Abstract
Diversity in the expression of K+ channels among neurons allows a wide range of excitability, growth, and functional regulation. Ether-à-go-go (EAG), a voltage-gated K+ channel, was first characterized in Drosophila mutants by spontaneous firing in nerve terminals and enhanced neurotransmitter release. Although diverse functions have been ascribed to this protein, its role within neurons remains poorly understood. The aim of this study was to characterize the function of EAG in situ in Drosophila larval motoneurons. Whole cell patch-clamp recordings performed from the somata revealed a decrease in IAv and IKv K+ currents in eag mutants and with targeted eag RNAi expression. Spontaneous spike-like events were observed in eag mutants but absent in wild-type motoneurons. Thus our results provide evidence that EAG represents a unique K+ channel contributing to multiple K+ currents in motoneurons helping to regulate excitability, consistent with previous observations in the Drosophila larval muscle.
Keywords: spontaneous firing, potassium channel subunit, heteromultimer
the wide variety, density, and distribution of K+ channels in neurons help to regulate the resting membrane potential, action potential shape, firing rate, and synaptic release from nerve terminals. In recent years, a wealth of information has emerged regarding the novel interactions among ion channels and their ability to influence signaling pathways (for reviews see Kaczmarek 2006). Ether-à-go-go (EAG), a member of the voltage-gated K+ channel family, was identified as a Drosophila mutant exhibiting abnormal leg shaking phenotype and later shown to display repetitive spontaneous firing of nerve terminals (Ganetzky and Wu 1983; Kaplan and Trout 1969). DNA sequence and protein alignment analysis of EAG reveal a putative K+ channel subunit, which is similar to the Shaker family of K+ channels. Both form tetramers, and each monomer has six putative transmembrane domains, including a voltage-sensing domain (S4) and a pore-forming domain that is selective to K+. Drosophila shaker and eag double mutants in vivo display synergistic effects in increasing spontaneous activity at the larval neuromuscular junction. Earlier studies using voltage-clamp recordings from Drosophila larval muscles demonstrated a reduction of voltage-activated transient K+ current (IAv) and voltage-dependent delayed rectifier K+ current (IKv) in shaker and eag mutants, respectively (Wu et al. 1983). Different allelic mutants of EAG were later shown to reduce different components of K+ currents (Zhong and Wu 1991, 1993). Ex vivo voltage-clamp recordings from Xenopus oocytes containing eag cDNA revealed outward sustained K+ current (Robertson et al. 1996). Coexpression of Shaker and eag RNA in oocytes led to faster inactivation of transient currents, suggesting a possible heteromultimeric channel assembly. However, this association is dependent on the concentration of the RNA used and the developmental time of the oocytes (Chen et al. 1996, 2000; Tang et al. 1998). Both these approaches have led to the speculation that EAG could form heteromultimers with other K+ channels. A definitive proof of heteromultimeric EAG channel formation in vivo remains to be explored.
Unlike the Shaker family, EAG has a cyclic nucleotide binding domain in the carboxy (COOH) terminus, similar to cyclic nucleotide-gated channels (Guy et al. 1991; Warmke et al. 1991). In addition to functioning as a voltage-gated ion channel, EAG displays numerous non-ion-conducting roles. Modulation of multiple K+ currents by cGMP and calmodulin antagonist (W7) is reduced in eag mutants (Zhong and Wu 1993). The COOH terminus of EAG contains a Ca2+/calmodulin-dependent protein kinase II (CaMKII) binding site. Similar to eag mutants, CAMKII inhibitor (Ala) also increases spontaneous repetitive firing (Griffith et al. 1994). Ca2+ or calmodulin is necessary for binding of CaMKII to EAG. Phosphorylation of EAG by CaMKII regulates EAG function (Wang et al. 2002). In turn, the binding domain of CaMKII in EAG dislodges the autoinhibition of CaMKII, thereby enabling it to be stably bound to EAG for a long time even in the absence of substrates (Sun et al. 2004). In addition to the above, the voltage sensor of nonconducting EAG channels has been shown to activate p38 mitogen-activated protein kinase (MAPK) signaling, leading to cell proliferation (Hegle et al. 2006).
Yet another novel function of EAG is its emerging role in transcription. eag transcripts are alternatively spliced to produce an 80-kDa protein (EAG80), which contains NH2 and COOH termini leaving the channel forming transmembrane regions of the protein. Synthesis of EAG80 can be triggered in vitro by Ca2+ influx and by activation of PKA or PKC through MAPK signaling, bringing about changes in cell morphology. The COOH terminus of EAG has a nuclear localization signal that enables it to translocate to the nucleus. This nuclear translocation, although necessary for MAPK signaling, does not serve directly as a transcriptional activator (Sun et al. 2009). Higher levels of EAG are found in cancerous cells, linking EAG to cell cycle regulation and proliferation (for reviews see Camacho 2006).
EAG represents a class of ion channels whose functional role in cellular excitability and regulation remains less understood. A wealth of information regarding the varied potential functions of EAG has been obtained from experiments using oocytes and cell culture systems. It has yet to be determined whether these functional roles are observed in the nervous system. Therefore, the aim of this study is to provide an electrophysiological characterization of the role of EAG in an identified Drosophila larval motoneuron using in situ whole cell patch-clamp techniques and genetic manipulations. Our results indicate that EAG contributes to diverse K+ currents, including transient and sustained voltage-activated currents, and in its absence increases excitability.
METHODS
Drosophila stocks.
Even-skipped (EVE) promoter GAL4 lines w−;noc/sco;RRA-GAL4, UAS-CD8GFP (Fujioka et al. 2003) and a flip-out strategy using w−;RN2-GAL4 (w−; RN2-GAL4, UAS-mCD8-GFP; ACT5C≪CD2≪GAL4,UAS-FLP; Hartwig et al. 2008) were used to label aCC and RP2 motoneurons in the larval ganglion. w−,ELAV-GAL4;+;UAS-GFP was used to misexpress UAS-eag RNA interference (RNAi) in all neurons. UAS-eag RNAi (transformant ID: 9127) and UAS-Dicer 1 (transformant ID: 24667) were obtained from the Vienna Drosophila RNAi Center. For misexpression studies, UAS-eag RNAi and UAS-Dicer 1 stocks were combined and then crossed to appropriate GAL4 lines. A 1.6666-fold decrease in eag mRNA was observed in the ganglion of ELAV GAL4:: UAS-eag RNAi ; UAS-Dicer1 larvae compared with control using real-time quantitative PCR. w−; P{tubP-GAL80ts}20; TM2/TM6B, Tb1 (no. 7019; Bloomington Stock Center, Indiana University, Bloomington, IN) was used to block GAL4 expression. eag mutants Df (1)eagx-6/FM7a, lethal; Dp(1:2)X-6/SM6b,Cy (hereafter, eagx-6) and In(1)sc29, wa (hereafter, eagsc29) were obtained from Dr. B. Ganetzky (University of Wisconsin, Madison, WI). Canton-S and w1118 were used as wild-type controls for mutants, and appropriate parental GAL4 lines were used as controls for RNAi studies.
Electrophysiology.
Wandering third instar Drosophila larvae were dissected in Ca++-A solution (containing, in mM, 118 NaCl, 2 NaOH, 2 KCl, 4 MgCl2, 5 trehalose, and 5 HEPES, pH 7.1–7.2 and osmolarity adjusted to 295 mosmol/l using sucrose) and used as the external recording solution during electrophysiological experiments. Procedures for dissection and preparations for performing electrophysiological experiments were carried out as described previously (Srinivasan et al. 2012). Male larvae from eag mutants were used for electrophysiological experiments. aCC motoneurons in the first thoracic segment were identified by intracellular dye filling using dextran-rhodamine (3,000 MW; catalog no. D-3307; Life Technologies). The position of the cell body in the ganglion and the bilateral dendritic arborization and ipsilateral axon were used to confirm aCC (Srinivasan et al. 2012).
Protocols and pharmacology.
Glass micropipettes of 2.5–5 MΩ (in bath) were filled with potassium gluconate internal solution (containing, in mM, 120 K-gluconate, 20 KCl, 1 MgCl2, 0.5 CaCl2, 5 EGTA, and 10 HEPES; pH was adjusted to 7.1–7.2 and osmolarity to 285–290 mosmol/l using glucose). Whole cell path-clamp recordings were performed using the Multiclamp 700B amplifier (Axon Instruments, Molecular Devices, Sunnyvale, CA). Clampex acquisition software (pCLAMP 10; Molecular Devices) was used to initiate and record voltage and current commands. Voltage-clamp protocols were used to isolate voltage-dependent K+ currents. The cells were held at −80 mV, and command steps of 20 mV were provided from −120 up to 60 mV in 200-ms duration. To visualize sustained K+ currents in isolation (see Fig. 2), the cells were held at −20 mV to inactivate the transient currents. Measurements of sustained currents (see graphs in Figs. 2–4) were made near the end of the 200-ms-long voltage step from cells held at −80 mV so that the voltage dependence of activation could be observed more accurately. A current-clamp protocol was used to study firing properties. A series of 10-pA, 500-ms current steps ranging from −10 to 100 pA was administered. To measure firing frequency, since resting membrane potential varied across cells, a bias current was injected to normalize the resting membrane potential to −60 mV in all cells. Extended periods of motor neuron activity were monitored using the gap-free protocol, and bias current was used to hold the cell at different membrane potentials. Na+ currents were blocked using external application of 1 μM tetrodotoxin (TTX; Sigma-Aldrich, St. Louis, MO). Ca2+ currents were blocked using external application of 500 μM CdCl (Acros Organics, Geel, Belgium) in TTX containing Ca++-A solution. Chemical reagents were obtained from Sigma-Aldrich unless specified otherwise.
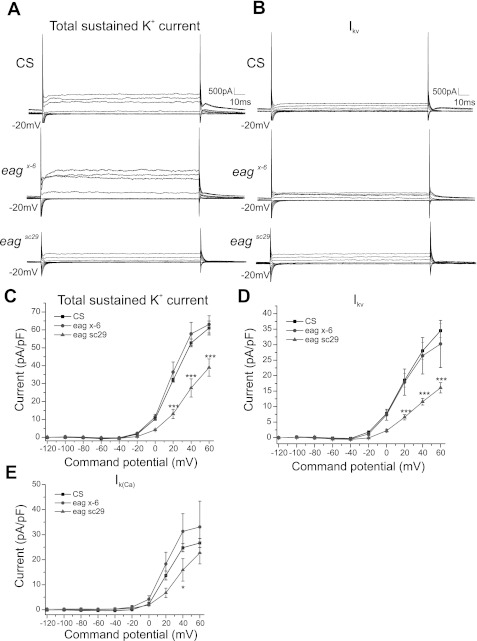
Fig. 2.
EAG contributes to voltage-dependent sustained K+ currents. A and B: voltage-clamp records from CS, eagx-6, and eagsc29. Cells were held at −20-mV holding potential, and 20-mV command steps were provided to obtain voltage-dependent sustained K+ currents. Leak subtraction was performed in all recordings. A: total sustained K+ currents were clearly visible from 0 mV. C: command potential vs. sustained K+ current density in CS, eagx-6, and eagsc29. Total sustained K+ current was reduced in eagsc29 but not in eagx-6. B: voltage-activated sustained K+ current (IKv) was isolated using CdCl to block Ca2+ channels from the same cell. D: command potential vs. IKv in CS, eagx-6, and eagsc29. IKv was reduced in eagsc29 but not in eagx-6. Note: A and B are representative voltage-clamp records obtained from a −20-mV holding potential to visualize sustained K+ currents; sustained K+ current measurements (C and D) were made from the same cells held at −80-mV holding potential. E: command potential vs. Ca2+-sensitive [IK(Ca)] current density in CS, eagx-6, and eagsc29. IK(Ca) was measured by subtracting IKv in B from total sustained K+ current in A. A significant reduction in IK(Ca) in eagsc29 was observed at 40 mV, but not in eagx-6. Two-way ANOVA followed by Bonferroni posttest was used to recognize significance. ***P < 0.001, CS vs. eagsc29. Scale bar: 500 pA, 10 ms.
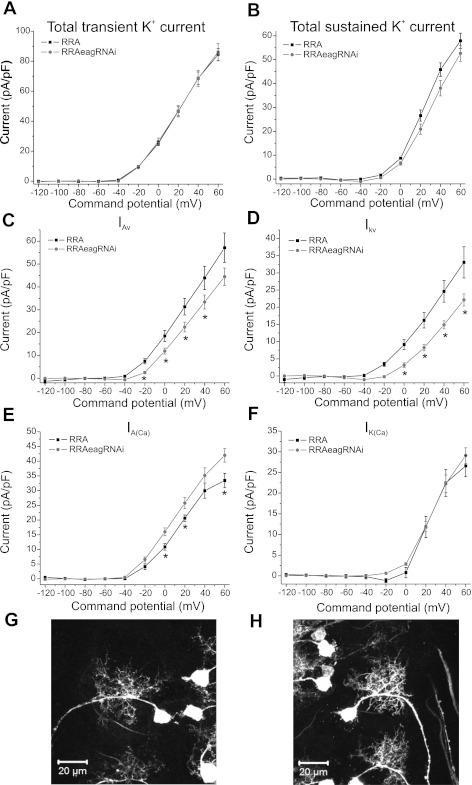
Fig. 3.
Targeted reduction of EAG in motoneurons. A: total transient K+ current densities in RRA-GAL4 (RRA, control) and EAG knockdown (RRAeagRNAi). Total transient K+ current was similar in eag RNAi manipulations compared with control. B: total sustained K+ current densities in RRA-GAL4 and EAG knockdown. No reduction was observed. C: IAv in RRA-GAL4 and EAG knockdown. IAv was significantly reduced in EAG manipulation compared with control. D: IKv in RRA-GAL4 and EAG knockdown. IKv was significantly reduced only in the EAG manipulation. E: IA(Ca) was upregulated in EAG manipulation, whereas IK(Ca) was unchanged (F). *P < 0.05. G and H: confocal images of single RP2 motoneurons in third instar larval CNS. G: control larval RP2 cell with normal dendrites. H: RP2 cell expressing eag RNAi. Note that there are no gross differences apparent between the dendritic structures of the motoneuron in control and eag RNAi individuals. Detailed description of the genotypes is provided in methods. Scale bar: 20 μm.
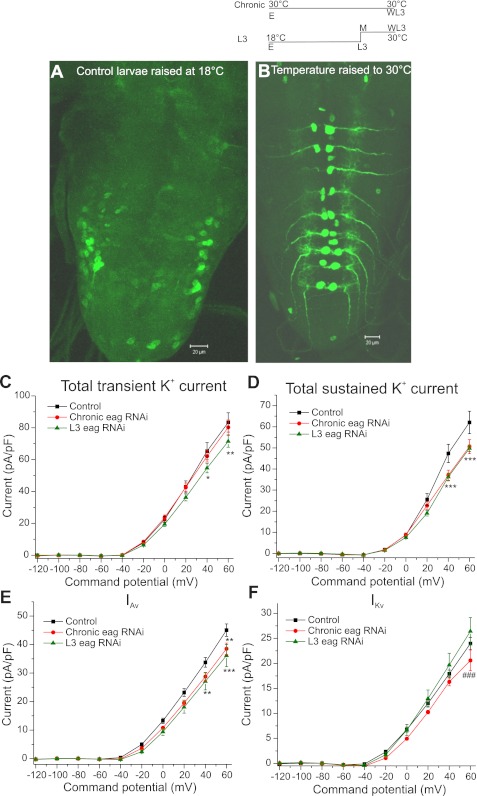
Fig. 4.
Larval and chronic knockdown of EAG. Temperature-sensitive GAL80 was used to reduce EAG levels in T1aCC. Temperature shift paradigm is shown at top right. Chronic indicates eag RNAi knockdown from embryo (E) to wandering third instar larval stage (WL3); L3 indicates eag RNAi knockdown from mid (M) third instar larvae to wandering third instar, when recordings were performed. Parental control larvae were raised in the L3 temperature shift paradigm. A: temperature-sensitive GAL80 blocked GAL4 expression, leading to no expression of green fluorescent protein (GFP) in dorsomedial motoneurons at 18°C. B: GAL80 was blocked at higher temperatures (30°C), leading to expression of GFP in dorsomedial motoneurons. Scale bar: 20 μm. C: total transient K+ current densities in control, chronic, and L3 knockdown. Total transient K+ current was significantly reduced in the larval eag RNAi manipulations compared with control. D: total sustained K+ current densities in control, chronic, and L3 knockdown. Both chronic and L3 eag RNAi knockdown showed a significant reduction compared with control. E: IAv in control, chronic, and L3 knockdown. IAv was significantly reduced in both L3 and chronic manipulations compared with control. F: IKv in control, chronic, and L3 knockdown. IKv was reduced only in the chronic manipulation at 60 mV compared with the L3 eag RNAi knockdown. No change was observed between control and chronic knockdown. Two-way ANOVA followed by Bonferroni posttest was used to recognize significance. *P < 0.05; **P < 0.01; ***P < 0.001, control vs. knockdown. ###P < 0.001, chronic vs. L3 eag RNAi knockdown.
Quantification.
Input resistance was measured using the most hyperpolarizing voltage step (−40 mV) in voltage-clamp experiments using Clampfit 10.1 (Molecular Devices). Values of input resistance were used to perform leak subtraction. All voltage-clamp records were leak subtracted unless otherwise indicated in the text. Series resistance was measured using the charging transient generated with a hyperpolarizing voltage step. Current densities are reported.
Confocal microscopy.
For imaging isolated RP2 motoneurons, w−; RN2-GAL4, UAS-mCD8-GFP; ACT5C≪CD2≪GAL4,UAS-FLP was crossed with w1118 to obtain w−; RN2-GAL4, UAS-mCD8-GFP/+; ACT5C≪CD2≪GAL4,UAS-FLP/+. To visualize the effect of eag RNAi manipulations on motoneuron dendrites, w−; RN2-GAL4, UAS-mCD8-GFP; ACT5C≪CD2≪GAL4,UAS-FLP was crossed with w−;UAS-EAG RNAi; UAS-Dicer 1 to obtain w−; RN2-GAL4, UAS-mCD8-GFP/UAS-eagRNAi; ACT5C≪CD2≪GAL4,UAS-FLP/UAS-Dicer 1. Third instar larvae were dissected in phosphate-buffered saline (PBS). The preparation was mounted in 80% glycerol (Sigma-Aldrich; diluted in dH2O). Live images were taken with a Ziess 510 Meta laser scanning confocal microscope at ×40 with a 1.8 zoom. Images were postprocessed using Ziess LSM Image Browser version 4.2.0.121 (see Fig. 3, G and H).
Temperature-sensitive experiments.
w−; P{tubP-GAL80ts}20;RRA-GAL4,UAS-CD8GFP females were crossed to w−;UAS-eag RNAi;UAS-Dicer 1 males. For L3 knockdown experiments, progeny were raised at 18°C until the early to mid third instar and then transferred to 30°C. Recordings were performed 1.5 days later in wandering late third instar. In the chronic experiments, eag RNAi was expressed throughout development by raising the progeny at 30°C until the wandering third instar stage. Parental line (w−; P{tubP-GAL80ts}20;RRA-GAL4,UAS-CD8GFP) reared at the same temperatures served as the control. Green fluorescent protein (GFP) expression in the late third instar larvae was used as an indicator for GAL80 suppression (see Fig. 4, A and B).
Statistical analysis.
Student's t-test was used to recognize significance (Excel 4.0; Microsoft, Redmond, WA). Two-way ANOVA followed by Bonferroni posttest was used to compare multiple genotypes and command potential (GraphPad Prism 5). Current densities are reported as means ± SE.
RESULTS
EAG contributes to transient K+ currents.
Our overall aim was to characterize the in vivo role of EAG in the nervous system by performing in situ whole cell patch-clamp recordings on the somata of aCC motoneurons in the first thoracic segment (T1aCC). Active and passive properties of this motoneuron have been described previously (Srinivasan et al. 2012). We tested the contribution of EAG to K+ currents using two well-studied mutants in the larval neuromuscular junction, eagx-6 and eagsc29. eagx-6 is a gamma ray-induced mutation resulting in the deletion of the chromosomal fragment between the locus 13A1-2 and 13E4-8 in the X chromosome and insertion of the fragment at locus 21E in the second chromosome, resulting in two DNA bands in in situ hybridization (Drysdale et al. 1991). eagsc29 is an inversion mutation resulting in a break point at 12F-13A in the X chromosome (Drysdale et al. 1991). Northern blot analysis from wild type, eagx-6, and eagsc29 revealed that a 10-kb fragment present in wild-type extracts was missing in the two mutants, but smaller fragments were observed (Drysdale et al. 1991). EAG protein is observed in the synaptic neuropil in wild-type larvae but absent in eagsc29 (Sun et al. 2004).
In the Drosophila larval muscle, allele-specific reduction of eag resulted in a decrease in IAv, IA(Ca), IKv, and IK(Ca), with eagx-6 selectively reducing IAv and IKv (Zhong and Wu 1991). Whole cell patch-clamp recordings performed on the somata of aCC motoneurons revealed total transient K+ currents clearly visible in wild type Canton-S (CS), eagx-6, and eagsc29 when depolarized above −20 mV (Fig. 1, A and C). Two-way ANOVA comparing total transient K+ current in CS, eagx-6, and eagsc29 revealed overall main effects of genotype [F(2, 130) = 99.03, P < 0.0001] and command potential [F(9, 130) = 378.9, P < 0.0001] (Fig. 1, A and C). The Bonferroni posttest revealed that eagsc29 showed a significant reduction (P < 0.001) from 0 to 60 mV compared with CS (Fig. 1C). No significant reduction was observed between CS and eagx-6 (Fig. 1, A and C). However, a significant difference (P < 0.001) was observed from 0 to 60 mV between eagsc29 and eagx-6.
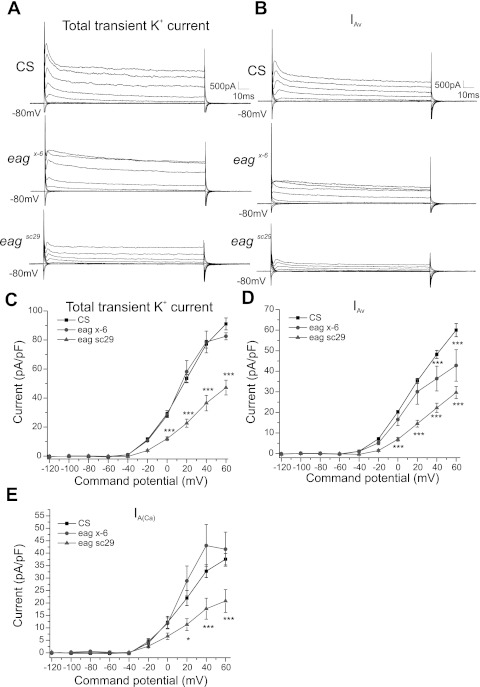
Fig. 1.
Ether-à-go-go (EAG) contributes to voltage-activated transient K+ current. A and B: voltage-clamp records from wild type Canton-S (CS) and eag mutants eagx-6 and eagsc29. Cells were held at −80 and 20 mV, and 200-ms command steps ranging from −120 up to 60 mV were provided in tetrodotoxin (TTX)-containing Ca++-A external solution. Leak subtraction was performed in all recordings. A: total K+ currents in aCC motoneurons in the first thoracic segment (T1aCC). Peak transient K+ currents were clearly visible from −20 mV. C: command potential vs. peak transient K+ current density in CS, eagx-6, and eagsc29. Total transient K+ current was reduced in eag sc29 but not in eagx-6. B: voltage-activated transient K+ current (IAv) was isolated using CdCl to block Ca2+ channels from the same cell using the same voltage-clamp protocol. D: graph of command potential vs. IAv current density in CS, eagx-6, and eagsc29. IAv was significantly reduced in eagsc29 but only at 40 and 60 mV in eagx-6. E: command potential vs. Ca2+-sensitive [IA(Ca)] current density in CS, eagx-6, and eagsc29. IA(Ca) was measured by subtracting IAv in B from total transient K+ current in A. IA(Ca) was reduced in eagsc29 from 20 up to 60 mV, but not in eagx-6. Two-way ANOVA followed by Bonferroni posttest was used to recognize significance. ***P < 0.001. Scale bar: 500 pA, 10 ms.
We then tested the specific contribution of EAG to IAv. IAv was isolated by bath application of CdCl to block Ca2+ channels in the same cell in all genotypes (Fig. 1B). IAv currents were visible in response to command steps from −20 through 60 mV (Fig. 1B). Similar to the total transient K+ currents, two-way ANOVA comparing IAv in CS, eagx-6, and eagsc29 revealed overall main effects of genotype [F(2, 130) = 65.8, P < 0.0001] and command potential [F(9, 130) = 205.5, P < 0.0001] (Fig. 1, B and D). The Bonferroni posttest revealed that eagsc29 showed a significant reduction (P < 0.001) from 0 to 60 mV, and eagx-6 also showed a reduction at 40 and 60 mV (P < 0.001), compared with CS (Fig. 1D). In addition, a significant difference (P < 0.05) was observed from 0 to 60 mV between eagsc29 and eagx-6.
We estimated the amplitude of Ca2+-activated transient K+ currents by subtracting IAv currents from the total K+ currents. There were overall effects of both genotype [F(2, 130) = 17.40, P < 0.0001] and command potential [F(9, 130) = 78.71, P < 0.0001]. The Bonferroni posttest indicated that there were significant differences (P < 0.05) between eagsc29 and control from 20 to 60 mV, but no significant differences were observed between eagx-6 and control. Similarly, there were significant differences between eagsc29 and eagx-6 from 20 to 60 mV (P < 0.001) (Fig. 1E). Thus IA(Ca) was significantly reduced in eagsc29 mutants. Total transient K+ current and IA(Ca) in eagx-6 appeared to plateau at 60 mV (Fig. 1, C and E). It is possible that the reversal potential of Ca2+ currents is altered in this mutant.
EAG contributes to sustained K+ currents.
Total sustained K+ current was isolated from the same cell using a −20-mV voltage-clamp protocol to inactivate transient K+ currents (Fig. 2A). Sustained K+ currents were activated from 0 to 60 mV (see methods). Two-way ANOVA comparing total sustained K+ current in CS, eagx-6, and eagsc29 revealed overall main effects of genotype [F(2, 130) = 40.16, P < 0.0001] and command potential [F(9, 130) = 253.7, P < 0.0001] (Fig. 2, A and C). The Bonferroni posttest revealed that total sustained K+ current was significantly reduced (P < 0.001) from 20 to 60 mV in eagsc29 compared with CS (Fig. 2, A and C). A significant reduction (P < 0.001) in total sustained K+ current was observed in eagsc29 compared with eagx-6 from 20 to 60 mV (Fig. 2C).
Further isolation of IKv (after application of CdCl) revealed overall effects of genotype [F(2, 130) = 27.51, P < 0.0001 ] and command potential [F(9, 130) = 94.53, P < 0.0001] (Fig. 2B). The Bonferroni posttest confirmed that IKv was significantly reduced in eagsc29 compared with CS from 20 to 60 mV (P < 0.001) (Fig. 2D). eagx-6 showed no clear reduction in IKv. A significant difference (P < 0.001) was also observed from 20 to 60 mV between eagsc29 and eagx-6. The level of the Ca2+-sensitive component of IK was estimated through subtraction as described above. Again, there were overall effects of both genotype [F(2, 130) = 6.944, P < 0.01] and command potential [F(9, 130) = 65.90, P < 0.0001]. The Bonferroni posttest revealed that there was a significant reduction in eagsc29 compared with CS, only at 40 mV (P < 0.05) (Fig. 2E). There were no significant differences observed between CS and eagx-6. Together, the results show that eagsc29 demonstrated a reduction in total transient and sustained K+ currents and also in IAv, IA(Ca) and IKv. The eagx-6 allele, on the other hand, showed a reduction only in IAv.
Targeted reduction of EAG in motoneurons.
To further study the effect of EAG on K+ currents only in motoneurons, eag RNAi was misexpressed in even-skipped promoter expressing GAL4 line, RRA. No reduction in total transient K+ current was observed when eag RNAi was misexpressed compared with the GAL4 control (Fig. 3A). However, further isolation of IAv revealed that eag RNAi caused a reduction in the current from −20 up to 60 mV (Fig. 3C). Interestingly, IA(Ca) was significantly increased in eag RNAi misexpression compared with control (Fig. 3E). In effect, eag RNAi misexpression caused reduction similar to eagx-6 in transient K+ current. eag RNAi did not cause a significant reduction in total sustained K+ current (Fig. 3B); however, a significant reduction was observed in IKv from 0 to 60 mV (Fig. 3D). No reduction in IK(Ca) was observed (Fig. 3F). We did not observe any difference in the gross morphology of dendrites and cell body of motoneurons with these manipulations, although dendritic branching has yet to be analyzed in depth (Fig. 3, G and H).
Embryonic contribution of EAG to K+ currents.
Reduction in EAG function in mutants and eag RNAi manipulations lead to increased excitability and perhaps other changes throughout development. Therefore, functional changes that are measured late in the larval stage may be influenced by the embryonic role of EAG protein. To eliminate the embryonic role, eag RNAi was expressed under the control of RRA-GAL4, and a temperature-sensitive GAL80, a blocker of GAL4, was used to conditionally block expression of eag RNAi in embryonic and early larval stages. Temperature-sensitive GAL80 functions at lower temperatures (18°C) to prevent GAL4 expression but is blocked at higher temperatures (30°C), thereby allowing GAL4 expression (McGuire et al. 2003). Thus, by controlling the rearing temperature, eag RNAi expression was allowed only in the third instar larval stage and not in the embryonic, first, and second instar larvae. GFP expression was absent at 18°C, confirming the activation of GAL80, and present at 30°C, confirming suppression of GAL80 (Fig. 4, A and B).
K+ currents were measured from larvae in which eag RNAi was expressed chronically (from embryo to L3) and only in the late larval stage (shifted at mid L3) and compared with controls (raised in a similar temperature shift paradigm as the larval knockdown). Two-way ANOVA comparing total transient K+ current in chronic and larval eag RNAi knockdown with control revealed overall main effects of genotype [F(2, 220) = 6.189, P < 0.01] and command potential [F(9, 220) = 490.7, P < 0.0001] (Fig. 4C). The Bonferroni posttest revealed that total transient K+ current was significantly reduced (P < 0.05) in larval eag RNAi compared with control from 40 to 60 mV. As in the first set of eag RNAi experiments, however, chronic eag RNAi showed no significant reduction in transient K+ current compared with control. A significant reduction (P < 0.05) was also observed between chronic and larval eag RNAi knockdown at 60 mV (Fig. 4C).
Looking specifically at the voltage-dependent component of the transient current, overall effects of genotype [F(2, 220) = 11.68, P < 0.0001] and command potential [F(9, 220) = 412.5, P < 0.0001] on IAv were observed between chronic or larval eag knockdown compared with control. The Bonferroni posttest indicated that IAv was significantly reduced (P < 0.01) in chronic knockdown at 60 mV and larval knockdown at 40 and 60 mV compared with control. No difference in IAv was observed between chronic and larval eag RNAi knockdown (Fig. 4E). In addition, no reduction was observed in IA(Ca) in these temperature manipulations (data not shown).
Overall effects of genotype [F(2, 220) = 9.102, P < 0.001] and command potential [F(9, 220) = 455.4, P < 0.0001] on total sustained K+ current were observed between chronic and larval eag knockdown compared with control. The Bonferroni posttest indicated that total sustained K+ current was significantly reduced (P < 0.001) in both chronic and larval knockdown at 40 and 60 mV (Fig. 4D). No difference was observed between chronic and larval eag knockdown. IKv also showed overall effects of genotype [F(2, 220) = 7.215, P < 0.0001] and command potential [F(9, 220) = 267.3, P < 0.0001] in these temporal manipulations. The Bonferroni posttest indicated a significant reduction (P < 0.001) at 60 mV between chronic and larval eag RNAi manipulations. No reduction was observed in chronic and larval eag RNAi manipulations compared with control (Fig. 4F). Both chronic and larval manipulations reduced IK(Ca) significantly only at the 40-mV command (data not shown).
Together, these results demonstrate the role of EAG in larval stages without its embryonic contribution. Failure to observe a reduction in IKv in the larval manipulation suggests a possible requirement of EAG during development. Surprisingly, total transient K+ current was reduced in the larval knockdown but not in the chronic knockdown, suggesting embryonic compensation. IAv was reduced in all manipulations, demonstrating that EAG contributes acutely to K+ currents in larval T1aCC. We cannot rule out the possibility that there was some degree of K+ current compensation by other K+ channels in larval eag RNAi knockdown, however, so our measure of K+ current reduction in larval eag RNAi manipulations may be an underestimation.
EAG influences firing properties in motoneurons.
We observed a decrease in diverse K+ currents in eag mutants and in eag RNAi manipulation. We hypothesized the reduction in multiple K+ currents may influence firing behavior of motoneurons. A current-clamp protocol was used to obtain firing frequency plots from control lines and eag mutants. Depolarizing steps of current injection were administered to CS (control) to evoke action potentials. The voltage threshold for firing was −34.85 ± 2.5 mV. The average resting membrane potential of T1aCC was −49.2 ± 1 mV, and the average input resistance was 383.16 ± 49 MΩ (Table 1). A delay to the first spike was observed following current injection (Fig. 5A). Baseline synaptic activity was also observed (Fig. 5A). The average resting membrane potential in eagx-6 and eagsc29 was more depolarized than in wild type at −34.5 and −37.7 mV, respectively, but the average input resistance was not affected (396.73 ± 88 and 378.79 ± 10 MΩ, respectively; Table 1).
Table 1.
Firing properties
| Frequency of Physiological Phenotypes |
||||||
|---|---|---|---|---|---|---|
| Genotype | n | Resting Membrane Potential, mV | Input Resistance, MΩ | Normal firing | Increased amplitude of EPSPs‡ | Spontaneous events§ |
| CS | 8 | −49.2 ± 1† | 383.16 ± 49 | 8/8 | 0/8 | 0/8 |
| eagx-6 | 10 | −34.5 ± 2† | 396.73 ± 88 | 1/10 | 5/10 | 3/10 |
| eagsc29 | 16 | −37.7 ± 1 | 378.79 ± 10 | 7/16 | 3/16 | 4/16 |
| RRA | 7 | −45.1 ± 1 | 345.32 ± 19 | 7/7 | 0/8 | 0/8 |
| RRA:: eag RNAi | 10 | −42.9 ± 3 | 409.54 ± 19 | 7/10 | 2/10 | 0/8 |
| ELAV::eag RNAi | 9 | −37 ± 3* | 286.93 ± 8 | 5/9 | 0/9 | 3/9 |
Summary of the resting membrane potential, input resistance (measured from voltage-clamp experiments), and frequency of physiological phenotypes in various genotypes tested (see text for details). Student's t-test was used to recognize significance.
P < 0.05, ELAV::eag RNAi vs. RRA.
P < 0.05, mutants vs. wild type Canon-S (CS).
Example excitatory postsynaptic potentials (EPSPs) in solid box in Fig. 5.
Examples in dashed boxes in Fig. 5.
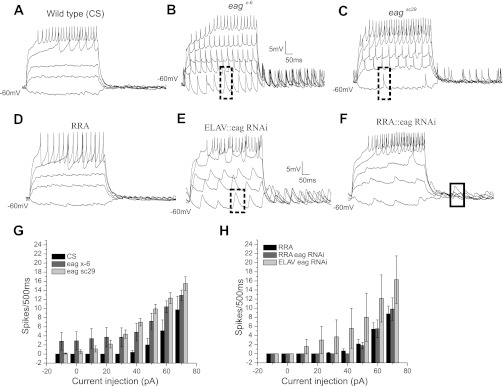
Fig. 5.
Influence of eag mutants and eag RNAi manipulations on firing properties. Representative current-clamp recordings are shown from CS (A), eagx-6 (B), eagsc29 (C), RRA-GAL4 (D), ELAV::eag RNAi (E), and RRA::eag RNAi (F) in T1aCC from a normalized membrane potential of −60 mV. Dashed boxes in B, C, and E denote spontaneous spike-like events, absent in CS (A). Solid box in F denotes large excitatory postsynaptic potentials (EPSPs). G and H: firing frequency plots of eag mutants (G) and eag RNAi manipulations (H) compared with CS and RRA, respectively. Evoked firing was comparable in all genotypes. Spontaneous spike-like events were observed in eagx-6, eagsc29, and ELAV::eag RNAi but not in CS, RRA, and RRA::eag RNAi.
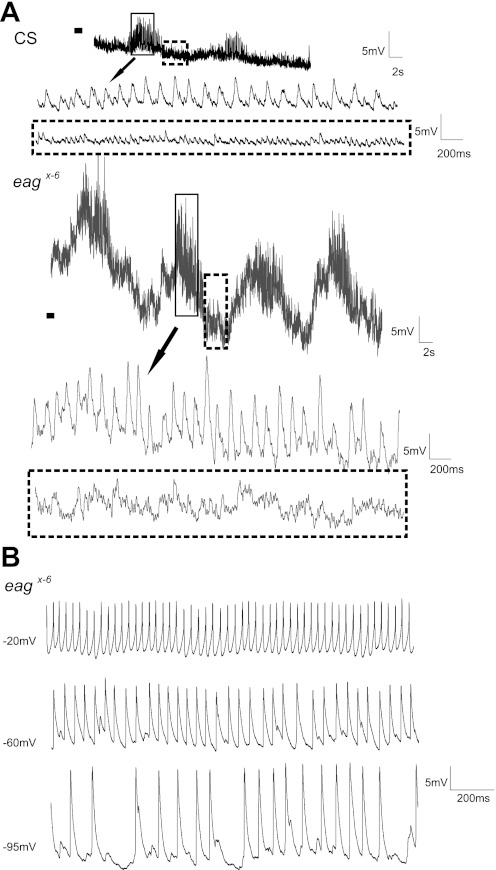
In the most striking phenotype of eagx-6 and eagsc29, large spontaneous spike-like events were observed, intermittently with little or no current injection, in some recordings (Table 1; dashed boxes in Fig. 5, B and C). These spontaneous events did not cause an increase in the frequency of evoked firing following current injection (Fig. 5G, current step 70 pA). However, a decrease in the delay to first spike following current injection was observed when spontaneous spike-like events occurred in mutants (Fig. 5, B and C). The spontaneous events in eag mutants were sometimes broader in shape, similar to those observed in Fig. 5E. Hyperpolarizing current injection increased the amplitude of the spontaneous events, as expected for excitatory postsynaptic potentials or action potentials. In addition, the frequency of the events decreased with hyperpolarization, suggesting a contribution of voltage-gated currents (Fig. 6B). In the example from an eag mutant shown in Fig. 6B, action potentials of ∼10 mV in amplitude with a prominent after-spike hyperpolarization were observed at a depolarized membrane potential of −20 mV. Upon hyperpolarization of the cell to −60 mV, spontaneous events of ∼11 mV in amplitude were observed with no visible after-spike hyperpolarization. Such spontaneous events were never observed in wild type (CS) at −60 mV. At −95 mV in the mutant cell, a further increase in amplitude to about 18 mV and a decrease in frequency were observed (Fig. 6B). In some recordings from the mutants, large excitatory postsynaptic potentials (EPSPs; Table 1) and an increased frequency of EPSPs were also observed (Fig. 6A). As expected of EPSPs, these events increased in amplitude, but did not decrease in frequency, with membrane hyperpolarization.
Fig. 6.
Influence of synaptic input in CS and eagx-6. A: extended periods of rhythmic motoneuron activity in CS and eagx-6. Solid box denotes rhythmic synaptic input (expanded timescale shown below, trace indicated by arrow), and dashed box denotes baseline synaptic activity (highlighted in expanded dashed box below) in both CS and eagx-6. Black bar denotes −60 mV in both sets of traces. B: bias current was injected to hyperpolarize the membrane in eagx-6 to observe the effects of membrane potential on spontaneous spike-like events. At −20 mV, action potentials with clear after-spike hyperpolarization were observed. At −60 mV, spontaneous spike-like events (absent in CS) were observed with no visible after-spike hyperpolarization. At −95 mV, these events became larger in amplitude but reduced in frequency.
The spontaneous events in mutants could reflect increased synaptic drive and/or altered intrinsic excitability of the motoneuron. We first attempted to phenocopy the eag mutants by using ELAV-GAL4 to express eag RNAi in all neurons. Similar to eag mutants, ELAV::eag RNAi displayed a more depolarized resting membrane potential of −37 ± 3 mV compared with control, but the average input resistance was not affected (286.93 ± 8 MΩ; Table 1). Spontaneous events, similar to eag mutants, were also observed in ELAV::eag RNAi manipulations (Table 1; dashed box in Fig. 5E). ELAV::eag RNAi motoneurons displayed a nonsignificant increase in frequency of evoked firing compared with control (Fig. 5F).
To help distinguish the effects of synaptic input from intrinsic changes in excitability, eag RNAi was driven in a small population of motoneurons by RRA-GAL4. CS and RRA-GAL4 controls displayed identical firing frequency characteristics (Fig. 5, G and H). No significant change in resting membrane potential or input resistance was observed between RRA-GAL4 control and RRA::eag RNAi. Spontaneous spike-like events were not observed in RRA::eag RNAi motoneurons, and evoked firing frequency was comparable to control. However, in both ELAV and RRA::eag RNAi manipulations, large EPSPs were sometimes observed (Table 1, box in Fig. 5F). Table 1 represents the variability in the frequency of physiological phenotypes.
EAG mutants display increased frequency and amplitude of EPSPs during rhythmic activity.
In some recordings it was possible to observe extended periods of rhythmic synaptic input to motoneurons (Fig. 6A). In CS, bouts of rhythmic synaptic activity lasting 3–4 s with compounding EPSPs of ∼6 mV in amplitude (solid box) and baseline synaptic activity composed of small EPSPs roughly 2 mV in amplitude (dashed box) were observed. The latter were also observed in the absence of rhythmic activity (as shown in Fig. 5A). In eagx-6, longer bouts of rhythmic activity (6–8 s) with larger EPSPs (∼12 mV, solid box) and a higher frequency of baseline synaptic input were observed (dashed box).
DISCUSSION
EAG contributes to diverse K+ currents.
The effects of EAG manipulation on motoneuron function are summarized in Table 2. eag mutants, eagsc29 and eagx-6, and eag RNAi manipulations showed a reduction in IAv and IKv. An increased excitability was also observed in eag mutants. K+ currents play important roles in regulating excitability. IAv are important in maintaining the shape and timing of action potentials. The delay to first spike observed in wild-type T1aCC was reduced in eag mutants displaying spontaneous spike-like events. IAv is responsible for the delay to spike observed in larval motoneurons (Choi et al. 2004). Shal, a voltage-activated K+ channel subunit, contributes to IAv in the adult flight motoneuron MN5 and is probably the predominant IAv in the somatodendritic compartment of embryonic and thoracic larval aCC and RP2 motoneurons (Baines and Bate 1998; Ryglewski and Duch 2009; Schaefer et al. 2010). A prominent reduction in IAv and a decreased delay to spike in Shal RNAi experiments has been observed (Schaefer et al. 2010; Srinivasan et al. 2012). In addition to Shal, EAG subunit also contributes to IAv (present results; Zhong and Wu 1991). However, unlike Shal, eag manipulations also reduce IKv (present results; Zhong and Wu 1991). Shaker, in addition to Shal, contributes to IAv in the adult motoneuron MN5 (Ryglewski and Duch 2009), but ordinarily not in the somatodendritic compartment of larval abdominal motoneurons (Choi et al. 2004). Shaker is predominantly expressed in the presynaptic terminal and contributes to IAv in the larval neuromuscular junction (Ganetzky and Wu 1982; Jan et al. 1977; Singh and Wu 1990; Wu et al. 1983). Mutations in eag also reduce IAv in larval muscle, where synergistic effects of Shaker and EAG have been observed (Ganetzky and Wu 1982). Therefore, EAG contributes to IAv in the soma (present results) and in the presynaptic terminal.
Table 2.
Summary of effects of eag manipulations on K+ currents
| Genotype | Total Transient K+ Current | IAv | Total Sustained K+ Current | IKv | IA(Ca) | IK(Ca) |
|---|---|---|---|---|---|---|
| eagx-6 (compared with CS) | No change | Reduced | No change | No change | No change | Increased |
| eagsc29 (compared with CS) | Reduced | Reduced | Reduced | Reduced | Reduced | Reduced |
| NC: eag RNAi (compared with RRA) | No change | Reduced | No change | Reduced | Increased | No change |
| C: L3 eag RNAi (compared with control) | Reduced | Reduced | Reduced | No change | No change | Reduced |
| C: chronic eag RNAi (compared with control) | No change | Reduced | Reduced | Reduced | No change | Reduced |
| C: chronic vs. late larval eag RNAi | Reduced | No change | No change | Reduced | No change | Reduced |
Comparison of the effect of eag manipulations in total transient K+ current, total sustained K+ current, voltage-activated transient K+ current (IAv), voltage-dependent sustained K+ current (IKv), and Ca2+-sensitive components of transient [IA(Ca)] and sustained currents [IK(Ca)]. Reduction indicates results from the posttest performed on data described in Figs. 1–4. NC, nonconditional eag RNAi experiments in Fig. 3. C, temperature-sensitive “conditional” experiments in Fig. 4.
Heteromultimeric channel assembly.
The diversity of effects may reflect interactions of EAG with other channel subunits. It is unclear whether EAG forms a homomeric channel or is capable of forming heteromultimers with other K+ channel subunits. Evidence of EAG subunits interacting with other K+ channels has been demonstrated previously. In Xenopus oocytes, Shaker and EAG channels interact functionally, and this interaction is dependent on developmental time and expression levels (Chen et al. 2000; Tang et al. 1998). Hyperkinetic, a Shaker β-subunit homolog, and EAG show synergistic interactions in the Drosophila larval neuromuscular junction and also demonstrate a physical interaction (Wilson et al. 1998). Therefore, one possible reason for diverse K+ currents reduced in eag mutants and eag RNAi manipulations could be the heteromultimeric assembly of EAG with other K+ channels. Given our recording situation, the effects of EAG that we observed can be related to the somatodendritic regions of the neuron but not to the presynaptic terminals. In the soma of larval motoneurons, Shal seems to play a dominant role in contributing to IAv (Bergquist et al. 2010; Schaefer et al. 2010; Srinivasan et al. 2012). Since EAG also contributes to IAv (present results), we hypothesize that Shal and EAG could form heteromultimers in the somatic compartment. In the presynaptic terminal, Shaker and EAG contribute to IAv. Therefore, Shaker and EAG could form heteromultimers in the presynaptic terminal. Apart from IAv, IKv is reduced in eagsc29 and EAG RNAi experiments. Shal RNAi manipulations reduce IAv but not IKv, suggesting that the reduction in multiple K+ currents is observed only in EAG manipulations, further supporting the formation of heteromultimers.
Other possible roles of EAG.
We observed allele-specific reduction in K+ currents, as documented elsewhere (Zhong and Wu 1991, 1993). Multiple reasons may be given for this observation. First, the eag mutants used in the study can produce different partial or disrupted protein fragments (as demonstrated by Drysdale et al. 1991). Second, the COOH terminus of EAG can be modulated by cyclic nucleotides and CAMKII (Griffith et al. 1994; Sun et al. 2004; Wang et al. 2002; Zhong and Wu 1993). Direct modulation of EAG channels by cyclic nucleotides has been demonstrated in oocytes (Bruggemann et al. 1993). Allele-specific differences in modulation of IAv and IKv by cGMP have also been observed in Drosophila larval muscles (Bhattacharya et al. 1999; Zhong and Wu 1993). Since we performed in situ whole cell recordings, it is possible that disruption of the membrane altered modulation of EAG channels during measurement of K+ currents. Third, the reduction of EAG can result in upregulation of other K+ channels, such as Shaker and Shal (Peng and Wu 2007). Increased expression of a Shal homolog, Kv4, in the lobster stomatogastric ganglion leads to an increase in hyperpolarization-activated cyclic nucleotide-gated channels (MacLean et al. 2003). In Drosophila larvae, reduction of Shal leads to upregulation of Shaker (Bergquist et al. 2010).
We have demonstrated an embryonic requirement of EAG. IKv was significantly reduced in chronic eag RNAi knockdown compared with larval knockdown, suggesting that the expression of these EAG currents is required throughout embryonic and larval stages. By contrast, IAv was reduced in both chronic and larval manipulations, suggesting contribution of EAG to the current in third instar motoneurons. Interestingly, the total transient current was reduced by larval, but not chronic, eag RNAi expression, suggesting that compensation may have occurred in the latter case. In fact, no reduction in IA(Ca) was observed in eagx-6 or in temperature manipulations, but an upregulation was observed in nonconditional eag RNAi. It is possible that IA(Ca) is upregulated to compensate for the reduction observed in IAv. If such compensations occurred, however, this did not restore normal firing, since we observed the spontaneous events and reduced delay to spike following eag knockdown.
In our experiments we observed discrepancies in the reduction of K+ currents in different EAG manipulations. eagsc29 reduced total transient, IAv and IA(Ca). By contrast, in nonconditional eag RNAi and chronic temperature-sensitive EAG manipulations, which should mimic eagsc29 and eagx-6, only IAv was reduced, not total transient current and IA(Ca). Such variability in knockdown has been observed among eag mutants in the larval muscle and suggests that multiple K+ currents are influenced by EAG (Zhong and Wu 1991). Similarly, in nonconditional eag RNAi, no reduction in total sustained current or IK(Ca) was observed, whereas in chronic EAG manipulations, total sustained K+ current was reduced.
EAG influences excitability in motoneurons by altering firing properties.
Variable physiological phenotypes were observed in eag mutants. Large spontaneous spike-like events were often observed in mutants with little or no current injection. These events may reflect spontaneous action potentials invading the cell body from the spike-initiating region and/or action potentials evoked by large EPSPs. This phenotype could, therefore, result from both pre- and postsynaptic absence of EAG. To distinguish these effects, eag RNAi was expressed in all neurons to mimic eag mutants. Large spontaneous events were also observed in ELAV::eag RNAi. EAG is found in the synaptic neuropil and is absent in eagsc29 (Sun et al. 2004). Thus, similar to eag mutants, ELAV::eag RNAi manipulation could also result in increased synaptic drive to motoneurons. Spontaneous events were not observed, however, when eag RNAi was expressed in motoneurons only, further suggesting the contribution of increased synaptic drive. It should be noted, however, that ELAV-GAL4 is a stronger driver than RRA-GAL4. Therefore, the differences observed between the two eag RNAi manipulations could reflect differences in the degree of knockdown.
Hyperpolarizing the cell increased the amplitude of spontaneous events, consistent with increased driving force for both EPSPs and action potentials, but the reduction in frequency suggests the involvement of voltage-gated currents in the postsynaptic motoneuron. Although RRA-GAL4 manipulation did not cause spontaneous spiking, large EPSPs were sometimes observed, and there were clear effects on K+ currents. A reduction in A-type K+ current in the dendritic region could be responsible for the large EPSPs. On the other hand, a decreased delay to the first spike was observed in eag mutants, suggesting reduction in the density of K+ channels in the spike-initiating zone. aCC motoneurons in eag mutants also have a more depolarized resting membrane potential than wild type. Thus the different phenotypes observed following EAG manipulations could reflect changes in the density and distribution of EAG in various regions of the neuron. The COOH terminus of EAG has been shown to translocate to the nucleus (Sun et al. 2009), which may produce pleiotropic effects. Therefore, the variability observed in the firing phenotypes and K+ currents could also reflect downstream regulation of transcription by EAG (and see below). EAG performs multiple roles in regulating excitability in the cell; therefore, the interpretation of the manifested phenotypes in relationship to specific mechanistic roles becomes challenging.
EAG senses and regulates neuronal excitability.
Given the wealth of information regarding the differential functions of EAG, it is possible that EAG could act as a sensor of excitability. Several lines of evidence support such speculation. The protein structure of EAG suggests it is an amalgam of inward rectifier, depolarization-activated, and cyclic nucleotide-gated channels. The COOH terminus of EAG has numerous sites for modulation. Synthesis of a non-ion-conducting EAG80 splice variant is triggered by Ca2+ influx and activation of PKA or PKC. Translocation of EAG80 to the nucleus provides yet another interesting link between transcriptional regulation and excitability (Sun et al. 2009). Another example is provided by the translocation of COOH-terminal fragment of Cav1.2 channels to the nucleus. Similar to EAG, intracellular Ca2+ triggers the synthesis of Ca2+ channel-associated transcription regulator (CCAT), which regulates transcription (Gomez-Ospina et al. 2006). Sensory neurons in the mouse vomeronasal organ showed stimulus-dependent regulation of ether-à-go-go-related gene expression (ERG) (Hagendorf et al. 2009). Depending on the sensory stimulus, ERG expression levels and resultant K+ current were altered, providing the first in vivo evidence for EAG-related genes as sensors of excitability (Hagendorf et al. 2009). We provide evidence that EAG regulates excitability and firing patterns in Drosophila larval motoneurons and contributes to multiple K+ currents in T1aCC. The variability in firing phenotypes we report in eag mutants is consistent with the possibility that EAG subunits could coassemble with diverse K+ channels, depending on the state of excitability of a neuron.
As demonstrated in this study, EAG represents a class of ion channels that performs multiple roles to regulate excitability. Unlike other K+ channels, mutations in EAG do not cause a single defect, but produce variable phenotypes. Participation of EAG in signaling mechanisms exacerbates these phenotypes. Translocation of EAG to nucleus provides yet another avenue for translating changes in excitability to gene expression. Studies in oocytes and cell culture systems cannot fully test the potential function of EAG, since these systems lack the complete gamut of proteins, signaling systems, and external stimulus that neurons experience. It would be worthwhile to test these possibilities using high-resolution in vivo imaging to monitor localization and translocation of EAG.
GRANTS
This study was supported by National Institute of Neurological Disorders and Stroke Grant NS28495 (to R. B. Levine).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.S. and R.B.L. conception and design of research; S.S. and K.L. performed experiments; S.S. and K.L. analyzed data; S.S. and R.B.L. interpreted results of experiments; S.S. and K.L. prepared figures; S.S. drafted manuscript; S.S., K.L., and R.B.L. approved final version of manuscript; S.S. and R.B.L. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Dr. B. Ganetzky for the generous donation of eag mutants.
REFERENCES
- Baines and Bate, 1998. Baines RA, Bate M. Electrophysiological development of central neurons in the Drosophila embryo. J Neurosci 18: 4673–4683, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist et al., 2010. Bergquist S, Dickman DK, Davis GW. A hierarchy of cell intrinsic and target-derived homeostatic signaling. Neuron 66: 220–234, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya et al., 1999. Bhattacharya A, Gu GG, Singh S. Modulation of dihydropyridine-sensitive calcium channels in Drosophila by a cAMP-mediated pathway. J Neurobiol 39: 491–500, 1999 [DOI] [PubMed] [Google Scholar]
- Bruggemann et al., 1993. Bruggemann A, Pardo LA, Stuhmer W, Pongs O. Ether-à-go-go encodes a voltage-gated channel permeable to K+ and Ca2+ and modulated by cAMP. Nature 365: 445–448, 1993 [DOI] [PubMed] [Google Scholar]
- Camacho, 2006. Camacho J. Ether à go-go potassium channels and cancer. Cancer Lett 233: 1–9, 2006 [DOI] [PubMed] [Google Scholar]
- Chen et al., 1996. Chen ML, Hoshi T, Wu CF. Heteromultimeric interactions among K+ channel subunits from Shaker and eag families in Xenopus oocytes. Neuron 17: 535–542, 1996 [DOI] [PubMed] [Google Scholar]
- Chen et al., 2000. Chen ML, Hoshi T, Wu CF. Sh and eag K+ channel subunit interaction in frog oocytes depends on level and time of expression. Biophys J 79: 1358–1368, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi et al., 2004. Choi JC, Park D, Griffith LC. Electrophysiological and morphological characterization of identified motor neurons in the Drosophila third instar larva central nervous system. J Neurophysiol 91: 2353–2365, 2004 [DOI] [PubMed] [Google Scholar]
- Drysdale et al., 1991. Drysdale R, Warmke J, Kreber R, Ganetzky B. Molecular characterization of eag: a gene affecting potassium channels in Drosophila melanogaster. Genetics 127: 497–505, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka et al., 2003. Fujioka M, Lear BC, Landgraf M, Yusibova GL, Zhou J, Riley KM, Patel NH, Jaynes JB. Even-skipped, acting as a repressor, regulates axonal projections in Drosophila. Development 130: 5385–5400, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganetzky and Wu, 1982. Ganetzky B, Wu CF. Drosophila mutants with opposing effects on nerve excitability: genetic and spatial interactions in repetitive firing. J Neurophysiol 47: 501–514, 1982 [DOI] [PubMed] [Google Scholar]
- Ganetzky and Wu, 1983. Ganetzky B, Wu CF. Neurogenetic analysis of potassium currents in Drosophila: synergistic effects on neuromuscular transmission in double mutants. J Neurogenet 1: 17–28, 1983 [DOI] [PubMed] [Google Scholar]
- Gomez-Ospina et al., 2006. Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R. The C terminus of the L-type voltage-gated calcium channel CaV1.2 encodes a transcription factor. Cell 127: 591–606, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith et al., 1994. Griffith LC, Wang J, Zhong Y, Wu CF, Greenspan RJ. Calcium/calmodulin-dependent protein kinase II and potassium channel subunit eag similarly affect plasticity in Drosophila. Proc Natl Acad Sci USA 91: 10044–10048, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy et al., 1991. Guy HR, Durell SR, Warmke J, Drysdale R, Ganetzky B. Similarities in amino acid sequences of Drosophila eag and cyclic nucleotide-gated channels. Science 254: 730, 1991 [DOI] [PubMed] [Google Scholar]
- Hagendorf et al., 2009. Hagendorf S, Fluegge D, Engelhardt C, Spehr M. Homeostatic control of sensory output in basal vomeronasal neurons: activity-dependent expression of ether-à-go-go-related gene potassium channels. J Neurosci 29: 206–221, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig et al., 2008. Hartwig CL, Worrell J, Levine RB, Ramaswami M, Sanyal S. Normal dendrite growth in Drosophila motor neurons requires the AP-1 transcription factor. Dev Neurobiol 68: 1225–1242, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegle et al., 2006. Hegle AP, Marble DD, Wilson GF. A voltage-driven switch for ion-independent signaling by ether-à-go-go K+ channels. Proc Natl Acad Sci USA 103: 2886–2891, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan et al., 1977. Jan YN, Jan LY, Dennis MJ. Two mutations of synaptic transmission in Drosophila. Proc R Soc Lond B Biol Sci 198: 87–108, 1977 [DOI] [PubMed] [Google Scholar]
- Kaczmarek, 2006. Kaczmarek LK. Non-conducting functions of voltage-gated ion channels. Nat Rev Neurosci 7: 761–771, 2006 [DOI] [PubMed] [Google Scholar]
- Kaplan and Trout, 1969. Kaplan WD, Trout WE., 3rd The behavior of four neurological mutants of Drosophila. Genetics 61: 399–409, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean et al., 2003. MacLean JN, Zhang Y, Johnson BR, Harris-Warrick RM. Activity-independent homeostasis in rhythmically active neurons. Neuron 37: 109–120, 2003 [DOI] [PubMed] [Google Scholar]
- McGuire et al., 2003. McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science 302: 1765–1768, 2003 [DOI] [PubMed] [Google Scholar]
- Peng and Wu, 2007. Peng IF, Wu CF. Differential contributions of Shaker and Shab K+ currents to neuronal firing patterns in Drosophila. J Neurophysiol 97: 780–794, 2007 [DOI] [PubMed] [Google Scholar]
- Robertson et al., 1996. Robertson GA, Warmke JM, Ganetzky B. Potassium currents expressed from Drosophila and mouse eag cDNAs in Xenopus oocytes. Neuropharmacology 35: 841–850, 1996 [DOI] [PubMed] [Google Scholar]
- Ryglewski and Duch, 2009. Ryglewski S, Duch C. Shaker and Shal mediate transient calcium-independent potassium current in a Drosophila flight motoneuron. J Neurophysiol 102: 3673–3688, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer et al., 2010. Schaefer JE, Worrell JW, Levine RB. Role of intrinsic properties in Drosophila motoneuron recruitment during fictive crawling. J Neurophysiol 104: 1257–1266, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh and Wu, 1990. Singh S, Wu CF. Properties of potassium currents and their role in membrane excitability in Drosophila larval muscle fibers. J Exp Biol 152: 59–76, 1990 [DOI] [PubMed] [Google Scholar]
- Srinivasan et al., 2012. Srinivasan S, Lance K, Levine RB. Segmental differences in firing properties and potassium currents in Drosophila larval motoneurons. J Neurophysiol 107: 1356–1365, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun et al., 2009. Sun XX, Bostrom SL, Griffith LC. Alternative splicing of the eag potassium channel gene in Drosophila generates a novel signal transduction scaffolding protein. Mol Cell Neurosci 40: 338–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun et al., 2004. Sun XX, Hodge JJ, Zhou Y, Nguyen M, Griffith LC. The eag potassium channel binds and locally activates calcium/calmodulin-dependent protein kinase II. J Biol Chem 279: 10206–10214, 2004 [DOI] [PubMed] [Google Scholar]
- Tang et al., 1998. Tang CY, Schulteis CT, Jimenez RM, Papazian DM. Shaker and ether-à-go-go K+ channel subunits fail to coassemble in Xenopus oocytes. Biophys J 75: 1263–1270, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al., 2002. Wang Z, Wilson GF, Griffith LC. Calcium/calmodulin-dependent protein kinase II phosphorylates and regulates the Drosophila eag potassium channel. J Biol Chem 277: 24022–24029, 2002 [DOI] [PubMed] [Google Scholar]
- Warmke et al., 1991. Warmke J, Drysdale R, Ganetzky B. A distinct potassium channel polypeptide encoded by the Drosophila eag locus. Science 252: 1560–1562, 1991 [DOI] [PubMed] [Google Scholar]
- Wilson et al., 1998. Wilson GF, Wang Z, Chouinard SW, Griffith LC, Ganetzky B. Interaction of the K channel beta subunit, Hyperkinetic, with eag family members. J Biol Chem 273: 6389–6394, 1998 [DOI] [PubMed] [Google Scholar]
- Wu et al., 1983. Wu CF, Ganetzky B, Haugland FN, Liu AX. Potassium currents in Drosophila: different components affected by mutations of two genes. Science 220: 1076–1078, 1983 [DOI] [PubMed] [Google Scholar]
- Zhong and Wu, 1991. Zhong Y, Wu CF. Alteration of four identified K+ currents in Drosophila muscle by mutations in eag. Science 252: 1562–1564, 1991 [DOI] [PubMed] [Google Scholar]
- Zhong and Wu, 1993. Zhong Y, Wu CF. Modulation of different K+ currents in Drosophila: a hypothetical role for the Eag subunit in multimeric K+ channels. J Neurosci 13: 4669–4679, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]