Summary
Background and objectives
Acute rejection remains a problem in renal transplantation. This study sought to determine the utility of a noninvasive cytokine assay in screening of acute rejection.
Design, setting, participants, & measurements
In this observational cross-sectional study, 64 patients from two centers were recruited upon admission for allograft biopsy to investigate acute graft dysfunction. Blood was collected before biopsy and assayed for a panel of 21 cytokines secreted by PBMCs. Patients were classified as acute rejectors or nonrejectors according to a classification rule derived from an initial set of 32 patients (training cohort) and subsequently validated in the remaining patients (validation cohort).
Results
Although six cytokines (IL-1β, IL-6, TNF-α, IL-4, GM-CSF, and monocyte chemoattractant protein-1) distinguished acute rejectors in the training cohort, logistic regression modeling identified a single cytokine, IL-6, as the best predictor. In the validation cohort, IL-6 was consistently the most accurate cytokine (area under the receiver-operating characteristic curve, 0.85; P=0.006), whereas the application of a prespecified cutoff level, as determined from the training cohort, resulted in a sensitivity and specificity of 92% and 63%, respectively. Secondary analyses revealed a strong association between IL-6 levels and acute rejection after multivariate adjustment for clinical characteristics (P<0.001).
Conclusions
In this pilot study, the measurement of a single cytokine can exclude acute rejection with a sensitivity of 92% in renal transplant recipients presenting with acute graft dysfunction. Prospective studies are needed to determine the utility of this simple assay, particularly for low-risk or remote patients.
Introduction
Although the introduction of calcineurin inhibitors has considerably reduced the incidence of acute rejection in renal transplant recipients, the 1-year risk still ranges between 10% and 15% worldwide (1). Early recognition of an acute rejection episode is crucial because delayed diagnosis leads to loss of graft function. Such loss has been associated with shorter graft survival, especially when rejection occurs late in the clinical course and when treatment fails to instigate a return to baseline function (2,3). Formal diagnosis requires needle-core biopsy, a costly and invasive procedure associated with such risks as hemorrhage, obstruction, and, rarely, graft loss.
Despite recent advances in new technologies, such as proteomics and gene expression profiling, we still lack a noninvasive tool to identify rejection in renal transplant recipients. To be clinically applicable, a noninvasive test needs to provide a result quickly and be simple to perform. In addition to reducing the need for invasive biopsies (4), such a test could eventually allow safe, serial monitoring of the rejection status of the allograft (5). There is increasing interest in cellular assays that measure cytokine production by PBMCs after ex vivo incubation. We and others have recently demonstrated associations between cellular cytokine levels and clinical conditions in renal transplant recipients (6–9).
The aim of the present study was to determine the utility of a cellular cytokine assay in the screening of acute rejection in renal transplant recipients. We hypothesized that the measurement of a single or a limited number of cytokines could discriminate between acute rejectors and non–acute rejectors in patients presenting with an acute decline in graft function.
Materials and Methods
Study Population
Between February 2009 and October 2010, 65 patients were recruited (Figure 1). Patients were invited to participate in this two-center, observational, cross-sectional study upon their admission to the hospital, under the approved guidelines of the institutional review boards. The training cohort included 32 patients, all of whom were recruited at Brigham and Women’s Hospital. Of the 32 patients in the validation cohort, 17 were enrolled at Brigham and Women’s Hospital and 15 at Lahey Clinic, both in Boston, Massachusetts. Patients were eligible for inclusion in the study if they were admitted at least 14 days after transplantation to undergo graft biopsy for investigation of an acute increase in serum creatinine that prompted clinical suspicion of an acute allograft rejection. The decision to perform a biopsy was made by the treating physician. All invited patients agreed to participate in the study. One patient was excluded because the biopsy was canceled. Patients were asked to provide a follow-up sample at 3 months after the initial blood collection; 33 patients agreed. In all cases, routine urine analysis and culture was performed; all results were negative for an infection.
Figure 1.
Flow of patients through the study.
The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul, as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Pathologic Classification
Sections of formalin-fixed, paraffin-embedded tissue were evaluated using hematoxylin and eosin, periodic acid-Schiff, Jones silver methenamine, and trichrome stains. These biopsy samples were read by the local attending pathologist and graded according to the Banff classification (10,11). Pathologists were blinded to the results of the cellular cytokine assays. Patients were classified according to the final pathologic diagnosis provided on the biopsy report as acute rejectors or nonrejectors (Figure 1). One patient had a diagnosis of polyoma (BK) virus concurrent with acute cellular rejection and remained in the study.
Cell Isolation and Cytokine Assay
Blood samples were collected on the day of biopsy, before any modification of immunosuppression. PBMCs were isolated from heparinized blood by density gradient centrifugation using Ficoll-Paque (GE Healthcare Biosciences AB, Uppsala, Sweden), washed twice with phosphate-buffered saline, counted, and stored in liquid nitrogen. Cells were thawed by slow reconstitution with RPMI 1640 medium (Cambrex Bioscience, Walkersville, MD), then incubated overnight without stimulation, as described elsewhere (7). The production of cytokines by peripheral cells was measured by examining supernatants from cell cultures using a 21-plex cytokine Milliplex panel (Millipore Corp., Billerica, MA). Acquisition was performed on a Luminex 100 platform. Experiments were performed in five separate batches.
Statistical Analyses
Area under the receiver-operating characteristic (ROC) curve (AUC) was used to evaluate the ability of the cytokines to discriminate between acute rejectors and nonrejectors. Power size calculations for ROC analysis revealed that 16 patients per group were required so that the analysis would have a power of 90% to detect an AUC of 0.9 (12).
Candidate cytokines were first identified in the training cohort using a threshold P value < 0.05 for the AUC. Stepwise logistic regression modeling was then performed to determine whether a classification rule based on a combination of cytokines would have greater accuracy in predicting acute rejection than individual cytokines. Data from the validation cohort were analyzed after completion of all analyses from the training cohort. Using log-transformed cytokine values, and after adjustment for clinical variables, multiple linear regression modeling was performed on the whole cohort of 64 patients to evaluate the relationship between the classification rule and acute rejection. ROC curve was used to study the relationship between the level of IL-6 and the severity of rejection. All P values were two-tailed. Statistical analyses were performed using Stata software, version 11.0 (Stata Corp, College Station, TX), and SPSS, version 16.0 (SPSS Inc., Chicago, IL).
Results
Study Population
A total of 64 samples from an equal number of patients were examined (Figure 1). All patients invited to participate to the study gave consent. One patient was excluded before blood collection because the biopsy had been canceled after enrollment. Rejectors were younger, were more likely to be male and to have received a living unrelated donor, and had a shorter time after transplantation (Table 1); these differences were not statistically significant. Induction and maintenance immunosuppressive regimens did not differ between the groups. At the time of recruitment, none of the patients had signs of active infection or systemic inflammatory condition. The mean ± SD absolute increase in serum creatinine was 0.69±44 mg/dl, which represents a percentage increase of 36%±27% from the stable baseline values; the mean ± SD number of days between the last stable creatinine and the admission for the biopsy was 33±19.
Table 1.
Clinical characteristics of the study population
| Characteristic | Rejectors (n=29) | Nonrejectors (n=35) | P Value |
|---|---|---|---|
| Mean age (yr) | 50±15 | 54±13 | 0.29 |
| Men | 13 (45) | 10 (29) | 0.20 |
| Ethnic group | 0.40 | ||
| white | 18 (62) | 27 (77) | |
| Hispanic | 9 (31) | 6 (17) | |
| black | 2 (7) | 2 (6) | |
| Donor type | 0.07 | ||
| deceased | 5 (17) | 11 (31) | |
| living related | 14 (48) | 20 (57) | |
| living unrelated | 10 (35) | 4 (11) | |
| Median time after transplant (mo) | 4 (1–27) | 9 (1–48) | 0.34 |
| Time after transplant | 0.82 | ||
| 0–6 mo | 15 (52) | 14 (40) | |
| 6–12 mo | 3 (10) | 5 (14) | |
| 12–24 mo | 3 (10) | 4 (11) | |
| >24 mo | 8 (28) | 12 (34) | |
| Mean serum creatinine at admission (mg/dl) | 2.4±1.0 | 2.5±1.2 | 0.69 |
| Induction therapy | 0.36 | ||
| no induction | 1 (3) | 0 (0) | |
| thymoglobulin | 26 (90) | 30 (86) | |
| IL-2 receptor inhibitor | 2 (7) | 5 (14) | |
| Maintenance immunosuppression | |||
| corticosteroids | 16 (55) | 18 (53) | 1.00 |
| calcineurin inhibitor | 27 (93) | 31 (89) | 0.68 |
| antimetabolite | 27 (93) | 29 (83) | 0.28 |
| rapamycin | 0 (0) | 1 (3) | 1.00 |
Unless otherwise noted, data are the number (percentage) of patients. Data with a plus/minus sign are mean ± SD. Medians are expressed with 25th–75th percentile. Comparisons were performed using unpaired t test, Fisher exact test, chi-squared test, or Mann-Whitney U test.
Cytokine Levels and Histologic Diagnosis in the Training Cohort
By design of the training cohort, 16 patients had a histologic diagnosis of acute rejection: acute cellular rejection in 7, acute antibody-mediated rejection (ABMR) in 4, and borderline changes in 5 (Figure 1). Of the 16 nonrejectors, 4 had a histologic diagnosis of acute tubular injury, 9 of chronic allograft damage, and 3 of recurring GN. ROC analysis identified six cytokines as potential predictors of rejection status: TNF-α, IL-1β, IL-6, IL-4, monocyte chemoattractant protein-1 (MCP-1), and GM-CSF (Table 2). The first three cytokines were strongly correlated with each other (all Spearman correlation coefficients ≥ 0.75; all P<0.001) and moderately correlated with MCP-1 and GM-CSF (Spearman correlation coefficients between 0.55 and 0.77; all P<0.01). There was no correlation between IL-4 and the other cytokines.
Table 2.
Cytokine levels and receiver-operating characteristic analysis of individual cytokines to discriminate acute rejectors in the training cohort
| Cytokine | Median Cytokine Levels (25th–75th Percentile) (pg/ml) | P Valuea | ROC Analysis (n=32) | ||
|---|---|---|---|---|---|
| Acute Rejectors (n=16) | Nonrejectors (n=16) | AUC (95% CI) | P Value | ||
| Training cohort | |||||
| TNF-α | 192.0 (60.5–2052.6) | 32.4 (14.9–56.0) | 0.001 | 0.86 (0.72–1.00) | 0.001 |
| IL-1β | 376.0 (32.5–1995.3) | 11.5 (4.1–27.8) | 0.003 | 0.81 (0.65–0.97) | 0.003 |
| IL-6 | 9120.7 (148.1–11831.8) | 122.3 (26.9–493.9) | 0.005 | 0.79 (0.63–0.95) | 0.005 |
| IL-4 | 1.5 (1.5–2.9) | 1.0 (1.0–1.5) | 0.006 | 0.76 (0.59–0.93) | 0.01 |
| MCP-1 | 9069.6 (7605.7–9712.2) | 8008.1 (1090.3–8467.1) | 0.03 | 0.73 (0.55–0.91) | 0.03 |
| GM-CSF | 9.0 (4.4–157.5) | 4.3 (1.5–7.9) | 0.03 | 0.73 (0.55–0.90) | 0.03 |
| IL-7 | 4.1 (3.0–5.6) | 3.0 (3.0–3.0) | 0.03 | 0.69 (0.50–0.88) | 0.07 |
| IL-10 | 99.8 (1.5–537.9) | 6.8 (2.1–32.1) | 0.11 | 0.67 (0.46–0.87) | 0.11 |
| IL-8 | 12,000.0 (11,251.8–12,000.0) | 10,959.6 (86,52.1–12,000.0) | 0.12 | 0.65 (0.46–0.85) | 0.14 |
| IFN-γ | 1.2 (1.0– 8.9) | 1.0 (1.0–1.0) | 0.18 | 0.62 (0.43–0.82) | 0.23 |
| IL-13 | 1.8 (1.0–4.9) | 1.0 (1.0–3.1) | 0.20 | 0.62 (0.42–0.82) | 0.24 |
| IL1-Ra | 3598.0 (491.6–8604.7) | 2177.6 (131.6–4997.0) | 0.39 | 0.59 (0.39–0.79) | 0.39 |
| IL-2 | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.27 | 0.57 (0.37–0.77) | 0.52 |
| IL-12(p40) | 1.0 (1.0–2.1) | 1.0 (1.0–1.0) | 0.35 | 0.57 (0.36–0.77) | 0.52 |
| IL-17 | 1.0 (1.0–2.6) | 1.0 (1.0–1.4) | 0.48 | 0.56 (0.36–0.77) | 0.55 |
| IL-5 | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.15 | 0.56 (0.36–0.76) | 0.55 |
| IL-9 | 3.0 (3.0–3.0) | 3.0 (3.0–3.0) | 0.32 | 0.53 (0.33–0.73) | 0.76 |
| IL-15 | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.72 | 0.52 (0.32–0.73) | 0.82 |
| VEGF | 7.8 (3.0–65.8) | 3.3 (3.0–79.8) | 0.97 | 0.50 (0.30–0.71) | 0.97 |
| IP10 | 242.9 (40.9–1875.0) | 292.7 (86.7–950.4) | 0.99 | 0.50 (0.30–0.71) | 0.99 |
| IL-12(p70) | 1.0 (1.0–1.8) | 1.0 (1.0–2.8) | 0.60 | 0.46 (0.25–0.66) | 0.68 |
| Validation cohort | |||||
| IL-6 | 507.0 (211.4–2918.7) | 70.1 (19.1–174.7) | 0.001 | 0.85 (0.71–0.99) | 0.001 |
| TNF-α | 57.4 (37.0–77.8) | 20.8 (15.3–31.9) | 0.02 | 0.75 (0.55–0.95) | 0.02 |
| MCP-1 | 7106.5 (6523.0–8738.3) | 5728.5 (1048.2–6905.6) | 0.03 | 0.73 (0.53–0.92) | 0.03 |
| IL-1β | 34.6 (20.1–178.9) | 7.8 (4.6–49.4) | 0.04 | 0.72 (0.54–0.89) | 0.04 |
| GM-CSF | 11.6 (6.7–30.6) | 6.8 (5.5–10.4) | 0.06 | 0.70 (0.50–0.90) | 0.06 |
| IL-4 | 1.0 (1.0–1.2) | 1.0 (1.0–1.0) | 0.38 | 0.56 (0.35–0.77) | 0.56 |
ROC, receiver-operating characteristic; AUC, area under ROC curve; MCP-1, monocyte chemoattractant protein-1; VEGF, vascular endothelial growth factor.
Comparison was performed using Mann-Whitney U test.
In a logistic regression analysis, the six candidate cytokines were used in a stepwise selection algorithm to determine whether a combination of cytokines would have greater diagnostic performance than a single cytokine. The analysis did not identify a multivariable model as the best classifier, however; rather, it indicated that IL-6 alone was the best predictor (P=0.009). On the basis of these results, a cutoff value for IL-6, determined by the coordinate points of the ROC curve of the training cohort, was selected for further validation (Table 3). Because the clinical value of such a screening test lies more in excluding than in confirming rejection, the chosen cutoff value was more stringent for sensitivity. A level of 85 pg/ml was selected, with a corresponding sensitivity and specificity of 88% and 50%, respectively.
Table 3.
Coordinate points and corresponding sensitivity and 1−specificity of the receiver-operating characteristic curve for IL-6 in the training cohort
| IL-6 Level (pg/ml) | Sensitivity | 1−Specificity |
|---|---|---|
| 0 | 1.00 | 1.00 |
| 3 | 1.00 | 0.88 |
| 13 | 1.00 | 0.81 |
| 31 | 1.00 | 0.75 |
| 45 | 0.94 | 0.75 |
| 49 | 0.94 | 0.69 |
| 60 | 0.94 | 0.63 |
| 70 | 0.88 | 0.63 |
| 78 | 0.88 | 0.56 |
| 85 | 0.88 | 0.50 |
| 104 | 0.81 | 0.50 |
| 141 | 0.75 | 0.50 |
| 182 | 0.75 | 0.44 |
| 215 | 0.75 | 0.38 |
| 229 | 0.69 | 0.38 |
| 274 | 0.69 | 0.31 |
| 435 | 0.69 | 0.25 |
| 693 | 0.69 | 0.19 |
| 1593 | 0.63 | 0.19 |
| 2520 | 0.56 | 0.19 |
| 3632 | 0.56 | 0.13 |
| 6749 | 0.56 | 0.06 |
| 9121 | 0.50 | 0.06 |
| 9939 | 0.44 | 0.06 |
| 10,940 | 0.38 | 0.06 |
| 11,504 | 0.31 | 0.06 |
| 11,749 | 0.31 | 0.00 |
| 11,826 | 0.25 | 0.00 |
| 11,919 | 0.19 | 0.00 |
| 12,001 | 0.00 | 0.00 |
Validation of the Candidate Cytokines and Cutoff Level for IL-6
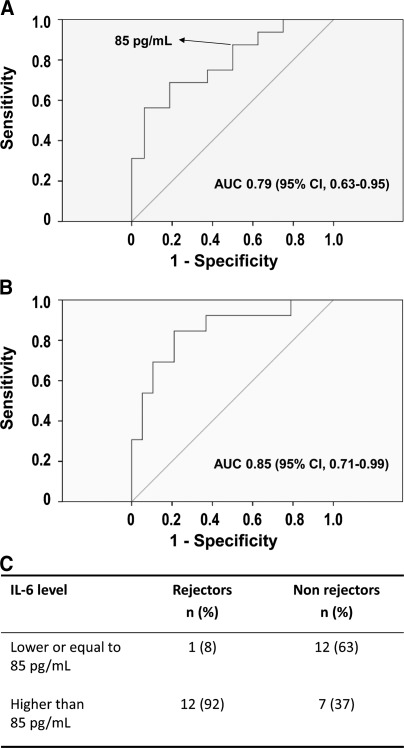
Among the 32 patients included in the validation cohort, 13 were classified in the rejectors group: 5 with acute cellular rejection, 3 with ABMR, and 5 with borderline changes. Of the 19 nonrejectors, 2 were diagnosed with borderline changes, 11 with chronic allograft damage, 5 with GN, and 1 with calcineurin inhibitor toxicity. The diagnostic performance of the individual candidate cytokines identified in the training cohort was evaluated separately in the validation cohort (Table 2). Consistent with the logistic regression analysis, the inflammatory cytokine IL-6 showed the highest discriminatory capacity (AUC, 0.85 [95% confidence interval, 0.71–0.99]; P=0.001; Figure 2), whereas IL-1β, TNF-α, GM-CSF, and MCP-1 each displayed a moderate ability to discriminate between acute rejectors and nonrejectors (AUC, 0.70–0.75).
Figure 2.
Receiver-operating-characteristic (ROC) curves according to IL-6 cutoff level selected in the training cohort. ROC curves show the sensitivity against 1−specificity for IL-6 levels. (A) The area under the curve (AUC; c-statistic) in the training cohort indicated that IL-6 had significant capacity to discriminate between rejection and nonrejection status (AUC, 0.79 [95% confidence interval (CI), 0.63–0.95]; P=0.005). (B) These results were consistent in the validation cohort (AUC, 0.85 [95% CI, 0.71–0.99]; P=0.001). (C) A cutoff level of 85 pg/ml, defined in the training cohort (sensitivity, 88%; specificity, 50%), correctly identified 12 of 13 rejectors and 12 of 19 nonrejectors in the validation cohort (sensitivity, 92%; specificity, 63%; P=0.003 by Fisher exact test).
Application of the prespecified IL-6 cutoff level of 85 pg/ml in the validation cohort revealed it to have a sensitivity of 92% and a specificity of 63% for the diagnosis of acute rejection (P=0.003 by Fisher exact test) (Figure 2). Clinical use of this assay as a screening test to decide whether to perform a biopsy would therefore have led to the performance of a biopsy in 19 of the 32 (59%) patients. One patient with a diagnosis of ABMR was falsely classified as a nonrejector; this patient had received induction with Thymoglobulin, and blood was drawn on day 15 after transplantation. The course of this was complicated by delayed graft function. The histologic diagnoses of the seven patients with false-positive findings were as follows: acute tubular injury in one, chronic allograft damage in two, and GN in three. In this validation cohort, in which the prevalence of acute rejection was 40% (13 of 32), the negative and positive predictive values of the assay were 92% and 63%, respectively.
Multivariate Correlates of Rejection and Cytokine Levels
In secondary analyses, we examined the relationship between IL-6 levels and rejection status in the complete cohort of 64 patients using multiple linear regression modeling, adjusting for the following covariates: age, gender, ethnicity, donor type, time after transplantation, induction, maintenance therapy, and experimental batch. After multivariate adjustment, there was a strong association between cytokine levels and rejection status (P<0.001).
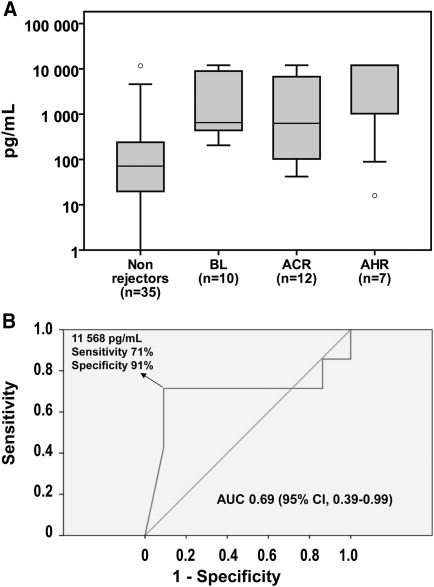
IL-6 levels were further analyzed according to the type of rejection (Figure 3). IL-6 levels varied widely within the borderline-change group. Notably, four patients had IL-6 levels exceeding 1000 pg/ml; one showed signs of glomerulitis and another had positive arteriolar C4d staining, both histologic findings potentially triggered by humoral alloreactivity (13). Post hoc analysis of the contrast ABMR versus borderline-change/acute cellular rejection patients revealed that IL-6 levels could potentially discriminate ABMR from cellular rejection with high specificity (91%) and moderate sensitivity (71%) (Figure 3; AUC, 0.69 [95% confidence interval, 0.39–0.99]; P=0.14; median values [25th–75th percentiles]: borderline change/acute cellular rejection, 632.7 [214.4–9 020.5] pg/ml vs. ABMR, 11,837.9 [89.1–12,000.0] pg/ml; P=0.14 by Mann-Whitney U test).
Figure 3.
IL-6 levels according to rejection type and severity. Allograft biopsy specimens were graded according to the Banff 07 classification of renal allograft pathology. (A) Box plots (10th, 25th, 50th, 75th, and 90th percentiles) of IL-6 levels are shown for each rejection type; nonrejectors are also shown. Circles represent outliers. (B) The receiver-operating characteristic curve shows the capacity of IL-6 to discriminate antibody-mediated rejection (ABMR) from borderline changes/acute cellular rejection. The area under the receiver-operating characteristic curve (AUC) is 0.69 (95% confidence interval [CI], 0.39–0.99; P=0.14) and the sensitivity and specificity to identify acute ABMR are 91% and 71%, respectively, at a cutoff level of 11,568 pg/ml. ACR, acute cellular rejection; BL, borderline rejection.
IL-6 Levels at 3-Month Follow-up Visit
Follow-up samples were available for 14 rejectors and 19 nonrejectors. These samples were collected at a mean ± SD duration of 2.8±1.4 months after initial blood sampling. Compared with the initial measurement, IL-6 levels at follow-up were lower in rejectors (P=0.05 by paired, Wilcoxon sign-rank test; Supplemental Figure 1). In contrast, nonrejectors showed no difference in IL-6 levels (P=0.18).
Discussion
In this pilot study, we found that the measurement of a single cytokine, IL-6, could distinguish patients with acute rejection or borderline changes from patients with no rejection, with a sensitivity of 92% and specificity of 63%. Logistic regression analysis did not show any improvement in diagnostic accuracy when a combination of cytokines was used. According to our results, the use of IL-6 levels as a predictive tool has a diagnostic performance similar to that initially attributed to other peripheral blood markers since adopted in clinical medicine. For instance, the validation study that followed a preliminary report describing the use of the plasma d-dimer test to exclude pulmonary embolism reported a sensitivity and specificity of 98% and 39%, respectively (14,15). More recently, the B-type natriuretic peptide used to predict the presence or absence of congestive heart failure showed a sensitivity of 90% and a specificity of 76% (16).
From a methodologic perspective, it is essential that a new clinical test or rule be validated in a different cohort of participants from that in which it was derived in order to obtain a realistic estimate of its diagnostic performance (17,18). No noninvasive clinical assay currently meets such requirement for the diagnosis of acute rejection in renal transplantation. In their landmark paper, Li et al. (19) described how the measurement of messenger RNA (mRNA) for perforin and granzyme B in the urine had a sensitivity/specificity of 83%/83% and 79%/77%, respectively for the prediction of acute rejection. In a recent review, Hartono et al. cited 23 studies, with sample sizes ranging from 15 to 177 patients, that evaluated the accuracy of various noninvasive assays, predominantly mRNA profiles assays based on urinary or peripheral blood cells, in predicting acute rejection (20). Of note, all but one of the studies presented results based on the whole cohort of patients recruited, in the absence of internal or external validation. Using a urinary peptide biomarker panel, Ling et al. recently reported a sensitivity of 80% and specificity of 83% in detecting acute rejection in a test set of 24 participants; the main caveat was that the nonrejectors were patients with stable renal function or patients with BK nephropathy, a control group relevant for research but not for clinical application of the test (21).
That the levels of the candidate cytokines secreted by PBMCs correlate strongly with each other suggests a plausible biologic connection between these inflammatory markers and the allograft rejection process. Furthermore, it explains why a combination of cytokines was not superior to IL-6 alone in predicting acute rejection. From a statistical perspective, the correlation between predictors is known as collinearity, which means that predictors share the same information about a given outcome. The main consequence of collinearity is to produce numeric instability in the prediction model, which may lead to inaccurate statistical inference (22). Whenever possible, it is preferable to limit the numbers of predictors to a minimum in order to develop a robust clinical decision rule.
Post hoc multivariate analysis showed a strong association between IL-6 levels and rejection status after adjustment for clinical factors. In addition, levels of IL-6 were higher in patients with antibody-mediated rejection than in those with acute cellular rejection; however, this association was not statistically significant because the study was not powered to examine the difference in cytokine levels between subtypes of rejectors. Acute allograft rejection is a T- or B-cell–mediated process associated with a stereotyped, inflammatory response involving cells of the adaptive and innate immune systems (23–25). Experiments in animal models showed that although acute rejection is initiated by T cells, the damage to the allograft parenchyma appears concurrent with monocyte recruitment and massive inflammation (26). Because monocytes are known to secrete inflammatory cytokines, notably IL-1β, IL-6, and TNF-α, in renal transplant recipients (8), it follows that the inflammatory process occurring during an acute rejection episode would translate into measurable changes in the peripheral blood.
The major clinical interest of a noninvasive test for acute transplant rejection clearly lies more in its ability to exclude than to confirm rejection (4). Although this study was not designed to conduct a bayesian analysis evaluating a pretest and post-test probability of acute rejection, this assay is likely to be most useful where the pretest probability of rejection is low, especially when the differential diagnosis includes transient causes of graft dysfunction, such as dehydration or acute tubular injury. In such instances, a negative test result would reinforce a clinical decision to pursue supportive therapy with repeat testing within a few days. This is in agreement with a recent report in cardiac transplantation, which indicated that gene-expression profiling, performed on a single blood test, was not inferior to endomyocardial biopsy in monitoring for allograft rejection (4). In this study, patients in the noninvasive group underwent six-fold fewer biopsies per person-year than did those in the standard group. The cellular assay described here has the potential to similarly reduce the requirement for renal allograft biopsies while using simpler laboratory methods.
As previously mentioned, a further potential benefit of such a reliable, noninvasive test would be the facilitation of repeated, longitudinal monitoring of the allograft (3,27). The increase in the prevalence of subclinical rejection over time, combined with the strong negative effect of late acute rejection episodes on graft survival, suggest that a more aggressive strategy to monitor renal transplant recipients for late acute rejection might enhance long-term outcomes, which have improved little in the last decade (2,28,29). This is especially true in the context of a growing proportion of kidney transplant recipients now followed up in satellite clinics, which often lack the facilities to perform onsite allograft biopsies (30).
Our study has obvious limitations. Although the study was adequately powered, the sample size is small. The analysis is strengthened by the study design, which included a training cohort and internal validation cohort comprising patients from two centers. One important limitation described for high-throughput technologies is the batch effect, a bias introduced by systematic differences between the different single experiments, affecting the reproducibility of the assay (31). The Luminex platform used here to measure cytokines evaluates a negligible number of variables compared with the thousands of variables measured by other platforms (such as gene arrays or proteomic analysis); however, we still accounted for potential bias due to this effect by showing a persistent relationship between IL-6 levels and rejection status in multivariate analysis where the experimental batch was included as a covariate.
In conclusion, the results of this pilot study indicate that measurement of IL-6 levels after overnight PBMC incubation under resting, nonstimulated conditions can discriminate acute rejectors from non–acute rejectors in patients presenting with acute allograft dysfunction. The obvious strength of this simple test is that, in contrast to more complex assays, it could easily be implemented in the setting of a tissue-typing laboratory using technologies already available and provide a decisive advantage in the daily management of renal transplant recipients, particularly those attending remote or satellite units. These findings now need to be confirmed in a larger, prospective trial.
Disclosures
None.
Acknowledgments
We thank all the patients who participated in the study.
S.A.D.S. is the recipient of a Kidney Research Scientist Core Education and National Training (KRESCENT) Program Post-Doctoral Fellowship Award from Kidney Foundation of Canada and a McLaughlin’s Dean Scholarship from Université Laval. A.C. and N.N. received support from the Clinical Trials in Organ Transplantation cooperative research program through the National Institutes of Health Grant U01 AI 063623.
The data reported within this paper were presented in an oral communication at the American Transplant Congress, May 3, 2011, Philadelphia, Pennsylvania.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11051011/-/DCSupplemental.
References
- 1.Nankivell BJ, Alexander SI: Rejection of the kidney allograft. N Engl J Med 363: 1451–1462, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B: Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 4: 378–383, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Opelz G, Döhler B, Collaborative Transplant Study Report : Influence of time of rejection on long-term graft survival in renal transplantation. Transplantation 85: 661–666, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Pham MX, Teuteberg JJ, Kfoury AG, Starling RC, Deng MC, Cappola TP, Kao A, Anderson AS, Cotts WG, Ewald GA, Baran DA, Bogaev RC, Elashoff B, Baron H, Yee J, Valantine HA, IMAGE Study Group : Gene-expression profiling for rejection surveillance after cardiac transplantation. N Engl J Med 362: 1890–1900, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Anglicheau D, Suthanthiran M: Noninvasive prediction of organ graft rejection and outcome using gene expression patterns. Transplantation 86: 192–199, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Boogaardt DE, van Miert PP, de Vaal YJ, de Fijter JW, Claas FH, Roelen DL: The ratio of interferon-gamma and interleukin-10 producing donor-specific cells as an in vitro monitoring tool for renal transplant patients. Transplantation 82: 844–848, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Najafian N, Salama AD, Fedoseyeva EV, Benichou G, Sayegh MH: Enzyme-linked immunosorbent spot assay analysis of peripheral blood lymphocyte reactivity to donor HLA-DR peptides: Potential novel assay for prediction of outcomes for renal transplant recipients. J Am Soc Nephrol 13: 252–259, 2002 [DOI] [PubMed] [Google Scholar]
- 8.De Serres SA, Vadivel N, Mfarrej BG, Grafals M, DeJoseph M, Dyer C, Magee CN, Chandraker A, Gallon LG, Najafian N: Monocyte-secreted inflammatory cytokines are associated with transplant glomerulopathy in renal allograft recipients. Transplantation 91: 552–559, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Poggio ED, Augustine JJ, Clemente M, Danzig JM, Volokh N, Zand MS, Hricik DE, Heeger PS: Pretransplant cellular alloimmunity as assessed by a panel of reactive T cells assay correlates with acute renal graft rejection. Transplantation 83: 847–852, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y: The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713–723, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M: Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant 8: 753–760, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Hanley JA, McNeil BJ: The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143: 29–36, 1982 [DOI] [PubMed] [Google Scholar]
- 13.Kikić Z, Regele H, Nordmeyer V, Wahrmann M, Kletzmayr J, Bartel G, Böhmig GA: Significance of peritubular capillary, glomerular, and arteriolar C4d staining patterns in paraffin sections of early kidney transplant biopsies. Transplantation 91: 440–446, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Bounameaux H, Cirafici P, de Moerloose P, Schneider PA, Slosman D, Reber G, Unger PF: Measurement of D-dimer in plasma as diagnostic aid in suspected pulmonary embolism. Lancet 337: 196–200, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Bounameaux H, Slosman D, de Moerloose P, Reber G: Diagnostic value of plasma D-dimer in suspected pulmonary embolism. Lancet 2: 628–629, 1988 [DOI] [PubMed] [Google Scholar]
- 16.Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA, Breathing Not Properly Multinational Study Investigators : Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 347: 161–167, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Laupacis A, Sekar N, Stiell IG: Clinical prediction rules. A review and suggested modifications of methodological standards. JAMA 277: 488–494, 1997 [PubMed] [Google Scholar]
- 18.Altman DG, Royston P: What do we mean by validating a prognostic model? Stat Med 19: 453–473, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Li B, Hartono C, Ding R, Sharma VK, Ramaswamy R, Qian B, Serur D, Mouradian J, Schwartz JE, Suthanthiran M: Noninvasive diagnosis of renal-allograft rejection by measurement of messenger RNA for perforin and granzyme B in urine. N Engl J Med 344: 947–954, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Hartono C, Muthukumar T, Suthanthiran M: Noninvasive diagnosis of acute rejection of renal allografts. Curr Opin Organ Transplant 15: 35–41, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ling XB, Sigdel TK, Lau K, Ying L, Lau I, Schilling J, Sarwal MM: Integrative urinary peptidomics in renal transplantation identifies biomarkers for acute rejection. J Am Soc Nephrol 21: 646–653, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleinbaum DG: Applied Regression Analysis and Other Multivariable Methods, Belmont, CA, Brooks/Cole, 2007 [Google Scholar]
- 23.Halloran PF: T cell-mediated rejection of kidney transplants: A personal viewpoint. Am J Transplant 10: 1126–1134, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Girlanda R, Kleiner DE, Duan Z, Ford EA, Wright EC, Mannon RB, Kirk AD: Monocyte infiltration and kidney allograft dysfunction during acute rejection. Am J Transplant 8: 600–607, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colvin RB: Antibody-mediated renal allograft rejection: Diagnosis and pathogenesis. J Am Soc Nephrol 18: 1046–1056, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Einecke G, Mengel M, Hidalgo L, Allanach K, Famulski KS, Halloran PF: The early course of kidney allograft rejection: Defining the time when rejection begins. Am J Transplant 9: 483–493, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Rush D: Can protocol biopsy better inform our choices in renal transplantation? Transplant Proc 41[Suppl]: S6–S8, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Rush D, Arlen D, Boucher A, Busque S, Cockfield SM, Girardin C, Knoll G, Lachance JG, Landsberg D, Shapiro J, Shoker A, Yilmaz S: Lack of benefit of early protocol biopsies in renal transplant patients receiving TAC and MMF: A randomized study. Am J Transplant 7: 2538–2545, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Lamb KE, Lodhi S, Meier-Kriesche HU: Long-term renal allograft survival in the United States: A critical reappraisal. Am J Transplant 11: 450–462, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Keough-Ryan TM, Prasad GV, Hewlett T, Shapiro RJ, Canadian Community Nephrology Study Group : Similar outcomes for Canadian renal transplant recipients followed up in transplant centers and satellite clinics. Transplantation 90: 591–596, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Luo J, Schumacher M, Scherer A, Sanoudou D, Megherbi D, Davison T, Shi T, Tong W, Shi L, Hong H, Zhao C, Elloumi F, Shi W, Thomas R, Lin S, Tillinghast G, Liu G, Zhou Y, Herman D, Li Y, Deng Y, Fang H, Bushel P, Woods M, Zhang J: A comparison of batch effect removal methods for enhancement of prediction performance using MAQC-II microarray gene expression data. Pharmacogenomics J 10: 278–291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]