Summary
ESRD has become an important problem for elderly patients. The segment of the ESRD population age 65 years or older has grown considerably, and this growth is expected to accelerate in coming years. Nephrologists caring for the elderly with advanced kidney disease will encounter patients with comorbid conditions common in younger patients, as well as physical, psychological, and social challenges that occur with increased frequency in the aging population. These challenging factors must be addressed to help inform decisions regarding the option to initiate dialysis, the choice of dialysis modality, whether to pursue kidney transplantation, and end-of-life care. This article will highlight some common problems encountered by elderly patients with ESRD and review data on the clinical outcomes of elderly patients treated with different modalities of dialysis, outcomes of kidney transplantation in the elderly, and nondialytic management of CKD stage 5.
Introduction
ESRD is becoming a geriatric condition. Among incident patients, those older than age 75 years outnumber those age 65–74 years and have the highest incident growth rate (1). Contributing factors include increased prevalence of diabetes and hypertension, improved life expectancy, and increased willingness to initiate dialysis therapy in the elderly. As the Baby Boomer generation ages, this growth should accelerate.
This review identifies challenges encountered by the elderly that complicate their ESRD care and examines data on dialysis modality and transplantation outcomes to help inform decisions on initiation of renal replacement therapy (RRT). We explore what is known about nondialytic management, discuss end-of-life issues, and offer suggestions for future work.
Although “elderly” and “geriatric” generally refer to those 65 years of age or older, there is heterogeneity in function, lifestyle, and life expectancy; some younger patients have worse functional impairment than certain octogenarians. The literature also varies in the definition of what ages are considered “elderly.” In this review, “elderly” refers to those age 65 years or older unless otherwise specified.
Geriatric Challenges
Geriatric syndromes are multifactorial constellations of signs, symptoms, and events involving multiple organ systems that predispose to increased morbidity and mortality. Examples include frailty, falls, and functional impairment. The presence of cognitive impairment and uremia may accentuate these issues in elderly patients with advanced CKD and ESRD.
Frailty
The term “frailty” often appears in the literature as an adjective describing patients with diminished reserve. However, frailty is a phenotype associated with increased risk for falls (hazard ratio [HR], 2.06 [95% confidence interval (CI), 1.64–2.59]), disability (HR, 5.61 [95% CI, 4.50–7.00]), hospitalization (HR, 2.25 [95% CI, 1.94–2.62]), and death (HR, 6.47 [95% CI, 4.63–9.03]) (2). Among the general population, 7% of those older than age 65 and 40% of those older than age 80 meet criteria for frailty (Table 1) (2,3).
Table 1.
Criteria for frailty
| Unintentional weight loss |
| Slow walking speed |
| Weakness |
| Exhaustion |
| Low physical activity |
Data obtained from reference 2.
In a retrospective analysis of the Third National Health and Nutrition Examination Survey, Wilhelm-Leen and colleagues found two-fold higher age-adjusted risk for frailty with mild CKD, which increased to a six-fold greater risk with an estimated GFR (eGFR) <45 ml/min per 1.73 m2. These findings persisted after correction for multiple comorbid conditions (3). Frailty and CKD were associated with increased mortality, and the combination was additive (3). Johansen et al. reported a higher rate of frailty in older patients with ESRD (age < 40 years, 44.4%; age 60–70 years, 74.2%; age >80 years, 78.8%) (4). For those with frailty, after 1 year adjusted HRs were 2.24 (95% CI, 1.60–3.15) for mortality and 1.56 (95% CI, 1.36–1.79) for hospitalization (4).
Although frailty increases with age, chronologic age is not a criterion. CKD is associated with frailty in populations unselected for age, suggesting that patients with CKD are prone to the physical and functional impairments that comprise frailty. Nephrologists should actively assess for these impairments so that corrective rehabilitative efforts can be pursued.
Falls
Studies suggest that >45% of elderly patients undergoing dialysis have one or more falls per year (5,6). Falls are an important risk factor for fractures, future falls, and death in geriatric populations. Frequency of syncope, presyncope, or dizziness did not differ between hemodialysis (HD) patients age 65 years or older, but those older than age 65 were more likely to fall (38% versus 4%) (7). There was a greater pre- to post-dialysis decrease in standing systolic BP in older than younger patients that did not vary between older patients who fell and those who did not, suggesting that orthostatic hypotension cannot fully explain falls. Increased risk for falls in the elderly may be due to unmeasured differences in functional impairment.
In HD patients (mean age, 75 years), mortality increased with at least one fall (HR, 1.63 [95% CI, 1.02–2.28]) (8). Risk increased with advanced age, comorbidity, and recent initiation of HD (8). Although falls from acute illnesses were excluded from that study to limit confounding, it is still not clear whether falls play a direct mechanistic role in mortality or are simply a surrogate marker of disease burden.
Functional Impairment
Studies demonstrated significant functional impairment (disability) among elderly dialysis patients (9,10). Functional impairment is the decreased ability to perform activities of daily living (ADLs) or instrumental activities of daily living (IADLs) (Table 2).
Table 2.
Activities of daily living and instrumental activities of daily living
| Activities of Daily Living | Instrumental Activities of Daily Living |
|---|---|
| Eating | Medication management |
| Dressing | Maintaining personal finances |
| Toileting | Cooking |
| Maintaining personal hygiene | Driving |
| Transferring | Shopping |
| Bed mobility | Telephone use |
| Walking | Care of pets |
Of 162 HD patients older than age 65 years, 8 had no disability, 69 had impaired IADLs, and 85 had impaired ADLs and IADLs (11). Factors associated with impairment included increased prescription drugs, decreased mobility, and lower educational level (11).
Using U.S. Renal Data System (USRDS) and information on functional impairment from the Minimum Data Set Activities of Daily Living, Kurella Tamura et al. reported marked decline in functional status in 3702 nursing home patients (mean age, 73.4 years) 3 months before initiation of maintenance dialysis (12). Only 39% maintained baseline function 3 months after initiation, and only 13% did so by 12 months (12). Twelve-month mortality was 58% after initiation (12). A smaller, retrospective study of mostly community-dwelling elderly persons (mean age, 84.5 years) also reported that 30% experienced significant decline in function 6 months after initiation (13).
Together, these studies suggest that dialysis initiation in the setting of functional impairment is unlikely to reverse disability. The time surrounding dialysis initiation is associated with risk for functional decline and represents a target for rehabilitation. Evidence from Canada suggests that functionally impaired HD patients achieved improved function and independence through inpatient rehabilitation (10).
Cognitive Impairment
An association between renal failure and cognitive impairment has been reported (14–16). The point prevalence of cognitive impairment and dementia was two-fold higher in patients with ESRD than in those without ESRD (17). In 825 participants of the Chronic Renal Insufficiency Cohort Study age 55 years or older, lower eGFR was associated with cognitive impairment, especially at an eGFR < 30 ml/min per 1.73 m2 (18). Mean 4-year cognitive decline was more pronounced with lower baseline eGFR (mean age, 74 years) (17). In 145 dialysis patients, cognitive impairment was independently associated with mortality (19).
Causality between cognitive impairment and renal failure is unclear. A single-center study of HD patients (mean age, 63 years) found no effect of dialysis adequacy on cognition (20). In addition, cognitive impairment complicates assessment of uremia and affects the decision to initiate dialysis. Among nursing home patients (mean age, 74 years), cognitive decline was associated with early (eGFR > 15 ml/min per 1.73 m2) initiation (21). These studies do not address the layer of complexity that cognitive impairment may add to decision-making capacity regarding dialysis initiation. Moreover, the potential effect of slowing cognitive decline on ESRD outcomes requires further investigation.
Decision to Initiate RRT
The decision to initiate RRT in the elderly is complicated by more challenges than in younger patients. Beyond geriatric syndromes, they are more likely to have problems with nonmedical barriers, including limited transportation, family support, and income (22,23). The elderly also have more cardiovascular and overall comorbid conditions and reduced life expectancy compared with younger patients (22,23).
Timing of dialysis initiation must be considered. The IDEAL (Initiating Dialysis Early and Late) study found no benefit for early initiation (24), and another study suggested that it may be associated with harm in the elderly, perhaps because of accelerated loss of residual renal function (25). Therefore, it becomes important to provide patients and families with prognostic information regarding timing of initiation, further complicated by assessing the competing mortality risk (likelihood of death in some elderly before ESRD is reached) (26).
A recent clinical practice guideline aims to aid decision-making for dialysis initiation (27). Shared decision-making, wherein patient values and preferences are strongly considered, is emphasized (27). Advanced care planning is highlighted through identification of preferences and use by some states of specific documents (i.e., Physician Orders for Life-Sustaining Treatment) (27). The guideline emphasizes communication skills for breaking bad news and conflict resolution (27). Time-limited dialysis trials are useful in cases with uncertain prognosis or no consensus (27–29).
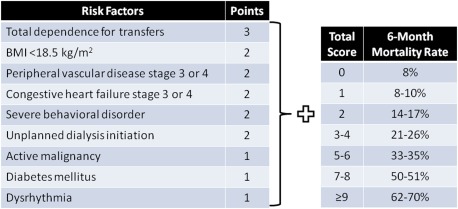
Prognostic tools to guide decision-making for elderly are available. Couchoud et al. validated a 6-month tool for incident elderly dialysis patients using clinical factors (Figure 1) (30). The highest-risk group (score ≥9) had a 6-month mortality rate of 70%, compared with 8% for the lowest-risk group (score, 0) (30).
Figure 1.
Six-month prognostic risk score in patients ≥75 years who initiate dialysis. Adapted from a prognostic model developed and validated by Couchoud et al. (30) using the French Renal Epidemiology and Information Network registry to predict 6-month mortality in ESRD patients ≥75 years who initiated dialysis. BMI, body mass index.
Cohen validated an integrated 6-month tool among prevalent dialysis patients featuring the “surprise” question: “Would I be surprised if this patient died in the next 12 months?” (31,32). The model uses five variables and has been incorporated into a web-based application (Table 3) (32).
Table 3.
Variables in the prognostic model to predict 6-month survival for patients undergoing maintenance hemodialysis
| “Surprise” questiona (yes or no) |
| Dementia (yes or no) |
| Peripheral vascular disease (yes or no) |
| Age |
| Serum albumin |
Data obtained from reference 32. The model is available as an online calculator: http://www.qxmd.com/apps/calculate-by-qxmd.
“Would I be surprised if this patient died in the next year?”
Maximal Nondialytic Conservative Management
Some patients with ESRD do not pursue RRT. A nondialytic strategy known in Europe as maximum conservative management (MCM) may be used (33–37). This intensive CKD approach is undertaken in a multidisciplinary clinic staffed by dietitians, social workers, and other support personnel. Patients receive the same care as they do in earlier CKD stages, with emphasis on supportive, symptomatic care.
Wong et al. analyzed 73 patients with CKD stage 5 who did not pursue dialysis (38). Most had been advised against initiation because of lack of expected mortality benefit. The 1-year survival rate was 65% (median survival duration, 1.95 years). In 2 years, 60% of patients had no hospitalizations. Among patients who died, 71% died at home.
Murtagh et al. retrospectively analyzed patients older than age 75 years in an MCM clinic (39). After study criteria were applied, 129 patients were analyzed: 52 who chose dialysis and 77 who chose MCM. None receiving MCM switched to dialysis. Dialysis withdrawal rates and hospice use were not discussed. Dialysis patients achieved a 2-year survival rate of 76% compared with 47% for those receiving MCM. However, mortality benefit was not observed for patients with cardiovascular disease or high comorbidity.
Smith and colleagues retrospectively analyzed a similar cohort, demonstrating similar survival benefit with dialysis (40). These studies may have been confounded by selection bias because MCM patients had significantly higher comorbidity and functional impairment than dialysis patients (40). A subset of patients for whom MCM had been recommended chose HD, with equivalent median survival (40).
Despite a potential survival benefit, dialysis may not improve quality of life; 65% of deaths among dialysis patients occurred while they were hospitalized, compared with 27% for those receiving MCM (40). Carson et al. also reported that dialysis patients lived longer than MCM patients (median survival duration, 37.8 months versus 13.9 months, respectively) but spent more time in or at the hospital (173 days per patient-year versus 16) (41). Those receiving MCM were four-fold more likely to die at home or with hospice (41).
Across studies, patients receiving MCM have fewer hospitalizations and are more likely to receive hospice services. Such information is helpful in counseling patients and families. Functional status and comorbidity must be considered, and individualized patient goals beyond mortality estimates (i.e., lifestyle changes, hospitalization, and loss of independence) must be deliberately addressed (26,27).
There are no United States outcomes data for maximal nondialytic conservative management. The nondialytic option is not commonly discussed before initiation. Caution is advised in extrapolating data because of observed cultural, religious, and social differences in advance care planning and dialysis withdrawal between the United States and Europe (27,42,43). In addition, available studies are limited by retrospective design and small sample sizes. Clearly this is an area warranting further investigation.
Choice of RRT
HD versus Peritoneal Dialysis
Survival of elderly dialysis patients is poor (Table 4) (44). The increase in relative life expectancy for the two oldest groups (Table 4) is probably influenced by decreased life expectancy in the age-matched general population (44). The all-cause hospitalization rate was 2.1 admissions per patient-year-at-risk for dialysis patients older than age 75 and 1.8 for those age 45–64 years (1). Cardiovascular disease is the leading cause of hospitalization and death for older patients and occurs more frequently in that group than in younger patients (1).
Table 4.
Percentage of remaining life expectancy for each age group with ESRD compared to the age-matched general population
| Age Group (yr) | Life Expectancy Compared with Age-Matched General Population (%) |
|---|---|
| 65–69 | 22.4 |
| 70–74 | 23.7 |
| 75–79 | 25.7 |
| 80–84 | 28.8 |
| ≥85 | 44.2 |
USRDS data obtained from reference 44.
Permanent vascular access is often problematic in older HD patients. A meta-analysis demonstrated increased failure of radiocephalic arteriovenous fistulas in older compared with younger patients (45). Age varied among reviewed studies but was typically older than 60 years (45). Lok and colleagues found a similar fistula survival rate between older (>65 years) and younger patients, although older patients primarily had brachiocephalic fistulas (46). Number of access-related interventions did not significantly differ (46).
In elderly HD patients, tunneled catheters are associated with increased mortality (47). However, catheter-based HD is the only option for many. One study found no difference in catheter use, infection rates, or flow-related malfunction in older (>75 years) versus younger patients (40–60), suggesting that although catheters are not ideal, outcomes with their use can be similar to those in younger patients (48).
Although HD may be an effective and attractive option (supervised, not done daily), elderly patients are generally more sensitive to fluid shifts because of increased cardiovascular comorbidity and may not tolerate ultrafiltration (49,50). In addition, problems with transportation and vascular access can limit success. Peritoneal dialysis (PD) may be advantageous because it provides gentler ultrafiltration, can be performed at home, and eliminates vascular access. However, barriers may exist because of functional limitations and insufficient social support; these factors, although present in younger patients, are more frequent and problematic in elderly (49,51).
Concerns exist regarding technique survival and peritonitis. One study reported equivalent technique survival for all ages at 5 years (52). Peritonitis data are variable (53–56). Some studies show an increased rate, some a decreased rate, and others the same rate for elderly and younger patients. There was a similar likelihood of remaining peritonitis-free in patients with diabetes older than age 70 years versus those age 40–60, but more total episodes of peritonitis occurred in the elderly, suggesting increased risk for recurrence (56).
Multiple European studies examined outcomes of elderly patients undergoing PD versus HD. There was no difference in 1-year mortality in 125 patients older than age 70 years in the North Thames Dialysis Study (57). The BOLDE (Broadening Options for Long-term Dialysis in the Elderly) study found similar quality of life for PD versus HD in prevalent elderly (>65 years) patients (49). In 3512 incident French dialysis patients older than 75 years (Renal Epidemiology and Information Network registry), the 2-year PD survival rate was 64%, similar to that seen with HD (58).
Analysis of 1615 French PD patients older than age 75 revealed survival similar to that found in the Renal Epidemiology and Information Network registry. Mean initiation age was 82 years, and 75% of patients required assisted PD (a nurse or family member helps with exchanges) (59–61). Survival was better for those with fewer comorbid conditions (59) and those not requiring assistance, probably reflecting selection bias (59). Strategies such as nocturnal or short daily dialysis may provide advantages similar to those seen with PD, such as gentler ultrafiltration. Nocturnal dialysis has not been studied in the elderly. A geriatric rehabilitation unit used six 2-hour HD sessions per week (10). Patients tolerated treatment with adequate ultrafiltration and preferred this schedule (10).
Given available data, dialysis modality choice in elderly patients should be individualized, with consideration of comorbid conditions, cognitive function, social support, and functional status.
Transplantation
Transplantation remains the treatment of choice for ESRD (62). There is no age at which a patient should not be considered for transplantation, provided they are in otherwise good health and do not have significant comorbid conditions (62,63). However, the growth of elderly patients on dialysis in the United States is not matched by a proportional increase in transplantation, although the transplantation rate is increasing (62).
A study examining the survival benefit of transplantation versus remaining wait-listed was performed using USRDS data on 252,358 patients (64). Patients older than age 70 were excluded (64). Significant survival benefit was observed for all ages, but the magnitude was less for those older than age 60 (64). A more recent study of 2000 deceased-donor recipients older than 70 years found a 41% mortality reduction compared with the rate in those who remained wait-listed (65). A substantial component of the survival benefit may be attributable to selection bias.
Expanded-criteria donor (ECD) kidneys are often unsuitable for younger patients because they are not expected to function as long as standard-criteria donor kidneys. Ethical concerns exist because the best outcomes for elderly recipients are from live donors, followed by standard-criteria donors, then ECDs (65,66). However, a 25% mortality reduction compared with remaining wait-listed was observed for elderly ECD recipients (65,66). Similar outcomes have been observed elsewhere (67–69). The main benefit of ECD kidneys is less wait time, and they are recommended for older patients who are diabetic, have no living donor, or have an anticipated long wait (65).
The Organ Procurement and Transplant Network recently proposed a new allocation system that attempts to maximize graft function for each kidney by matching graft life expectancy with recipients (70). Kidneys would be evaluated on a continuum rather than as standard-criteria donor/ECD (70). Factors for recipients are age, dialysis time, previous organ transplantation, and diabetes (70). A decreased percentage of transplants but no change in average years of benefit for patients older than age 50 are predicted (70).
Transplant outcomes improve with less dialysis time (71,72). In a Norwegian study, the worst outcomes were for patients older than age 70 with more than 1 year of dialysis (71). Survival was decreased for patients age 60–69 years with more than 1 year of dialysis, equivalent to the rate in patients older than age 70 with less than 1 year of dialysis (71). Unfortunately, patients older than age 65 are less likely to receive a deceased-donor kidney within 1 year (73). In addition, the pre-emptive transplantation rate is very low, especially in the elderly (73). With prompt evaluation (for living- and deceased-donor candidacy), properly selected elderly dialysis patients can achieve very favorable transplant outcomes.
Palliative Care and Hospice
Dialysis discontinuation occurs more frequently among older patients and may be particularly appropriate for those with severe functional or cognitive impairment (27,74). USRDS data demonstrate increased withdrawal with age (Table 5) (75). Survival varies, with most deaths attributable to comorbid conditions rather than uremia (74). Anuric patients usually die in 8–12 days (74). With residual function, some may survive several months (74).
Table 5.
Rates of withdrawal from dialysis by age group among prevalent patients
| Age Group (yr) | Percentage Withdrawing from Dialysis (%) |
|---|---|
| 0–44 | 12 |
| 45–64 | 16 |
| 65–74 | 23 |
| 75–84 | 30 |
| ≥85 | 35 |
Data obtained from reference 75.
Symptoms encountered after dialysis discontinuation, which may predate dialysis withdrawal, include pain, myoclonus, hunger, thirst, and dyspnea (74). World Health Organization guidelines for pain management should be followed. Fentanyl and hydromorphone have metabolic profiles superior to that of morphine for impaired renal function, and meperidine is contraindicated. Low-dose benzodiazepines may be used for myoclonus. Diet should be liberalized for hunger and thirst. For dyspnea, opioids can be considered.
Murtagh et al. studied symptoms before death in 74 patients with CKD stage 5 in whom dialysis was not initiated (76,77). Symptoms were similar to those in patients with end-stage cancer (76). Degree of distress correlated with frequency, except for dyspnea (76). A sharp increase in distress and health-related concerns occurred 2 months before death (77).
Hospice services are underused for patients with ESRD (78–82). USRDS data for deceased patients reveals that only 13.5% used hospice; the rate was 42.9% in those who withdrew dialysis (82). Significant regional differences were observed (82). Among those who withdrew, hospice patients had lower health care–related costs in the last week of life and were three-fold more likely to die at home (82). There is clear need for increased focus on end-of-life symptom management and hospice use (27). In addition, palliative care should be used when appropriate throughout CKD stages (27).
Conclusions and Future Directions
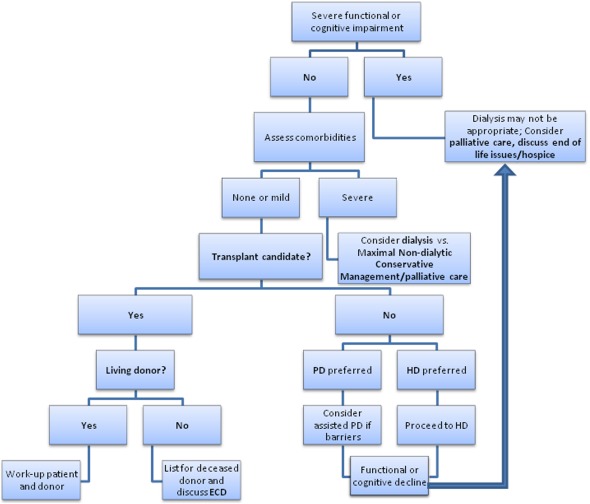
RRT decision-making in the elderly requires consideration of factors more common in this population, including functional and cognitive impairment and cardiovascular disease (Figure 2). If severe impairment exists, nondialytic management, including palliative care and hospice, may be appropriate. In those with less impairment but severe comorbidity, MCM (in conjunction with palliative care) is an alternative. Transplantation is an option in those with less comorbidity. If there is no living donor, careful evaluation for a deceased-donor transplant (possibly with ECD listing) should be pursued. PD and HD are acceptable RRT modalities, and individual preferences and social support must be considered. After initiation, patients should be monitored for functional or cognitive decline. If such decline is present, RRT withdrawal and palliative or hospice services may be reasonable.
Figure 2.
Approach to the elderly patient with ESRD. The model uses such factors as cognitive impairment, functional impairment, and the severity of comorbid conditions to help guide the clinical thought process. The model emphasizes circumstances in which nondialytic management, including palliative care, may be appropriate, as well as the need for continual assessment of patients on dialysis to determine whether changes requiring reassessment of the appropriateness of continuing renal replacement therapy have occurred. ECD, expanded-criteria donor; HD, hemodialysis; PD, peritoneal dialysis.
Underuse of PD presents a potential area for growth. Assisted PD can remove nonmedical barriers and increase use (83). Extension of prognostic models to earlier CKD stages could provide information to predict CKD progression and which patients should pursue RRT. Multidisciplinary clinics and incorporation of tools assessing function may assist in delivering appropriate care. It is undetermined whether earlier recognition of functional and cognitive decline and use of rehabilitation can slow progression and improve ESRD outcomes.
Disclosures
None.
Acknowledgments
This work was supported by O'Brien Kidney Research Core Center Grant P30DK079328, awarded to the University of Texas Southwestern Medical Center.
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.U.S. Renal Data System : USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End Stage Renal Disease in the United States, Bethesda, MD, National Institute of Health, National Institute of Diabetes and Digestive and Kidney Disease, 2010 [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research Group : Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56: M146–M156, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Wilhelm-Leen ER, Hall YN, K Tamura M, Chertow GM: Frailty and chronic kidney disease: The third National Health and Nutrition Evaluation Survey. Am J Med 122: 664–671, e2, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansen KL, Chertow GM, Jin C, Kutner NG: Significance of frailty among dialysis patients. J Am Soc Nephrol 18: 2960–2967, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Kutner NG: Promoting functioning and well-being in older CKD patients: review of recent evidence. Int Urol Nephrol 40: 1151–1158, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Odden MC: Physical functioning in elderly persons with kidney disease. Adv Chronic Kidney Dis 17: 348–357, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Roberts R, Jeffrey C, Carlisle G, Brierley E: Prospective investigation of the incidence of falls, dizziness and syncope in haemodialysis patients. Int Urol Nephrol 39: 275–279, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Li M, Tomlinson G, Naglie G, Cook WL, Jassal SV: Geriatric comorbidities, such as falls, confer an independent mortality risk to elderly dialysis patients. Nephrol Dial Transplant 23: 1396–1400, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Saito GK, Jassal SV: The ‘Sit-to-Scale’ score—a pilot study to develop an easily applied score to follow functional status in elderly dialysis patients. Nephrol Dial Transplant 22: 3318–3321, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Li M, Porter E, Lam R, Jassal SV: Quality improvement through the introduction of interdisciplinary geriatric hemodialysis rehabilitation care. Am J Kidney Dis 50: 90–97, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook WL, Jassal SV: Functional dependencies among the elderly on hemodialysis. Kidney Int 73: 1289–1295, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE: Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 361: 1539–1547, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jassal SV, Chiu E, Hladunewich M: Loss of independence in patients starting dialysis at 80 years of age or older. N Engl J Med 361: 1612–1613, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Kurella Tamura M, Larive B, Unruh ML, Stokes JB, Nissenson A, Mehta RL, Chertow GM, Frequent Hemodialysis Network Trial Group : Prevalence and correlates of cognitive impairment in hemodialysis patients: The Frequent Hemodialysis Network trials. Clin J Am Soc Nephrol 5: 1429–1438, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurella M, Mapes DL, Port FK, Chertow GM: Correlates and outcomes of dementia among dialysis patients: The Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant 21: 2543–2548, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Murray AM: Cognitive impairment in the aging dialysis and chronic kidney disease populations: An occult burden. Adv Chronic Kidney Dis 15: 123–132, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurella M, Chertow GM, Fried LF, Cummings SR, Harris T, Simonsick E, Satterfield S, Ayonayon H, Yaffe K: Chronic kidney disease and cognitive impairment in the elderly: The health, aging, and body composition study. J Am Soc Nephrol 16: 2127–2133, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Yaffe K, Ackerson L, Kurella Tamura M, Le Blanc P, Kusek JW, Sehgal AR, Cohen D, Anderson C, Appel L, Desalvo K, Ojo A, Seliger S, Robinson N, Makos G, Go AS, Chronic Renal Insufficiency Cohort Investigators : Chronic kidney disease and cognitive function in older adults: Findings from the Chronic Renal Insufficiency Cohort Cognitive study. J Am Geriatr Soc 58: 338–345, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griva K, Stygall J, Hankins M, Davenport A, Harrison M, Newman SP: Cognitive impairment and 7-year mortality in dialysis patients. Am J Kidney Dis 56: 693–703, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Giang LM, Weiner DE, Agganis BT, Scott T, Sorensen EP, Tighiouart H, Sarnak MJ: Cognitive function and dialysis adequacy: No clear relationship. Am J Nephrol 33: 33–38, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurella Tamura M, O’Hare AM, McCulloch CE, Johansen KL: Signs and symptoms associated with earlier dialysis initiation in nursing home residents. Am J Kidney Dis 56: 1117–1126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buemi M, Lacquaniti A, Bolignano D, Donato V, Fazio MR, Campo S, Coppolino G, Sturiale A: Dialysis and the elderly: An underestimated problem. Kidney Blood Press Res 31: 330–336, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Kurella Tamura M: Incidence, management, and outcomes of end-stage renal disease in the elderly. Curr Opin Nephrol Hypertens 18: 252–257, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, Harris A, Johnson DW, Kesselhut J, Li JJ, Luxton G, Pilmore A, Tiller DJ, Harris DC, Pollock CA, IDEAL Study : A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 363: 609–619, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Rosansky SJ, Eggers P, Jackson K, Glassock RJ, Clark WF: Early start of hemodialysis may be harmful. Arch Intern Med 171: 396–403, 2011 [DOI] [PubMed] [Google Scholar]
- 26.O’Hare AM: The management of older adults with a low eGFR: moving toward an individualized approach. Am J Kidney Dis 53: 925–927, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renal Physicians Association : Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis, 2nd Ed., Rockville, MD, Renal Physicians Association, 2010 [Google Scholar]
- 28.Germain MJ, Davison SN, Moss AH: When enough is enough: The nephrologist’s responsibility in ordering dialysis treatments. Am J Kidney Dis 58: 135–143, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Quill TE, Holloway R: Time-limited trials near the end of life. JAMA 306: 1483–1484, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Couchoud C, Labeeuw M, Moranne O, Allot V, Esnault V, Frimat L, Stengel B, French Renal Epidemiology and Information Network (REIN) registry : A clinical score to predict 6-month prognosis in elderly patients starting dialysis for end-stage renal disease. Nephrol Dial Transplant 24: 1553–1561, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moss AH, Ganjoo J, Sharma S, Gansor J, Senft S, Weaner B, Dalton C, MacKay K, Pellegrino B, Anantharaman P, Schmidt R: Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol 3: 1379–1384, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen LM, Ruthazer R, Moss AH, Germain MJ: Predicting six-month mortality for patients who are on maintenance hemodialysis. Clin J Am Soc Nephrol 5: 72–79, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burns A, Davenport A: Maximum conservative management for patients with chronic kidney disease stage 5. Hemodial Int 14[Suppl 1]: S32–S37, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Busuioc M, Gusbeth-Tatomir P, Covic A: Dialysis or not in the very elderly ESRD patient. Int Urol Nephrol 40: 1127–1132, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Burns A, Carson R: Maximum conservative management: A worthwhile treatment for elderly patients with renal failure who choose not to undergo dialysis. J Palliat Med 10: 1245–1247, 2007 [DOI] [PubMed] [Google Scholar]
- 36.De Biase V, Tobaldini O, Boaretti C, Abaterusso C, Pertica N, Loschiavo C, Trabucco G, Lupo A, Gambaro G: Prolonged conservative treatment for frail elderly patients with end-stage renal disease: The Verona experience. Nephrol Dial Transplant 23: 1313–1317, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Visser A, Dijkstra GJ, Kuiper D, de Jong PE, Franssen CFM, Gansevoort RT, Izaks GJ, Jager KJ, Reijneveld SA: Accepting or declining dialysis: considerations taken into account by elderly patients with end-stage renal disease. J Nephrol 22: 794–799, 2009 [PubMed] [Google Scholar]
- 38.Wong CF, McCarthy M, Howse ML, Williams PS: Factors affecting survival in advanced chronic kidney disease patients who choose not to receive dialysis. Ren Fail 29: 653–659, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Murtagh FEM, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE: Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant 22: 1955–1962, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Smith C, Da Silva-Gane M, Chandna S, Warwicker P, Greenwood R, Farrington K: Choosing not to dialyse: Evaluation of planned non-dialytic management in a cohort of patients with end-stage renal failure. Nephron Clin Pract 95: c40–c46, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Carson RC, Juszczak M, Davenport A, Burns A: Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol 4: 1611–1619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jassal SV, Kelman EE, Watson D: Non-dialysis care: An important component of care for elderly individuals with advanced stages of chronic kidney disease. Nephron Clin Pract 119[Suppl 1]: c5–c9, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Kurella Tamura M, Goldstein MK, Pérez-Stable EJ: Preferences for dialysis withdrawal and engagement in advance care planning within a diverse sample of dialysis patients. Nephrol Dial Transplant 25: 237–242, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.U.S. Renal Data System : USRDS 2007 Annual Data Report: Atlas of Chronic Kidney Disease and End Stage Renal Disease in the United States, Bethesda, MD, National Institute of Health, National Institute of Diabetes and Digestive and Kidney Disease, 2007 [Google Scholar]
- 45.Lazarides MK, Georgiadis GS, Antoniou GA, Staramos DN: A meta-analysis of dialysis access outcome in elderly patients. J Vasc Surg 45: 420–426, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Lok CE, Oliver MJ, Su J, Bhola C, Hannigan N, Jassal SV: Arteriovenous fistula outcomes in the era of the elderly dialysis population. Kidney Int 67: 2462–2469, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Xue JL, Dahl D, Ebben JP, Collins AJ: The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis 42: 1013–1019, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Forauer AR, McNulty NJ, Kaneko TM: Tunneled hemodialysis catheter outcomes in elderly patients. J Vasc Interv Radiol 20: 467–471, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Brown EA, Johansson L, Farrington K, Gallagher H, Sensky T, Gordon F, Da Silva-Gane M, Beckett N, Hickson M: Broadening Options for Long-term Dialysis in the Elderly (BOLDE): differences in quality of life on peritoneal dialysis compared to haemodialysis for older patients. Nephrol Dial Transplant 25: 3755–3763, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindsay RM, Heidenheim PA, Nesrallah G, Garg AX, Suri R, Daily Hemodialysis Study Group London Health Sciences Centre : Minutes to recovery after a hemodialysis session: a simple health-related quality of life question that is reliable, valid, and sensitive to change. Clin J Am Soc Nephrol 1: 952–959, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Finkelstein FO, Afolalu B, Wuerth D, Finkelstein SH: The elderly patient on CAPD: Helping patients cope with peritoneal dialysis. Perit Dial Int 28: 449–451, 2008 [PubMed] [Google Scholar]
- 52.Li PKT, Law MC, Chow KM, Leung CB, Kwan BCH, Chung KY, Szeto CC: Good patient and technique survival in elderly patients on continuous ambulatory peritoneal dialysis. Perit Dial Int 27[Suppl 2]: S196–S201, 2007 [PubMed] [Google Scholar]
- 53.Yang X, Fang W, Kothari J, Khandelwal M, Naimark D, Jassal SV, Bargman JM, Oreopoulos DG: Clinical outcomes of elderly patients undergoing chronic peritoneal dialysis: experiences from one center and a review of the literature. Int Urol Nephrol 39: 1295–1302, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Nessim SJ, Bargman JM, Austin PC, Story K, Jassal SV: Impact of age on peritonitis risk in peritoneal dialysis patients: An era effect. Clin J Am Soc Nephrol 4: 135–141, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szeto CC, Kwan BCH, Chow KM: Peritonitis risk for older patients on peritoneal dialysis. Perit Dial Int 28: 457–460, 2008 [PubMed] [Google Scholar]
- 56.De Vecchi AF, Maccario M, Braga M, Scalamogna A, Castelnovo C, Ponticelli C: Peritoneal dialysis in nondiabetic patients older than 70 years: Comparison with patients aged 40 to 60 years. Am J Kidney Dis 31: 479–490, 1998 [DOI] [PubMed] [Google Scholar]
- 57.Lamping DL, Constantinovici N, Roderick P, Normand C, Henderson L, Harris S, Brown E, Gruen R, Victor C: Clinical outcomes, quality of life, and costs in the North Thames Dialysis Study of elderly people on dialysis: A prospective cohort study. Lancet 356: 1543–1550, 2000 [DOI] [PubMed] [Google Scholar]
- 58.Couchoud C, Moranne O, Frimat L, Labeeuw M, Allot V, Stengel B: Associations between comorbidities, treatment choice and outcome in the elderly with end-stage renal disease. Nephrol Dial Transplant 22: 3246–3254, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Castrale C, Evans D, Verger C, Fabre E, Aguilera D, Ryckelynck JP, Lobbedez T: Peritoneal dialysis in elderly patients: Report from the French Peritoneal Dialysis Registry (RDPLF). Nephrol Dial Transplant 25: 255–262, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Dimkovic N, Oreopoulos DG: Assisted peritoneal dialysis as a method of choice for elderly with end-stage renal disease. Int Urol Nephrol 40: 1143–1150, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Povlsen JV, Ivarsen P: Assisted peritoneal dialysis: Also for the late referred elderly patient. Perit Dial Int 28: 461–467, 2008 [PubMed] [Google Scholar]
- 62.Huang E, Segev DL, Rabb H: Kidney transplantation in the elderly. Semin Nephrol 29: 621–635, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hartmann EL, Wu C: The evolving challenge of evaluating older renal transplant candidates. Adv Chronic Kidney Dis 17: 358–367, 2010 [DOI] [PubMed] [Google Scholar]
- 64.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 65.Rao PS, Merion RM, Ashby VB, Port FK, Wolfe RA, Kayler LK: Renal transplantation in elderly patients older than 70 years of age: Results from the Scientific Registry of Transplant Recipients. Transplantation 83: 1069–1074, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Chuang FP, Novick AC, Sun GH, Kleeman M, Flechner S, Krishnamurthi V, Modlin C, Shoskes D, Goldfarb DA: Graft outcomes of living donor renal transplantations in elderly recipients. Transplant Proc 40: 2299–2302, 2008 [DOI] [PubMed] [Google Scholar]
- 67.Basic-Jukic N, Furic-Cunko V, Kes P, Bubic-Filipi L, Pasini J, Hudolin T, Juric I: Outcome after renal transplantation in a “senior” program: The Croatian experience. Transplant Proc 40: 3418–3421, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Ghafari A, Ardalan MR: Renal transplantation in elderly recipients: A single-center experience. Transplant Proc 40: 238–239, 2008 [DOI] [PubMed] [Google Scholar]
- 69.Shah T, Bunnapradist S, Hutchinson I, Pravica V, Cho YW, Mendez R, Mendez R, Takemoto SK: The evolving notion of “senior” kidney transplant recipients. Clin Transplant 22: 794–802, 2008 [DOI] [PubMed] [Google Scholar]
- 70.The Organ Procurement and Transplantation Network : Concepts for Kidney Allocation, Richmond, VA, : The Organ Procurement and Transplantation Network, 2011 [Google Scholar]
- 71.Heldal K, Hartmann A, Leivestad T, Svendsen MV, Foss A, Lien B, Midtvedt K: Clinical outcomes in elderly kidney transplant recipients are related to acute rejection episodes rather than pretransplant comorbidity. Transplantation 87:1045–1051, 2009 [DOI] [PubMed] [Google Scholar]
- 72.Heldal K, Leivestad T, Hartmann A, Svendsen MV, Lien BH, Midtvedt K: Kidney transplantation in the elderly—the Norwegian experience. Nephrol Dial Transplant 23: 1026–1031, 2008 [DOI] [PubMed] [Google Scholar]
- 73.U.S. Renal Data System : USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End Stage Renal Disease in the United States, Bethesda, MD, National Institute of Health, National Institute of Diabetes and Digestive and Kidney Disease, 2011 [Google Scholar]
- 74.Cohen LM, Germain MJ, Poppel DM: Practical considerations in dialysis withdrawal: “To have that option is a blessing”. JAMA 289: 2113–2119, 2003 [DOI] [PubMed] [Google Scholar]
- 75.U.S. Renal Data System : USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End Stage Renal Disease in the United States, Bethesda, MD, National Institute of Health, National Institute of Diabetes and Digestive and Kidney Disease, 2008 [Google Scholar]
- 76.Murtagh FEM, Addington-Hall J, Edmonds P, Donohoe P, Carey I, Jenkins K, Higginson IJ: Symptoms in the month before death for stage 5 chronic kidney disease patients managed without dialysis. J Pain Symptom Manage 40: 342–352, 2010 [DOI] [PubMed] [Google Scholar]
- 77.Murtagh FEM, Sheerin NS, Addington-Hall J, Higginson IJ: Trajectories of illness in stage 5 chronic kidney disease: A longitudinal study of patient symptoms and concerns in the last year of life. Clin J Am Soc Nephrol 6: 1580–1590, 2011 [DOI] [PubMed] [Google Scholar]
- 78.Brown EA: Epidemiology of renal palliative care. J Palliat Med 10: 1248–1252, 2007 [DOI] [PubMed] [Google Scholar]
- 79.Cohen LM, Ruthazer R, Germain MJ: Increasing hospice services for elderly patients maintained with hemodialysis. J Palliat Med 13: 847–854, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Germain M, Cohen LM: Renal supportive care: view from across the pond: the United States perspective. J Palliat Med 10: 1241–1244, 2007 [DOI] [PubMed] [Google Scholar]
- 81.Murtagh FEM, Higginson IJ: Death from renal failure eighty years on: How far have we come? J Palliat Med 10: 1236–1238, 2007 [DOI] [PubMed] [Google Scholar]
- 82.Murray AM, Arko C, Chen SC, Gilbertson DT, Moss AH: Use of hospice in the United States dialysis population. Clin J Am Soc Nephrol 1: 1248–1255, 2006 [DOI] [PubMed] [Google Scholar]
- 83.Oliver MJ, Quinn RR, Richardson EP, Kiss AJ, Lamping DL, Manns BJ: Home care assistance and the utilization of peritoneal dialysis. Kidney Int 71: 673–678, 2007 [DOI] [PubMed] [Google Scholar]