Summary
Background and objectives
Linear epitopes on the Goodpasture autoantigen involved in human anti-glomerular basement membrane (GBM) disease are not fully defined. This study investigated the linear epitopes recognized by circulating antibodies in anti-GBM patients, aiming to identify the potential nephrogenic linear epitopes and their clinical significance.
Design, setting, participants, & measurements
Sixty-eight patients with anti-GBM disease were enrolled. Twenty-four overlapping linear peptides were synthesized across the whole sequence of the human Goodpasture autoantigen. ELISA detected circulating antibodies against linear epitopes. Their associations with clinical features were further analyzed.
Results
Antibodies against linear peptides were detected in sera from 55 patients (80.9%). Three major epitopes with high frequencies were identified: P14 (41%), P16 (36.8%), and P18 (57%). P14, a formerly defined T cell epitope, was a mutual B cell epitope. Antibodies against P14 were frequently detected in patients with positive antineutrophil cytoplasmic antibodies (39.3% versus 12.5%; P=0.01). Patients with anti-P16 antibodies presented with higher serum creatinine on diagnosis (665.5±227.2 versus 443.7±296.8 μmol/L; P=0.001) and worse renal outcome during follow-up (hazard ratio, 2.10; 95% confidence interval, 1.10–3.90; P=0.02). The level of anti-P18 antibodies positively correlated with the percentage of crescents in glomeruli (r=0.54; P=0.008). Recognition of P22 was an independent predictor for patient death (hazard ratio, 3.02; 95% confidence interval, 1.20–7.57; P=0.02).
Conclusions
Antibodies against linear epitopes on the Goodpasture autoantigen could be detected in human anti-GBM disease and were associated with kidney injury. P14 was a mutual T and B cell epitope, implying its nephrogenic role in disease initiation.
Introduction
Anti-glomerular basement membrane (GBM) disease, also known as Goodpasture’s disease, is an autoimmune disorder characterized by rapidly progressive GN and a high risk for alveolar hemorrhage (1,2). It has been regarded as a prototypical example of autoantibody-mediated disease with a well documented autoantigen, which has been identified as the noncollagenous domain (NC1) of α3 chain of type IV collagen (α3(IV)NC1) (3–6). Two major immunodominant regions, EA and EB, have been mapped to residues 17–31 and 127–141 of the α3(IV)NC1 (7). Further studies define them as conformational epitopes that are sequestrated in the quaternary structure of GBM dependent on a critical sulfilimine bond (8,9).
The pathogenic role of circulating antibodies against the Goodpasture autoantigen has been demonstrated in passive transfer studies (10–13). However, there is now compelling evidence for the importance of T cell–mediated autoimmunity in the initiation of disease. In vitro studies have shown that peripheral CD4+ T cells in anti-GBM patients proliferated with the same autoantigen of α3(IV)NC1 recognized by anti-GBM antibodies (14). In animal models either lacking T cells or with an interrupted B7/CD28 co-stimulation pathway, experimental crescentic GN is alleviated (15–17). Direct evidence comes from the passive transfer studies showing that in the absence of anti-GBM antibodies, the antigen-specific CD4+ T cells per se could initiate kidney injury (18). Subsequent studies have successfully identified a 13-mer peptide, pCol(28–40), that could induce autoantibodies against the peptide itself, autoreactive CD4+ T cell proliferation, and severe crescentic GN in Wistar Kyoto rats (19). Moreover, this pathogenic linear epitope could also trigger a diversified anti-GBM antibody response through intramolecular and intermolecular B cell epitope spreading (20).
Nonetheless, it has proven difficult to define the fine pathogenic linear epitopes on α3(IV)NC1 molecules recognized by autoreactive T cells in humans, because mapping studies require a large quantity of peripheral blood to isolate T cells that is not feasible in clinical practice. Interestingly, a recent study on experimental autoimmune GN identified a similar 15-mer linear peptide, pCol(24–38), from rat α3(IV)NC1 as a mutual B and T cell epitope, which could initiate GN (21). The finding of a mutual linear epitope shared by both T and B cells may enable us to explore the nephrogenic T cell epitopes in human anti-GBM disease by examining linear peptides recognized by B cells. We speculated that in patients with anti-GBM disease, certain linear epitopes might be involved in disease initiation, whereas others might act as risk epitope(s) to induce severe kidney injury. In this study, we designed a panel of overlapping synthetic linear peptides covering the whole sequence of human α3(IV)NC1. In a large cohort of anti-GBM patients, circulating antibodies against linear peptides were detected and their associations with clinical features were investigated, aiming to explore the role of linear epitopes of human α3(IV)NC1 in the pathogenesis of anti-GBM disease.
Materials and Methods
Sera and Patients
Sera from 68 patients with anti-GBM disease, diagnosed at Peking University First Hospital from 1997 to 2008, were collected and preserved at −20°C until use. On diagnosis, sera from all patients were positive for anti-GBM autoantibodies by ELISA using purified bovine α(IV)NC1 and recombinant human α3(IV)NC1 as solid phase antigens. Antineutrophil cytoplasmic antibodies (ANCAs) were screened by indirect immunofluorescence assay (Euroimmun, Lubeck, Germany) and antigen-specific ELISA for antibodies against myeloperoxidase (MPO) and proteinase 3 (PR3). The sera were collected on diagnosis before immunosuppressive treatment and plasmapheresis. Clinical data were collected at the time of diagnosis as well as during follow-up. Renal pathology data included examinations of light microscopy and direct immunofluorescence microscopy. Twenty-four sera obtained from healthy blood donors were used as normal controls. Informed consent was obtained for tissue and blood sampling.
Preparation of Antigens
The published sequence of human α3(IV)NC1 was used to synthesize peptides (7). A panel of 24 peptides was synthesized covering the whole sequence of human α3(IV)NC1, based on a series of 20-mer peptides overlapping with 10 amino acids. Peptides were synthesized on an automatic peptide synthesizer using Fmoc (9-fluorenyl-methyloxycarbonyl) chemistry (Beijing Scilight Biotechnology Ltd Co, Beijing, China), and purified by a reverse-phase CIS column on a preparative HPLC. Purified peptides were analyzed by HPLC for purity and mass spectrometry for the correct sequence. Peptides with purity >98% were used for further tests.
Sequences of each peptide are listed in Table 1. Among them, P3 and P14 contained the sequences that constitute conformational B cell immunodominant regions EA and EB (7), respectively.
Table 1.
Sequences of linear peptides on α3(IV)NC1 and frequencies and levels of antibodies against them in sera from patients with anti-glomerular basement membrane disease
| Peptide | Sequence from N-Terminal | Position from N-Terminal | Recognition, n (%) | Mean Levels of Antibodies |
|---|---|---|---|---|
| P1 | SPATWTTRGFVFTRHSQTTA | 1–20 | 0 | — |
| P2 | VFTRHSQTTAIPSCPEGTVPL | 11–31 | 1 (1.5) | 0.10 |
| P3 | TAIPSCPEGTVPLYSGFSFLFV | 19–40 | 6 (8.8) | 0.51 |
| P4 | LYSGFSFLFVQGNQRAHGQD | 31–50 | 6 (8.8) | 0.18 |
| P5 | QGNQRAHGQDLGTLGSCLQR | 41–60 | 0 | — |
| P6 | LGTLGSCLQRFTTMPFLFCN | 51–70 | 4 (5.9) | 0.30 |
| P7 | FTTMPFLFCNVNDVCNFASR | 61–80 | 4 (5.9) | 0.30 |
| P8 | VNDVCNFASRNDYSYWLSTPA | 71–91 | 4 (5.9) | 0.18 |
| P9 | NDYSYWLSIPALMPMNMAPI | 81–100 | 4 (5.9) | 0.92 |
| P10 | ALMPMNMAPITGRALEPYIS | 91–110 | 2 (2.9) | 0.44 |
| P11 | TGRALEPYISRCTVCEGPAI | 101–120 | 1 (1.5) | 0.79 |
| P12 | RCTVCEGPAIAIAVHSQTTD | 111–130 | 0 | — |
| P13 | AIAVHSQTTDIPPCPHGWIS | 121–140 | 4 (5.9) | 0.35 |
| P14 | TDIPPCPHGWISLWKGFSFIMF | 129–150 | 28 (41.2) | 0.29 |
| LWKGFSFIMFTSAGSEGTGQa | 141–160 | |||
| P15 | TSAGSEGTGQALASPGSCLE | 151–170 | 7 (10.3) | 0.79 |
| P16 | ALASPGSCLEEFRASPFLEC | 161–180 | 25 (36.8) | 0.72 |
| P17 | EFRASPFLECHGRGTCNYYS | 171–190 | 5 (7.4) | 0.50 |
| P18 | HGRGTCNYYSNSYSFWLASL | 181–200 | 39 (57.4) | 1.29 |
| P19 | NSYSFWLASLNPERMFRKPI | 191–210 | 4 (5.9) | 0.09 |
| P20 | NPERMFRKPIPSTVKAGELE | 201–220 | 3 (4.4) | 0.27 |
| P21 | PSTVKAGELEKIISRCQVCM | 211–230 | 7 (10.3) | 0.46 |
| P22 | KIISRCQVCMKKRH | 221–234 | 15 (22.1) | 0.38 |
| P23 | KGFSFIMFTSAGSEb | 143–156 | — | — |
| P24 | FIMFTSAGSEGTGQ | 157–160 | 2 ( 2.9) | 0.18 |
N=68 patients.
This sequence was not available for peptide synthesis due to synthetic difficulties. Two smaller peptides derived from it were then designed and successfully synthesized as P23 and P24.
P23 was insoluble and could not be tested in ELISA.
Detection of Linear Epitope Specificity
Each peptide was diluted at 10 μmol/L with 0.05 mol/L bicarbonate buffer (pH 9.6) and coated onto half of the wells of a polystyrene microtiter plate (Nunc, Roskild, Denmark). The other half of the wells were coated with bicarbonate buffer alone as antigen-free wells to exclude nonspecific binding. The volume of each well was 50 μl. Incubation was carried out at 37°C for 60 minutes. Test sera were diluted at 1:100 in PBS containing 0.05% Tween-20 and 0.2% BSA (PBST-BSA) and added in duplication to both antigen-coated wells and antigen-free wells at 37°C for 30 minutes. After washing thrice, alkaline phosphatase–conjugated goat antihuman IgG (Fc specific; Sigma, St. Louis, MO) diluted 1:4000 was added. Incubation resumed at 37°C for 30 minutes. After washing, P-nitrophenyl phosphate (1 mg/ml; Sigma) in substrate buffer (1 mol/L diethanolamine, 0.5 mmol/L MgCl2, pH 9.8) was used as substrate, and color development was measured spectrophotometrically at 405 nm (Bio-Rad, Tokyo, Japan) 30 minutes later. Samples were re-examined when SEMs >10% were found. The net absorbance values were calculated (the absorbance values of peptide-coated wells minus the absorbance values of antigen-free wells) for each testing serum on each individual peptide, respectively. Twenty-four sera from healthy blood donors diluted at 1:100 were used to build up the cutoff values using mean + 2 SD.
Cross-Reaction between Antibodies against Linear Peptides and Intact a3(IV)NC1
Cross-reaction between antibodies against the linear peptides and intact α3(IV)NC1 was investigated using inhibition ELISA. In brief, polystyrene microtiter plates were coated with α3(IV)NC1. The diluted sera were preincubated with either soluble α3(IV)NC1 from 0.1 to 10 μg/ml or linear peptides from 0.1 to 10 μmol/L at 37°C for 60 minutes, respectively. The mixtures were then transferred to the α3(IV)NC1-coated microtiter plates and the bound antibodies were detected with alkaline phosphatase–conjugated secondary antibodies, as described above.
Statistical Analyses
Differences in quantitative parameters were assessed using the t test for data that were normally distributed or the nonparametric test for data that were not normally distributed. Differences in qualitative data were compared using the chi-squared test and Fisher’s exact test. Pearson and Spearman rank correlations were performed to analyze the relationship between the levels of antibodies against linear peptides and the clinical data. Peptides with recognition <5% were excluded from further statistical analysis. Kaplan–Meier curves were used to analyze patient survival and renal survival. Univariate survival analysis was performed using the log-rank test. Multivariate survival analysis was performed using the Cox regression model. Results were expressed as hazard ratios (HRs) with 95% confidence intervals (95% CIs). A P value <0.05 was considered significant.
Results
Demographic Features and Clinical Data of Patients
This study enrolled 68 patients with anti-GBM disease. The demographic and clinical data of patients are shown in Table 2. Of the 68 patients, 41 patients (60.3%) progressed to ESRD and 19 patients (27.9%) died during follow-up. Forty-seven patients received a kidney biopsy, all of which were demonstrated with linear IgG deposition along GBM, with or without C3 by direct immunofluorescence. ESRD patients and very elderly individuals (>75 years old) could not undergo kidney biopsy. The disease diagnosis was confirmed by recombinant human α3(IV)NC1-specific ELISA with dual antigen-coated and antigen-free wells.
Table 2.
Demographic data and clinical and pathologic parameters of patients with anti-glomerular basement membrane disease
| Parameter | n (%) |
|---|---|
| Age (yr) | 42.9±19.9 |
| Sex (male/female) | 47/21 |
| Prodromal infection | 36 (52.9) |
| Hydrocarbon exposure | 6 (8.8) |
| Smoking | 25 (36.8) |
| Hemoptysis | 35 (51.5) |
| Hemoglobin (g/L) | 85.0±24.0 |
| Oliguria/anuria | 17 (25) |
| Urinary protein (g/24 h) | 3.6±3.4 |
| Nephrotic syndrome | 14 (20.6) |
| Gross hematuria | 17 (25) |
| Serum creatinine on diagnosis (μmol/L) | 525.3±292.1 |
| Positive ANCA | 16 (23.5) |
| MPO/PR3-ANCA | 14/2 |
| Level of anti-GBM antibodies (U/ml) | 59.3±36.6 |
| Crescents in glomeruli (%) | 71.9±34.3 |
| Renal survival at 1 yr | 23 (33.8) |
| Patient survival at 1 yr | 52 (76.5) |
N=68 patients. ANCA, antineutrophil cytoplasmic antibody; MPO, myeloperoxidase; PR3, proteinase 3; GBM, glomerular basement membrane.
Frequency of Serum Antibodies Recognizing Linear Peptides
We synthesized 24 overlapping linear peptides across the whole sequence of human α3(IV)NC1. However, peptide 23 was insoluble and could not be used for further tests.
Of the 68 patients, sera from 55 patients (80.9%) showed at least one of the linear peptides. Patients with antibodies against linear peptides presented with higher serum creatinine on diagnosis than those without the antibodies (564.8±284.8 versus 358.0±271.8 μmol/L; P=0.03). No other significant differences were found between patients with and without antibodies against linear peptides (P>0.05).
Frequencies of Serum Antibodies Recognizing Each Linear Peptide
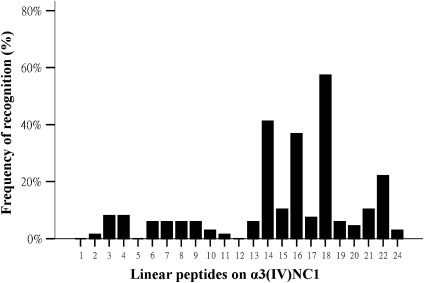
Of the 23 linear peptides tested, 20 peptides were found in the sera from patients with anti-GBM disease. High frequencies of recognition were found for three linear peptides: P14 (41.2%), P16 (36.8%), and P18 (57.4%) (Table 1, Figure 1). Among them, P14 contained the immunodominant region EB (7) and was proven to be one of the T cell epitopes in patients with Goodpasture disease (22). The recognition for other linear peptides was lower, ranging from 1.5% to 22.1%. No recognition was found for P1, P5, and P12.
Figure 1.
Frequencies of antibodies against each linear peptide in patients with anti-glomerular basement membrane disease. α3(IV)NC1, noncollagenous domain NC1 of α3 chain of type IV collagen.
There were 16 patients with positive ANCA results; 14 of whom had sera specific to MPO and 2 had sera specific to PR3. We found that patients with antibodies against P14 presented with a significantly higher prevalence of positive ANCA (39.3% versus 12.5%; P=0.01). For antigen specificity of ANCA, the association with P14 was significant for MPO-ANCA (34.6% versus 12.5%; P=0.03) but not for PR3-ANCA.
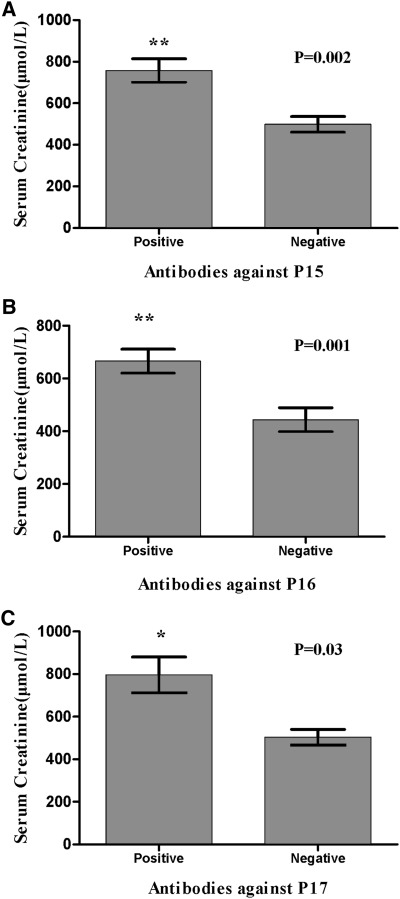
Patients with antibodies against P15, P16, or P17 presented with higher levels of serum creatinine on diagnosis, which were as follows: 757.4±148.4 versus 498.6±293.4 μmol/L for P15 (P=0.002), 665.5±227.2 versus 443.7±296.8 μmol/L for P16 (P=0.001), and 796.4±188.5 versus 503.8±289.0 μmol/L for P17 (P=0.03) (Figure 2). Moreover, when recognition of P15–P17 was combined (defined as detection of antibodies against either of P15, P16, or P17), we found that patients with antibodies against P15–17 had a significantly higher percentage of crescents in glomeruli (83.5%±25.2% versus 61.6%±38.2%; P=0.03). Patients with antibodies against P19 had a higher frequency of hemoptysis (100% versus 33.3%; P=0.03).
Figure 2.
Mean levels of serum creatinine on diagnosis compared among patients with and without antibodies against P15, P16, and P17. Comparisons between patients with and without antibodies against (A) P15 (P=0.002), (B) P16 (P=0.001), and (C) P17 (P=0.03).
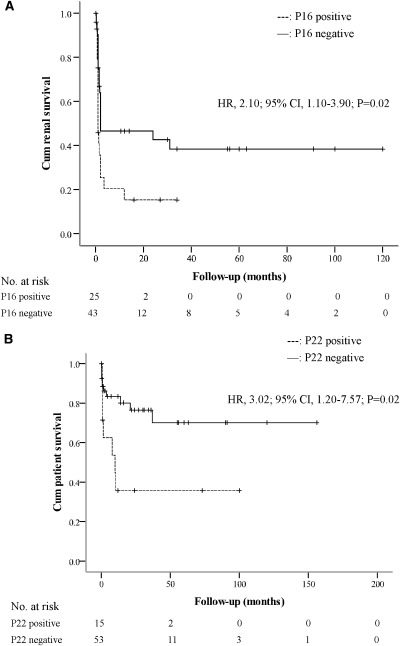
We used log-rank tests and Kaplan–Meier curves for univariate survival analysis to assess renal outcome of patients during follow-up, and we found that the recognition of P16 was a risk factor for the end stage of renal failure (HR, 2.10; 95% CI, 1.10–3.90; P=0.02) (Figure 3A). For patient outcomes, the recognition of P22 was the independent predictor for patient death (HR, 3.02; 95% CI, 1.20–7.57; P=0.02) (Figure 3B).
Figure 3.
Renal and patient outcome in patients with and without antibodies against P16 and P22, respectively. Kaplan–Meier analysis for renal (A) and patient (B) survival compared between patients with and without antibodies against P16 and P22, respectively. HR, hazard ratio; 95% CI, 95% confidence interval.
Levels of Serum Antibodies against Each Linear Peptide
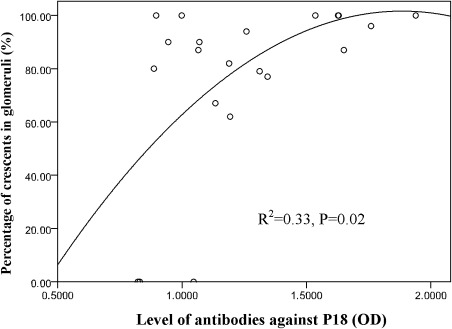
The mean levels of antibodies against each linear peptide are shown in Table 1. We found that the level of antibodies against P21 was closely correlated with the serum level of antibodies against α3(IV)NC1 (r=0.82; P=0.02). The level of antibodies against P18 was positively correlated with the percentage of crescents in glomeruli (r=0.54; P=0.008). Furthermore, the level of antibodies against P18 was an independent determinant factor to predict the percentage of glomerular crescents on kidney biopsy (R2=0.33; P=0.02) (Figure 4). It was suggested that a higher level of antibodies against P18 predicted a higher percentage of glomerular crescents.
Figure 4.
Correlation between the level of antibodies against P18 and the percentage of crescents in glomeruli. A positive correlation was shown between the level of antibodies against P18 and the percentage of crescent formation in glomeruli (R2=0.33; P=0.02).
Number of Linear Peptides Recognized by Serum from Each Patient
The number of linear peptides recognized by serum from each patient was different, ranging from 0 to 9. The association between the number of linear peptides recognized by each patient and the clinical data were further investigated. The number of peptide recognitions per patient was positively correlated with the serum level of antibodies against α3(IV)NC1 (r=0.31; P=0.02).
Cross-Reaction of Antibodies against Linear Peptides and Intact α3(IV)NC1
Because P14 contained the sequence constituting a conformational epitope EB on α3(IV)NC1 and the level of antibodies against P21 was positively correlated with the serum level of antibodies against α3(IV)NC1, inhibition ELISA was performed to detect whether there was cross-reaction between antibodies against the linear peptides and intact α3(IV)NC1. Binding of α3(IV)NC1 IgG was strongly inhibited by soluble α3(IV)NC1 but not the peptides. The absence of cross-reaction of antibodies against α3(IV)NC1 and the peptides indicated that they were two distinct populations.
Discussion
Three novel observations in this study are described here. First, antibodies against linear epitopes on the Goodpasture autoantigen were detected in patients with anti-GBM disease. Three major linear epitopes were identified with high frequencies of recognition: P14, P16, and P18. Second, a mutual T and B cell epitope, P14, was revealed in patients with anti-GBM disease, which was not only a target antigen of circulating antibodies found in this study but was also a formerly defined T cell epitope in Goodpasture patients (22). Third, the frequencies and levels of antibodies against linear epitopes were found, for the first time, to be associated with the severity of kidney injury of anti-GBM patients.
In this study, we reported a novel panel of circulating anti-GBM antibodies with target antigens of linear epitopes on the Goodpasture autoantigen. These antibodies could be detected in 80.9% of a large cohort of 68 patients, which implied their common existence in anti-GBM disease. Linear epitopes of anti-GBM antibodies were previously reported. Levy et al. (23) identified nine reactive regions on α3(IV)NC1, whereas their results were not confirmed by Hellmark et al. (24). Given the different designs of overlapping peptides and the relatively small groups of patients, no conclusions could be drawn from these studies. In this study, the high detection rate from a large cohort of patients indicated that antibodies against linear epitopes on the Goodpasture autoantigen do exist in human anti-GBM disease. Furthermore, no cross-reaction was demonstrated between antibodies against linear peptides and α3(IV)NC1, indicating that antibodies against linear epitopes were distinct from those against conformational epitopes on α3(IV)NC1.
P14, amino acid residues 129–150, was formerly defined as a T cell epitope that could stimulate peripheral CD4+ T cell proliferation in Goodpasture patients (22). Previous studies have demonstrated that P14 contained the core sequence with highest predicted binding affinity with HLA-DR15 (25,26), which was proven to be the genetic marker for susceptibility to anti-GBM disease. The finding that a mutual T and B cell epitope exists in human anti-GBM disease, in accordance with the results from animal models, favors the hypothesis that certain nephrogenic linear epitopes might initiate the disease by stimulating both humoral and cellular responses.
Intramolecular epitope spreading of B cells has been demonstrated in Wistar Kyoto rats with immunization by a nephrogenic T cell epitope that not only induced antibodies specific for this linear peptide but also triggered classic antibodies against conformational epitopes on α3(IV)NC1 (20). Meanwhile, evidence for intermolecular epitope spreading from α3(IV)NC1 to α1–5(IV)NC1 has been revealed in disease initiation and progression in human anti-GBM disease (27). The linear epitope recognized by both T and B cells might be the starting and key point of epitope spreading. The current finding of a mutual T and B cell epitope in anti-GBM patients, for the first time, provides underpinnings for a hypothesis on intramolecular epitope spreading in human anti-GBM disease. We also noticed that, in each patient, the number of recognized linear epitopes was positively correlated with the level of antibodies against intact α3(IV)NC1. It is possible that patients with a broader linear epitope spectrum have undergone a more active immune stimulus resulting in higher level of immune response.
Our results showed that 20%–40% of patients with anti-GBM disease also had ANCA-positive sera. The mechanism for the coexistence of these two sets of autoantibodies is still inconclusive. No cross-reaction has been demonstrated between them at the B cell level; however, whether there is T cell cross-reaction remains unclear (28,29). In this study, antibodies against P14 were found strongly associated with positive ANCA. As a mutual T and B cell epitope, P14 might be a possible link between anti-GBM disease and ANCA-associated vasculitis in cellular immune response. A T cell cross-reaction against P14 is speculated in the pathogenesis of these double positive patients; further studies are needed to prove this hypothesis.
The recognition for the cluster of amino acid residues 151–190 was associated with the severity of crescentic GN in human anti-GBM disease. P16 was in the center of the cluster and recognized with the highest frequency. The recognition for P16 was identified to be associated with higher concentration of serum creatinine on diagnosis and was a poor prognostic indicator for renal outcome. We noticed that P16 contained one of the core sequences on α3(IV)NC1 that could be naturally processed and presented by antigen presenting cells (25,30). It is likely that these autoreactive B cells against P16 may serve as professional antigen presenting cells to stimulate autoreactive T cells. Thus, both autoantibodies and autoreactive T cells were generated, potentially leading to a sustained autoimmune response and worse kidney injury (31).
In this study, it is interesting that antibodies against linear epitopes were associated with not only the severity of kidney injury but also with the clinical phenotypes and patient prognosis, such as P19 recognition with pulmonary hemorrhage and P22 with patient death. Patients with anti-P22 antibodies were significantly older (55.2±22.5 versus 39.4±17.8 years, P=0.006), which could be an explanation for worse outcomes (32). However, for P19, the low frequency of recognition prevented us drawing any conclusions from it.
The association between clinical features and classic anti-GBM antibodies against different epitopes or antigens has been fully proven (27,33). The differences in linear epitope recognition, as the starting point of intramolecular and intermolecular epitope spreading, may contribute to the production of different patterns of autoimmune reaction and lead to different clinical phenotypes. Therefore, some linear epitopes such as P14 may be associated with disease initiation, whereas others such as P16 or P22 may be associated with disease progression or may be called risk epitopes. These risk epitopes and their associations with clinical presentation may help us to understand the pathogenesis of anti-GBM disease. Further studies are needed to fully elucidate the disease pathogenesis.
A limitation of this study is that not all patients had a kidney biopsy, especially patients with ESRD or very elderly individuals (>75 years old). For those patients, the diagnosis was confirmed by using the recombinant human α3(IV)NC1-specific ELISA with dual antigen-coated and antigen-free wells. Furthermore, we re-analyzed the data excluding patients without kidney biopsy and found that the results were highly comparable with our earlier results.
In conclusion, circulating antibodies against linear epitopes on the Goodpasture autoantigen could be detected commonly in patients with anti-GBM disease. P14 might be the mutual nephrogenic epitope for both T and B cells, implying intramolecular epitope spreading in the initiation of human anti-GBM disease.
Disclosures
None.
Acknowledgments
The authors greatly appreciate the technical support provided by Ying Zhang.
This work was supported by grants from the Chinese 973 Program (2012CB517702), the Natural Science Fund of China to the Innovation Research Group (81021004), and the National Natural Science Fund of China (81170645).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Goodpasture EW: The significance of certain pulmonary lesions in relation to the etiology of influenza. Am J Med Sci 158: 863–870, 1919 [DOI] [PubMed] [Google Scholar]
- 2.Wilson CB, Dixon FJ: Anti-glomerular basement membrane antibody-induced glomerulonephritis. Kidney Int 3: 74–89, 1973 [DOI] [PubMed] [Google Scholar]
- 3.Saus J, Wieslander J, Langeveld JP, Quinones S, Hudson BG: Identification of the Goodpasture antigen as the alpha 3(IV) chain of collagen IV. J Biol Chem 263: 13374–13380, 1988 [PubMed] [Google Scholar]
- 4.Turner N, Mason PJ, Brown R, Fox M, Povey S, Rees A, Pusey CD: Molecular cloning of the human Goodpasture antigen demonstrates it to be the alpha 3 chain of type IV collagen. J Clin Invest 89: 592–601, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steblay RW, Rudofsky U: In vitro and in vivo properties of autoantibodies eluted from kidneys of sheep with autoimmune glomerulonephritis. Nature 218: 1269–1271, 1968 [DOI] [PubMed] [Google Scholar]
- 6.Edgington TS, Glassock RJ, Dixon FJ: Autologous immune-complex pathogenesis of experimental allergic glomerulonephritis. Science 155: 1432–1434, 1967 [DOI] [PubMed] [Google Scholar]
- 7.Borza DB, Netzer KO, Leinonen A, Todd P, Cervera J, Saus J, Hudson BG: The goodpasture autoantigen. Identification of multiple cryptic epitopes on the NC1 domain of the alpha3(IV) collagen chain. J Biol Chem 275: 6030–6037, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Pedchenko V, Bondar O, Fogo AB, Vanacore R, Voziyan P, Kitching AR, Wieslander J, Kashtan C, Borza DB, Neilson EG, Wilson CB, Hudson BG: Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N Engl J Med 363: 343–354, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanacore R, Ham AJ, Voehler M, Sanders CR, Conrads TP, Veenstra TD, Sharpless KB, Dawson PE, Hudson BG: A sulfilimine bond identified in collagen IV. Science 325: 1230–1234, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalluri R, Danoff TM, Okada H, Neilson EG: Susceptibility to anti-glomerular basement membrane disease and Goodpasture syndrome is linked to MHC class II genes and the emergence of T cell-mediated immunity in mice. J Clin Invest 100: 2263–2275, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sado Y, Naito I, Okigaki T: Transfer of anti-glomerular basement membrane antibody-induced glomerulonephritis in inbred rats with isologous antibodies from the urine of nephritic rats. J Pathol 158: 325–332, 1989 [DOI] [PubMed] [Google Scholar]
- 12.Kohda T, Okada S, Hayashi A, Kanzaki S, Ninomiya Y, Taki M, Sado Y: High nephritogenicity of monoclonal antibodies belonging to IgG2a and IgG2b subclasses in rat anti-GBM nephritis. Kidney Int 66: 177–186, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Reynolds J, Albouainain A, Duda MA, Evans DJ, Pusey CD: Strain susceptibility to active induction and passive transfer of experimental autoimmune glomerulonephritis in the rat. Nephrol Dial Transplant 21: 3398–3408, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Salama AD, Chaudhry AN, Ryan JJ, Eren E, Levy JB, Pusey CD, Lightstone L, Lechler RI: In Goodpasture’s disease, CD4(+) T cells escape thymic deletion and are reactive with the autoantigen alpha3(IV)NC1. J Am Soc Nephrol 12: 1908–1915, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Li S, Holdsworth SR, Tipping PG: Antibody independent crescentic glomerulonephritis in mu chain deficient mice. Kidney Int 51: 672–678, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Tipping PG, Huang XR, Qi M, Van GY, Tang WW: Crescentic glomerulonephritis in CD4- and CD8-deficient mice. Requirement for CD4 but not CD8 cells. Am J Pathol 152: 1541–1548, 1998 [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds J, Tam FW, Chandraker A, Smith J, Karkar AM, Cross J, Peach R, Sayegh MH, Pusey CD: CD28-B7 blockade prevents the development of experimental autoimmune glomerulonephritis. J Clin Invest 105: 643–651, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Hicks J, Borillo J, Glass WF, 2nd, Lou YH: CD4(+) T cells specific to a glomerular basement membrane antigen mediate glomerulonephritis. J Clin Invest 109: 517–524, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J, Borillo J, Glass WF, Hicks J, Ou CN, Lou YH: T-cell epitope of alpha3 chain of type IV collagen induces severe glomerulonephritis. Kidney Int 64: 1292–1301, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Bolton WK, Chen L, Hellmark T, Wieslander J, Fox JW: Epitope spreading and autoimmune glomerulonephritis in rats induced by a T cell epitope of Goodpasture’s antigen. J Am Soc Nephrol 16: 2657–2666, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Reynolds J, Haxby J, Juggapah JK, Evans DJ, Pusey CD: Identification of a nephritogenic immunodominant B and T cell epitope in experimental autoimmune glomerulonephritis. Clin Exp Immunol 155: 311–319, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cairns LS, Phelps RG, Bowie L, Hall AM, Saweirs WW, Rees AJ, Barker RN: The fine specificity and cytokine profile of T-helper cells responsive to the alpha3 chain of type IV collagen in Goodpasture’s disease. J Am Soc Nephrol 14: 2801–2812, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Levy JB, Coulthart A, Pusey CD: Mapping B cell epitopes in Goodpasture’s disease. J Am Soc Nephrol 8: 1698–1705, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Hellmark T, Brunmark C, Trojnar J, Wieslander J: Epitope mapping of anti-glomerular basement membrane (GBM) antibodies with synthetic peptides. Clin Exp Immunol 105: 504–510, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phelps RG, Jones VL, Coughlan M, Turner AN, Rees AJ: Presentation of the Goodpasture autoantigen to CD4 T cells is influenced more by processing constraints than by HLA class II peptide binding preferences. J Biol Chem 273: 11440–11447, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Phelps RG, Jones V, Turner AN, Rees AJ: Properties of HLA class II molecules divergently associated with Goodpasture’s disease. Int Immunol 12: 1135–1143, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Zhao J, Cui Z, Yang R, Jia XY, Zhang Y, Zhao MH: Anti-glomerular basement membrane autoantibodies against different target antigens are associated with disease severity. Kidney Int 76: 1108–1115, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Jayne DR, Marshall PD, Jones SJ, Lockwood CM: Autoantibodies to GBM and neutrophil cytoplasm in rapidly progressive glomerulonephritis. Kidney Int 37: 965–970, 1990 [DOI] [PubMed] [Google Scholar]
- 29.Short AK, Esnault VL, Lockwood CM: Anti-neutrophil cytoplasm antibodies and anti-glomerular basement membrane antibodies: Two coexisting distinct autoreactivities detectable in patients with rapidly progressive glomerulonephritis. Am J Kidney Dis 26: 439–445, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Phelps RG, Turner AN, Rees AJ: Direct identification of naturally processed autoantigen-derived peptides bound to HLA-DR15. J Biol Chem 271: 18549–18553, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Lin RH, Mamula MJ, Hardin JA, Janeway CA, Jr: Induction of autoreactive B cells allows priming of autoreactive T cells. J Exp Med 173: 1433–1439, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui Z, Zhao J, Jia XY, Zhu SN, Zhao MH: Clinical features and outcomes of anti-glomerular basement membrane disease in older patients. Am J Kidney Dis 57: 575–582, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Yang R, Hellmark T, Zhao J, Cui Z, Segelmark M, Zhao MH, Wang HY: Levels of epitope-specific autoantibodies correlate with renal damage in anti-GBM disease. Nephrol Dial Transplant 24: 1838–1844, 2009 [DOI] [PubMed] [Google Scholar]