Abstract
A common modulation of gene expression in aneuploids is an inverse correlation of the monitored gene with the dosage of another segment of the genome. Such effects can be reduced to the action of single genes. One gene previously found to modulate leaky alleles of the white eye color gene in Drosophila is Inverse regulator-a (Inr-a). Heterozygotes of mutations increase the expression of white about 2-fold, and trisomic regions surrounding the gene reduce the expression to about two-thirds of the normal diploid level. Further cytological definition of the location of this gene on the second chromosome led to a candidate pre-mRNA cleavege complex II protein (Pcf11) as the only gene in the remaining region whose mutations exhibit recessive lethality as do alleles of Inr-a. The product of Pcf11 has been implicated in transcriptional initiation, elongation, and termination reactions. Four mutant alleles showed molecular lesions predicted to lead to nonfunctional products of Pcf11. The identification of the molecular lesion of Inr-a provides insight into the basis of this common aneuploidy effect.
Keywords: Drosophila, inverse effect, dosage effect, aneuploidy, gene balance hypothesis
Over three decades ago, Birchler (1979) studied the expression of several enzymes in a dosage series of the long arm of chromosome 1 in maize. Some of the gene products that were not encoded on this chromosome arm were negatively correlated in amount with the dosage of the chromosome arm. The range of effect was within the limits of an inverse correlation, and hence, this effect became known as the “inverse effect.” Subsequent studies on protein profiles in different dosage series of maize indicated that any one protein could be modulated in this way by several regions of the genome (Birchler and Newton 1981). Any one region would modulate some fraction of the total detectable proteins. In addition to inverse effects, there were also direct correlations of protein levels that operated in trans (i.e. variation of a particular chromosome arm would modulate the expression of a protein encoded elsewhere in the genome). Different chromosome arms produced a few to many effects. Further studies indicated that these effects operate on the mRNA level (Birchler et al. 1990; Guo and Birchler 1994). Also, Guo and Birchler (1994) found that the magnitude of these effects was within the limits of direct and inverse correlations of expression with dosage in the triploid endosperm of maize, suggesting that the relative dosage was critical to the response.
An examination of the literature with regard to gene expression in segmental trisomics indicated that the inverse effect was quite prevalent in various organisms, including Datura, barley, Drosophila, and later in human cell lines (Altug-Teber et al. 2007; Huettel et al. 2008; Prandini et al. 2007; Aït Yahya-Graison et al. 2007; Kahlem et al. 2004; McDaniel and Ramage 1970; O’Brien and Gethman 1973; Pipkin et al. 1977; Rawls and Lucchesi 1974; Detwiler and Macintyre 1978; Hall and Kankel 1976; Hodgetts 1975; Moore and Sullivan 1978; Oliver et al. 1978; Smith and Conklin 1975; Nawata et al. 2011; Birchler et al. 1989; Devlin et al. 1988; Birchler 1992; Sun and Birchler 2009). Some of these studies noted these modulations, whereas in others, they are obvious in the presented data. The fact that several regions of the genome modulated the same gene product perhaps led many authors to discount these effects, together with the fact that, in the context of the times, gene regulation was thought to operate by a basically positively acting mechanism, despite the fact that an inverse effect would not be indicative of negative regulation as usually defined. However, the studies in maize (Birchler 1979; Birchler and Newton 1981; Guo and Birchler 1994) included corresponding monosomics and trisomics in their analyses and so it was clear that these effects were not a spandrel of detrimental aneuploid syndromes. Rather, they were negative correlations with chromosomal dosage and not a secondary effect of the aneuploid condition.
Because many different segments of the genome can produce an inverse dosage effect upon any one gene product, it is often the case that the structural gene for a monitored product and any segment that inversely modulates it are varied together in larger aneuploids. When this occurs the gene dosage effect and an inverse dosage effect are of such magnitude that they will cancel each other and generate dosage compensation for the monitored gene product in a dosage series (Birchler 1979, 1981; Birchler and Newton 1981; Devlin et al. 1982; Birchler et al. 1990). That dosage compensation results from such a combination was demonstrated by dissecting larger aneuploid regions into smaller ones and finding the two types of effects as separate entities (Birchler 1981; Birchler et al. 1990).
A mutagenesis screen was developed with the goal of testing whether single gene mutations could mimic the dosage effects that are found in segmental aneuploids. A phenotypic reporter was used in which slight modulations of either an increase or decrease in expression could be recognized. For this, leaky alleles of the white eye color mutation in Drosophila were used. In particular, the white-apricot allele produces an amount of pigment for which changes in the 2-fold range had been classically used (Muller 1932). The rationale of the mutagenesis was that if a mutation is generated that knocks out the expression of a gene responsible for these effects anywhere in the genome, then as a heterozygote, the eye color could be recognized as different from the norm. The individual flies with these changes could then be bred to test the heritability and to study further the nature of the effect and genes involved. In December of 1982, the first such mutation was recovered from a hybrid dysgenesis screen and eventually acquired the name, Inverse regulator-a[hd1] (Rabinow et al. 1991).
This mutation increased the expression of white-apricot about 2-fold as a heterozygote (Rabinow et al. 1991). It was located to chromosome 2 and found that homozygotes were recessive lethal. Additional alleles were recovered based on their failure to complement the recessive lethality. A trisomic region spanning the genetic location of Inr-a reduced the expression of apricot, and the mutation in a triploid increased the pigment levels by a ratio of 3/2 and thus conformed to an inverse relationship. The mutations were found to modulate the white locus on the mRNA level in some developmental stages.
Through a variety of mutageneses and other approaches, eventually 47 modifiers of the white gene were identified, and for many, the molecular identification of a predicted function was made. These include transcription factors, signal transduction components, and chromatin proteins and their modifiers. From a variety of types of evidence, the basis of their dosage effects have been attributed to their involvement in macromolecular complexes (Birchler et al. 2001, 2005; Veitia et al. 2008). In particular, the kinetics of assembly of macromolecular complexes contributes to their dosage sensitivity (Veitia 2002). The kinetics can account for both the positive and negative correlations with dosage (Veitia et al. 2008) and is a potential explanation for why such a diverse set of functions produce similar types of dosage effects.
Yet, despite these advances, the molecular identification of the first identified inversely acting single gene, Inr-a, remained unknown. Here, we describe genetic and molecular analyses that indicate that Inr-a is synonymous with pre-mRNA cleavage complex II protein (Pcf11). Pcf11 has been studied in yeast, Drosophila, and mammalian cells (e.g. Amrani et al. 1997; Sadowski et al. 2003; Zhang et al. 2005; Zhang and Gilmour 2006; West and Proudfoot 2008). Its initially identified function involved transcriptional termination reactions, but subsequent studies have implicated this protein in other aspects of transcription, such as processivity of RNA polymerase II (Zhang et al. 2007) and the recycling of transcription factors for initiation (Mapendano et al. 2010).
Materials and Methods
Stocks
The Inr-a mutations have previously been described (Rabinow et al. 1991). The Inr-ahd1 allele was recovered from a hybrid dysgenesis screen for mutations that as heterozygotes would increase or decrease the amount of color of wa. The Inr-aEMS-2 was independently recovered from an ethyl methane sulfonate chemical mutagenesis on a marked second chromosome based on the failure to complement Inr-ahd1 and then tested for an effect on wa . The Inr-aγC allele was recovered from a gamma irradiation mutagenesis based on using the same approach as for Inr-ahd1. The strain y w67c23; P{w+mC=lacW}Pcf11k08015/CyO is from the Bloomington Stock Center (#10756). The stocks carrying a P or piggyBac element with a FRT site are from Exelixis Inc. The strains e02114 (insertion at 2R: 10740461, genome sequence version R5.42), f05586 (2R: 10740433.0.10740458), and d03333 (2R: 10761429) were used for Pcf11 deletions (Pdel-1 and Pdel-2). The strains f03590 (2R: 10740461), e00756 (2R: 10660106), and d00997 (2R: 10769492) were used for the duplication (Pdup-3 and Pdup-4). Two other strains P{hsFLP}, w1118; Adv/CyO and w1118; wgSp-1/CyO; sensLy-1/TM6B, Tb were also used to generate deletion and duplication strains.
Genetic analysis and FLP-FRT recombination
Deletion and duplication strains were generated according to the company (Exelixis)-provided methods (Parks et al. 2004).
Total RNA and genomic DNA isolation and gDNA and cDNA sequencing
Young adult flies (∼50) were used to prepare genomic DNA (gDNA) with a quick gDNA preparation method (Drosophila Protocols, p431–432). Total RNA from ∼50 late 3rd instar larvae was isolated by TRIzol reagent (GibicoGRL). The RNA was first treated with Turbo DNase (Ambion), then reverse-transcribed using M-MLV reverse transcriptase from Promega. PCR primers were designed and synthesized to amplify fragments of Pcf11. Sequencing was performed at the MU DNA core facility.
Fluorescence in situ hybridization (FISH)
Larvae at the late 3rd instar stage were dissected for salivary glands in 0.7% NaCl. The glands were transferred to a drop of the same solution (∼20 µl) on a cover slip. Then the solution was removed, and 20 µl of acetic acid solution (5 vol. of glacial acetic acid plus 3 vol. of water) was immediately added to cover the glands. After ∼10 min of incubation at room temperature, the salivary glands were squashed and fixed by UV cross-linking (Pardue 2000). Labeling the probes and hybridization conditions were as described (Kato et al. 2011).
Results
Identification of Inr-a mutations
Previously, multiple mutations showing an inverse dosage effect on the white eye color gene were isolated by various genetic mutagenesis screens (Rabinow et al. 1991). Several of them that were located to chromosome 2 showed recessive lethality and failed to complement each other. They were assumed to be one locus and designated as Inr-a.
In an attempt to identify this gene, the Inr-ahd1 mutation was genetically mapped by recombination (Rabinow et al. 1991). The most likely genetic position was 72.5 cM. Using the available deficiency strains at the time with deletions from 46B to 52D to do the complementation tests, no deletion was found to remove the Inr-a mutation. However, three chromosome regions were not covered in the deletions: 46D-47D, 50A-50F, and 51B-51E (Rabinow et al. 1991). The region 51B–51E contains the gene knot mapped at 72.3 cM; thus Inr-a may be located in this region.
The gene Pcf11 seemed to be a candidate of interest in this region (∼80 genes total) because its mutations are recessive lethal. A transposon insertion mutant of Pcf11, l(2)K08015 from the Bloomington Stock Center, was tested and failed to complement the recessive lethality of any Inr-a mutation, suggesting Pcf11 and Inr-a are the same gene. This insertion carries the mini-white marker gene, and thus, it cannot be tested for phenotypic modulation of white alleles.
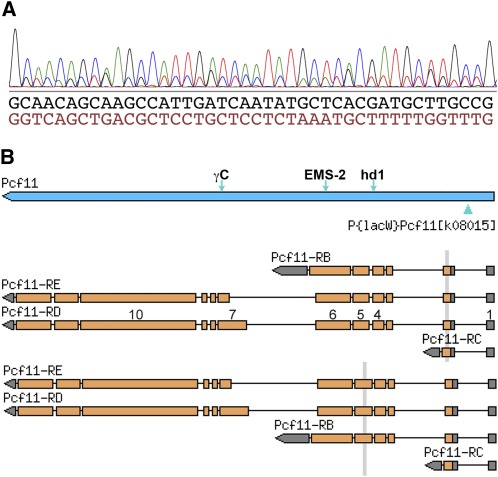
To confirm that the P-element insertion in intron 1 of Pcf11 caused dysfunction of the gene in the l(2)K08015 allele, cDNA was prepared from the heterozygote strain and amplified for sequencing. The sequencing data showed that DNA polymorphisms at exon 10 were present in the cDNA sequence. However, the sequence at the 5′-end (exons 1–6) was mostly double peaked (Figure 1A), indicating two different sequences. After manually reading the sequence trace, both sequences were clear. Blasting the genomic sequence with the 41 bp sequences shown in Figure 1A, they matched exons 2 and 5 (Figure 1B), indicating that the transcript from the P-inserted allele skips exons 2–4, likely due to alternative splicing.
Figure 1.
The P-element insertion in Pcf11 intron 1 causes alternative splicing. (A) The cDNA was prepared from strain l(2) K08015/+; the 5′-end sequencing result is partly shown. The double-peaked graph can be read in two sequences. (B) The BLAST search results indicate that the two sequences match exons 2 and 5. Some exons in RD are numbered on the shaded boxes (open reading frame with brown color). The vertical bars show the matching regions. The green arrowhead below the blue bar representing the Pcf11 gene denotes the position of the P insertion. The other three arrows above the bar denote the positions of the other mutations analyzed in this study.
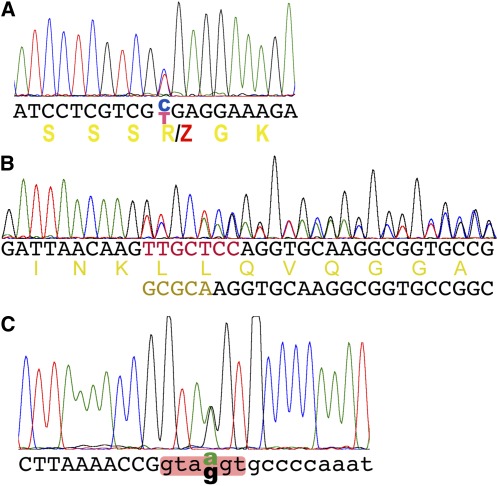
Next, we amplified the genomic DNA and sequenced the Pcf11 gene of three Inr-a heterozygous mutants available in our lab stocks described in Materials and Methods. A stop codon mutation was found in the mutation Inr-aEMS-2, which changes 456R (according to Pcf11-PD) to a stop codon (CGA to UGA in exon 6) (Figure 2A; see Figure 1B for its location in the gene). A frame-shift mutation was found in Inr-aγC: seven base pairs (bp)(TTGCTCC) are replaced by five (GCGCA) in exon 7; thus the amino acid sequence is changed after 656 K (see Figure 2B; see Figure 1B for its location in the gene).
Figure 2.
Lesions identified in the Pcf11 gene from the Inr-a mutations. (A) A stop codon found in the Inr-aEMS-2/+ heterozygote. A base pair of C:G was changed to T:A by EMS mutagenesis, thus changing 456R to a stop codon (Z). The double peaks indicate a nucleotide change. DNA sequence and its translation are shown. (B) A replacement of 7 bp with 5 bp (colored in DNA sequences) was found in the Inr-aγC/+ heterozygote. A series of double peaks is shown. The original and new DNA sequences and the translation of the original one are indicated. (C). Double peaks were found in Pcf11 intron 4 in the Inr-ahd1/+ heterozygote. This change is in the conserved splicing donor site (shaded). DNA sequences of the exon intron were shown as upper- and lowercased letters.
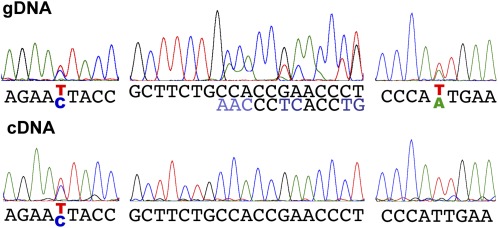
A point mutation was found in the consensus splicing donor site of intron 4 in Inr-ahd1 (see Figure 2C; see Figure 1B for its location in the gene). To confirm that this mutation causes alternative splicing, first we attempted to PCR the cDNA from the heterozygous strain with primers anchored in exon 4 and exon 5, but we failed to see polymorphisms. Then we attempted to detect the alternative splicing by taking advantage of polymorphisms between the DNA sequences of the two alleles. Single-base polymorphisms and a 6 bp deletion are distributed along the codon sequence. By sequencing the cDNA and gDNA of the same heterozygous strain, we observed cDNA polymorphisms (double peaks in the sequencing traces) at the 5′ end before intron 4 but not at the 3′ end including the polymorphisms of the deletion (a series of double peaks after the deletion point) (Figure 3). This result indicates that the mutant copy of Pcf11 expressed a truncated mRNA unable to function. Taken together, we conclude that Inr-a is synonymous with Pcf11.
Figure 3.
The mRNA of both alleles of Pcf11 was detected at the 5′ end, but only one allele was detected at the 3′ end in the Inr-ahd1/+ heterozygote. Upper row shows the genomic DNA polymorphisms (double peaks). Lower row shows the cDNA sequences at the corresponding positions. When the polymorphisms disappear, this indicates that only one allele is expressed. Left, single nucleotide polymorphism at position 1089; middle, deletion of six nucleotides starts at position 7869; right, single nucleotide polymorphism at position 8696. DNA sequences are shown with paired colored letters for double peaks.
Short cytological regions containing Pcf11 demonstrate an inverse dosage effect
The dosage effect of Inr-a was demonstrated previously by using mutations, which presumably disrupt a copy of the gene (confirmed by our study), and a large duplication (44C–50B). Because the content in this large fragment is complex and many genes within are functionally unknown, the question arises whether the expression effect detected was caused by an extra dose of Inr-a or the collectively varied genes.
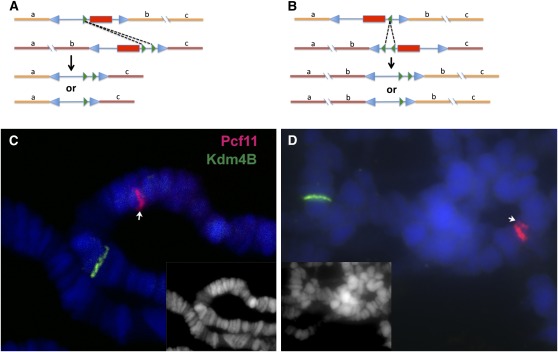
To clarify this point and further confirm Pcf11 as an inverse-dosage gene, we tried to delete and duplicate Pcf11 in a small region using the FLP-FRT system. P- or piggyback-element–containing FRT sites were previously transformed into the fly genome, and collections of stocks, each with an element located variously ,were generated by Exelixis Inc. Strains were carefully chosen so that a deletion or duplication could be generated from these strains covering the gene Pcf11 without the mini-white marker (Figure 4, A and B). These deletion or duplication strains could be used to test the dosage effect on white-apricot (wa) later.
Figure 4.
Generation of deletion and duplication of Pcf11 by FLP-FRT–mediated recombination. (A) Sketch for the strategy of making the deletion Pdel-1. The piggyback element RB and P-element XP (the blue arrowheads indicate the repeats at the ends) carrying FRT sequences (green arrowheads) are shown inserted in two copies of the chromosome, in which the sequence orders are indicated by letters a, b, and c. Fragment b contains the Pcf11 gene. The mini-white genes are shown as red rectangles. After recombination, two possibilities of deletions without mini-white are shown. (B) Similar sketch for generating the duplication Pdup-3. Here piggyback WH and XP are shown. (C, D) Probes for detecting Pcf11 and Kdm4B were labeled with Texas-Red or Alexa Fluor 488 (green). The genes were detected on salivary gland polytene chromosomes. Heterozygous deletion of Pcf11 Pdel-1/+ was confirmed in panel C with a half band in red (arrow). Heterozygous duplication Pdup-3/+ was confirmed in panel D with one-and-a-half bands (arrow). The DAPI staining channel is shown in insets.
Two deletion strains, Pdel-1 and Pdel-2, were generated spanning Pcf11 flanked with six genes. The sizes of the deletions are ∼21.0 kb, and they share one end point (2R: 10761429, genome sequence version R5.42); the other ends are very close (2R: 10740461 and 2R: 10740433). Both strains showed a dosage effect on wa and were recessive lethal. We focused on Pdel-1 for later experiments.
Also, a tandem duplication of ∼29 kb, Pdup-3, was generated, including Pcf11 and another 10 genes (from 2R 10740461 to 10769492). Another tandem duplication, Pdup-4, was generated with the same start point downstream but extending farther upstream with a size of 109 kb (from 2R: 10660106 to 10769492). Both duplications showed similar dosage effects and were lethal as homozygotes. The shorter duplication, Pdup-3, was used for further analyses.
To confirm the deletions and duplications, we first sequenced the original stocks to insure the transposons were present and inserted at the right loci. Then using Pcf11 and a “control” gene Kdm5B (which is located ∼1 Mb upstream) as probes, we applied FISH to the polytene chromosomes of salivary glands. The deletions were confirmed by half-bands of Pcf11 (Figure 4C), which represent a signal on only one of the two homologs present. The duplication Pdup-3 was more difficult to be detected by FISH. Occasionally when the homologous chromosomes were separated, we noticed the brightness of the Pcf11 band was doubled on one homolog compared with the other. In rare cases, double bands could be seen on one homolog but not on the other; that is, a half band and a whole band together when the homologs were not separated (Figure 4D).
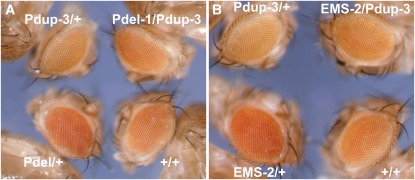
To test the dosage effects of the deletion and duplication, we crossed these mutants with the wa marker. This reporter allele was previously shown to be effective to indicate the dosage effect. As expected, wa expression as shown by the eye color was increased in the deletion strain (1 copy of Pcf11) and reduced in the duplication strain (three copies) compared with the wild-type (two copies) (Figure 5A). When the deletion and duplication is combined (two total copies), the eye color is restored to a similar level as the wild-type (Figure 5A). Pdup-3 can also cancel the dosage effect of Inr-aEMS-2, as expected (Figure 5B), indicating a copy number rescue of the mutant phenotype. Because wa has a copia retrotransposon insertion into white, an additional point mutation allele, wa2, was tested as previously to confirm that the effects are not specific to apricot (Rabinow et al. 1991). The allele Inr-aEMS-2 and Pdel1/+ increased wa2 eye color above normal and Pdup3/+ reduced it (Figure S1). Therefore, the results are consistent with the inverse dosage effect being caused by a single gene copy number change in the genome.
Figure 5.
The Pcf11 deletion and duplication mutations show dosage effects on white-apricot (wa). The eye color indicates the expression level of wa. The genotypes of the eyes are briefly indicated (“EMS-2” denotes “Inr-aEMS-2”). One copy of Pcf11 (“Pdel-1/+” in panel A and “EMS-2/+” in panel B) produced the strongest color and three copies (“Pdup-3/+” in both panels A and B) produced the weakest color. When the deletion or the mutation were combined with the duplication, the eye color was largely restored to the wild-type (“Pdel-1/Pdup-3” in panel A and “EMS-2/Pdup-3” in panel B compared with “+/+”).
Discussion
In this study, a more precise localization of Inr-a was conducted. With this information, only a few possible genes remained to associate the inverse effect phenotype with a DNA sequence. The alleles of Inr-a exhibit a recessive lethality, and only one gene in the cytologically defined region did the same: Pcf11. Four different types of alleles of Inr-a, namely, a P-element insertion, an allele from hybrid dysgenesis, an EMS-induced allele, and a gamma irradiation–induced allele were examined for lesions in Pcf11. Through comparisons of the cDNA and genomic DNA from heterozygous stocks, all four of these Inr-a mutations possess a molecular lesion in Pcf11 that would be predicted to lead to a nonfunctional product.
As noted above, Pcf11 has been implicated in affecting transcriptional initiation, elongation, and termination reactions. We hope the finding that a dosage series of this gene that produces an inverse effect on a reporter target gene will serve to more fully understand how Pcf11 gene functions. Alternatively, the inverse dosage effect is a common modulation of gene expression (Sabl and Birchler 1993; Guo and Birchler 1994) found in aneuploids in diverse organisms, so the identification of an example single gene with this response will provide a system in which to learn more about this effect.
Supplementary Material
Acknowledgments
This research was supported by National Institutes of Health Grant RO1-GM-068042.
Footnotes
Communicating editor: K. S. McKim
Literature Cited
- Aït Yahya-Graison E., Aubert J., Dauphinot L., Rivals I., Prieur M., et al. , 2007. Classification of human chromosome 21 gene-expression variations in Down Syndrome: impact on disease phenotypes. Am. J. Hum. Genet. 81: 475–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altug-Teber O., Bonin M., Walter M., Mau-Holzman U. A., Dufke A., et al. , 2007. Specific transcriptional changes in human fetuses with autosomal trisomies. Cytogenet. Genome Res. 119: 171–184 [DOI] [PubMed] [Google Scholar]
- Amrani N., Minet M., Wyers F., Dufour M. E., Aggerbeck L. P., et al. , 1997. PCF11 encodes a third protein component of yeast cleavage and polyadenylation factor I. Mol. Cell. Biol. 17: 1102–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler J. A., 1979. A study of enzyme activities in a dosage series of the long arm of chromosome one in maize. Genetics 92: 1211–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler J. A., 1981. The genetic basis of dosage compensation of alcohol dehydrogenase-1 in maize. Genetics 97: 625–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler J. A., 1992. Expression of cis-regulatory mutants of the white locus in metafemales of Drosophila melanogaster. Genet. Res. 59: 11–18 [DOI] [PubMed] [Google Scholar]
- Birchler J. A., Newton K. J., 1981. Modulation of protein levels in chromosomal dosage series of maize: the biochemical basis of aneuploid syndromes. Genetics 99: 247–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler J. A., Hiebert J. C., Krietzman M., 1989. Gene expression in adult metafemales of Drosophila melanogaster. Genetics 122: 869–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler J. A., Hiebert J. C., Paigen K., 1990. Analysis of autosomal dosage compensation involving the Alcohol dehydrogenase locus in Drosophila melanogaster. Genetics 124: 677–686 [PMC free article] [PubMed] [Google Scholar]
- Birchler J. A., Bhadra U., Pal Bhadra M., Auger D. L., 2001. Dosage dependent gene regulation in multicellular eukaryotes: Implications for dosage compensation, aneuploid syndromes and quantitative traits. Dev. Biol. 234: 275–288 [DOI] [PubMed] [Google Scholar]
- Birchler J. A., Riddle N. C., Auger D. L., Veitia R. A., 2005. Dosage balance in gene regulation: biological implications. Trends Genet. 21: 219–226 [DOI] [PubMed] [Google Scholar]
- Devlin R. H., Holm D. G., Grigliatti T. A., 1982. Autosomal dosage compensation in Drosophila melangaster strains trisomic for the left arm of chromosome 2. Proc. Natl. Acad. Sci. USA 79: 1200–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin R. H., Holm D. G., Grigliatti T. A., 1988. The influence of whole-arm trisomy on gene expression in Drosophila. Genetics 118: 87–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J., Kankel D. R., 1976. Genetics of acetylcholinesterase in D. melanogaster. Genetics 83: 517–533 [PMC free article] [PubMed] [Google Scholar]
- Hodgetts R. B., 1975. Response of DOPA decarboxylase activity variations in gene dosage in Drosophila: a possible location of the structural gene. Genetics 79: 45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel B., Kreil D. P., Matzke M., Matzke A. J. M., 2008. Effects of aneuploidy on genome structure, expression and interphase organization in Arabidopsis thaliana. PLoS Genet. 4: e1000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detwiler C., MacIntyre R., 1978. A genetic and developmental analysis of an acid deoxyribonuclease in Drosophila melanogaster. Biochem. Genet. 16: 1113–1134 [DOI] [PubMed] [Google Scholar]
- Guo M., Birchler J. A., 1994. Trans-acting dosage effects on the expression of model gene systems in maize aneuploids. Science 266: 1999–2002 [DOI] [PubMed] [Google Scholar]
- Kahlem P., Sultan M., Herwig R., Steinfath M., Balzereit D., et al. , 2004. Transcript level alterations reflect gene dosage effects across multiple tissues in a mouse model of Down syndrome. Genome Res. 14: 1258–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A., Lamb J. C., Albert P. S., Danilova T., Han F., et al. , 2011. Chromosome painting for plant biotechnology, pp. 67–96 Plant Chromosome Engineering, edited by Birchler J. A. Humana Press, New York: [DOI] [PubMed] [Google Scholar]
- Mapendano C. K., Lykke-Andersen S., Kjems J., Bertrand E., Jensen T. H., 2010. Crosstalk between mRNA 3 end processing and transcription initiation. Mol. Cell 40: 410–422 [DOI] [PubMed] [Google Scholar]
- McDaniel R. G., Ramage R. T., 1970. Genetics of primary trisomic series in barley: identification by protein identification. Can. J. Genet. Cytol. 12: 155–167 [Google Scholar]
- Moore G. P., Sullivan D. T., 1978. Biochemical and genetic characterization of kynurenine formamidase from Drosophila melanogaster. Biochem. Genet. 16: 619–634 [DOI] [PubMed] [Google Scholar]
- Muller H. J., 1932. Further studies on the nature and causes of gene mutations. Proc. 6th Int. Congr. Genet. 1: 213–255 [Google Scholar]
- Nawata H., Kashino G., Tano K., Daino K., Shimada Y., et al. , 2011. Dysregulation of gene expression in the artificial human trisomy cells of chromosome 8 associated with transformed cell phenotypes. PLoS ONE 6: e25319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien S. J., Gethman R. C., 1973. Segmental aneuploidy as a probe for structural genes in Drosophila: mitochondrial membrane enzymes. Genetics 75: 155–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver M. J., Huber R. E., Williamson J. W., 1978. Genetic and biochemical aspects of trehalase from Drosophila melanogaster. Biochem. Genet. 16: 927–940 [DOI] [PubMed] [Google Scholar]
- Pardue M.-L., 2000. In situ hybridization to polytene chromosomes, pp. 119–127 in Drosophila Protocols, edited by Sullivan W., Ashburner M., Hawley R. S. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Parks A. L., Cook K. R., Belvin M., Dompe N. A., Fawcett R., et al. , 2004. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat. Genet. 36: 288–292 [DOI] [PubMed] [Google Scholar]
- Pipkin S. B., Chakrabartty P. K., Bremner T. A., 1977. Location and regulation of Drosophila fumarase. J. Hered. 68: 245–252 [Google Scholar]
- Prandini P., Deutsch S., Lyle R., Gagnebin M., Vivier C. D., et al. , 2007. Natural gene-expression variation in Down syndrome modulates the outcome of gene-dosage imbalance. Am. J. Hum. Genet. 812: 252–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinow L., Nguyen-Huynh A. T., Birchler J. A., 1991. A trans-acting regulatory gene that inversely affects the expression of the white, brown and scarlet loci in Drosophila. Genetics 129: 463–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls J. M., Lucchesi J. C., 1974. Regulation of enzyme activities in Drosophila. I. The detection of regulatory loci by gene dosage responses. Genet. Res. 24: 59–72 [DOI] [PubMed] [Google Scholar]
- Sabl J. F., Birchler J. A., 1993. Dosage dependent modifiers of white alleles in Drosophila melanogaster. Genet. Res. 62: 15–22 [DOI] [PubMed] [Google Scholar]
- Sadowski M., Dichtl B., Hübner W., Keller W., 2003. Independent functions of yeast Pcf11p in pre-mRNA 3′ end processing and in transcription termination. EMBO J. 22: 2167–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. H., Conklin M. E., 1975. Effects of gene dosage on peroxidase isozymes in Datura stramonium trisomics, pp. 603–618 in Isozymes, Vol. 3, edited by Markert C. L. Academic Press, New York [Google Scholar]
- Sun X., Birchler J. A., 2009. Interaction study of the male specific lethal (MSL) complex and trans-acting dosage effects in metafemales of Drosophila melanogaster. Cytogenet. Genome Res. 124: 298–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veitia R. A., 2002. Exploring the etiology of haploinsufficiency. Bioessays 24: 175–184 [DOI] [PubMed] [Google Scholar]
- Veitia R. A., Bottani S., Birchler J. A., 2008. Cellular reactions to gene dosage imbalance: genomic, transcriptomic and proteomic effects. Trends Genet. 24: 390–397 [DOI] [PubMed] [Google Scholar]
- West S., Proudfoot N. J., 2008. Human Pcf11 enhances degradation of RNA polymerase II-associated nascent RNA and transcriptional termination. Nucleic Acids Res. 36: 905–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Gilmour D. S., 2006. Pcf11 is a termination factor in Drosophila that dismantles the elongation complex by bridging the CTD of RNA polymerase II to nascent transcript. Mol. Cell 21: 65–74 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Fu J., Gilmour D. S., 2005. CTD-dependent dismantling of the RNA polymerase II elongation complex by the pre-mRNA 3′end processing factor, Pcf11. Genes Dev. 19: 1572–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Klatt A., Henderson A. J., Gilmour D. S., 2007. Transcription termination factor Pcf11 limits the processivity of Pol II on an HIV provirus to repress gene expression. Genes Dev. 21: 1609–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.