Google Scholar]
- Lowe et al., 2007. Lowe AS, Beech JS, Williams SC. Small animal, whole brain fMRI: innocuous and nociceptive forepaw stimulation. Neuroimage 35: 719–728, 2007 [DOI] [PubMed] [Google Scholar]
- Lui et al., 2008. Lui F, Duzzi D, Corradini M, Serafini M, Baraldi P, Porro CA. Touch or pain? Spatio-temporal patterns of cortical fMRI activity following brief mechanical stimuli. Pain 138: 362–374, 2008 [DOI] [PubMed] [Google Scholar]
- Maihofner and Kaltenhauser, 2009. Maihofner C, Kaltenhauser M. Quality discrimination for noxious stimuli in secondary somatosensory cortex: a MEG-study. Eur J Pain 13: 1048.e1–e7, 2009 [DOI] [PubMed] [Google Scholar]
- Malisza et al., 2003. Malisza KL, Gregorash L, Turner A, Foniok T, Stroman PW, Allman AA, Summers R, Wright A. Functional MRI involving painful stimulation of the ankle and the effect of physiotherapy joint mobilization. Magn Reson Imaging 21: 489–496, 2003 [DOI] [PubMed] [Google Scholar]
- Mehagnoul-Schipper et al., 2002. Mehagnoul-Schipper DJ, van der Kallen BF, Colier WN, van der Sluijs MC, van Erning LJ, Thijssen HO, Oeseburg B, Hoefnagels WH, Jansen RW. Simultaneous measurements of cerebral oxygenation changes during brain activation by near-infrared spectroscopy and functional magnetic resonance imaging in healthy young and elderly subjects. Hum Brain Mapp 16: 14–23, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr et al., 2005. Mohr C, Binkofski F, Erdmann C, Buchel C, Helmchen C. The anterior cingulate cortex contains distinct areas dissociating external from self-administered painful stimulation: a parametric fMRI study. Pain 114: 347–357, 2005 [DOI] [PubMed] [Google Scholar]
- Morrow et al., 1998. Morrow TJ, Paulson PE, Danneman PJ, Casey KL. Regional changes in forebrain activation during the early and late phase of formalin nociception: analysis using cerebral blood flow in the rat. Pain 75: 355–365, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa et al., 1990. Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A 87: 9868–9872, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasley et al., 2007. Pasley BN, Inglis BA, Freeman RD. Analysis of oxygen metabolism implies a neural origin for the negative BOLD response in human visual cortex. Neuroimage 36: 269–276, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos and Watson, 1998. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 1998 [Google Scholar]
- Peng et al., 1996a. Peng YB, Lin Q, Willis WD. Effects of GABA and glycine receptor antagonists on the activity and PAG-induced inhibition of rat dorsal horn neurons. Brain Res 736: 189–201, 1996a [DOI] [PubMed] [Google Scholar]
- Peng et al., 1996b. Peng YB, Lin Q, Willis WD. The role of 5-HT3 receptors in periaqueductal gray-induced inhibition of nociceptive dorsal horn neurons in rats. J Pharmacol Exp Ther 276: 116–124, 1996b [PubMed] [Google Scholar]
- Peyron et al., 1999. Peyron R, Garcia-Larrea L, Gregoire MC, Costes N, Convers P, Lavenne F, Mauguiere F, Michel D, Laurent B. Haemodynamic brain responses to acute pain in humans: sensory and attentional networks. Brain 122: 1765–1780, 1999 [DOI] [PubMed] [Google Scholar]
- Peyron et al., 2000. Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol Clin 30: 263–288, 2000 [DOI] [PubMed] [Google Scholar]
- Pitcher and Henry, 2002. Pitcher GM, Henry JL. Second phase of formalin-induced excitation of spinal dorsal horn neurons in spinalized rats is reversed by sciatic nerve block. Eur J Neurosci 15: 1509–1515, 2002 [DOI] [PubMed] [Google Scholar]
- Rose and Woolsey, 1948. Rose JE, Woolsey CN. The orbitofrontal cortex and its connections with the mediodorsal nucleus in rabbit, sheep and cat. Res Publ Assoc Res Nerv Ment Dis. 27: 210–232, 1948 [PubMed] [Google Scholar]
- Rovati et al., 2007. Rovati L, Salvatori G, Bulf L, Fonda S. Optical and electrical recording of neural activity evoked by graded contrast visual stimulus. Biomed Eng Online 6: 28, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakatani et al., 2007. Sakatani K, Murata Y, Fujiwara N, Hoshino T, Nakamura S, Kano T, Katayama Y. Comparison of blood-oxygen-level-dependent functional magnetic resonance imaging and near-infrared spectroscopy recording during functional brain activation in patients with stroke and brain tumors. J Biomed Opt 12: 062110, 2007 [DOI] [PubMed] [Google Scholar]
- Schridde et al., 2008. Schridde U, Khubchandani M, Motelow JE, Sanganahalli BG, Hyder F, Blumenfeld H. Negative BOLD with large increases in neuronal activity. Cereb Cortex 18: 1814–1827, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter et al., 2006. Schroeter ML, Kupka T, Mildner T, Uludag K, von Cramon DY. Investigating the post-stimulus undershoot of the BOLD signal–a simultaneous fMRI and fNIRS study. Neuroimage 30: 349–358, 2006 [DOI] [PubMed] [Google Scholar]
- Seifert and Maihofner, 2009. Seifert F, Maihofner C. Central mechanisms of experimental and chronic neuropathic pain: findings from functional imaging studies. Cell Mol Life Sci 66: 375–390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiyama et al., 2004. Seiyama A, Seki J, Tanabe HC, Sase I, Takatsuki A, Miyauchi S, Eda H, Hayashi S, Imaruoka T, Iwakura T, Yanagida T. Circulatory basis of fMRI signals: relationship between changes in the hemodynamic parameters and BOLD signal intensity. Neuroimage 21: 1204–1214, 2004 [DOI] [PubMed] [Google Scholar]
- Shah et al., 2005. Shah YB, Haynes L, Prior MJ, Marsden CA, Morris PG, Chapman V. Functional magnetic resonance imaging studies of opioid receptor-mediated modulation of noxious-evoked BOLD contrast in rats. Psychopharmacology (Berl) 180: 761–773, 2005 [DOI] [PubMed] [Google Scholar]
- Shmuel et al., 2006. Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci 9: 569–577, 2006 [DOI] [PubMed] [Google Scholar]
- Slater et al., 2006. Slater R, Cantarella A, Gallella S, Worley A, Boyd S, Meek J, Fitzgerald M. Cortical pain responses in human infants. J Neurosci 26: 3662–3666, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strangman et al., 2002. Strangman G, Culver JP, Thompson JH, Boas DA. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Neuroimage 17: 719–731, 2002 [PubMed] [Google Scholar]
- Takeuchi et al., 2009. Takeuchi M, Hori E, Takamoto K, Tran AH, Satoru K, Ishikawa A, Ono T, Endo S, Nishijo H. Brain cortical mapping by simultaneous recording of functional near infrared spectroscopy and electroencephalograms from the whole brain during right median nerve stimulation. Brain Topogr 22: 197–214, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian et al., 2009a. Tian F, Alexandrakis G, Liu H. Optimization of probe geometry for diffuse optical brain imaging based on measurement density and distribution. Appl Opt 48: 2496–2504, 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian et al., 2009b. Tian F, Chance B, Liu H. Investigation of the prefrontal cortex in response to duration-variable anagram tasks using functional near-infrared spectroscopy. J Biomed Opt 14: 054016, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann et al., 2001. Timmermann L, Ploner M, Haucke K, Schmitz F, Baltissen R, Schnitzler A. Differential coding of pain intensity in the human primary and secondary somatosensory cortex. J Neurophysiol 86: 1499–1503, 2001 [DOI] [PubMed] [Google Scholar]
- Toronov et al., 2007. Toronov VY, Zhang X, Webb AG. A spatial and temporal comparison of hemodynamic signals measured using optical and functional magnetic resonance imaging during activation in the human primary visual cortex. Neuroimage 34: 1136–1148, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey and Mantyh, 2007. Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron 55: 377–391, 2007 [DOI] [PubMed] [Google Scholar]
- Villringer et al., 1993. Villringer A, Planck J, Hock C, Schleinkofer L, Dirnagl U. Near infrared spectroscopy (NIRS): a new tool to study hemodynamic changes during activation of brain function in human adults. Neurosci Lett 154: 101–104, 1993 [DOI] [PubMed] [Google Scholar]
- Wallace et al., 1996. Wallace MT, Wilkinson LK, Stein BE. Representation and integration of multiple sensory inputs in primate superior colliculus. J Neurophysiol 76: 1246–1266, 1996 [DOI] [PubMed] [Google Scholar]
- Zimmermann, 1983. Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16: 109–110, 1983 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.